Abstract
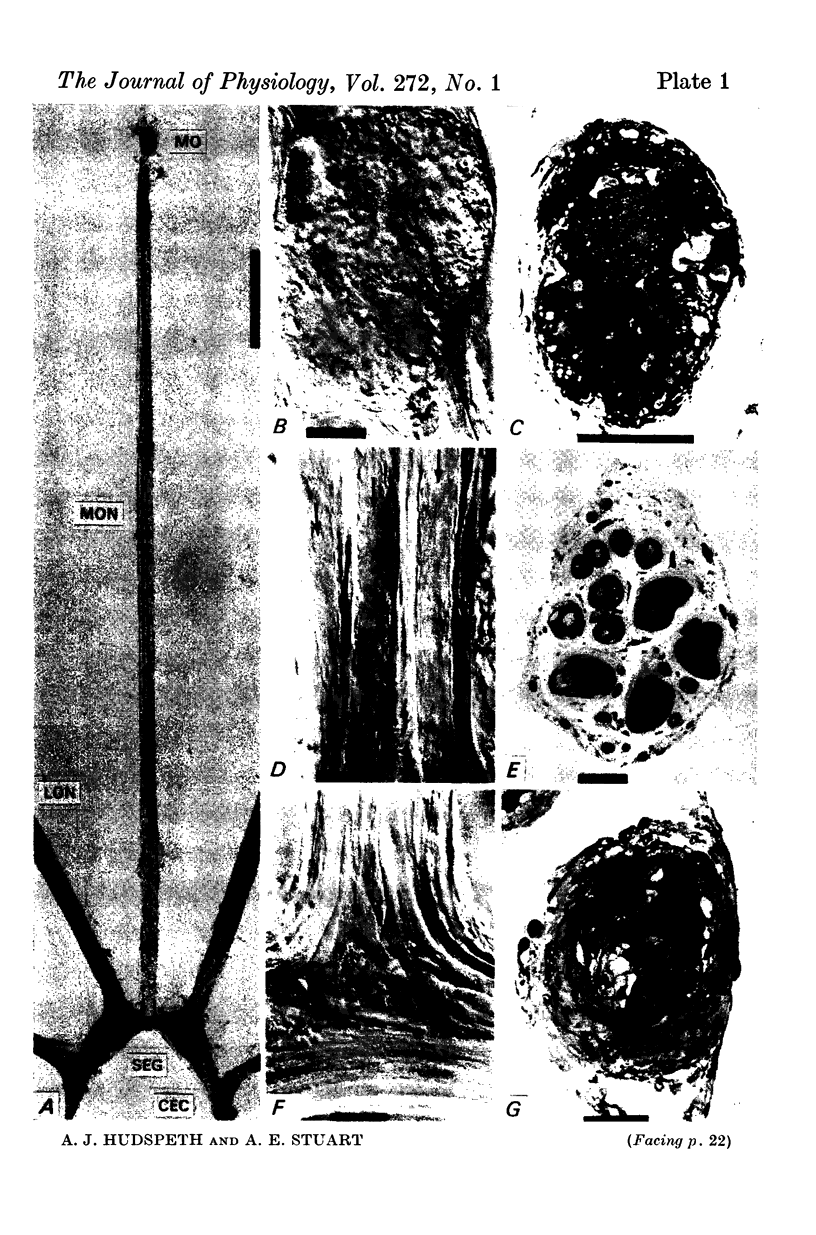
1. The median eye of the giant barnacle, B. nubilus, comprises four large photoreceptor neurones which are visible under the dissecting microscope for almost their entire length. We have studied the structure of, and the responses to light recorded in, the somata, axons, and terminal regions of these neurones.
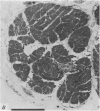
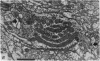
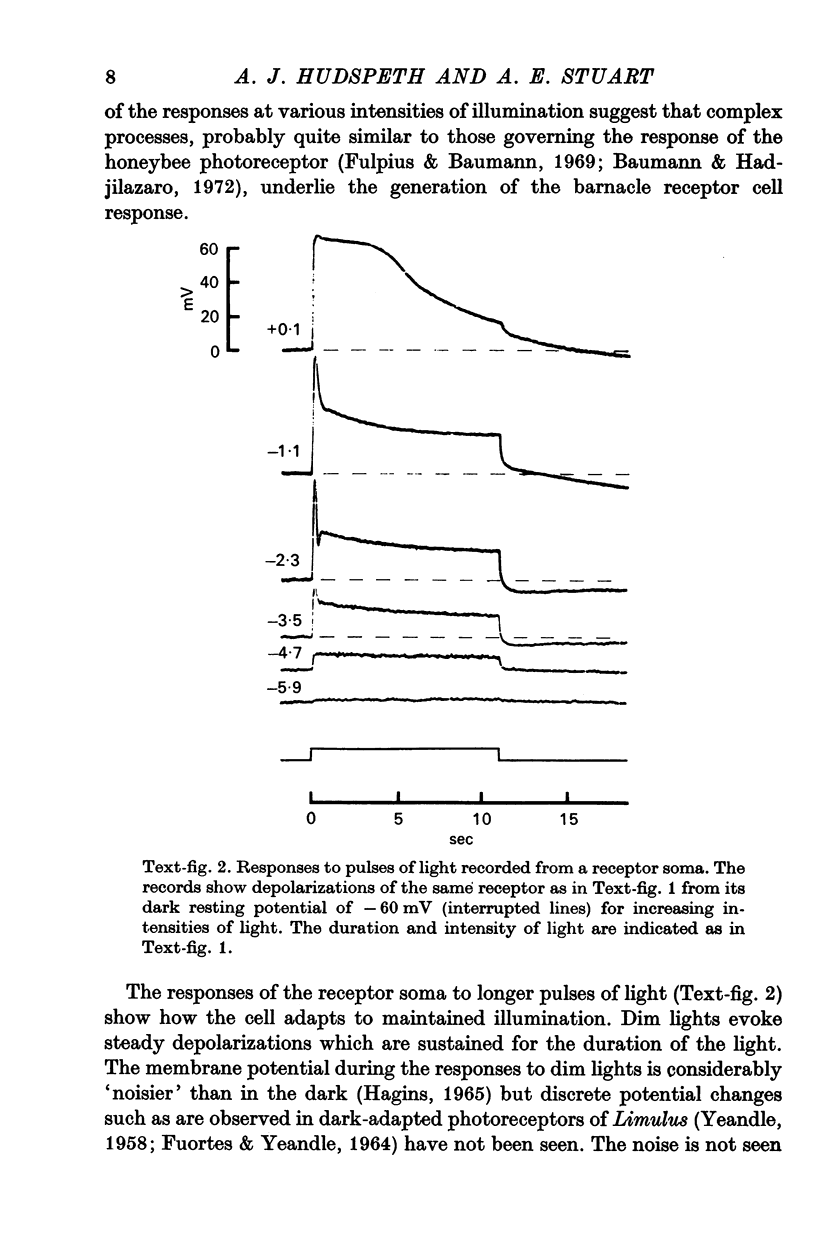
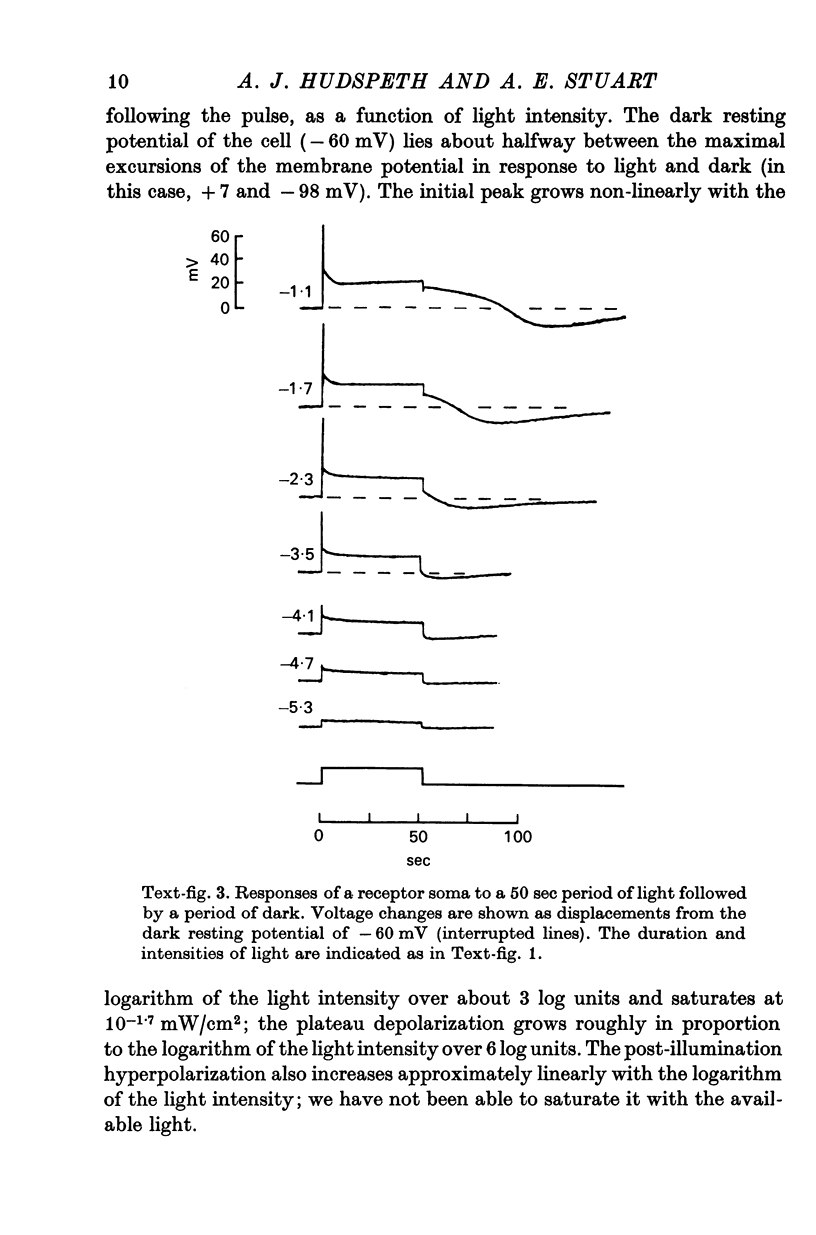
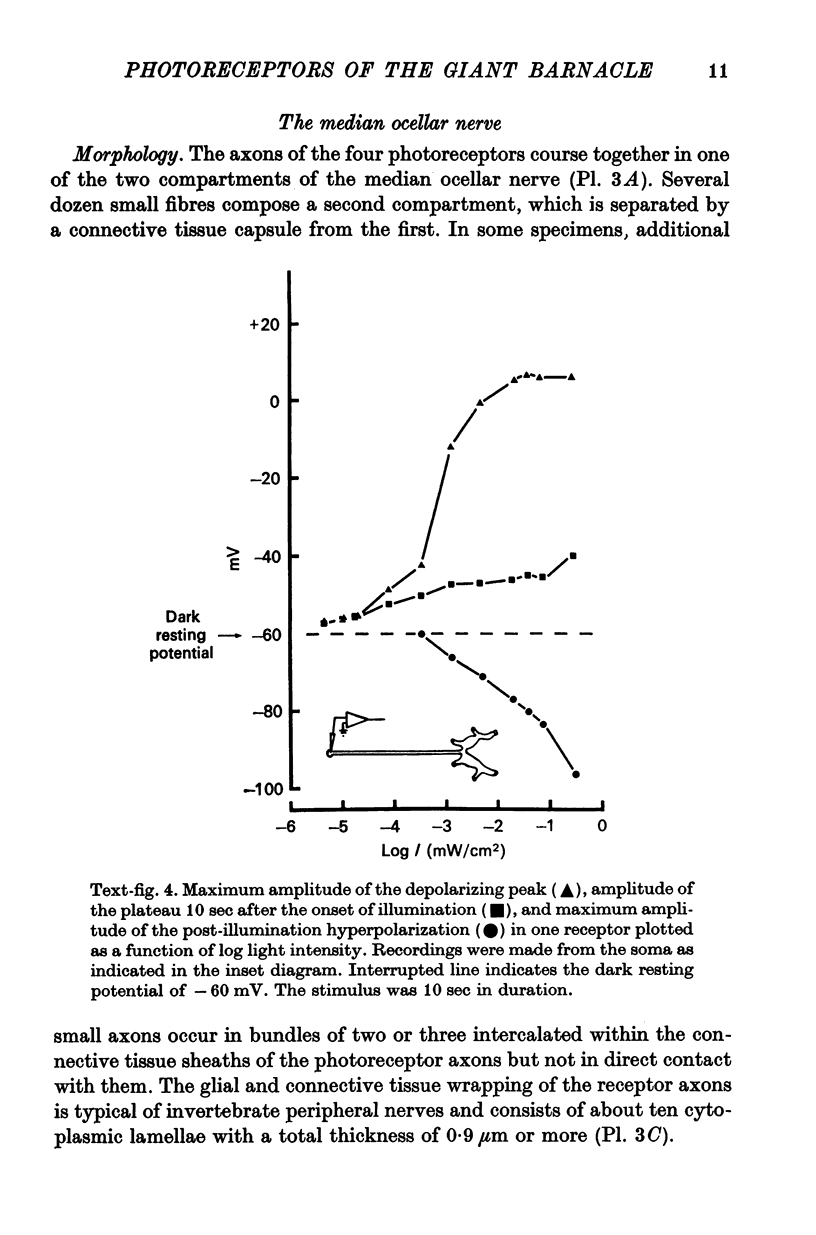
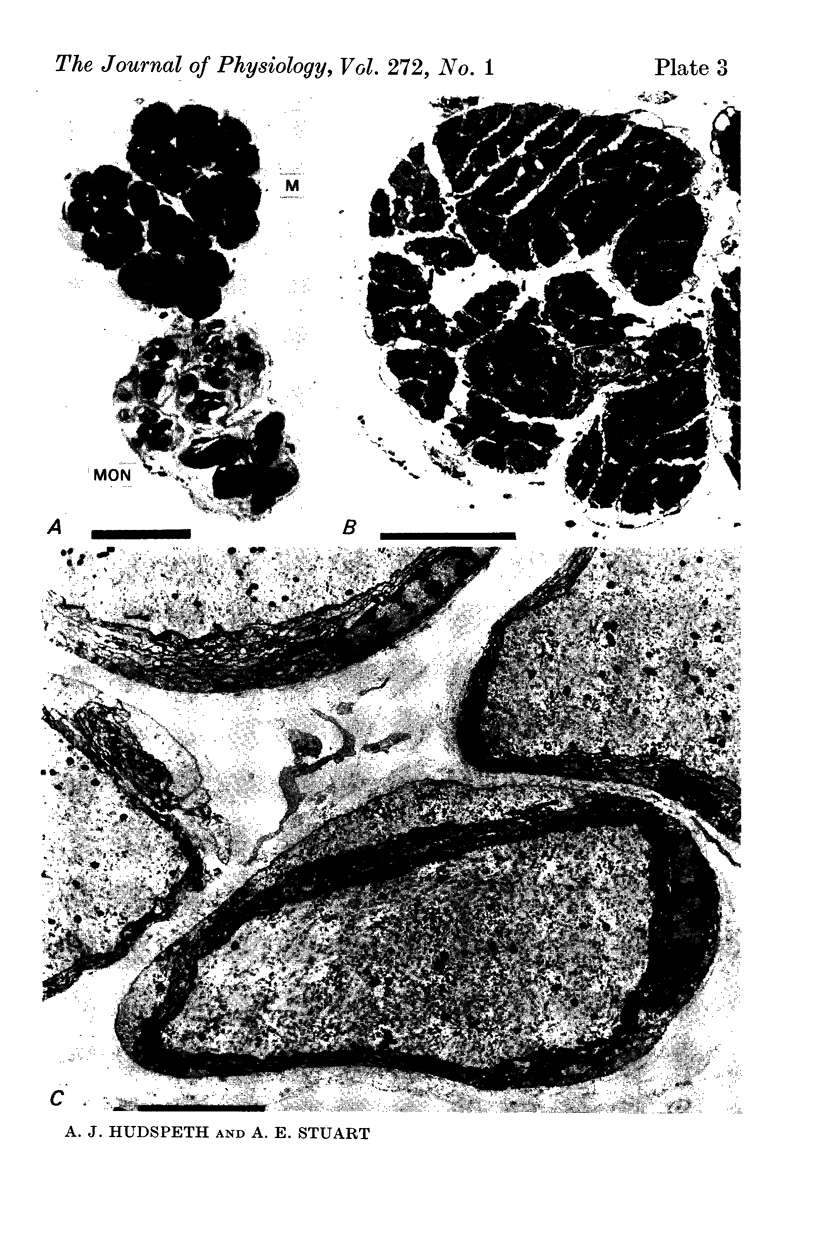
2. The photoreceptor somata, each 40-70 μm in diameter, extend numerous light-sensitive dendritic processes whose membranes form rhabdomeric microvilli. Recordings from the soma show that dim light evokes a steady, noisy depolarization; brighter light elicits a transient depolarization which decays to a maintained plateau, followed by a hyperpolarization when the light is turned off.
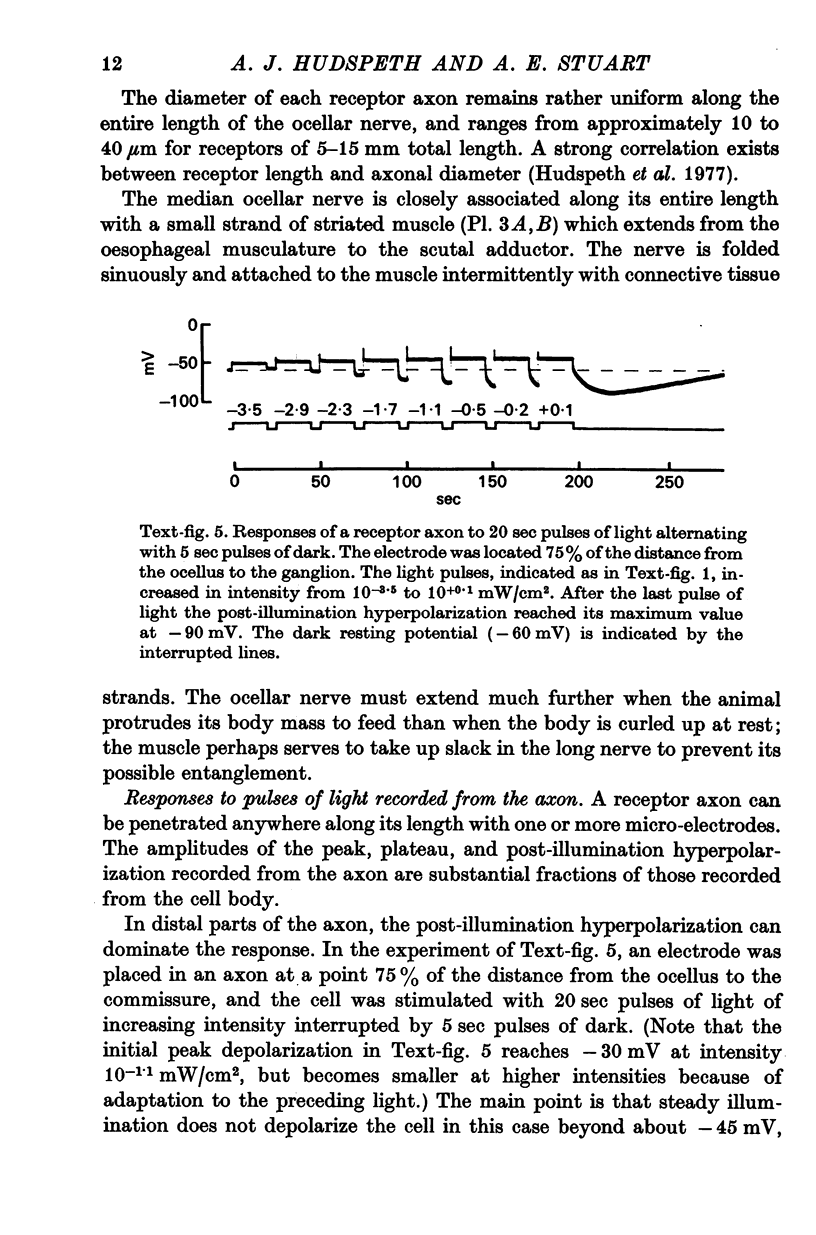
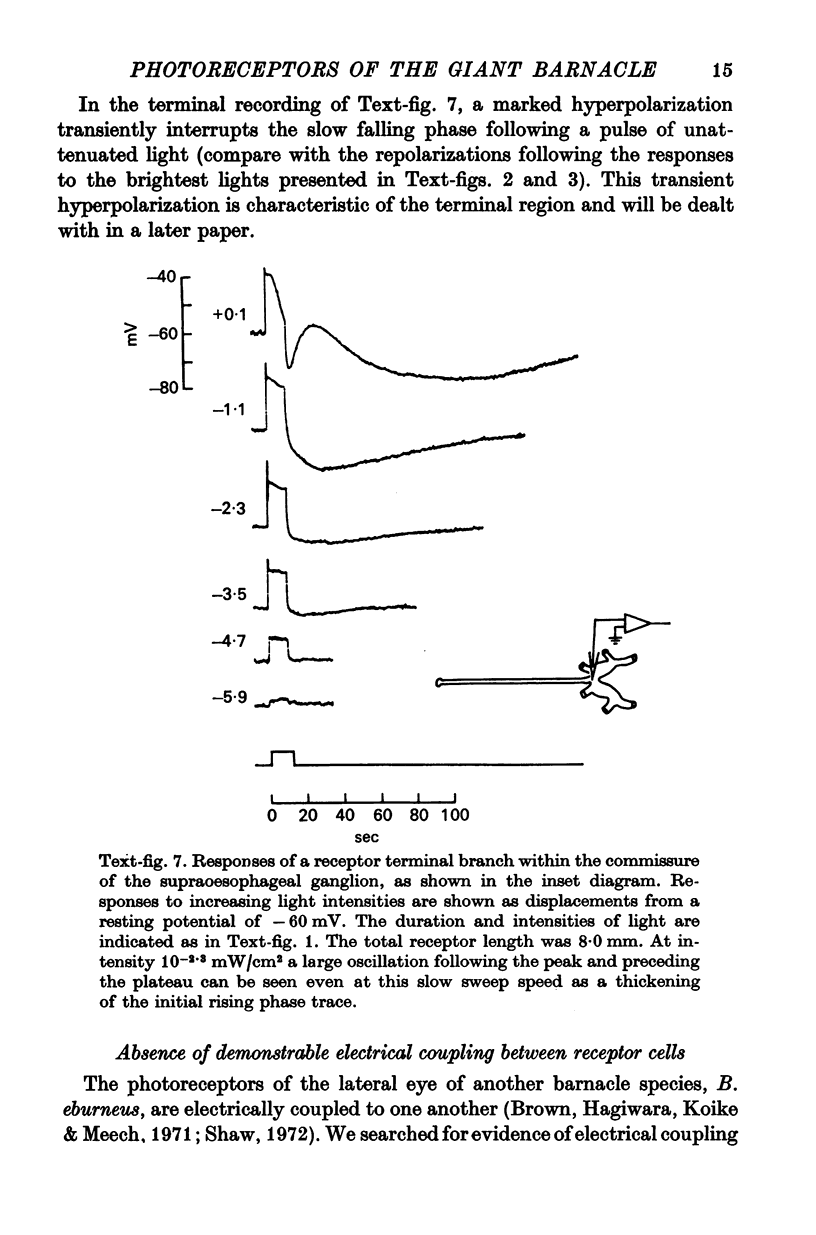
3. Light-induced voltage changes spread decrementally along the photoreceptor axons, which average 10 mm in length and 25 μm in diameter. In distal parts of the axon, near the presynaptic terminals, depolarizations and hyperpolarizations can be as large as 50% or more of their values in the soma.
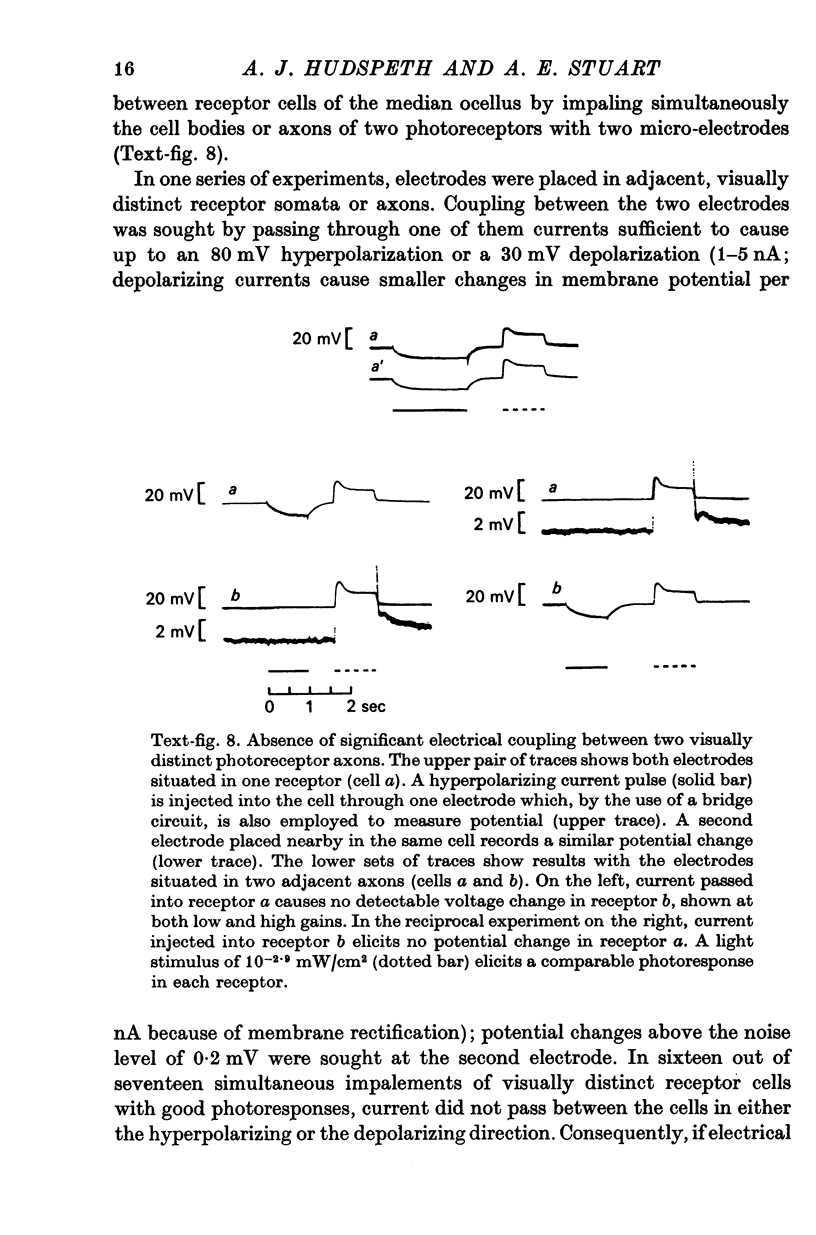
4. There is no demonstrable electrical coupling between photoreceptor neurones as shown by simultaneous recordings from two receptor somata or axons.
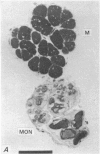
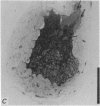
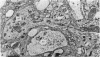
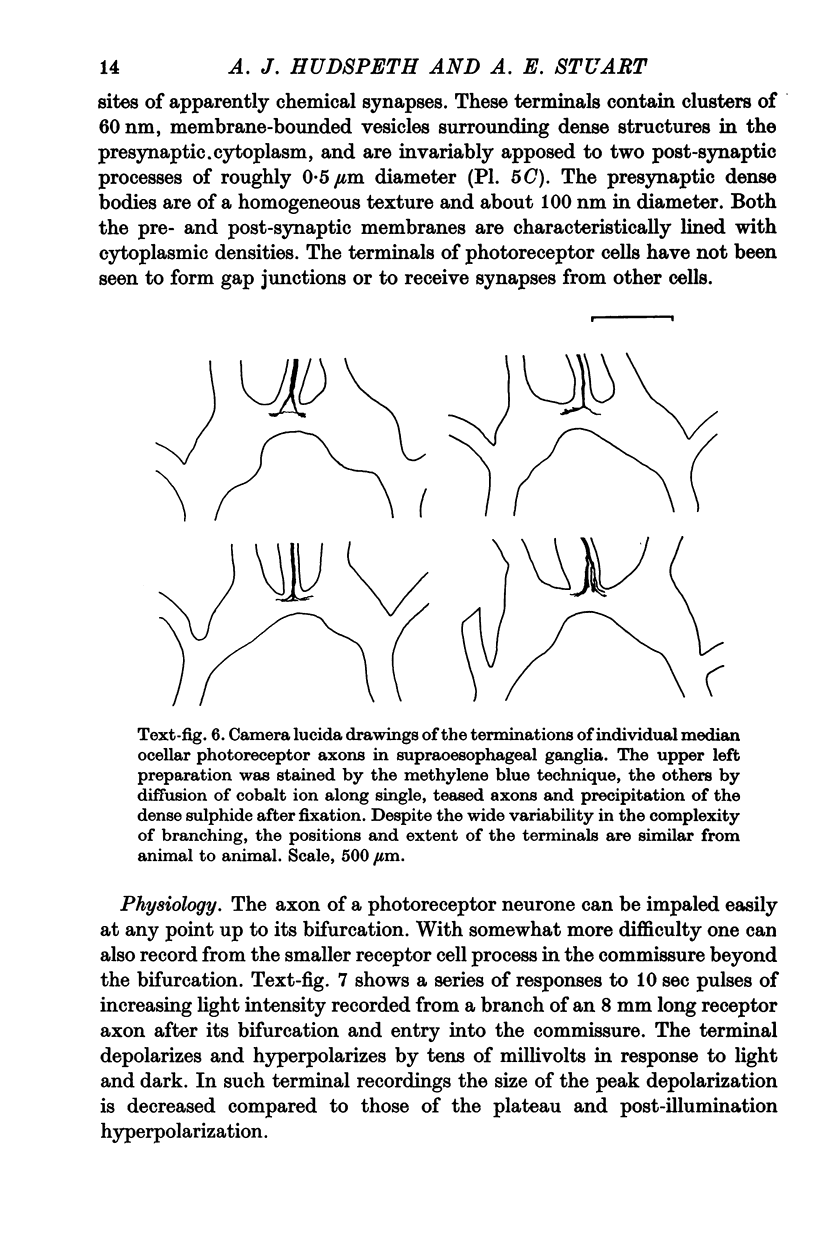
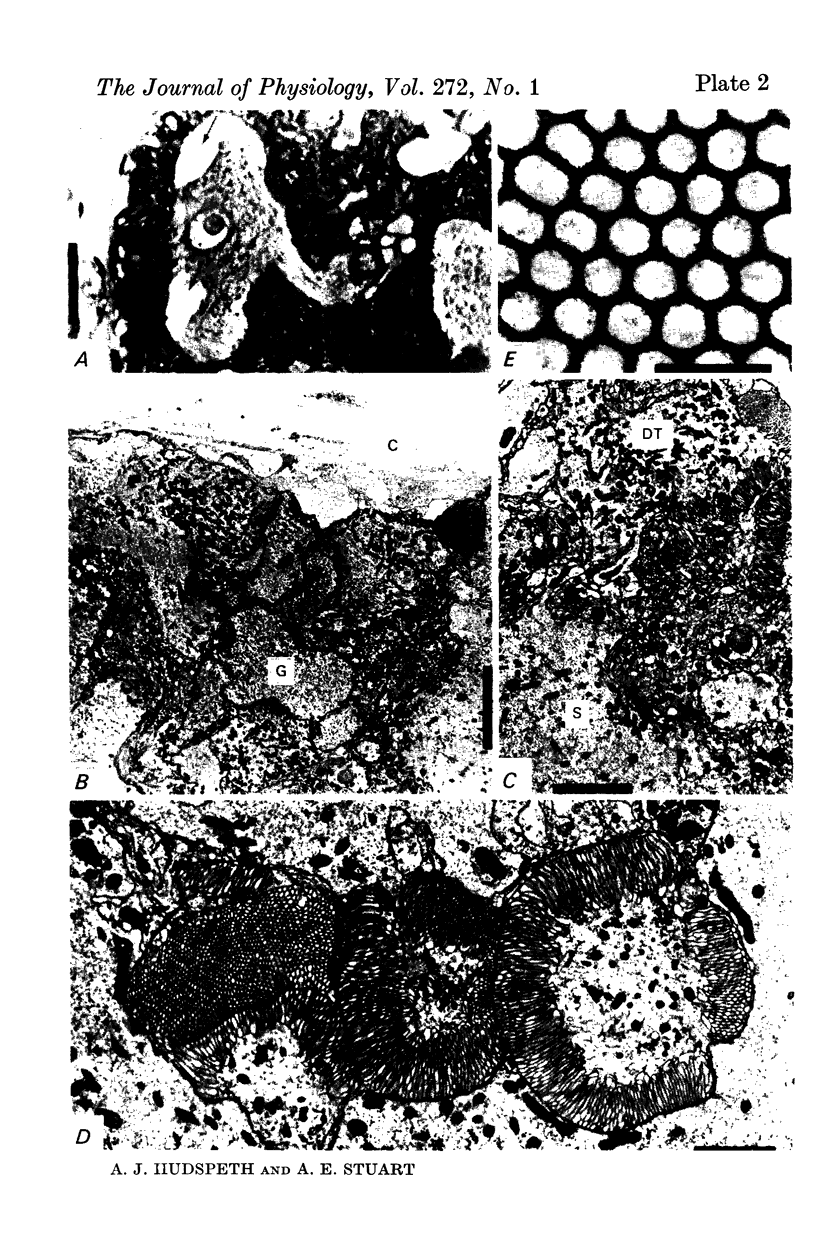
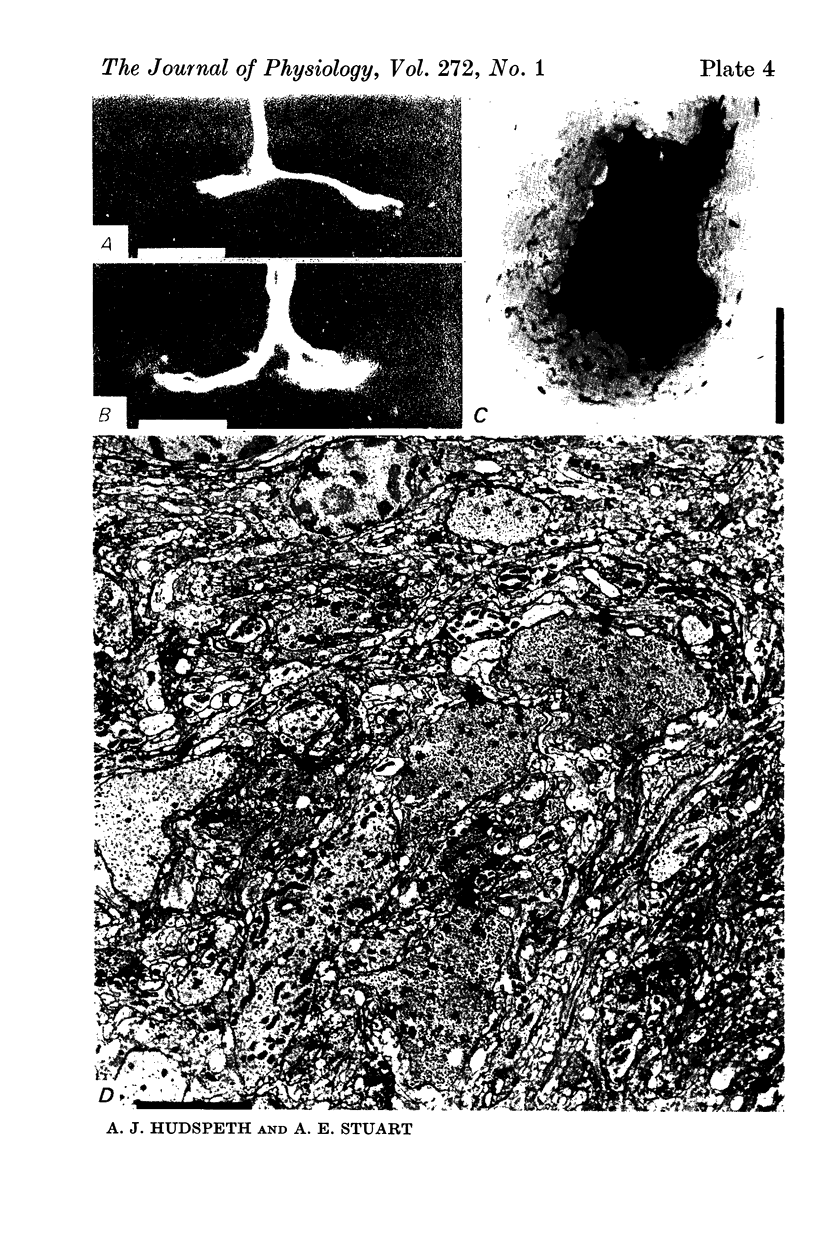
5. Each photoreceptor axon enters the mid line commissure of the supraoesophageal ganglion, bifurcates, and arborizes in a restricted zone of neuropil in each hemiganglion. The large size of the terminal processes of these neurones and their characteristic cytoplasmic inclusions enable one to trace them with the electron microscope as they branch in the neuropil.
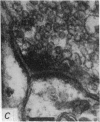
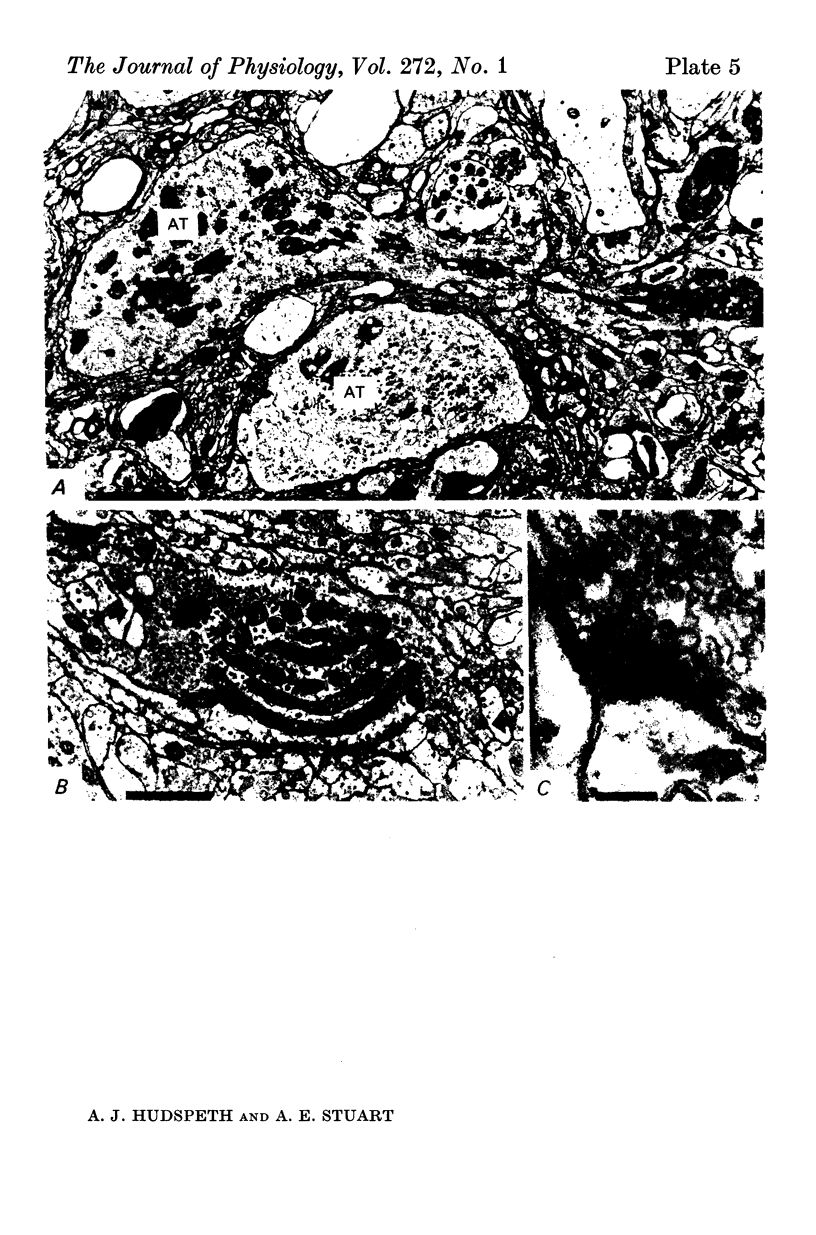
6. The terminal processes subdivide and end in 1-3 μm diameter branches which are the sites of apparently chemical synapses. Vesicle-containing, presynaptic loci on these processes of the receptor cell are invariably apposed to two post-synaptic processes from cells as yet unidentified.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C., Baumann F., Bertrand D. Role of intracellular calcium and sodium in light adaptation in the retina of the honey bee drone (Apis mellifera, L). J Gen Physiol. 1976 Apr;67(4):475–491. doi: 10.1085/jgp.67.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann F., Hadjilazaro B. A depolarizing aftereffect of intense light in the drone visual receptor. Vision Res. 1972 Jan;12(1):17–31. doi: 10.1016/0042-6989(72)90134-4. [DOI] [PubMed] [Google Scholar]
- Baumann F. Slow and spike potentials recorded from retinula cells of the honeybee drone in response to light. J Gen Physiol. 1968 Dec;52(6):855–875. doi: 10.1085/jgp.52.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsellino A., Fuortes M. G., Smith T. G. Visual responses in Limulus. Cold Spring Harb Symp Quant Biol. 1965;30:429–443. doi: 10.1101/sqb.1965.030.01.042. [DOI] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Hagiwara S., Koike H., Meech R. W. Electrical characteristics of a barnacle photoreceptor. Fed Proc. 1971 Jan-Feb;30(1):69–78. [PubMed] [Google Scholar]
- Brown J. E., Lisman J. E. An electrogenic sodium pump in Limulus ventral photoreceptor cells. J Gen Physiol. 1972 Jun;59(6):720–733. doi: 10.1085/jgp.59.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell R. L., Dowling J. E. Neural organization of the median ocellus of the dragonfly. I. Intracellular electrical activity. J Gen Physiol. 1972 Aug;60(2):121–147. doi: 10.1085/jgp.60.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Coupling between rod photoreceptors in a vertebrate retina. Nature. 1976 Mar 4;260(5546):57–59. doi: 10.1038/260057a0. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Chappell R. L. Neural organization of the median ocellus of the dragonfly. II. Synaptic structure. J Gen Physiol. 1972 Aug;60(2):148–165. doi: 10.1085/jgp.60.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHRENBACK W. H. THE MICROMORPHOLOGY OF SOME SIMPLE PHOTORECEPTORS. Z Zellforsch Mikrosk Anat. 1965 Apr 8;66(2):233–254. [PubMed] [Google Scholar]
- FUORTES M. G., YEANDLE S. PROBABILITY OF OCCURRENCE OF DISCRETE POTENTIAL WAVES IN THE EYE OF LIMULUS. J Gen Physiol. 1964 Jan;47:443–463. doi: 10.1085/jgp.47.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulpius B., Baumann F. Effects of sodium, potassium, and calcium ions on slow and spike potentials in single photoreceptor cells. J Gen Physiol. 1969 May;53(5):541–561. doi: 10.1085/jgp.53.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwilliam G. F., Bradbury J. C. Activity patterns in the isolated central nervous system of the barnacle and their relation to behavior. Biol Bull. 1971 Dec;141(3):502–513. doi: 10.2307/1540264. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J., Poo M. M., Stuart A. E. Passive signal propagation and membrane properties in median photoreceptors of the giant barnacle. J Physiol. 1977 Oct;272(1):25–43. doi: 10.1113/jphysiol.1977.sp012032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H., Brown H. M., Hagiwara S. Hyperpolarization of a barnacle photoreceptor membrane following illumination. J Gen Physiol. 1971 Jun;57(6):723–737. doi: 10.1085/jgp.57.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. Light-induced changes of sensitivity in Limulus ventral photoreceptors. J Gen Physiol. 1975 Oct;66(4):473–488. doi: 10.1085/jgp.66.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulus ventral photoreceptors. J Gen Physiol. 1972 Jun;59(6):701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Kuffler S. W. Visual identification of synaptic boutons on living ganglion cells and of varicosities in postganglionic axons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):485–508. doi: 10.1098/rspb.1971.0044. [DOI] [PubMed] [Google Scholar]
- Muller K. J. Photoreceptors in the crayfish compound eye: electrical interactions between cells as related to polarized-light sensitivity. J Physiol. 1973 Aug;232(3):573–595. doi: 10.1113/jphysiol.1973.sp010286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKA K. I. Recording of retinal action potentials from single cells in the insect compound eye. J Gen Physiol. 1961 Jan;44:571–584. doi: 10.1085/jgp.44.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S., Hagiwara S., Nicolaysen K., Stuart A. E. Signal transmission from photoreceptors to ganglion cells in the visual system of the giant barnacle. Cold Spring Harb Symp Quant Biol. 1976;40:563–570. doi: 10.1101/sqb.1976.040.01.052. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of bipolar cells in the retina of the turtle. J Physiol. 1974 Jan;236(1):211–224. doi: 10.1113/jphysiol.1974.sp010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Rod-rod interaction in the retina of the turtle. J Physiol. 1975 Apr;246(3):617–638. doi: 10.1113/jphysiol.1975.sp010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S. R. Decremental conduction of the visual signal in barnacle lateral eye. J Physiol. 1972 Jan;220(1):145–175. doi: 10.1113/jphysiol.1972.sp009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S. R. Interreceptor coupling in ommatidia of drone honeybee and locust compound eyes. Vision Res. 1969 Sep;9(9):999–1029. doi: 10.1016/0042-6989(69)90044-3. [DOI] [PubMed] [Google Scholar]
- Trujillo-Cenóz O. Some aspects of the structural organization of the arthropod eye. Cold Spring Harb Symp Quant Biol. 1965;30:371–382. doi: 10.1101/sqb.1965.030.01.037. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]