Abstract
1. Properties of neurones in the trigeminal nuclei principalis and oralis responding to movements of facial sinus hairs were studied in cats anaesthetized by I. V. infusion of pentobarbitone.
2. Using electrophysiological methods trigeminal neurones were classified into primary afferent fibres, trigeminothalamic relay neurones, interneurones and other unspecified higher order neurones.
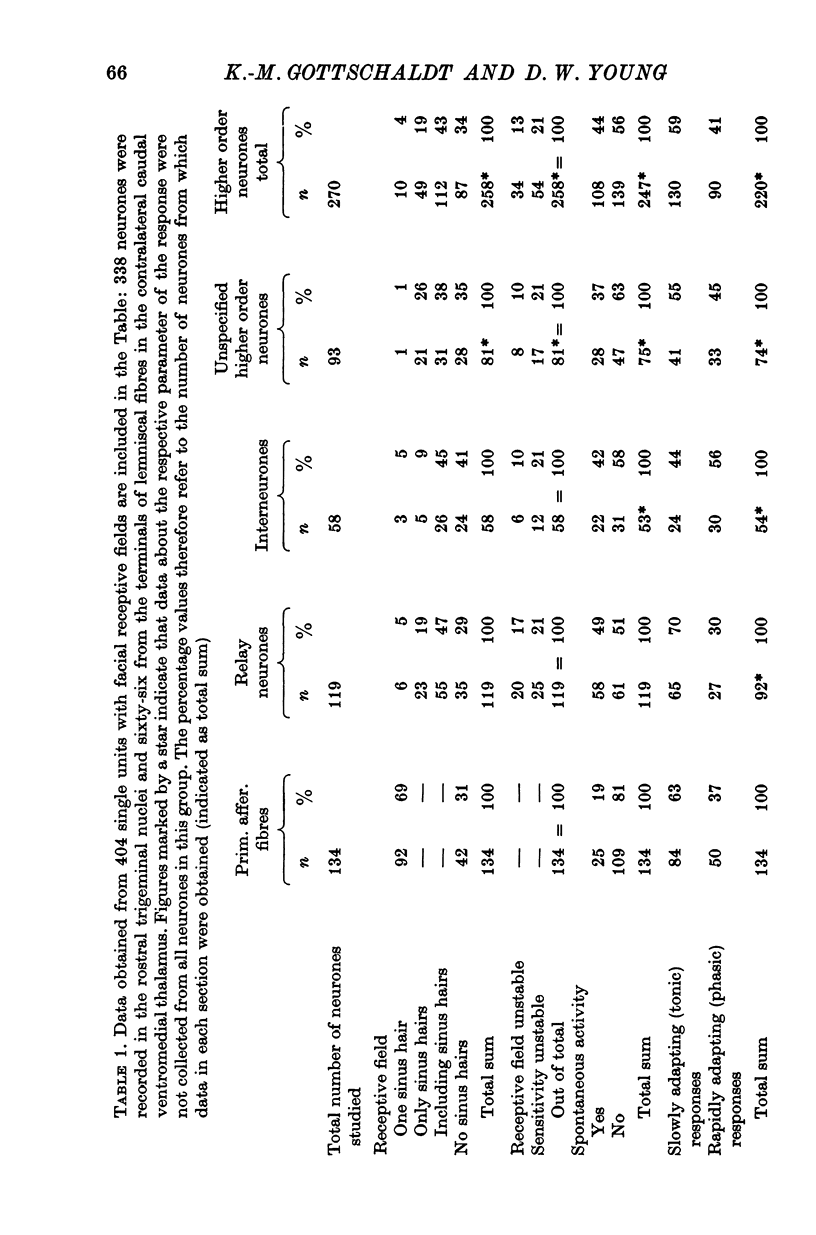
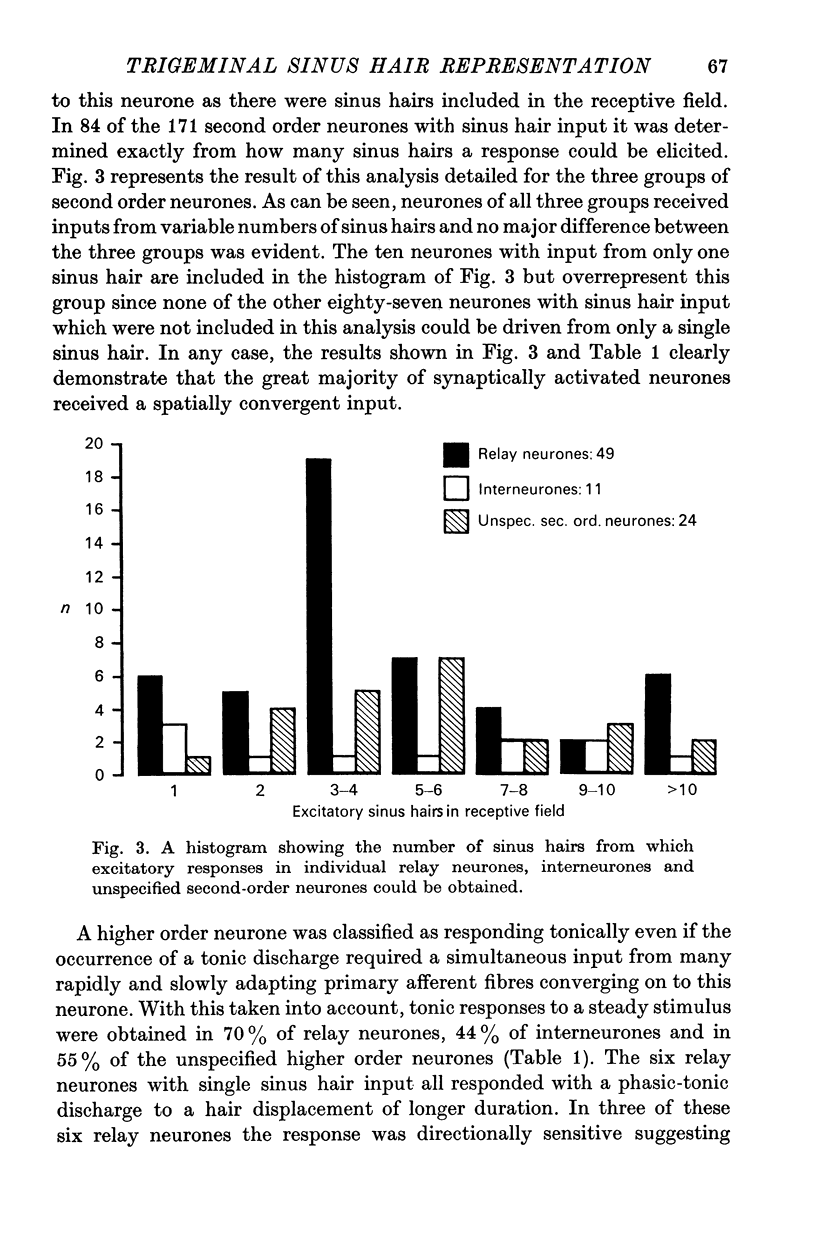
3. When receptive fields of synaptically activated neurones were compared with those of primary afferent fibres, an often extensive convergence from first order on to higher order neurones was established. Out of 119 relay neurones six received input from one sinus hair only. Spontaneous activity was encountered about twice as often in synaptically activated neurones than in primary afferent fibres.
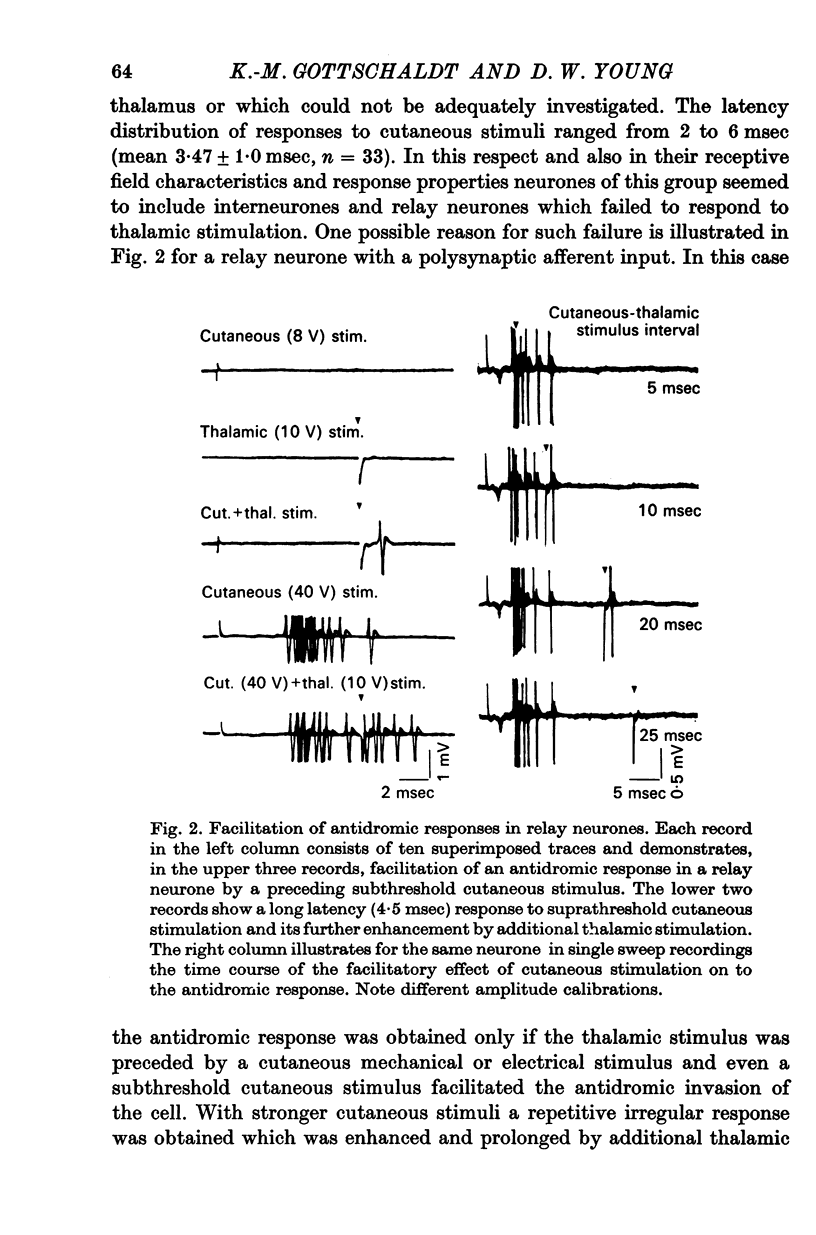
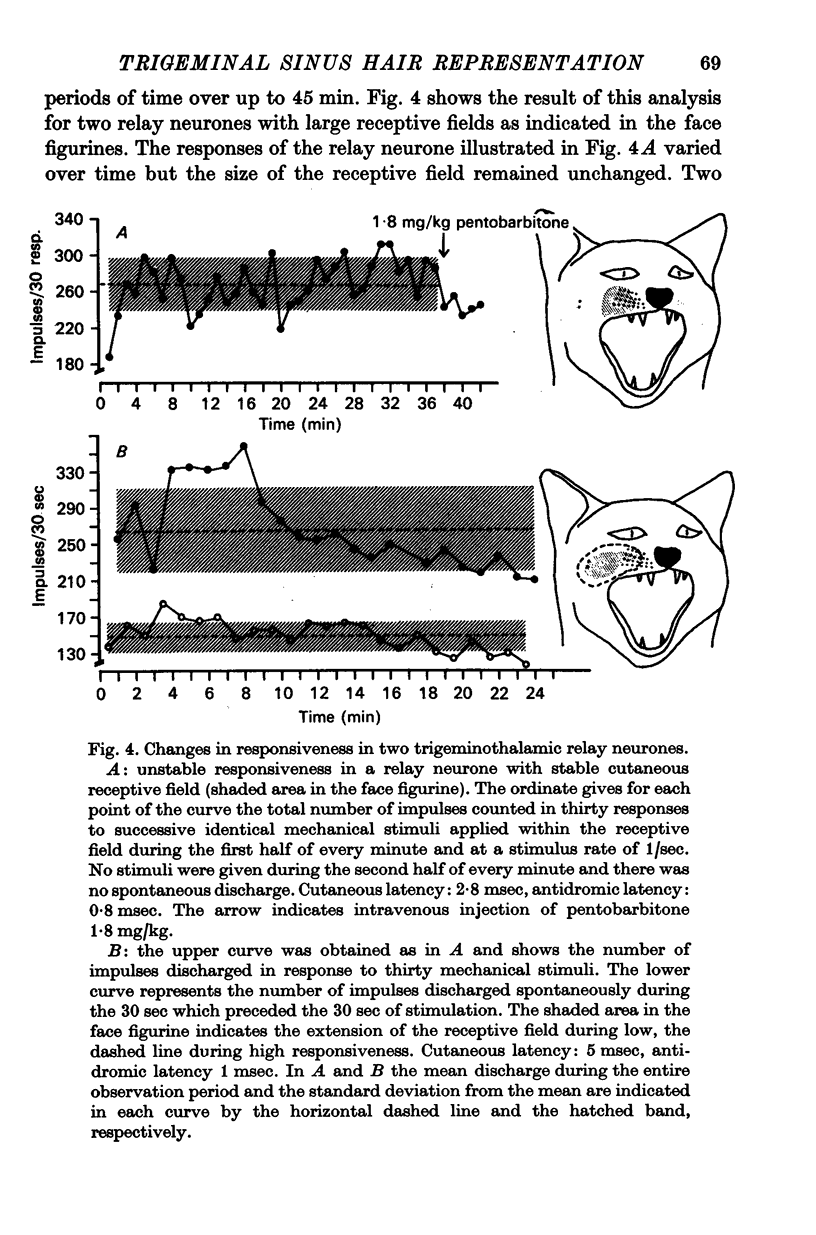
4. The responsiveness of single neurones was unstable over time in about one fifth of the population and then the total number of impulses discharged in successive responses could vary by as much as 500%. Unstable responsiveness occurred sometimes alone but was often accompanied by marked changes in the size or the configuration of the receptive field. Such instabilities were observed in all kinds of synaptically activated neurones but not in primary afferent fibres.
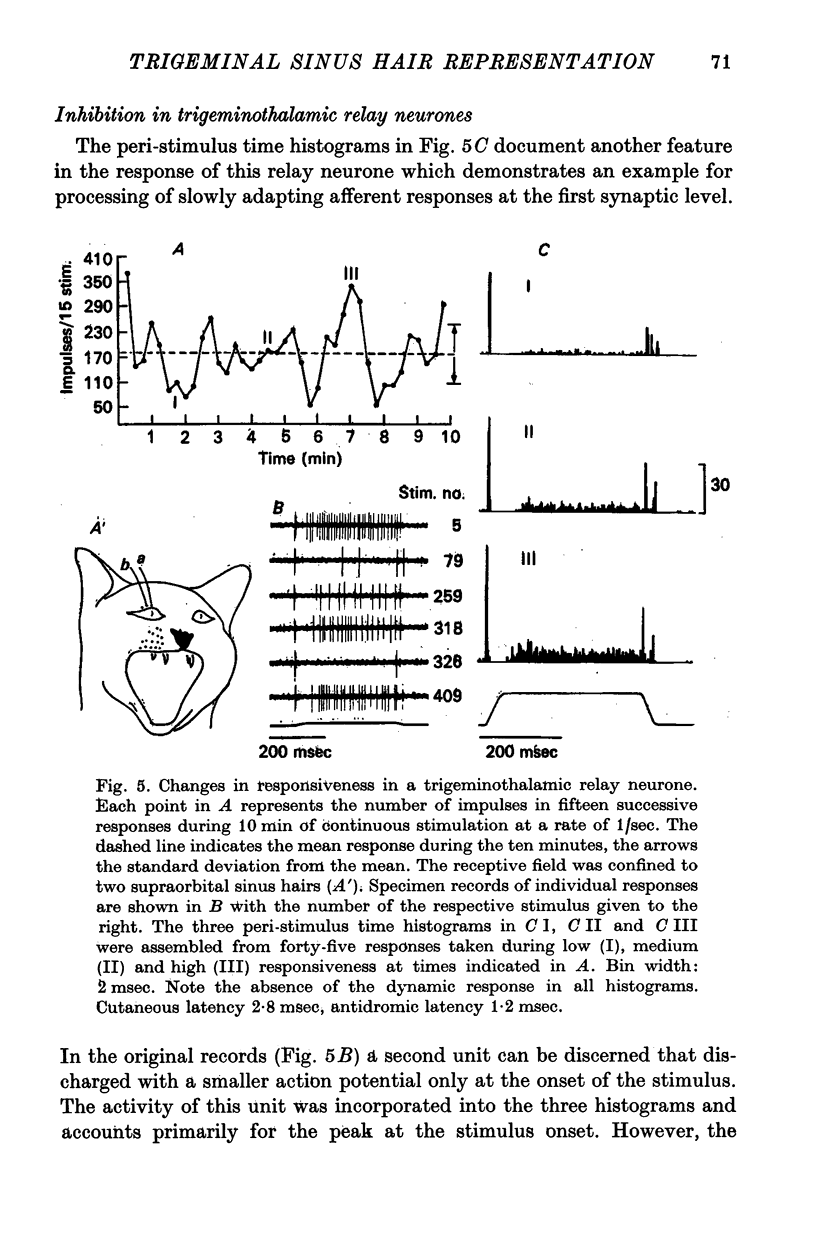
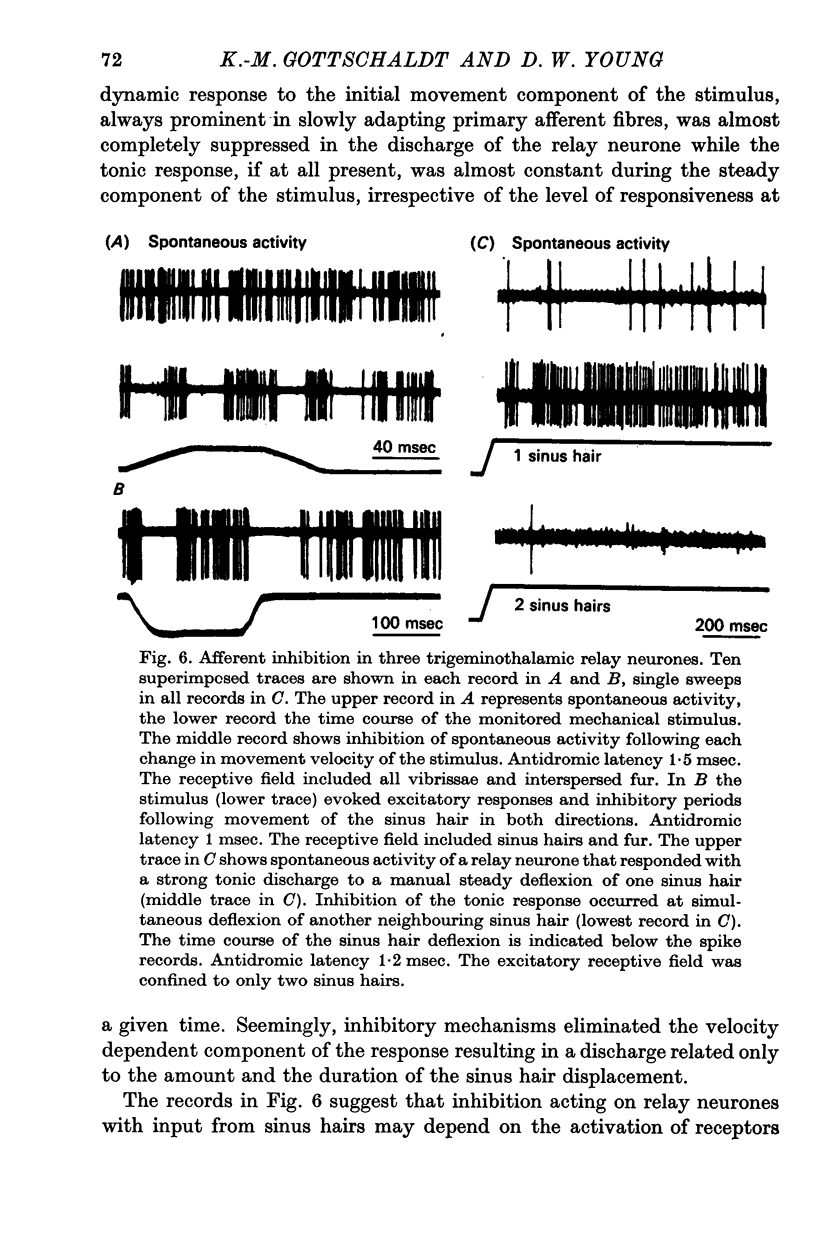
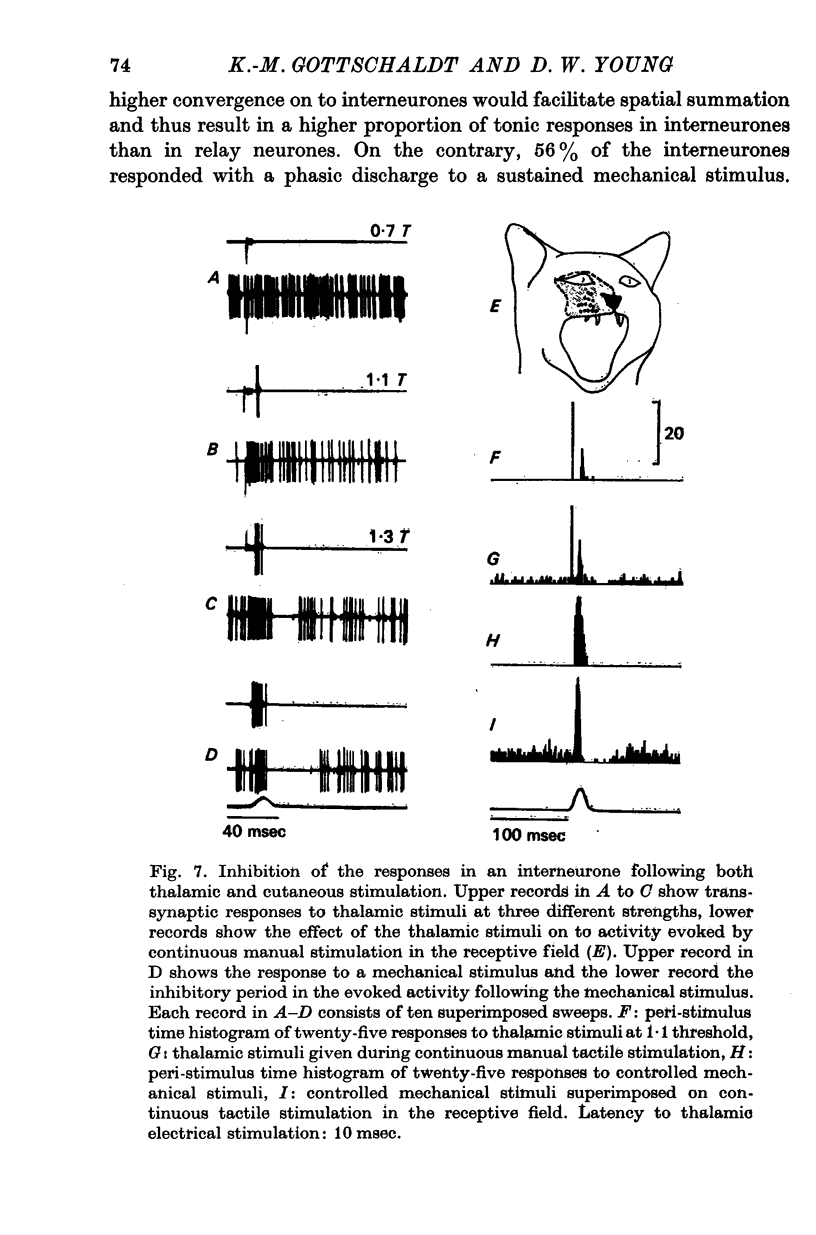
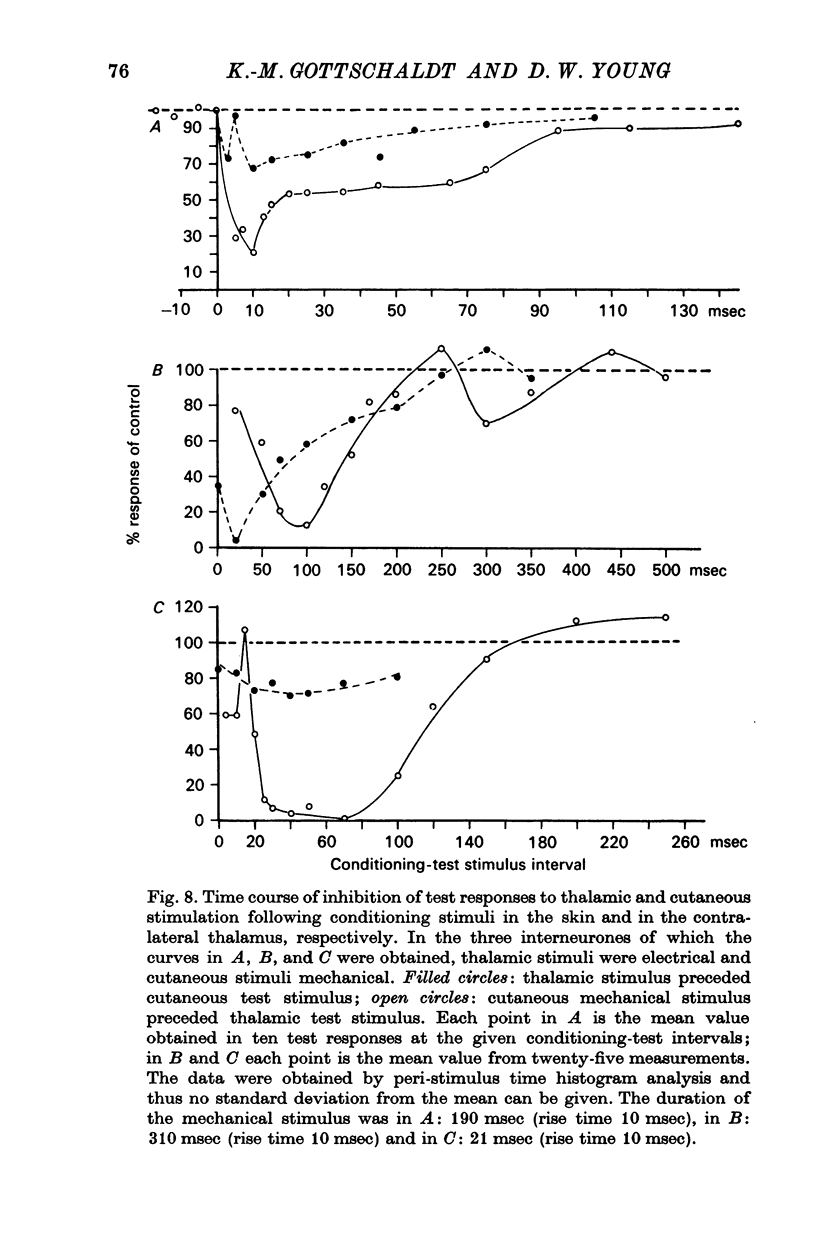
5. Afferent inhibition in relay neurones could be elicited from within the excitatory receptive field and appeared to be related to the activation of distinct receptor populations responding to specific stimulus parameters. Inhibition was also seen in interneurones following both mechanical stimulation of the skin and electrical stimulation of lemniscal fibre terminals in the contralateral ventromedial thalamus.
6. The results are discussed and compared with previous findings about sinus hair representation in the trigeminal nucleus and the ascending lemniscal projection. The findings indicate that the concept of the `static properties' of relay neurones is not adequate for all trigeminothalamic relay neurones and may require a critical reconsideration.
7. It is suggested that the afferent input from sinus hairs is effectively controlled at the level of the rostral trigeminal nuclei. This control may affect the spatial input to relay neurones, the temporal components of their responses and the intensity dimension of their transmission capacity. It is postulated that by these mechanisms tactile information from the sinus hair system is modulated according to the instantaneous sensory requirements of the behaving cat.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. IDENTIFICATION OF RELAY CELLS AND INTERNEURONS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1080–1095. doi: 10.1152/jn.1964.27.6.1080. [DOI] [PubMed] [Google Scholar]
- Baldissera F., Broggi G., Mancia M. Depolarization of trigeminal afferents induced by stimulation of brain-stem and peripheral nerves. Exp Brain Res. 1967;4(1):1–17. doi: 10.1007/BF00235213. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Gordon G., Kay R. H. A study of single axons in the cat's medial lemniscus. J Physiol. 1974 Jan;236(1):225–246. doi: 10.1113/jphysiol.1974.sp010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARPENTER M. B., HANNA G. R. Fiber projections from the spinal trigeminal nucleus in the cat. J Comp Neurol. 1961 Aug;117:117–131. doi: 10.1002/cne.901170110. [DOI] [PubMed] [Google Scholar]
- Carmody J., Rowe M. Inhibition within the trigeminal nucleus induced by afferent inputs and its influence on stimulus coding by mechanosensitive neurones. J Physiol. 1974 Nov;243(1):195–210. doi: 10.1113/jphysiol.1974.sp010749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARIAN-SMITH I. CORTICAL PROJECTIONS OF THALAMIC NEURONES EXCITED BY MECHANICAL STIMULATION OF THE FACE OF THE CAT. J Physiol. 1964 Jun;171:339–360. doi: 10.1113/jphysiol.1964.sp007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARIAN-SMITH I., MAYDAY G. Somatotopic organization within the brain-stem trigeminal complex of the cat. Exp Neurol. 1960 Jun;2:290–309. doi: 10.1016/0014-4886(60)90015-7. [DOI] [PubMed] [Google Scholar]
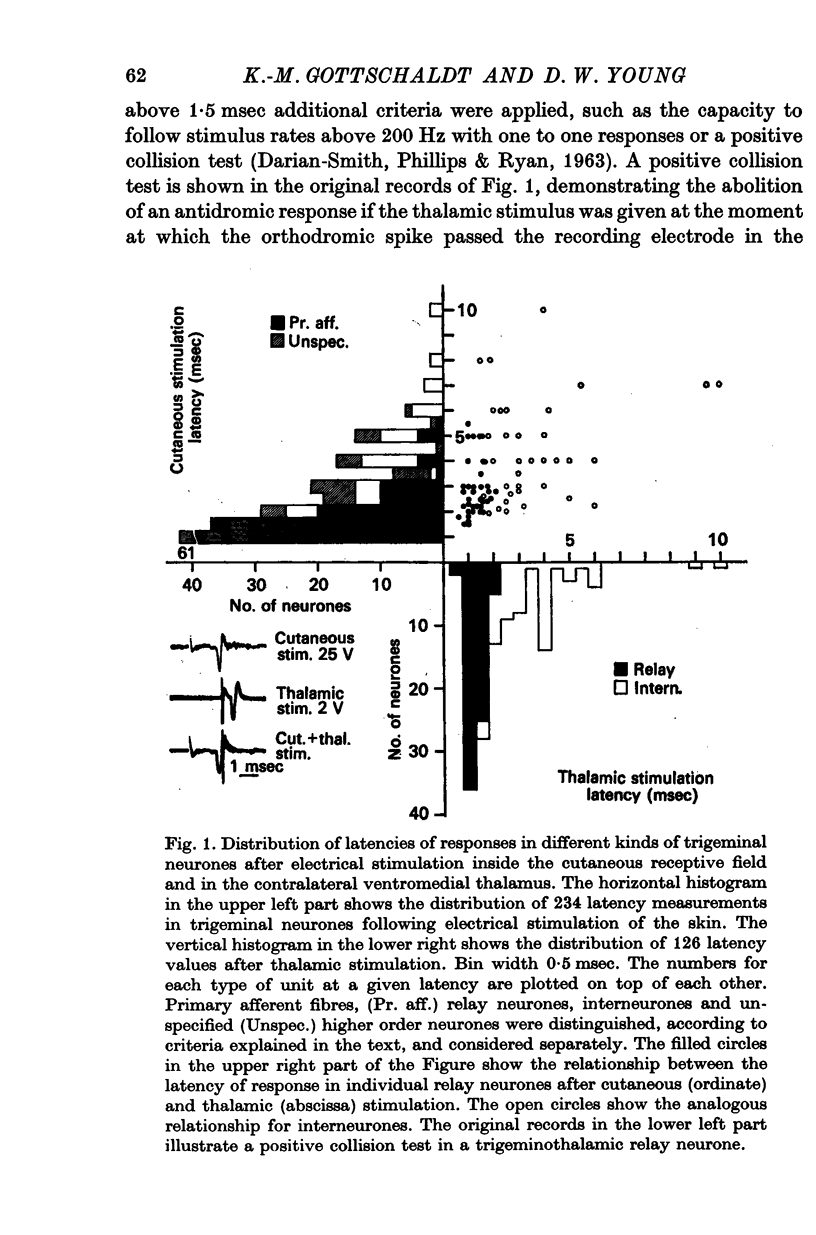
- DARIAN-SMITH I., PHILLIPS G., RYAN R. D. FUNCTIONAL ORGANIZATION IN THE TRIGEMINAL MAIN SENSORY AND ROSTRAL SPINAL NUCLEI OF THE CAT. J Physiol. 1963 Aug;168:129–146. doi: 10.1113/jphysiol.1963.sp007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARIAN-SMITH I., PHILLIPS G. SECONDARY NEURONES WITHIN A TRIGEMINO-CEREBELLAR PROJECTION TO THE ANTERIOR LOBE OF THE CEREBELLUM IN THE CAT. J Physiol. 1964 Jan;170:53–68. doi: 10.1113/jphysiol.1964.sp007313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARIAN-SMITH I., PROCTOR R., RYAN R. D. A SINGLE-NEURONE INVESTIGATION OF SOMATOTOPIC ORGANIZATION WITHIN THE CAT'S TRIGEMINAL BRAIN-STEM NUCLEI. J Physiol. 1963 Aug;168:147–157. doi: 10.1113/jphysiol.1963.sp007183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I., Isbister J., Mok H., Yokota T. Somatic sensory cortical projection areas excited y tactile stimulation of the cat: a triple representation. J Physiol. 1966 Feb;182(3):671–689. doi: 10.1113/jphysiol.1966.sp007844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny-Brown D., Yanagisawa N. The function of the descending root of the fifth nerve. Brain. 1973 Dec;96(4):783–814. doi: 10.1093/brain/96.4.783. [DOI] [PubMed] [Google Scholar]
- Dykes R. W. Afferent fibers from mystacial vibrissae of cats and seals. J Neurophysiol. 1975 May;38(3):650–662. doi: 10.1152/jn.1975.38.3.650. [DOI] [PubMed] [Google Scholar]
- EISENMAN J., FROMM G., LANDGREN S., NOVIN D. THE ASCENDING PROJECTIONS OF TRIGEMINAL NEURONES IN THE CAT, INVESTIGATED BY ANTIDROMIC STIMULATION. Acta Physiol Scand. 1964 Apr;60:337–350. doi: 10.1111/j.1748-1716.1964.tb02896.x. [DOI] [PubMed] [Google Scholar]
- EMMERS R. ORGANIZATION OF THE FIRST AND THE SECOND SOMESTHETIC REGIONS (SI AND SII) IN THE RAT THALAMUS. J Comp Neurol. 1965 Apr;124:215–227. doi: 10.1002/cne.901240207. [DOI] [PubMed] [Google Scholar]
- Fitzgerald O. Discharges from the sensory organs of the cat's vibrissae and the modification in their activity by ions. J Physiol. 1940 May 14;98(2):163–178. doi: 10.1113/jphysiol.1940.sp003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., LANDGREN S., SEED W. A. The functional characteristics of single cells in the caudal part of the spinal nucleus of the trigeminal nerve of the cat. J Physiol. 1961 Oct;158:544–559. doi: 10.1113/jphysiol.1961.sp006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschaldt K-M, Young D. W. Quantitative aspects of responses in trigeminal relay neurones and interneurones following mechanical stimulation of sinus hairs and skin in the cat. J Physiol. 1977 Oct;272(1):85–103. doi: 10.1113/jphysiol.1977.sp012035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschaldt K. M., Iggo A., Young D. W. Functional characteristics of mechanoreceptors in sinus hair follicles of the cat. J Physiol. 1973 Dec;235(2):287–315. doi: 10.1113/jphysiol.1973.sp010388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood L. F., Sessle B. J. Inputs to trigeminal brain stem neurones from facial, oral, tooth pulp and pharyngolaryngeal tissues: II. Role of trigeminal nucleus caudalis in modulating responses to innocuous and noxious stimuli. Brain Res. 1976 Nov 26;117(2):227–238. doi: 10.1016/0006-8993(76)90732-0. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ-PEON R., HAGBARTH K. E. Interaction between afferent and cortically induced reticular responses. J Neurophysiol. 1955 Jan;18(1):44–55. doi: 10.1152/jn.1955.18.1.44. [DOI] [PubMed] [Google Scholar]
- Hayward J. N. Response of ventrobasal thalamic cells to hair displacement on the face of the waking monkey. J Physiol. 1975 Sep;250(2):385–407. doi: 10.1113/jphysiol.1975.sp011061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellweg F. C., Schultz W., Creutzfeldt O. D. Extracellular and intracellular recordings from cat's cortical whisker projection area: thalamocortical response transformation. J Neurophysiol. 1977 May;40(3):463–479. doi: 10.1152/jn.1977.40.3.463. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schoultz T., Spencer W. A. Temporal and spatial parameters of excitation and afferent inhibition in cuneothalamic relay neurons. J Neurophysiol. 1977 Jul;40(4):822–835. doi: 10.1152/jn.1977.40.4.822. [DOI] [PubMed] [Google Scholar]
- KERR F. W., LYSAK W. R. SOMATOTOPIC ORGANIZATION OF TRIGEMINAL-GANGLION NEURONES. Arch Neurol. 1964 Dec;11:593–602. doi: 10.1001/archneur.1964.00460240025003. [DOI] [PubMed] [Google Scholar]
- KRUGER L., MICHEL F. A morphological and somatotopic analysis of single unit activity in the trigeminal sensory complex of the cat. Exp Neurol. 1962 Feb;5:139–156. doi: 10.1016/0014-4886(62)90030-4. [DOI] [PubMed] [Google Scholar]
- Kerr F. W., Kruger L., Schwassmann H. O., Stern R. Somatotopic organization of mechanoreceptor units in the trigeminal nuclear complex of the macaque. J Comp Neurol. 1968 Oct;134(2):127–144. doi: 10.1002/cne.901340202. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick D. B., Kruger L. Physiological properties of neurons in the principal sensory trigeminal nucleus of the cat. Exp Neurol. 1975 Sep;48(3 Pt 1):664–690. doi: 10.1016/0014-4886(75)90022-9. [DOI] [PubMed] [Google Scholar]
- Mosso J. A., Kruger L. Receptor categories represented in spinal trigeminal nucleus caudalis. J Neurophysiol. 1973 May;36(3):472–488. doi: 10.1152/jn.1973.36.3.472. [DOI] [PubMed] [Google Scholar]
- Nilsson B. Y. Structure and function of the tactile hair receptors on the cat's foreleg. Acta Physiol Scand. 1969 Dec;77(4):396–416. doi: 10.1111/j.1748-1716.1969.tb04584.x. [DOI] [PubMed] [Google Scholar]
- Nord S. G. Receptor field characteristics of single cells in the rat spinal trigeminal complex. Exp Neurol. 1968 Jun;21(2):236–243. doi: 10.1016/0014-4886(68)90142-8. [DOI] [PubMed] [Google Scholar]
- Pubols B. H., Jr, Donovick P. J., Pubols L. M. Opossum trigeminal afferents associated with vibrissa and rhinarial mechanoreceptors. Brain Behav Evol. 1973;7(5):360–381. doi: 10.1159/000124423. [DOI] [PubMed] [Google Scholar]
- Pubols B. H., Jr, Pubols L. M., DiPette D. J., Sheely J. C. Opossum somatic sensory cortex: a microelectrode mapping study. J Comp Neurol. 1976 Jan 15;165(2):229–245. doi: 10.1002/cne.901650208. [DOI] [PubMed] [Google Scholar]
- Rowe M. J., Sessle B. J. Responses of trigeminal ganglion and brain stem neurones in the cat to mechanical and thermal stimulation of the face. Brain Res. 1972 Jul 20;42(2):367–384. doi: 10.1016/0006-8993(72)90537-9. [DOI] [PubMed] [Google Scholar]
- Rowe M. J., Sessle B. J. Somatic afferent input to posterior thalamic neurones and their axon projection to the cerebral cortex in the cat. J Physiol. 1968 May;196(1):19–35. doi: 10.1113/jphysiol.1968.sp008491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W., Galbraith G. C., Gottschaldt K. M., Creutzfeldt O. D. A comparison of primary afferent and cortical neurone activity coding sinus hair movements in the cat. Exp Brain Res. 1976 Feb 26;24(4):365–381. doi: 10.1007/BF00235004. [DOI] [PubMed] [Google Scholar]
- Scibetta C. J., King R. B. Hyperpolarizing influence of trigeminal nucleus caudalis on primary afferent preterminals in trigeminal nucleus oralis. J Neurophysiol. 1969 Mar;32(2):229–238. doi: 10.1152/jn.1969.32.2.229. [DOI] [PubMed] [Google Scholar]
- Sessle B. J., Dubner R. Presynaptic excitability changes of trigeminothalamic and corticothalamic afferents. Exp Neurol. 1971 Feb;30(2):239–250. doi: 10.1016/s0014-4886(71)80004-3. [DOI] [PubMed] [Google Scholar]
- Sessle B. J., Greenwood L. F. Inputs to trigeminal brain stem neurones from facial, oral, tooth pulp and pharyngolaryngeal tissues: I. Responses to innocuous and noxious stimuli. Brain Res. 1976 Nov 26;117(2):211–226. doi: 10.1016/0006-8993(76)90731-9. [DOI] [PubMed] [Google Scholar]
- Shipley M. T. Response characteristics of single units in the rat's trigeminal nuclei to vibrissa displacements. J Neurophysiol. 1974 Jan;37(1):73–90. doi: 10.1152/jn.1974.37.1.73. [DOI] [PubMed] [Google Scholar]
- WALL P. D., TAUB A. Four aspects of trigeminal nucleus and a paradox. J Neurophysiol. 1962 Jan;25:110–126. doi: 10.1152/jn.1962.25.1.110. [DOI] [PubMed] [Google Scholar]
- Waite P. M. The responses of cells in the rat thalamus to mechanical movements of the whiskers. J Physiol. 1973 Jan;228(2):541–561. doi: 10.1113/jphysiol.1973.sp010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971 Mar 5;26(2):259–275. [PubMed] [Google Scholar]
- Welker C. Receptive fields of barrels in the somatosensory neocortex of the rat. J Comp Neurol. 1976 Mar 15;166(2):173–189. doi: 10.1002/cne.901660205. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M. The pyramidal tract: recent investigations on its morphology and function. Ergeb Physiol. 1969;61:72–136. doi: 10.1007/BFb0111447. [DOI] [PubMed] [Google Scholar]
- Young D. W., Iggo A. Neurones in the spinal trigeminal nucleus of the cat responding to movement of the vibrissae. Exp Brain Res. 1977 Jul 15;28(5):457–467. doi: 10.1007/BF00236470. [DOI] [PubMed] [Google Scholar]
- Young R. F., King R. B. Excitability changes in trigeminal primary afferent fibers in response to noxious and nonnoxious stimuli. J Neurophysiol. 1972 Jan;35(1):87–95. doi: 10.1152/jn.1972.35.1.87. [DOI] [PubMed] [Google Scholar]
- Zucker E., Welker W. I. Coding of somatic sensory input by vibrissae neurons in the rat's trigeminal ganglion. Brain Res. 1969 Jan;12(1):138–156. doi: 10.1016/0006-8993(69)90061-4. [DOI] [PubMed] [Google Scholar]


