Abstract
1. Stimulus-response relationships in discharges of trigeminal relay- and interneurones were investigated in the barbiturate anaesthetized cat using controlled sinus hair or skin displacements.
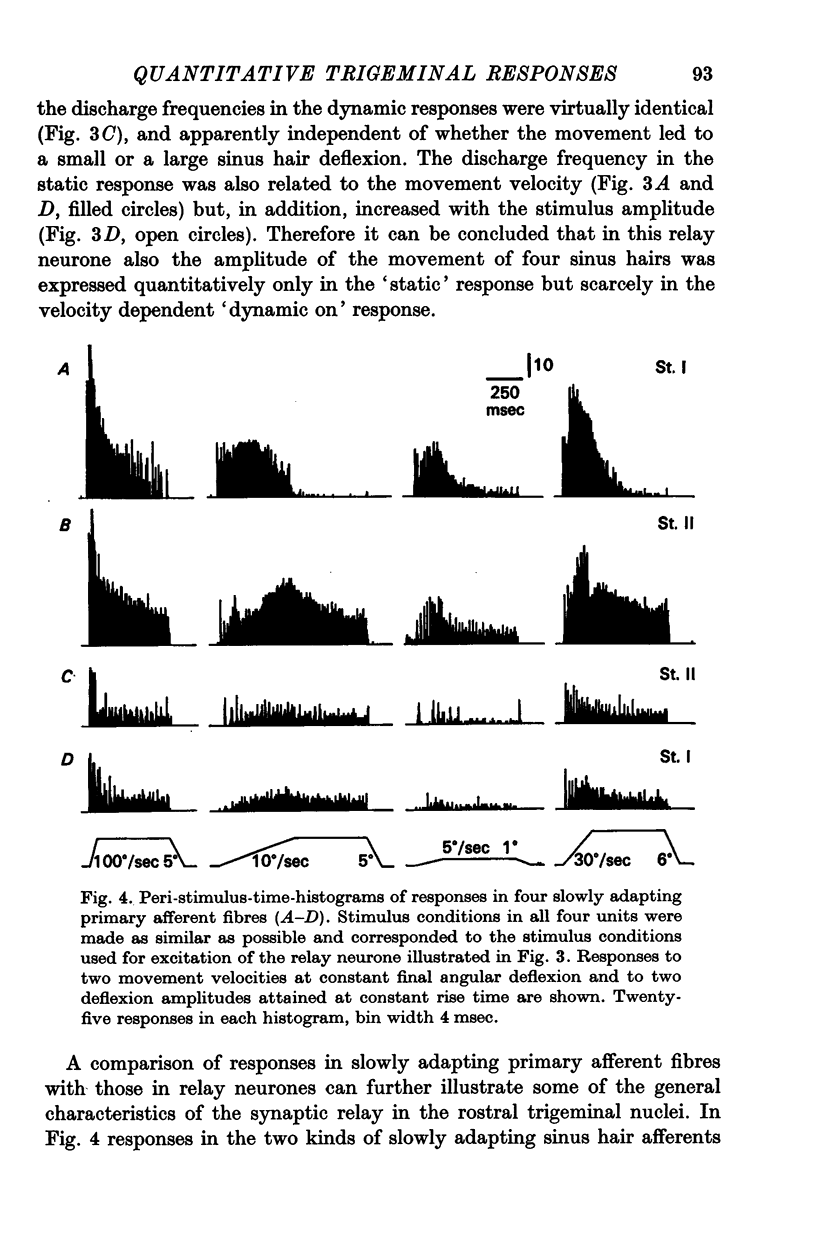
2. In comparison with discharges in slowly adapting primary afferent fibres the responses in all higher order neurones were considerably reduced in firing rate and often revealed modifications suggesting the interaction of mechanisms actively modulating the afferent input.
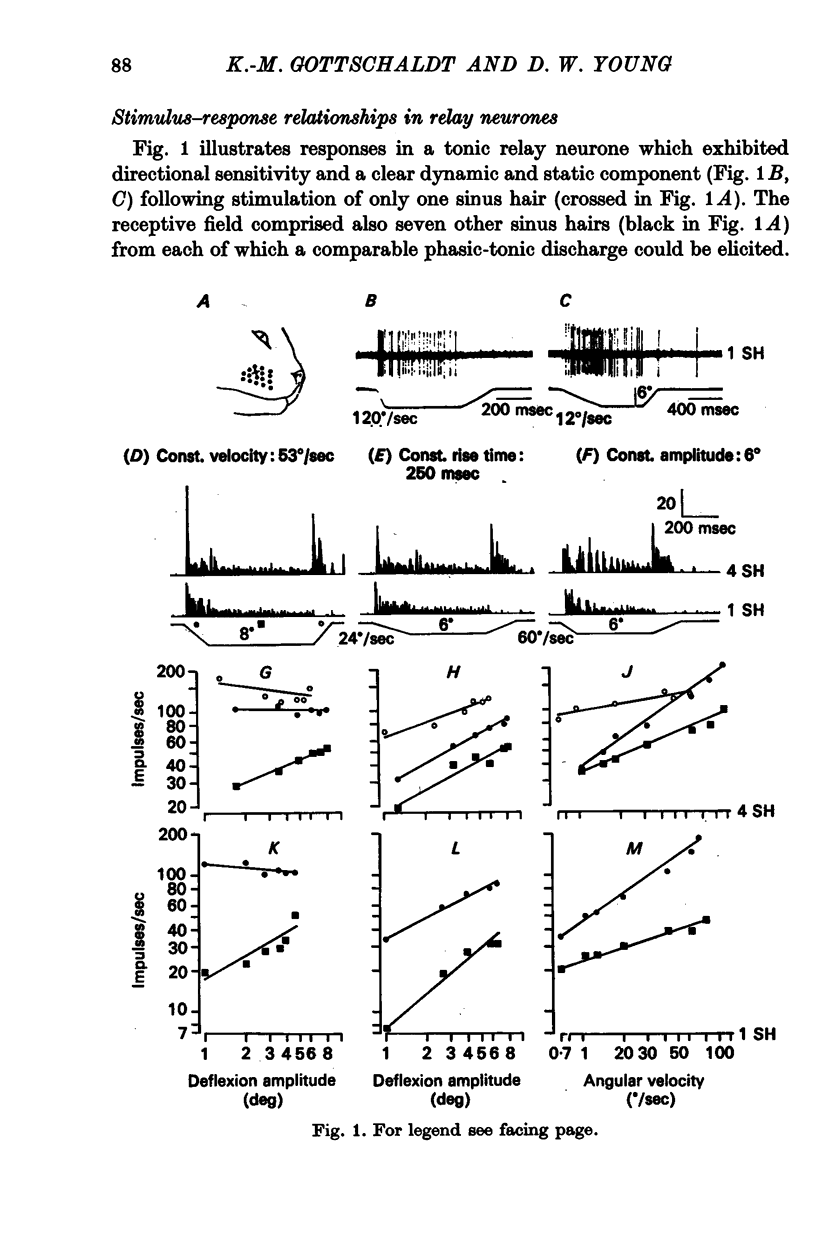
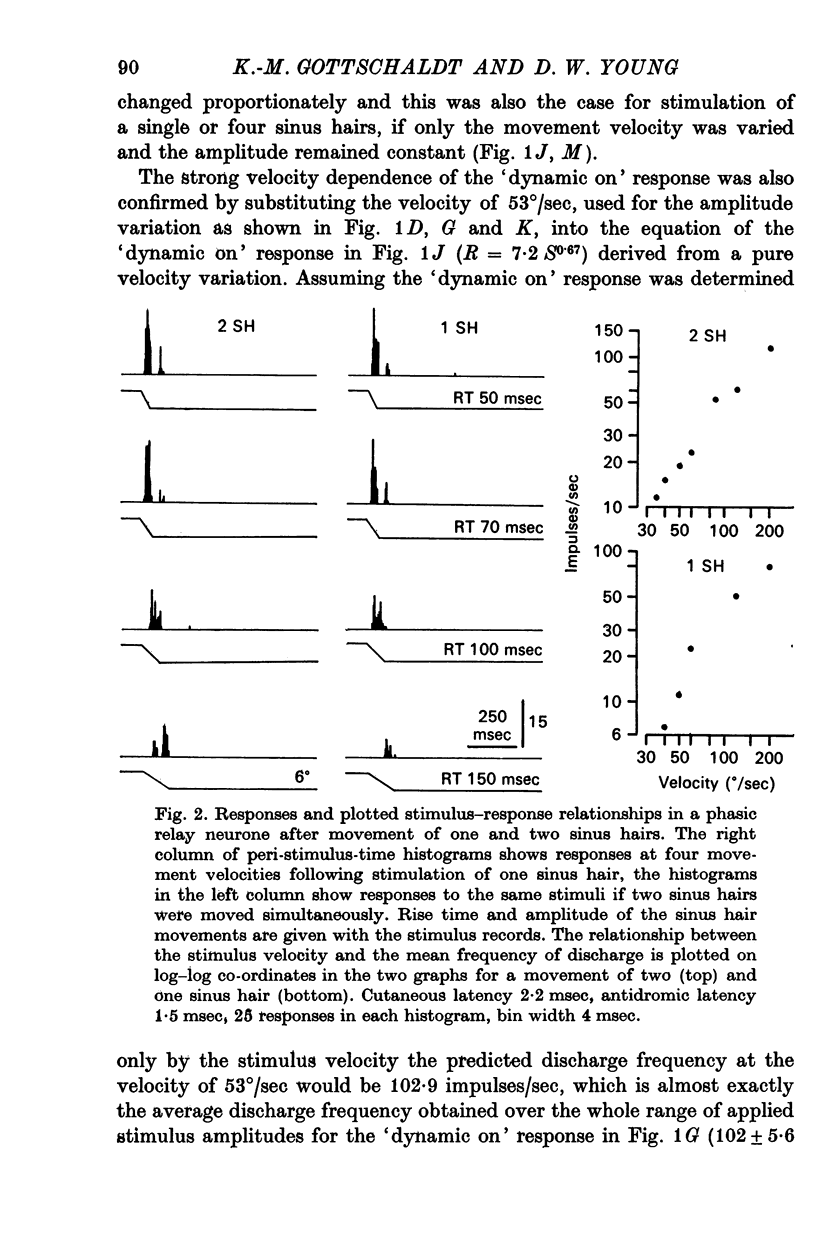
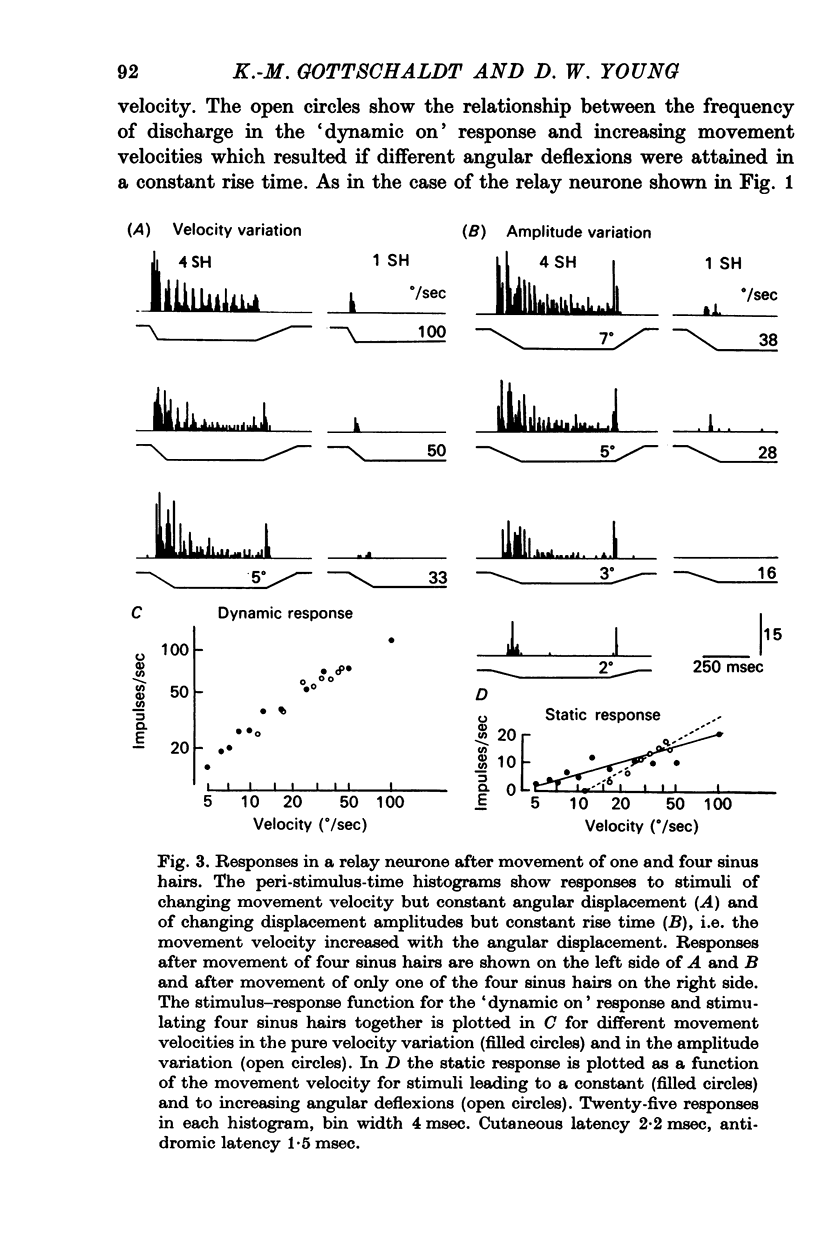
3. In relay neurones with or without a tonic discharge component the `dynamic on' response during a trapezoidal displacement of sinus hairs was found to be determined entirely or predominantly by the movement velocity and to be independent of the deflexion angle of a stimulus. In contrast, the static response in tonic relay neurones was determined by both the movement velocity and the displacement amplitude.
4. Spatial summation of afferent input caused either only quantitative changes in the responses of relay neurones leaving the general discharge properties unaltered or caused both qualitative and quantitative changes in the responses.
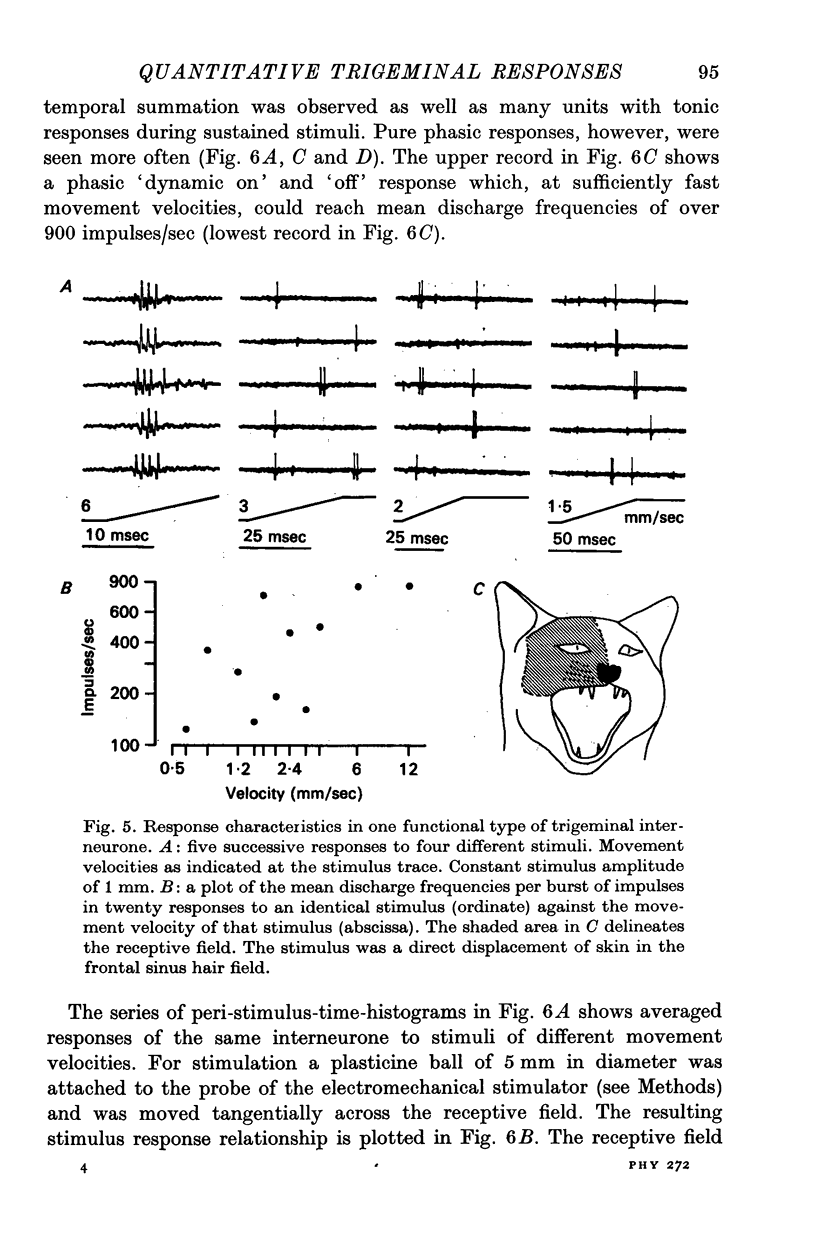
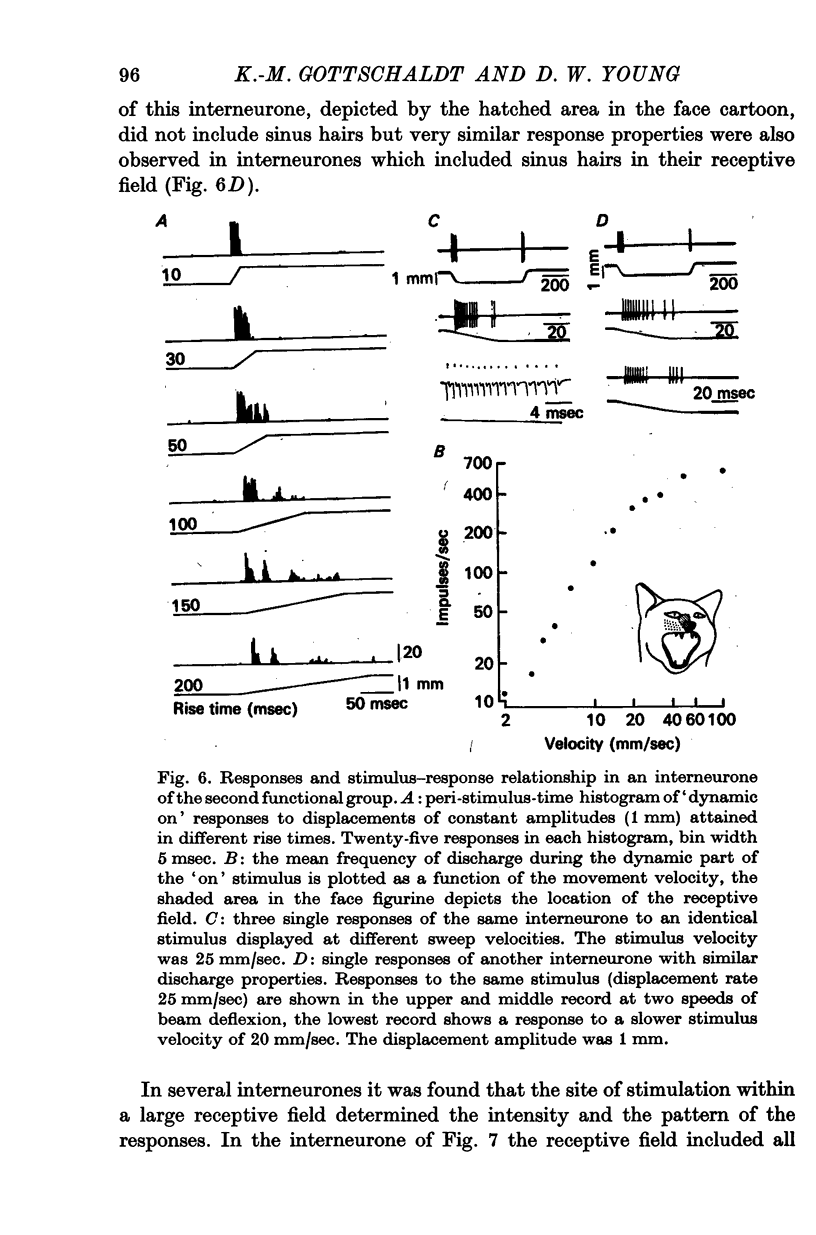
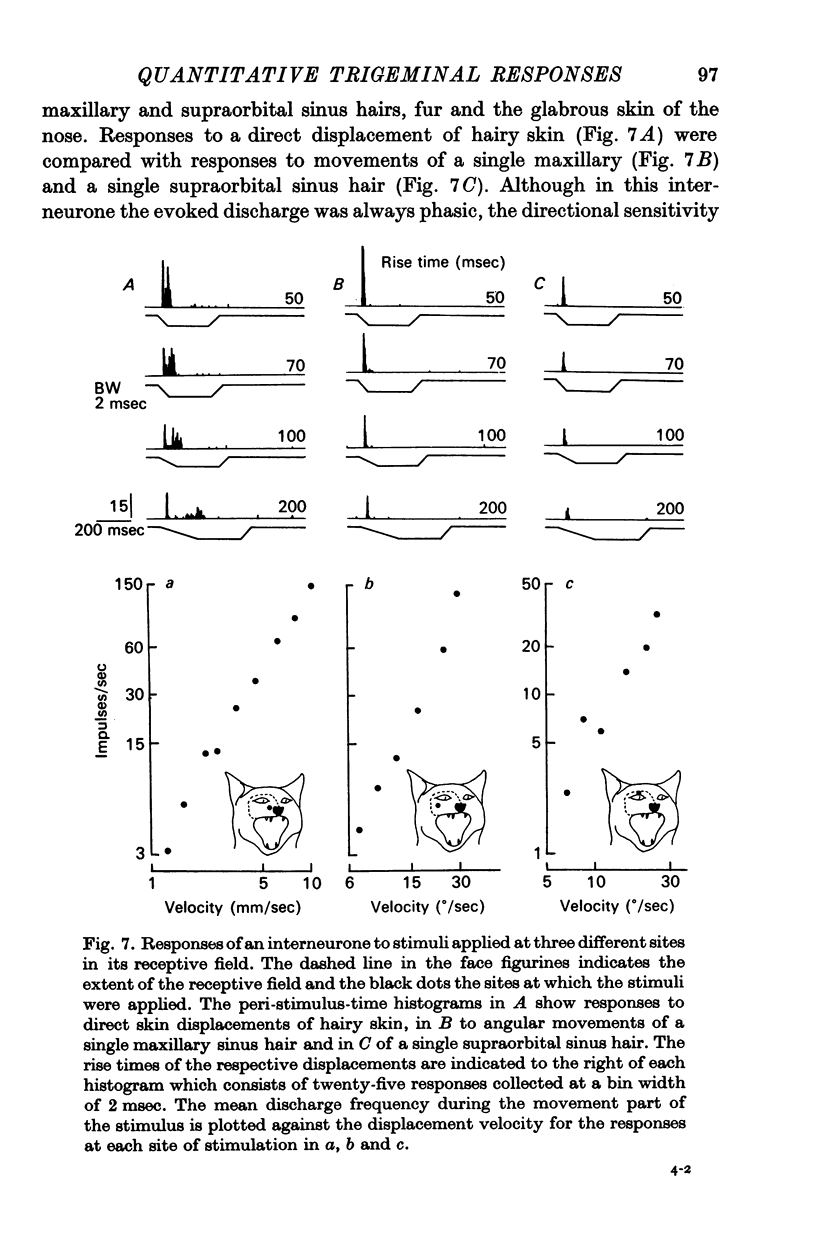
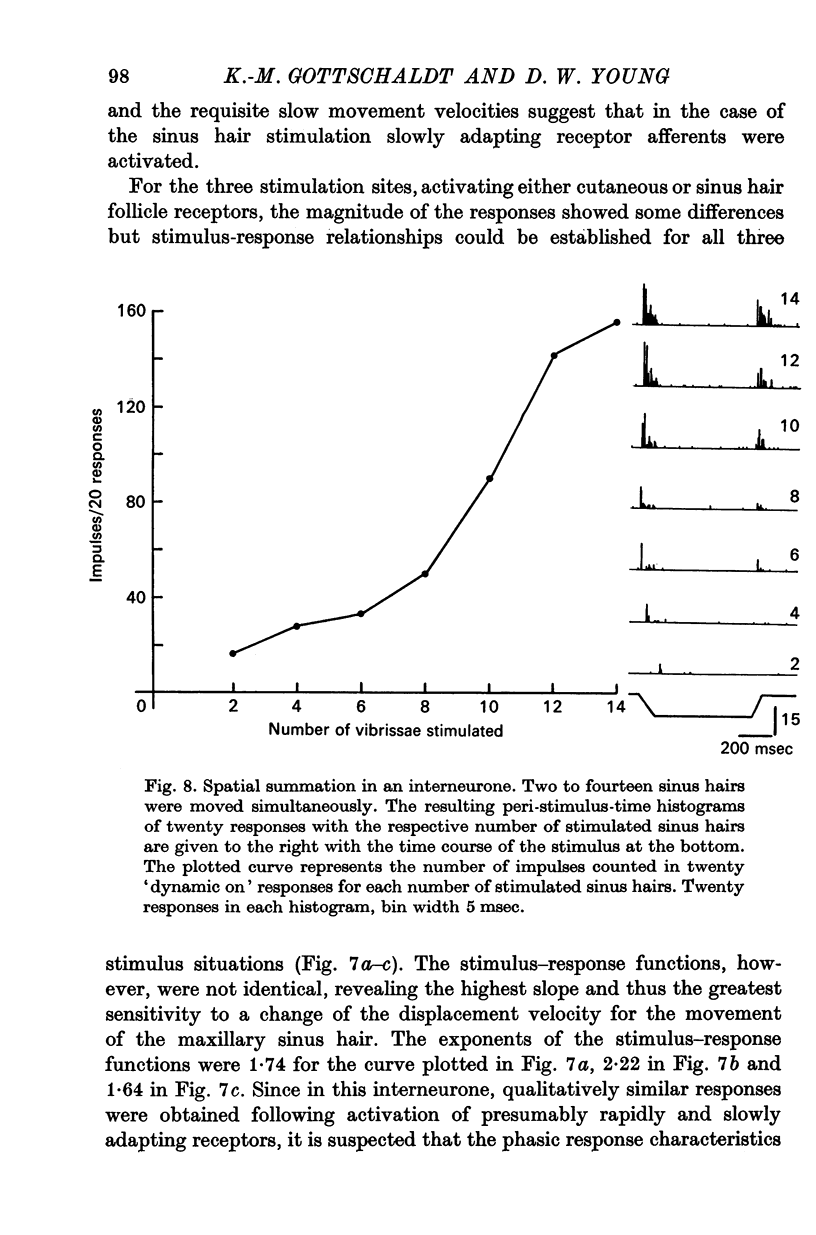
5. Interneurones consisted of two functional groups. In about 25% of them the responses were not or only slightly dependent on the intensity of the applied stimulus, often burstlike and of an all or nothing character. In the second group of interneurones the responses showed a quantitative dependence on the applied stimuli. In this group of interneurones responses often increased with the spatial extension of the peripheral stimulus revealing spatial summation of the afferent input.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armett C. J., Gray J. A., Hunsperger R. W., Lal S. The transmission of information in primary receptor neurones and second-order neurones of a phasic system. J Physiol. 1962 Dec;164(3):395–421. doi: 10.1113/jphysiol.1962.sp007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. B. Response of cat dorsal horn cells to variations of intensity. location, and area of cutaneous stimuli. Exp Neurol. 1969 Feb;23(2):249–265. doi: 10.1016/0014-4886(69)90061-2. [DOI] [PubMed] [Google Scholar]
- Carmody J., Rowe M. Inhibition within the trigeminal nucleus induced by afferent inputs and its influence on stimulus coding by mechanosensitive neurones. J Physiol. 1974 Nov;243(1):195–210. doi: 10.1113/jphysiol.1974.sp010749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers M. R., Andres K. H., von Duering M., Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I., Rowe M. J., Sessle B. J. "Tactile" stimulus intensity: information transmission by relay neurons in different trigeminal nuclei. Science. 1968 May 17;160(3829):791–794. doi: 10.1126/science.160.3829.791. [DOI] [PubMed] [Google Scholar]
- Dykes R. W. Afferent fibers from mystacial vibrissae of cats and seals. J Neurophysiol. 1975 May;38(3):650–662. doi: 10.1152/jn.1975.38.3.650. [DOI] [PubMed] [Google Scholar]
- Gardner E. P., Spencer W. A. Sensory funneling. II. Cortical neuronal representation of patterned cutaneous stimuli. J Neurophysiol. 1972 Nov;35(6):954–977. doi: 10.1152/jn.1972.35.6.954. [DOI] [PubMed] [Google Scholar]
- Gottschaldt K-M, Young D. W. Properties of different functional types of neurones in the cat's rostral trigeminal nuclei responding to sinus hair stimulation. J Physiol. 1977 Oct;272(1):57–84. doi: 10.1113/jphysiol.1977.sp012034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschaldt K. M., Iggo A., Young D. W. Functional characteristics of mechanoreceptors in sinus hair follicles of the cat. J Physiol. 1973 Dec;235(2):287–315. doi: 10.1113/jphysiol.1973.sp010388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood L. F., Sessle B. J. Inputs to trigeminal brain stem neurones from facial, oral, tooth pulp and pharyngolaryngeal tissues: II. Role of trigeminal nucleus caudalis in modulating responses to innocuous and noxious stimuli. Brain Res. 1976 Nov 26;117(2):227–238. doi: 10.1016/0006-8993(76)90732-0. [DOI] [PubMed] [Google Scholar]
- Hahn J. F. Stimulus-response relationships in first-order sensory fibres from cat vibrissae. J Physiol. 1971 Feb;213(1):215–226. doi: 10.1113/jphysiol.1971.sp009377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Schoultz T., Spencer W. A. Temporal and spatial parameters of excitation and afferent inhibition in cuneothalamic relay neurons. J Neurophysiol. 1977 Jul;40(4):822–835. doi: 10.1152/jn.1977.40.4.822. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick D. B., Kruger L. Physiological properties of neurons in the principal sensory trigeminal nucleus of the cat. Exp Neurol. 1975 Sep;48(3 Pt 1):664–690. doi: 10.1016/0014-4886(75)90022-9. [DOI] [PubMed] [Google Scholar]
- Mosso J. A., Kruger L. Receptor categories represented in spinal trigeminal nucleus caudalis. J Neurophysiol. 1973 May;36(3):472–488. doi: 10.1152/jn.1973.36.3.472. [DOI] [PubMed] [Google Scholar]
- Pubols B. H., Pubols L. M. Coding of mechanical stimulus velocity and indentation depth by squirrel monkey and raccoon glabrous skin mechanoreceptors. J Neurophysiol. 1976 Jul;39(4):773–787. doi: 10.1152/jn.1976.39.4.773. [DOI] [PubMed] [Google Scholar]
- Rowe M. J., Sessle B. J. Responses of trigeminal ganglion and brain stem neurones in the cat to mechanical and thermal stimulation of the face. Brain Res. 1972 Jul 20;42(2):367–384. doi: 10.1016/0006-8993(72)90537-9. [DOI] [PubMed] [Google Scholar]
- Schultz W., Galbraith G. C., Gottschaldt K. M., Creutzfeldt O. D. A comparison of primary afferent and cortical neurone activity coding sinus hair movements in the cat. Exp Brain Res. 1976 Feb 26;24(4):365–381. doi: 10.1007/BF00235004. [DOI] [PubMed] [Google Scholar]
- Shipley M. T. Response characteristics of single units in the rat's trigeminal nuclei to vibrissa displacements. J Neurophysiol. 1974 Jan;37(1):73–90. doi: 10.1152/jn.1974.37.1.73. [DOI] [PubMed] [Google Scholar]
- Wilson H. R., Cowan J. D. Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J. 1972 Jan;12(1):1–24. doi: 10.1016/S0006-3495(72)86068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T. C., Williams W. J. Dynamic response and transfer characteristics of joint neurons in somatosensory thalamus of the cat. J Neurophysiol. 1976 May;39(3):582–600. doi: 10.1152/jn.1976.39.3.582. [DOI] [PubMed] [Google Scholar]
- Zucker E., Welker W. I. Coding of somatic sensory input by vibrissae neurons in the rat's trigeminal ganglion. Brain Res. 1969 Jan;12(1):138–156. doi: 10.1016/0006-8993(69)90061-4. [DOI] [PubMed] [Google Scholar]


