Abstract
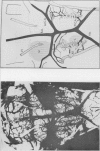
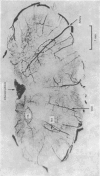
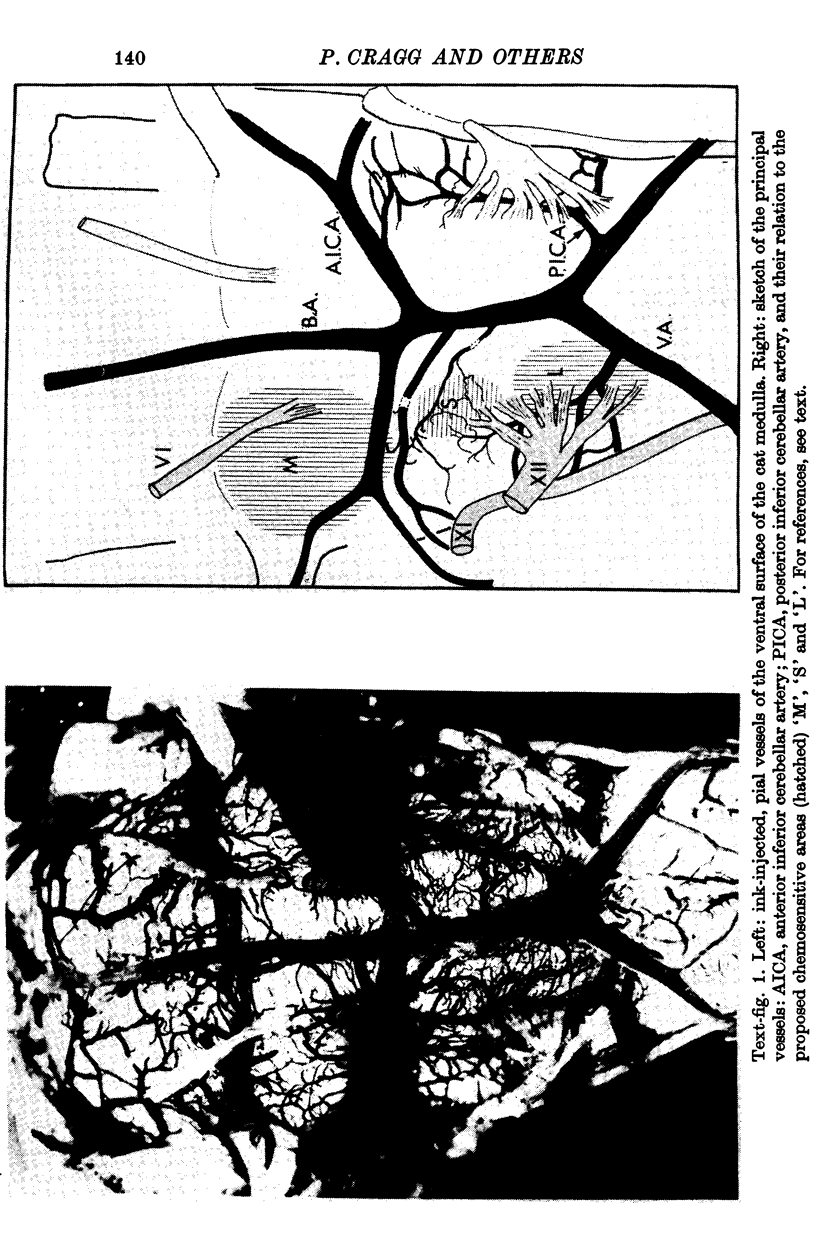
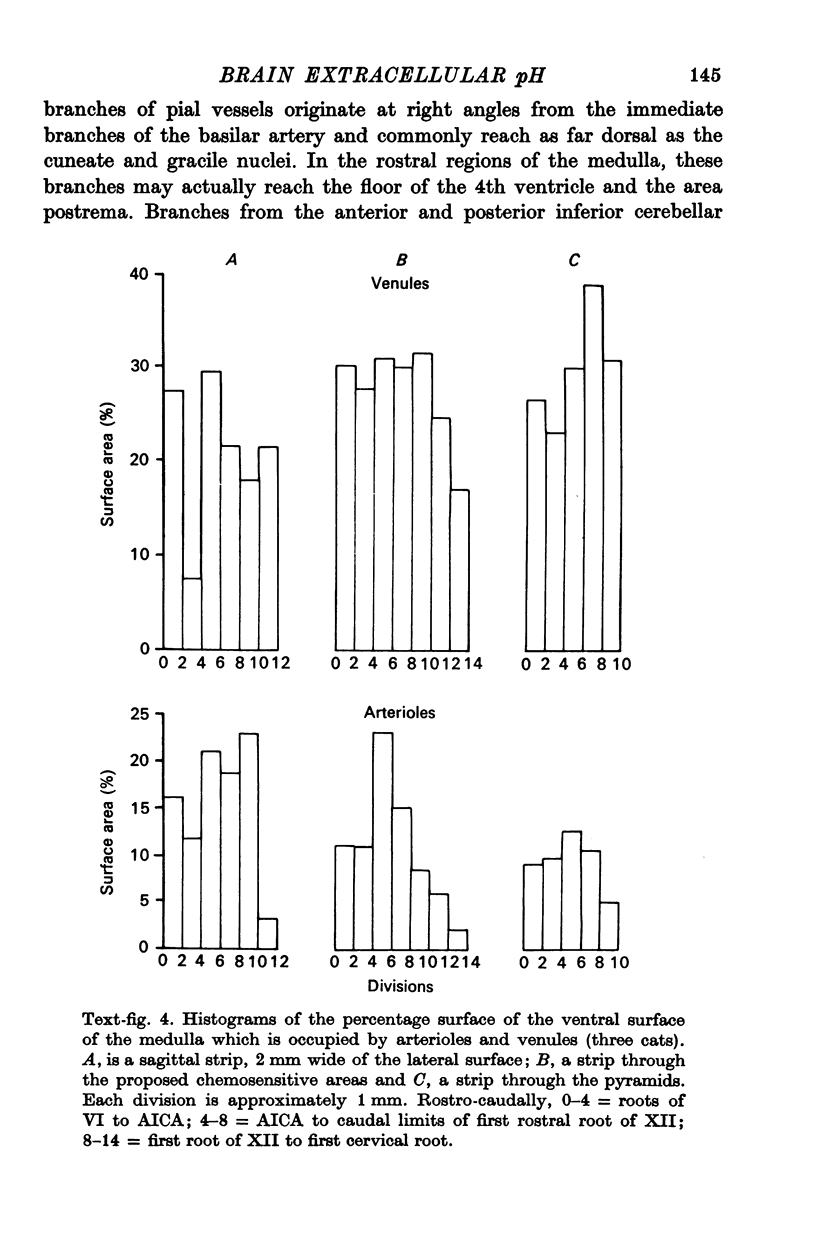
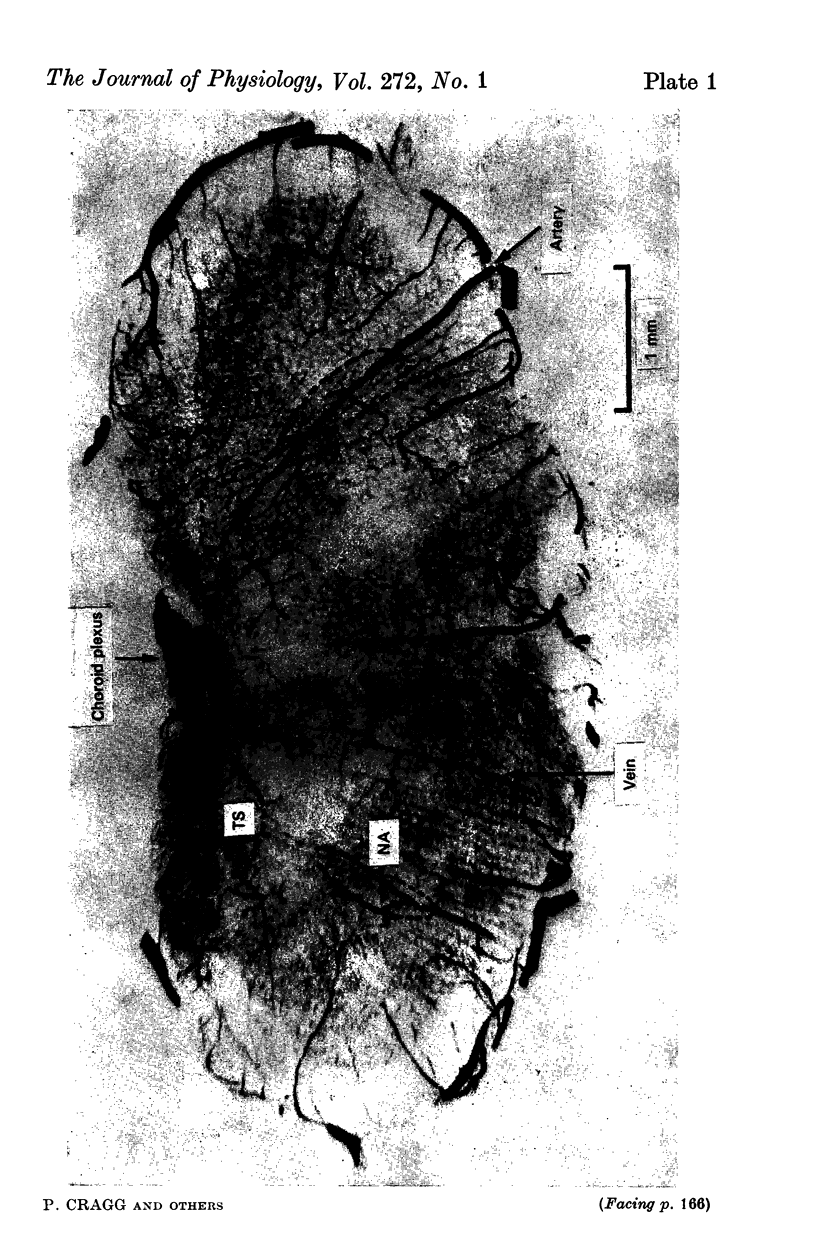
1. The blood supply to the medulla was determined by the injection of indian ink via the vertebral arteries. Virtually the whole medulla was supplied by penetrating vessels from the ventral surface. The highest density of small arterioles and venules was found close to the roots of XII and on the ventrolateral surface.
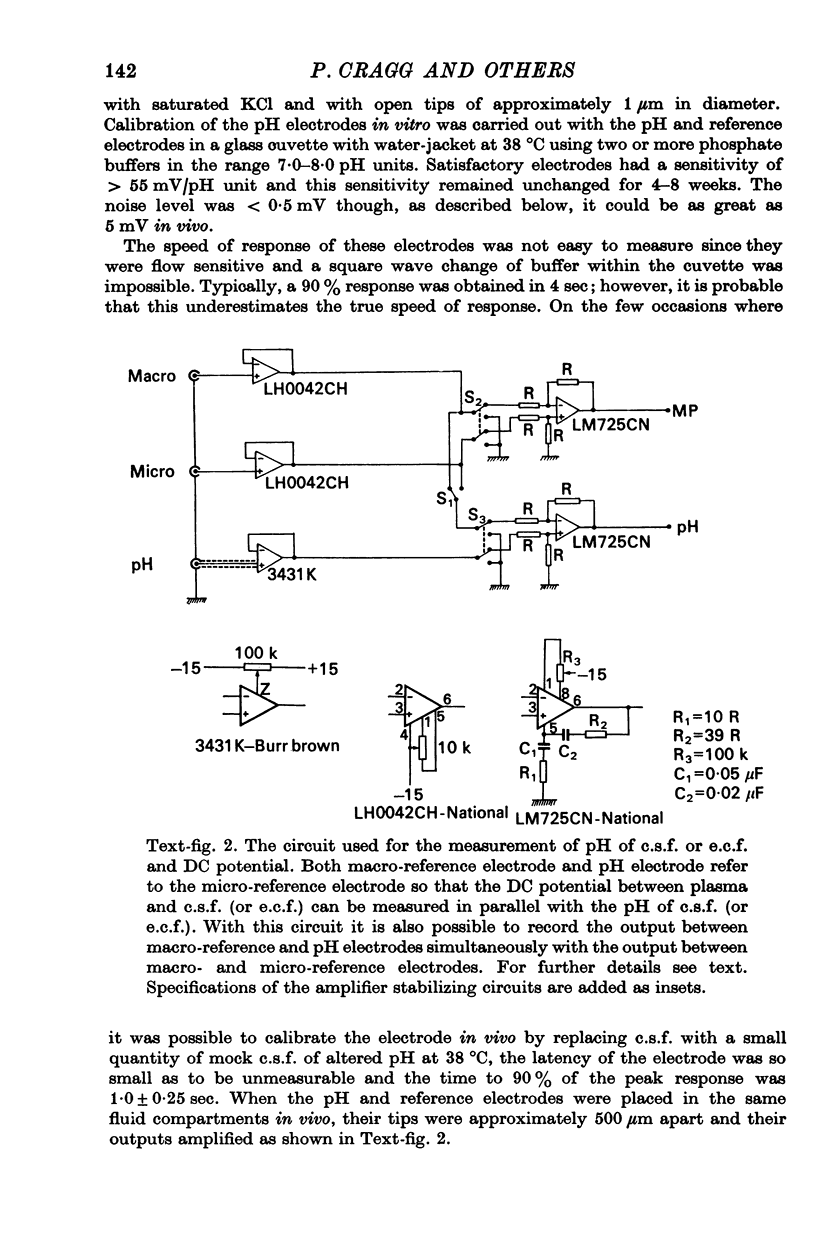
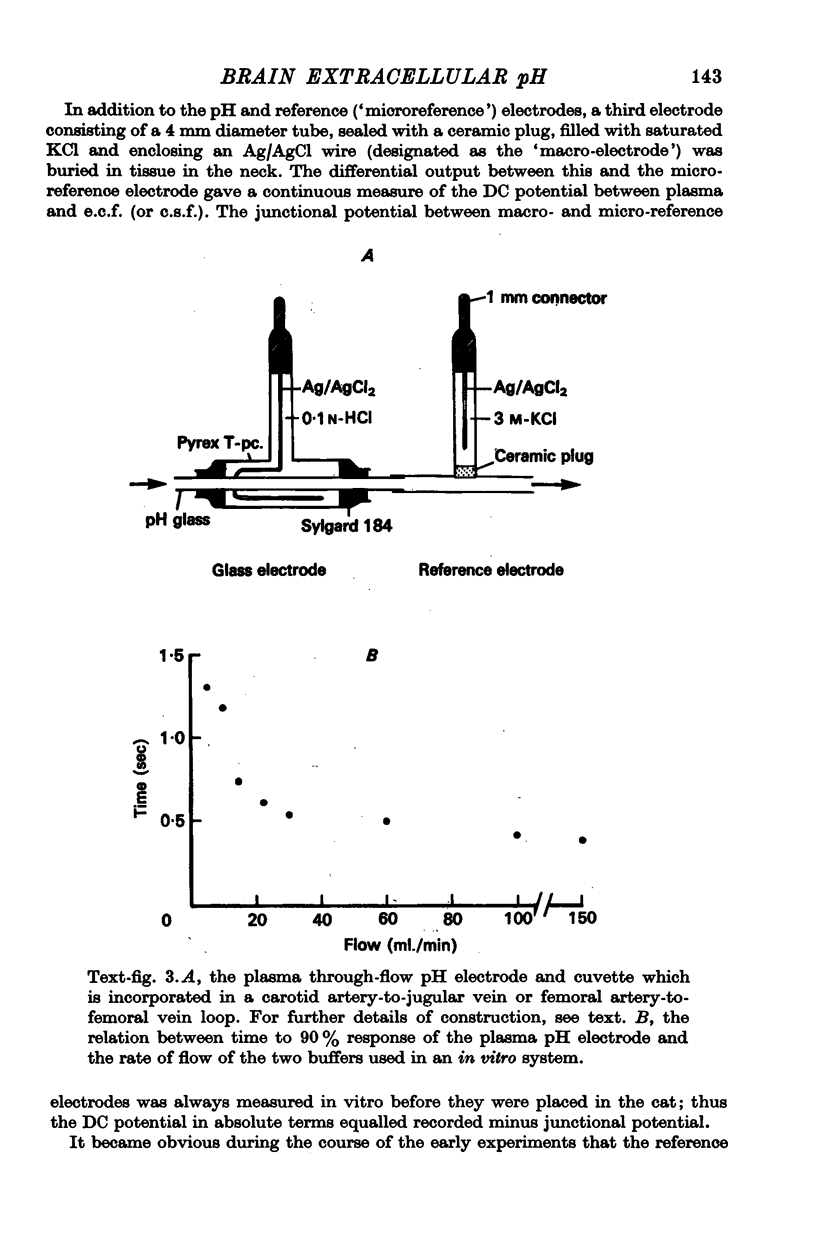
2. The pH of extracellular fluid (pHe.c.f.) was measured with pH microelectrodes of tip size 1-3 μm in cortex and medulla in seventeen cats, anaesthetized with pentobarbitone or a chloralose—urethane mixture. Parallel measurements were made of the pH of c.s.f. and plasma, the DC potential between plasma and brain and ventilation or phrenic nerve discharge.
3. In the majority of tests under steady conditions, the pH of e.c.f. was found to be lower than that of c.s.f. by between 0·03 and 0·08 units. No systematic pH gradients could be found to a depth of 5 mm beneath the surface of either medulla or cortex.
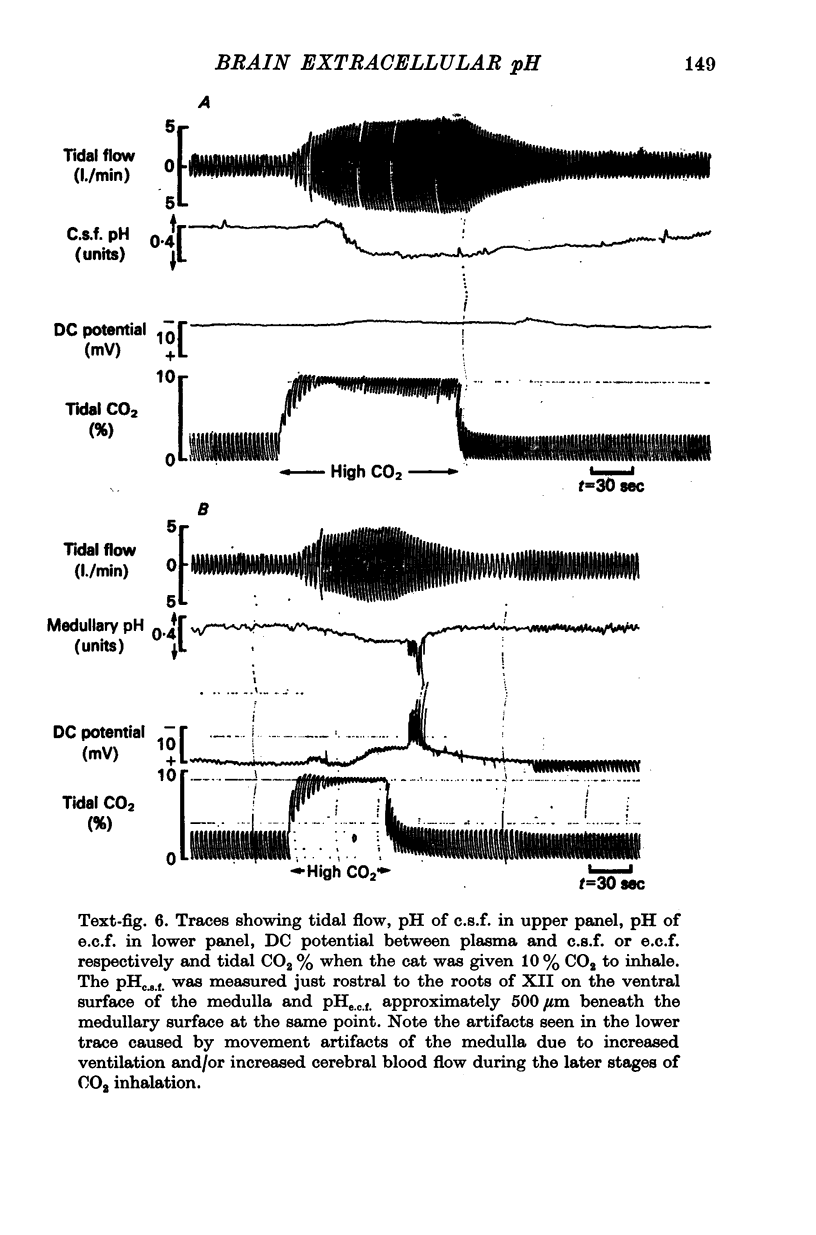
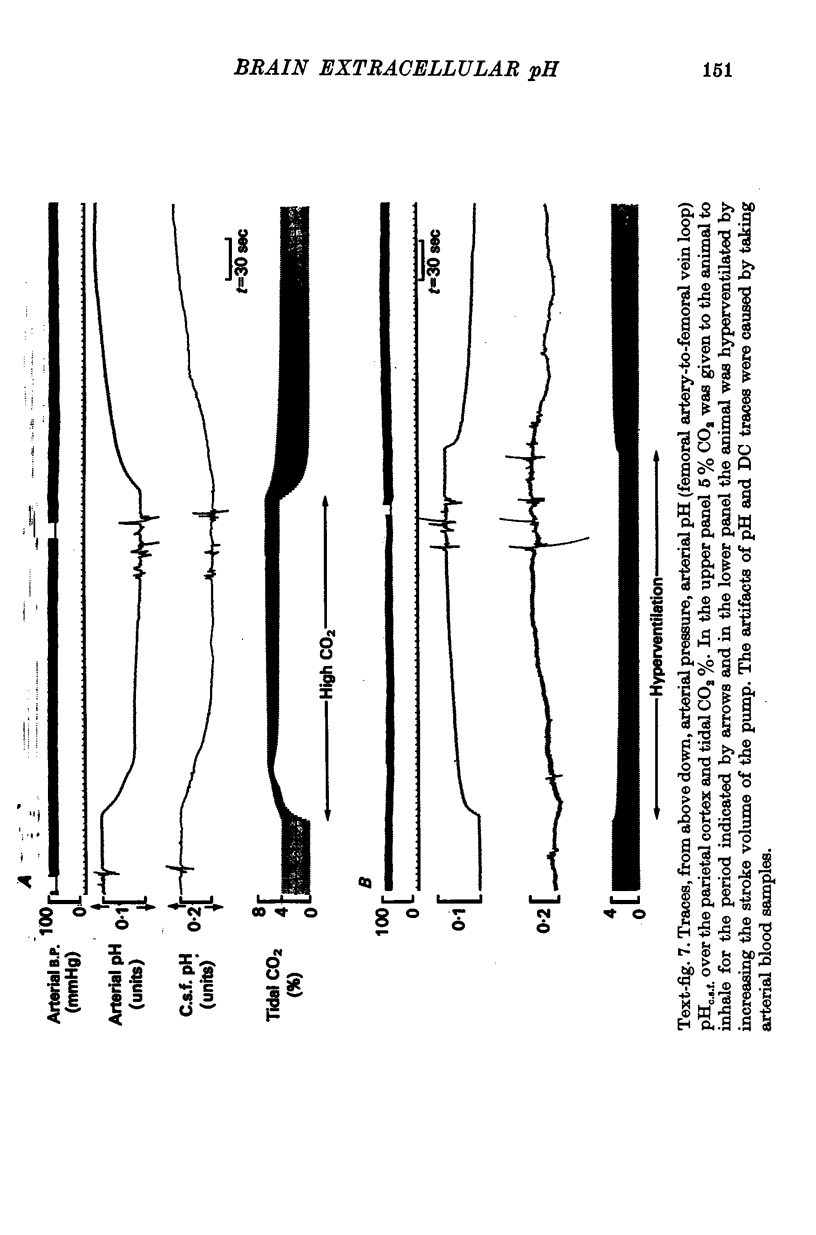
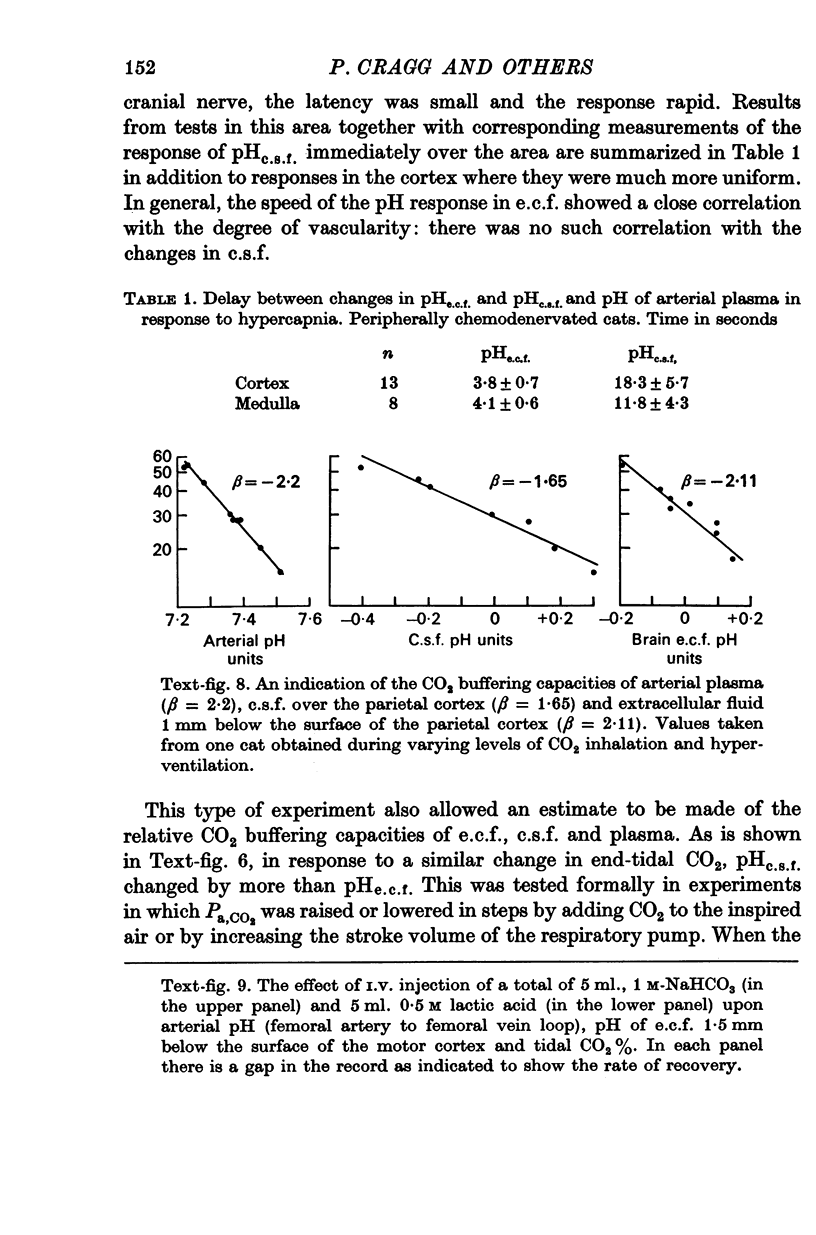
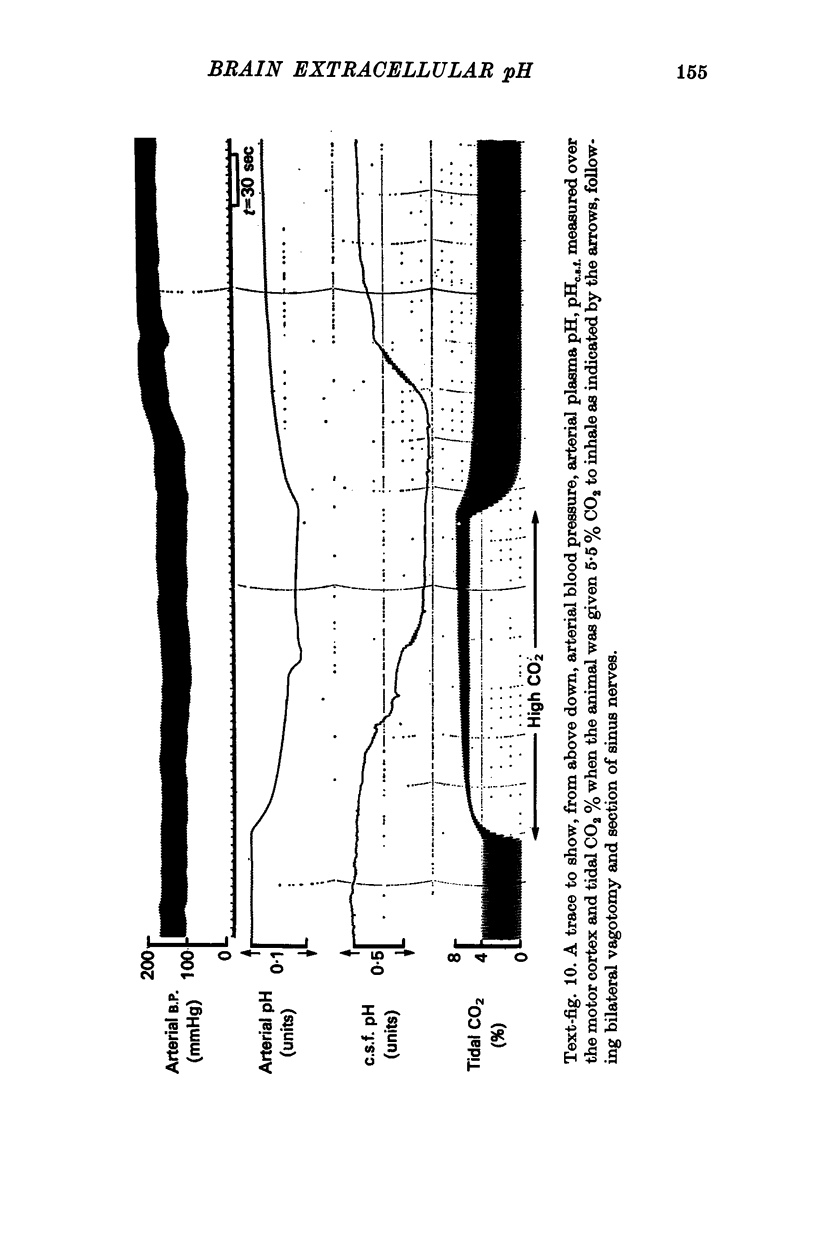
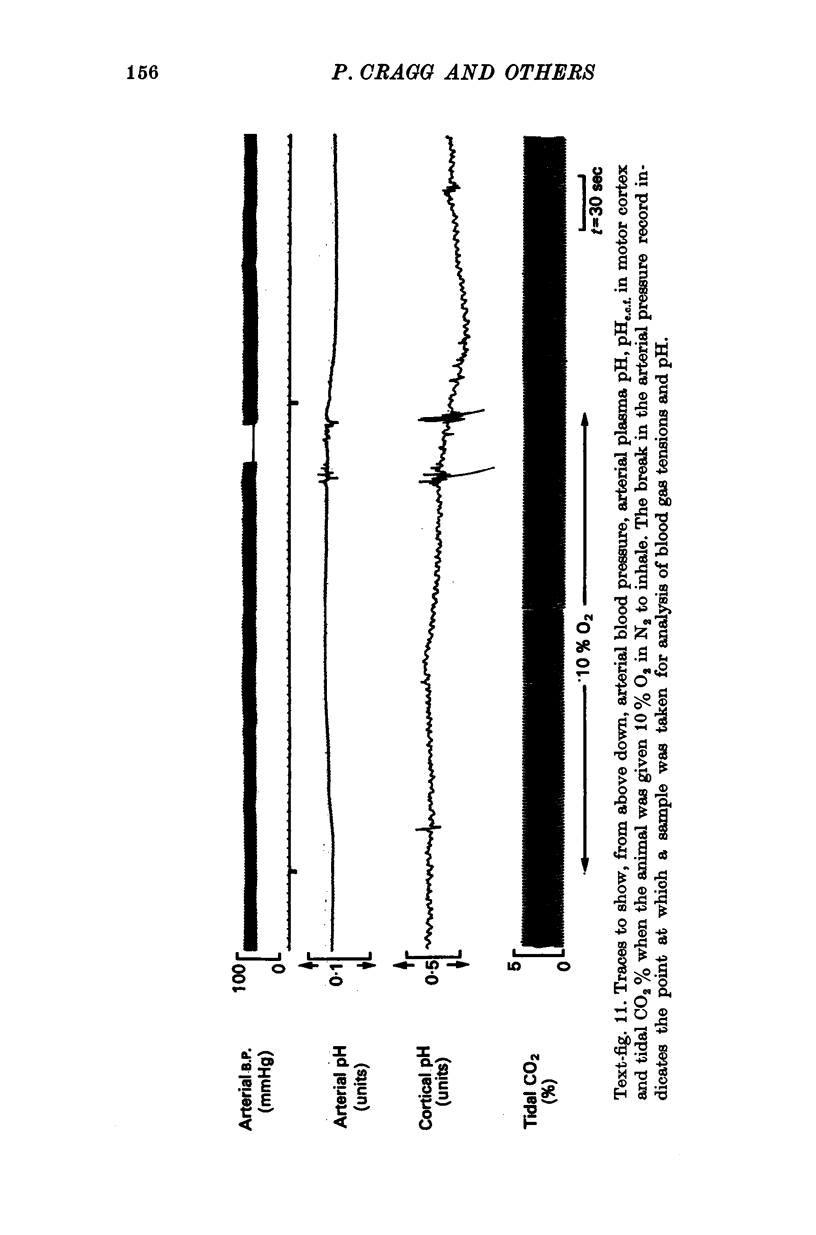
4. When plasma PCO2 was altered, pHe.c.f. changed with a latent period and speed of response related to the density of blood vessels. In vascular areas of the medulla and in the cortex, the latent period of 4 sec and the change of pHe.c.f. coincided with changes in ventilation. Changes in pHc.s.f. over the same areas were invariably slower.
5. CO2 buffering capacities were in the order plasma > e.c.f. > c.s.f. Typical values were respectively, -2·2, -2·1 and -1·6.
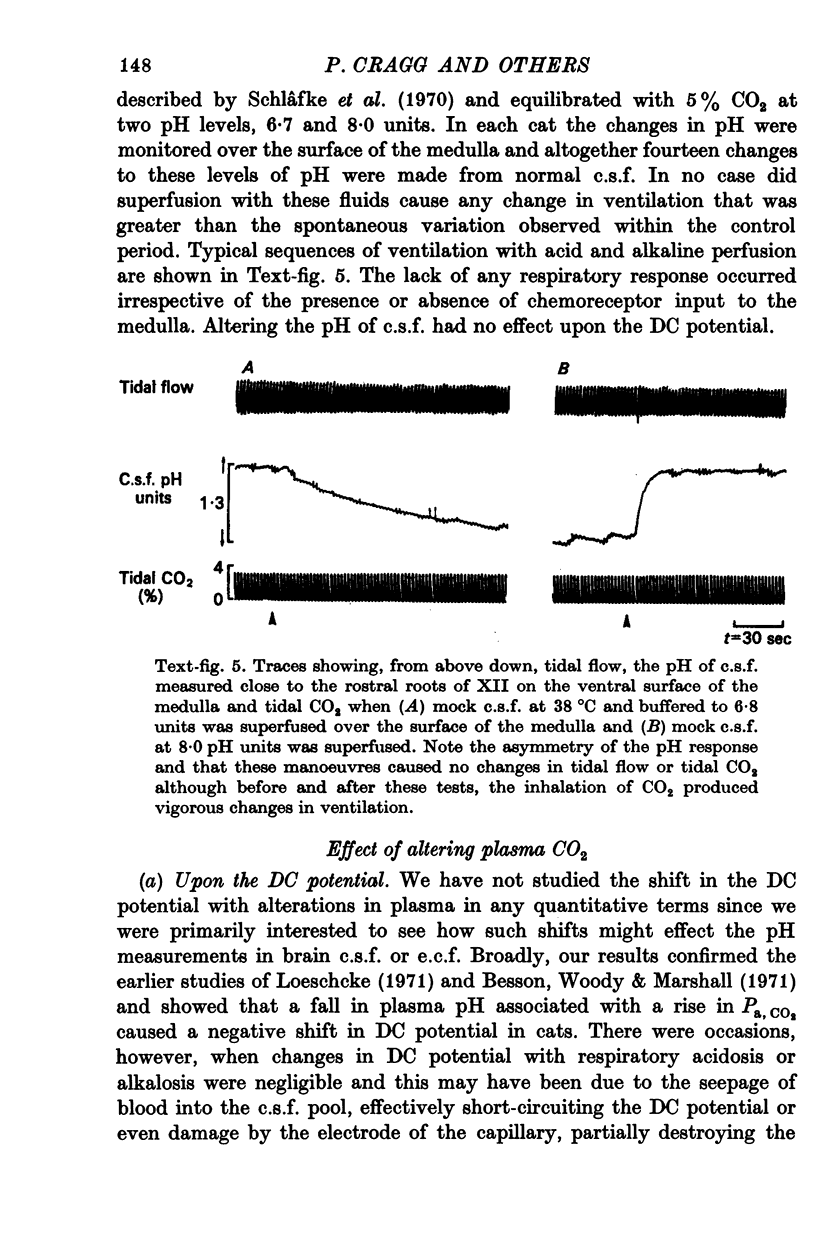
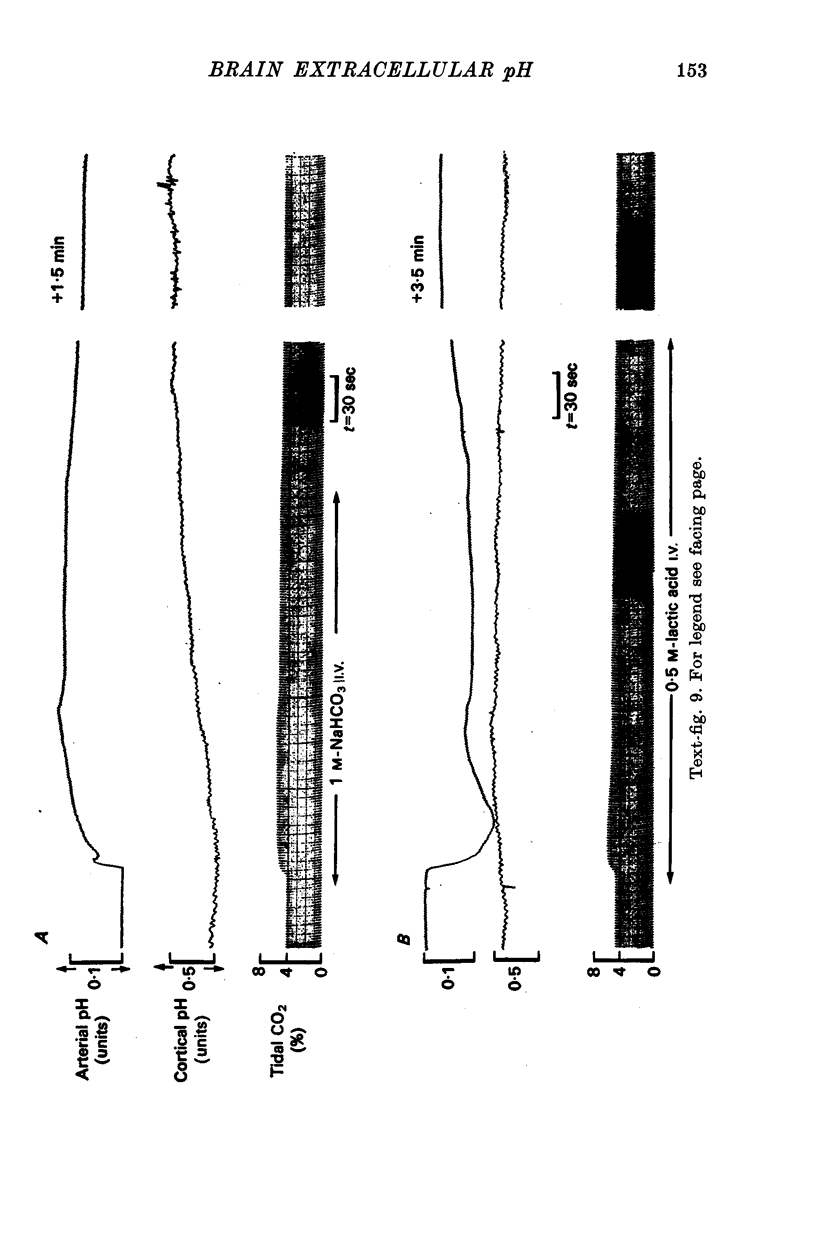
6. The pH of e.c.f. was unaffected by the intravenous injection of H+ and only slowly by the injection of HCO3-. Only up to a depth of 1 mm beneath the surface was pHe.c.f. affected by superfusion of mock c.s.f. in the range 6·8-8·0 units. This response had a latent period of 2-3 min and was complete in 15 min.
7. The pH of e.c.f. fell with hypoxia after a latent period of > 1 min and if all vasosensory nerves had been cut, pHe.c.f. was markedly affected by changes of blood pressure.
8. These results indicate that even under steady conditions, the pH of e.c.f. and c.s.f. is not identical, that pHe.c.f. is more obviously affected by changes in Pa, CO2 than pHc.s.f. and that putative H+ sensors which drive respiratory neurones are likely to be similarly affected.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biscoe T. J., Purves M. J. Observations on the rhythmic variation in the cat carotid body chemoreceptor activity which has the same period as respiration. J Physiol. 1967 Jun;190(3):389–412. doi: 10.1113/jphysiol.1967.sp008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. B., Jr, Clancy R. L. In vivo and in vitro CO2 blood buffer curves. J Appl Physiol. 1965 Sep;20(5):885–889. doi: 10.1152/jappl.1965.20.5.885. [DOI] [PubMed] [Google Scholar]
- Fencl V., Miller T. B., Pappenheimer J. R. Studies on the respiratory response to disturbances of acid-base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol. 1966 Mar;210(3):459–472. doi: 10.1152/ajplegacy.1966.210.3.459. [DOI] [PubMed] [Google Scholar]
- HELD D., FENCL V., PAPPENHEIMER J. R. ELECTRICAL POTENTIAL OF CEREBROSPINAL FLUID. J Neurophysiol. 1964 Sep;27:942–959. doi: 10.1152/jn.1964.27.5.942. [DOI] [PubMed] [Google Scholar]
- Heuser D., Astrup J., Lassen N. A., Betz B. E. Brain carbonic acid acidosis after acetazolamide. Acta Physiol Scand. 1975 Mar;93(3):385–390. doi: 10.1111/j.1748-1716.1975.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Hori T., Roth G. I., Yamamoto W. S. Respiratory sensitivity of rat brain-stem surface to chemical stimuli. J Appl Physiol. 1970 Jun;28(6):721–724. doi: 10.1152/jappl.1970.28.6.721. [DOI] [PubMed] [Google Scholar]
- Hornbein T. F., Pavlin E. G. Distribution of H+ and HCO3 minus between CSF and blood during respiratory alkalosis in dogs. Am J Physiol. 1975 Apr;228(4):1149–1154. doi: 10.1152/ajplegacy.1975.228.4.1149. [DOI] [PubMed] [Google Scholar]
- James I. M., MacDonell L. A. The role of baroreceptors and chemoreceptors in the regulation of the cerebral circulation. Clin Sci Mol Med. 1975 Nov;49(5):465–471. doi: 10.1042/cs0490465. [DOI] [PubMed] [Google Scholar]
- KIBLER R. F., O'NEILL R. P., ROBIN E. D. INTRACELLULAR ACID-BASE RELATIONS OF DOG BRAIN WITH REFERENCE TO THE BRAIN EXTRACELLULAR VOLUME. J Clin Invest. 1964 Mar;43:431–443. doi: 10.1172/JCI104928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., RANDIC M., SIESJOE B. K. CORTICAL CO2 TENSION AND NEURONAL EXCITABILITY. J Physiol. 1965 Jan;176:105–122. doi: 10.1113/jphysiol.1965.sp007538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjällquist A., Nardini M., Siesjö B. K. The regulation of extra- and intracellular acid-base parameters in the rat brain during hyper- and hypocapnia. Acta Physiol Scand. 1969 Aug;76(4):485–494. doi: 10.1111/j.1748-1716.1969.tb04495.x. [DOI] [PubMed] [Google Scholar]
- Kjällquist A., Siesjö B. K. The CSF-blood potential in sustained acidosis and alkalosis in the rat. Acta Physiol Scand. 1967 Oct-Nov;71(2):255–256. doi: 10.1111/j.1748-1716.1967.tb03732.x. [DOI] [PubMed] [Google Scholar]
- Kjällquist A. The CSF-blood potential in sustained acid-base changes in the rat. With calculations of electrochemical potential differences for H+ and HCO3. Acta Physiol Scand. 1970 Jan;78(1):85–93. doi: 10.1111/j.1748-1716.1970.tb04642.x. [DOI] [PubMed] [Google Scholar]
- Leniger-Follert E., Lübbers D. W., Wrabetz W. Regulation of local tissue PO2 of the brain cortex at different arterial O2 pressures. Pflugers Arch. 1975 Aug 29;359(1-2):81–95. doi: 10.1007/BF00581279. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. T., Boyarsky L. L. Neurophysiological investigations of medullary chemosensitive areas of respiration. Respir Physiol. 1972 Dec;16(3):362–376. doi: 10.1016/0034-5687(72)90065-5. [DOI] [PubMed] [Google Scholar]
- Loeschcke H. H., De Lattre J., Schläfke M. E., Trouth C. O. Effects on respiration and circulation of electrically stimulating the ventral surface of the medulla oblongata. Respir Physiol. 1970 Sep;10(2):184–197. doi: 10.1016/0034-5687(70)90082-4. [DOI] [PubMed] [Google Scholar]
- Loeschcke H. H., Sugioka K. pH of cerebrospinal fluid in the cisterna Magna and on the surface of the choroid plexus of the 4th ventricle and its effect on ventilation in experimental disturbances of acid base balance. Transients and steady states. Pflugers Arch. 1969;312(4):161–188. doi: 10.1007/BF00586927. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Carman C. T., Severinghaus J. W., Richardson B. W., Singer M. M., Shnider S. Stability of cerebrospinal fluid pH in chronic acid-base disturbances in blood. J Appl Physiol. 1965 May;20(3):443–452. doi: 10.1152/jappl.1965.20.3.443. [DOI] [PubMed] [Google Scholar]
- Mithoefer J. C., Karetzky M. S., Porter W. F. The in vivo carbon dioxide titration curve in the presence of hypoxia. Respir Physiol. 1968 Jan;4(1):15–23. doi: 10.1016/0034-5687(68)90003-0. [DOI] [PubMed] [Google Scholar]
- POSNER J. B., SWANSON A. G., PLUM F. ACID-BASE BALANCE IN CEREBROSPINAL FLUID. Arch Neurol. 1965 May;12:479–496. doi: 10.1001/archneur.1965.00460290035006. [DOI] [PubMed] [Google Scholar]
- Pannier J. L., Weyne J., Leusen I. The CDF-blood potential and the regulation of the bicarbonate concentration of CSF during acidosis in the cat. Life Sci I. 1971 Mar 1;10(5):287–300. doi: 10.1016/0024-3205(71)90316-x. [DOI] [PubMed] [Google Scholar]
- Pavlin E. G., Hornbein ttf Distribution of H+ and HCO3 minus between CSF and blood during metabolic alkalosis in dogs. Am J Physiol. 1975 Apr;228(4):1141–1144. doi: 10.1152/ajplegacy.1975.228.4.1141. [DOI] [PubMed] [Google Scholar]
- Pavlin E. G., Hornbein T. F. Distribution of H+ and HCO3 minus between CSF and blood during metabolic acidosis in dogs. Am J Physiol. 1975 Apr;228(4):1134–1140. doi: 10.1152/ajplegacy.1975.228.4.1134. [DOI] [PubMed] [Google Scholar]
- Pavlin E. G., Hornbein T. F. Distribution of H+ and HCO3 minus between CSF and blood during respiratory acidosis in dogs. Am J Physiol. 1975 Apr;228(4):1145–1148. doi: 10.1152/ajplegacy.1975.228.4.1145. [DOI] [PubMed] [Google Scholar]
- Pelligrino D. A., Dempsey J. A. Dependence of CSF on plasma bicarbonate during hypocapnia and hypoxemic hypocapnia. Respir Physiol. 1976 Feb;26(1):11–26. doi: 10.1016/0034-5687(76)90048-7. [DOI] [PubMed] [Google Scholar]
- Persson L., Hansson H. A., Sourander P. Extravasation, spread and cellular uptake of Evans blue-labelled albumin around a reproducible small stab-wound in the rat brain. Acta Neuropathol. 1976 Mar 15;34(2):125–136. doi: 10.1007/BF00684663. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ W. B., RELMAN A. S. A critique of the parameters used in the evaluation of acid-base disorders. "Whole-blood buffer base" and "standard bicarbonate" compared with blood pH and plasma bicarbonate concentration. N Engl J Med. 1963 Jun 20;268:1382–1388. doi: 10.1056/NEJM196306202682503. [DOI] [PubMed] [Google Scholar]
- Schlaefke M. E., See W. R., Loeschcke H. H. Ventilatory response to alterations of H+ ion concentration in small areas of the ventral medullary surface. Respir Physiol. 1970 Sep;10(2):198–212. doi: 10.1016/0034-5687(70)90083-6. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Pontén U. Acid-base changes in the brain in nonrespiratory acidosis and alkalosis. Exp Brain Res. 1966;2(2):176–190. doi: 10.1007/BF00240405. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Pontén U. The buffer capacity of brain tissue and of equivalent systems. Ann N Y Acad Sci. 1966 Apr 1;133(1):180–194. doi: 10.1111/j.1749-6632.1966.tb50724.x. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Symposium on acid-base homeostasis. The regulation of cerebrospinal fluid pH. Kidney Int. 1972 May;1(5):360–374. doi: 10.1038/ki.1972.47. [DOI] [PubMed] [Google Scholar]
- Silver I. A. Some observations on the cerebral cortex with an ultramicro, membrane-covered, oxygen electrode. Med Electron Biol Eng. 1965 Oct;3(4):377–387. doi: 10.1007/BF02476132. [DOI] [PubMed] [Google Scholar]
- Sorensen S. C., Severinghaus J. W. Effect of cerebral acidosis on the CSF-blood potential difference. Am J Physiol. 1970 Jul;219(1):68–71. doi: 10.1152/ajplegacy.1970.219.1.68. [DOI] [PubMed] [Google Scholar]
- Stopford J. S. The Arteries of the Pons and Medulla Oblongata. J Anat Physiol. 1916 Jan;50(Pt 2):131–164. [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M., Deetjen P., Thurau K., Ingvar D. H., Lassen N. A. Micropuncture evaluation of the importance of perivascular pH for the arteriolar diameter on the brain surface. Pflugers Arch. 1970;316(2):152–163. doi: 10.1007/BF00586483. [DOI] [PubMed] [Google Scholar]
- Weyne J., Demeester G., Leusen I. Bicarbonate and chloride shifts in rat brain during acute and prolonged respiratory acid-base changes. Arch Int Physiol Biochim. 1968 Jul;76(3):415–433. doi: 10.3109/13813456809058715. [DOI] [PubMed] [Google Scholar]
- Weyne J., Pannier J. L., Demeester G., Leusen I. Bicarbonate and chloride of rat brain during infusion-induced changes in bicarbonate concentration of blood. Pflugers Arch. 1970;320(1):45–63. doi: 10.1007/BF00588456. [DOI] [PubMed] [Google Scholar]