Abstract
1. The responses of single units in the cat's primary visual cortex to moving bars have been examined quantitatively as a function of bar length.
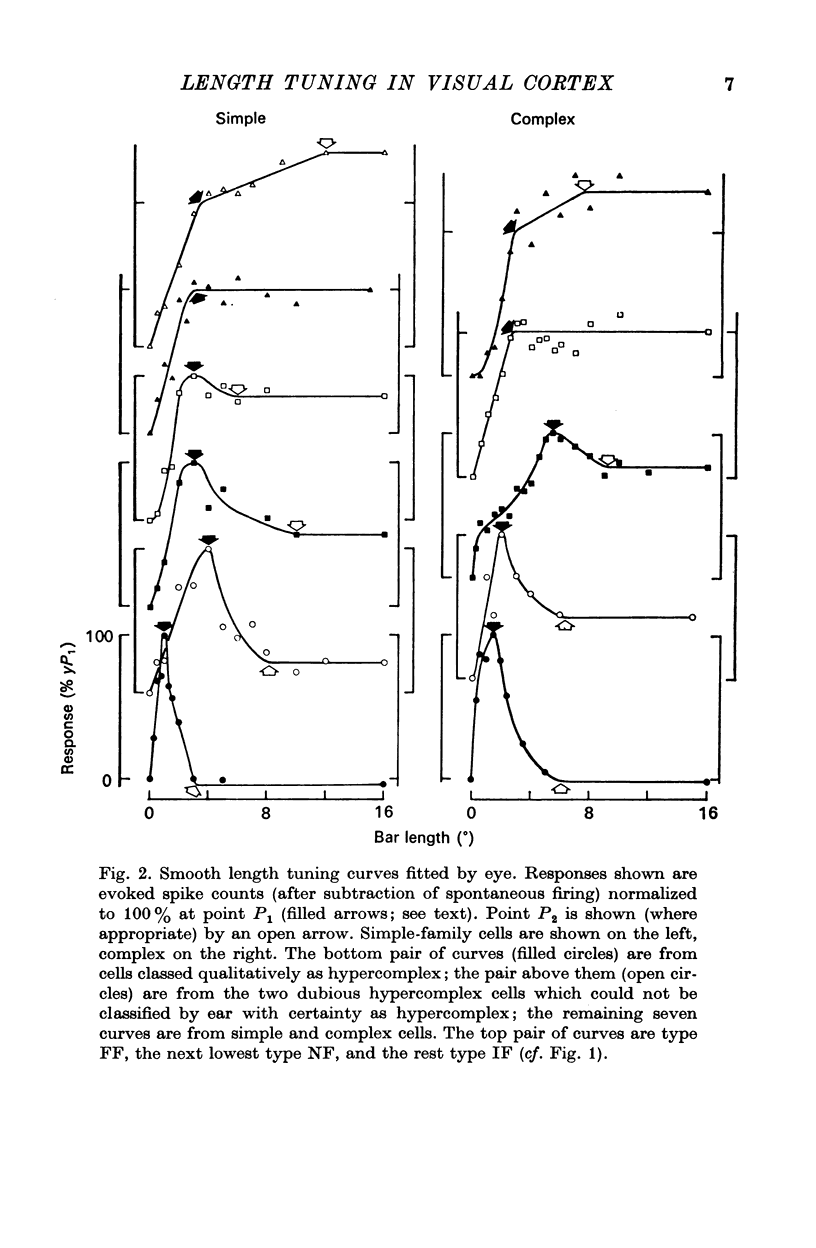
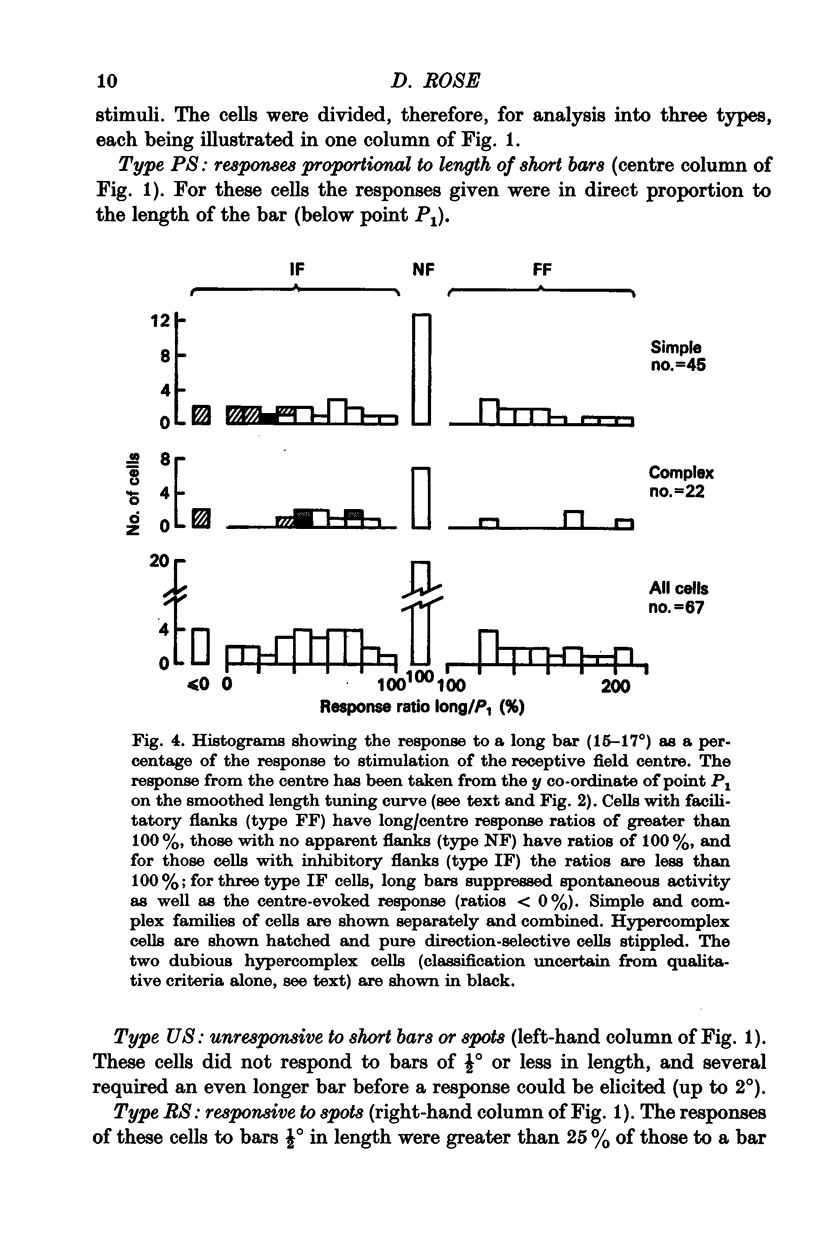
2. For about half the cells studied, very long bars evoked weaker responses than short bars, implying that there were inhibitory regions flanking the receptive field centre. In another quarter of the cell sample, there was evidence of flanking regions which were facilitatory in effect.
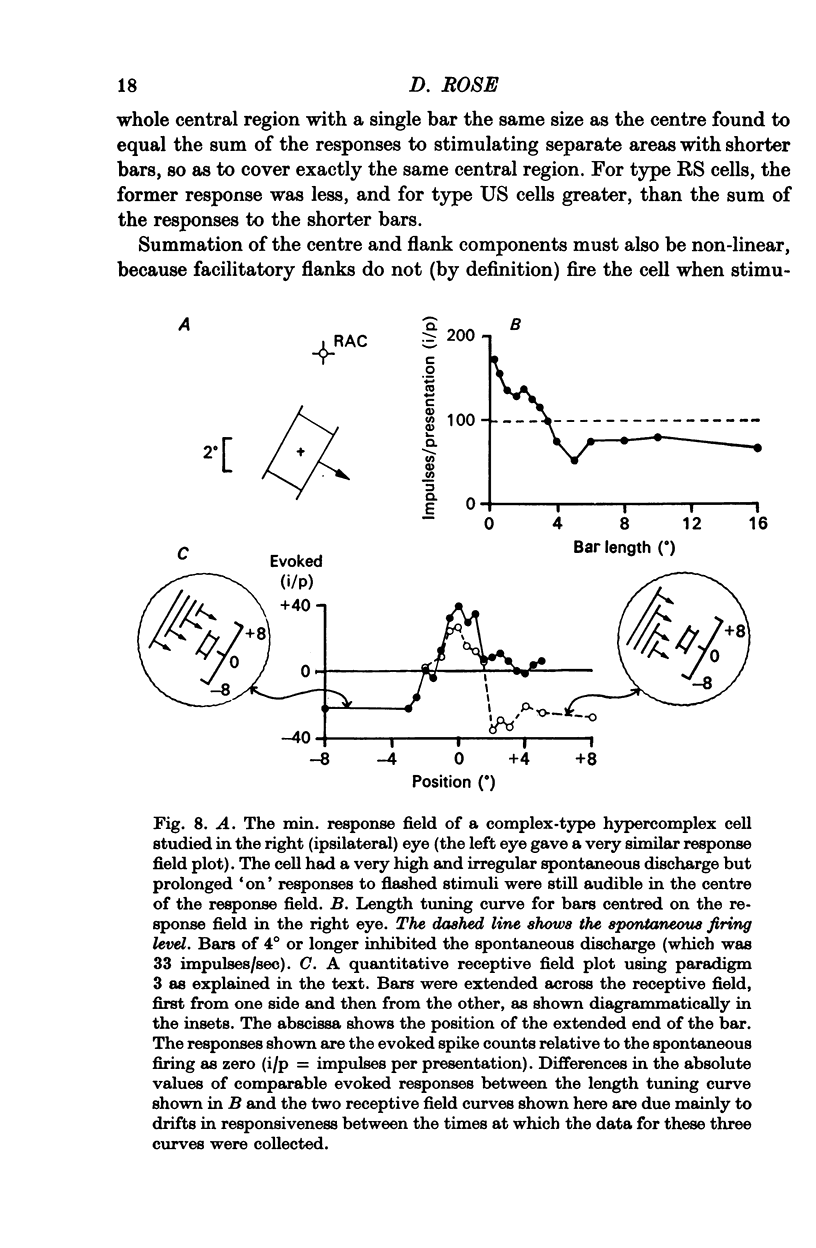
3. The strength of the flanking regions was found to vary from cell to cell and there was no sudden transition between cells which were `hyper-complex' and those which were not.
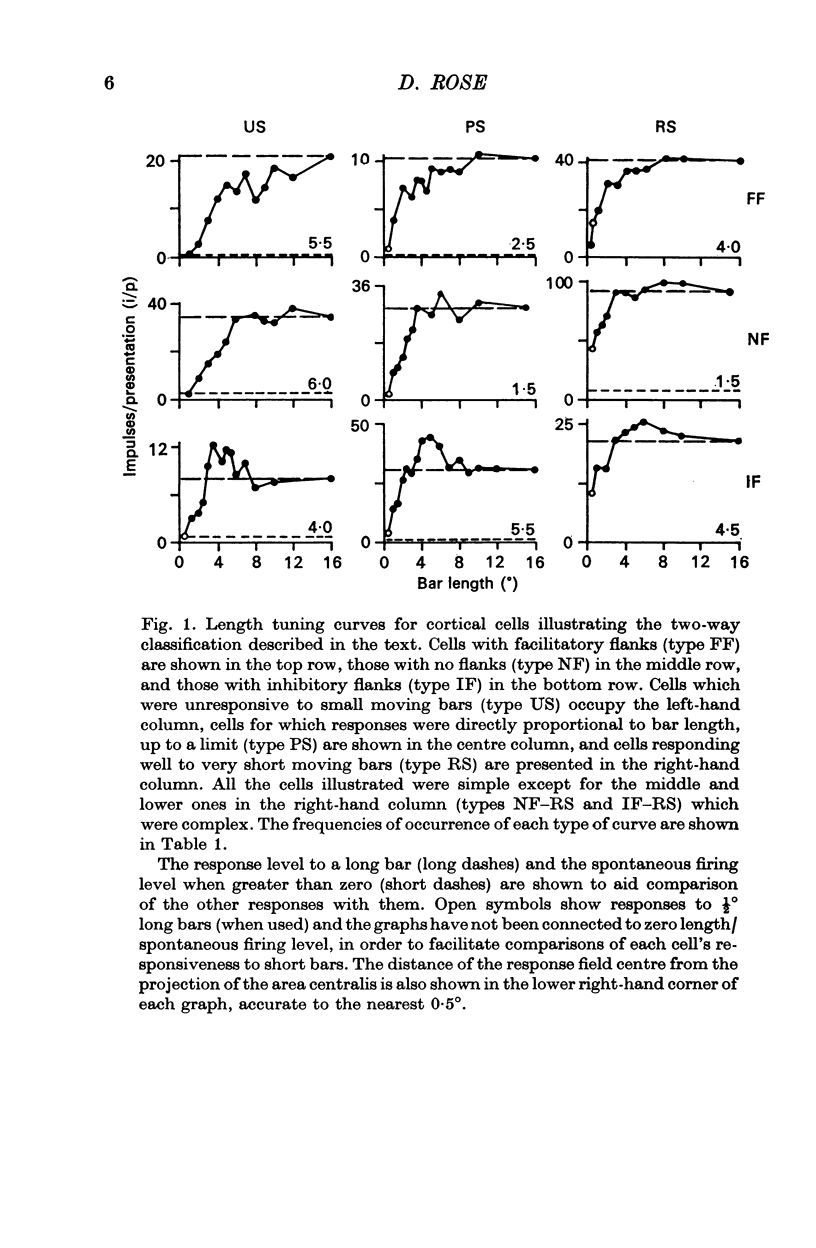
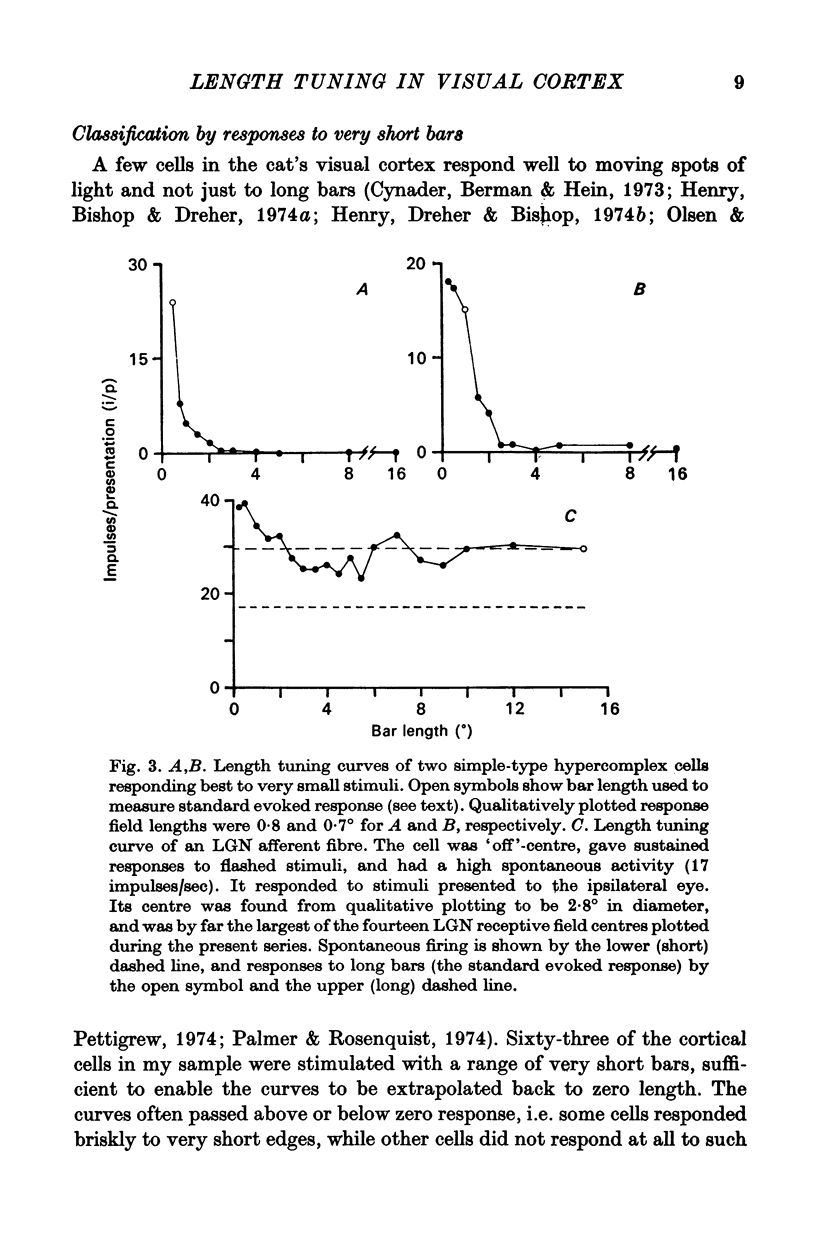
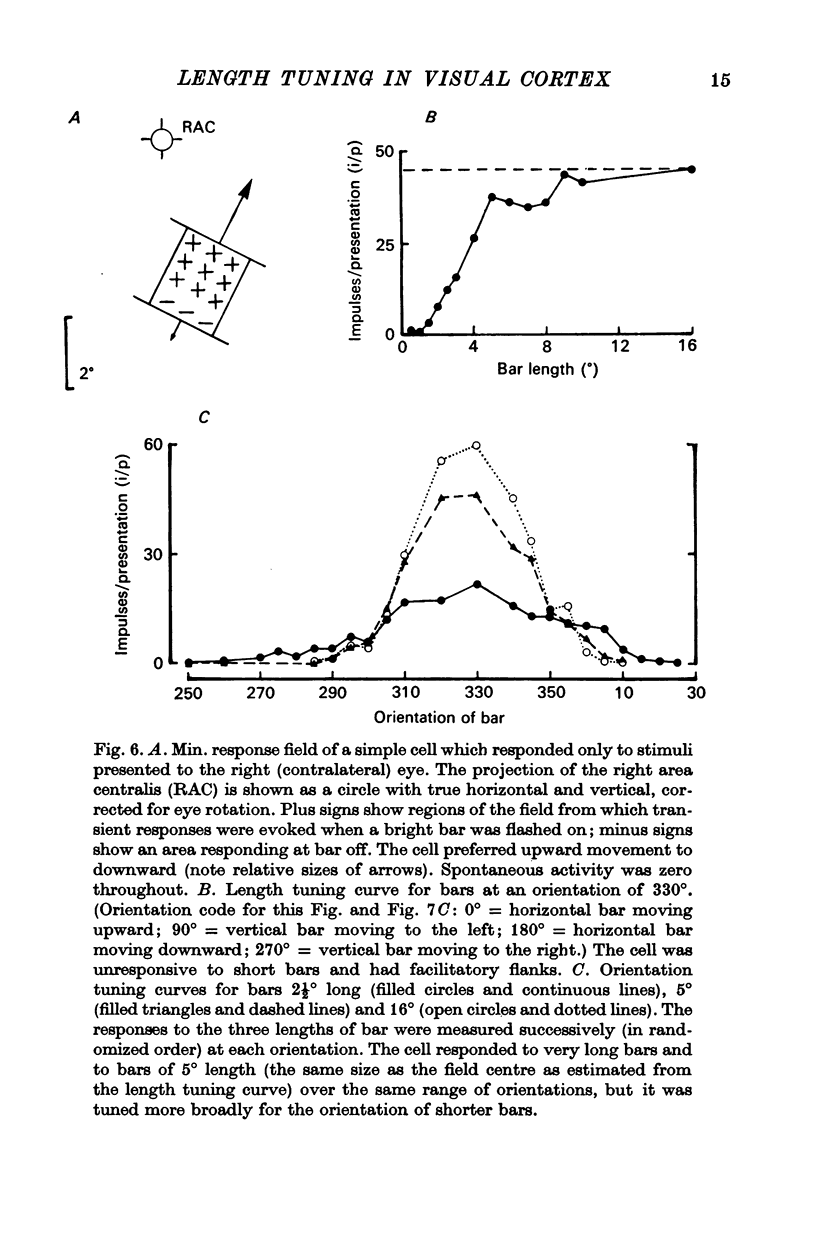
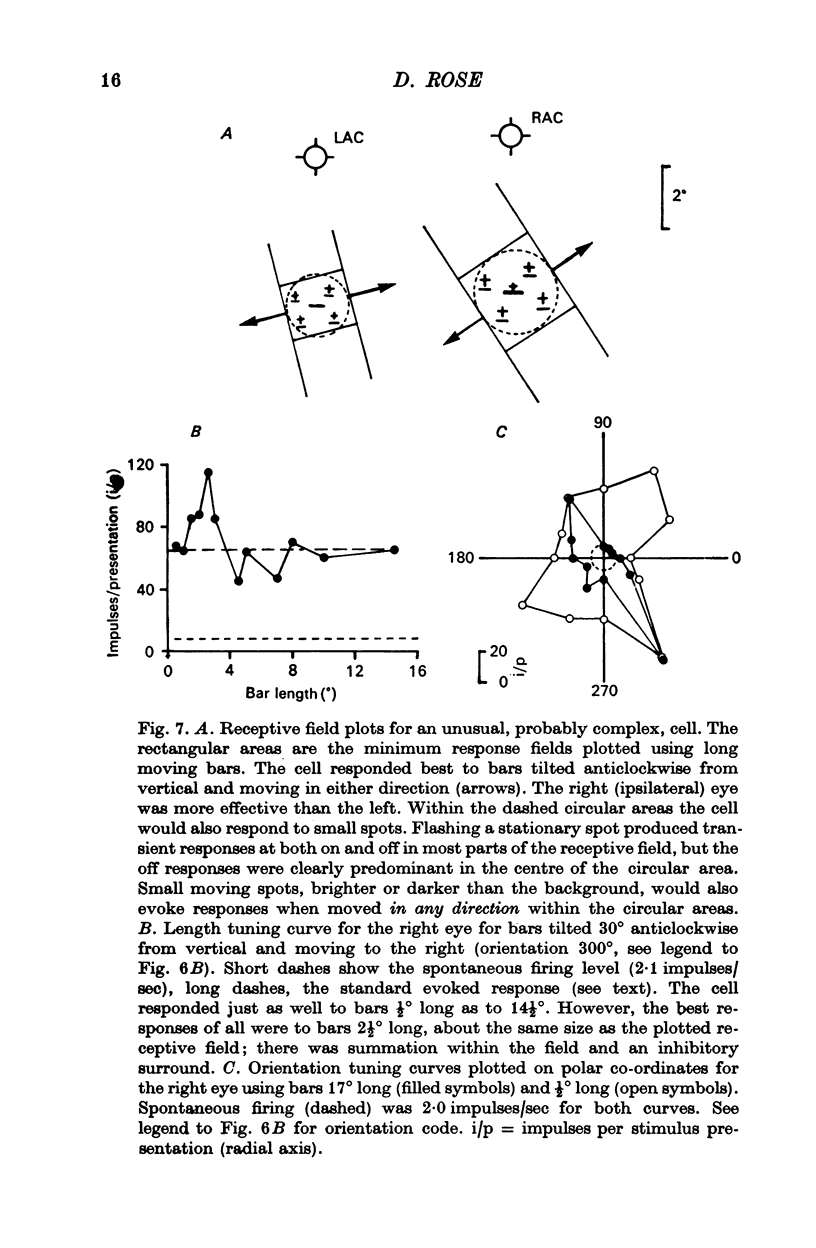
4. Within the central region of the receptive field, the responses of most (but not all) cells increased with bar length. About half the cells responded to very short bars or spots of light, but about one in six would not respond at all to short bars.
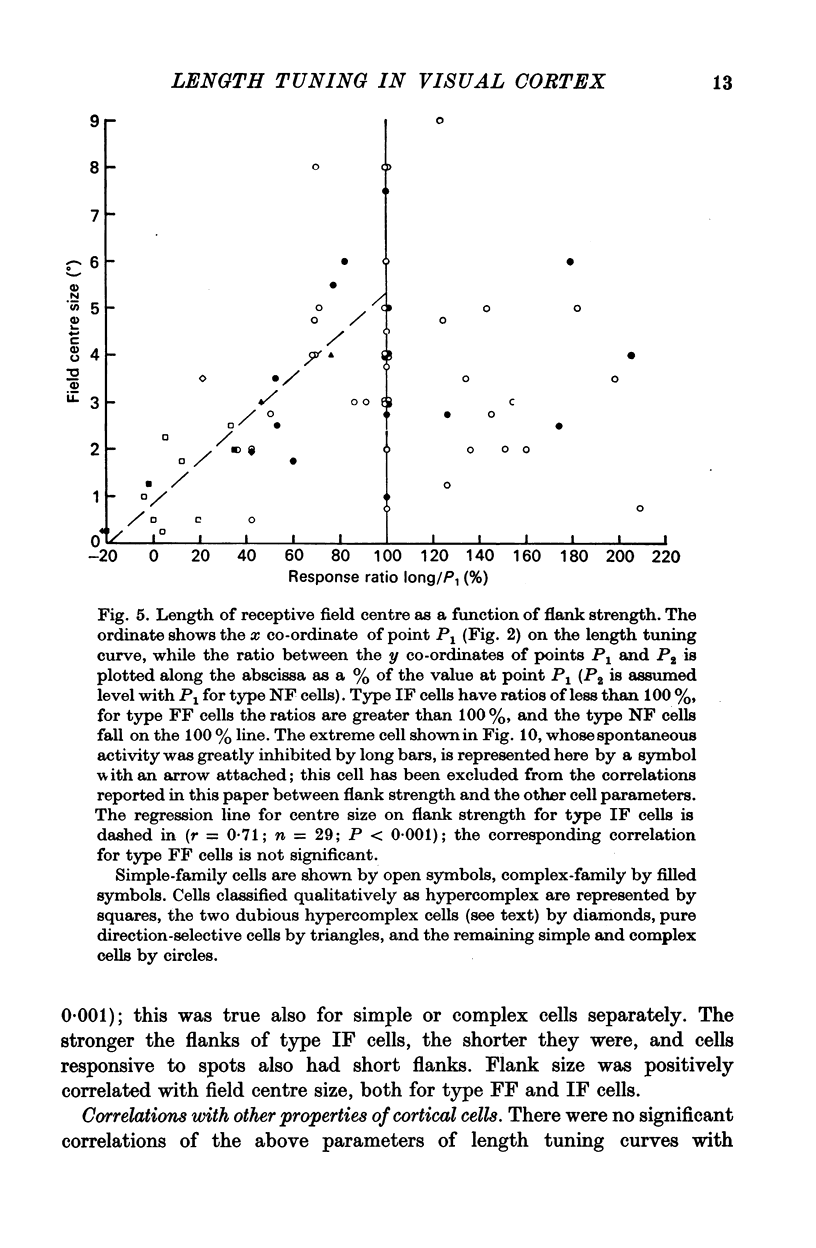
5. Correlations were sought between the properties of cells as simple or complex, their responsiveness to moving spots of light, the size of their receptive field centre and the polarity, strength and size of their receptive field flanks. Simple and complex cells with small receptive fields were more likely to respond well to spots, and to have strong inhibitory flanks.
6. Correlations were also sought between the above properties and several other parameters of cell behaviour. Cells with strong inhibitory flanks were found to be more broadly tuned for orientation. Individual cells were also more broadly tuned for the orientation of short bars than of long bars.
7. Evidence was obtained that spatial summation can be linear or non-linear for different cells.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Receptive fields of simple cells in the cat striate cortex. J Physiol. 1973 May;231(1):31–60. doi: 10.1113/jphysiol.1973.sp010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H. Striate neurons: receptive field concepts. Invest Ophthalmol. 1972 May;11(5):346–354. [PubMed] [Google Scholar]
- Blakemore C., Tobin E. A. Lateral inhibition between orientation detectors in the cat's visual cortex. Exp Brain Res. 1972;15(4):439–440. doi: 10.1007/BF00234129. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Reversal of the physiological effects of monocular deprivation in kittens: further evidence for a sensitive period. J Physiol. 1974 Feb;237(1):195–216. doi: 10.1113/jphysiol.1974.sp010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodis-Wollner I. G., Pollen D. A., Ronner S. F. Responses of complex cells in the visual cortex of the cat as a function of the length of moving slits. Brain Res. 1976 Nov 5;116(2):205–216. doi: 10.1016/0006-8993(76)90900-8. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Kuhnt U., Benevento L. A. An intracellular analysis of visual cortical neurones to moving stimuli: response in a co-operative neuronal network. Exp Brain Res. 1974;21(3):251–274. doi: 10.1007/BF00235746. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O., Innocenti G. M., Brooks D. Vertical organization in the visual cortex (area 17) in the cat. Exp Brain Res. 1974;21(3):315–336. doi: 10.1007/BF00235750. [DOI] [PubMed] [Google Scholar]
- Cynader M., Berman N., Hein A. Cats reared in stroboscopic illumination: effects on receptive fields in visual cortex. Proc Natl Acad Sci U S A. 1973 May;70(5):1353–1354. doi: 10.1073/pnas.70.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B. Hypercomplex cells in the cat's striate cortex. Invest Ophthalmol. 1972 May;11(5):355–356. [PubMed] [Google Scholar]
- Dreher B., Sanderson K. J. Receptive field analysis: responses to moving visual contours by single lateral geniculate neurones in the cat. J Physiol. 1973 Oct;234(1):95–118. doi: 10.1113/jphysiol.1973.sp010336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Dreher B. Orientation, axis and direction as stimulus parameters for striate cells. Vision Res. 1974 Sep;14(9):767–777. doi: 10.1016/0042-6989(74)90141-2. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Tupper R. M., Dreher B. Orientation specificity and response variability of cells in the striate cortex. Vision Res. 1973 Sep;13(9):1771–1779. doi: 10.1016/0042-6989(73)90094-1. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Dreher B., Bishop P. O. Orientation specificity of cells in cat striate cortex. J Neurophysiol. 1974 Nov;37(6):1394–1409. doi: 10.1152/jn.1974.37.6.1394. [DOI] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Proceedings: Properties of sustained-X, transient-Y and transient-X cells in the cat's lateral geniculate nucleus. J Physiol. 1976 Jan;254(1):65P–66P. [PubMed] [Google Scholar]
- Innocenti G. M., Fiore L. Post-synaptic inhibitory components of the responses to moving stimuli in area 17. Brain Res. 1974 Nov 8;80(1):122–126. doi: 10.1016/0006-8993(74)90728-8. [DOI] [PubMed] [Google Scholar]
- Kelly J. P., Van Essen D. C. Cell structure and function in the visual cortex of the cat. J Physiol. 1974 May;238(3):515–547. doi: 10.1113/jphysiol.1974.sp010541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The unresponsive regions of visual cortical receptive fields. Vision Res. 1976;16(10):1131–1139. doi: 10.1016/0042-6989(76)90253-4. [DOI] [PubMed] [Google Scholar]
- Movshon J. A. The velocity tuning of single units in cat striate cortex. J Physiol. 1975 Aug;249(3):445–468. doi: 10.1113/jphysiol.1975.sp011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson C. R., Pettigrew J. D. Single units in visual cortex of kittens reared in stroboscopic illumination. Brain Res. 1974 Apr 19;70(2):189–204. doi: 10.1016/0006-8993(74)90312-6. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Rose D., Blakemore C. An analysis of orientation selectivity in the cat's visual cortex. Exp Brain Res. 1974 Apr 30;20(1):1–17. doi: 10.1007/BF00239014. [DOI] [PubMed] [Google Scholar]
- Rose D., Blakemore C. Effects of bicuculline on functions of inhibition in visual cortex. Nature. 1974 May 24;249(455):375–377. doi: 10.1038/249375a0. [DOI] [PubMed] [Google Scholar]
- Rose D. Proceedings: The hypercomplex cell classification in the cat's striate cortex. J Physiol. 1974 Oct;242(2):123P–125P. [PubMed] [Google Scholar]
- Shapley R., Hochstein S. Visual spatial summation in two classes of geniculate cells. Nature. 1975 Jul 31;256(5516):411–413. doi: 10.1038/256411a0. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975 Sep;250(2):305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M., Versiani V. Synaptic mechanisms contributing to the lenght preference of hypercomplex cells [proceedings]. J Physiol. 1976 Dec;263(1):171P–172P. [PubMed] [Google Scholar]
- Singer W., Tretter F., Cynader M. Organization of cat striate cortex: a correlation of receptive-field properties with afferent and efferent connections. J Neurophysiol. 1975 Sep;38(5):1080–1098. doi: 10.1152/jn.1975.38.5.1080. [DOI] [PubMed] [Google Scholar]
- Toyama K., Maekawa K., Takeda T. An analysis of neuronal circuitry for two types of visual cortical neurones classified on the basis of their responses to photic stimuli. Brain Res. 1973 Oct 26;61:395–399. doi: 10.1016/0006-8993(73)90545-3. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Sherman S. M. Receptive-field characteristics of neurons in cat striate cortex: Changes with visual field eccentricity. J Neurophysiol. 1976 May;39(3):512–533. doi: 10.1152/jn.1976.39.3.512. [DOI] [PubMed] [Google Scholar]



