Abstract
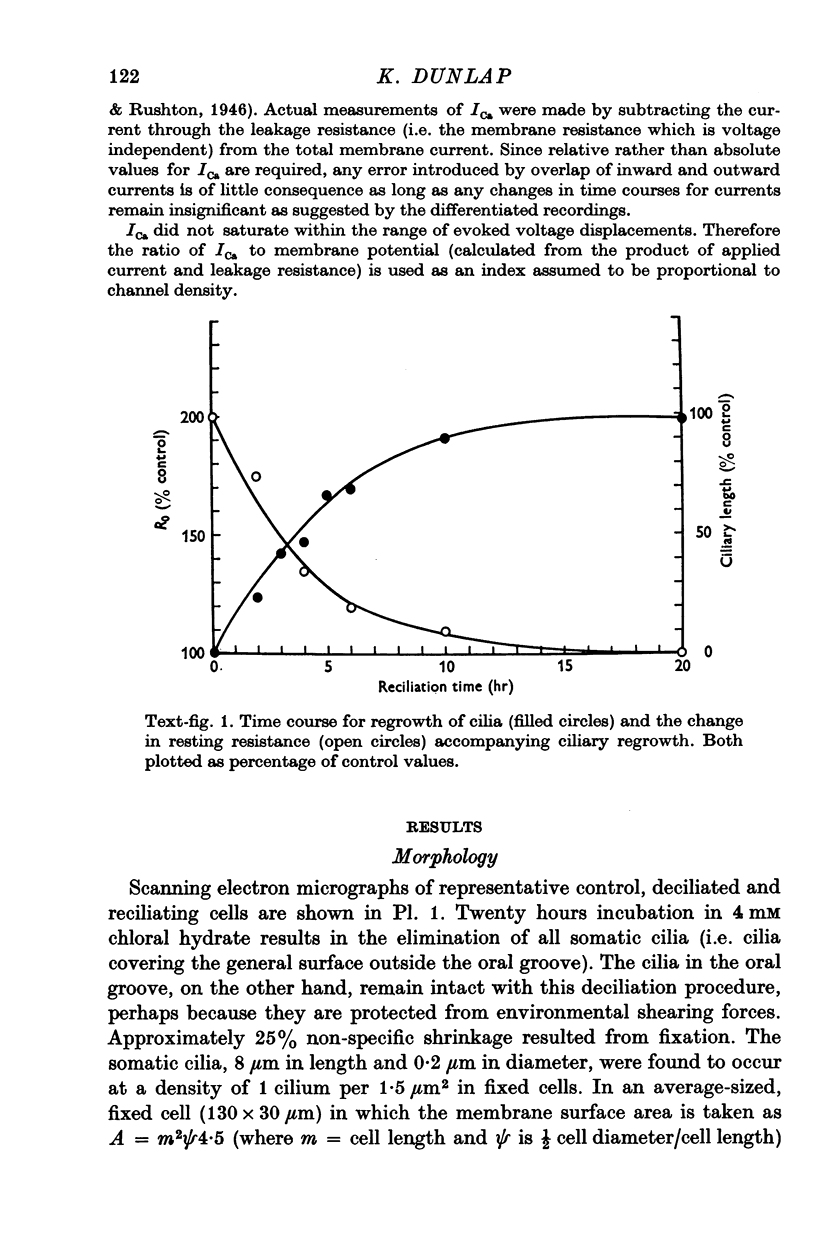
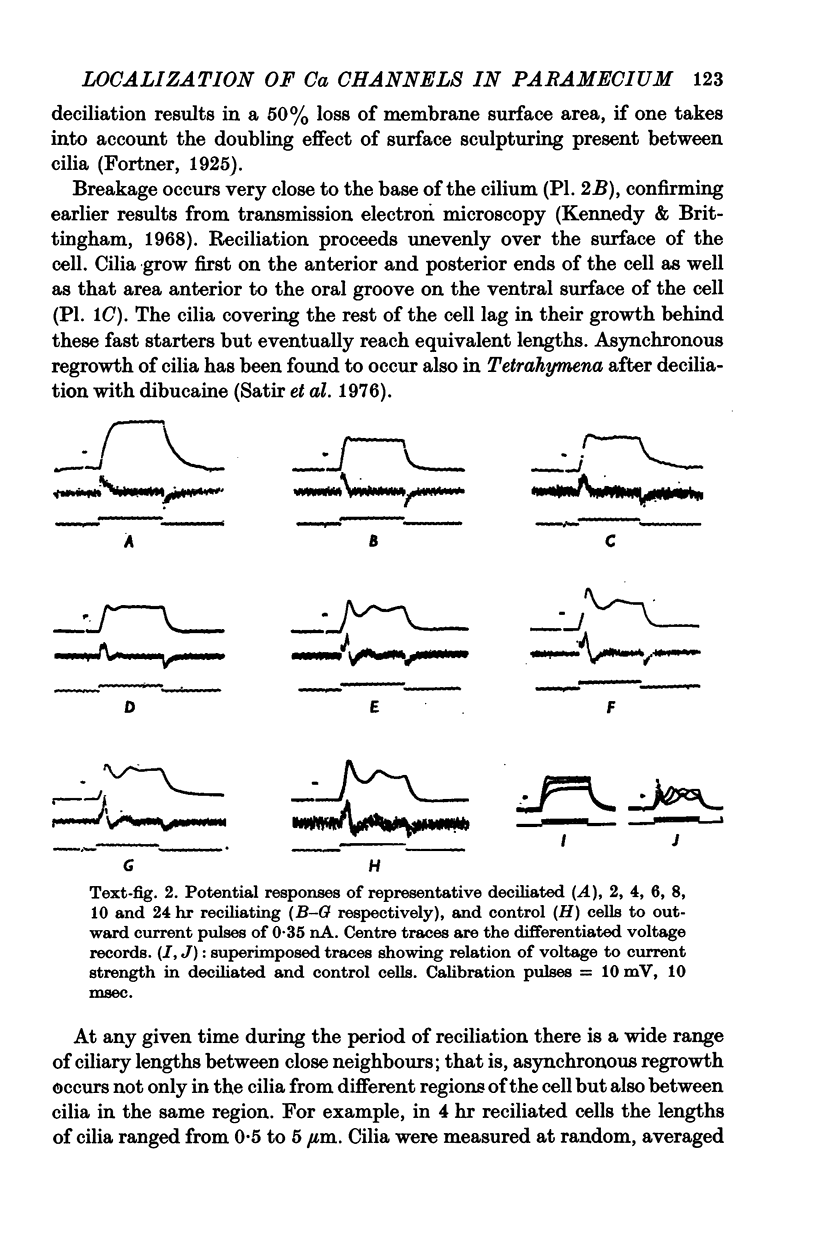
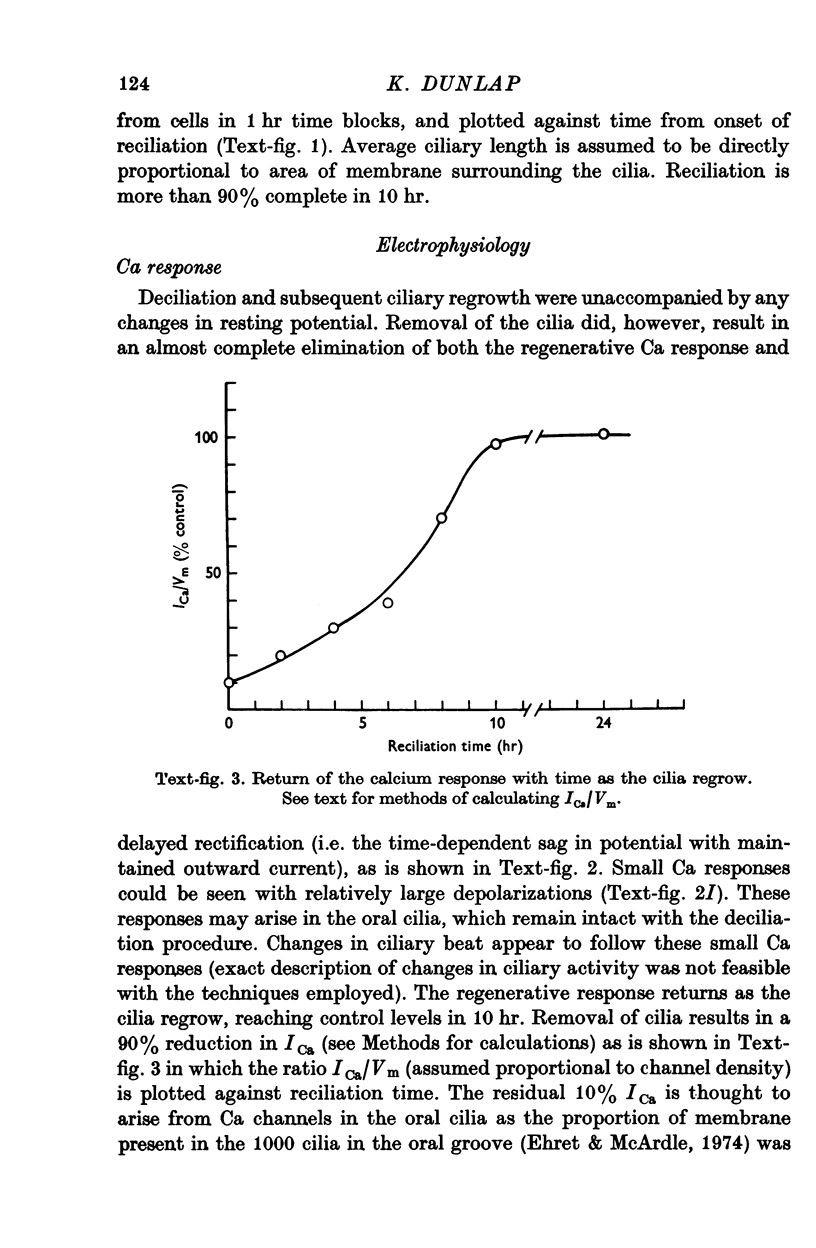
1. Electrical recordings from Paramecium caudatum were made after removal of the cilia with chloral hydrate and during ciliary regrowth to study the electrical properties of that portion of the surface membrane enclosing the ciliary axoneme. 2. Removal of the somatic cilia (a 50% reduction in membrane surface area) results in an almost complete elimination of the regenerative Ca response, all-or-none Ba2+ spike, and delayed rectification. 3. A twofold increase in input resistance resulted from the 50% reduction in membrane surface area. 4. The electrical properties remained unchanged, despite prolonged exposure to the chloral hydrate, until the cilia were mechanically removed. 5. Restoration of the Ca response accompanied ciliary regrowth, so that complete excitability returns when the cilia regain their original lengths. 6. It is concluded that the voltage-sensitive Ca channels are localized to that portion of surface membrane surrounding the cilia. 7. Measurements of membrane constants before and after deciliation and estimations of the cable constants of a single cilium suggest that the cilia of Paramecium may be fully isopotential along their length and with the major cell compartment.
Full text
PDF






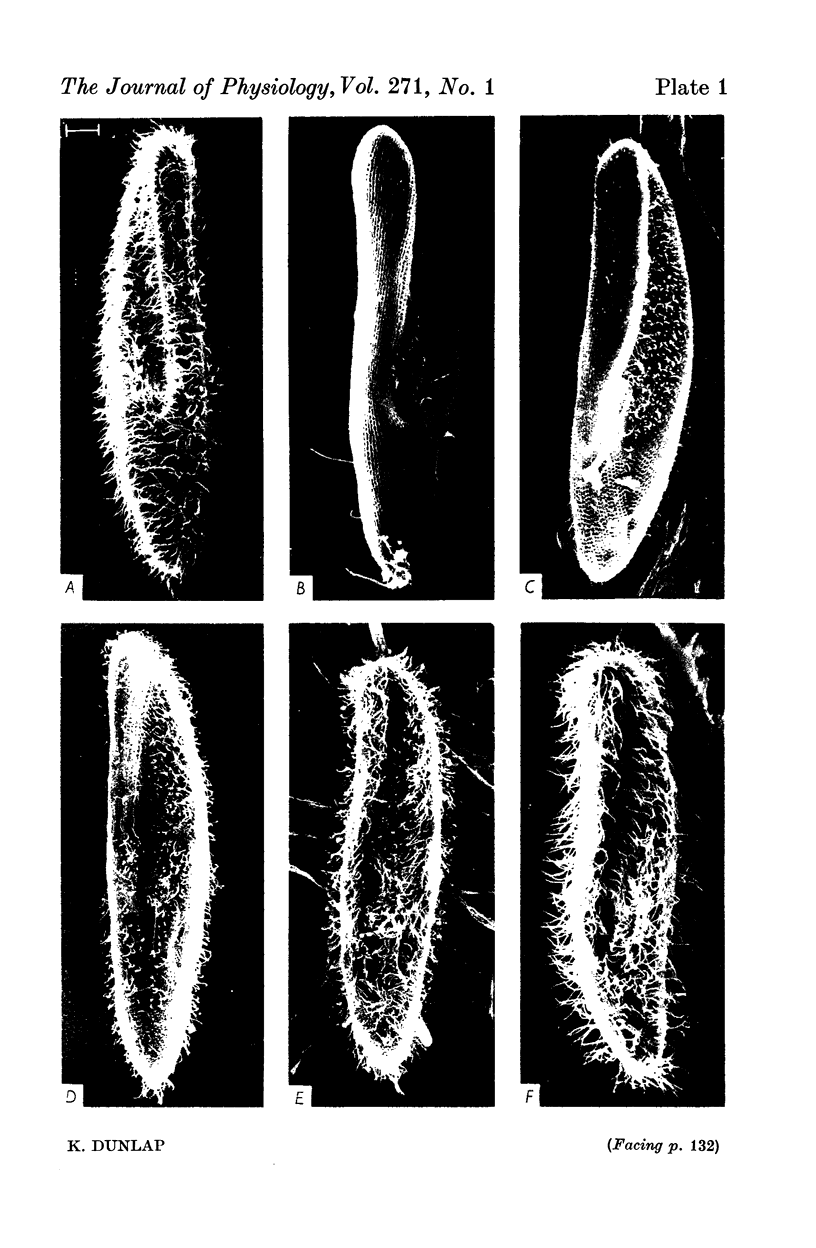
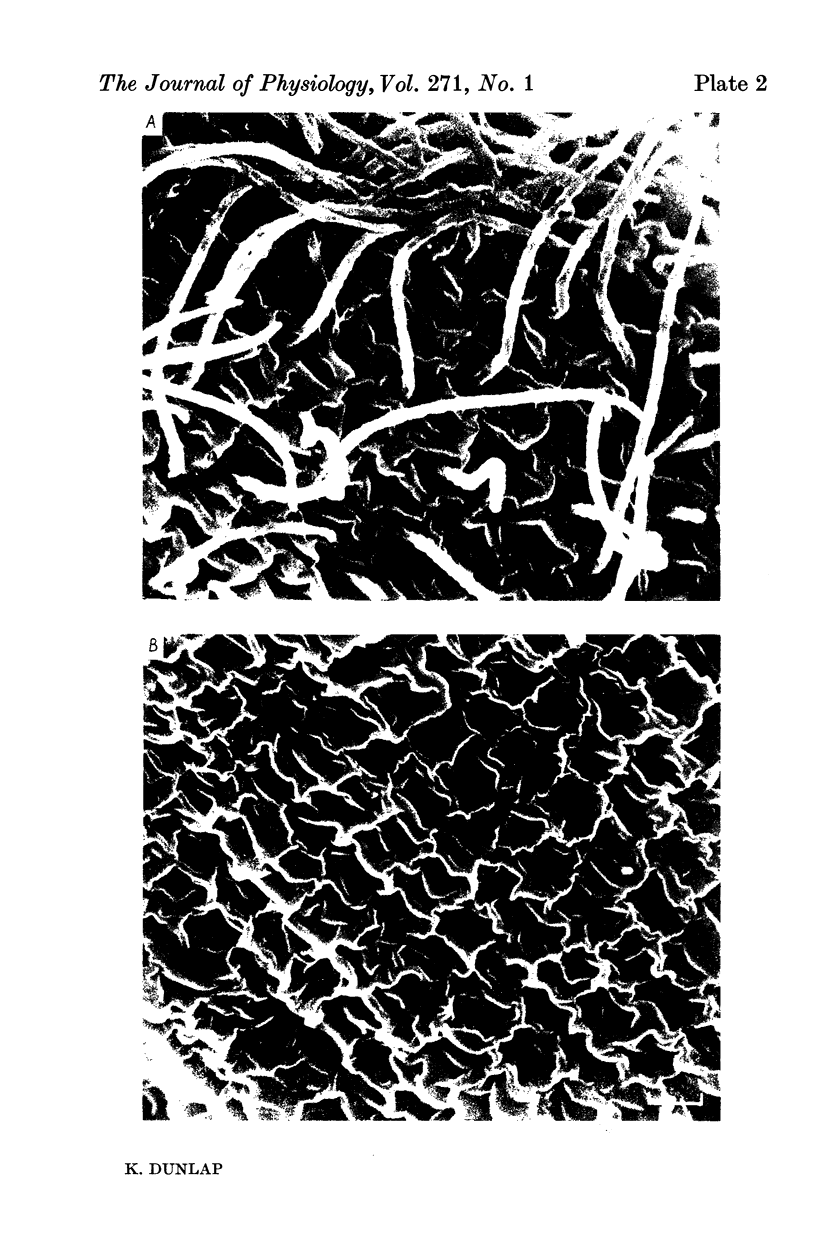

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., CURTIS D. R. THE EXCITATION OF THALAMIC NEURONES BY ACETYLCHOLINE. Acta Physiol Scand. 1964 May-Jun;61:85–99. doi: 10.1111/j.1748-1716.1964.tb02945.x. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., SEARS T. A. THE ROLE OF INHIBITION IN THE PHASING OF SPONTANEOUS THALAMO-CORTICAL DISCHARGE. J Physiol. 1964 Oct;173:459–480. doi: 10.1113/jphysiol.1964.sp007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Dingledine R., Kanazawa I., Kelly J. S. Inhibitory effects of acetylcholine on neurones in the feline nucleus reticularis thalami. J Physiol. 1976 Oct;261(3):647–671. doi: 10.1113/jphysiol.1976.sp011579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Kanazawa I., Kelly J. S. Exclusively inhibitory action of iontophoretic acetylcholine on single neurones of feline thalamus. Nature. 1976 Jan 29;259(5541):327–330. doi: 10.1038/259327a0. [DOI] [PubMed] [Google Scholar]
- Bowsher D. Diencephalic projections from the midbrain reticular formation. Brain Res. 1975 Sep 23;95(2-3):211–220. doi: 10.1016/0006-8993(75)90102-x. [DOI] [PubMed] [Google Scholar]
- Brownstein M., Kobayashi R., Palkovits M., Saavedra J. M. Choline acetyltransferase levels in diencephalic nuclei of the rat. J Neurochem. 1975 Jan;24(1):35–38. doi: 10.1111/j.1471-4159.1975.tb07624.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. The synaptic excitation of Renshaw cells. Exp Brain Res. 1966;2(1):81–96. doi: 10.1007/BF00234362. [DOI] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Eckert R., Naitoh Y., Friedman K. Sensory mechanisms in Paramecium. I. Two components of the electric response to mechanical stimulation of the anterior surface. J Exp Biol. 1972 Jun;56(3):683–694. doi: 10.1242/jeb.56.3.683. [DOI] [PubMed] [Google Scholar]
- Eckert R., Naitoh Y. Passive electrical properties of Paramecium and problems of ciliary coordination. J Gen Physiol. 1970 Apr;55(4):467–483. doi: 10.1085/jgp.55.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. B., de Olmos J. S. Autoradiographic studies of the projections of the midbrain reticular formation: ascending projections of nucleus cuneiformis. J Comp Neurol. 1976 Feb 15;165(4):417–431. doi: 10.1002/cne.901650403. [DOI] [PubMed] [Google Scholar]
- Filion M., Lamarre Y., Cordeau J. P. Neuronal discharges of the ventrolateral nucleus of the thalamus during sleep and wakefulness in the cat. II. Evoked activity. Exp Brain Res. 1971 Jun 29;12(5):499–508. doi: 10.1007/BF00234245. [DOI] [PubMed] [Google Scholar]
- Frigyesi T. L. Intracellular recordings from neurons in dorsolateral thalamic reticular nucleus during capsular, basal ganglia and midline thalamic stimulation. Brain Res. 1972 Dec 24;48:157–172. doi: 10.1016/0006-8993(72)90176-x. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Fuller J. H. Brain stem reticular units: some properties of the course and origin of the ascending trajectory. Brain Res. 1975 Jan 17;83(3):349–367. doi: 10.1016/0006-8993(75)90830-6. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. G. Some aspects of the organization of the thalamic reticular complex. J Comp Neurol. 1975 Aug 1;162(3):285–308. doi: 10.1002/cne.901620302. [DOI] [PubMed] [Google Scholar]
- Kataoka K., Nakamura Y., Hassler R. Habenulo-interpeduncular tract: a possible cholinergic neuron in rat brain. Brain Res. 1973 Nov 9;62(1):264–267. doi: 10.1016/0006-8993(73)90639-2. [DOI] [PubMed] [Google Scholar]
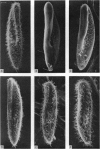
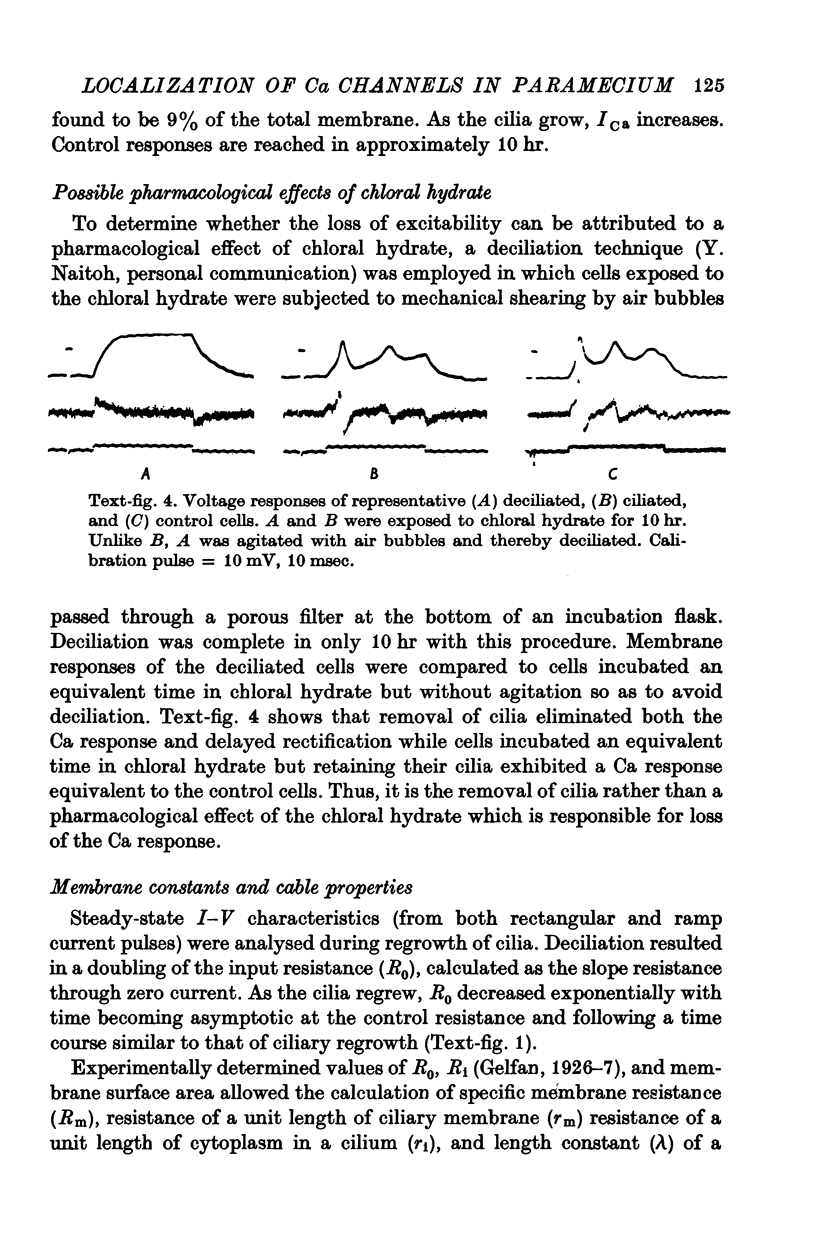
- Kennedy J. R., Jr, Brittingham E. Fine structure changes during chloral hydrate deciliation of Paramecium caudatum. J Ultrastruct Res. 1968 Mar;22(5):530–545. doi: 10.1016/s0022-5320(68)90039-7. [DOI] [PubMed] [Google Scholar]
- Kuhar M. J., DeHaven R. N., Yamamura H. I., Rommel-Spacher H., Simon J. R. Further evidence for cholinergic habenulo-interpeduncular neurons: pharmacologic and functional characteristics. Brain Res. 1975 Oct 31;97(2):265–275. doi: 10.1016/0006-8993(75)90449-7. [DOI] [PubMed] [Google Scholar]
- Kung C., Eckert R. Genetic modification of electric properties in an excitable membrane (paramecium-calcium conductance-electrophysiological measurements-membrane mutant). Proc Natl Acad Sci U S A. 1972 Jan;69(1):93–97. doi: 10.1073/pnas.69.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
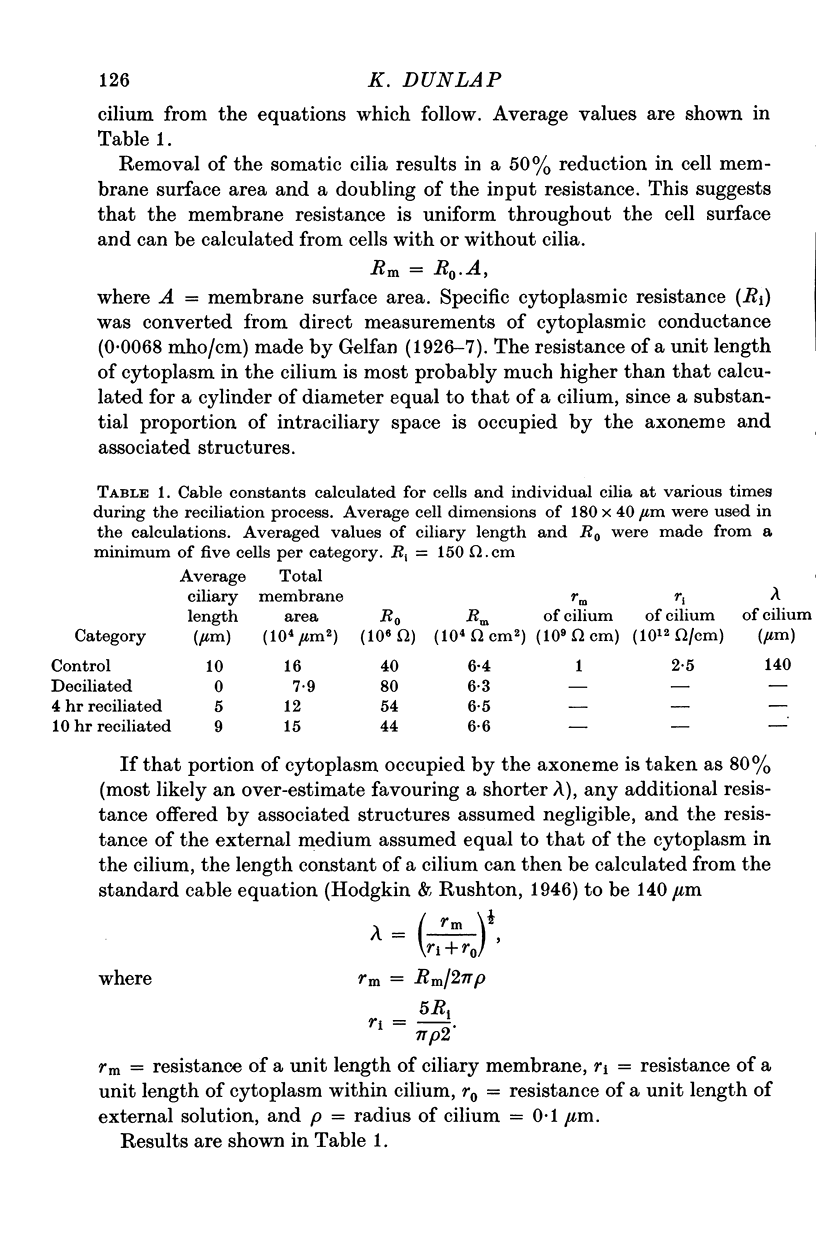
- Kung C. Genic mutants with altered system of excitation in Paramecium aurelia. II. Mutagenesis, screening and genetic analysis of the mutants. Genetics. 1971 Sep;69(1):29–45. doi: 10.1093/genetics/69.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarre Y., Filion M., Cordeau J. P. Neuronal discharges of the ventrolateral nucleus of the thalamus during sleep and wakefulness in the cat. I. Spontaneous activity. Exp Brain Res. 1971 Jun 29;12(5):480–498. doi: 10.1007/BF00234244. [DOI] [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C., Silver A. Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J Physiol. 1967 Jul;191(1):215–224. doi: 10.1113/jphysiol.1967.sp008246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léránth C. S., Brownstein M., Záborszky L., Járányi Z. S., Palkovits M. Morphological and biochemical changes in the rat interpeduncular nucleus following the transection of the habenulo-interpeduncular tract. Brain Res. 1975 Nov 28;99(1):124–128. doi: 10.1016/0006-8993(75)90613-7. [DOI] [PubMed] [Google Scholar]
- Margulis L., Banerjee S., White T. Colchicine-inhibited cilia regeneration: explanation for lack of effect in tris buffer medium. Science. 1969 Jun 6;164(3884):1177–1178. doi: 10.1126/science.164.3884.1177. [DOI] [PubMed] [Google Scholar]
- McCance I., Phillis J. W., Westerman R. A. Acetylcholine-sensitivity of thalamic neurones: its relationship to synaptic transmission. Br J Pharmacol Chemother. 1968 Mar;32(3):635–651. doi: 10.1111/j.1476-5381.1968.tb00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
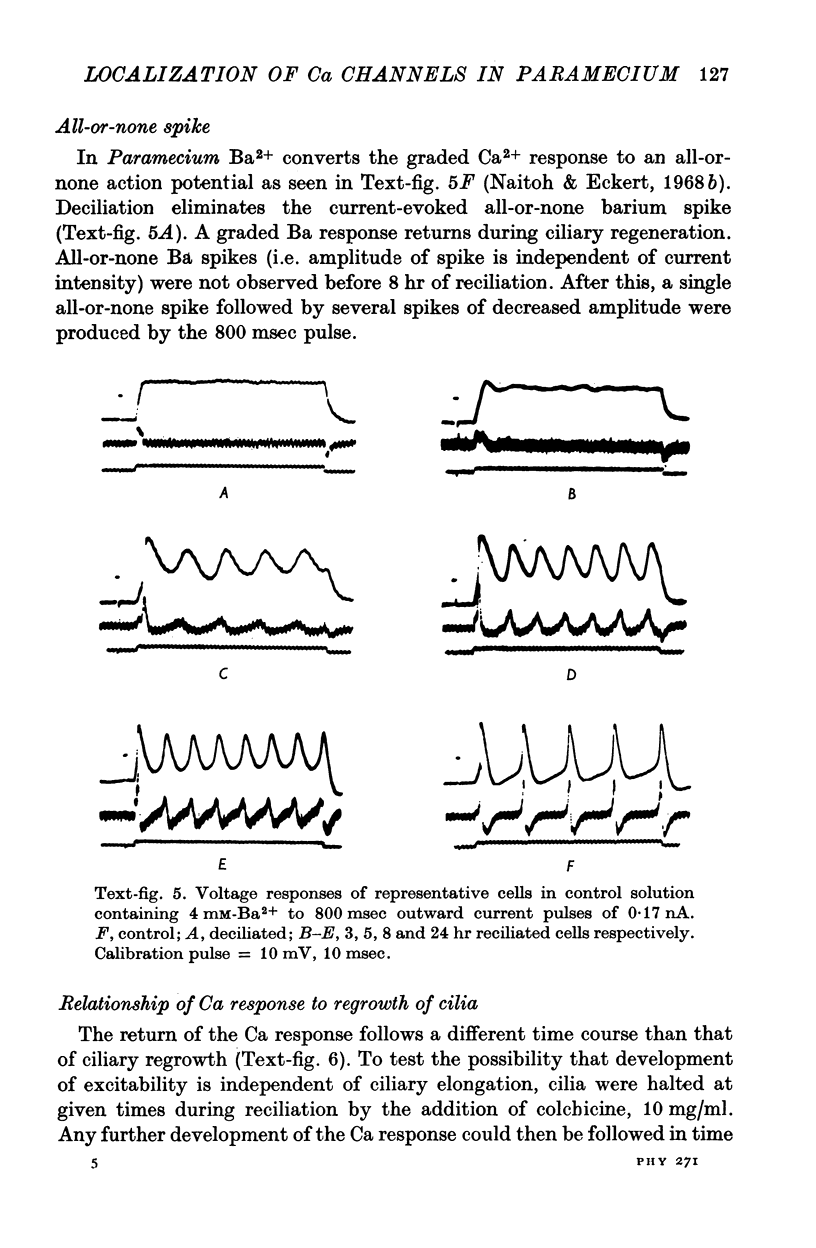
- Minderhoud J. M. An anatomical study of the efferent connections of the thalamic reticular nucleus. Exp Brain Res. 1971 May 26;112(4):435–446. doi: 10.1007/BF00234497. [DOI] [PubMed] [Google Scholar]
- Mukhametov L. M., Rizzolatti G., Tradardi V. Spontaneous activity of neurones of nucleus reticularis thalami in freely moving cats. J Physiol. 1970 Oct;210(3):651–667. doi: 10.1113/jphysiol.1970.sp009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
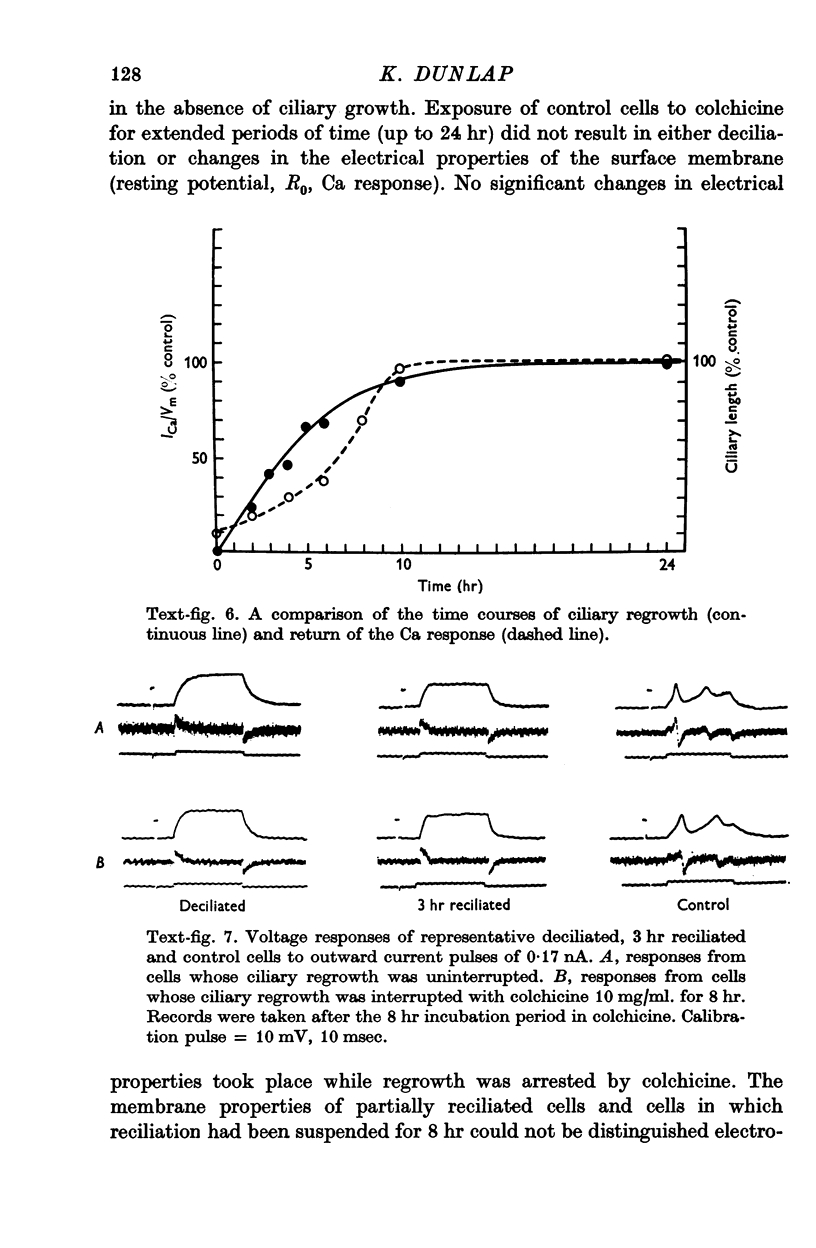
- Naito Y., Kaneko H. Reactivated triton-extracted models o paramecium: modification of ciliary movement by calcium ions. Science. 1972 May 5;176(4034):523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- Naitoh Y., Eckert R., Friedman K. A regenerative calcium response in Paramecium. J Exp Biol. 1972 Jun;56(3):667–681. doi: 10.1242/jeb.56.3.667. [DOI] [PubMed] [Google Scholar]
- Naitoh Y., Eckert R. Ionic mechanisms controlling behavioral responses of paramecium to mechanical stimulation. Science. 1969 May 23;164(3882):963–965. doi: 10.1126/science.164.3882.963. [DOI] [PubMed] [Google Scholar]
- New W., Jr Constant-current source for microelectrodes. J Appl Physiol. 1972 Jun;32(6):885–887. doi: 10.1152/jappl.1972.32.6.885. [DOI] [PubMed] [Google Scholar]
- Ogura A., Takahashi K. Artificial deciliation causes loss of calcium-dependent responses in Paramecium. Nature. 1976 Nov 11;264(5582):170–172. doi: 10.1038/264170a0. [DOI] [PubMed] [Google Scholar]
- Olivier A., Parent A., Poirier L. J. Identification of the thalamic nuclei on the basis of their cholinesterase content in the monkey. J Anat. 1970 Jan;106(Pt 1):37–50. [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., Tebecis A. K., York D. H. A study of cholinoceptive cells in the lateral geniculate nucleus. J Physiol. 1967 Oct;192(3):695–713. doi: 10.1113/jphysiol.1967.sp008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., York D. H. Cholinergic inhibition in the cerebral cortex. Brain Res. 1967 Aug;5(4):517–520. doi: 10.1016/0006-8993(67)90025-x. [DOI] [PubMed] [Google Scholar]
- Plattner H., Miller F., Bachmann L. Membrane specializations in the form of regular membrane-to-membrane attachment sites in Paramecium. A correlated freeze-etching and ultrathin-sectioning analysis. J Cell Sci. 1973 Nov;13(3):687–719. doi: 10.1242/jcs.13.3.687. [DOI] [PubMed] [Google Scholar]
- Purpura D. P., McMurtry J. G., Maekawa K. Synaptic events in ventrolateral thalamic neurons during suppression of recruiting responses by brain stem reticular stimulation. Brain Res. 1966 Jan;1(1):63–76. doi: 10.1016/0006-8993(66)90105-3. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. L., Carlson K. Cilia regeneration in Tetrahymena and its inhibition by colchicine. J Cell Biol. 1969 Feb;40(2):415–425. doi: 10.1083/jcb.40.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmoiraghi G. C., Stefanis C. N. A critique of iontophoretic studies of central nervous system neurons. Int Rev Neurobiol. 1967;10:1–30. doi: 10.1016/s0074-7742(08)60149-x. [DOI] [PubMed] [Google Scholar]
- Satir B., Sale W. S., Satir P. Membrane renewal after dibucaine deciliation of Tetrahymena. Freeze-fracture technique, cilia, membrane structure. Exp Cell Res. 1976 Jan;97:83–91. doi: 10.1016/0014-4827(76)90657-1. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Structural organization of nonspecific thalamic nuclei and their projection toward cortex. Brain Res. 1967 Sep;6(1):60–94. doi: 10.1016/0006-8993(67)90183-7. [DOI] [PubMed] [Google Scholar]
- Schlag J., Waszak M. Electrophysiological properties of units of the thalamic reticular complex. Exp Neurol. 1971 Jul;32(1):79–97. doi: 10.1016/0014-4886(71)90167-1. [DOI] [PubMed] [Google Scholar]
- Shute C. C., Lewis P. R. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967 Sep;90(3):497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- Singer W. The effect of mesencephalic reticular stimulation on intracellular potentials of cat lateral geniculate neurons. Brain Res. 1973 Oct 26;61:35–54. doi: 10.1016/0006-8993(73)90514-3. [DOI] [PubMed] [Google Scholar]
- Spehlmann R., Smathers C. C., Jr The effects of acetylcholine and of synaptic stimulation on the sensorimotor cortex of cats. II. Comparison of the neuronal responses to reticular and other stimuli. Brain Res. 1974 Jul 12;74(2):243–253. doi: 10.1016/0006-8993(74)90581-2. [DOI] [PubMed] [Google Scholar]
- Steriade M. Ascending control of thalamic and cortical responsiveness. Int Rev Neurobiol. 1970;12:87–144. doi: 10.1016/s0074-7742(08)60059-8. [DOI] [PubMed] [Google Scholar]
- Steriade M., Wyzinski P. Cortically elicited activities in thalamic reticularis neurons. Brain Res. 1972 Jul 20;42(2):514–520. doi: 10.1016/0006-8993(72)90552-5. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J. Choline acetyltransferase and acetylcholinesterase in fascia dentata following lesion of the entorhinal afferents. Brain Res. 1974 Nov 15;80(2):181–197. doi: 10.1016/0006-8993(74)90683-0. [DOI] [PubMed] [Google Scholar]
- Symmes D., Anderson K. V. Reticular modulation of higher auditory centers in monkey. Exp Neurol. 1967 Jun;18(2):161–176. doi: 10.1016/0014-4886(67)90038-6. [DOI] [PubMed] [Google Scholar]
- Waszak M. Effect of barbiturate anesthesia on discharge pattern in nucleus reticularis thalami. Pharmacol Biochem Behav. 1974 May;2(3):339–345. doi: 10.1016/0091-3057(74)90078-1. [DOI] [PubMed] [Google Scholar]
- Waszak M. Firing pattern of neurons in the rostral and ventral part of nucleus reticularis thalami during EEG spindles. Exp Neurol. 1974 Apr;43(1):38–58. doi: 10.1016/0014-4886(74)90132-0. [DOI] [PubMed] [Google Scholar]
- Yingling C. D., Skinner J. E. Regulation of unit activity in nucleus reticularis thalami by the mesencephalic reticular formation and the frontal granular cortex. Electroencephalogr Clin Neurophysiol. 1975 Dec;39(6):635–642. doi: 10.1016/0013-4694(75)90076-0. [DOI] [PubMed] [Google Scholar]