Abstract
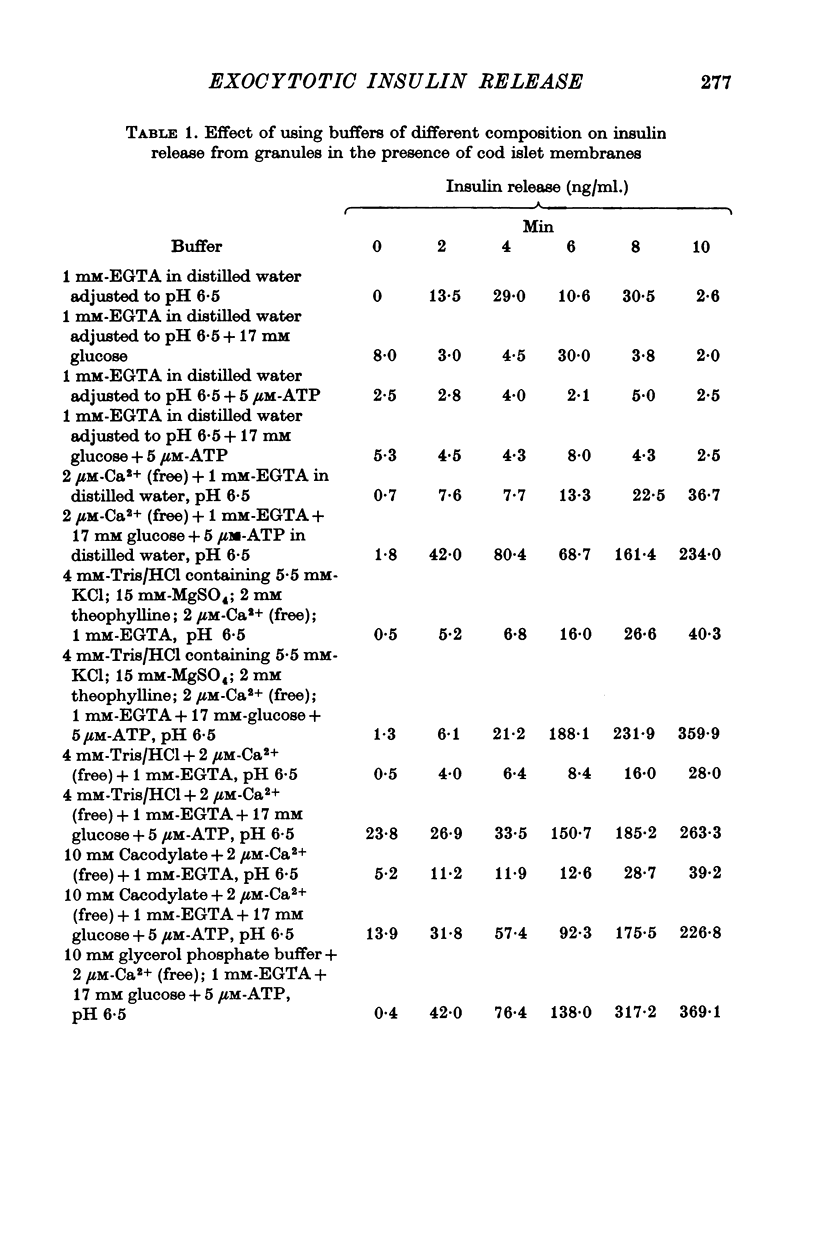
1. Investigation of the ionic requirements of the in vitro insulin release system, which consists of cod islet plasma membrane and rabbit islet granules incubated at pH 6.5, showed that the presence of Ca2+ was obligatory for the system to operate.
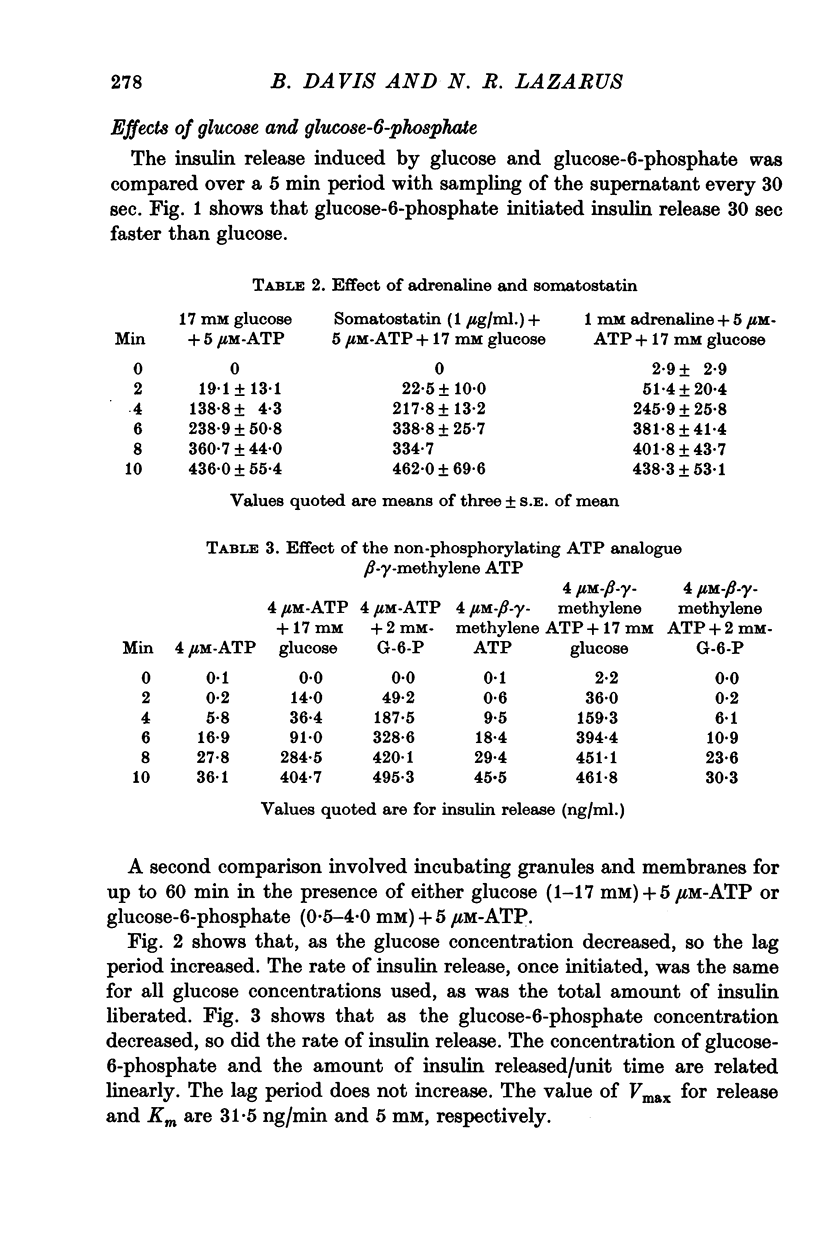
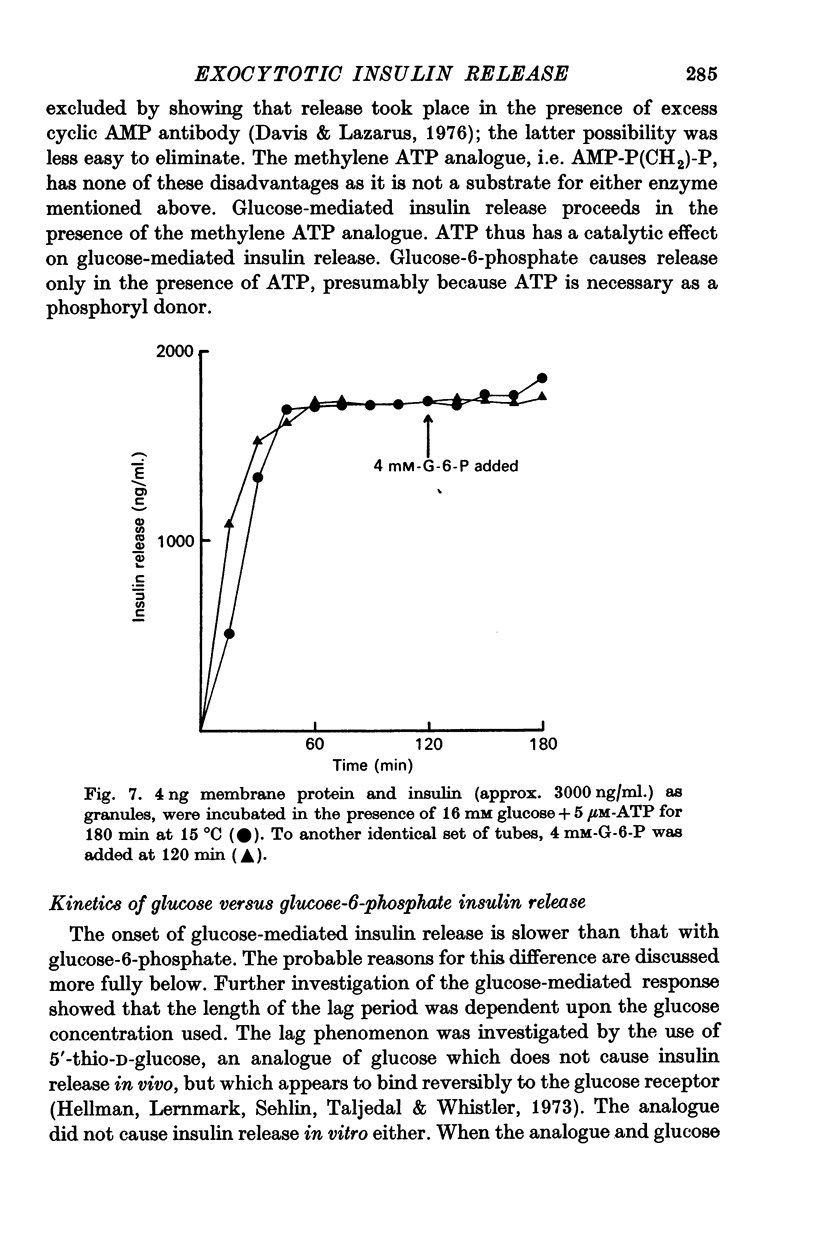
2. Glucose-initiated insulin release was as effective in the presence of β-γ-methylene ATP, as it was in the presence of ATP. This analogue of ATP is a substrate neither for adenylate cyclase nor for any known animal membrane proteases. The effect of ATP on glucose mediated release is allosteric.
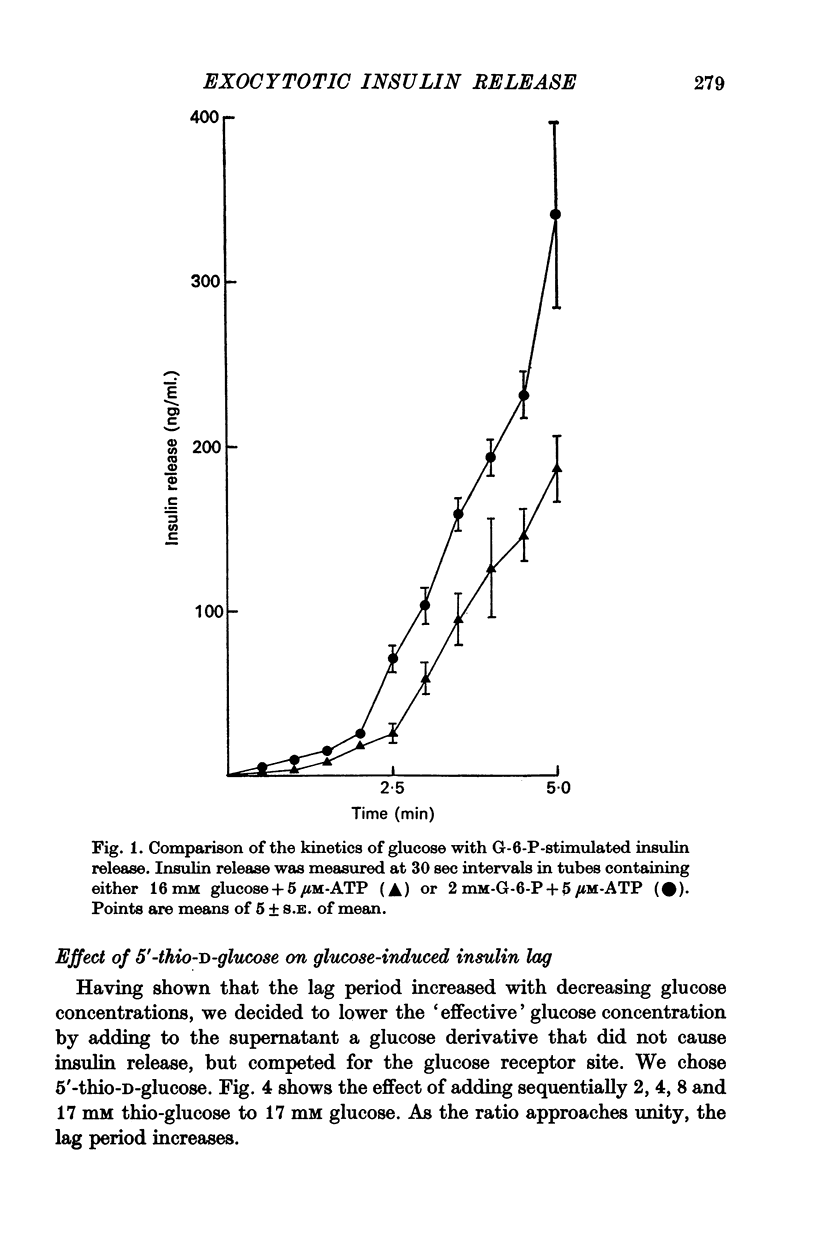
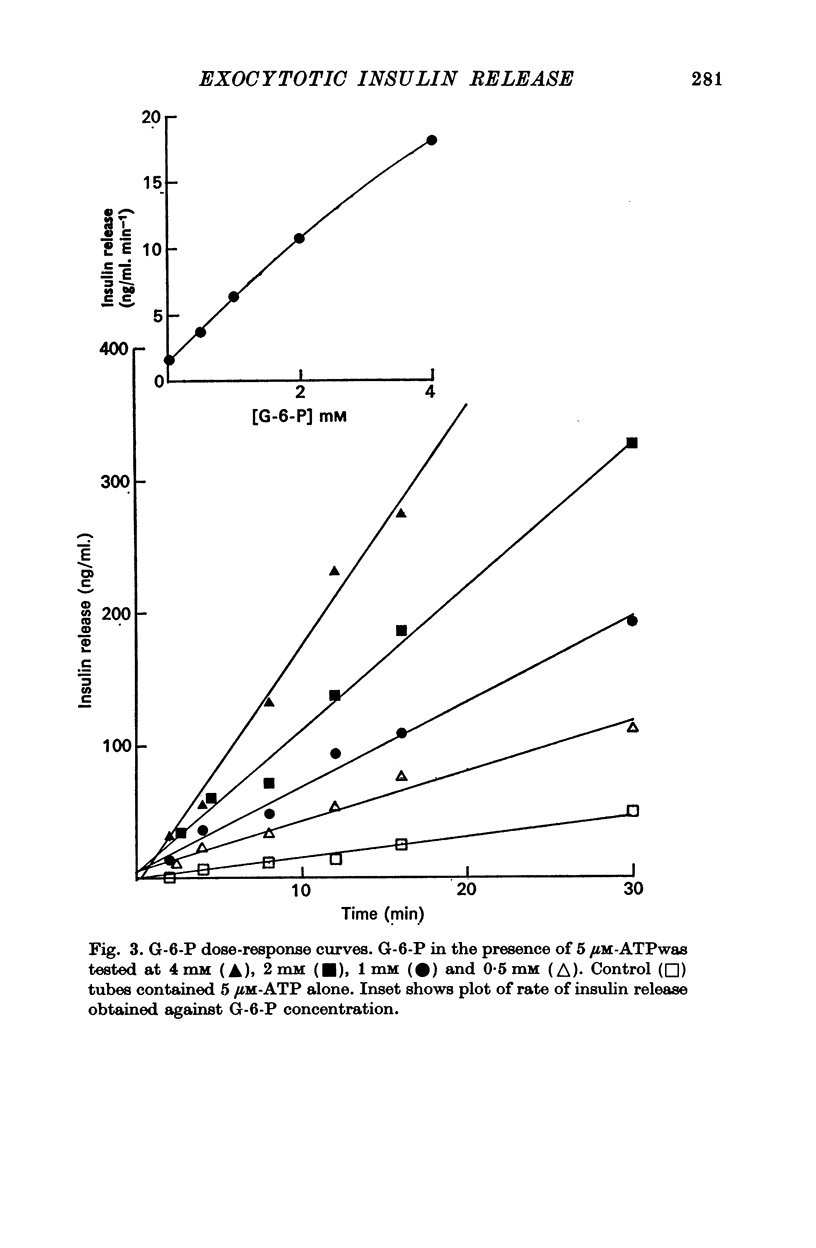
3. Glucose (16 mM)-initiated insulin release was slower than that induced by glucose-6-phosphate (4 mM); 150 and 120 sec, respectively.
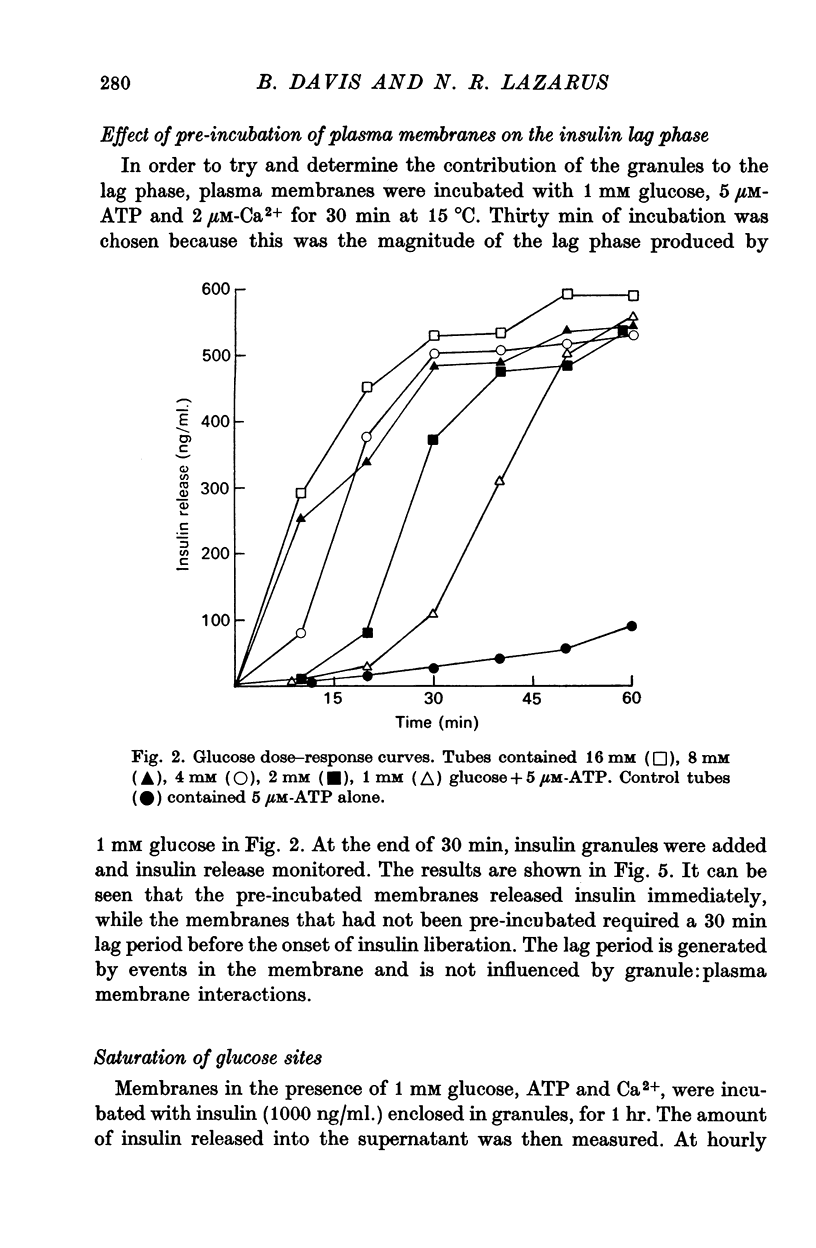
4. The lag found with glucose-mediated insulin release was dependent upon glucose concentration. The lower the glucose concentration, the longer the lag. With 1 mM glucose the lag extended to 30 min.
5. Once insulin release was initiated, the rate and amount of insulin release was independent of the glucose concentration.
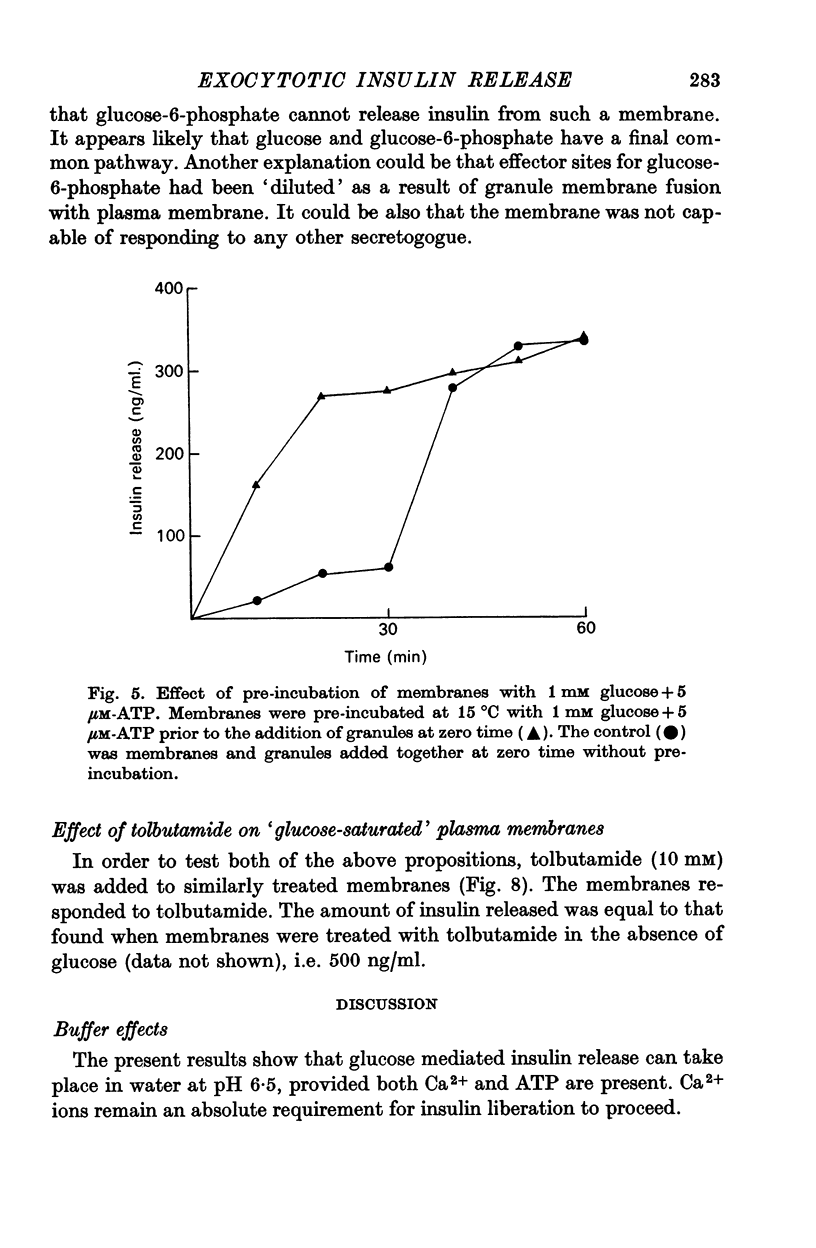
6. Pre-incubation of membranes with Ca2+, glucose and ATP prior to the addition of granules, abolished the extended lag that had been obtained with 1 mM glucose. Events in the plasma membrane are the major contributor to the generation of the extended lag.
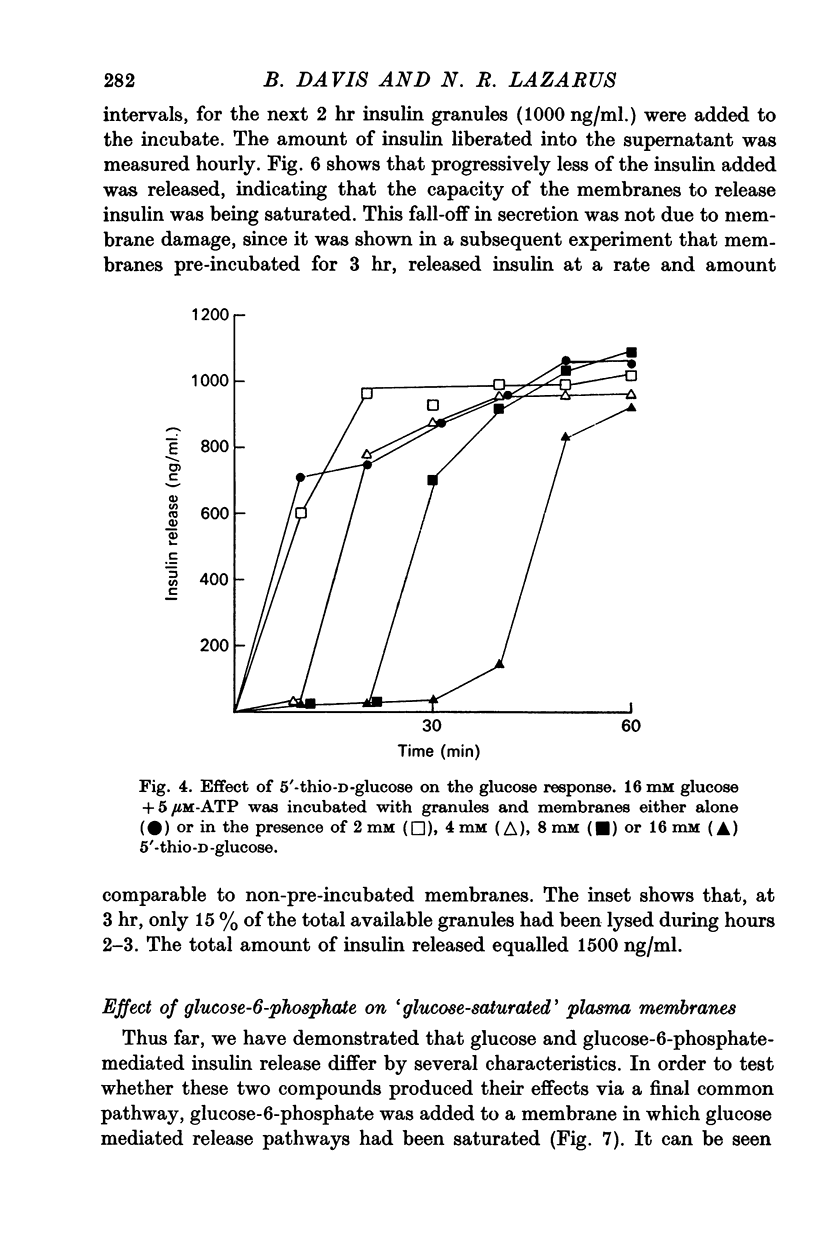
7. The glucose analogue 5′thio-D-glucose, although not able to release insulin, was shown to compete with glucose for the glucoreceptor. By increasing the ratio of analogue to glucose the lag time increased. Thus, the lag time is dependent upon the `effective' external glucose concentration.
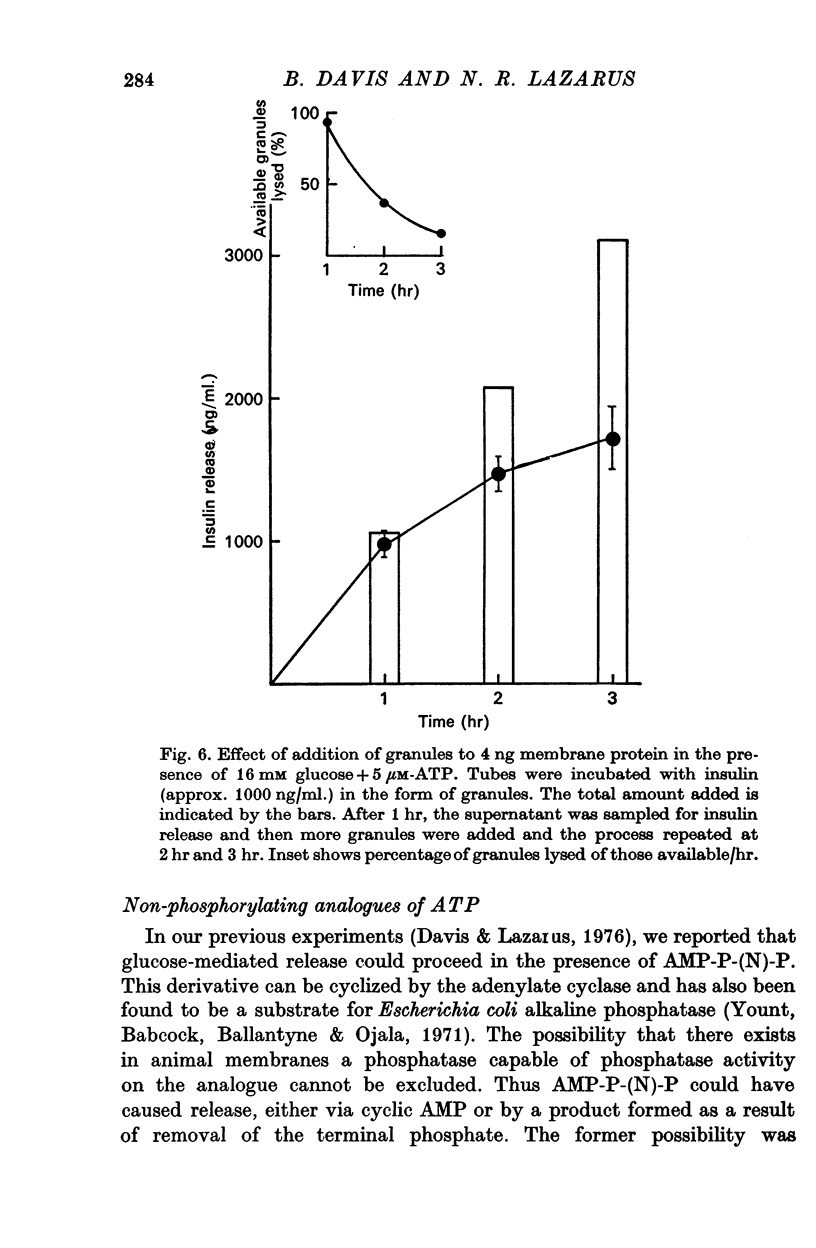
8. The max. amount of insulin released by 4 ng of membrane in the presence of glucose (16 mM) was 300 ng. The fact that membranes became refractory to glucose after this max. amount of insulin was released showed that recycling of release sites was not taking place in vitro and that granule: granule interactions were not occurring.
9. The 120 sec lag before glucose-6-phosphate-initiated release was independent of glucose-6-phosphate concentration. The rate of insulin release with glucose-6-phosphate was concentration dependent.
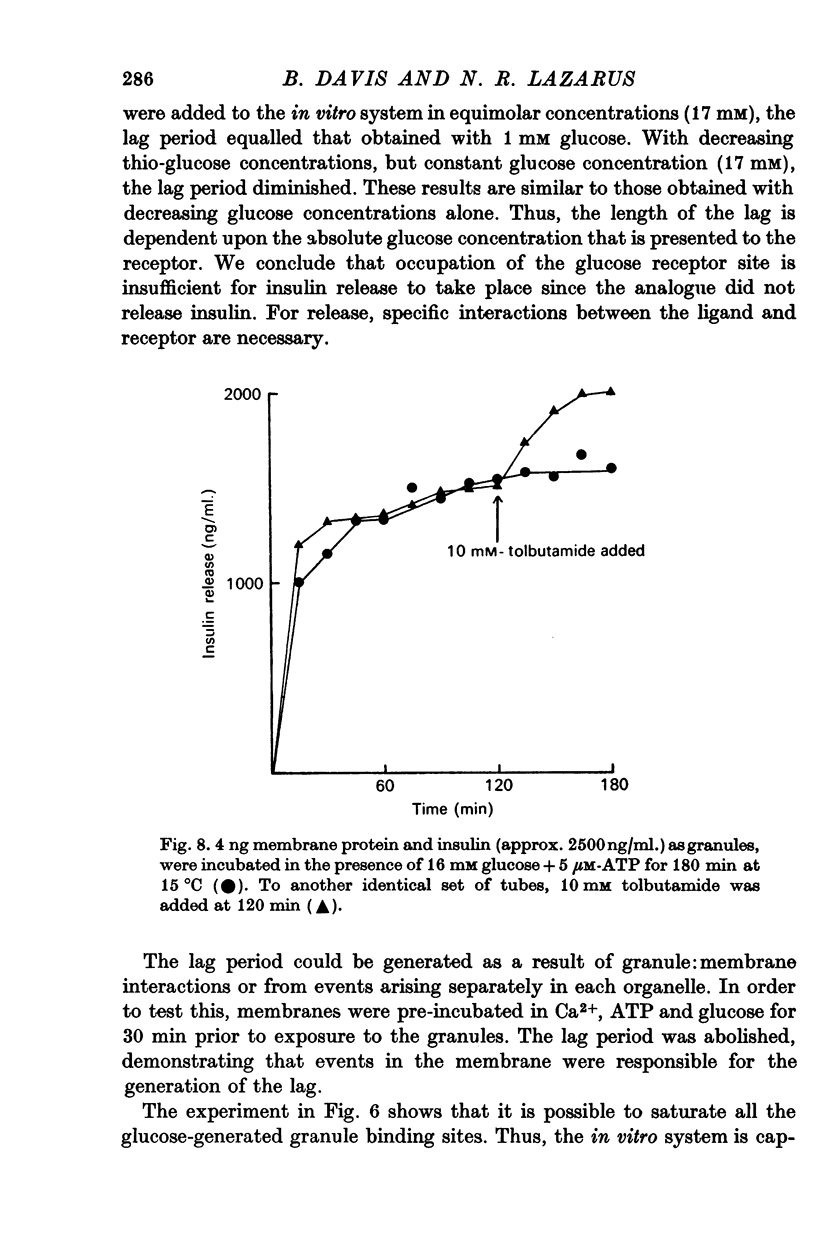
10. Glucose-6-phosphate did not cause further insulin release from a membrane that had released the max. amount of insulin it was capable of in the presence of glucose. The addition of tolbutamide (10 mM) to such a membrane did cause insulin release. This suggests that glucose and glucose-6-phosphate share a final common pathway.
11. Adrenaline and somatostatin did not inhibit glucose-mediated insulin release.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti K. G., Christensen N. J., Christensen S. E., Hansen A. P., Iversen J., Lundbaek K., Seyer-Hansen K., Orskov H. Inhibition of insulin secretion by somatostatin. Lancet. 1973 Dec 8;2(7841):1299–1301. doi: 10.1016/s0140-6736(73)92873-0. [DOI] [PubMed] [Google Scholar]
- DPAVIS B., Lazarus N. R. Regulation of 3',5'-cyclic AMP-dependent protein kinase in the plasma membrane of cod (Gadus callarius) and mouse islets. J Membr Biol. 1975;20(3-4):301–318. doi: 10.1007/BF01870640. [DOI] [PubMed] [Google Scholar]
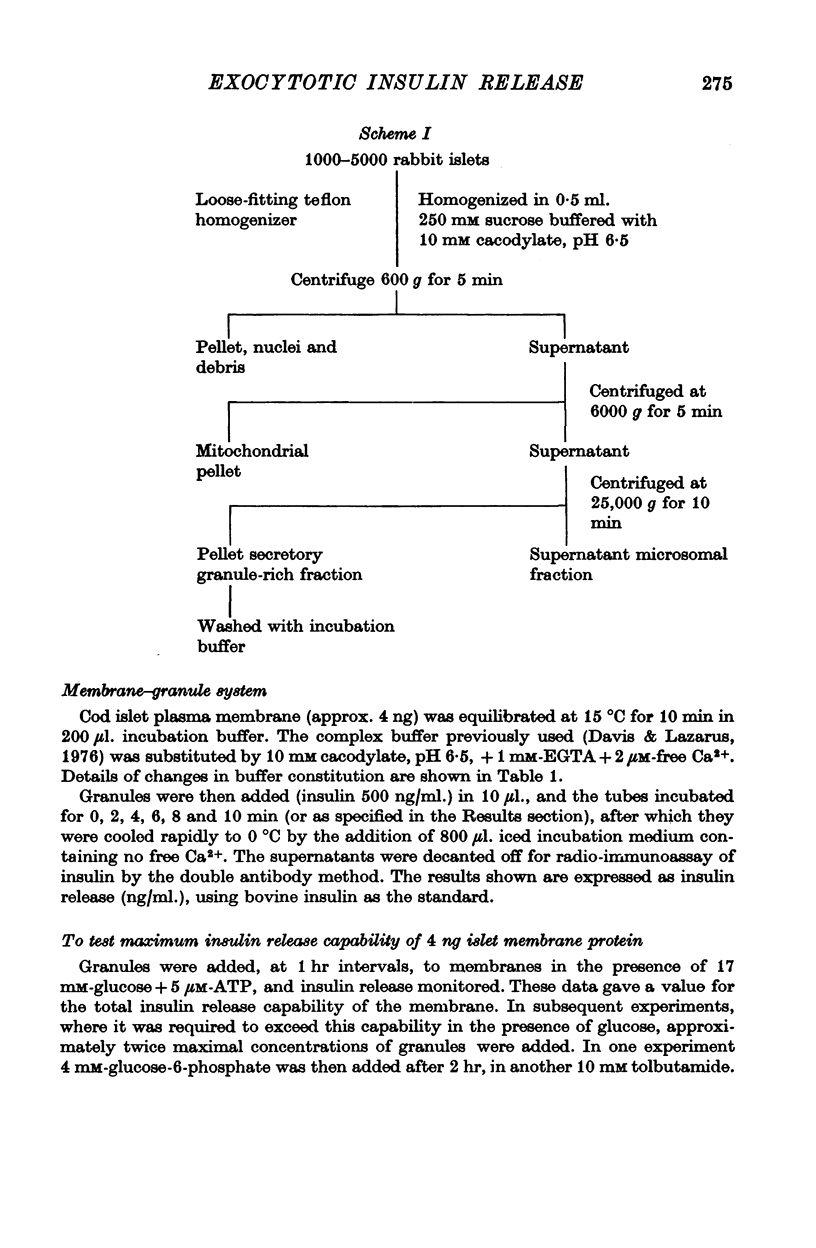
- Davis B., Lazarus N. R. An in Vitro system for studying insulin release caused by secretory granules-plasma membrane interaction: definition of the system. J Physiol. 1976 Apr;256(3):709–729. doi: 10.1113/jphysiol.1976.sp011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Lernmark A., Sehlin J., Täljedal I. B., Whistler R. L. The pancreatic beta-cell recognition of insulin secretagogues. 3. Effects of substituting sulphur for oxygen in the D-glucose molecule. Biochem Pharmacol. 1973 Jan 1;22(1):29–35. doi: 10.1016/0006-2952(73)90251-7. [DOI] [PubMed] [Google Scholar]
- Kiehn E. D., Holland J. J. Membrane and nonmembrane proteins of mammalian cells. Synthesis, turnover, and size distribution. Biochemistry. 1970 Apr 14;9(8):1716–1728. doi: 10.1021/bi00810a010. [DOI] [PubMed] [Google Scholar]
- Lazarus N. R., Davis B., O'Connor K. J. An approach to a molecular understanding of exocytotic insulin release. J Physiol (Paris) 1976 Nov;72(6):787–794. [PubMed] [Google Scholar]
- Porte D., Jr A receptor mechanism for the inhibition of insulin release by epinephrine in man. J Clin Invest. 1967 Jan;46(1):86–94. doi: 10.1172/JCI105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]


