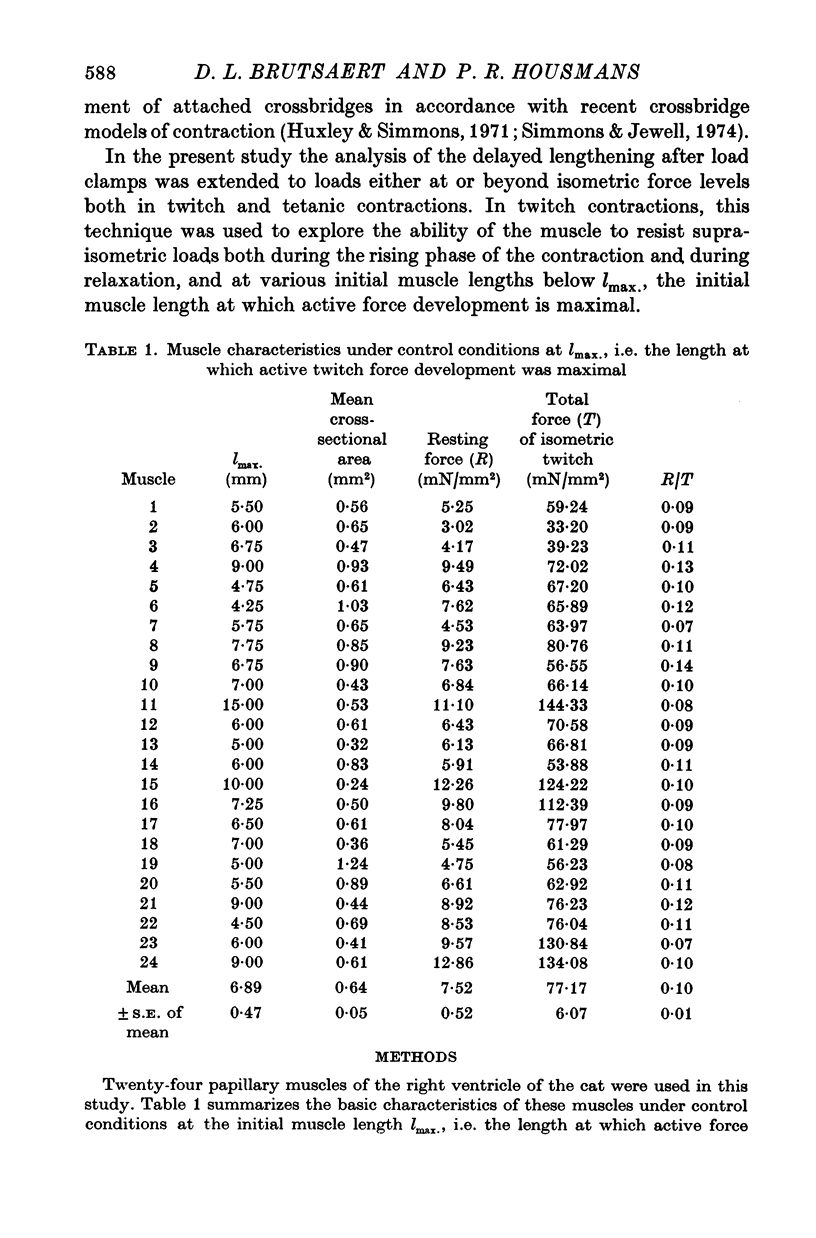
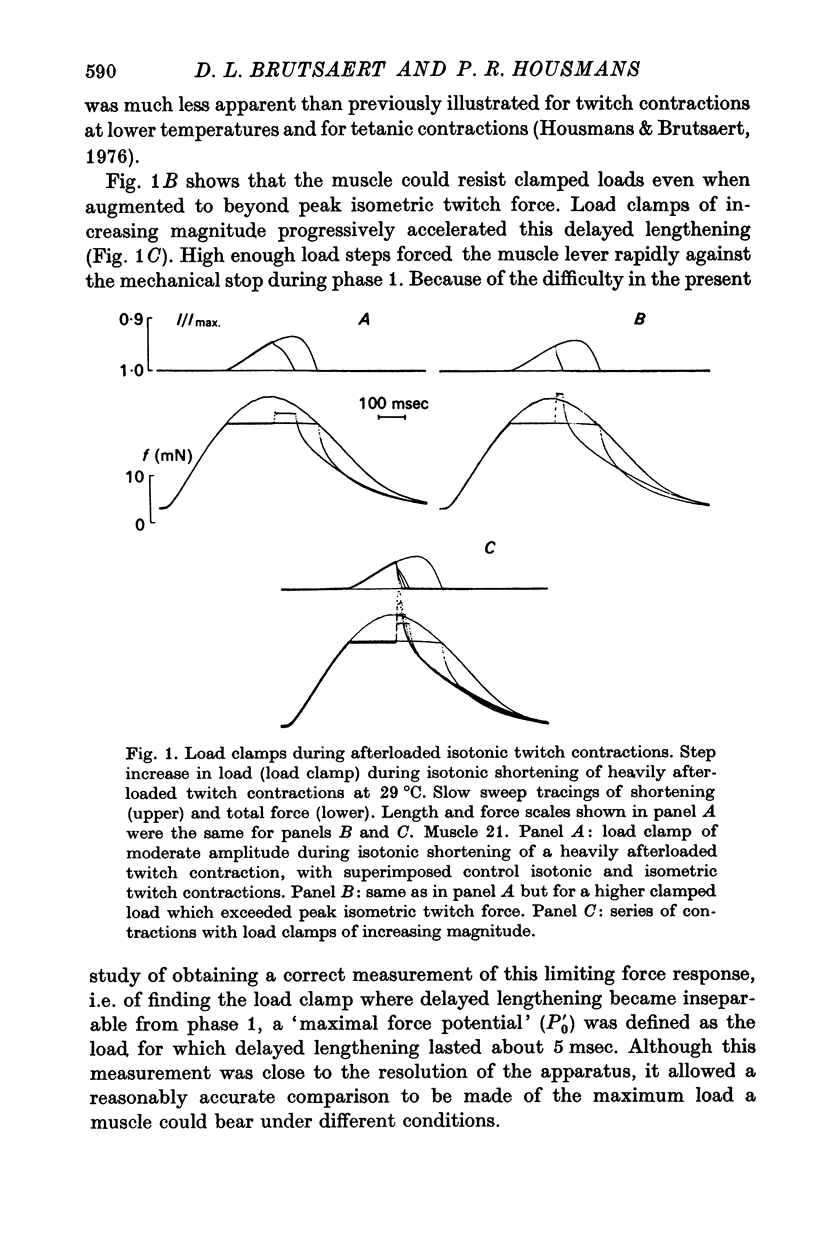
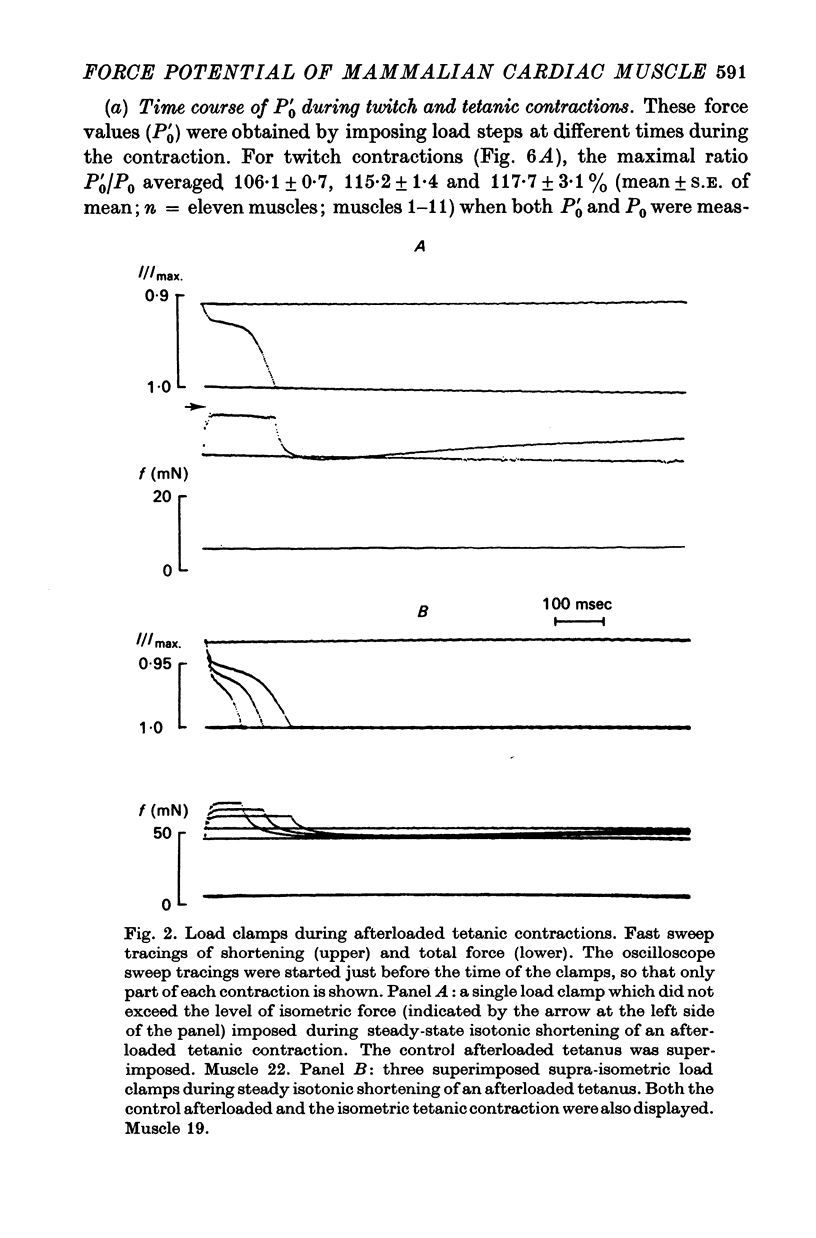
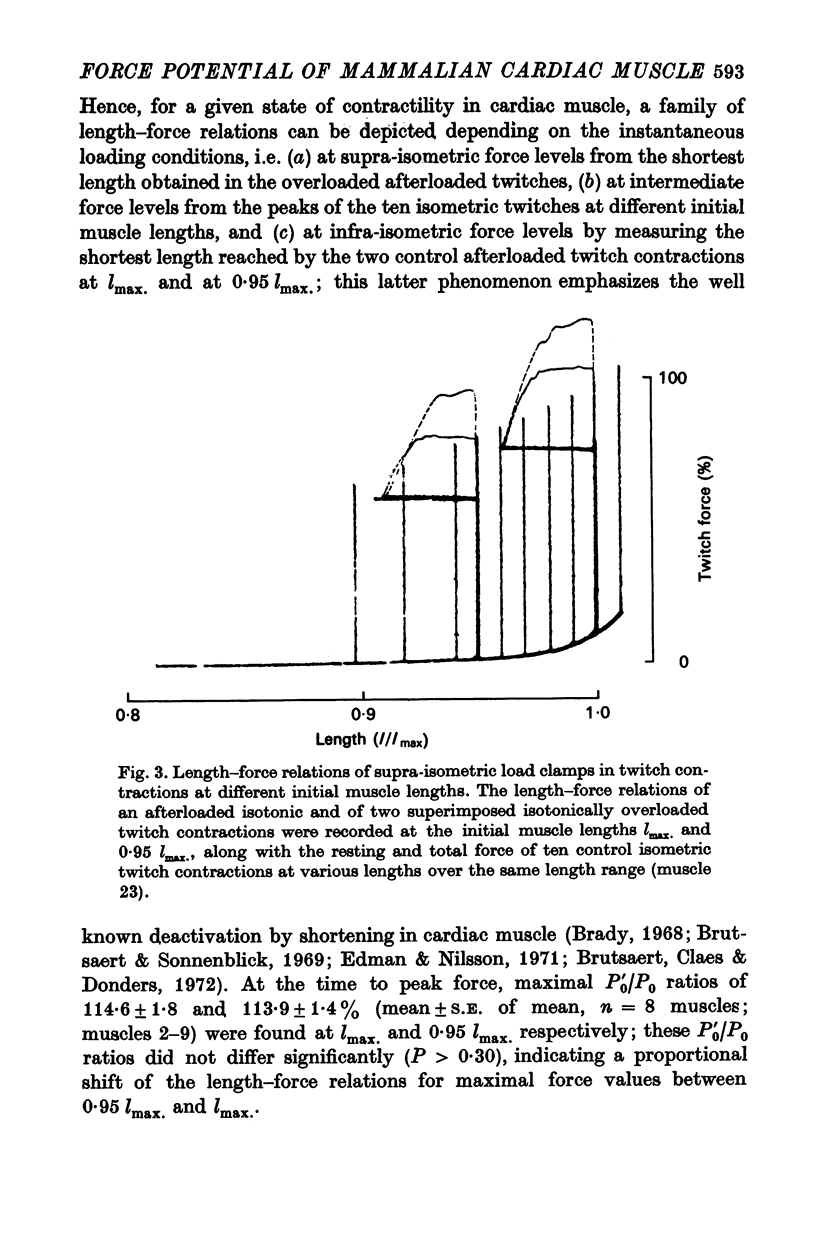
Abstract
1. Abrupt alterations in load (load clamps) have been imposed on cat papillary muscle during isotonic shortening and relaxation of afterloaded twitch and tetanic contractions, to assess the maximal force potential for a given contractile state. 2. These load clamps were accompanied by an initial fast lengthening reflecting an undamped series compliance. Even when exceeding isometric twitch and tetanic force, these loads could be borne for a considerable time, accompanied by a slower lengthening after the initial extension of the series compliance. At sufficiently high loads the muscle was pulled out very rapidly; a maximal supra-isometric force potential was defined as the load the muscle could bear momentarily and this was measured at times throughout contraction and relaxation. 3. This maximal force potential was determined at different initial muscle lengths. Depending on the instantaneous loading conditions, various length-force relations were obtained from : (a) peak force values of isometric twitches at different starting lengths, (b) the shortest length reached during after loaded isotonic twitches, and (c) the forces obtained in overloaded isotonic twitch contractions. 4. These results are consistent with a crossbridge model in which the delayed lengthening during isotonic overloading is due to back rotation and detachment of attached crossbridges and in which the inital phase of spontaneous isotonic relaxation is governed by the same mechanism.
Full text
PDF


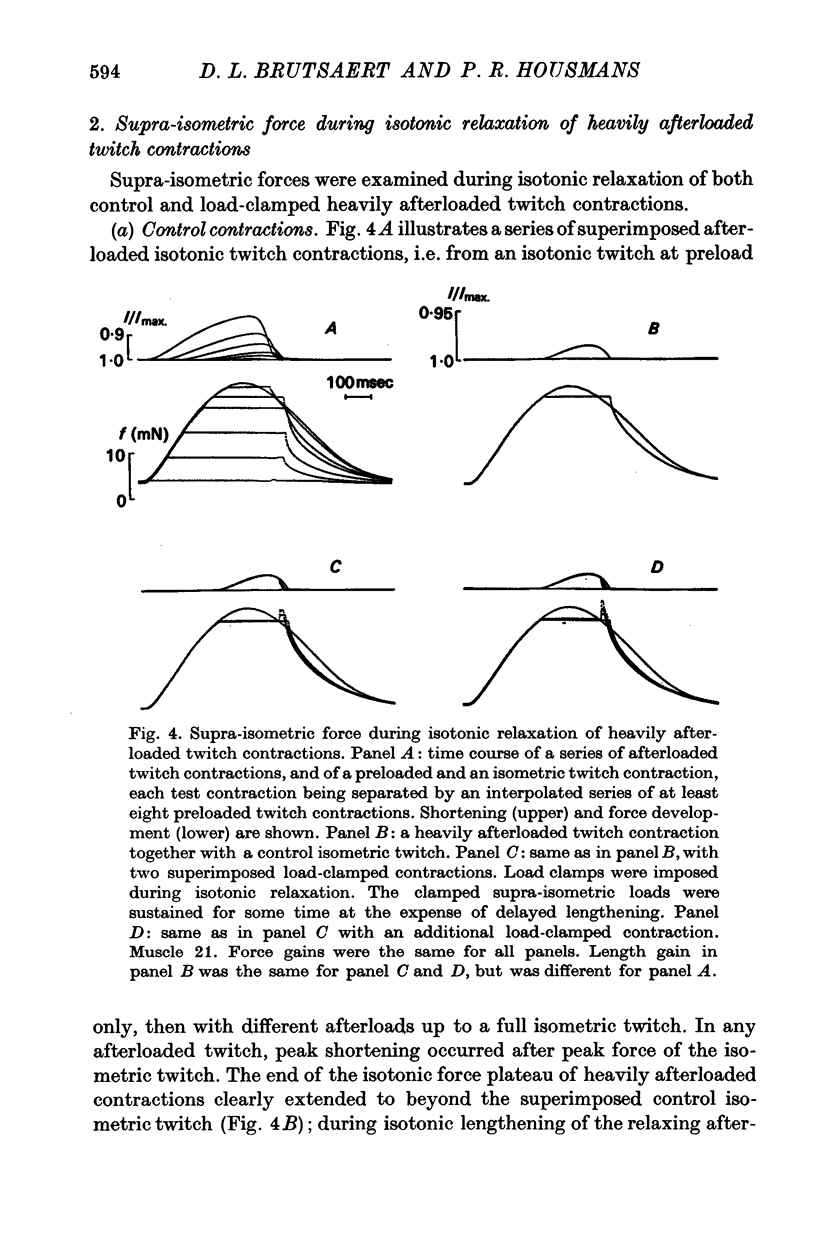






Selected References
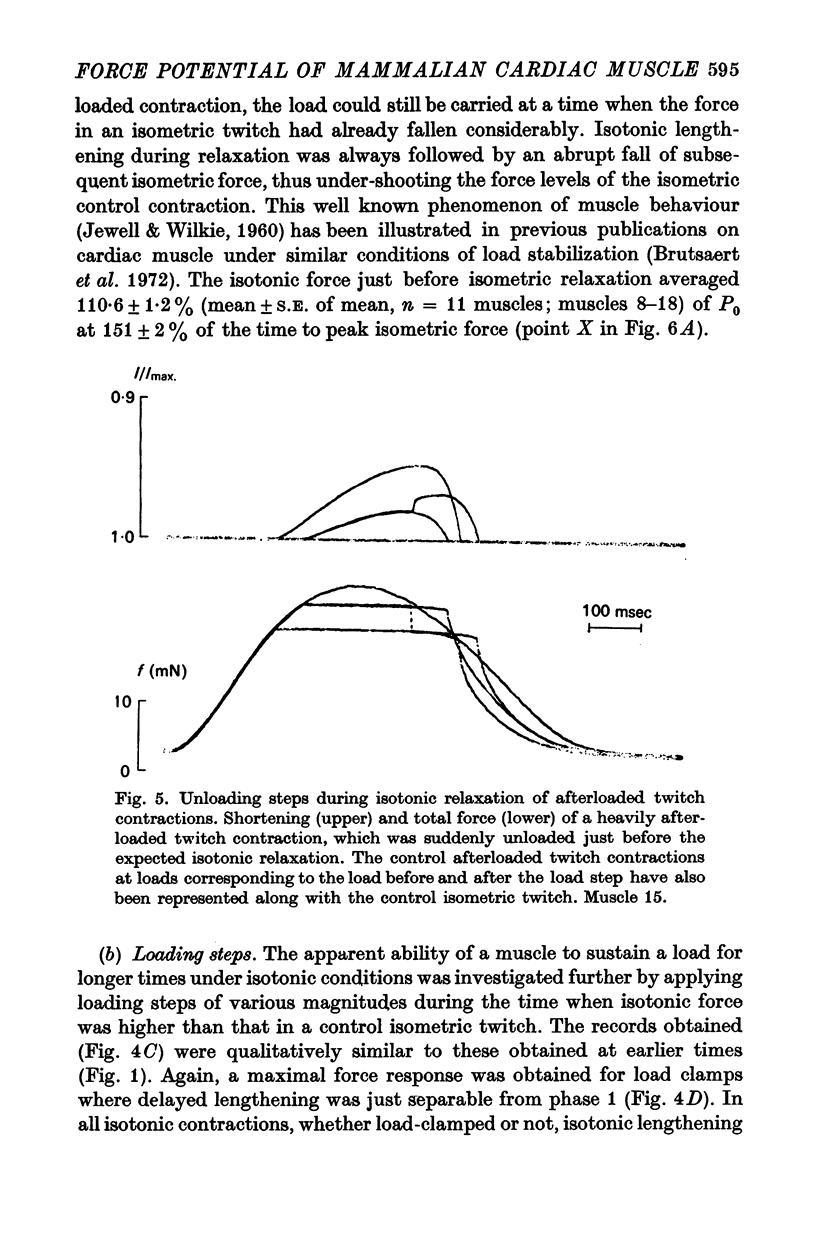
These references are in PubMed. This may not be the complete list of references from this article.
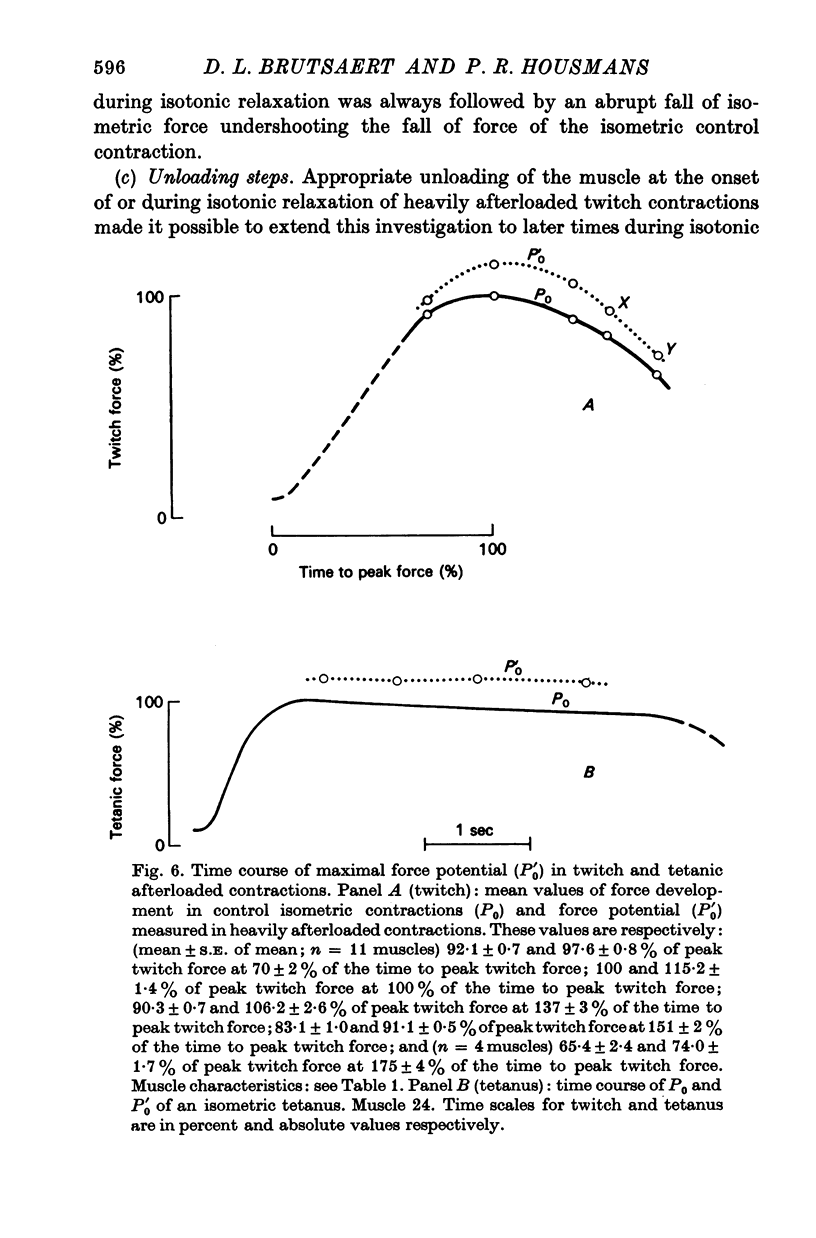
- Abbott B. C., Gordon D. G. A commentary on muscle mechanics. Circ Res. 1975 Jan;36(1):1–7. doi: 10.1161/01.res.36.1.1. [DOI] [PubMed] [Google Scholar]
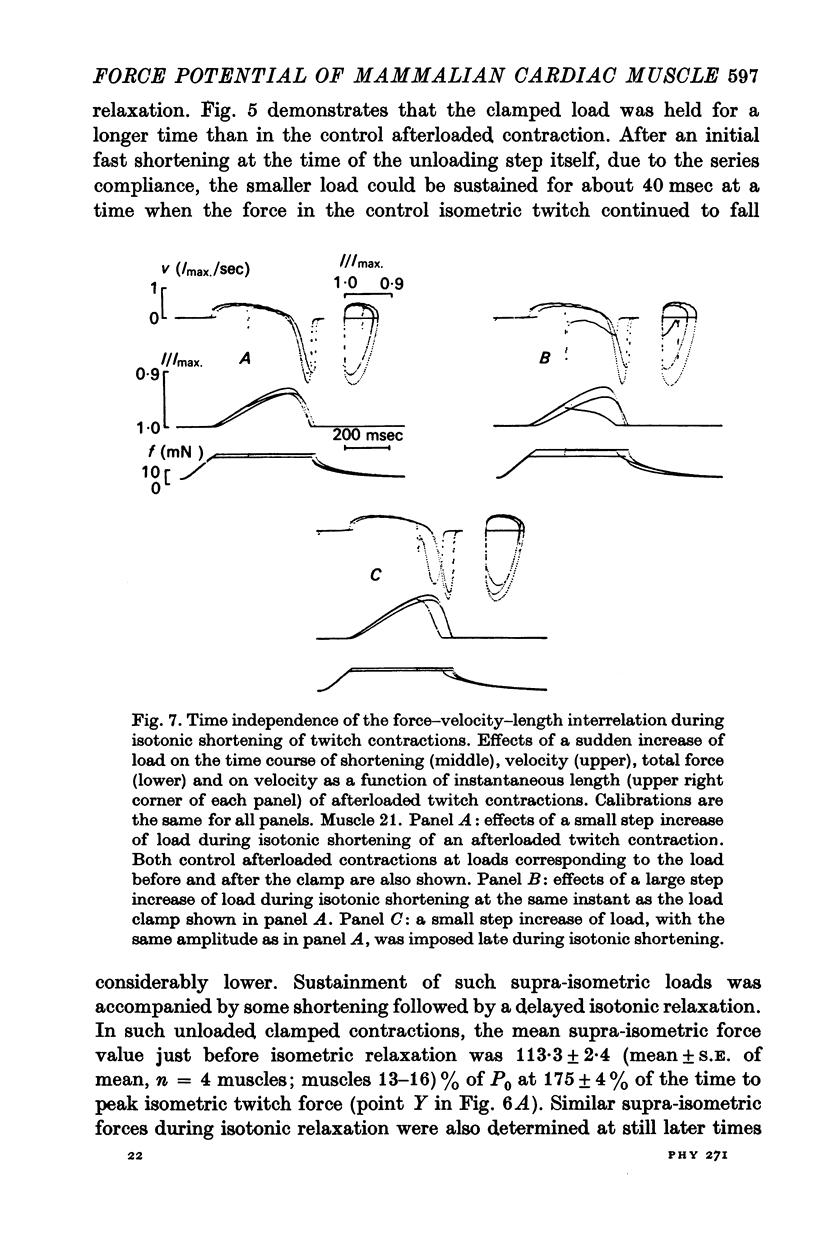
- Brady A. J. A measurement of the active state in heart muscle. Cardiovasc Res. 1971 Jul;Suppl 1:11–17. doi: 10.1093/cvr/5.supp1.11. [DOI] [PubMed] [Google Scholar]
- Brady A. J. Active state in cardiac muscle. Physiol Rev. 1968 Jul;48(3):570–600. doi: 10.1152/physrev.1968.48.3.570. [DOI] [PubMed] [Google Scholar]
- Brady A. J. Time and displacement dependence of cardiac contractility: problems in defining the active state and force-velocity relations. Fed Proc. 1965 Nov-Dec;24(6):1410–1420. [PubMed] [Google Scholar]
- Brutsaert D. L., Claes V. A., Donders J. J. Effects of controlling the velocity of shortening on force-velocity-length and time relations in cat papillary muscle. Velocity clamping. Circ Res. 1972 Mar;30(3):310–315. doi: 10.1161/01.res.30.3.310. [DOI] [PubMed] [Google Scholar]
- Brutsaert D. L., Claes V. A. Onset of mechanical activation of mammalian heart muscle in calcium- and strontium-containing solutions. Circ Res. 1974 Sep;35(3):345–357. doi: 10.1161/01.res.35.3.345. [DOI] [PubMed] [Google Scholar]
- Brutsaert D. L., Claes V. A., Sonnenblick E. H. Effects of abrupt load alterations on force-velocity-length and time relations during isotonic contractions of heart muscle: load clamping. J Physiol. 1971 Jul;216(2):319–330. doi: 10.1113/jphysiol.1971.sp009527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert D. L., Sonnenblick E. H. Force-velocity-length-time relations of the contractile elements in heart muscle of the cat. Circ Res. 1969 Feb;24(2):137–149. doi: 10.1161/01.res.24.2.137. [DOI] [PubMed] [Google Scholar]
- Claes V. A., Brutsaert D. L. Infrared-emitting diode and optic fibers for underwater force measurement in heart muscle. J Appl Physiol. 1971 Sep;31(3):497–498. doi: 10.1152/jappl.1971.31.3.497. [DOI] [PubMed] [Google Scholar]
- De Clerck N. M., Claes V. A., Brutsaert D. L. Force velocity relations of single cardiac muscle cells: calcium dependency. J Gen Physiol. 1977 Feb;69(2):221–241. doi: 10.1085/jgp.69.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Elzinga G., Noble M. I. Proceedings: Force enhancement induced by stretch of contracting single isolated muscle fibres of the frog. J Physiol. 1976 Jun;258(2):95P–96P. [PubMed] [Google Scholar]
- Endo M. Stretch-induced increase in activation of skinned muscle fibres by calcium. Nat New Biol. 1972 Jun 14;237(76):211–213. doi: 10.1038/newbio237211a0. [DOI] [PubMed] [Google Scholar]
- Flitney F. W., Hirst D. G. Proceedings: Tension responses and sarcomere movements during length changes applied to contracting frog's muscle. J Physiol. 1975 Sep;251(1):66P–68P. [PubMed] [Google Scholar]
- Henderson A. H., Brutsaert D. L. Force-velocity-length relationship in heart muscle: lack of time-independence during twitch contractions of frog ventricle strips with caffeine. Pflugers Arch. 1974 Apr 4;348(1):59–64. doi: 10.1007/BF00587739. [DOI] [PubMed] [Google Scholar]
- Henderson A. H., Claes V. A., Brutsaert D. L. Influence of caffeine and other inotropic interventions on the onset of unloaded shortening velocity in mammalian heart muscle. Time course of activation. Circ Res. 1973 Sep;33(3):291–302. doi: 10.1161/01.res.33.3.291. [DOI] [PubMed] [Google Scholar]
- Housmans P. R., Brutsaert D. L. Three-step yielding of load-clamped mammalian cardiac muscle. Nature. 1976 Jul 1;262(5563):56–58. doi: 10.1038/262056a0. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. The mechanical properties of relaxing muscle. J Physiol. 1960 Jun;152:30–47. doi: 10.1113/jphysiol.1960.sp006467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell B. R., Rovell J. M. Influence of previous mechanical events on the contractility of isolated cat papillary muscle. J Physiol. 1973 Dec;235(3):715–740. doi: 10.1113/jphysiol.1973.sp010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Sollins M. R. Sarcomere length-tension relations in living rat papillary muscle. Circ Res. 1975 Sep;37(3):299–308. doi: 10.1161/01.res.37.3.299. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann R. L., Lab M. J., Hennekes R., Krause H. Feedback interaction of mechanical and electrical events in the isolated mammalian ventricular myocardium (cat papillary muscle). Pflugers Arch. 1971;324(2):100–123. doi: 10.1007/BF00592656. [DOI] [PubMed] [Google Scholar]
- Krueger J. W., Pollack G. H. Myocardial sarcomere dynamics during isometric contraction. J Physiol. 1975 Oct;251(3):627–643. doi: 10.1113/jphysiol.1975.sp011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUIR A. R. FURTHER OBSERVATIONS ON THE CELLULAR STRUCTURE OF CARDIAC MUSCLE. J Anat. 1965 Jan;99:27–46. [PMC free article] [PubMed] [Google Scholar]
- Parmley W. W., Brutsaert D. L., Sonnenblick E. H. Effects of altered loading on contractile events in isolated cat papillary muscle. Circ Res. 1969 Apr;24(4):521–532. doi: 10.1161/01.res.24.4.521. [DOI] [PubMed] [Google Scholar]
- Ridgeway E. B., Gordon A. M. Muscle activation: effects of small length changes on calcium release in single fibers. Science. 1975 Sep 12;189(4206):881–884. doi: 10.1126/science.1154025. [DOI] [PubMed] [Google Scholar]


