Abstract
1. The earliest known change in rat fast muscle following denervation is a fall in resting membrane potential unaccompanied by change in membrane resistance. The present study tested the hypothesis that increased Na permeability (PNa) accounted for this early depolarization.
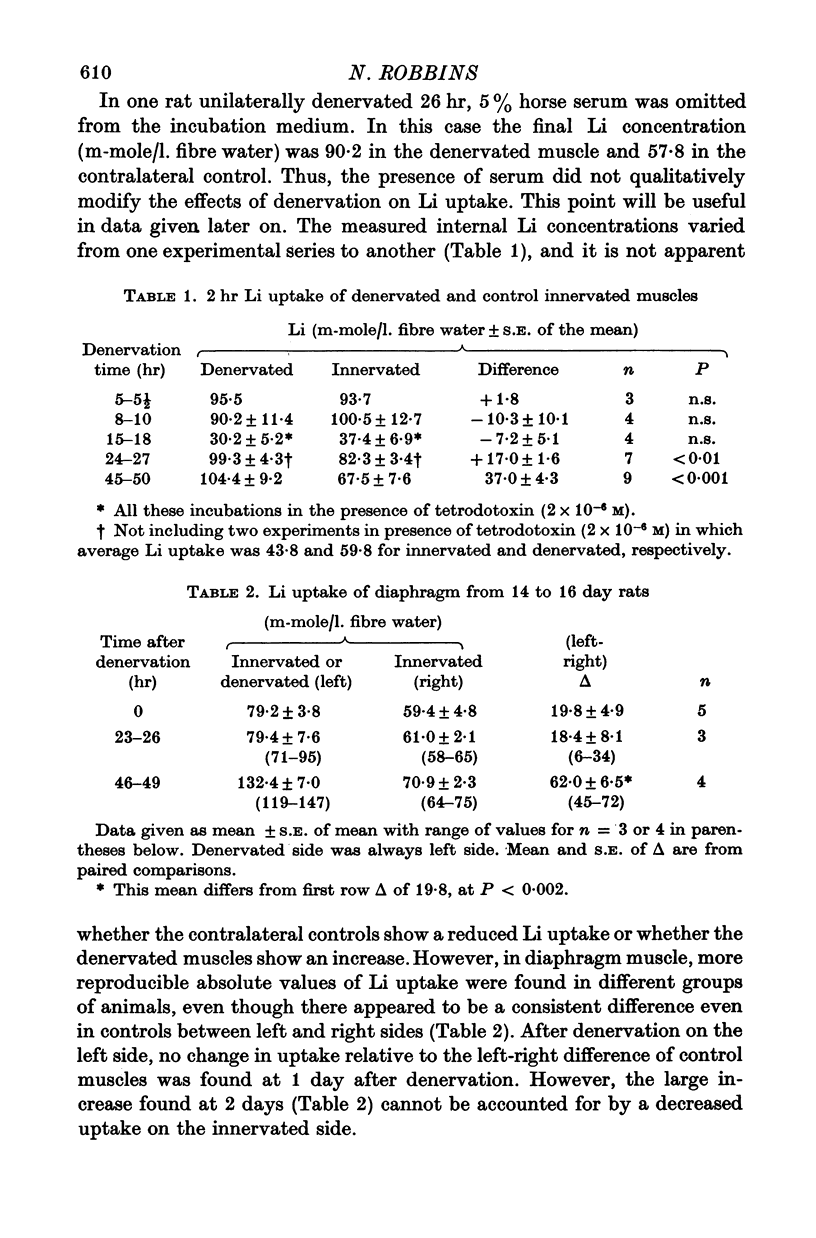
2. In all experiments, rat extensor digitorum longus muscles were studied in vitro at 25° C. Li uptake in vitro, used as a measure of PNa, was greater in 1- and 2-day denervated muscles (and in 2-day denervated diaphragm) than in paired controls.
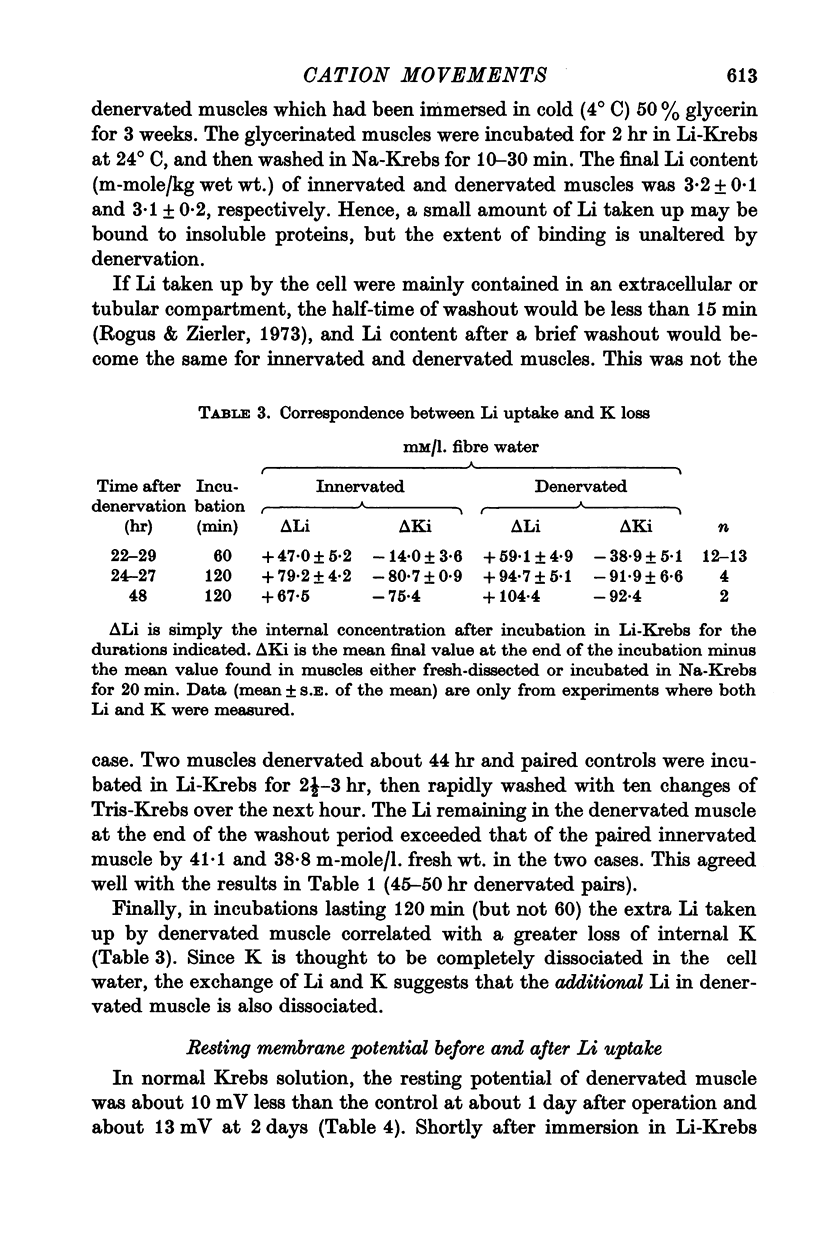
3. The extra Li taken up by denervated muscle was not sequestered in an extracellular or freely exchangeable compartment, nor was it irreversibly bound.
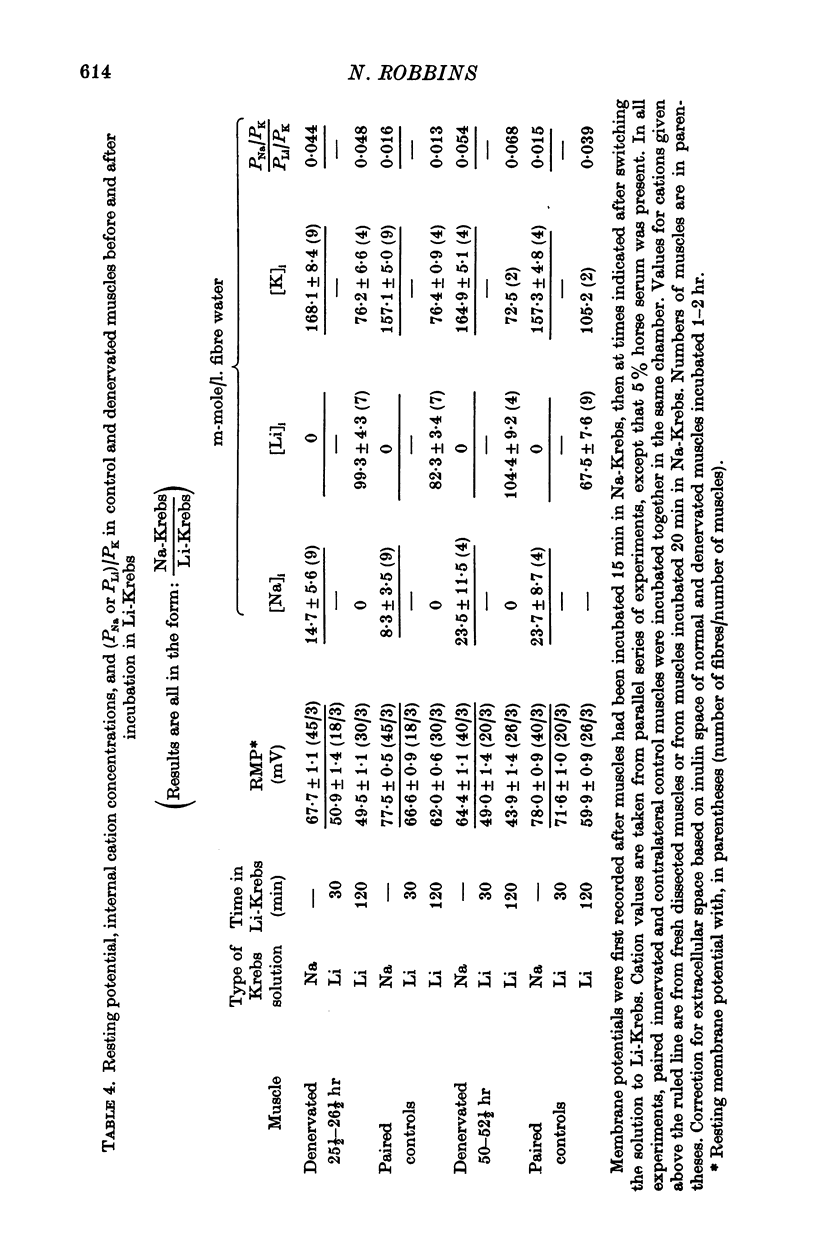
4. Measurements of resting membrane potential and of internal Na, K, and Li in Krebs solution before and 2 hr after replacement of NaCl by LiCl, were used to compute the ratios PNa/PK and PLi/PK for normal or denervated muscles. PNa and PLi were similar relative to PK within each class of muscle.
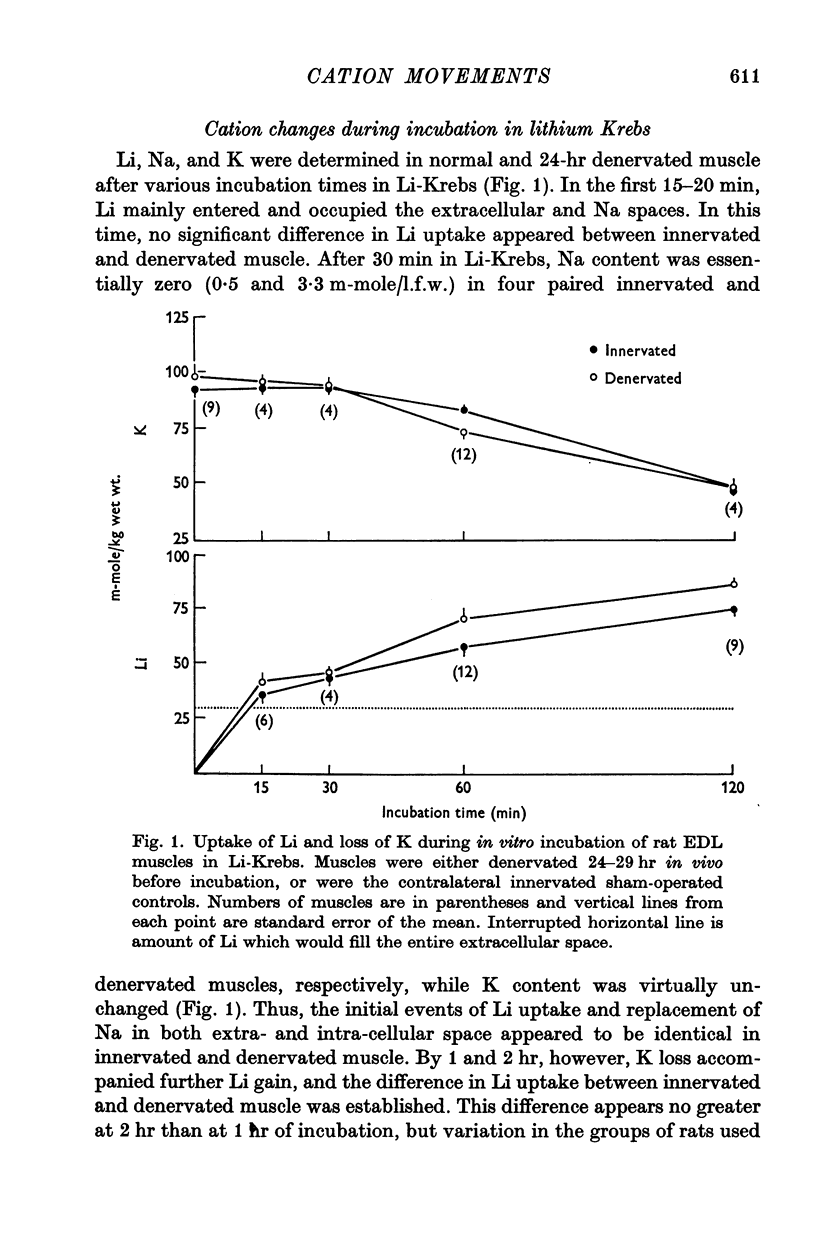
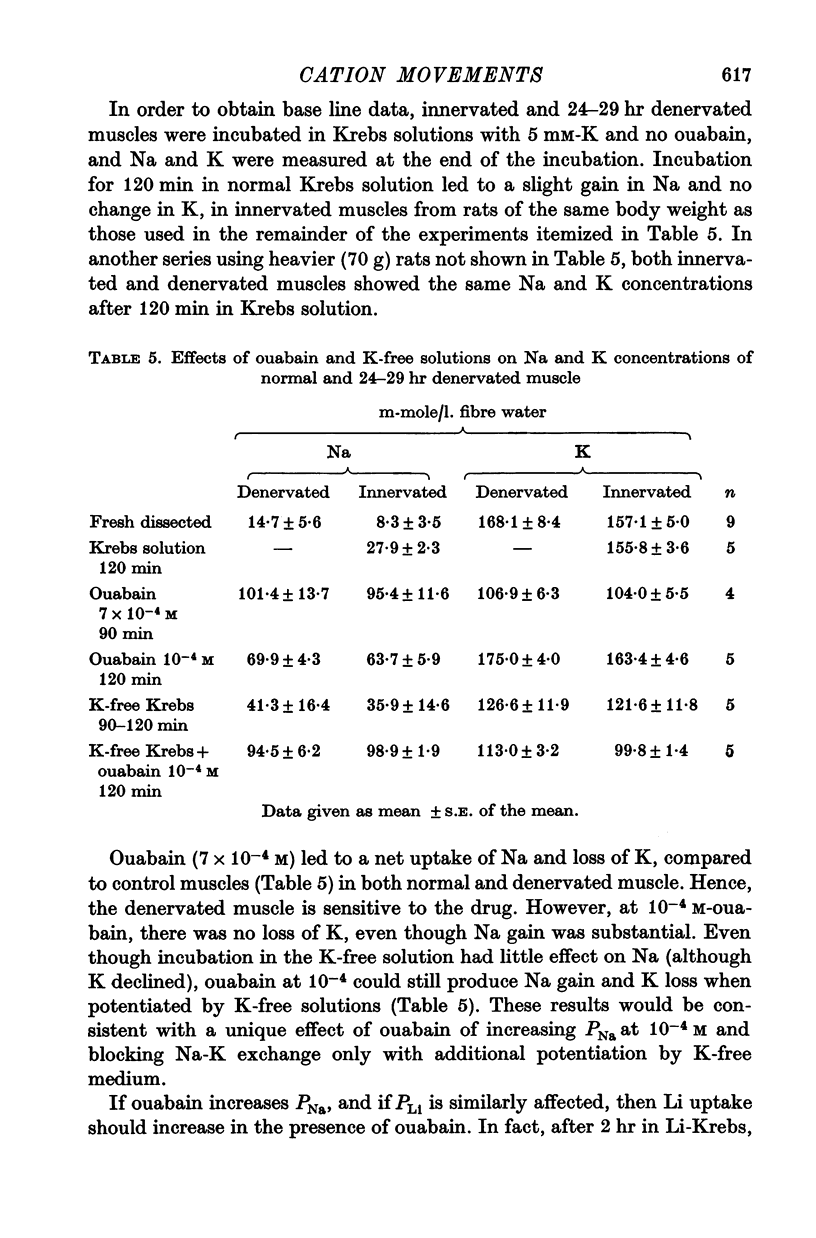
5. Both PNa/PK and PLi/PK ratios were elevated more than twofold in denervated muscle, as were most estimates of relative PLi approximated by the flux equation.
6. These data, and measurement of resting membrane potential of normal muscle in 1 mM external K-Krebs solution, support the view that an electrogenic Na—K pump does not substantially contribute to this potential of normal or denervated muscle, and that the early depolarization after denervation results from increased PNa.
7. The Na—K pump of denervated muscle was as sensitive to ouabain as normal muscle. An effect of ouabain on PNa may explain previously noted differential effects of ouabain on normal and denervated muscle.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N. Contribution of an electrogenic sodium pump to membrane potential in mammalian skeletal muscle fibres. J Physiol. 1975 Mar;245(3):499–520. doi: 10.1113/jphysiol.1975.sp010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T., Grundfest H. The hyperpolarization of frog skeletal muscle fibres induced by removing potassium from the bathing medium. J Physiol. 1971 Aug;217(1):33–60. doi: 10.1113/jphysiol.1971.sp009558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., McIsaac R. J. Fast and slow mammalian muscles after denervation. Exp Neurol. 1970 Jan;26(1):183–202. doi: 10.1016/0014-4886(70)90099-3. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Schuh F. T., Kauffman F. C. Early membrane depolarization of the fast mammalian muscle after denervation. Pflugers Arch. 1971;328(1):36–50. doi: 10.1007/BF00587359. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Thesleff S. A comparative study of membrane properties of innervated and chronically denervated fast and slow skeletal muscles of the rat. Acta Physiol Scand. 1968 Aug;73(4):471–480. doi: 10.1111/j.1365-201x.1968.tb10886.x. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Warnick J. E. The pharmacology of batrachotoxin. IV. Interaction with tetrodotoxin on innervated and chronically denervated rat skeletal muscle. J Pharmacol Exp Ther. 1972 Mar;180(3):683–697. [PubMed] [Google Scholar]
- Armstrong W. M., Lee C. O. Sodium and potassium activities in normal and "sodium-rich" frog skeletal muscle. Science. 1971 Jan 29;171(3969):413–415. doi: 10.1126/science.171.3969.413. [DOI] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. Membrane constants of mammalian muscle fibres. J Physiol. 1959 Oct;147:450–457. doi: 10.1113/jphysiol.1959.sp006255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray J. J., Hawken M. J., Hubbard J. I., Pockett S., Wilson L. The membrane potential of rat diaphragm muscle fibres and the effect of denervation. J Physiol. 1976 Mar;255(3):651–667. doi: 10.1113/jphysiol.1976.sp011301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREESE R., SCHOLES N. W., TAYLOR D. B. Temperature and resting potential of diaphragm muscle in a CO2-bicarbonate medium. J Pharmacol Exp Ther. 1958 Sep;124(1):47–52. [PubMed] [Google Scholar]
- Caldwell P. C. Factors governing movement and distribution of inorganic ions in nerve and muscle. Physiol Rev. 1968 Jan;48(1):1–64. doi: 10.1152/physrev.1968.48.1.1. [DOI] [PubMed] [Google Scholar]
- Camerino D., Bryant S. H. Effects of denervation and colchicine treatment on the chloride conductance of rat skeletal muscle fibers. J Neurobiol. 1976 May;7(3):221–228. doi: 10.1002/neu.480070305. [DOI] [PubMed] [Google Scholar]
- Creese R. Sodium fluxes in diaphragm muscle and the effects of insulin and serum proteins. J Physiol. 1968 Jul;197(2):255–278. doi: 10.1113/jphysiol.1968.sp008558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R., el-Shafie A. L., Vrbová G. Sodium movements in denervated muscle and the effects of antimycin A. J Physiol. 1968 Jul;197(2):279–294. doi: 10.1113/jphysiol.1968.sp008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. R. Electrophysiological activity of tetrodotoxin on the resting membrane of the squid giant axon. Comp Biochem Physiol A Comp Physiol. 1971 Sep 1;40(1):71–82. doi: 10.1016/0300-9629(71)90148-4. [DOI] [PubMed] [Google Scholar]
- Gauthier G. F., Padykula H. A. Cytological studies of fiber types in skeletal muscle. A comparative study of the mammalian diaphragm. J Cell Biol. 1966 Feb;28(2):333–354. doi: 10.1083/jcb.28.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinke J. A., Caillé J. P., Gayton D. C. Distribution and state of monovalent ions in skeletal muscle based on ion electrode, isotope, and diffusion analyses. Ann N Y Acad Sci. 1973 Mar 30;204:274–296. doi: 10.1111/j.1749-6632.1973.tb30785.x. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The permeability of frog muscle fibres to lithium ions. J Physiol. 1959 Oct;147:626–638. doi: 10.1113/jphysiol.1959.sp006265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLAUS W., LUELLMANN H., MUSCHOLL E. [Potassium flux of normal and denervated rat diaphragm]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;271:761–775. [PubMed] [Google Scholar]
- Khuri R. N., Hajjar J. J., Agulian S. K. Measurement of intracellular potassium with liquid ion-exchange microelectrodes. J Appl Physiol. 1972 Mar;32(3):419–422. doi: 10.1152/jappl.1972.32.3.419. [DOI] [PubMed] [Google Scholar]
- Klein R., Haddow J. E., Kind C., Cockburn F. Effect of cold on muscle potentials and electrolytes. Metabolism. 1968 Dec;17(12):1094–1103. doi: 10.1016/0026-0495(68)90088-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Yonemura K. The extracellular space in red and white muscles of the rat. Jpn J Physiol. 1967 Dec 15;17(6):698–707. doi: 10.2170/jjphysiol.17.698. [DOI] [PubMed] [Google Scholar]
- Kovács T., Vissy A., Went E. The effect of denervation on iontransport in tonic and tetanic skeletal muscles of the rat. Acta Physiol Acad Sci Hung. 1968;33(1):55–68. [PubMed] [Google Scholar]
- LI C. L. Effect of cooling on neuromuscular transmission in the rat. Am J Physiol. 1958 Jul;194(1):200–206. doi: 10.1152/ajplegacy.1958.194.1.200. [DOI] [PubMed] [Google Scholar]
- Locke S., Solomon H. C. Relation of resting potential of rat gastrocnemius and soleus muscles to innervation, activity, and the Na-K pump. J Exp Zool. 1967 Dec;166(3):377–386. doi: 10.1002/jez.1401660310. [DOI] [PubMed] [Google Scholar]
- McArdle J. J., Albuquerque E. X. Effects of ouabain on denervated and dystrophic muscles of the mouse. Exp Neurol. 1975 May;47(2):353–356. doi: 10.1016/0014-4886(75)90263-0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. Electrophysiology and electron-microscopy of rat neuromuscular junctions after nerve degeneration. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):289–306. doi: 10.1098/rspb.1968.0012. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T., Norris F. H., Jr Effect of local temperature on the resting membrane potential in rat muscle. Electroencephalogr Clin Neurophysiol. 1970 Jun;28(6):633–636. doi: 10.1016/0013-4694(70)90206-3. [DOI] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. II. The action of tetrodotoxin. Acta Physiol Scand. 1971 May;82(1):70–78. doi: 10.1111/j.1748-1716.1971.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Rogus E., Zierler K. L. Sodium and water contents of sarcoplasm and sarcoplasmic reticulum in rat skeletal muscle: effects of anisotonic media, ouabain and external sodium. J Physiol. 1973 Sep;233(2):227–270. doi: 10.1113/jphysiol.1973.sp010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salafsky B., Bell J., Prewitt M. A. Development of fibrillation potentials in denervated fast and slow skeletal muscle. Am J Physiol. 1968 Sep;215(3):637–643. doi: 10.1152/ajplegacy.1968.215.3.637. [DOI] [PubMed] [Google Scholar]
- Stonnington H. H., Engel A. G. Normal and denervated muscle. A morphometric study of fine structure. Neurology. 1973 Jul;23(7):714–724. doi: 10.1212/wnl.23.7.714. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Withrow C. D., Woodbury D. M. Effects of nephrectomy and KC1 on transmembrane potentials, intracellular electrolytes, and cell pH of rat muscle and liver in vivo. J Physiol. 1971 Jan;212(1):117–128. doi: 10.1113/jphysiol.1971.sp009313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A., Withrow C. D., Woodbury D. M. Effects of ouabain and diphenylhydantoin on transmembrane potentials, intracellular electrolytes, and cell pH of rat muscle and liver in vivo. J Physiol. 1971 Jan;212(1):101–115. doi: 10.1113/jphysiol.1971.sp009312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura K., Sato M. The resting membrane potential and cation movement in frog muscle fibers after exposure to lithium ions. Jpn J Physiol. 1967 Dec 15;17(6):678–697. doi: 10.2170/jjphysiol.17.678. [DOI] [PubMed] [Google Scholar]


