Abstract
1. The iontophoretic application of bicuculline, an antagonist of GABA, the putative inhibitory transmitter in the visual cortex, has been used to examine the contribution of post-synaptic inhibitory processes to the directional selectivity of simple, complex and hypercomplex cells in the cat's striate cortex.
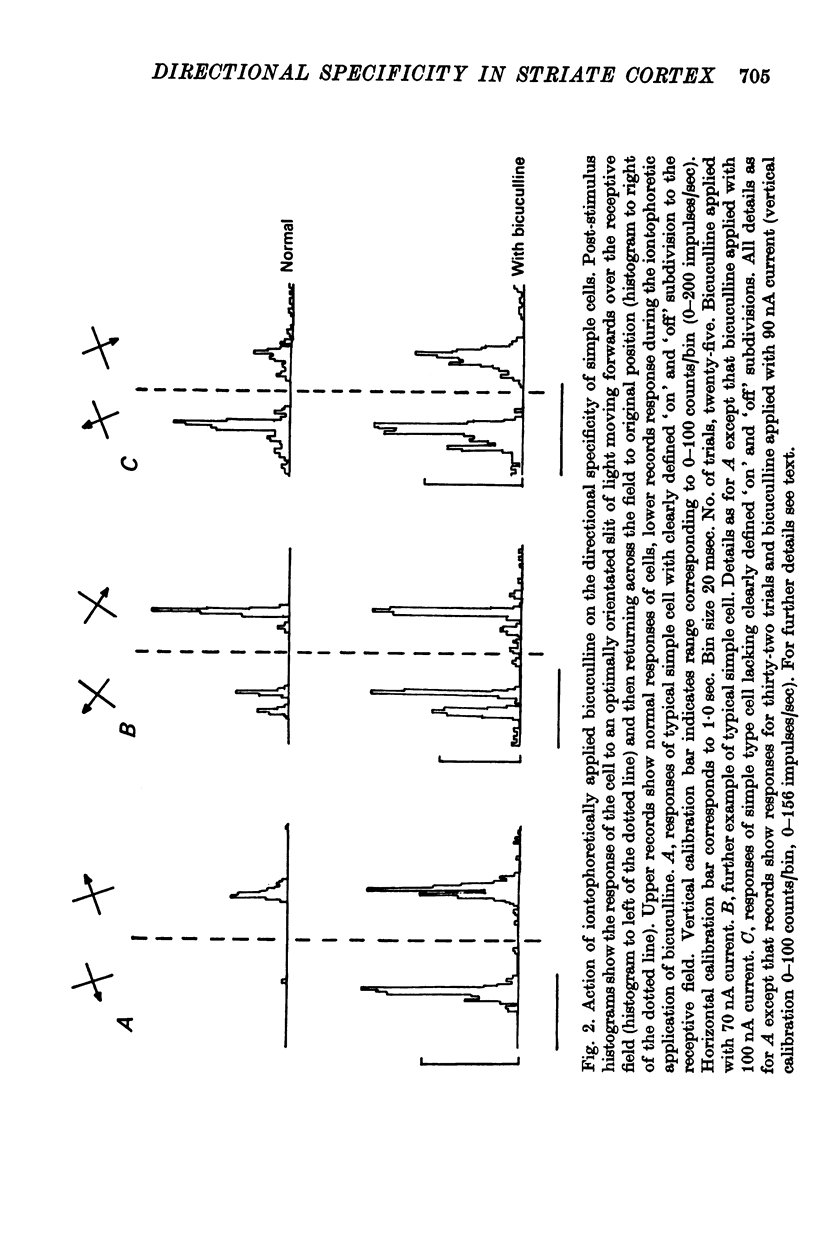
2. The directional selectivity of simple cells was significantly reduced or eliminated during the iontophoretic application of bicuculline. This supports the view that the selectivity is derived from the action of a GABA-mediated post-synaptic inhibitory input modifying their response to a non-directionally specific excitatory input.
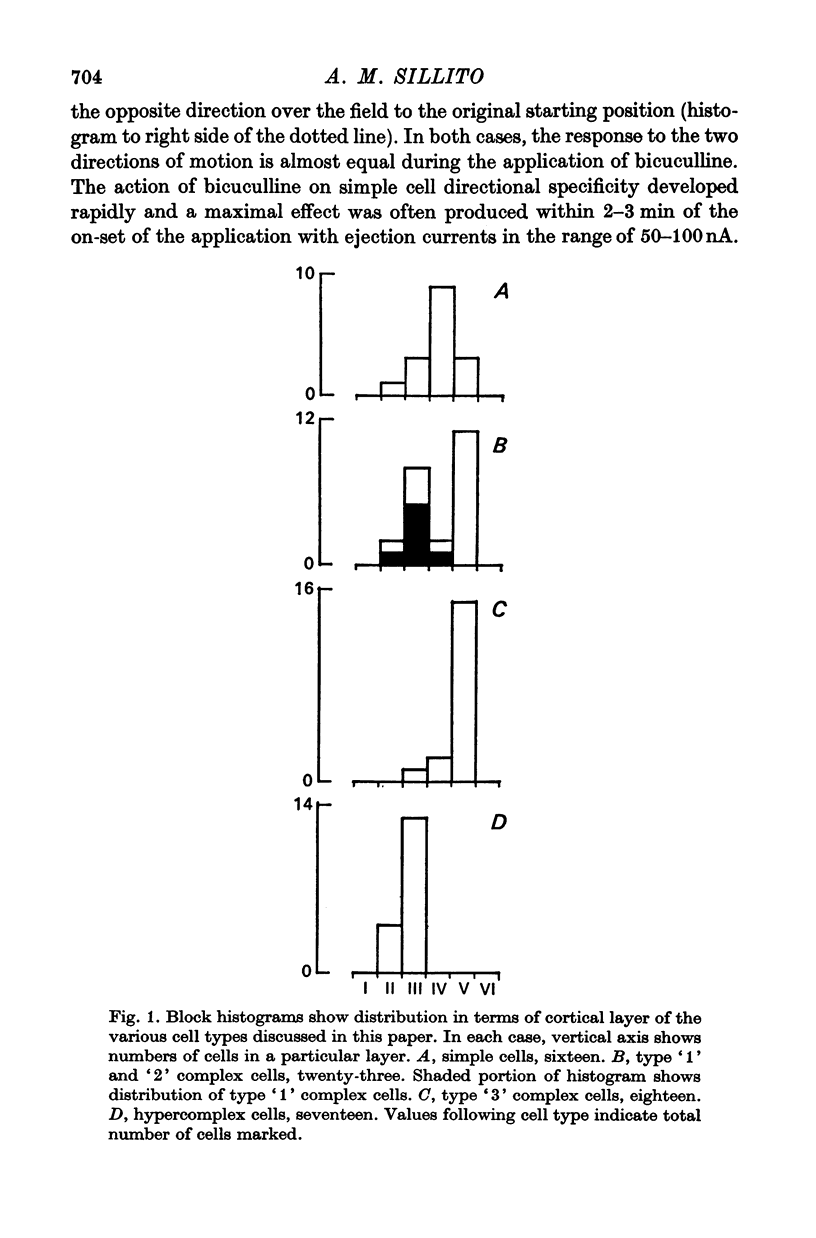
3. Complex cells were subdivided into three categories on the basis of the action of iontophoretically applied bicuculline on their directional selectivity, receptive field characteristics and distribution in terms of cortical layer. They are referred to as type `1', `2' and `3' complex cells.
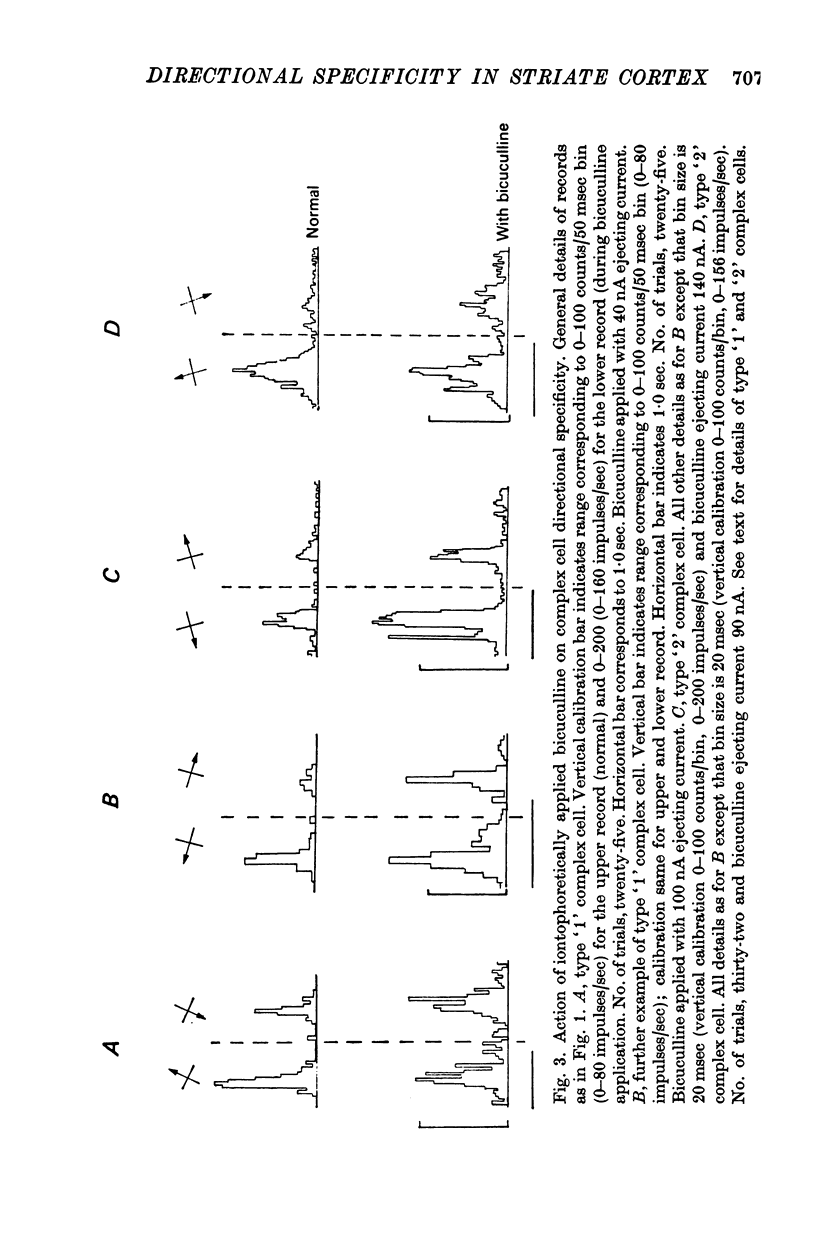
4. The directional specificity of type `1' complex cells was eliminated during the iontophoretic application of bicuculline. It seems likely, therefore, that they receive a non-directionally specific excitatory input and that, as for simple cells, the directional specificity derives from the action of a GABA-mediated post-synaptic inhibitory input. No type `1' complex cells were recorded below layer IV.
5. The directional specificity of type `2' complex cells was unaffected by the iontophoretic application of bicuculline, despite increases in response magnitude, a block of the action of iontophoretically applied GABA and, in some cases, changes in other receptive field properties. It is suggested that these cells receive a directionally specific excitatory input. The type `2' complex cells were found both superficial and deep to layer IV with the majority in layer V.
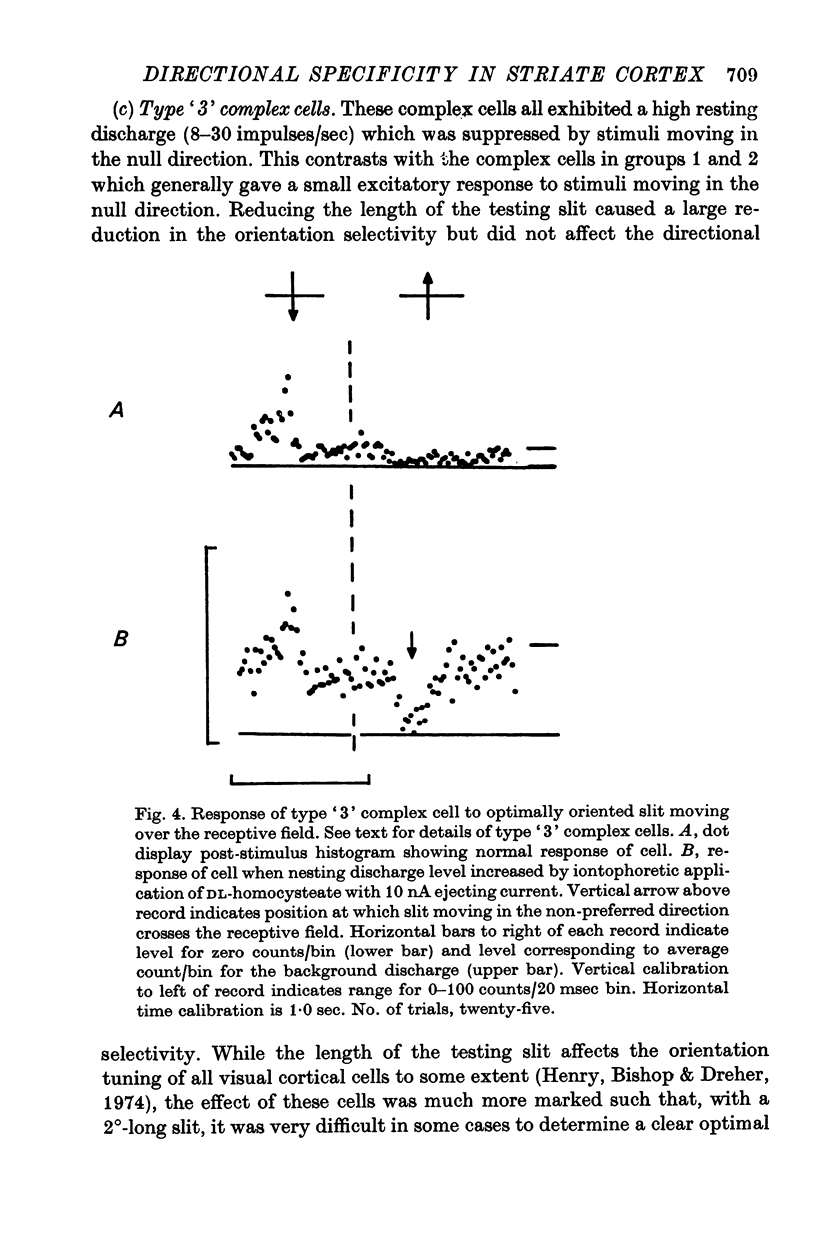
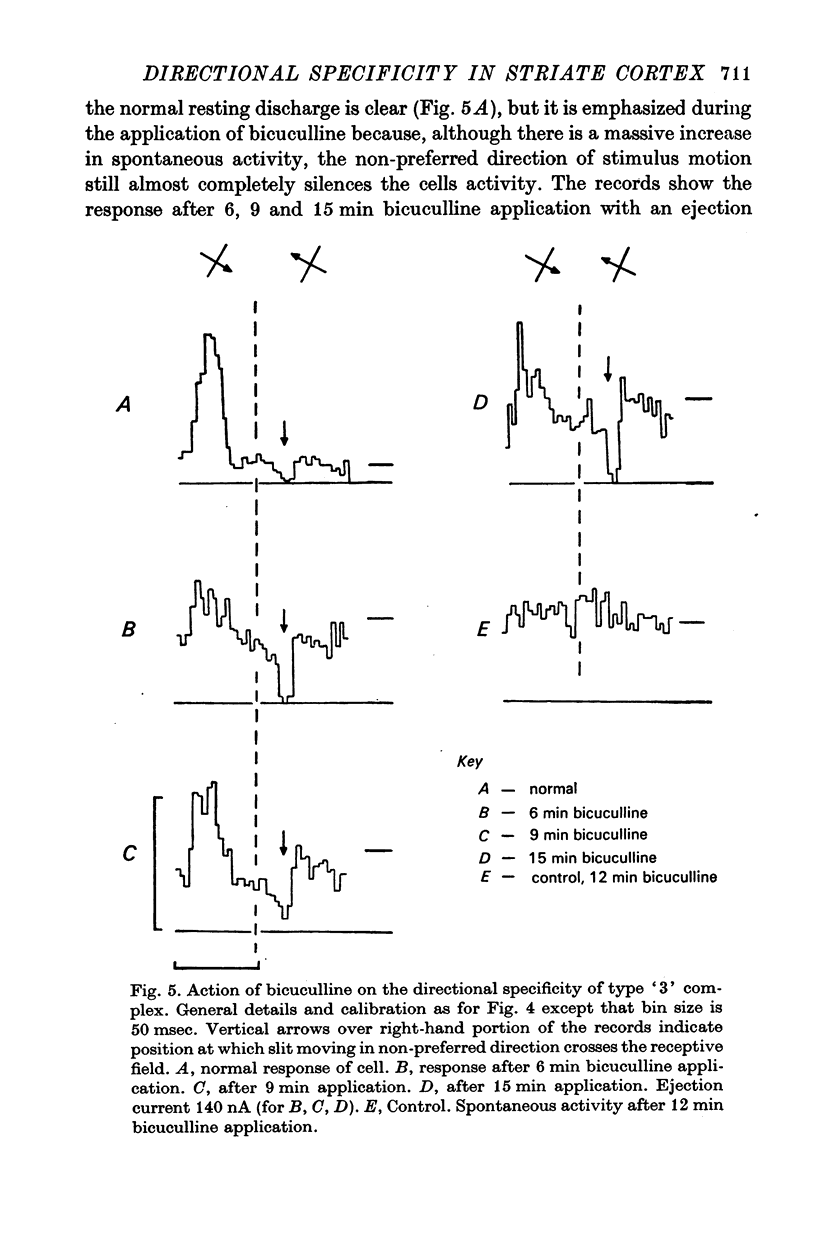
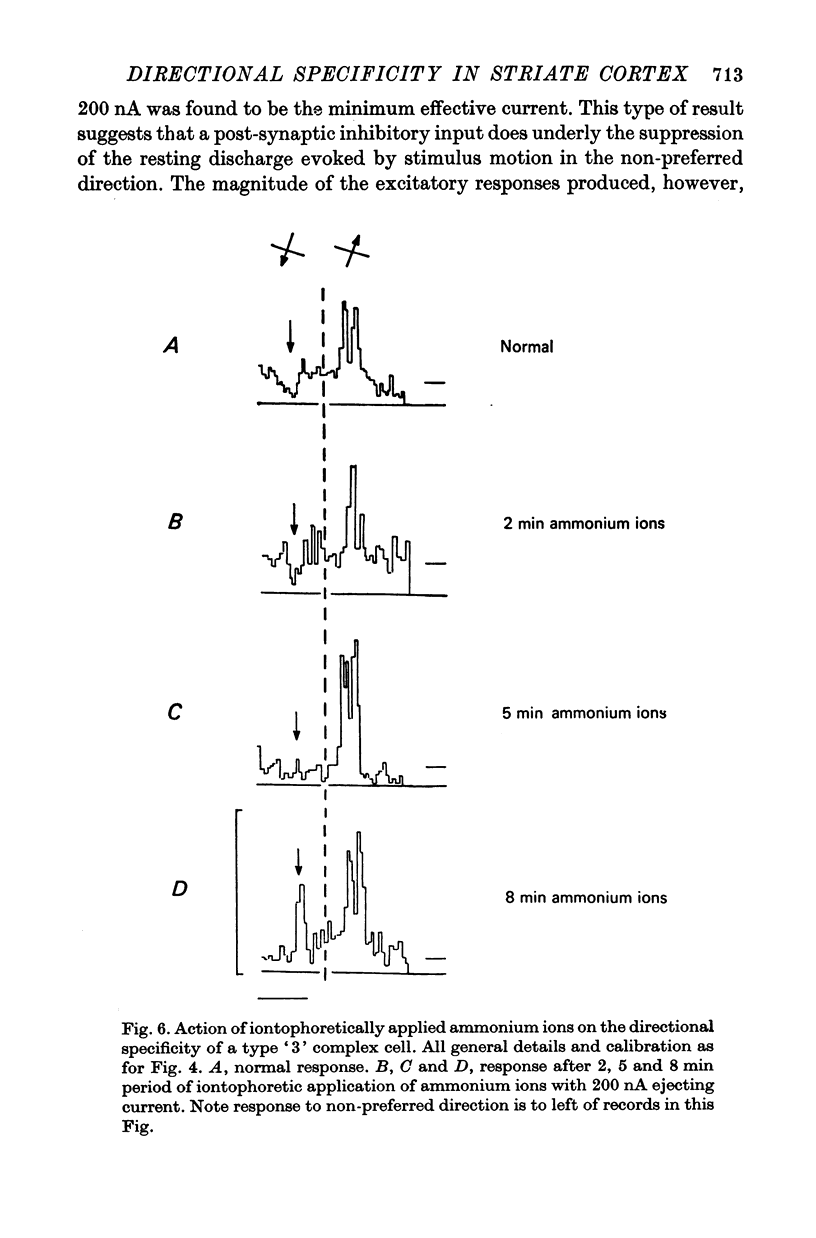
6. Type `3' complex cells appear to have very similar receptive field properties to those of the cells described by other workers as projecting to the superior colliculus. They were found predominantly in layer V. Their directional specificity was not eliminated by the iontophoretic application of bicuculline. However, they exhibited a powerful suppression of the resting discharge in response to stimulus motion in the non-preferred direction. Iontophoretic application of ammonium ions revealed a small excitatory response in place of the suppression. It appears from these observations that the directional specificity of the type `3' complex cells could be determined, at least in part, by an inhibitory process which is not GABA-mediated.
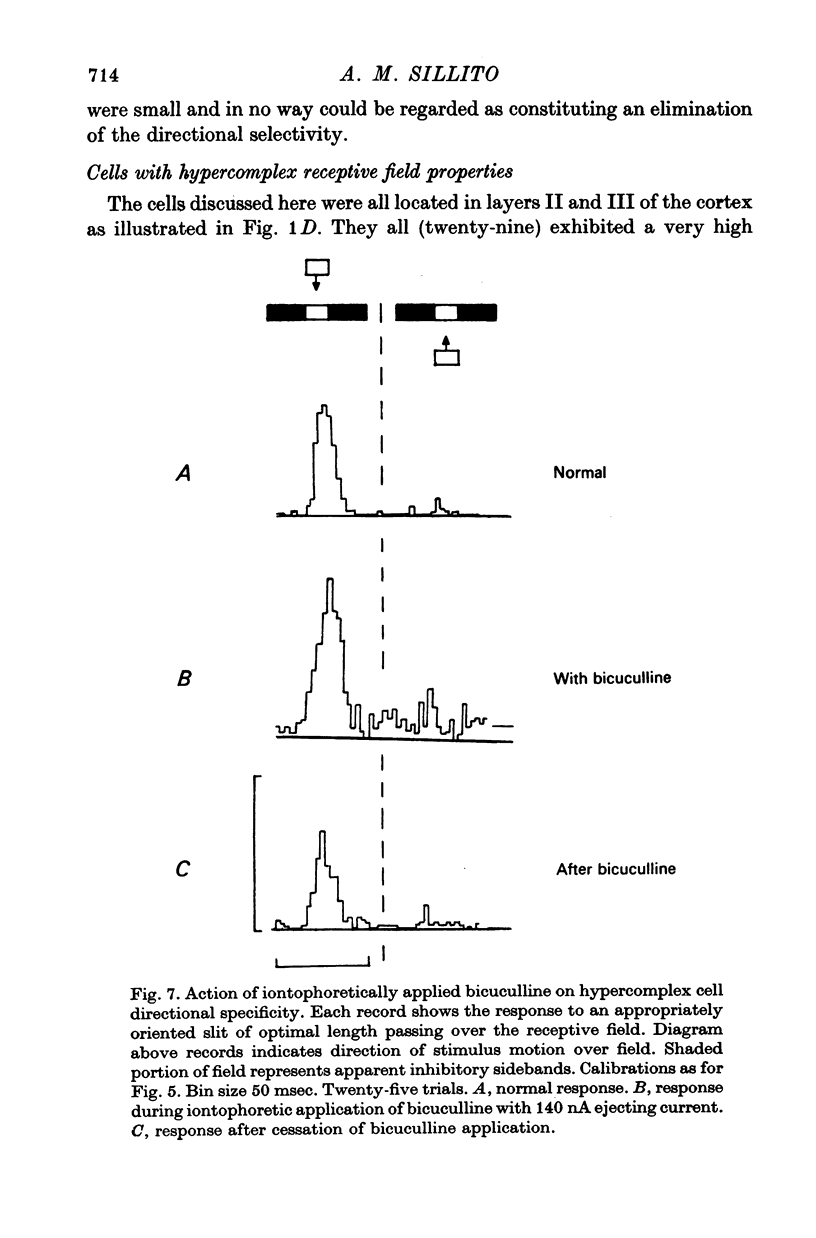
7. The directional specificity of hypercomplex cells found in layers II and III was unaffected by the iontophoretic application of bicuculline, and they showed no suppression of their background discharge level in response to stimulus motion in the non-preferred direction. This evidence is consistent with the view that they receive a directionally specific excitatory input.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benevento L. A., Creutzfeldt O. D., Kuhnt U. Significance of intracortical inhibition in the visual cortex. Nat New Biol. 1972 Jul 26;238(82):124–126. doi: 10.1038/newbio238124a0. [DOI] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H. Striate neurons: receptive field concepts. Invest Ophthalmol. 1972 May;11(5):346–354. [PubMed] [Google Scholar]
- Garey L. J. A light and electron microscopic study of the visual cortex of the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):21–40. doi: 10.1098/rspb.1971.0079. [DOI] [PubMed] [Google Scholar]
- Goodwin A. W., Henry G. H., Bishop P. O. Direction selectivity of simple striate cells: properties and mechanism. J Neurophysiol. 1975 Nov;38(6):1500–1523. doi: 10.1152/jn.1975.38.6.1500. [DOI] [PubMed] [Google Scholar]
- Goodwin A. W., Henry G. H. Direction selectivity of complex cells in a comparison with simple cells. J Neurophysiol. 1975 Nov;38(6):1524–1540. doi: 10.1152/jn.1975.38.6.1524. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Innocenti G. M., Fiore L. Post-synaptic inhibitory components of the responses to moving stimuli in area 17. Brain Res. 1974 Nov 8;80(1):122–126. doi: 10.1016/0006-8993(74)90728-8. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Mitchell J. F., Srinivasan V. The release of gamma-aminobutyric acid during inhibition in the cat visual cortex. J Physiol. 1971 Jan;212(2):519–534. doi: 10.1113/jphysiol.1971.sp009339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. P., Van Essen D. C. Cell structure and function in the visual cortex of the cat. J Physiol. 1974 May;238(3):515–547. doi: 10.1113/jphysiol.1974.sp010541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R., Baker R., Precht W. Blockage of inhibition by ammonium acetate action on chloride pump in cat trochlear motoneurons. J Neurophysiol. 1974 May;37(3):522–532. doi: 10.1152/jn.1974.37.3.522. [DOI] [PubMed] [Google Scholar]
- Movshon J. A. The velocity tuning of single units in cat striate cortex. J Physiol. 1975 Aug;249(3):445–468. doi: 10.1113/jphysiol.1975.sp011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTSUKA R., HASSLER R. [On the structure and segmentation of the cortical center of vision in the cat]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1962;203:212–234. doi: 10.1007/BF00352744. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Raabe W., Gumnit R. J. Disinhibition in cat motor cortex by ammonia. J Neurophysiol. 1975 Mar;38(2):347–355. doi: 10.1152/jn.1975.38.2.347. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Watkins D. W., Wilson J. R. Further differences in receptive field properties of simple and complex cells in cat striate cortex. Vision Res. 1976;16(9):919–927. doi: 10.1016/0042-6989(76)90221-2. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975 Sep;250(2):305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M. The effectiveness of bicuculline as an antagonist of GABA and visually evoked inhibition in the cat's striate cortex. J Physiol. 1975 Sep;250(2):287–304. doi: 10.1113/jphysiol.1975.sp011055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M., Versiani V. Synaptic mechanisms contributing to the lenght preference of hypercomplex cells [proceedings]. J Physiol. 1976 Dec;263(1):171P–172P. [PubMed] [Google Scholar]


