Abstract
The rdxBHIS gene cluster of Rhodobacter sphaeroides 2.4.1, located downstream of the ccoNOQP operon encoding the cbb3 cytochrome c oxidase, is required for the posttranscriptional modification of the cbb3 cytochrome c oxidase. The cbb3 cytochrome c oxidase is the main terminal oxidase under microaerobic conditions, as well as a component of the signal transduction pathway controlling photosynthesis gene expression. Because of the intimate functional and positional relationships of the ccoNOQP operon and the rdxBHIS gene cluster, we have examined the transcriptional activities of this DNA region in order to understand their expression and regulation. Northern blot analysis and reverse transcription-PCR, together with earlier complementation analysis, suggested that the ccoNOQP-rdxBHIS cluster is transcribed as ccoNOQP-, ccoNOQP-rdxBH-, rdxBH-, and rdxIS-specific transcripts. Multiple transcriptional start sites have been identified by primer extension analyses: five for ccoN, four for rdxB, and one for rdxI. Transcription from P1N of ccoN and P1B of rdxB is dependent on the presence of FnrL. LacZ fusion analysis support the above-described studies, especially the importance of FnrL. Expression of the cco-rdx cluster is closely related to photosynthesis gene expression, suggesting that transcript stoichiometry and presumably the stoichiometry of the gene products are critical factors in controlling photosynthesis gene expression.
In a series of published studies, we have demonstrated that the cbb3 cytochrome c oxidase of Rhodobacter sphaeroides 2.4.1, in addition to its role as a terminal oxidase, is also a critical component of the signal transduction pathway which regulates the onset of photosynthesis gene expression (15-18, 20, 23). Mutations of the ccoNOQP operon lead to the induction of photosynthesis gene expression under aerobic conditions, and as a related but secondary effect, such mutations also lead to altered carotenoid accumulations (15-18, 20). Since the photosystem is normally repressed under aerobic conditions, we have proposed that the cbb3 cytochrome c oxidase transduces an inhibitory signal as the result of electron transfer, which ultimately inhibits photosynthesis gene expression under high-oxygen conditions. We have also shown that the rate or volume of electron transfer through the cbb3 cytochrome oxidase is inversely related to the expression levels of the photosynthesis genes (18). Ultimately, this signal transduction pathway terminates with the global regulators PrrBA, comprising a two-component activation system (2, 4, 5, 15).
The rdxBHIS (ccoGHIS/fixGHIS) cluster is located immediately downstream of the ccoNOQP operon (20, 22). RdxB is believed to be a membrane-localized ferredoxin-like protein containing a 2[4Fe-4S] motif (by analogy to RdxA [14]). RdxI is a new subfamily of the CPx-type ATPase that functions to maintain the activity and integrity of the cbb3 cytochrome c oxidase, believed to be through maintenance of intracellular copper homeostasis (20). RdxH and RdxS possess no recognizable protein motifs or homologues (20). Inactivation of the rdxBHIS gene cluster also results in photosystem gene expression under aerobic conditions, suggesting that these proteins are involved, either directly or indirectly, in the same signal transduction pathway as the cbb3 cytochrome c oxidase (16, 17, 20). RdxH, -I, and -S are involved in the pathway by or through which the maturation and/or stability of the cbb3 cytochrome oxidase is maintained (20).
Previously, we reported that the RDXB1 mutant of R. sphaeroides 2.4.1, in which the rdxB gene was disrupted by insertion of a trimethoprim cassette, could be complemented only when both the rdxBH genes were present in trans (16). The rdxI and rdxS genes were not required for complementation of the RDXB1 mutant, suggesting the existence of an internal promoter for complete transcription of the rdxBHIS gene cluster.
Analysis of the fixGHIS (rdxBHIS) cluster of Sinorhizobium meliloti suggested that fixGHIS constitutes a single transcription unit (7). The genes of the ccoGHIS (rdxBHIS) cluster of Rhodobacter capsulatus were reported to be expressed independently from one another (9). However, the growth of a ccoGHIS deletion mutation of R. capsulatus was not fully complemented by the ccoGHIS cluster but only by both the ccoNOQP and ccoGHIS gene clusters (9).
Because of the importance of the rdxBHIS gene cluster to photosynthesis gene expression and carotenoid biosynthesis, its relation to the expression of the cbb3 cytochrome c oxidase, and the apparent disparity of homologous gene expression in other closely related bacteria, we have undertaken a thorough analysis of the transcriptional control of this region in R. sphaeroides 2.4.1.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The R. sphaeroides and Escherichia coli strains and plasmids used in this study are described in Table 1. E. coli strains were grown at 37°C on Luria-Bertani medium supplemented, when required, with tetracycline at 10 μg/ml and ampicillin, streptomycin, and spectinomycin at 50 μg/ml each. R. sphaeroides strains were grown at 30°C on Sistrom's minimal medium A containing succinate as a carbon source. Final concentrations of antibiotics were 1 μg/ml for tetracycline and 50 μg/ml for kanamycin, trimethoprim, streptomycin, and spectinomycin. Aerobic cultures were sparged with 30% O2-69% N2-1% CO2. Photosynthetic cultures were grown at a light intensity of 30 W/m2 and sparged with 95% N2-5% CO2. Growth of R. sphaeroides was monitored by measuring the optical density at 600 nm (OD600) with a Shimadzu UV-1601PC spectrophotometer. Plasmids pRK415 and pCF1010 were used for gene expression and as a promoter-cloning vector for R. sphaeroides, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| E. coli | ||
| DH5αphe | DH5α phe::Tn10dCm | 5 |
| S17-1 | C600::RP4-2(Tc::Mu)(Km::Tn7)thi pro hsdR recA Tra+ | 21 |
| R. sphaeroides | ||
| 2.4.1 | Wild type | W. Sistrom |
| CCOP1 | 2.4.1 derivative, ccoP::ΩTpr | 16 |
| JZ722 | 2.4.1 derivative, end of ccoN::Tn5TpMCS | 23 |
| JZ1678 | 2.4.1 derivative, ΔfnrL::ΩKmr | 22 |
| Plasmids | ||
| pRK415 | Tcr; Mob+lacZα IncP | 8 |
| pGEM-T | Apr; TA cloning vector | Promega |
| pCF1010 | Tcr; Smr; Spr; IncQ/IncP4, promoter cloning vector | 12 |
| pJR194 | pGEM-T::979 bp upstream of rdxB | This study |
| − 380 | pGEM-T::380 bp upstream of rdxB | This study |
| − 112 | pGEM-T::112 bp upstream of rdxB | This study |
| pUI1970 | pRK415::fnrL | 24 |
| pUI2803 | pRK415::4.7-kb BamHI + EcoRI containing ccoNOQP | 16 |
| pUI2805 | pRK415::4.2-kb PstI + BamHI containing rdxBHI′ | 16 |
| pJR103 | pRK415::4.9-kb PstI fragment containing rdxBHIS | This study |
| pJR235 | pRK415::8.3-kb BamHI + PstI containing ccoNOQP-rdxBHIS | This study |
| pJR471 | pRK415::2.9-kb EcoO109I + PstI containing rdxH'IS | This study |
| pJR473 | pRK415::8.1-kb BamHI fragment containing ccoNOQP-rdxBHI′ | This study |
| pJR316 | pCF1010::380 bp upstream of rdxB | This study |
| pJR319 | pCF1010::112 bp upstream of rdxB | This study |
| pJR320 | pCF1010::753 bp upstream of rdxH | This study |
| pJR321 | pCF1010::442 bp upstream of rdxI | This study |
| pJR322 | pCF1010::805 bp upstream of rdxS | This study |
DNA manipulation and analysis.
Standard protocols or manufacturer's instructions were followed to isolate plasmid DNA as well as for restriction enzyme treatment. DNA fragments containing the rdxBH, rdxIS, rdxBHIS, ccoNOQP, and ccoNOQP-rdxBH genes were subcloned under tet promoter control of pRK415 (Table 1). Plasmids were mobilized by biparental or triparental mating from E. coli strains into R. sphaeroides. Promoter regions for rdxBHIS::lacZ construction were amplified using PCR and subcloned into the pGEM-T plasmid (Promega). The plasmid pJR194 for rdxB was prepared with primer 52 (5′-CCG GAT CCC TGG GCC ATC GGC TAT TCG A-3′, extending from position −979 to −960 of rdxB) with a BamHI site and a reverse primer (5′-CCT CTA GAG ATC CAC CAC TTC AGC CTG C-3′, extending from position +102 to +83 of rdxB). This DNA construct was subjected to nested deletion analysis. The 5′-deletion constructs −380 and −112 were created by exonuclease III digestion of pJR194 linearized with BamHI and SacI by using the Erase-A-Base system (Promega) according to the manufacturer's instructions. For the rdxH::lacZ construction, primer 82 (5′-CCA TGC ATG ATG GAC GAG GAG ACG ATC A-3′, extending from position −753 to −732 of rdxH) and primer 81 (5′-CCT CTA GAG CAG GTT CAC CGC GAT GAT-3′, extending from position +76 to +58 of rdxH) were used. The rdxI::lacZ fusion was prepared with primer 83 (5′-CCC TGC AGC AAG GTT CTG GCG ATC ACC-3′, extending from position −442 to −423 of rdxI) and primer 84 (5′-CCA TGC ATG TTC GGC GGA AGG TGC GGC-3′, extending from position +94 to +76 of rdxI). The rdxS::lacZ fusion was constructed with primer 85 (5′-CCC TGC AGC ACC AAT CTC GCG ACG CTT-3′, extending from position −805 to −787 of rdxS) and primer 86 (CCA TGC ATA GGA AGA GCG AGA TCG GGA-3′, extending from position +41 to +23 of rdxS). The PCR-amplified DNA fragments were confirmed by sequencing and subsequently subcloned into pCF1010.
Enzyme assay.
Assays of β-galactosidase were performed on crude cell extracts of R. sphaeroides as previously described (20) at least twice, with standard deviations not exceeding 20%. Protein concentrations were determined with the Pierce (Rockford, Ill.) bicinchoninic acid protein assay reagent with bovine serum albumin as a standard.
RNA manipulation.
Total RNA from R. sphaeroides was isolated by the acidic hot-phenol method. R. sphaeroides grown with sparging as described above was harvested at 6,000 × g for 4 min after the addition of rifampin (200 μg/ml). Pellets were resuspended in 3 ml of lysis solution (0.15 M sucrose, 10 mM sodium acetate [pH 4.7], 1% sodium dodecyl sulfate), which was preheated at 65°C. The lysate was transferred immediately to a 15-ml disposable polypropylene tube containing 3 ml of acidic phenol (pH 4.3) preheated to 65°C. The lysis mixture was maintained at 65°C for 10 min and carefully inverted several times. After centrifugation at 6,000 × g for 15 min, the lysis mixture was subjected to repeated acidic phenol extraction as described above. After centrifugation, the supernatant was transferred to a new tube containing phenol-chloroform-isoamyl alcohol (125:24:1, pH 4.3), mixed, and centrifuged as described above. The upper phase was extracted with chloroform-isoamyl alcohol (24:1). RNA was ethanol precipitated after centrifugation. The RNA pellets were washed with 70% ethanol, slightly dried, and resuspended in diethylpyrocarbonate-treated H2O. To remove chromosomal DNA contamination, 40 U of RQ1 RNase-free DNase (Promega) was added to the RNA solution and incubated at 37°C for 1 h. RNA was precipitated by adding the same volume of 4 M LiCl after acidic phenol extraction as described above. Purified total RNA was separated by formaldehyde-containing agarose gel electrophoresis and transferred to a positively charged nylon membrane, BrightStar-Plus (Ambion). Strand-specific antisense RNA probes using [γ-32P]CTP were prepared according to the manufacturer's instructions with an RNA transcription kit (Promega). Labeled probes (106 cpm/ml) were added to QuickHyb hybridization solution (Stratagene). Membrane hybridization and washing were performed according to the manufacturer's instructions. A portion of the 23S rRNA (0.4-kb HindIII-PstI fragment) that is processed to 14S rRNA was used as a control for RNA normalization and standardization.
RT-PCR.
Coupled reverse transcription-PCR (RT-PCR) was performed to investigate transcription of the ccoNOQP-rdxBHIS gene cluster. To remove chromosomal DNA contamination, 100 μg of total purified RNA was further incubated with 40 U of RQ1 RNase-free DNase (Promega) at 37°C for 40 min. The reaction mixture was extracted with acidic phenol (pH 4.3), phenol-chloroform-isoamyl alcohol, and chloroform-isoamyl alcohol as described above for total RNA isolation. RNA was ethanol precipitated after the supernatant was transferred to a new tube. The RNA pellets were washed with 70% ethanol and resuspended in diethylpyrocarbonate-treated water. First-strand cDNA was synthesized from 10 μg of total RNA prepared as described above by using the SuperScript II RNase H− reverse transcriptase (Invitrogen) at 45°C for 1 h with oligonucleotide a (5′-AGG CTG CTG GTT TGG GTA TC-3′, extending from position +32 to +12 of the rdxB gene). One-fourth of the total cDNA was then PCR amplified with LA Taq polymerase (Takara) by using primers b (5′-CCG ATC CGC GCC GTG AAT GAT-3′, extending from position −112 to −92 of rdxB), c (5′-TCA CCC ACG GCA TCC GCA-3′, extending from position +470 to +488 of ccoP), d (5′-TAC AGC CTG CTG CGT GGC-3′, extending from position +10 to +27 of ccoQ), and e (5′-CCG TTC CCG CTG AGT GAA-3′, extending from position +1592 to +1609 of ccoN).
For each RT-PCR experiment, three control experiments were performed: one without template to detect contamination, one with the RNA template but without the reverse transcriptase to ensure that there was no trace DNA, and one with pRK415 containing the ccoNOQP-rdxBHIS genes (pJR235) as a positive control for PCR amplification.
Primer extension.
Total RNA was purified as described above and spectrophotometrically quantified. Primers for reverse transcription were end labeled with [γ-32P]ATP and T4 polynucleotide kinase (Invitrogen). The primer labeling reaction included 20 pmol of the primer in a mixture of 39 μl of H2O, 5 μl of [γ-32P]ATP (3,000 Ci/mmol), 5 μl of 10× buffer, and 1 μl of T4 polynucleotide kinase. The reaction mixture was incubated for 1 h at 37°C. The unincorporated ATP was separated from the primer by using a Micro Bio-Spin P-30 column (Bio-Rad). Labeled primer containing 106 cpm and 10 μg of total RNA was mixed in 24 μl of H2O, incubated at 70°C for 10 min, and chilled on ice. Reverse transcription was performed by mixing 8 μl of 5× reaction buffer, 4 μl of 0.1 M dithiothreitol, 2 μl of 1-mg/ml actinomycin D, 1 μl of 10 mM deoxynucleoside triphosphates, 1 μl of RNase inhibitor (SUPERase-In; Ambion), and 1 μl of SuperScript II RNase H− reverse transcriptase (Invitrogen). After 1 h of incubation at 45°C for extension, the enzyme was inactivated by heating at 70°C for 15 min and ethanol precipitated, and products were separated on an 8% sequencing gel next to a DNA sequencing reaction performed with the same primer. The specific signals revealed by Northern blotting as well as primer extension analyses were scanned and quantitated using NIH Image 1.62 software. Quantification was performed by subtracting the background level for each lane from the specific band.
RESULTS
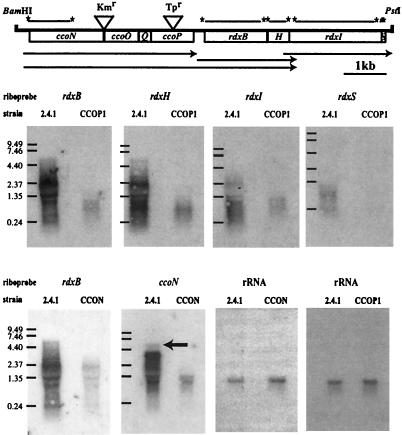
The ccoNOQP-rdxBHIS cluster constitutes a variable transcriptional unit. (i) The rdxBHIS cluster.
Although the rdxBHIS genes from R. sphaeroides 2.4.1 are tightly clustered and possess the same transcriptional polarity, we suggested previously that an internal promoter might exist within the rdxBHIS gene cluster (16). Complementation experiments showed that an RDXB1 mutant (rdxB::ΩTpr) could be complemented with an rdxBH-containing DNA fragment but not with the rdxB gene alone and thus did not require the rdxIS genes (16). To ascertain the transcriptional activity of the rdxBHIS gene cluster in R. sphaeroides 2.4.1, we performed Northern blot analysis using total RNA derived from cells grown under phototrophic conditions. As shown in Fig. 1, the rdxBHIS cluster in the wild type produced several different transcription products which hybridized with different probes from the region, suggesting that the rdxBHIS gene cluster does not form a simple transcriptional unit. Although the transcripts appeared to be relatively unstable, as noted by the extent of smearing, the largest transcript (∼5.7 kb) as well as a broad zone of degraded mRNA species from the wild type was observed with both the rdxB and rdxH riboprobes. The band at 5.7 kb was not observed with the rdxI or rdxS riboprobe. Extending the exposure with the rdxS probe revealed the same transcript pattern as that of the rdxI probe (data not shown). The rdxB, rdxH, rdxI, and rdxS probes hybridized to longer transcripts (approximately 5.7 kb for rdxB and rdxH and 2.5 kb for rdxI and rdxS) than their expected gene sizes (20), namely, 1.434, 458, 2,213, and 158 bp, respectively. Because the size of the rdxBHIS cluster is approximately 4.3 kb and the longest transcript which showed a signal with either the rdxB or rdxH probe was not detected with the rdxI and rdxS probes, we conclude that rdxBH and rdxIS are transcribed differentially. These results suggested that the longer transcripts containing rdxB and rdxH contain all or part of the ccoNOQP operon, which is located immediately upstream of the rdxBHIS cluster and which is in the same transcriptional orientation. If there is an rdxBHIS transcript, it is of low abundance and very unstable.
FIG. 1.
Northern blot analysis of the ccoNOQP-rdxBHIS gene cluster. Total RNA was isolated from R. sphaeroides 2.4.1 and the ccoN::Km (CCON) and ccoP::Tp (CCOP1) mutants grown under phototrophic conditions. Insertion of the Kmr and Tpr cassettes within the ccoN and ccoP genes, respectively, is indicated. Labeled lines with asterisks above the open bar designate the 5′ and 3′ ends of the riboprobes used. The arrows under the open bar indicate the deduced transcripts. The 3,434-bp ccoNOQP operon (1,608, 725, 204, and 873 bp, respectively) and the 4,268-bp rdxBHIS gene cluster (1,434, 459, 2,214, and 159 bp, respectively) are separated by 235 bp. Approximately 10 μg of total RNA was loaded onto each lane. The 32P-labeled riboprobes corresponding to the rdxB, rdxH, rdxI, rdxS, ccoN, and 23S rRNA genes were used for hybridization as described in Materials and Methods. Molecular size ladders are shown to the left. The arrow in the ccoN hybridization panel indicates the transcript corresponding to the position of the longest transcript which was observed with the rdxB and rdxH riboprobes.
(ii) ccoNOQP and rdxBHIS.
To determine if the ccoNOQP and the rdxBH genes form a cotranscript, Northern blot analysis using RNA extracted from the CCOP1 mutant (16), in which the ccoP gene is disrupted by an ΩTpr cassette (Fig. 1), was performed. As demonstrated in Fig. 1, insertion of an ΩTpr cassette into the ccoP gene resulted in a significantly reduced size and amount of the rdxBHIS transcript(s), thereby suggesting that transcription of the rdxBHIS genes is dependent primarily upon transcription that is initiated upstream of the ΩTpr cassette, i.e., within cco.
Because the longest (∼5.7-kb) transcript containing both the rdxB and rdxH genes has a size similar to the expected size of a transcript extending from the start of ccoN to the end of the rdxH protein-coding sequence (5,562 bp), we suggest that the ccoNOQP and rdxBH genes are for the most part transcribed as a single transcription unit. To confirm this hypothetical organization, transcriptional analysis was performed with total RNA isolated from the wild type and strain JZ722 (CCON), which contains a Tn5TpMCS cassette inserted between the ccoN and ccoO genes (23). We assumed that if cotranscription of ccoNOQP-rdxBH exists, the transcript size of the rdxB gene would be changed in mutant CCON, as was the case for the CCOP1 mutant, compared to the size of the transcript obtained by using both rdxB and ccoN as probes of the wild-type 2.4.1 RNA.
As shown in Fig. 1, when rdxB was used as a hybridization probe, significantly reduced transcript levels were observed in CCON. When the ccoN gene was used as a hybridization probe, we detected a 3.6-kb ccoNOQP transcript in the wild-type strain in addition to the same transcripts that were detected when rdxB was used as a probe. The 5.7-kb transcript detected with the ccoN and rdxB probes is best explained by cotranscription of ccoNOQP-rdxBH under photosynthetic conditions, again suggesting that expression of the rdx cluster is dependent primarily upon the upstream cco operon. The rRNA transcript was readily detected in relatively similar amounts in both strains.
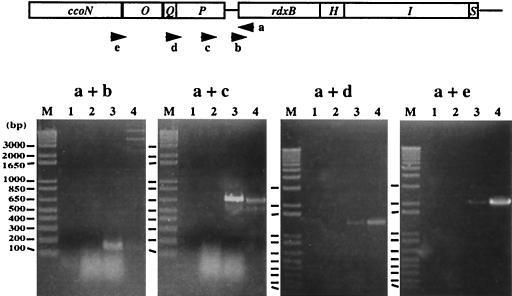
(iii) RT-PCR.
To further confirm the existence of a single transcript containing ccoNOQP-rdxBH, RT-PCR was performed with a set of oligonucleotides hybridizing to the 5′-untranslated region of rdxB (b) in addition to regions of the cco operon (c, d, and e) and oligonucleotide a, hybridizing to the rdxB gene (Fig. 2). PCR products of the expected sizes, i.e., 144 bp for primers a plus b, 670 bp for primers a plus c, 1,330 bp for primers a plus d, and 2,110 bp for primers a plus e, were obtained (Fig. 2). No PCR products were detected in any of the control reactions that were designed to detect DNA contamination. Although RT-PCR with the rdxH-specific primer was not performed, the Northern data showed that the rdxH gene was included in the longer (∼5.7-kb) transcript containing the ccoNOQP operon.
FIG. 2.
RT-PCR of R. sphaeroides 2.4.1 total RNA. The locations of the oligonucleotides used for RT-PCR are shown by the arrows. The PCR products amplified by using the primers a, b, c, d, and e were subjected to electrophoresis on a 1% agarose gel (PCR product with primers a plus d and a plus e) or a 2% agarose gel (PCR products with primers a plus b and a plus c). The positions and sizes of the 1-kb plus DNA ladder from Invitrogen are indicated to the left (lanes M). The RT-PCR lacking a template failed to detect any contamination (lane 1), and total RNA without reverse transcriptase also failed to detect DNA contamination in the template (lanes 2), with the cDNA sample (lanes 3), and with positive control DNA, i.e., pJR235 containing the ccoNOQP-rdxBHIS genes in pRK415 (lanes 4).
Cotranscription of the ccoNOQP-rdxBH genes was also supported from Northern blot analysis using total RNA of an RDXI mutant in which approximately 1.5 kb of the rdxI gene was deleted in frame (20). The 5.7-kb band which was observed with the rdxB, rdxH, and ccoN probes was still observed in the RDXI mutant (data not shown).
Because the RT-PCR, Northern blot analysis, and earlier complementation experiments all support cotranscription of ccoNOQP-rdxBH, we went on to characterize the promoter regions of these gene clusters in order to better understand their expression and regulation.
Promoter structure of the ccoNOQP operon, rdxB, and rdxI. (i) ccoNOQP.
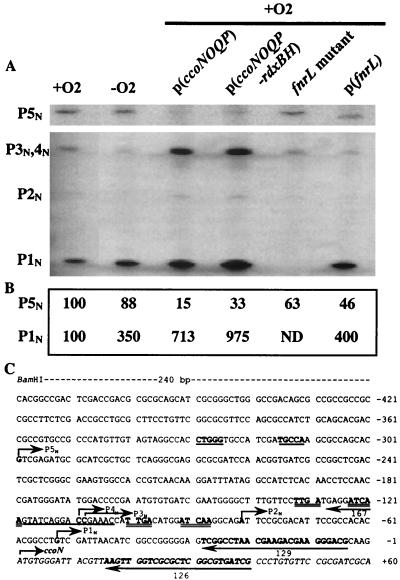
To begin to understand the promoter structure of the ccoNOQP operon, we performed primer extension analysis using total RNA isolated from wild-type cells grown under aerobic and photosynthetic conditions. We also used total RNA prepared from strains that contained the ccoNOQP (pUI2803) and ccoNOQP-rdxBH (pJR473) genes in the multicopy plasmid pRK415. The oligonucleotides used for these experiments are indicated in Fig. 3C. Transcriptional start points were determined when different primers were used to detect the same transcripts in the primer extension experiments and when transcript abundance was correlated to the presence of extra copies of the corresponding genes. Specific signals (P1N to P5N) for the ccoN gene were obtained with oligonucleotides 126 and 129. The results of the primer extension experiments demonstrated the presence of a major signal located at −53 bp (P1N) upstream of the ccoN translational start codon (Fig. 3A). An additional four 5′ ends, which showed weaker signals, were observed at −82 bp (P2N), −109 bp (P3N), −110 bp (P4N), and −300 bp (P5N). The 5′ ends were designated P1N, P2N, P3N, P4N, and P5N, in order, from the ccoN translational start codon (Fig. 3A and C). Primer 167 confirmed the presence of the P5N transcript (data not shown). As shown in Fig. 3A and B, an approximately 3.5-fold-increased level of P1N was obtained when total RNA was derived from cells grown under photosynthetic conditions (lane 2) rather than under aerobic conditions (lane 1). The bands for P2N, P3N, and P4N were faint under both conditions. P5N was expressed at significantly greater levels than P2N, P3N, and P4N, but much less than P1N, under both growth conditions. Introduction of extra copies of the ccoNOQP operon under aerobic conditions caused significantly increased levels of P1N, P3N, and P4N, but P5N decreased (Fig. 3A and B, lanes 3 and 4), perhaps suggesting an autoregulatory effect upon the P5N 5′ end. Thus, extra copies of ccoNOQP and ccoNOQP-rdxBH gave the same (but amplified) 5′ ends (except P5N) as when they were present in single copy.
FIG. 3.
Primer extension experiments and nucleotide sequence upstream of the ccoN gene. (A) Total RNAs isolated from the wild type grown under aerobic conditions (+O2) and under photosynthetic conditions (−O2), the wild type containing ccoNOQP in pRK415 [p(ccoNOQP)], the wild type containing ccoNOQP-rdxBH in pRK415 [p(ccoNOQP-rdxBH)], the fnrL mutant, and the wild type containing the fnrL gene in pRK415 [p(fnrL)] were used for primer extension experiments with primer 126. The 5′ ends (P1N to P5N) are indicated on the left. Sizes of the primer extension products were determined by comparison to the sequencing ladder obtained from the same primer. P3N, P4N, and P5N were confirmed by extended gel electrophoresis (data not shown). (B) Quantification of signal levels of P1N and P5N. Since the level of P2N was very low and the bands for P3N and P4N are indistinguishable, we did not quantitate these signals. The levels of P1N and P5N of wild-type cells grown aerobically were considered to be 100%, and other values are given relative to that value. ND, not detectable. (C) Nucleotide numbering is relative to the putative translational start site (designated +1) of the ccoN gene, which is shown in italics. The binding sites of the primers used in the primer extension experiments are underlined with arrows, and the number under the arrow is the primer number used for the experiment. The 5′ ends and directions of transcription are marked by arrows above the determined 5′ ends and are indicated as P1N, P2N, P3N, P4N, and P5N. The BamHI site was used in order to subclone the ccoNOQP operon into pRK415. Two putative FnrL binding sites are double underlined. A putative σ54 motif is indicated by an underline. The nucleotides mentioned above are in boldface, except the sequence for primer 167.
The ccoNOQP promoter region was analyzed to predict the possible DNA elements involved in its regulation. Previously, two putative FnrL binding motifs were described for the sequence upstream of the ccoN gene (13) (Fig. 3C). The FnrL binding motifs are centered at positions −73.5 and −41.5 from P1N. Since β-galactosidase activity for a ccoN::lacZ fusion was significantly reduced in the fnrL mutant, it was suggested that FnrL is a major regulator of ccoNOQP expression (13). When total RNA was prepared from the fnrL mutant (lane 5) as well as from an fnrL-overexpressing strain (lane 6), we observed that transcription from P1N was absolutely dependent on the presence of FnrL. This is consistent with a role for FnrL as an activator of ccoNOQP expression and further supports the role of the P1N start site.
(ii) rdxB and rdxI.
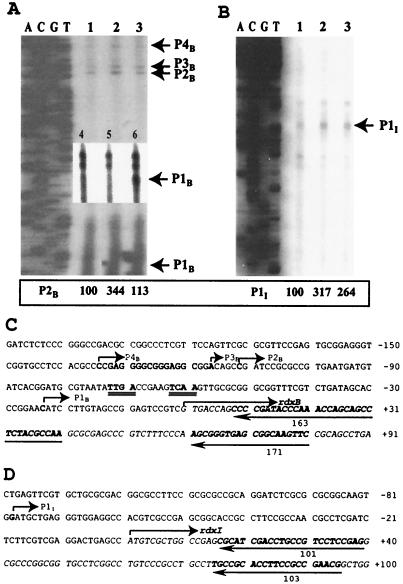
As demonstrated from the Northern blot analysis using CCOP1 and CCON in Fig. 1, transcription from the cco promoter constitutes the major regulatory region leading to the expression of rdxBH. In addition, the regions responsible for expression of rdxB and rdxI were also mapped by primer extension analysis because transcription of rdxBH and rdxIS was suggested earlier (Fig. 4A and B). Total RNA was prepared from strains containing plasmids pJR103, pUI2805, and pJR471, which contain the rdxBHIS, rdxBH, and rdxIS genes in pRK415, respectively, in addition to the wild-type strain. Four 5′ ends for rdxB were identified, at −23 bp (P1B), −111 bp (P2B), −116 bp (P3B), and −134 bp (P4B) upstream of the translational initiation site of the rdxB gene (Fig. 4C). Using extra copies of the rdxBH genes (Fig. 4A, lanes 2 and 6), we detected increased levels of these signals, supporting the results obtained for the wild type (Fig. 4A, lanes 1 and 4). Because the minor bands showed no alternations under any conditions, these were not considered significant. Total RNA isolated from the fnrL mutant (lanes 3 and 5) was also used to investigate the effect of FnrL on these 5′ ends, since a putative FnrL binding motif was found in the rdxB upstream region. The transcription level from P1B in the fnrL mutant was significantly reduced, suggesting positive regulation by FnrL, while the level was significantly increased with extra copies of rdxBH (lane 6).
FIG. 4.
Primer extension experiments and nucleotide sequence of the upstream regions of rdxB (A and C) and rdxI (B and D). (A) Total RNAs isolated from the wild type (lanes 1 and 4), the wild type containing the rdxBH genes in pRK415 (lanes 2 and 6), and the fnrL mutant (lanes 3 and 5) were used for primer extension experiments. The strains were grown under aerobic conditions. The levels of P2B and P1I of the wild type were considered to be 100%, and other levels are compared to these values. The P1B transcript from another primer extension experiment is shown by a separate gel (small box inside the gel). DNA sequence and transcripts (P1B to P4B) are indicated on the left and right, respectively. (B) Total RNAs isolated from the wild type (lane 1), the wild type containing the rdxBHIS genes in pRK415 (lane 2), and the wild type containing the rdxIS genes in pRK415 (lane 3) were used for primer extension experiments with primer 101. The strains were grown aerobically. DNA sequence and transcript (P1I) are indicated on the left and right, respectively. (C) The translational start site (designated +1) for rdxB is indicated with an arrow above the sequence which is shown in italics. A putative FnrL binding sequence is doubly underlined. (D) The translational start site (designated +1) for rdxI is indicated with an arrow above the sequence, which is shown in italics. The 5′ ends and directions are marked by arrows above the determined 5′ ends. The nucleotides mentioned above are in boldface. The binding sites of the primers used in the primer extension experiments are underlined with an arrow, and the numbers under the arrows indicate the primers used for the experiments.
Using the same approach with rdxBHIS and rdxIS, we determined a 5′ end at −79 bp upstream of the rdxI translational start (Fig. 4B). When extra copies of the rdxBHIS and rdxIS genes were used, the band for P1I increased approximately 2.5- to 3-fold (Fig. 4B, lanes 2 and 3) compared to wild type (lane 1), whereas there were no changes observed for the minor bands, which are not believed to be significant.
(iii) lacZ fusion analysis.
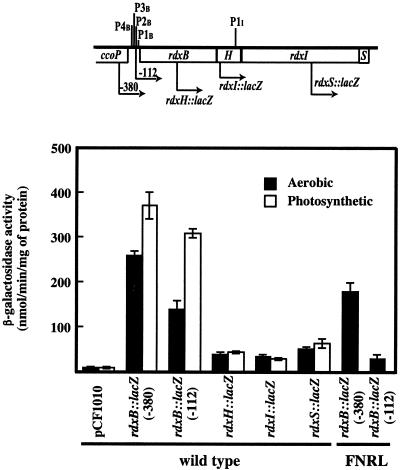
In light of the report that each gene of the ccoGHIS cluster of R. capsulatus is expressed separately (9), we constructed lacZ fusion plasmids carrying DNA sequences upstream of each gene as determined from the preceding experiments. For the rdxB::lacZ fusion, we made two fusion plasmids, pJR316 and pJR319, containing sequences for all reported 5′ ends and for P1B alone, respectively. The rdxB::lacZ, rdxH::lacZ, rdxI::lacZ, and rdxS::lacZ fusions were introduced into R. sphaeroides 2.4.1, and β-galactosidase activities were determined (Fig. 5). The results demonstrated that each of the rdxB, rdxH, rdxI, and rdxS genes could be individually expressed, as reported for the R. capsulatus ccoGHIS, although only the rdxB fusion showed high expression for both aerobically and photosynthetically grown cells, which agrees with both the Northern and 5′-end analyses. Also, the −112 fusion showed lower β-galactosidase activities than the −380 fusion under these same conditions. The lacZ activities for the rdxH, rdxI, and rdxS fusions were all very low and showed little variation when assayed in cells grown aerobically or photosynthetically. In the fnrL mutant, β-galactosidase activity for rdxB::lacZ(−112) was strongly suppressed, again suggesting FnrL regulation of the P1B transcriptional start. In the fnrL mutant, β-galactosidase activity for rdxB::lacZ(−380) was higher than that for rdxB::lacZ(−112). However, in the fnrL mutant grown aerobically, the −112 fusion showed the most significant decrease in β-galactosidase activity, suggesting the possible existence of a regulator(s) for aerobic activation of P1B in this region (between −380 and −112 bp) in addition to FnrL.
FIG. 5.
β-Galactosidase activities of the various rdxBHIS::lacZ fusions in the wild-type and fnrL mutant strains. The positions of relevant RNA 5′ ends and 5′ endpoints of lacZ fusions are indicated. Strains were grown aerobically (sparged with 69% N2-30% O2-1% CO2) and photosynthetically (sparged with 95% N2-5% CO2) to an OD600 of 0.4 ± 0.1. Activities are expressed as nanomoles of o-nitrophenyl-β-d-galactopyranoside hydrolyzed per minute per milligram of protein extract. Values with the ranges indicated are the average of two independent determinations. pCF1010 is the control plasmid vector containing the promoterless lacZ used to construct the rdxBHIS::lacZ fusions as described in Materials and Methods. Error bars indicate standard deviations.
Cotranscription is important to photosynthesis gene expression.
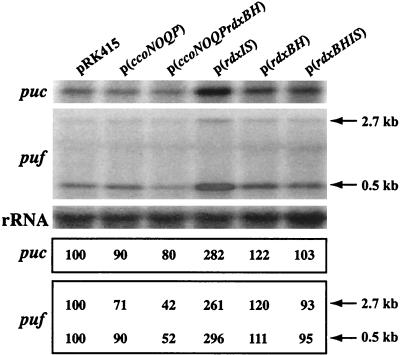
Our results strongly suggest that the ccoNOQP-rdxBHIS loci are transcribed predominantly as ccoNOQP, ccoNOQP-rdxBH, rdxBH, and rdxIS. To determine the potential role(s) of each transcript, plasmids which carried ccoNOQP, ccoNOQP-rdxBH, rdxBHIS, rdxBH, and rdxIS in pRK415 were constructed, and each plasmid was introduced into R. sphaeroides 2.4.1. R. sphaeroides 2.4.1 containing the rdxIS genes in pRK415 displayed a more intense red coloration under aerobic conditions than the other strains. Because we showed previously that colony coloration was related to the amount of spectral complexes formed under aerobic conditions (15-18, 20, 23), we determined the expression levels of the puf and the puc operons from these cells grown under aerobic conditions. As shown in Fig. 6, extra copies of rdxIS in trans (fourth lane) caused the highest expression levels for both puf and puc compared to the other strains. However, the effect of rdxIS overexpression was diminished by the presence of the rdxBH genes, which by themselves have very little effect (fifth and sixth lanes), suggesting that the stoichiometry of rdxBH and rdxIS expression is a critical factor in modulating photosynthesis gene expression. The levels of puc and puf expression were further decreased (Fig. 6) by overexpression of ccoNOQP-rdxBH compared to in the presence of ccoNOQP (17, 18).
FIG. 6.
Northern blot analysis of R. sphaeroides 2.4.1 grown under aerobic conditions. Total RNA was isolated from the wild type containing pRK415, the wild type containing ccoNOQP in pRK415 [p(ccoNOQP)], the wild type containing ccoNOQP-rdxBH in pRK415 [p(ccoNOQP-rdxBH)], the wild type containing rdxIS in pRK415 [p(rdxIS)], the wild type containing rdxBH in pRK415 [p(rdxBH)], and the wild type containing rdxBHIS in pRK415 [p(rdxBHIS)]. The strains were grown in Sistrom's minimal medium A containing tetracycline, by sparging with 69% N2-30% O2-1% CO2, to an OD600 of 0.3 ± 0.05. Approximately 10 μg of total RNA was loaded onto each lane. Riboprobes specific for puf, puc, and the 23S rRNA gene were used for hybridization as described in Materials and Methods. The 2.7- and 0.5-kb transcripts for puf are present (6, 11). The levels of puc and puf were normalized to the level of rRNA. The mRNA level in the wild type containing pRK415 was considered 100%, and other values are given relative to that value.
DISCUSSION
The ccoNOQP operon, encoding the cbb3 cytochrome c terminal oxidase, and the rdxBHIS gene cluster, whose gene products are involved in the structure and function of the cbb3-type cytochrome c oxidase, are adjacent and lie in the same transcriptional orientation on chromosome I of R. sphaeroides 2.4.1 (16, 20, 22). Earlier complementation experiments using an rdxB::Tp mutation suggested the presence of a promoter internal to the gene cluster rdxBHIS (16). In this study, using Northern blot analysis (Fig. 1) and RT-PCR (Fig. 2), we have shown that the ccoNOQP-rdxBHIS cluster is transcribed as a ccoNOQP-rdxBH, ccoNOQP, rdxBH, and rdxIS series of transcripts. Using lacZ fusions, we have shown that each of the rdxHIS genes can be expressed separately at very low levels whose physiologic relevance is questionable. We cannot completely exclude the possibility of the presence of a low-abundance, unstable rdxBHIS transcript. However, the transcript would be rare, if it exists, because the expected 4.3-kb rdxBHIS transcript was not detected in either the ccoN::Km or the ccoP::Tp mutant strains. Further, the demonstration of the presence of a ccoNOQP-rdxBH transcript supports the conclusion that these are likely to represent the major source of expression of the rdxBHIS cluster. These results also reveal that expression of these gene clusters in R. sphaeroides is quite different from the expression patterns observed for R. capsulatus (9) and S. meliloti (7).
Since the cbb3 cytochrome c oxidase is the main respiratory enzyme under microaerobic conditions and deficiency of the RdxHIS gene products causes a loss of the cbb3 cytochrome c oxidase activity (20), coordination of the expression of ccoNOQP-rdxBHIS is likely to be precisely regulated in order to optimize energy production under these conditions. Previously, we reported that expression of ccoNOQP was dependent upon FnrL and was highest under semiaerobic conditions, moderate under photosynthetic conditions, and lowest under aerobic conditions (13). We have earlier shown that FnrL is required for expression of the photosynthesis genes, such as puc, hemA, hemN, hemZ, and bchE, in addition to expression of ccoNOQP, rdxBHIS, and dorS, which in turn affects the presence of dimethyl sulfoxide reductase (13, 19, 22). Thus, an fnrL mutant is unable to grow anaerobically, either photosynthetically or in the dark using dimethyl sulfoxide as an electron acceptor (22).
To understand how FnrL is involved in the regulation of ccoNOQP expression, we performed primer extension experiments with both the wild-type and the fnrL mutant strain (Fig. 3). Five ccoNOQP 5′ ends, designated P1N, P2N, P3N, P4N, and P5N, were identified by primer extension analysis and are located at −53 bp, −82 bp, −109 bp, −110 bp, and −300 bp upstream of the ccoN translation start, respectively. Since β-galactosidase activity of a ccoN::lacZ fusion was significantly diminished in the fnrL mutant strain (13), in which the P1N transcript is absent, we suggest that P1N is both the major transcriptional start site for the ccoNOQP operon and positively regulated by FnrL. This pattern of cco transcription is entirely consistent with the previous determinations of β-galactosidase activity of ccoN::lacZ fusion-containing strains (13). Further, these data show that the R. sphaeroides FnrL is functional under aerobic conditions, although not as strongly as under microaerobic conditions. The aerobic activity of R. sphaeroides FnrL has been noted earlier and explained previously (22), when comparisons to a mutant form of the E. coli Fnr, which is oxygen stable, were made (10). R. sphaeroides FnrL possesses a histidine residue at position 29 and an alanine residue at position 154 that appear to influence oxygen stability of the E. coli Fnr.
Two FnrL binding motifs were centered at positions −41.5 and −73.5 relative to the P1N transcript site and at −11.5 and −43.5 from P2N. Although FnrL functions as a major regulator of P1N expression, P2N expression was not affected in the fnrL mutant. Because the second FnrL binding motif (−43.5) from P2N is very close to the typical class II position (−41.5), additional experiments involving site-directed mutagenesis will be required to determine how the two FnrL motifs participate in the regulation of ccoNOQP expression. Although the other four start sites were similarly expressed in the fnrL and the wild type, transcription from the P5N start site appeared to involve another, unknown regulator(s). Inspection of the DNA sequence immediately preceding the P5N start site reveals sequence similarity to the E. coli σ54 promoter consensus sequence (1) (Fig. 3C). Experiments to confirm whether R. sphaeroides σ54 participates in ccoNOQP expression are in progress.
rdxB also has an FnrL binding motif at −42.5 bp from P1B (Fig. 4). Primer extension experiments and β-galactosidase activity of an rdxB::lacZ fusion (−112) demonstrate that transcription from P1B is positively regulated by FnrL (Fig. 4 and 5). The β-galactosidase activity of an rdxB::lacZ fusion (−380) in the fnrL mutant strain was approximately 70% of that in the wild type. Together, these results suggest that FnrL plays a role in rdxB expression under these conditions. Because expression of the two rdxB::lacZ fusions was high under photosynthetic conditions but differentially expressed in the absence of FnrL under aerobic conditions, we suggest that the presence of some unknown regulator(s) is also involved in the aerobic expression of rdxB.
The presence of multiple promoters for ccoNOQP-rdxBHIS expression suggests that expression of the cco-rdx cluster is tightly coordinated, depending upon the growth conditions, in order to permit the cells to adapt rapidly to the onset of environmental changes and to ensure proper cellular levels of the cbb3 cytochrome c oxidase, which is essential for growth and regulation of photosynthesis gene expression under these different conditions.
Overexpression of rdxIS (Fig. 6) in R. sphaeroides 2.4.1 increased formation of the photosystem under aerobic conditions. Similarly, an rdxI in-frame deletion mutation showed spectral complexes under aerobic conditions, resulting from the instability of the cbb3 cytochrome oxidase (20). We suggested that RdxI is involved in the assembly and therefore the activity of the cbb3 cytochrome oxidase through the maintenance of copper homeostasis (20). RdxS was also shown to have an effect on the steady-state presence of the cbb3 cytochrome oxidase (20). Thus, either the absence or excess of the RdxIS gene products turns on photosynthesis gene expression under aerobic conditions, suggesting that these conditions can interfere with the structure-function of the cbb3 oxidase, which in turn gives rise to photosynthesis gene expression such as when the cco operon is inactivated. In the closely related bacterium R. capsulatus, a CcoS (RdxS) mutant gives rise to a form of the cbb3 cytochrome oxidase lacking heme b, heme b3, and the copper cofactors of subunit I (9). Thus, we assume that the phenotype resulting from rdxIS overexpression is related to the cofactor state of the cbb3 cytochrome oxidase.
Interestingly, rdxBHIS overexpression does not show the phenotype(s) which characterizes rdxIS overexpression (Fig. 6). This result suggests that rdxBH compensates for the effect of rdxIS overexpression, perhaps by establishing an appropriate stoichiometry for these gene products. In the rdxB in-frame deletion mutant, the cbb3 cytochrome c oxidase was not affected in either activity or protein composition (17, 20). This mutant, however, does show photosynthesis gene expression under aerobic conditions. Thus, the physiological roles of RdxB and RdxH, -I, and -S must be distinct. One intermediate which transmits the inhibitory signal from the cbb3 cytochrome oxidase to the PrrBA two-component system (2, 4, 5) in the presence of O2, namely, PrrC has been identified (3). Are there others? RdxB has high homology to the bacterial ferredoxins, most likely containing a 2[4Fe-4S] cluster and two potential half centers (20). Because overexpression of ccoNOQP-rdxBH further represses photosynthesis gene expression under aerobic conditions, it is possible that electrons may be transferred via the cbb3 cytochrome oxidase to RdxBH, which in turn may indirectly regulate PrrB activity.
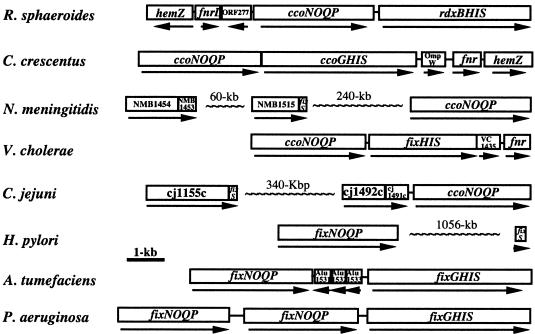
Finally, database searches revealed both similar and dissimilar gene organizations for the ccoNOQP-rdxBHIS cluster in a variety of bacteria (Fig. 7). This diversity in the structure of the ccoNOQP-rdxBHIS loci in these various bacteria is interesting in its own right and likely reflects specific physiological requirements. But more importantly from the data presented here, it is apparent that these diverse gene organizations may reflect the need to provide gene products in alternative forms and abundances.
FIG. 7.
Diagrams illustrating the genetic organization of the cco(fix)NOQP-rdxBHIS (fix/ccoGHIS) cluster and their flanking regions in different organisms. Homology searching was done with rdxH and rdxS because rdxB and rdxI have high homology to bacterial ferredoxin and CPx-type metal transporter which are normally found in many other bacteria. The DDBJ/EMBL/GenBank accession numbers of the sequences are as follows: R. sphaeroides, U58092 and AF202779; Caulobacter crescentus, AE005673; Neisseria meningitidis, AE002098 and AL162759; Vibrio cholerae, AE004222; Campylobacter jejuni, AL111168; Helicobacter pylori, AE001439 and AE000511; Agrobacterium tumefaciens, AE007869 and AE008688; and Pseudomonas aeruginosa, AE004091. C. crescentus OmpW is a putative outer membrane protein. The fnrL and fnr genes encode anaerobic transcriptional regulators. NMB1454 and NMB1453 are rdxBH homologues. VC1435 and Atu1531 are hypothetical proteins, and hemZ corresponds to the genes encoding isoenzymic forms of coproporphyrinogen III oxidase. NMB1515, which is composed of 436 amino acids, shows similarity to the central and carboxyl regions of the R. sphaeroides RdxI protein (737 amino acids) and is a putative transporter. cj1492c-cj1491c is a two-component regulatory system. cj1155c is an rdxI homologue. Atu1532 and Atu1533 are proteins related to glyoxylate and bleomycin resistance. The rdxB homologue of V. cholerae and the rdxB and rdxI homologues of H. pylori are not indicated. The rdxH homologue was not found for H. pylori.
Acknowledgments
This work was supported by a grant (GM15590) from the Public Health Service to S.K.
REFERENCES
- 1.Barrios, H., B. Valderrama, and E. Morett. 1999. Compilation and analysis of σ54-dependent promoter sequences. Nucleic Acids Res. 27:4305-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eraso, J. M., and S. Kaplan. 1996. Complex regulatory activities associated with the histidine kinase PrrB in expression of photosynthesis genes in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 178:7037-7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eraso, J. M., and S. Kaplan. 2000. From redox flow to gene regulation: role of the PrrC protein of Rhodobacter sphaeroides 2.4.1. Biochemistry 39:2052-2062. [DOI] [PubMed] [Google Scholar]
- 4.Eraso, J. M., and S. Kaplan. 1995. Oxygen-insensitive synthesis of the photosynthetic membranes of Rhodobacter sphaeroides: a mutant histidine kinase. J. Bacteriol. 177:2695-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eraso, J. M., and S. Kaplan. 1994. prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J. Bacteriol. 176:32-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong, L., J. K. Lee, and S. Kaplan. 1994. The Q gene of Rhodobacter sphaeroides: its role in puf operon expression and spectral complex assembly. J. Bacteriol. 176:2946-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn, D., M. David, O. Domergue, M. L. Daveran, J. Ghai, P. R. Hirsch, and J. Batut. 1989. Rhizobium meliloti fixGHI sequence predicts involvement of a specific cation pump in symbiotic nitrogen fixation. J. Bacteriol. 171:929-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 9.Koch, H. G., C. Winterstein, A. S. Saribas, J. O. Alben, and F. Daldal. 2000. Roles of the ccoGHIS gene products in the biogenesis of the cbb3-type cytochrome c oxidase. J. Mol. Biol. 297:49-65. [DOI] [PubMed] [Google Scholar]
- 10.Lazazzera, B. A., D. M. Bates, and P. J. Kiley. 1993. The activity of the Escherichia coli transcription factor FNR is regulated by a change in oligomeric state. Genes Dev. 7:1993-2005. [DOI] [PubMed] [Google Scholar]
- 11.Lee, J. K., B. S. DeHoff, T. J. Donohue, R. I. Gumport, and S. Kaplan. 1989. Transcriptional analysis of puf operon expression in Rhodobacter sphaeroides 2.4.1 and an intercistronic transcription terminator mutant. J. Biol. Chem. 264:19354-19365. [PubMed] [Google Scholar]
- 12.Lee, J. K., and S. Kaplan. 1995. Transcriptional regulation of puc operon expression in Rhodobacter sphaeroides. Analysis of the cis-acting downstream regulatory sequence. J. Biol. Chem. 270:20453-20458. [PubMed] [Google Scholar]
- 13.Mouncey, N. J., and S. Kaplan. 1998. Oxygen regulation of the ccoN gene encoding a component of the cbb3 oxidase in Rhodobacter sphaeroides 2.4.1T: involvement of the FnrL protein. J. Bacteriol. 180:2228-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neidle, E. L., and S. Kaplan. 1992. Rhodobacter sphaeroides rdxA, a homolog of Rhizobium meliloti fixG, encodes a membrane protein which may bind cytoplasmic [4Fe-4S] clusters. J. Bacteriol. 174:6444-6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Gara, J. P., J. M. Eraso, and S. Kaplan. 1998. A redox-responsive pathway for aerobic regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 180:4044-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Gara, J. P., and S. Kaplan. 1997. Evidence for the role of redox carriers in photosynthesis gene expression and carotenoid biosynthesis in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 179:1951-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh, J. I., and S. Kaplan. 1999. The cbb3 terminal oxidase of Rhodobacter sphaeroides 2.4.1: structural and functional implications for the regulation of spectral complex formation. Biochemistry 38:2688-2696. [DOI] [PubMed] [Google Scholar]
- 18.Oh, J. I., and S. Kaplan. 2000. Redox signaling: globalization of gene expression. EMBO J. 19:4237-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh, J. I., and S. Kaplan. 2001. Generalized approach to the regulation and integration of gene expression. Mol. Microbiol. 39:1116-1123. [DOI] [PubMed] [Google Scholar]
- 20.Roh, J. H., and S. Kaplan. 2000. Genetic and phenotypic analyses of the rdx locus of Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 182:3475-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1:37-45. [Google Scholar]
- 22.Zeilstra-Ryalls, J. H., and S. Kaplan. 1995. Aerobic and anaerobic regulation in Rhodobacter sphaeroides 2.4.1: the role of the fnrL gene. J. Bacteriol. 177:6422-6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeilstra-Ryalls, J. H., and S. Kaplan. 1996. Control of hemA expression in Rhodobacter sphaeroides 2.4.1: regulation through alterations in the cellular redox state. J. Bacteriol. 178:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeilstra-Ryalls, J. H., and S. Kaplan. 1998. Role of the fnrL gene in photosystem gene expression and photosynthetic growth of Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 180:1496-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]