Abstract
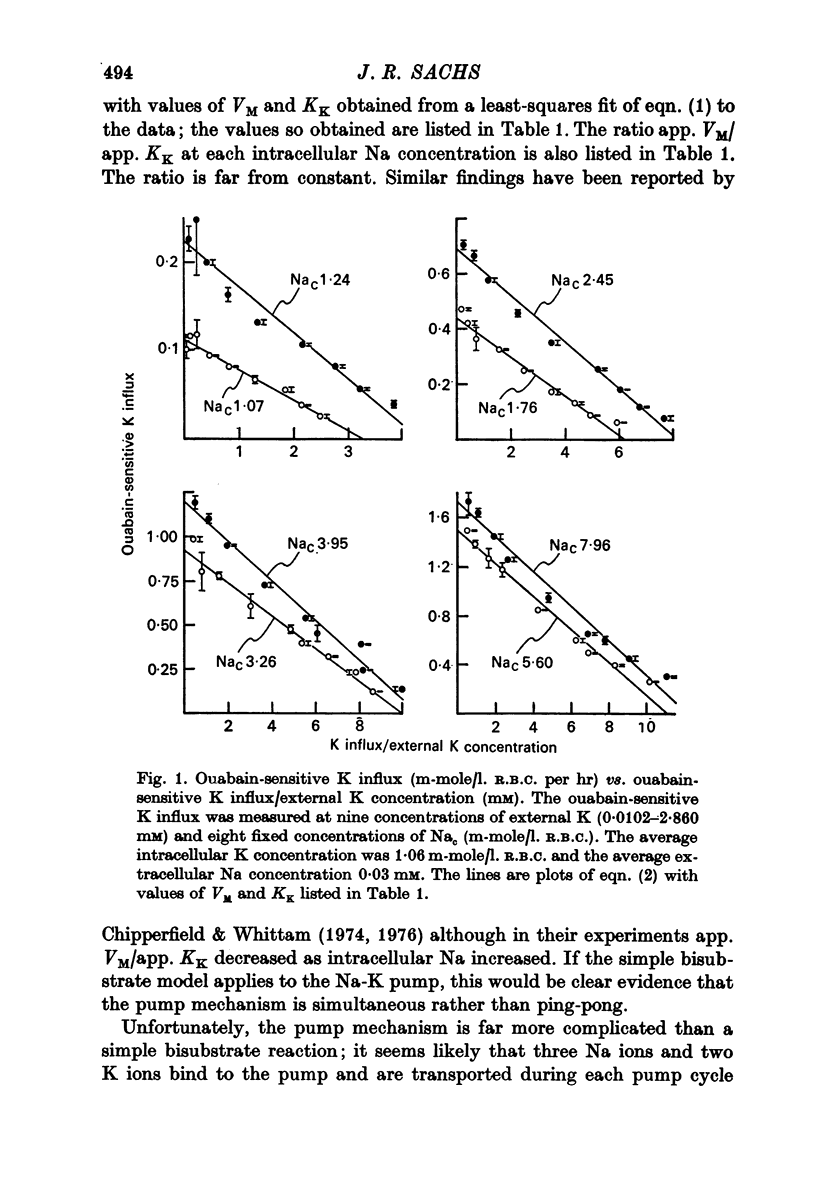
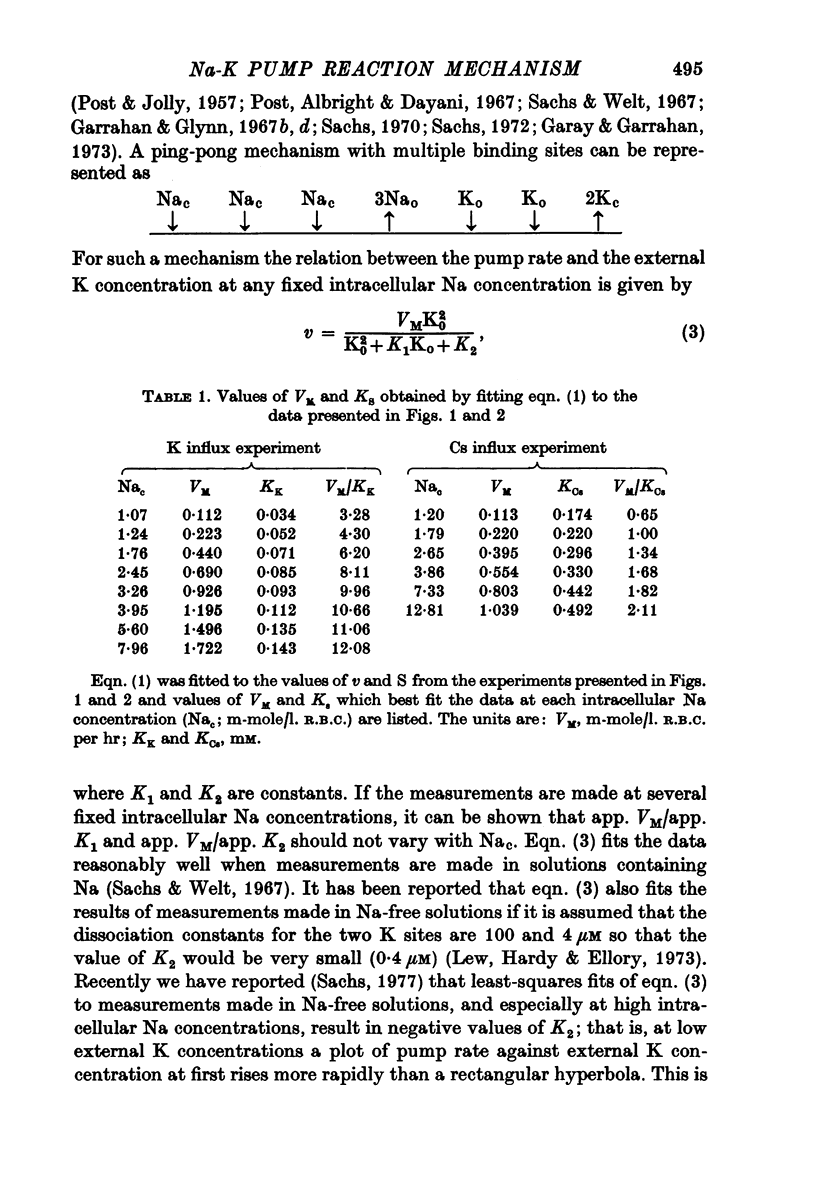
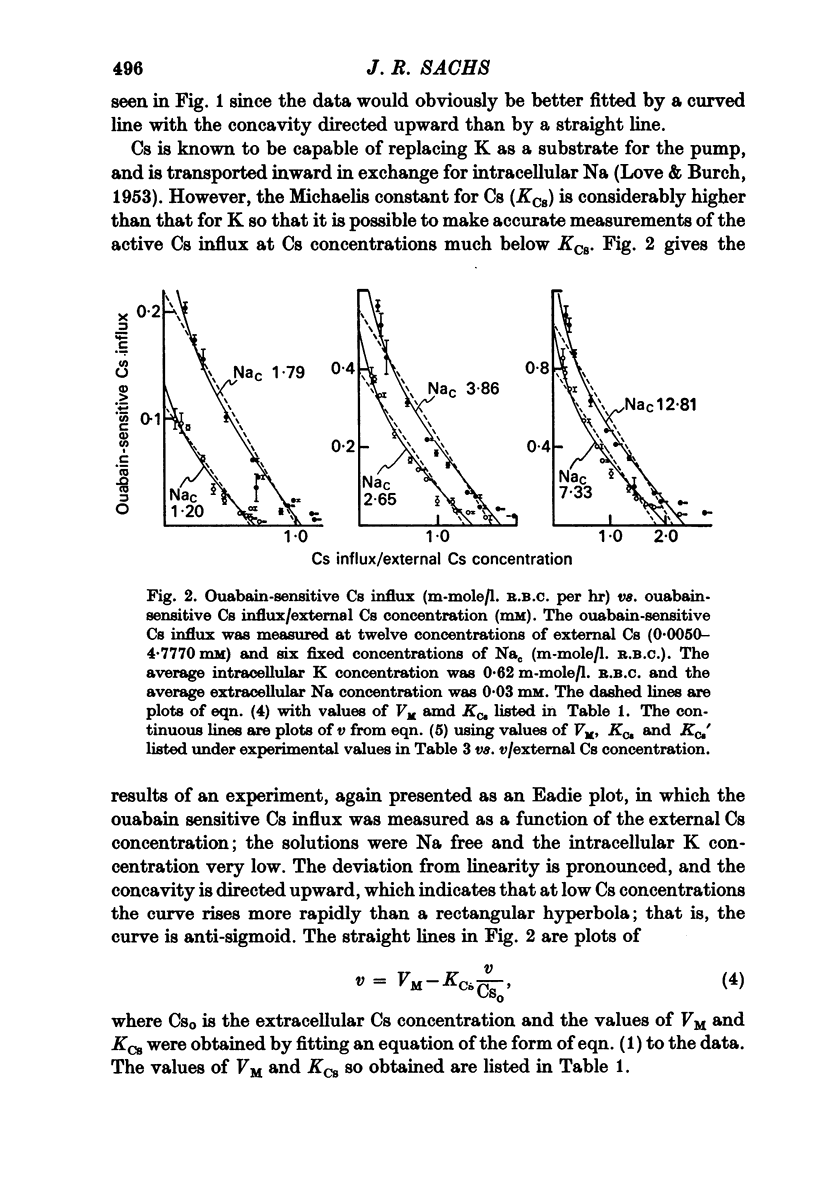
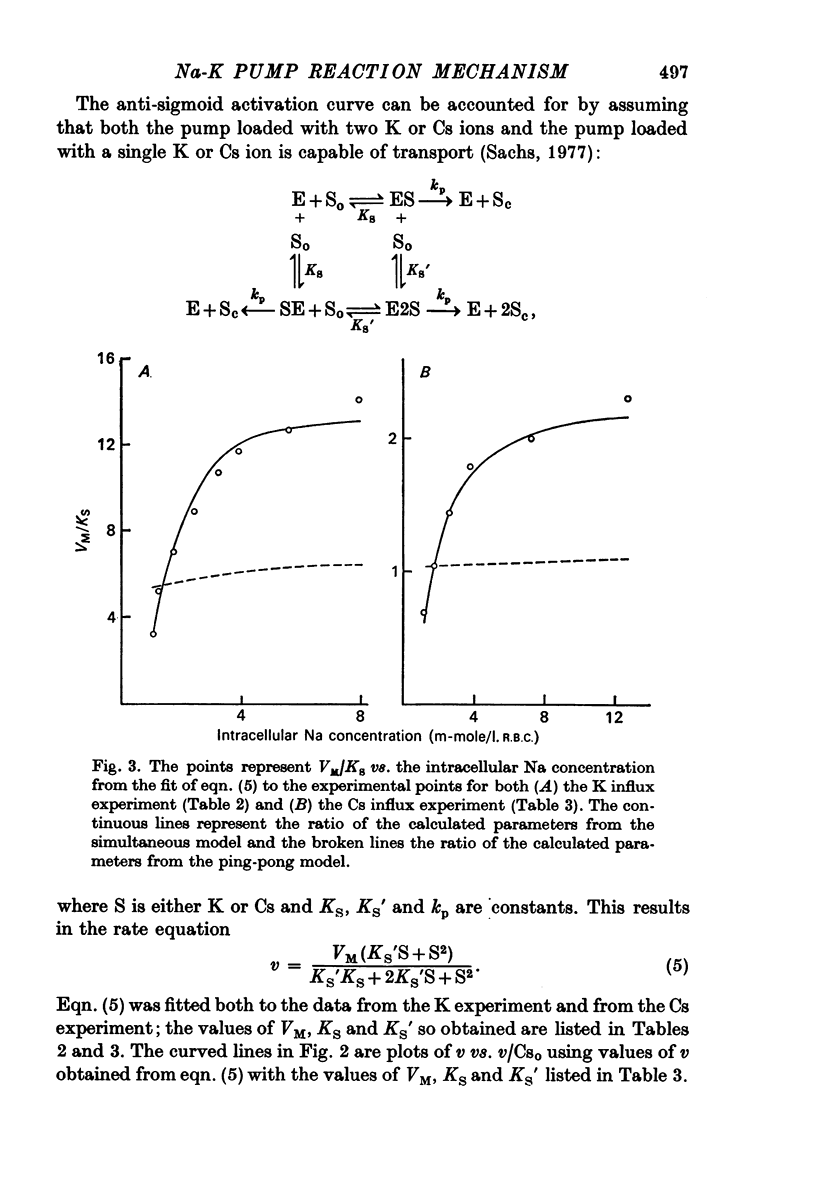
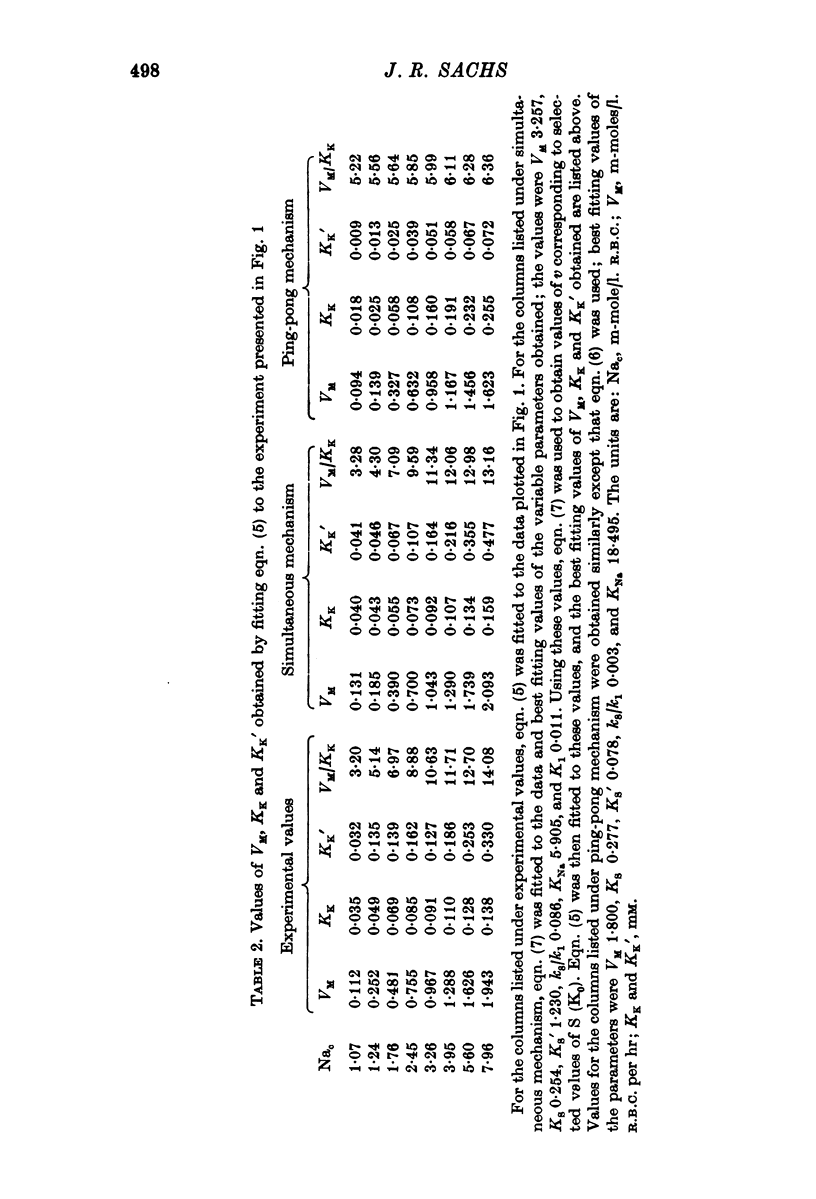
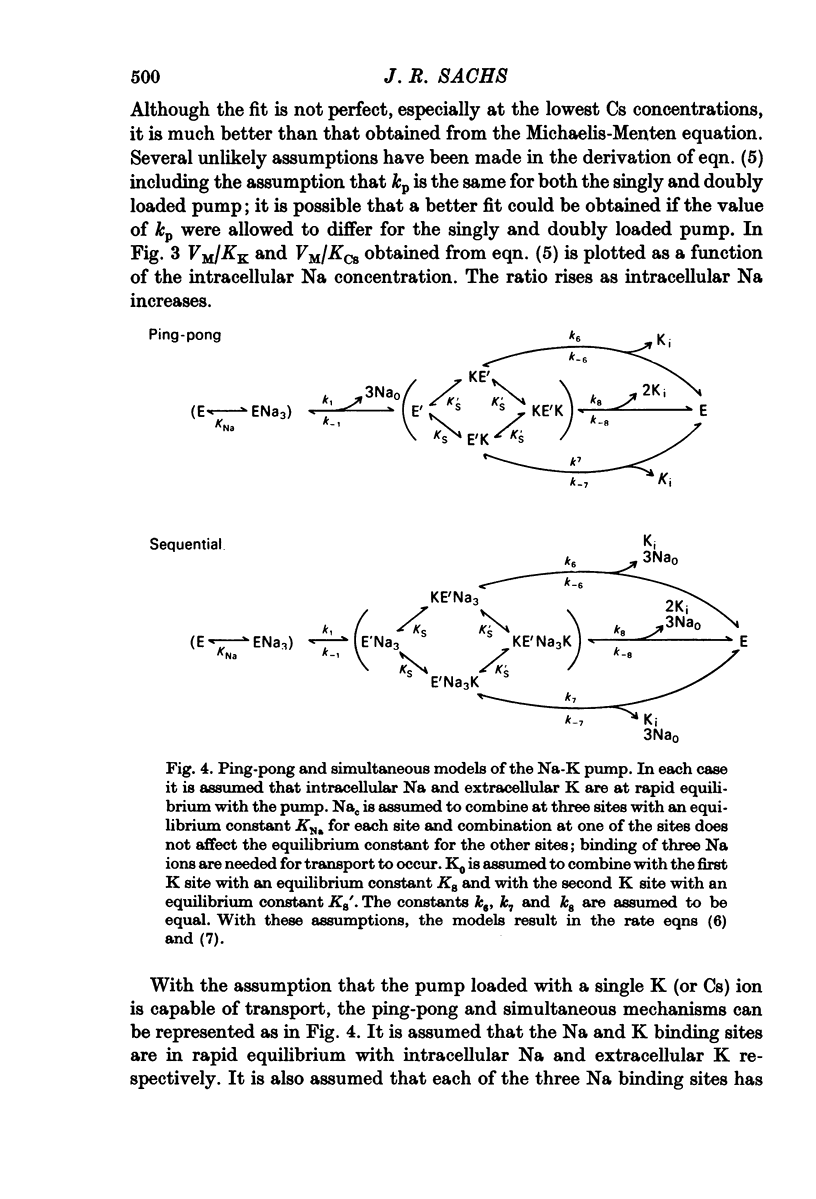
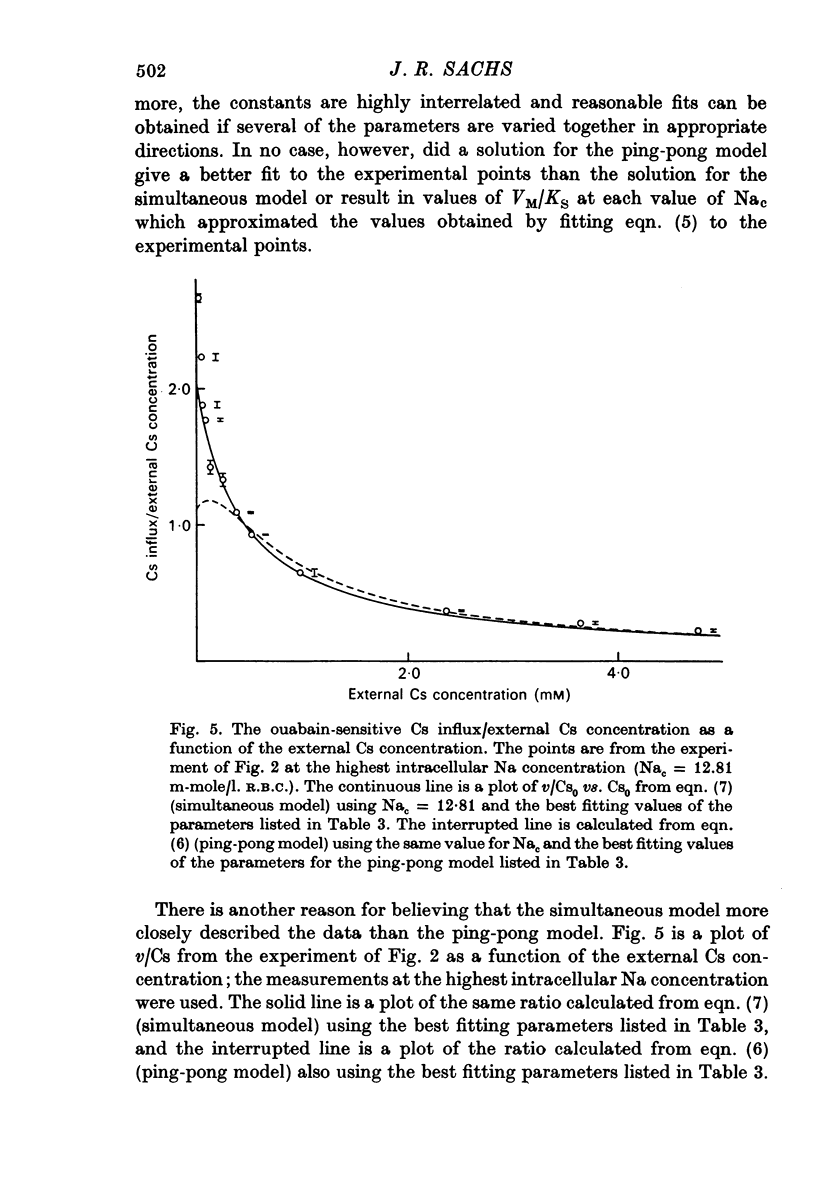
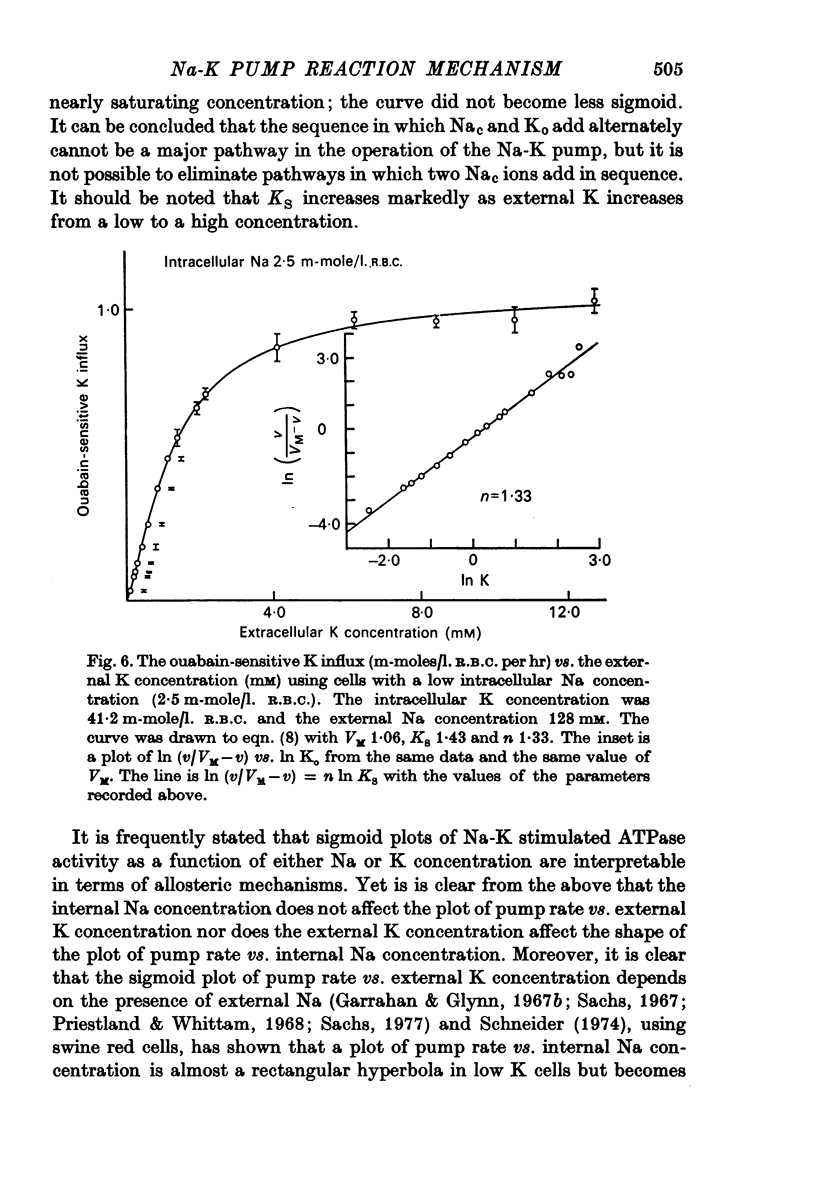
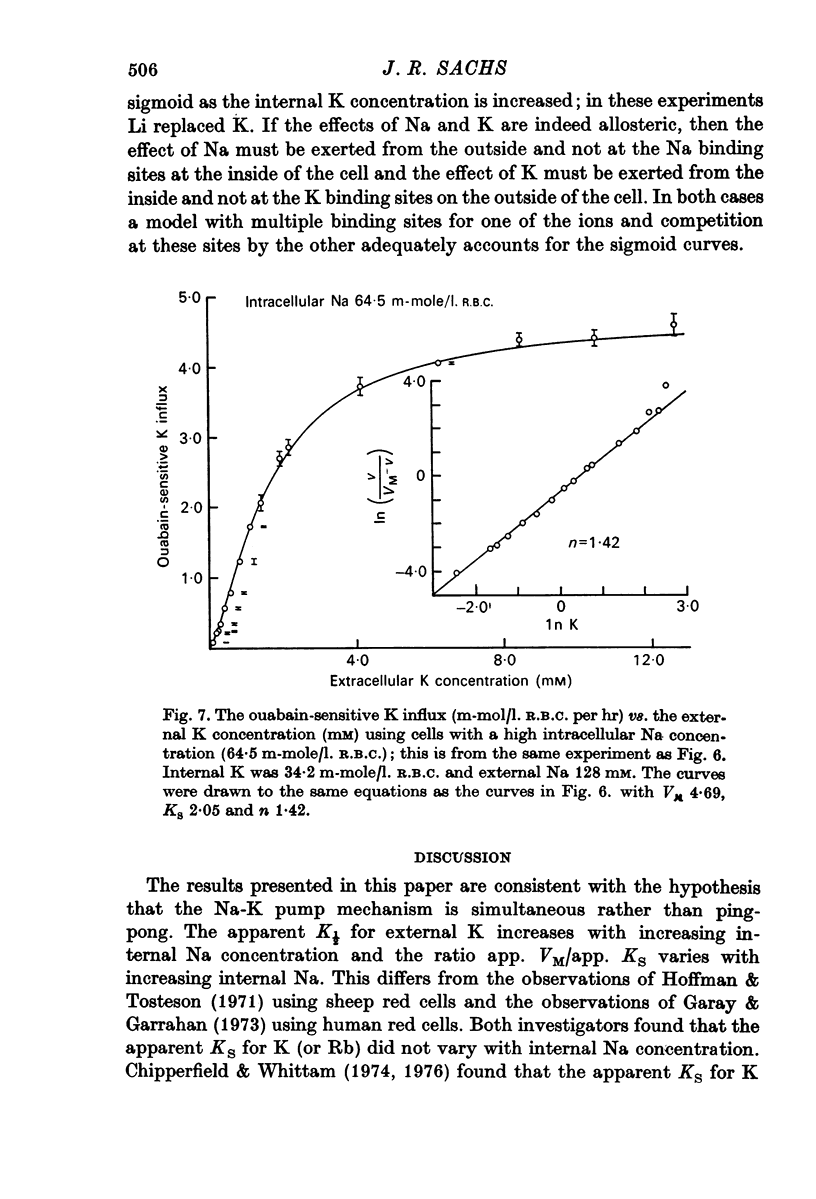
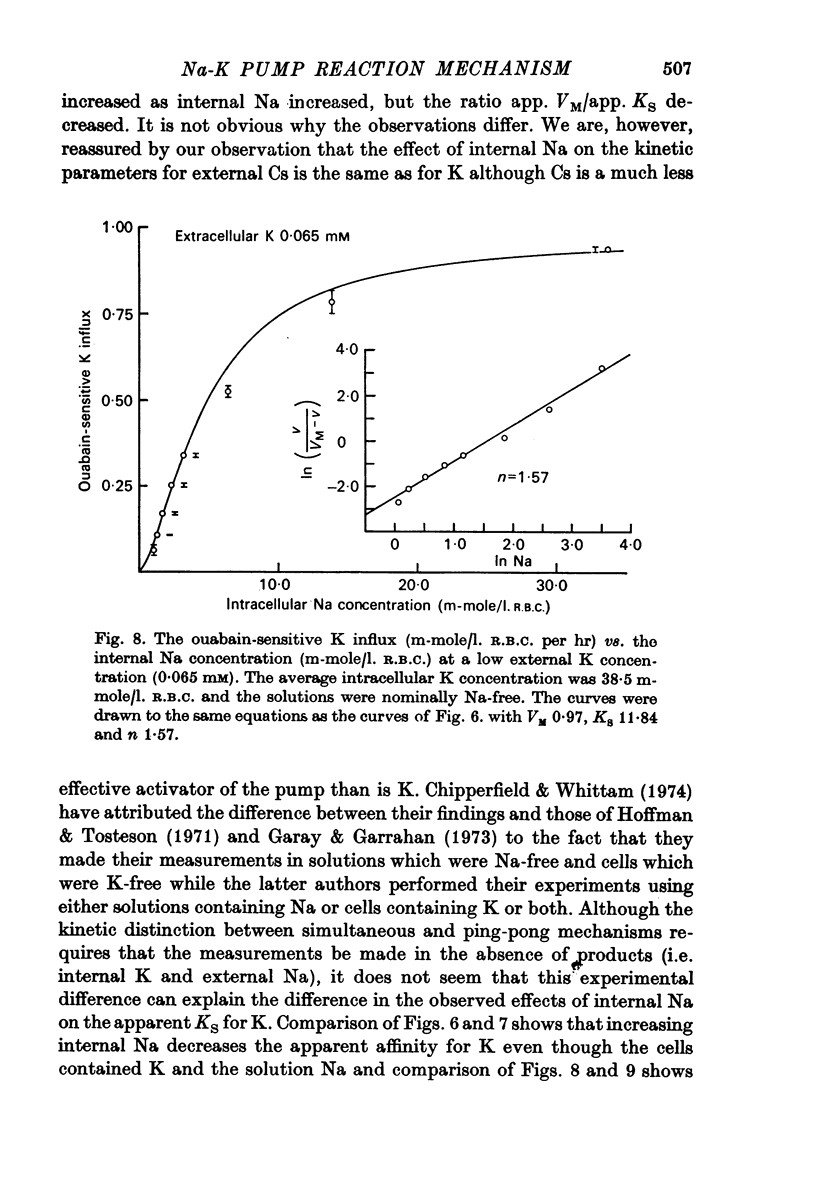
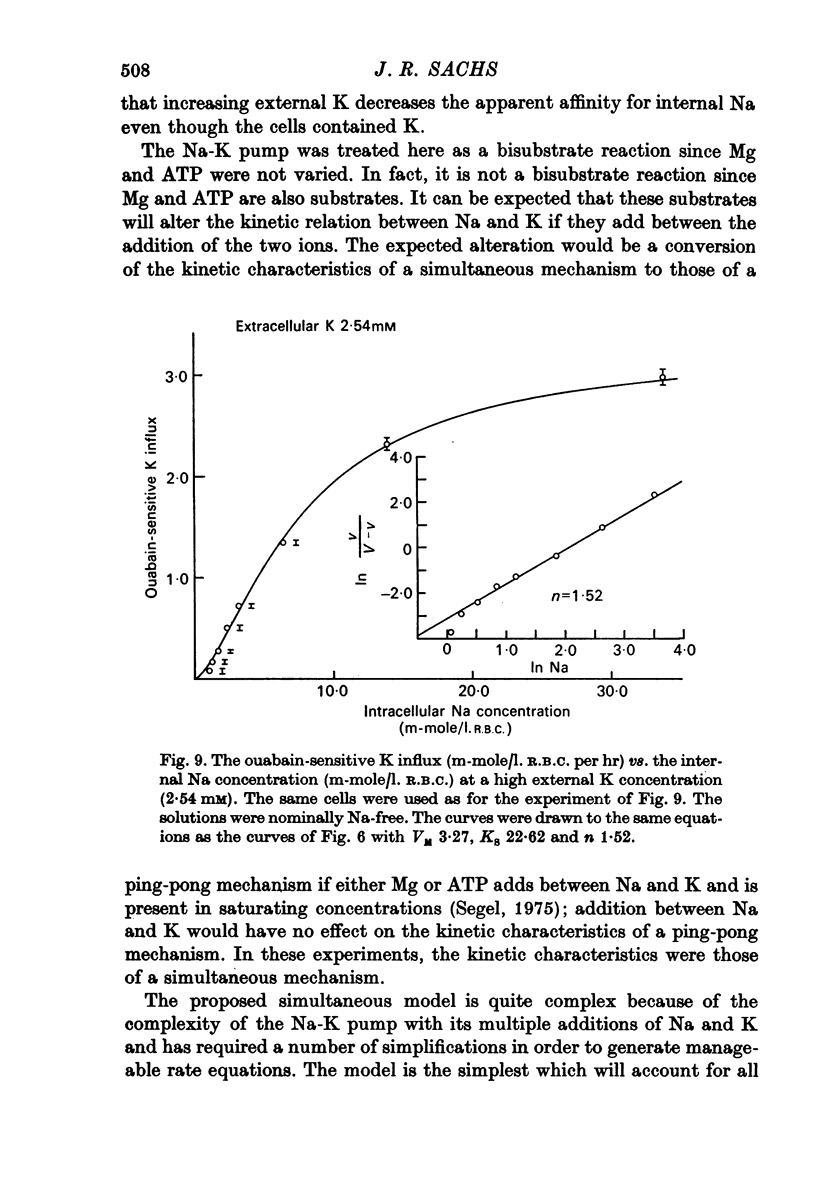
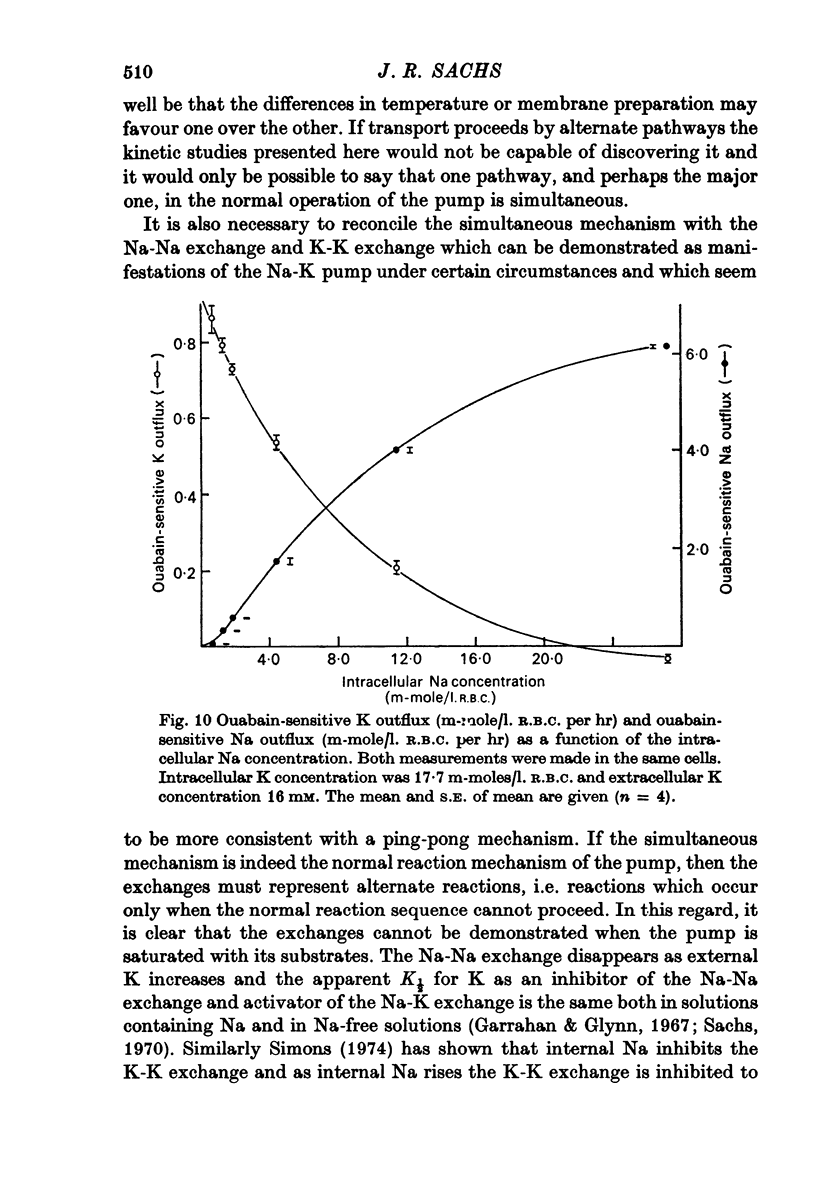
1. The ouabain-sensitive K influx was measured at varying external K concentrations ([K]o) and at several fixed internal Na concentrations ([Na]c). The cells were nominally K-free and the solutions Na-free. Both the apparent maximal velocity (VM) and the apparent Michaelis constant for K (KK) increased as Nac increased. The ratio app. VM/app. KK increased with increasing Nac. 2. The ouabain-sensitive Cs influx was measured at varying external Cs concentrations and at several fixed Nac in K-free cells and Na-free solutions. Both app. VM and app. Kcs increased as Nac increased and the ratio app. VM/app. Kcs increased with increasing Nac. 3. The data were evaluated in terms of ping-pong model and a simultaneous model for the pump reaction mechanism. The simultaneous model described the data adequately and the ping-pong models did not. 4. The K influx was measured at varying external K concentrations in solutions containing Na and at a low and high Nac; the cells contained K. The relation between the pump rate and the external K concentration was sigmoid. A Hill equation was fitted to the data. KK was higher in the high Nac cells, but the Hill coefficient (n) was not altered as Nac increased. 5. The K influx was measured at varying internal Na concentrations and two fixed external K concentrations; the cells contained K. The relation between the pump rate and Nac was sigmoid. When a Hill equation was fitted to the data, it was found that KNac was higher at the high external K concentration, but n was the same at both K concentrations.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Stone A. J. A kinetic method for investigating hypothetical models of the sodium pump. Biochim Biophys Acta. 1966 Oct 10;126(2):321–329. doi: 10.1016/0926-6585(66)90069-0. [DOI] [PubMed] [Google Scholar]
- Banerjee S. P., Wong S. M., Khanna V. K., Sen A. K. Inhibition of sodium- and potassium-dependent adenosine triphosphatase by N-ethylmaleimide. I. Effects on sodium-sensitive phosphorylation and potassium-sensitive dephosphorylation. Mol Pharmacol. 1972 Jan;8(1):8–17. [PubMed] [Google Scholar]
- Banerjee S. P., Wong S. M., Sen A. K. Inhibition of sodium- and potassium-dependent adenosine triphosphatase by N-ethylmaleimide. II. Effects of sodium-activated transphosphorylation. Mol Pharmacol. 1972 Jan;8(1):18–29. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Chipperfield A. R., Whittam R. The connexion between the ion-binding sites of the sodium pump. J Physiol. 1976 Sep;260(2):371–385. doi: 10.1113/jphysiol.1976.sp011520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms A. S., Boyer P. D. Occurrence and characteristics of 18 O exchange reactions catalyzed by sodium- and potassium-dependent adenosine triphosphatases. J Biol Chem. 1973 May 10;248(9):3155–3162. doi: 10.2172/4485348. [DOI] [PubMed] [Google Scholar]
- Fahn S., Koval G. J., Albers R. W. Sodium-potassium-activated adenosine triphosphatase of Electrophorus electric organ. I. An associated sodium-activated transphosphorylation. J Biol Chem. 1966 Apr 25;241(8):1882–1889. [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Facftors affecting the relative magnitudes of the sodium:potassium and sodium:sodium exchanges catalysed by the sodium pump. J Physiol. 1967 Sep;192(1):189–216. doi: 10.1113/jphysiol.1967.sp008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The behaviour of the sodium pump in red cells in the absence of external potassium. J Physiol. 1967 Sep;192(1):159–174. doi: 10.1113/jphysiol.1967.sp008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The sensitivity of the sodium pump to external sodium. J Physiol. 1967 Sep;192(1):175–188. doi: 10.1113/jphysiol.1967.sp008295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The stoicheiometry of the sodium pump. J Physiol. 1967 Sep;192(1):217–235. doi: 10.1113/jphysiol.1967.sp008297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Rega A. F. Cation loading of red blood cells. J Physiol. 1967 Nov;193(2):459–466. doi: 10.1113/jphysiol.1967.sp008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giotta G. J. Quaternary structure of (Na+ + K+)-dependent adenosine triphosphatase. J Biol Chem. 1976 Mar 10;251(5):1247–1252. [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L., Lüthi U. Reversal of the potassium entry mechanism in red cells, with and without reversal of the entire pump cycle. J Physiol. 1970 Apr;207(2):371–391. doi: 10.1113/jphysiol.1970.sp009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. G., Tosteson D. C. Active sodium and potassium transport in high potassium and low potassium sheep red cells. J Gen Physiol. 1971 Oct;58(4):438–466. doi: 10.1085/jgp.58.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins B. E., Wagner H., Jr, smith T. W. Sodium- and potassium-activated adenosine triphosphatase of the nasal salt gland of the duck (Anas platyrhynchos). Purification, characterization, and NH2-terminal amino acid sequence of the phosphorylating polypeptide. J Biol Chem. 1976 Jul 25;251(14):4365–4371. [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+, K+)-ATPase. V. Conformational changes in the enzyme Transitions between the Na-form and the K-form studied with tryptic digestion as a tool. Biochim Biophys Acta. 1975 Sep 2;401(3):399–415. doi: 10.1016/0005-2736(75)90239-4. [DOI] [PubMed] [Google Scholar]
- Kepner G. R., Macey R. I. Membrane enzyme systems. Molecular size determinations by radiation inactivation. Biochim Biophys Acta. 1968 Sep 17;163(2):188–203. doi: 10.1016/0005-2736(68)90097-7. [DOI] [PubMed] [Google Scholar]
- Kropp D. L., Sachs J. R. Kinetics of the inhibition of the Na-K pump by tetrapropylammonium chloride. J Physiol. 1977 Jan;264(2):471–487. doi: 10.1113/jphysiol.1977.sp011678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J. Properties of the two polypeptides of sodium- and potassium-dependent adenosine triphosphatase. J Biol Chem. 1972 Dec 10;247(23):7642–7649. [PubMed] [Google Scholar]
- LOVE W. D., BURCH G. E. A comparison of potassium 42, rubidium 86, and cesium 134 as tracers of potassium in the study of cation metabolism of human erythrocytes in vitro. J Lab Clin Med. 1953 Mar;41(3):351–362. [PubMed] [Google Scholar]
- Lew V. L., Hardy M. A., Jr, Ellory J. C. The uncoupled extrusion of Na+ through the Na+ pump. Biochim Biophys Acta. 1973 Oct 11;323(2):251–266. doi: 10.1016/0005-2736(73)90149-1. [DOI] [PubMed] [Google Scholar]
- McConaghey P. D., Maizels M. Cation exchanges of lactose-treated human red cells. J Physiol. 1962 Aug;162(3):485–509. doi: 10.1113/jphysiol.1962.sp006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Izumi F., Yoshida H. Studies on potassium dependent phosphatase; its distribution and properties. J Biochem. 1966 Mar;59(3):295–303. [PubMed] [Google Scholar]
- Nakao M., Nagano K., Nakao T., Mizuno N., Tashima Y., Fujita M., Maeda H., Matsudaira H. Molecular weight of Na, K-ATPase approximated by the radiation inactivation method. Biochem Biophys Res Commun. 1967 Nov 30;29(4):588–592. doi: 10.1016/0006-291x(67)90526-8. [DOI] [PubMed] [Google Scholar]
- POST R. L., JOLLY P. C. The linkage of sodium, potassium, and ammonium active transport across the human erythrocyte membrane. Biochim Biophys Acta. 1957 Jul;25(1):118–128. doi: 10.1016/0006-3002(57)90426-2. [DOI] [PubMed] [Google Scholar]
- POST R. L., SEN A. K., ROSENTHAL A. S. A PHOSPHORYLATED INTERMEDIATE IN ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT ACROSS KIDNEY MEMBRANES. J Biol Chem. 1965 Mar;240:1437–1445. [PubMed] [Google Scholar]
- Post R. L., Albright C. D., Dayani K. Resolution of pump and leak components of sodium and potassium ion transport in human erythrocytes. J Gen Physiol. 1967 May;50(5):1201–1220. doi: 10.1085/jgp.50.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repke K. R., Schön R., Henke W., Schönfeld W., Streckenbach B., Dittrick F. Experimental and theoretical examination of the flip-flop model of (Na, K)-ATPase function. Ann N Y Acad Sci. 1974;242(0):203–219. doi: 10.1111/j.1749-6632.1974.tb19091.x. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Affinity of the (Na+ plus K+)-dependent ATPase for Na+ measured by Na+-modified enzyme inactivation. FEBS Lett. 1974 Jan 15;38(3):325–328. doi: 10.1016/0014-5793(74)80083-9. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Variable affinity of the (Na+ + K+)-dependent adenosine triphosphatase for potassium. Studies using beryllium inactivation. Arch Biochem Biophys. 1973 May;156(1):232–243. doi: 10.1016/0003-9861(73)90361-5. [DOI] [PubMed] [Google Scholar]
- SHAW T. I. Potassium movements in washed erythrocytes. J Physiol. 1955 Sep 28;129(3):464–475. doi: 10.1113/jphysiol.1955.sp005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Competitive effects of some cations on active potassium transport in the human red blood cell. J Clin Invest. 1967 Sep;46(9):1433–1441. doi: 10.1172/JCI105635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Kinetics of the inhibition of the Na-K pump by external sodium. J Physiol. 1977 Jan;264(2):449–470. doi: 10.1113/jphysiol.1977.sp011677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Recoupling the Na-K pump. J Clin Invest. 1972 Dec;51(12):3244–3247. doi: 10.1172/JCI107151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Sodium movements in the human red blood cell. J Gen Physiol. 1970 Sep;56(3):322–341. doi: 10.1085/jgp.56.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R., Welt L. G. The concentration dependence of active potassium transport in the human red blood cell. J Clin Invest. 1967 Jan;46(1):65–76. doi: 10.1172/JCI105512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. P. Regulation of sodium transport in erythrocytes. Arch Biochem Biophys. 1974 Feb;160(2):552–560. doi: 10.1016/0003-9861(74)90431-7. [DOI] [PubMed] [Google Scholar]
- Stein W. D., Lieb W. R., Karlish S. J., Eilam Y. A modle for active transport of sodium and potassium ions as mediated by a tetrameric enzyme. Proc Natl Acad Sci U S A. 1973 Jan;70(1):275–278. doi: 10.1073/pnas.70.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Chipperfield A. R. The reaction mechanism of the sodium pump. Biochim Biophys Acta. 1975 Jun 30;415(2):149–171. doi: 10.1016/0304-4157(75)90001-5. [DOI] [PubMed] [Google Scholar]


