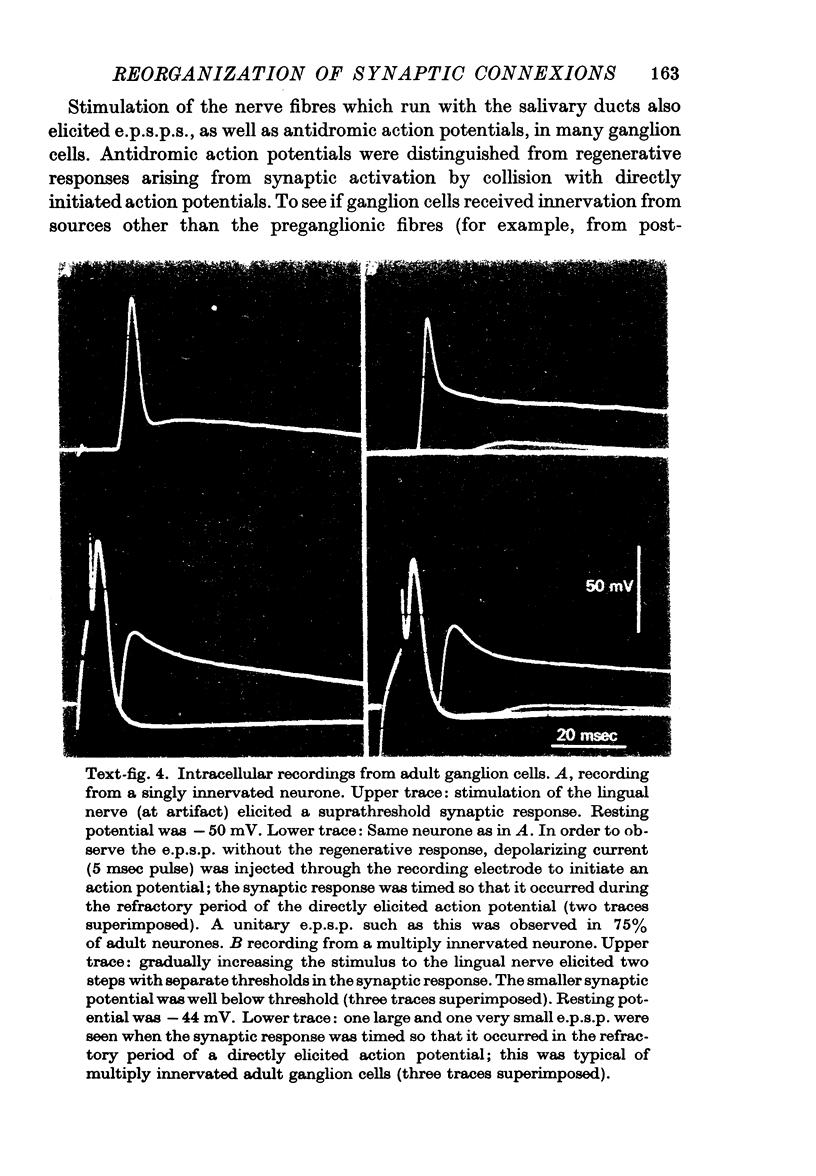
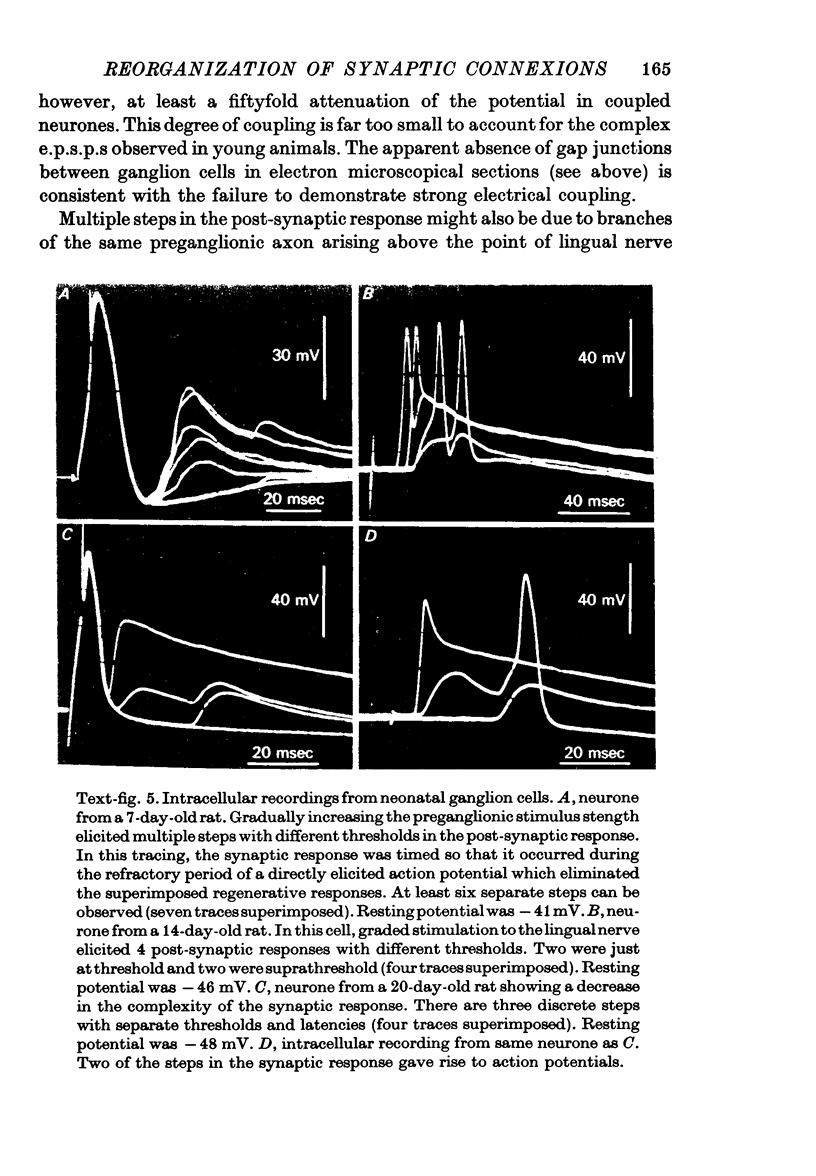
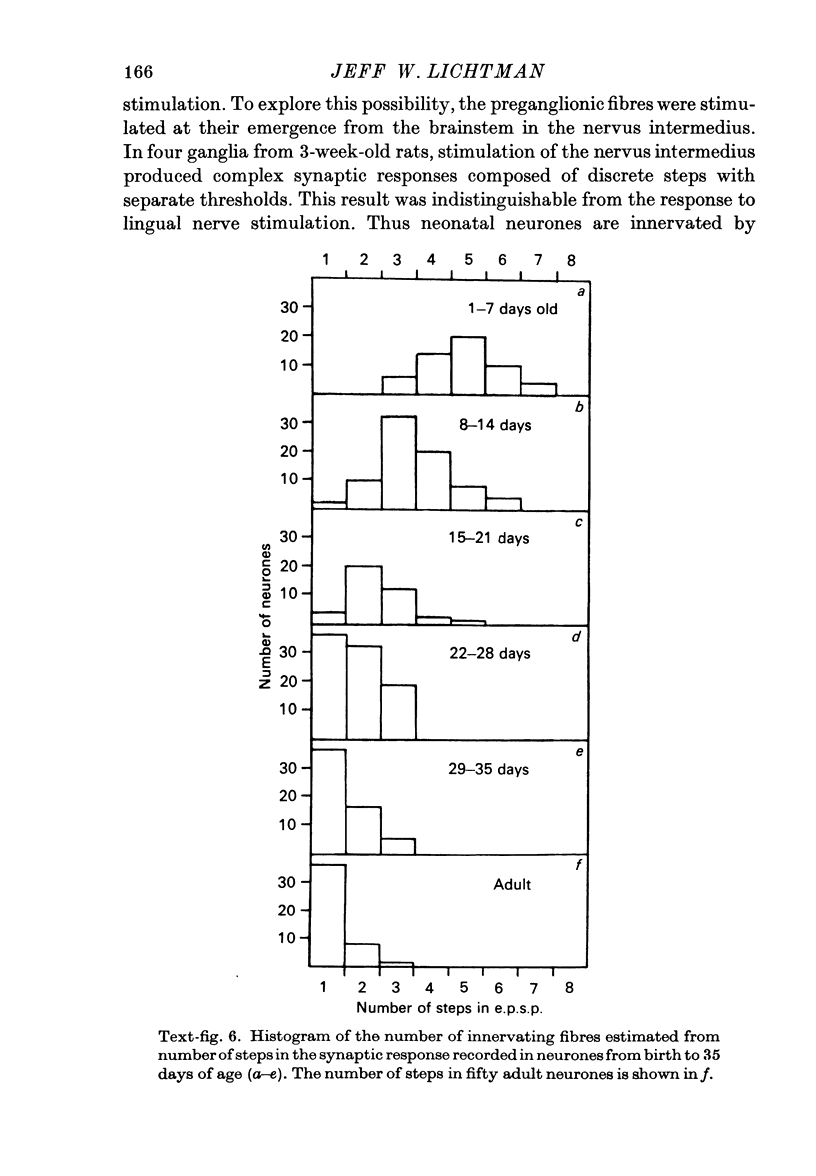
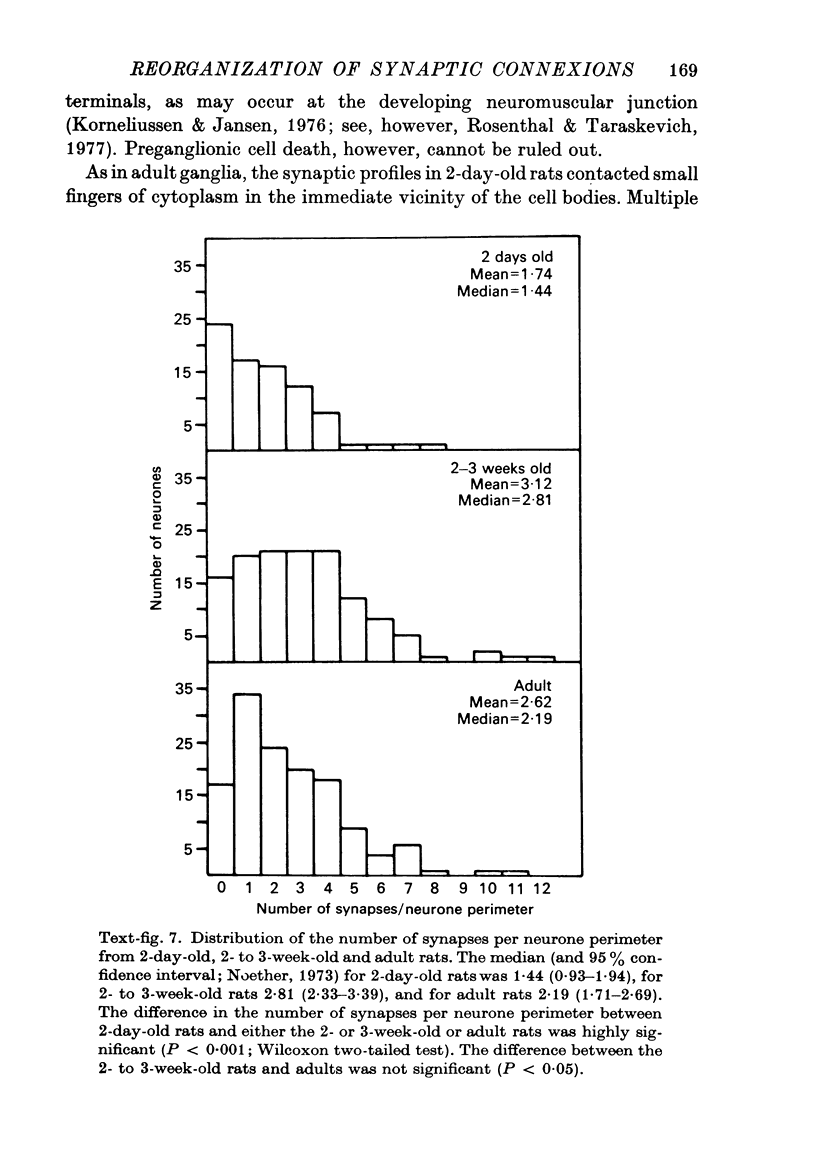
Abstract
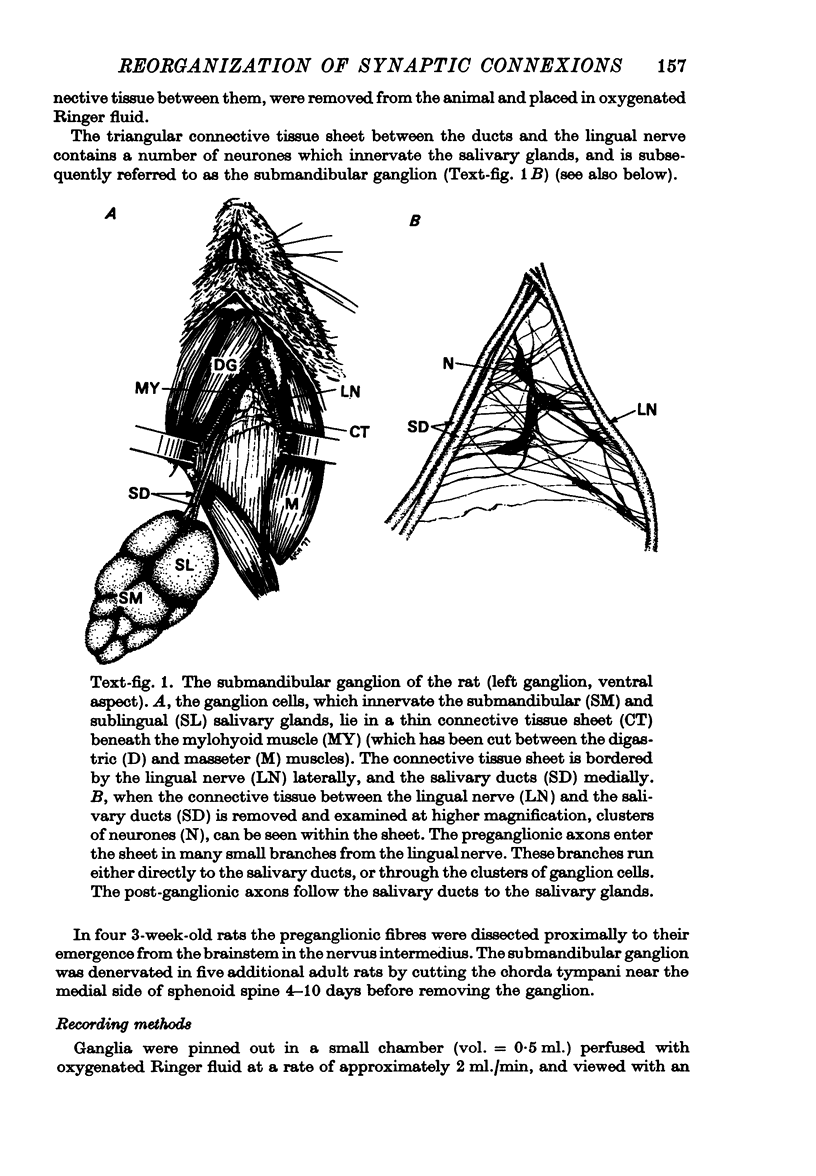
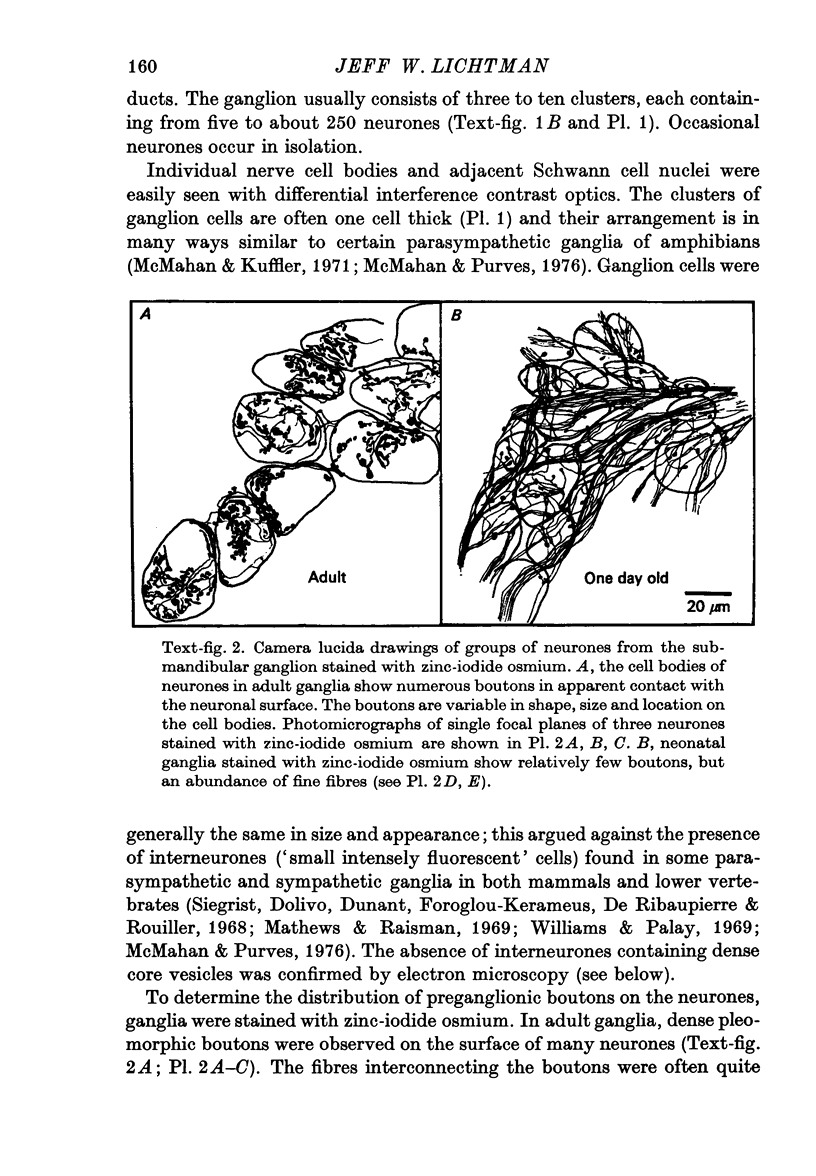
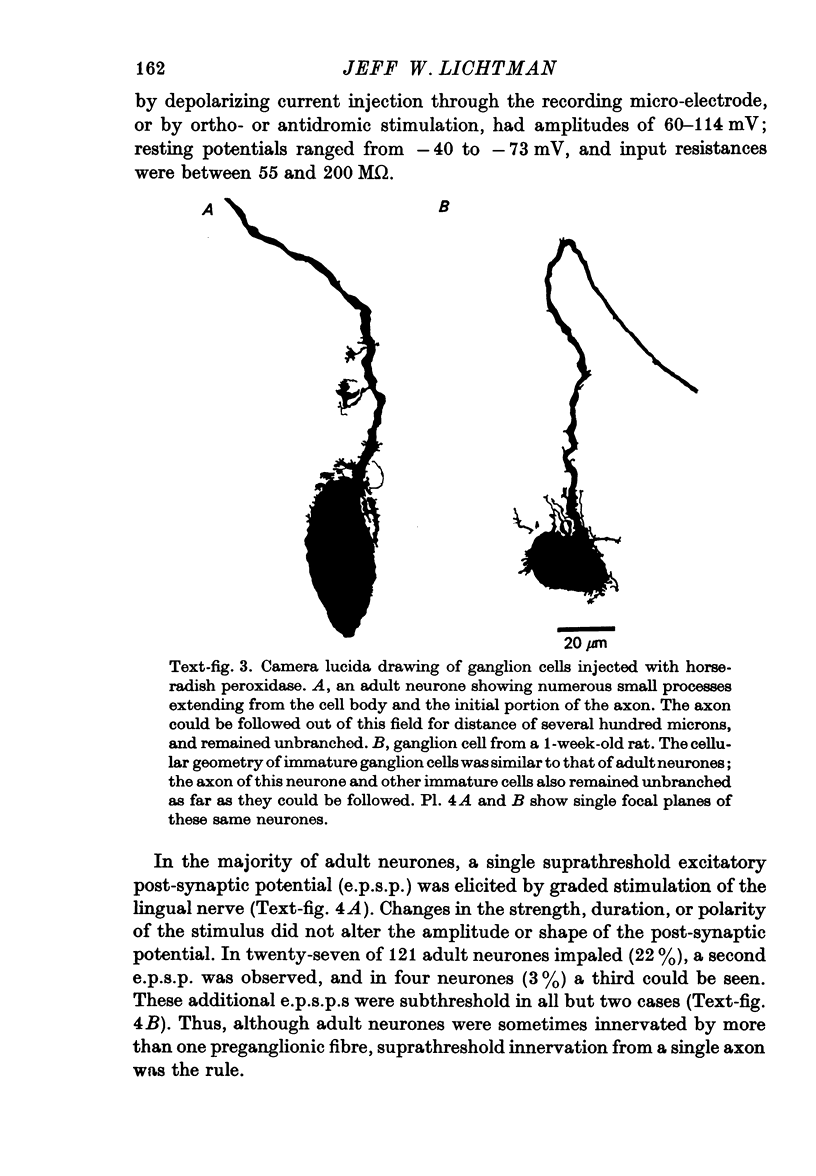
1. The innervation of neurones in the submandibular ganglion of neonatal and adult rats has been studied with intracellular recording, and light and electron microscopy. 2. Intracellular recordings from neurones in isolated ganglia from adult animals showed that about 75% of the ganglion cells are innervated by a single preganglionic fibre. 3. However multiple steps in the post-synaptic potential (about five on average) were elicited in ganglion cells from neonatal animals by graded stimulation of the preganglionic nerve. The same result was obtained when the preganglionic fibres were stimulated at their emergence from the brainstem, indicating that neonatal neurones are innervated by several different preganglionic nerve cells. 4. The number of preganglionic fibres innervating individual ganglion cells gradually decreased during the first few weeks of life, and by about 5 weeks each ganglion cell was generally contacted by a single preganglionic axon. 5. Synapses were made on short protuberances in the immediate vicinity of the neuronal cell bodies in both neonatal and adult ganglia as shown by staining presynaptic boutons with the zinc-iodide osmium method, injection of horseradish peroxidase into ganglion cells, and electron microscopical examination. 6. Electron microscopical counts of synaptic profiles per ganglion cell perimeter showed that the number of synaptic contacts made on ganglion cells actually increased during the first few post-natal weeks, when the number of axons innervating each neurone was decreasing. 7. These results show that in the rat submandibular ganglion there is a reorganization of neuronal connexions during the first few weeks of life which results in a transition from multiple to generally single innervation of ganglion cells.
Full text
PDF







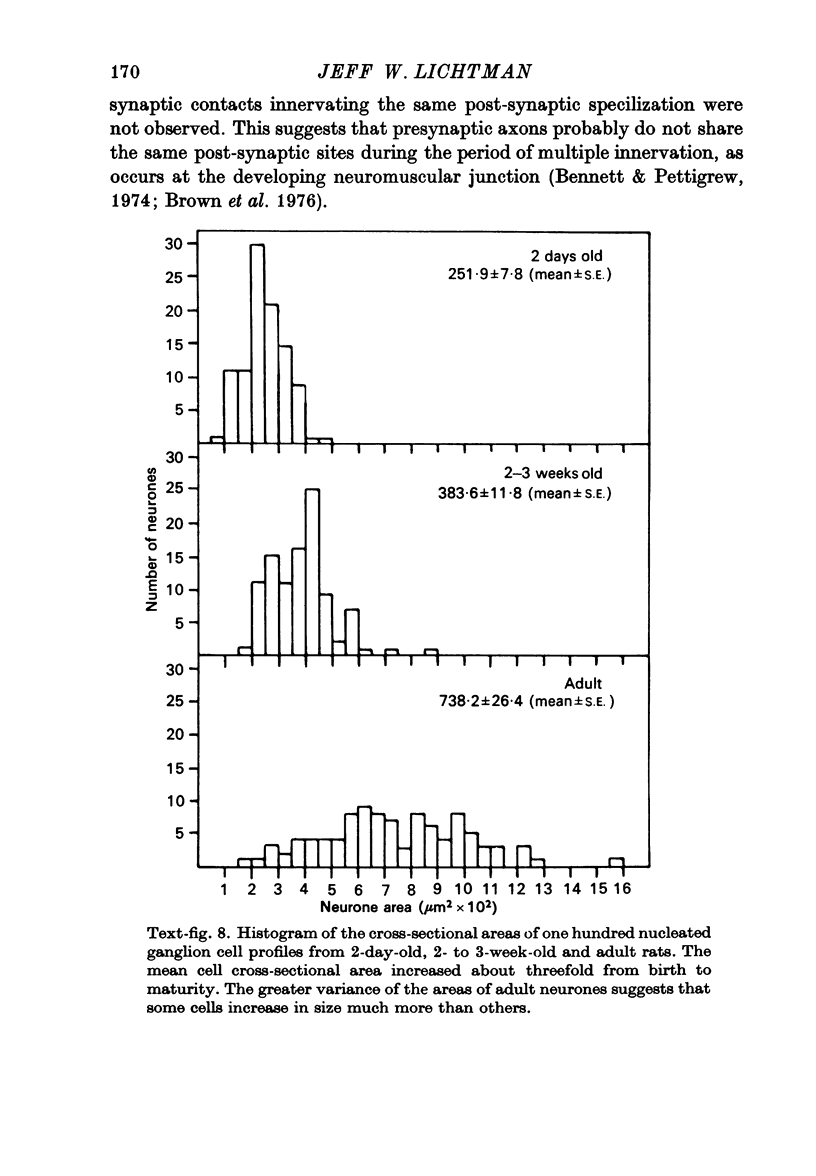
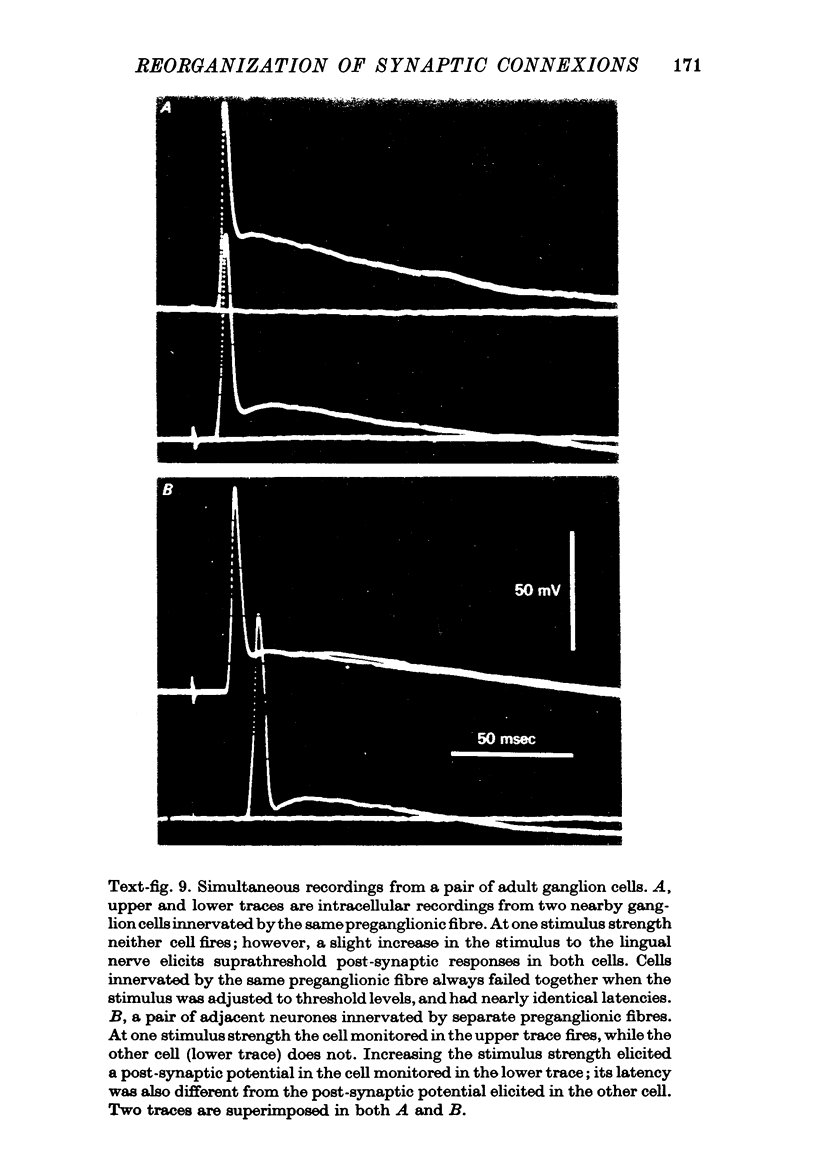









Images in this article
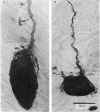
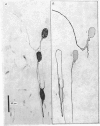
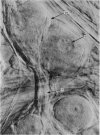
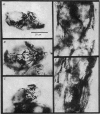
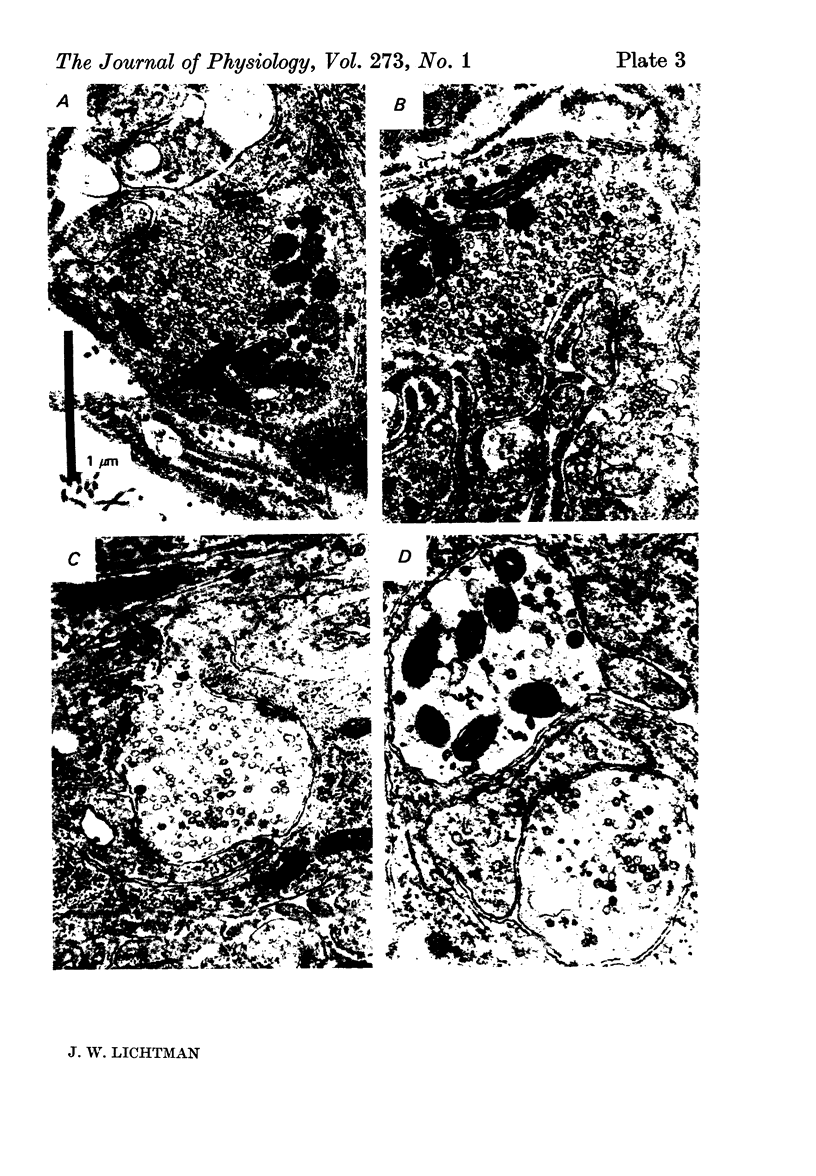
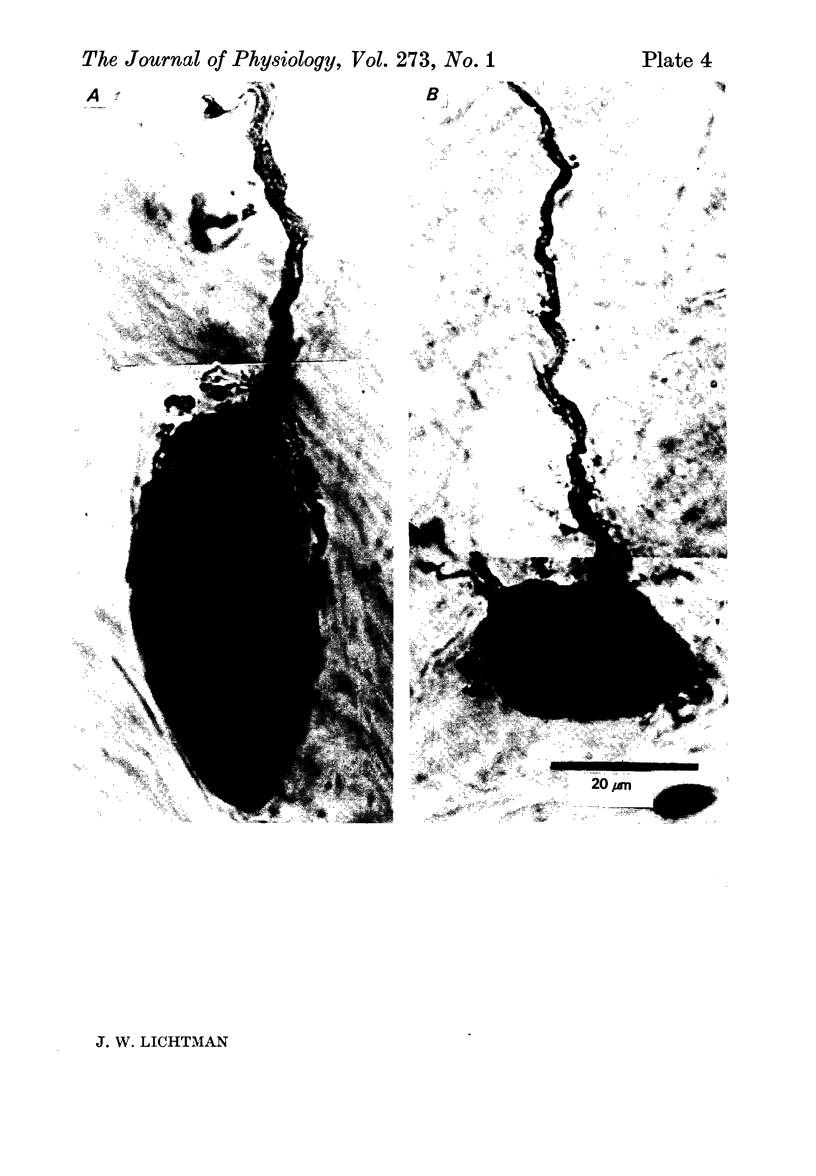
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagust J., Lewis D. M., Westerman R. A. Polyneuronal innervation of kitten skeletal muscle. J Physiol. 1973 Feb;229(1):241–255. doi: 10.1113/jphysiol.1973.sp010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of neuromuscular synapses. Cold Spring Harb Symp Quant Biol. 1976;40:409–424. doi: 10.1101/sqb.1976.040.01.039. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in striated muscle during development. J Physiol. 1974 Sep;241(2):515–545. doi: 10.1113/jphysiol.1974.sp010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Crowcroft P. J., Devine C. E., Holman M. E., Yonemura K. Transmission from pregnanglionic fibres in the hypogastric nerve to peripheral ganglia of male guinea-pigs. J Physiol. 1969 May;201(3):723–743. doi: 10.1113/jphysiol.1969.sp008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S. E., Warner A. E. Low resistance junctions between mesoderm cells during development of trunk muscles. J Physiol. 1976 Feb;255(1):209–230. doi: 10.1113/jphysiol.1976.sp011276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H. R., Johnson E. W. Physiological and morphological effects of post-ganglionic axotomy on presynaptic nerve terminals. J Physiol. 1976 Aug;260(1):143–158. doi: 10.1113/jphysiol.1976.sp011508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradi S., Skoglund S. Observations on the ultrastructure of the initial motor axon segment and dorsal root boutons on the motoneurons in the lumbosacral spinal cord of the cat during postnatal development. Acta Physiol Scand Suppl. 1969;333:53–76. [PubMed] [Google Scholar]
- Cowan W. M., Wann D. F. A computer system for the measurement of cell and nuclear sizes. J Microsc. 1973 Dec;99(3):331–348. doi: 10.1111/j.1365-2818.1973.tb04630.x. [DOI] [PubMed] [Google Scholar]
- Crepel F., Mariani J., Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976 Nov;7(6):567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Delhaye-Bouchaud N., Crepel F., Mariani J. Mise en évidence d'une multi-innervation temporaire des cellules de Purkinje du cervelet par les fibres grimpantes au cours du développement chez le rat. C R Acad Sci Hebd Seances Acad Sci D. 1975 Sep 29;281(13):909–912. [PubMed] [Google Scholar]
- Dennis M. J., Ort C. A. Physiological properties of nerve-muscle junctions developing in vivo. Cold Spring Harb Symp Quant Biol. 1976;40:435–442. doi: 10.1101/sqb.1976.040.01.041. [DOI] [PubMed] [Google Scholar]
- Hámori J., Láng E., Simon L. Experimental degeneration of the preganglionic fibers in the superior cervical ganglion of the cat. An electron microscope study. Z Zellforsch Mikrosk Anat. 1968;90(1):37–52. doi: 10.1007/BF00496701. [DOI] [PubMed] [Google Scholar]
- Kelly A. M., Zacks S. I. The histogenesis of rat intercostal muscle. J Cell Biol. 1969 Jul;42(1):135–153. doi: 10.1083/jcb.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneliussen H., Jansen J. K. Morphological aspects of the elimination of polyneuronal innervation of skeletal muscle fibres in newborn rats. J Neurocytol. 1976 Oct;5(8):591–604. doi: 10.1007/BF01175572. [DOI] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Fate of ganglionic synapses and ganglion cell axons during normal and induced cell death. J Cell Biol. 1976 Feb;68(2):357–374. doi: 10.1083/jcb.68.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Synaptic transmission and cell death during normal ganglionic development. J Physiol. 1974 Sep;241(3):737–749. doi: 10.1113/jphysiol.1974.sp010681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. The onset and development of transmission in the chick ciliary ganglion. J Physiol. 1972 May;222(3):691–713. doi: 10.1113/jphysiol.1972.sp009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinsky M. S. The development of nerve-muscle junctions in Rana catesbeiana tadpoles. Dev Biol. 1974 Sep;40(1):129–153. doi: 10.1016/0012-1606(74)90114-6. [DOI] [PubMed] [Google Scholar]
- MURRAY J. G., THOMPSON J. W. The occurrence and function of collateral sprouting in the sympathetic nervous system of the cat. J Physiol. 1957 Jan 23;135(1):133–162. doi: 10.1113/jphysiol.1957.sp005700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M. R., Nelson V. H. Detachment of structurally intact nerve endings from chromatolytic neurones of rat superior cervical ganglion during the depression of synaptic transmission induced by post-ganglionic axotomy. J Physiol. 1975 Feb;245(1):91–135. doi: 10.1113/jphysiol.1975.sp010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M. R., Raisman G. The ultrastructure and somatic efferent synapses of small granule-containing cells in the superior cervical ganglion. J Anat. 1969 Sep;105(Pt 2):255–282. [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Kuffler S. W. Visual identification of synaptic boutons on living ganglion cells and of varicosities in postganglionic axons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):485–508. doi: 10.1098/rspb.1971.0044. [DOI] [PubMed] [Google Scholar]
- McMahan U. J., Purves D. Visual identification of two kinds of nerve cells and their synaptic contacts in a living autonomic ganglion of the mudpuppy (Necturus maculosus). J Physiol. 1976 Jan;254(2):405–425. doi: 10.1113/jphysiol.1976.sp011238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K. J., McMahan U. J. The shapes of sensory and motor neurones and the distribution of their synapses in ganglia of the leech: a study using intracellular injection of horseradish peroxidase. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):481–499. doi: 10.1098/rspb.1976.0090. [DOI] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper S. An electrophysiological study of chemical and electrical synapses on neurones in the parasympathetic cardiac ganglion of the mudpuppy, Necturus maculosus: evidence for intrinsic ganglionic innervation. J Physiol. 1976 Jan;254(2):427–454. doi: 10.1113/jphysiol.1976.sp011239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J. L., Taraskevich P. S. Reduction of multiaxonal innervation at the neuromuscular junction of the rat during development. J Physiol. 1977 Sep;270(2):299–310. doi: 10.1113/jphysiol.1977.sp011953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S., McMahan U. J. Altered patterns of innervation in frog muscle after denervation. J Neurocytol. 1976 Dec;5(6):719–730. doi: 10.1007/BF01181583. [DOI] [PubMed] [Google Scholar]
- SNELL R. S. The histochemical appearances of cholinesterase in the parasympathetic nerves supplying the submandibular and sublingual salivary glands of the rat. J Anat. 1958 Oct;92(4):534–543. [PMC free article] [PubMed] [Google Scholar]
- STRAUS W. FACTORS AFFECTING THE CYTOCHEMICAL REACTION OF PEROXIDASE WITH BENZIDINE AND THE STABILITY OF THE BLUE REACTION PRODUCT. J Histochem Cytochem. 1964 Jun;12:462–469. doi: 10.1177/12.6.462. [DOI] [PubMed] [Google Scholar]
- SZENTAGOTHAI J. Zum elementaren Bau der interneuronalen Synapse. Acta Anat (Basel) 1957;30(1-4):827–841. [PubMed] [Google Scholar]
- Siegrist G., Dolivo M., Dunant Y., Foroglou-Kerameus C., De Ribaupierre F., Rouiller C. Ultrastructure and function of the chromaffin cells in the superior cervical ganglion of the rat. J Ultrastruct Res. 1968 Dec;25(5):381–407. doi: 10.1016/s0022-5320(68)80093-0. [DOI] [PubMed] [Google Scholar]
- Williams T. H., Palay S. L. Ultrastructure of the small neurons in the superior cervical ganglion. Brain Res. 1969 Sep;15(1):17–34. doi: 10.1016/0006-8993(69)90307-2. [DOI] [PubMed] [Google Scholar]