Abstract
1. Intracellular pH (pHi), Cl- and Na+ levels were recorded in snail neurones using ion-sensitive micro-electrodes, and the mechanism of the pHi recovery from internal acidification investigated.
2. Reducing the external HCO3- concentration greatly inhibited the rate of pHi recovery from HCl injection.
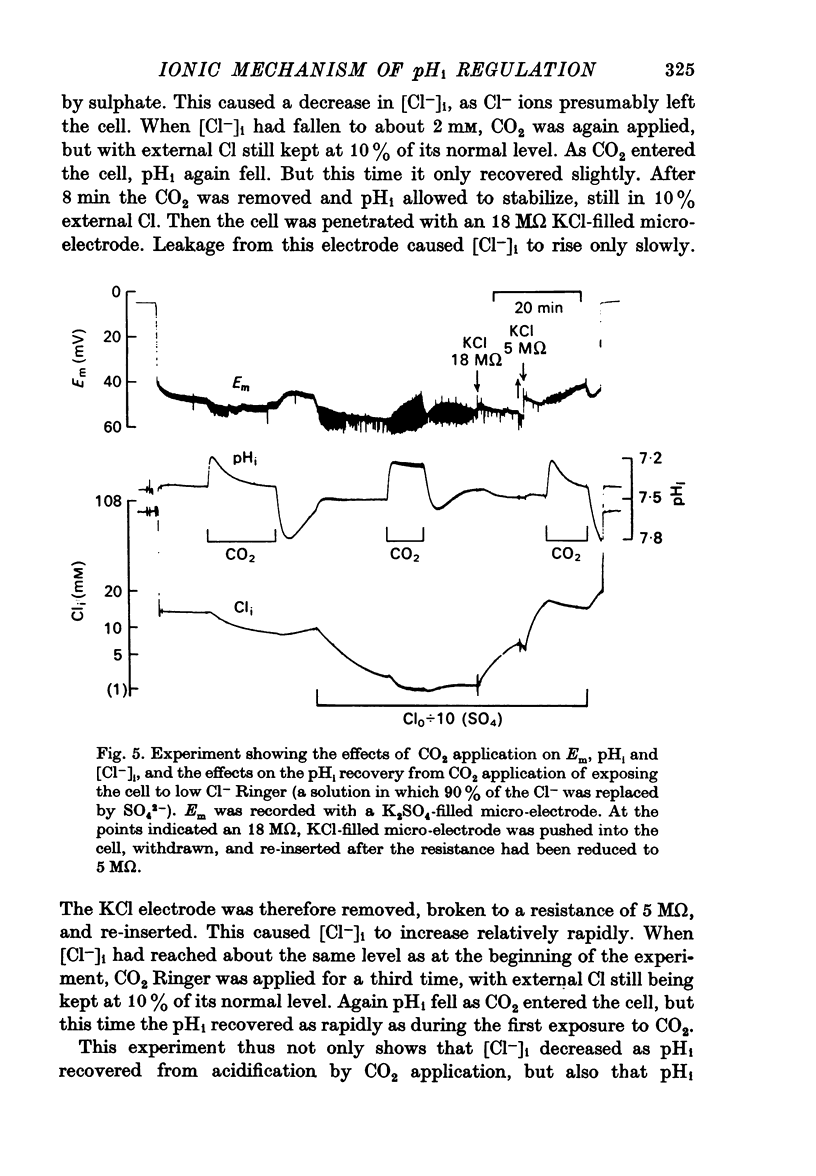
3. Reducing external Cl- did not inhibit pHi recovery, but reducing internal Cl-, by exposing the cell to sulphate Ringer, inhibited pHi recovery from CO2 application.
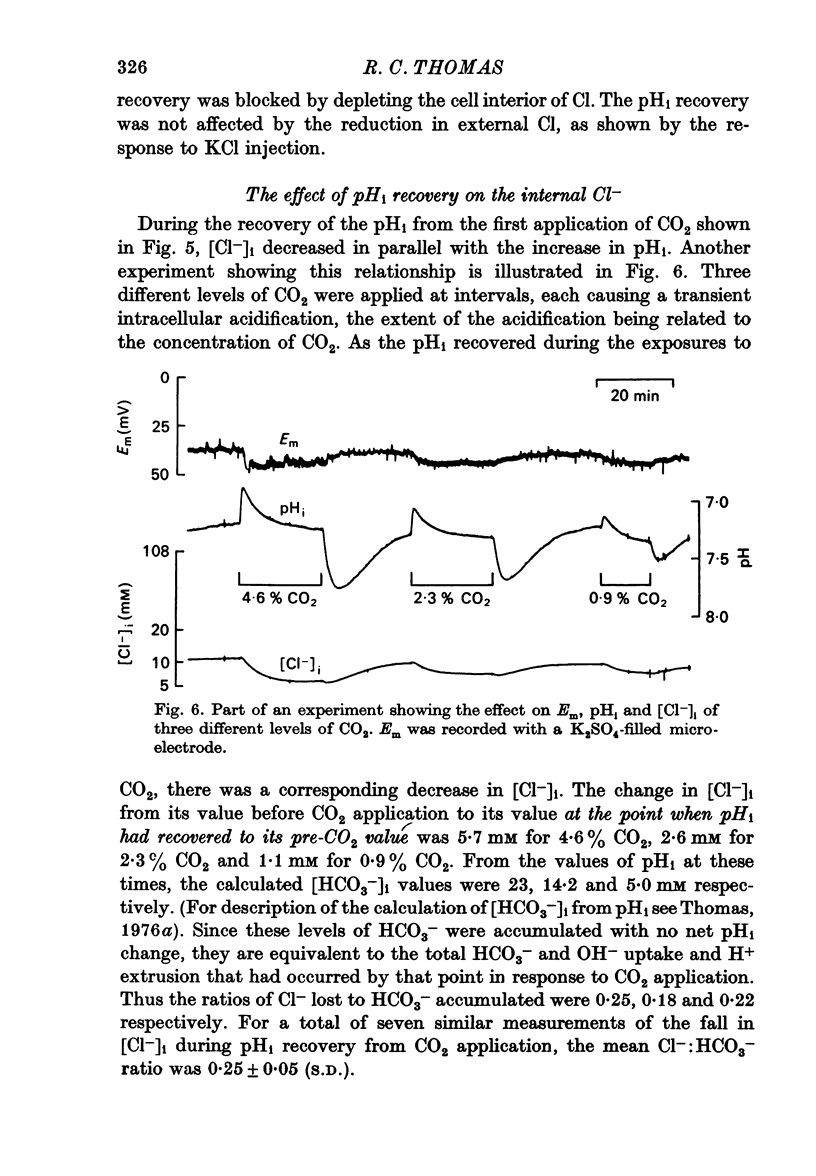
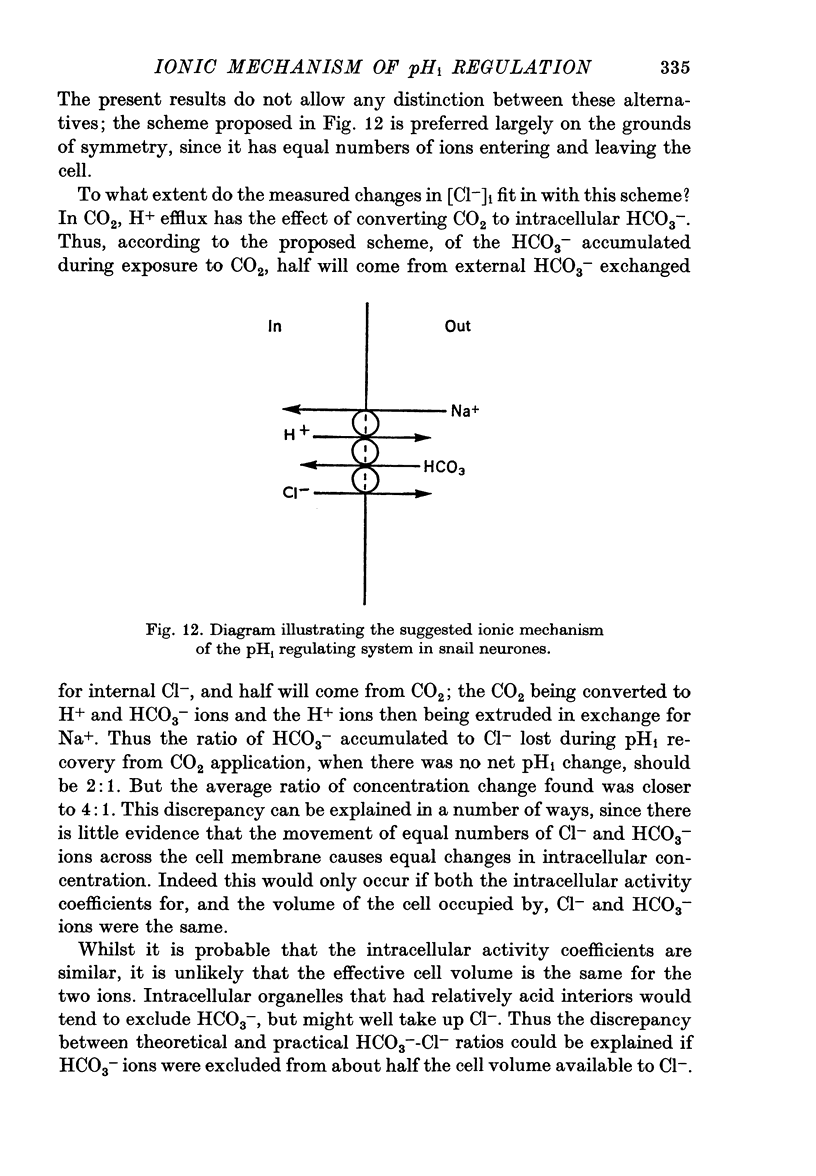
4. During pHi recovery from CO2 application the internal Cl- concentration decreased. The measured fall in internal Cl- concentration averaged about 25% of the calculated increase in internal HCO3-.
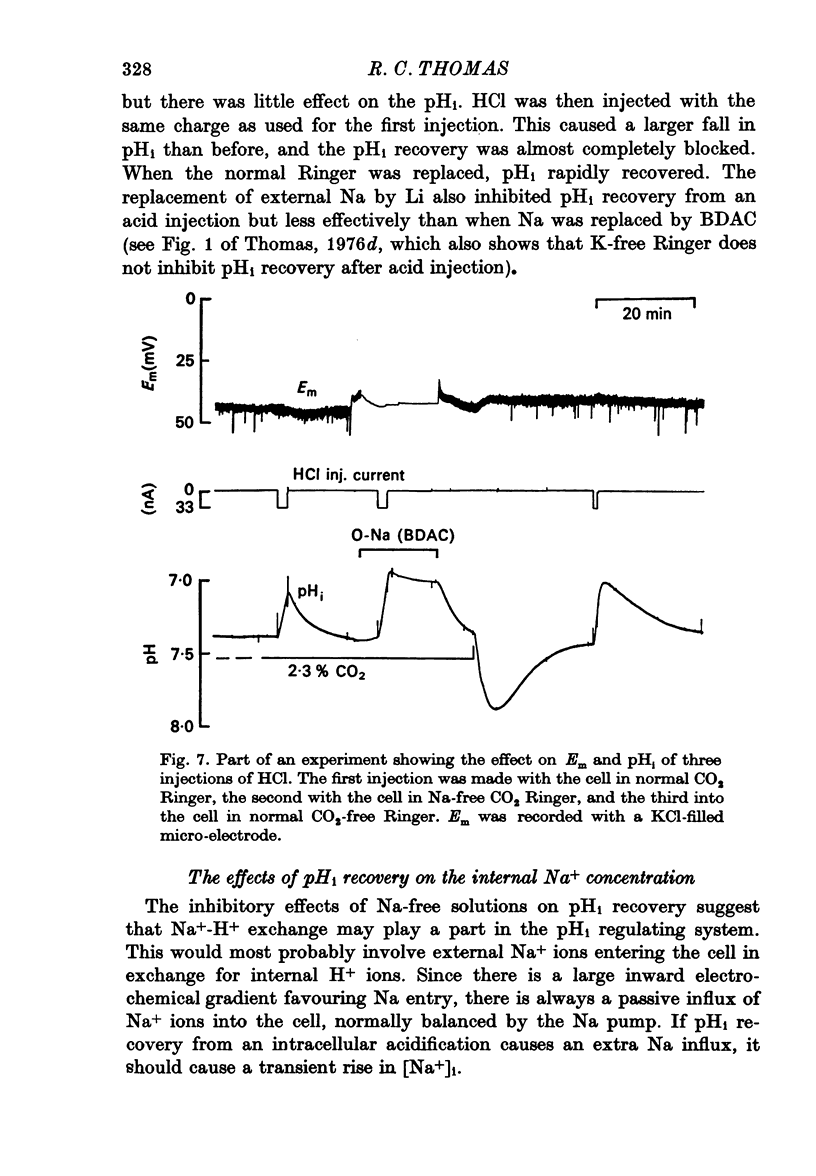
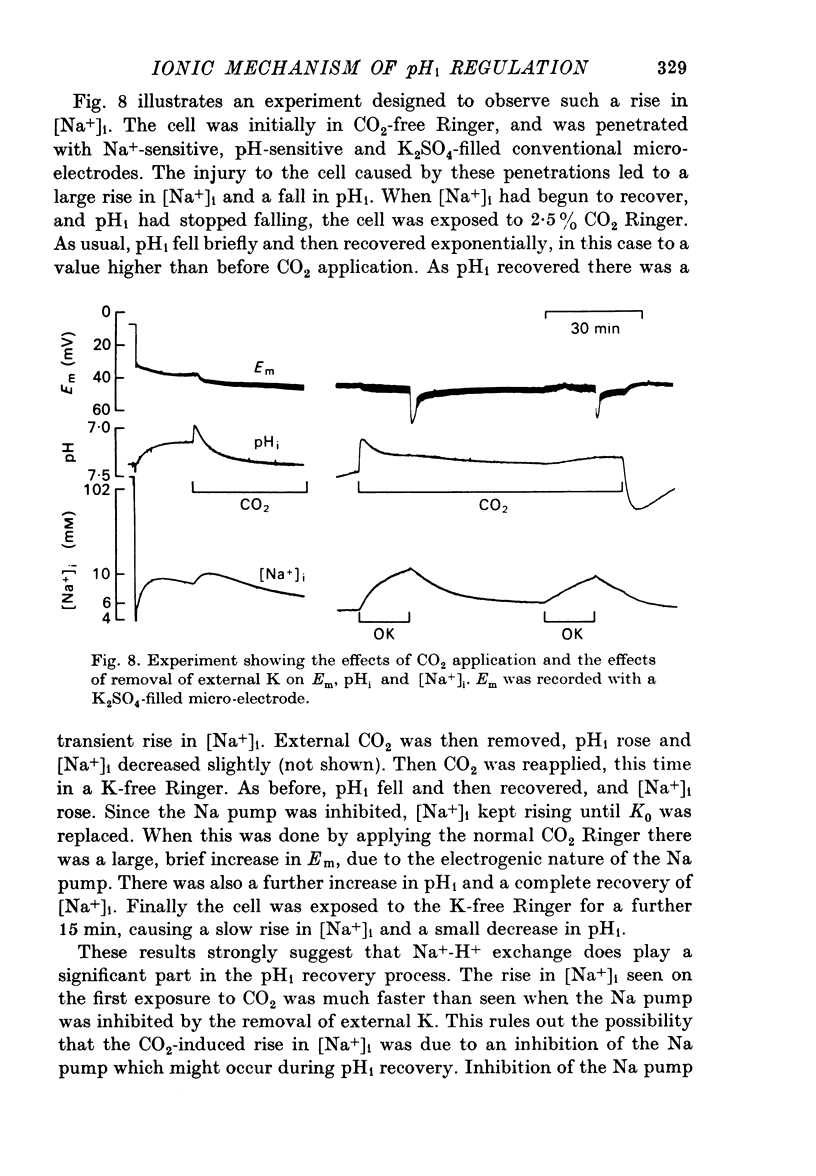
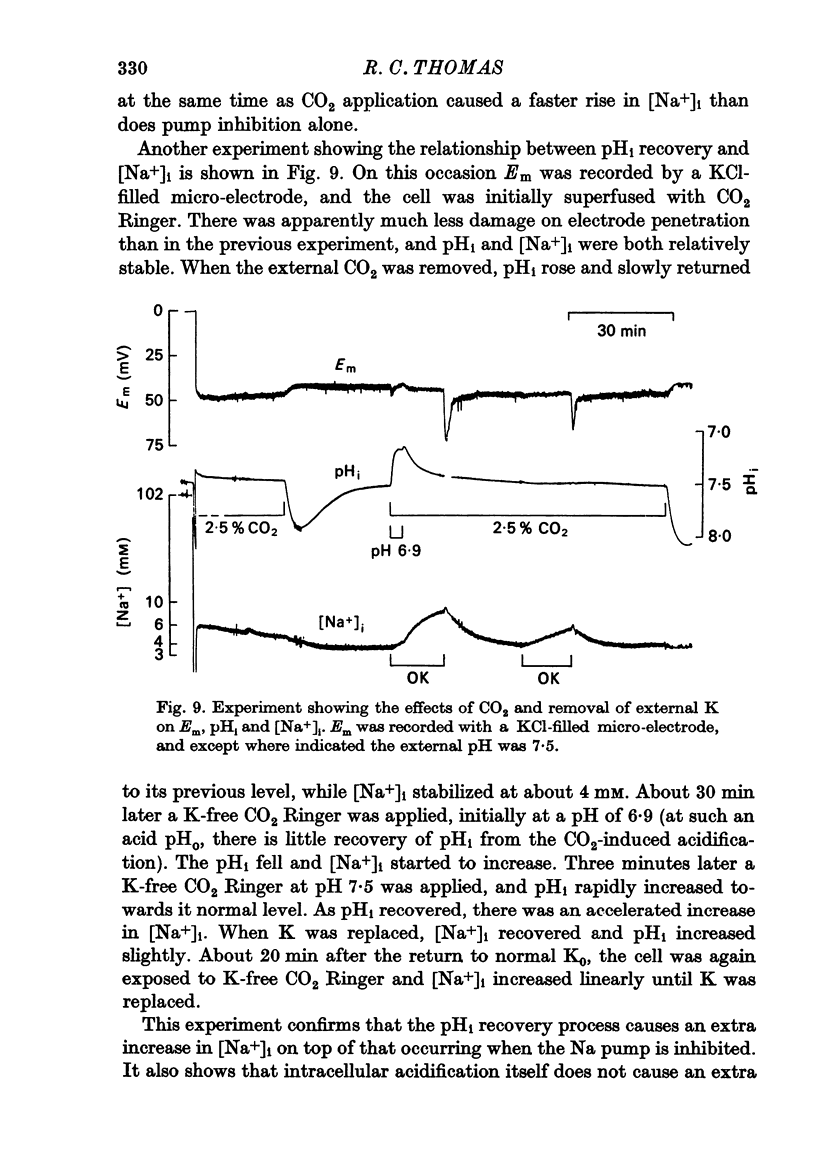
5. Removal of external Na inhibited the pHi recovery from either CO2 application or HCl injection.
6. During the pHi recovery from acidification there was an increase in the internal Na+ concentration ([Na+]i). The increase was larger than that occurring when the Na pump was inhibited by K-free Ringer.
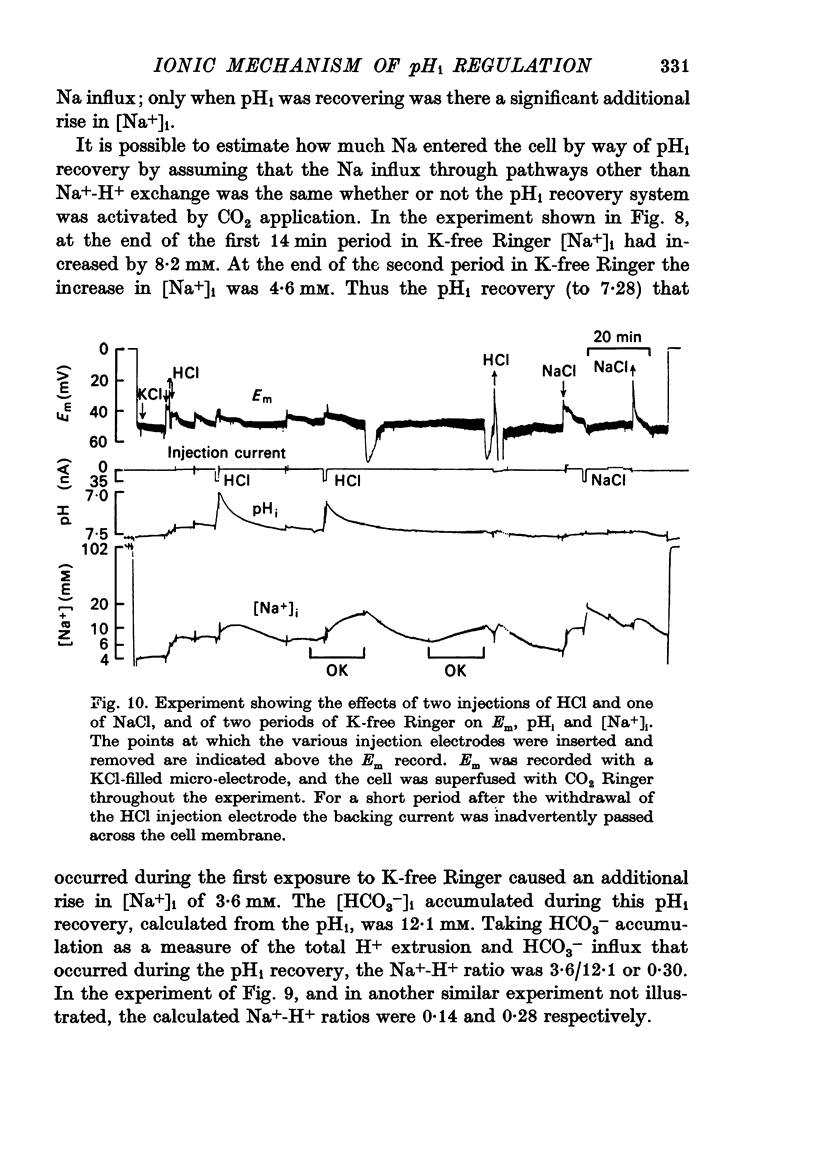
7. The increase in [Na+]i that occurred during pHi recovery from an injection of HCl was about half of that produced by a similar injection of NaCl.
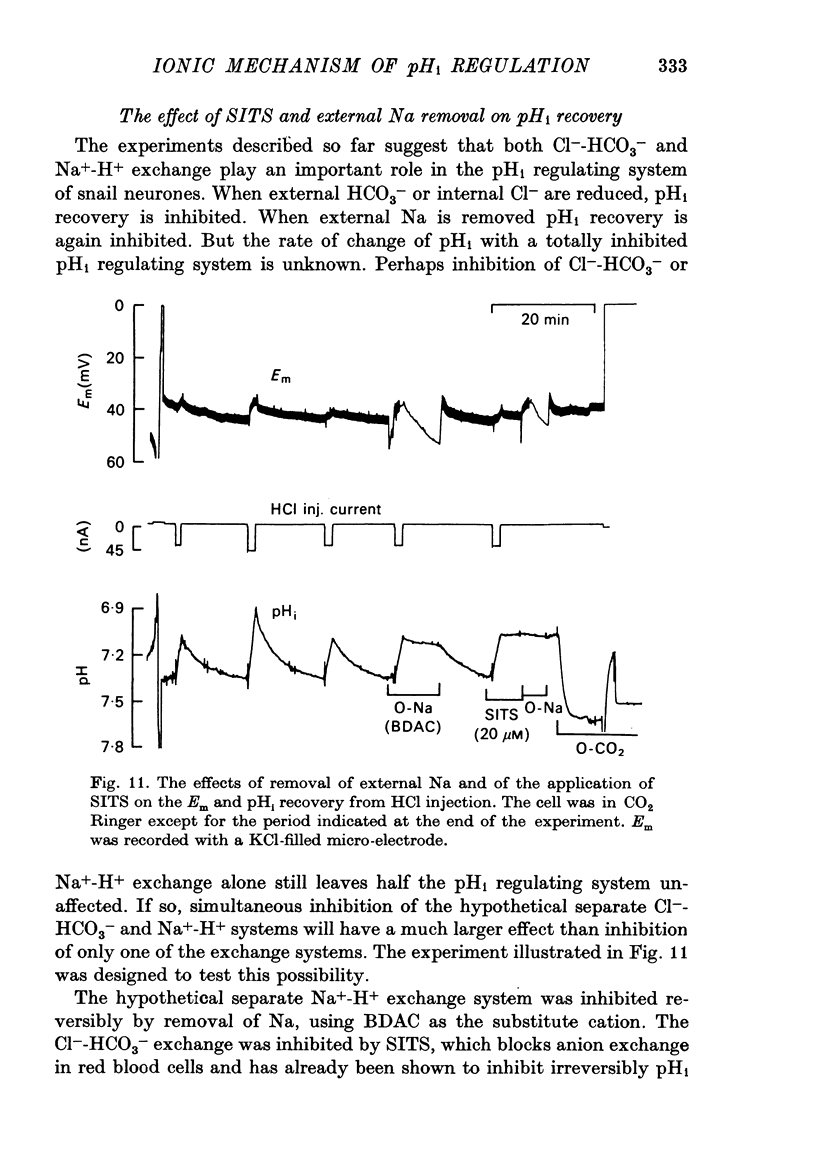
8. The inhibitory effects of Na-free Ringer and of the anion exchange inhibitor SITS on pHi recovery after HCl injection were not additive.
9. It is concluded that the pHi regulating system involves tightly linked Cl--HCO3- and Na+-H+ exchange, with Na entry down its concentration gradient probably providing the energy to drive the movement inwards of HCO3- and the movement outward of Cl- and H+ ions.
Full text
PDF


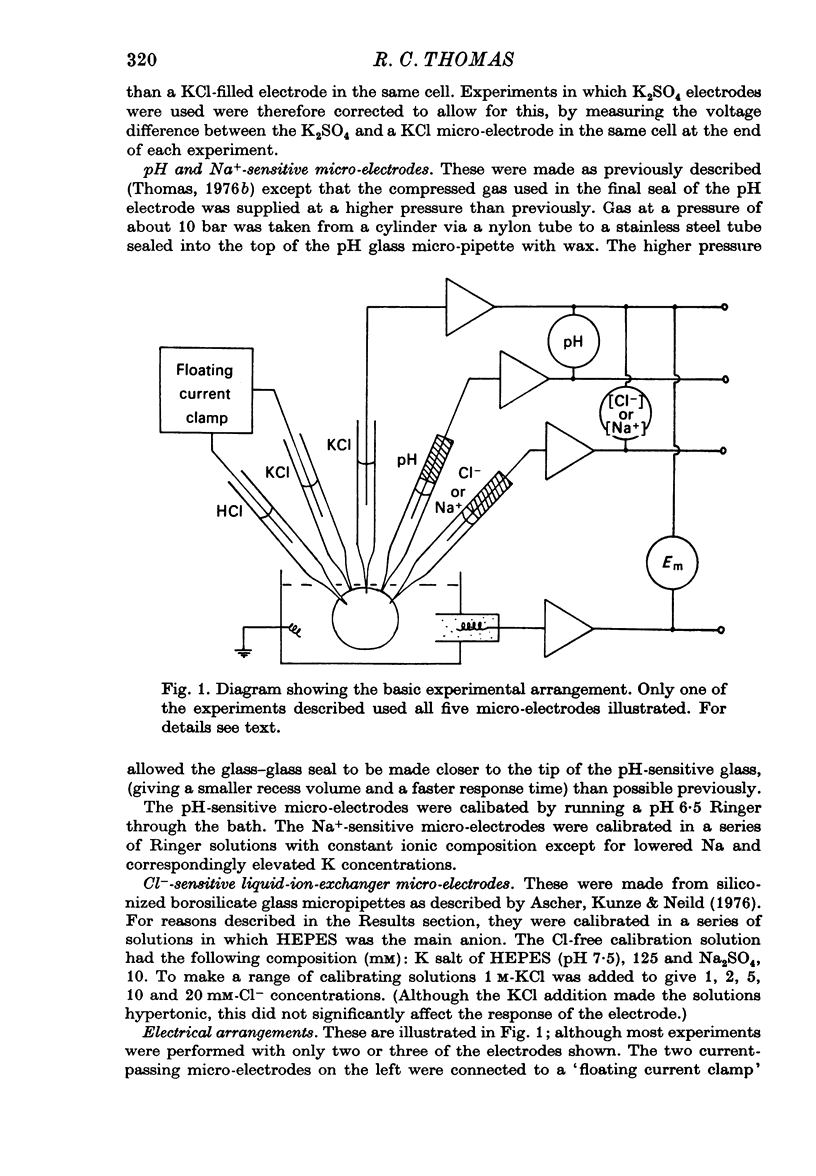
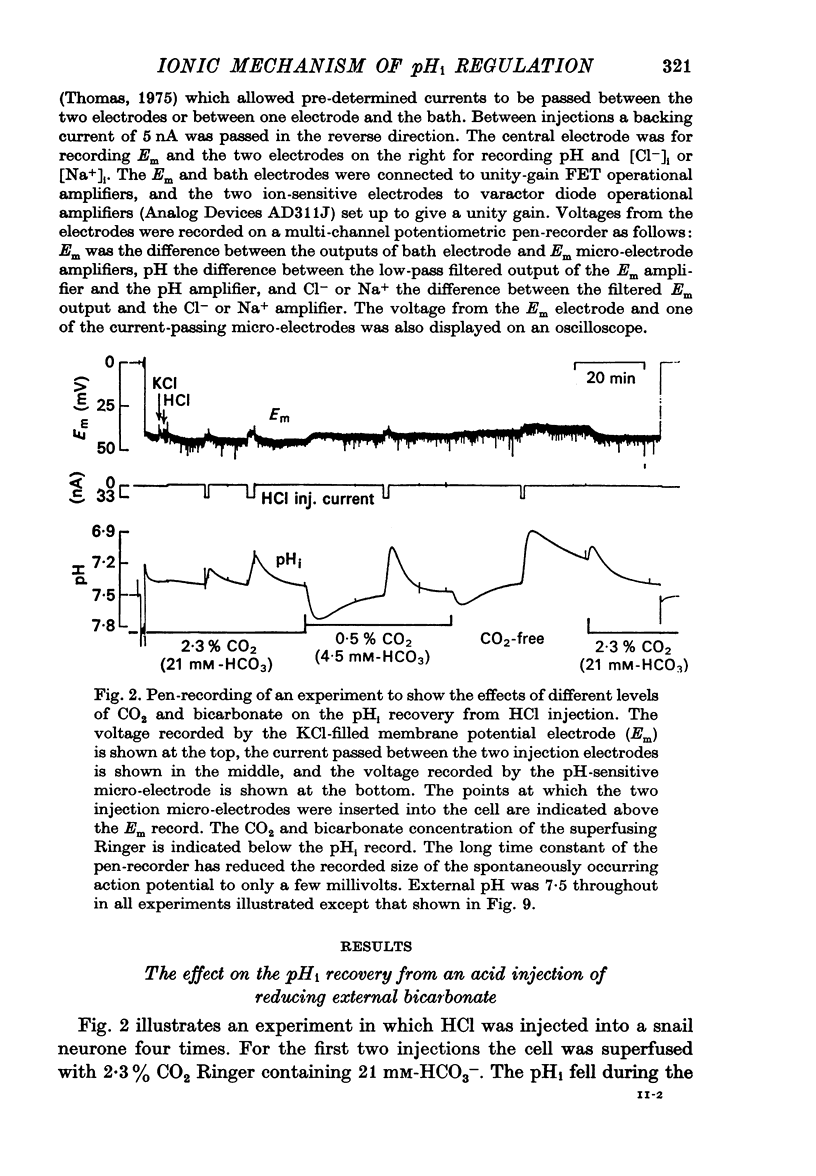
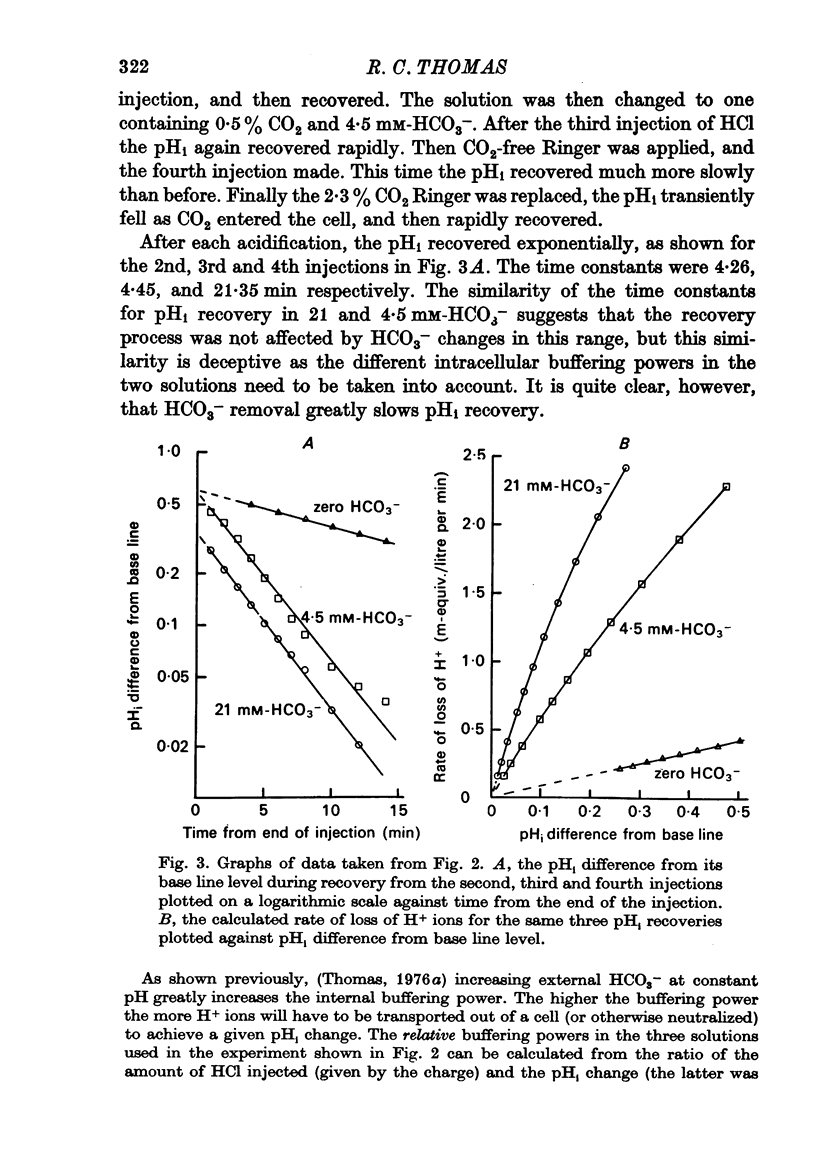

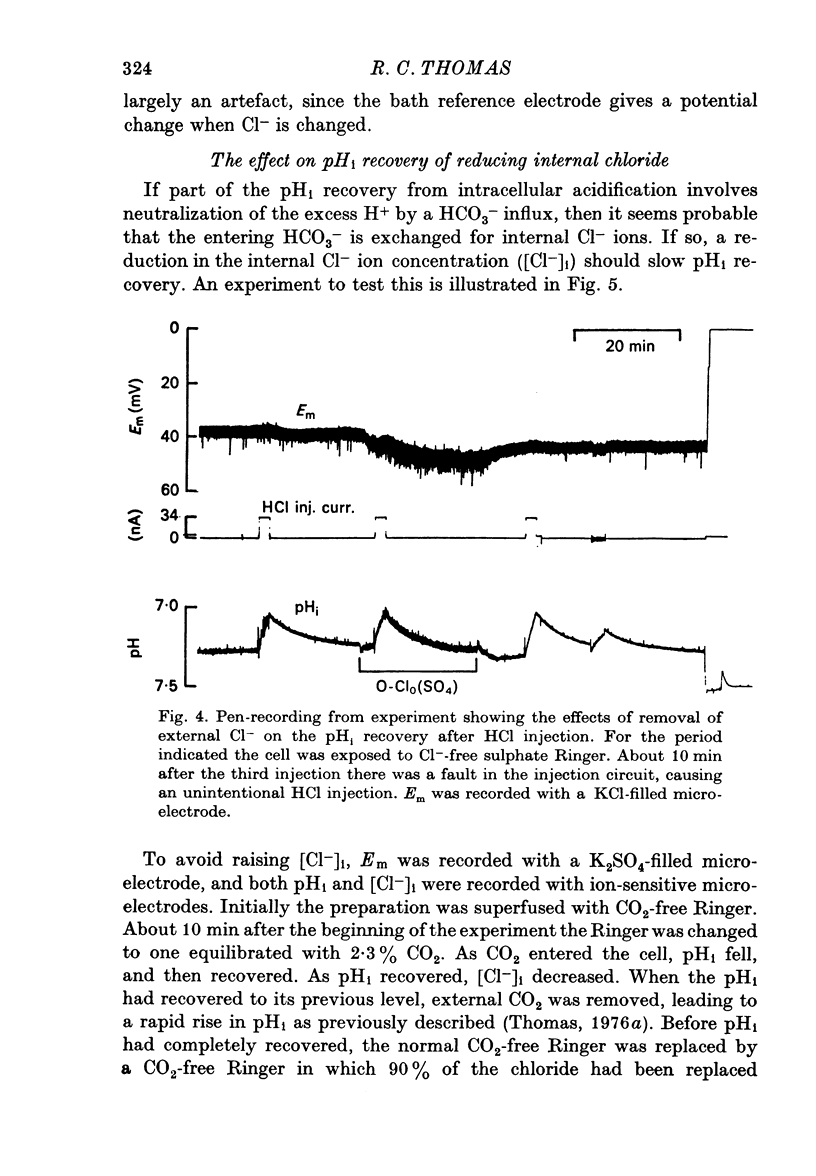






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Kunze D., Neild T. O. Chloride distribution in Aplysia neurones. J Physiol. 1976 Apr;256(2):441–464. doi: 10.1113/jphysiol.1976.sp011332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Active proton transport stimulated by CO2/HCO3-, blocked by cyanide. Nature. 1976 Jan 22;259(5540):240–241. doi: 10.1038/259240a0. [DOI] [PubMed] [Google Scholar]
- Cohen R. D., Iles R. A. Intracellular pH: measurement, control, and metabolic interrelationships. CRC Crit Rev Clin Lab Sci. 1975 Sep;6(2):101–143. doi: 10.3109/10408367509151567. [DOI] [PubMed] [Google Scholar]
- Ellis D., Thomas R. C. Direct measurement of the intracellular pH of mammalian cardiac muscle. J Physiol. 1976 Nov;262(3):755–771. doi: 10.1113/jphysiol.1976.sp011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L. D., Thomas R. C. A twelve-way rotary tap for changing physiological solutions. J Physiol. 1975 Feb;245(2):22P–23P. [PubMed] [Google Scholar]
- Russell J. M., Boron W. F. Role of choloride transport in regulation of intracellular pH. Nature. 1976 Nov 4;264(5581):73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. A floating current clamp for intracellular injection of salts by interbarrel iontophoresis. J Physiol. 1975 Feb;245(2):20P–22P. [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Ionic mechanism of the H+ pump in a snail neurone. Nature. 1976 Jul 1;262(5563):54–55. doi: 10.1038/262054a0. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]


