Abstract
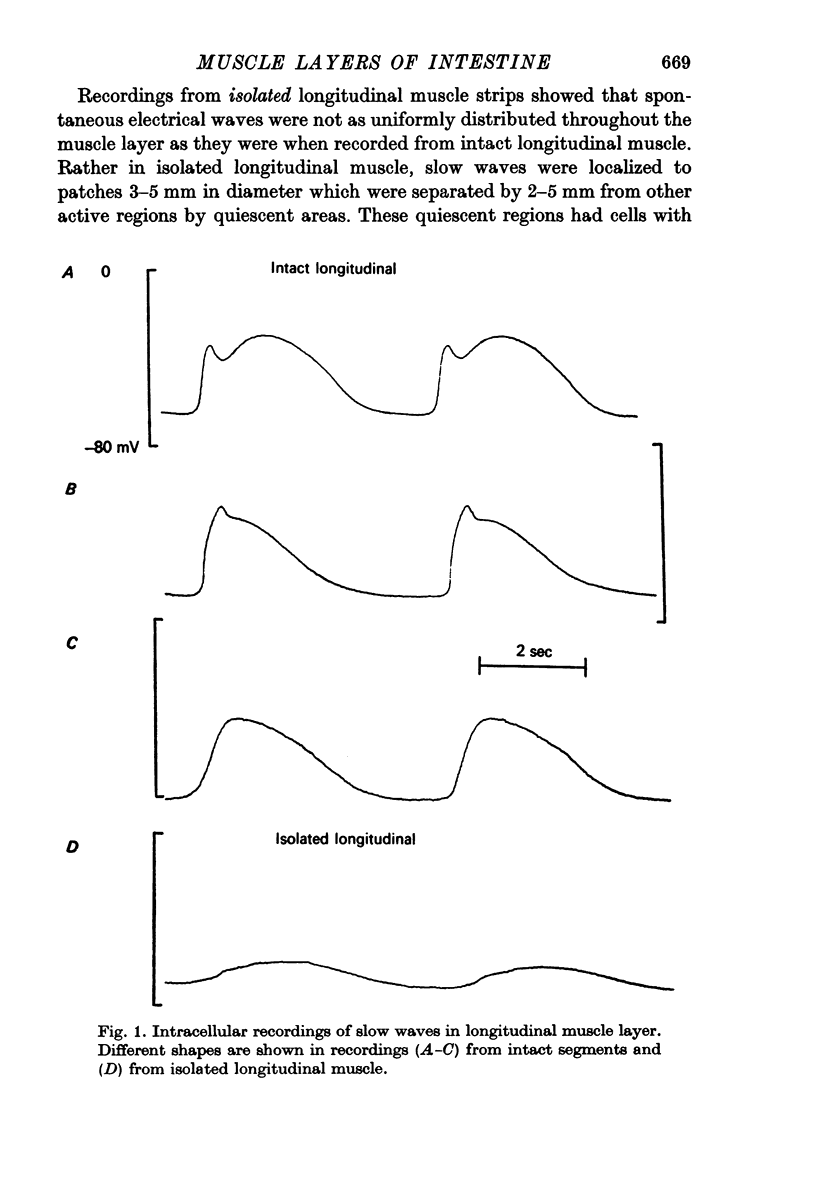
1. Slow waves recorded from isolated longitudinal muscle averaged 13 mV and had slow rate of rise (0.04 V/sec) whereas when recorded from intact segments the amplitude averaged 27 mV and the rate of rise was more rapid (0.09 V/sec), often with a notch between the initial peak and the plateau. Membrane potentials of longitudinal muscle were similar in isolated and intact preparations (- 66 mV). Resting potentials of circular muscle averaged - 67 mV.
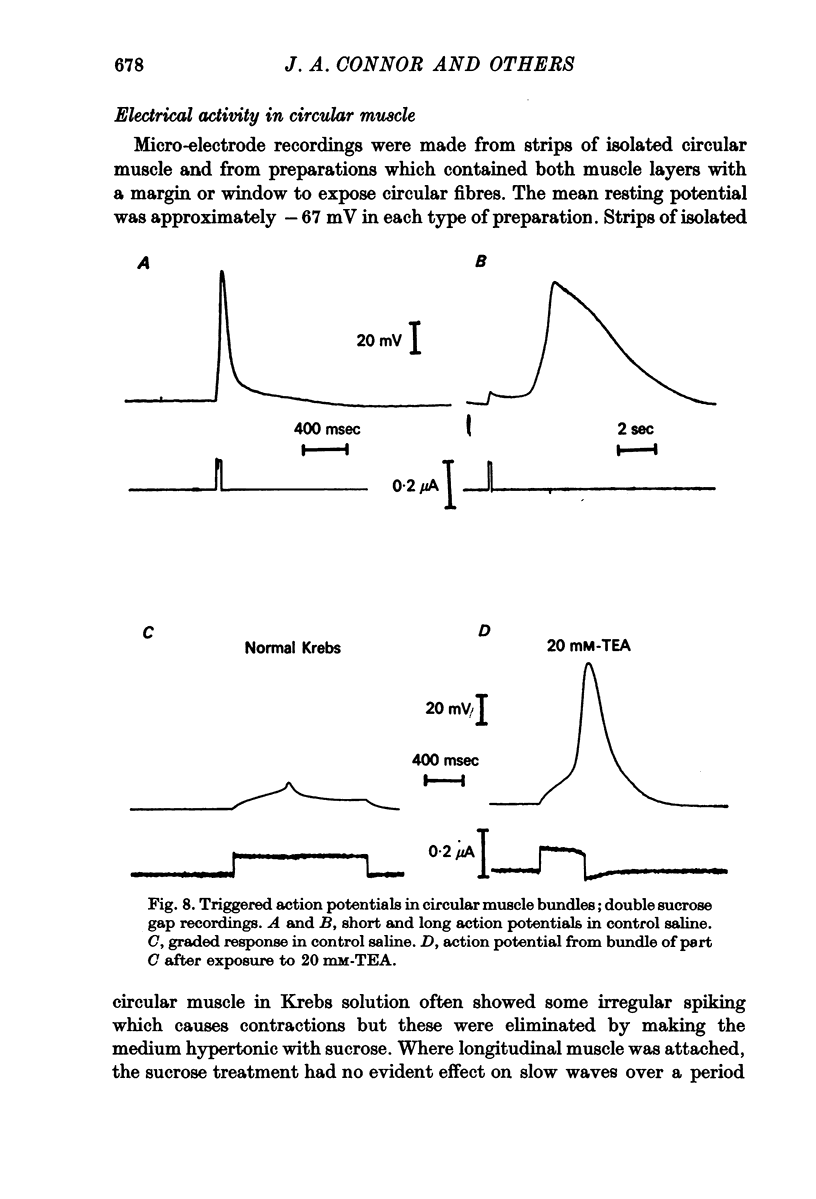
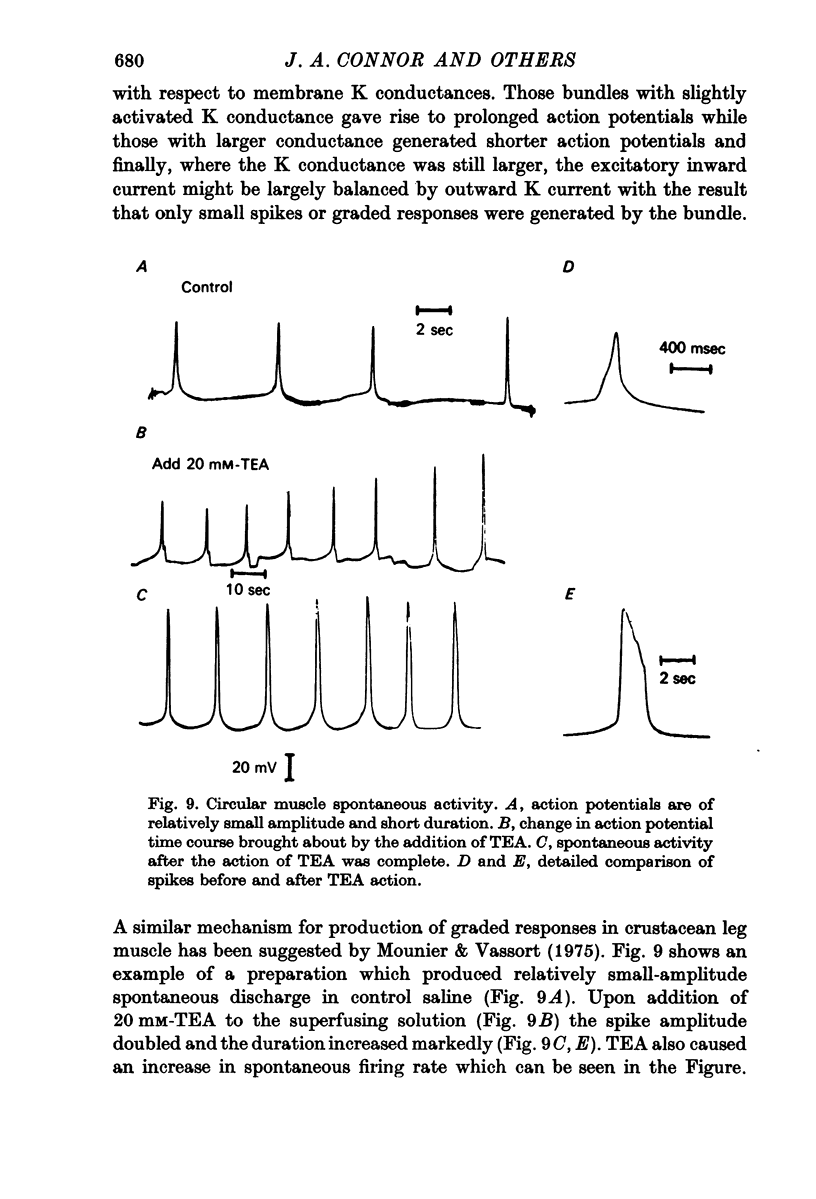
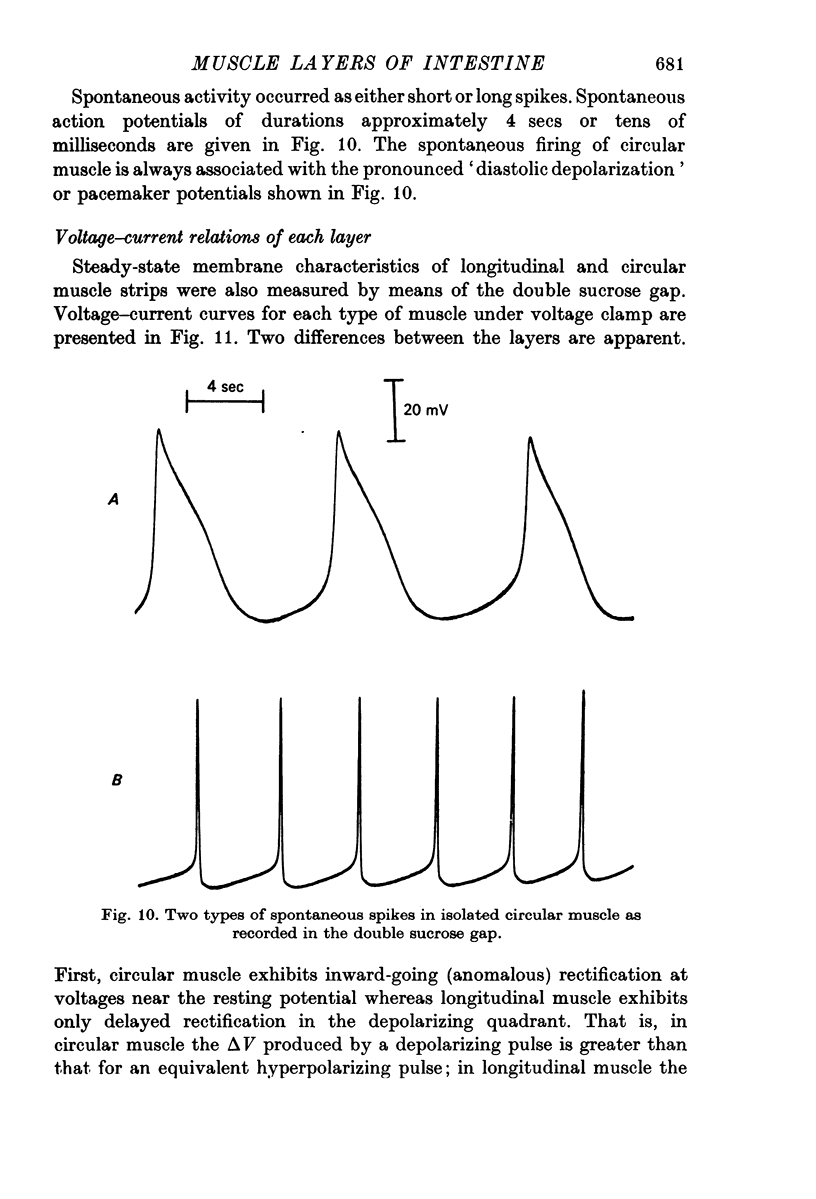
2. Small bundles of circular muscle tested in the double sucrose gap produced activity, either spontaneously or in response to stimulation, which fell into three categories: fast spikes (50-200 msec duration), slow spikes (1-5 sec duration), and small graded responses. The duration of fast spikes could be increased severalfold by the addition of TEA; the graded responses were converted to full-sized spikes by TEA.
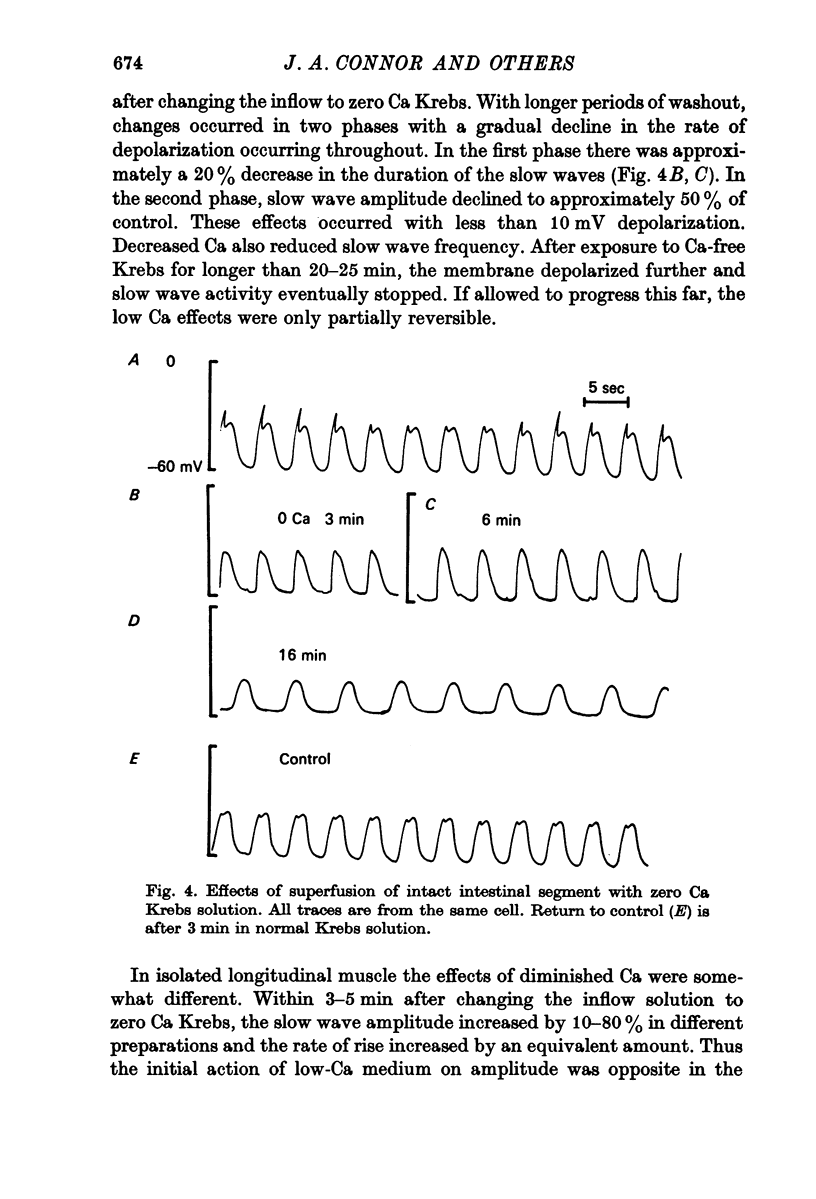
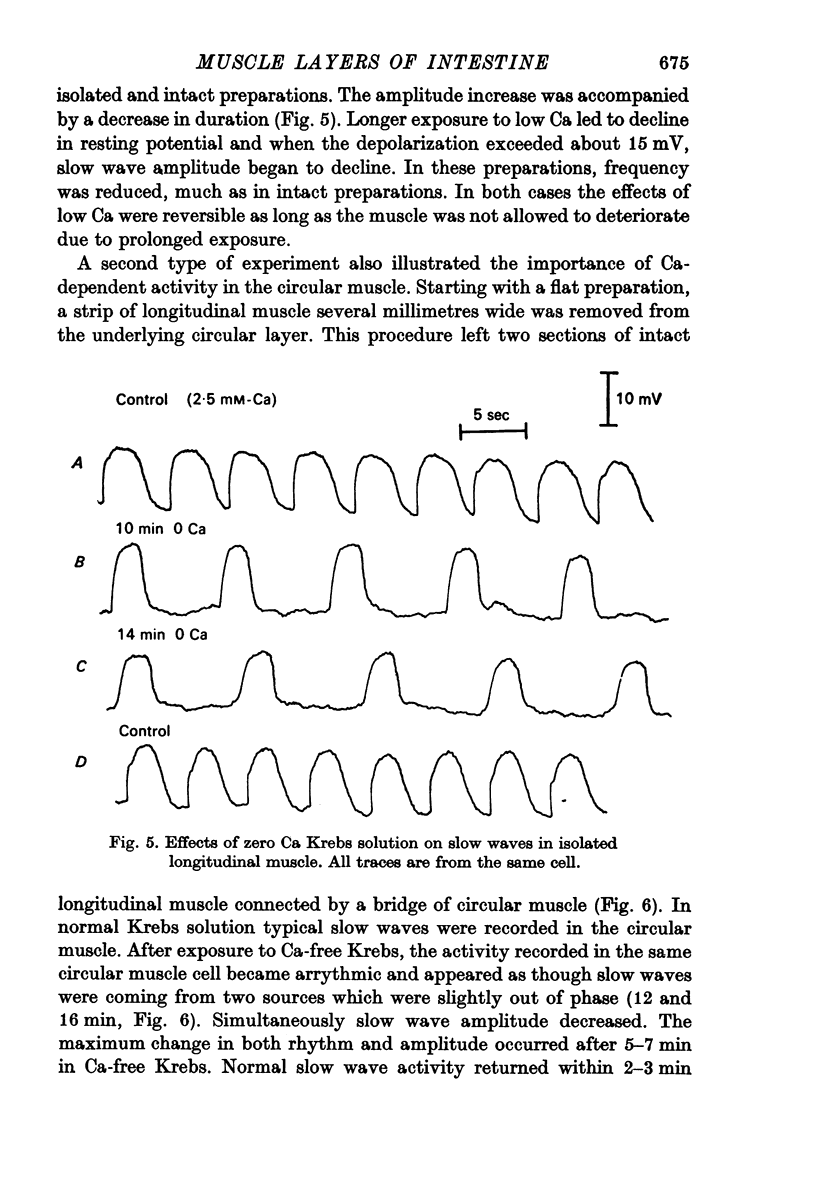
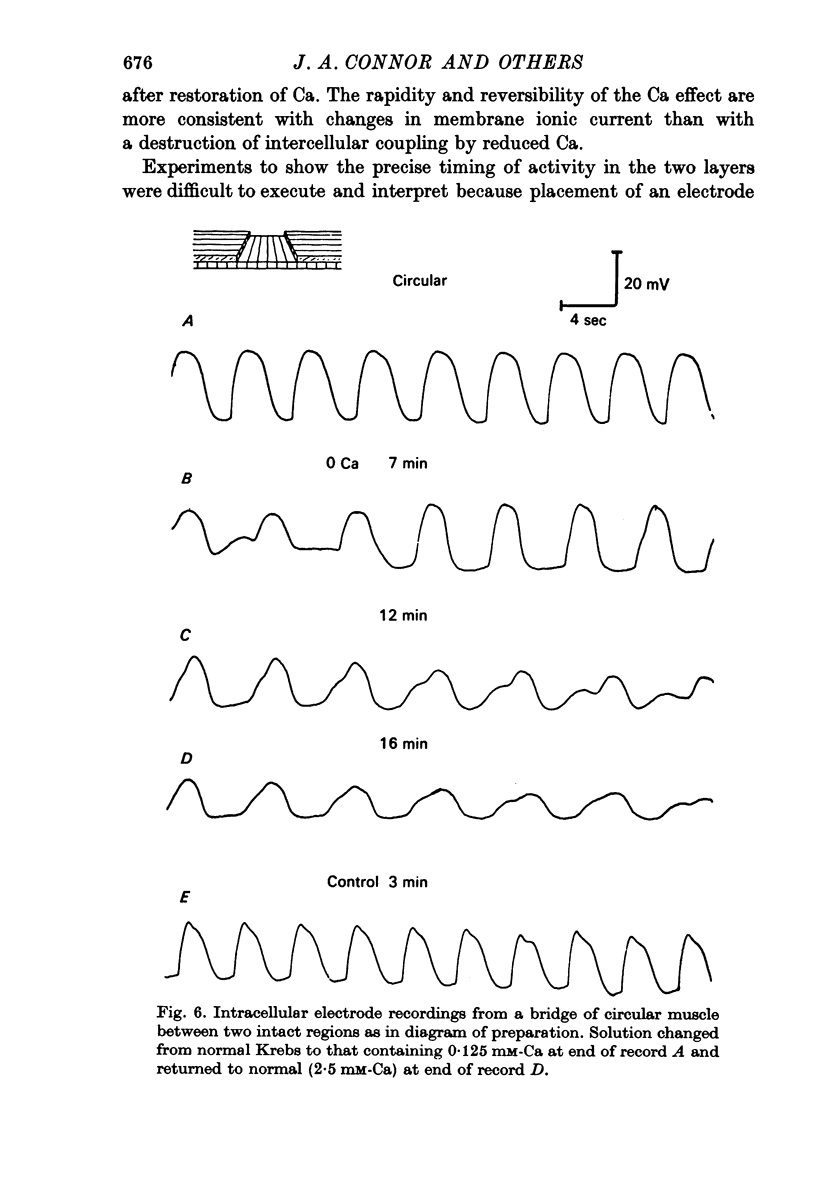
3. Treatment of circular muscle with Ca-free Krebs solution eliminated spikes, and in intact preparations reduced the amplitude and rate of rise of slow waves and eliminated the notch on slow waves.
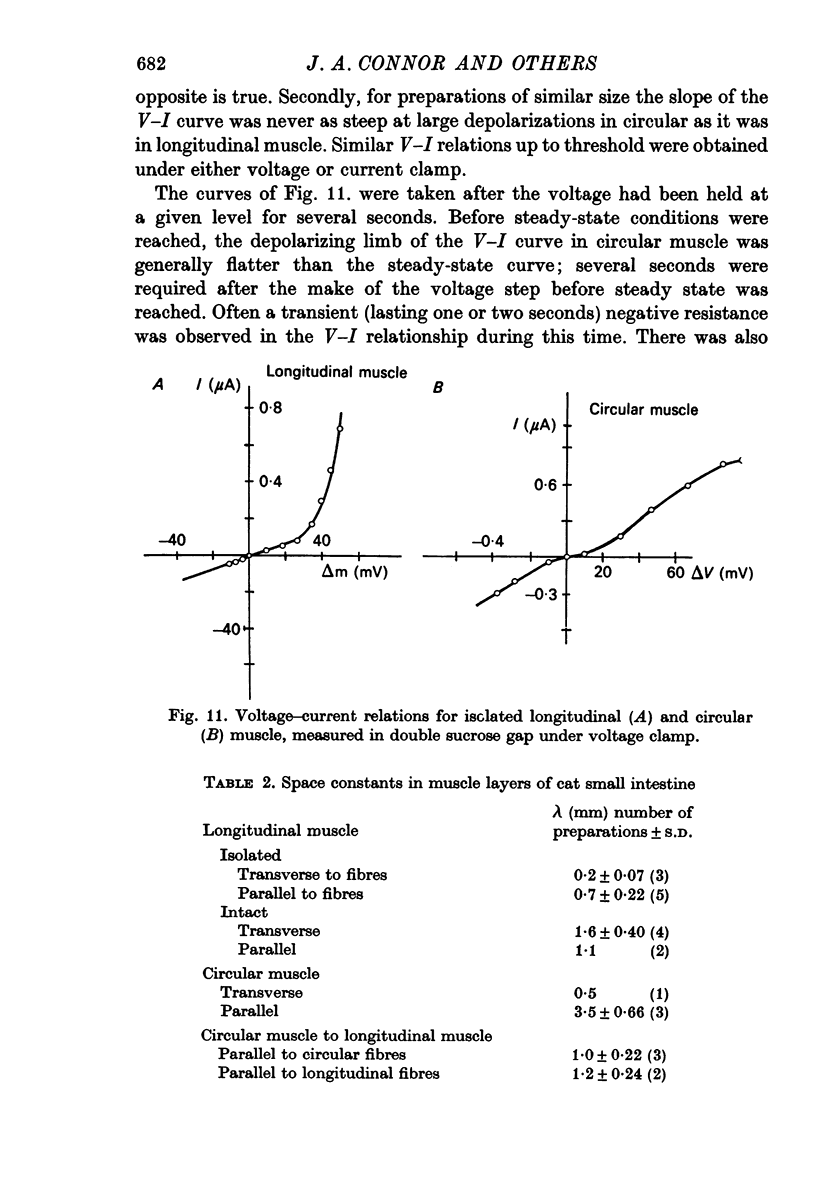
4. Current—voltage curves of longitudinal muscle show delayed rectification in the depolarizing quadrant; similar curves of circular muscle show anomalous rectification, i.e. a region where a very small current causes a large voltage change.
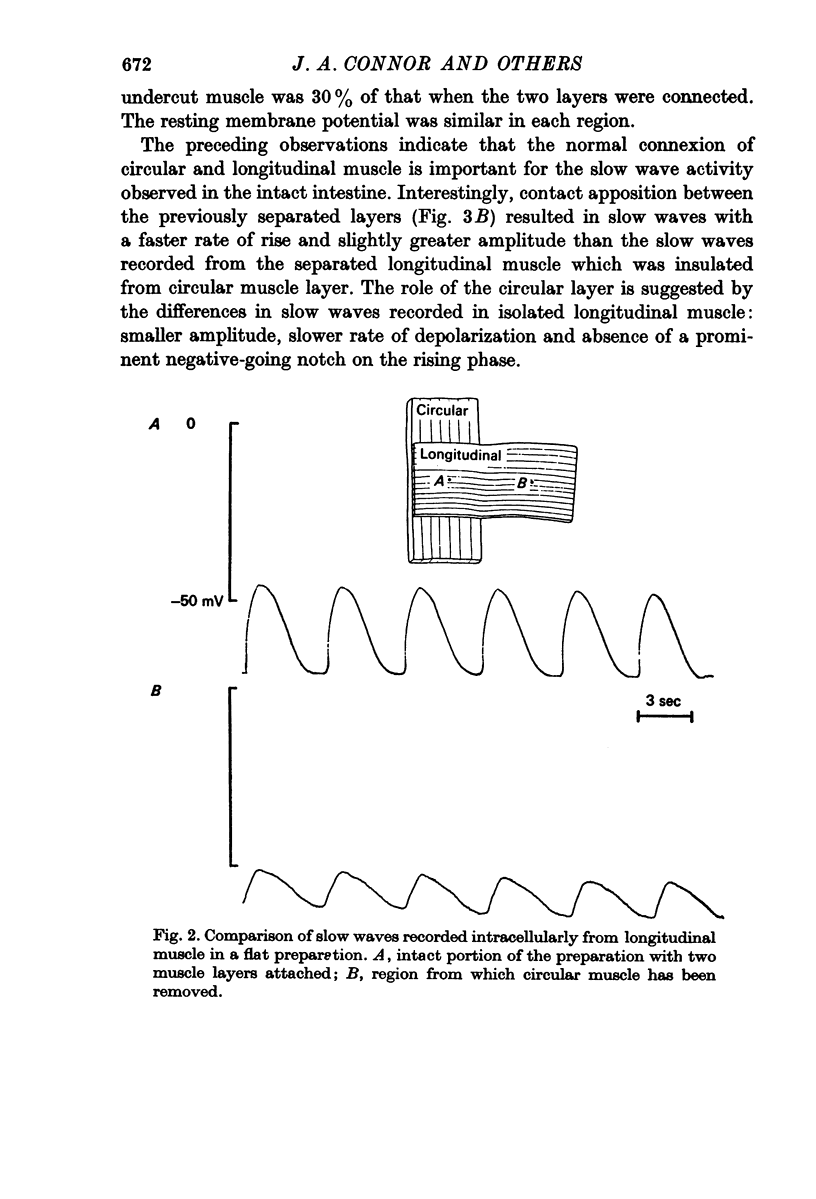
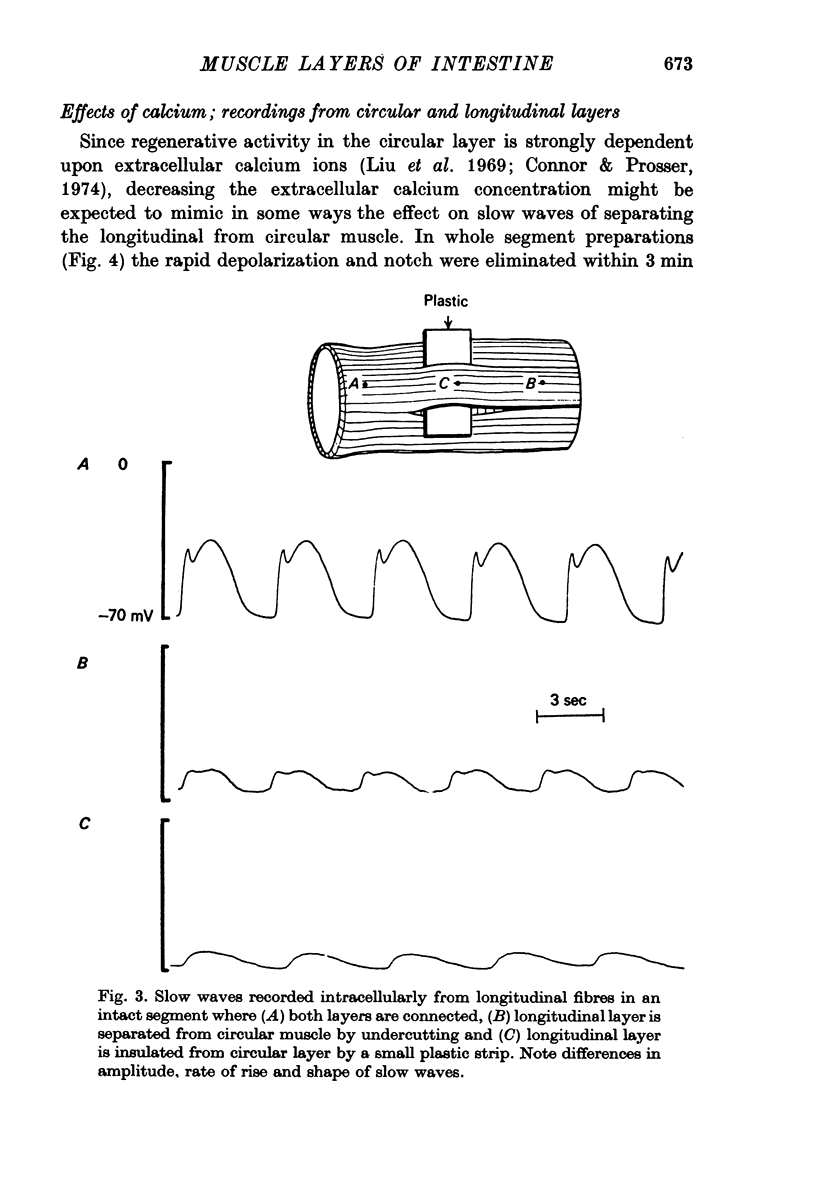
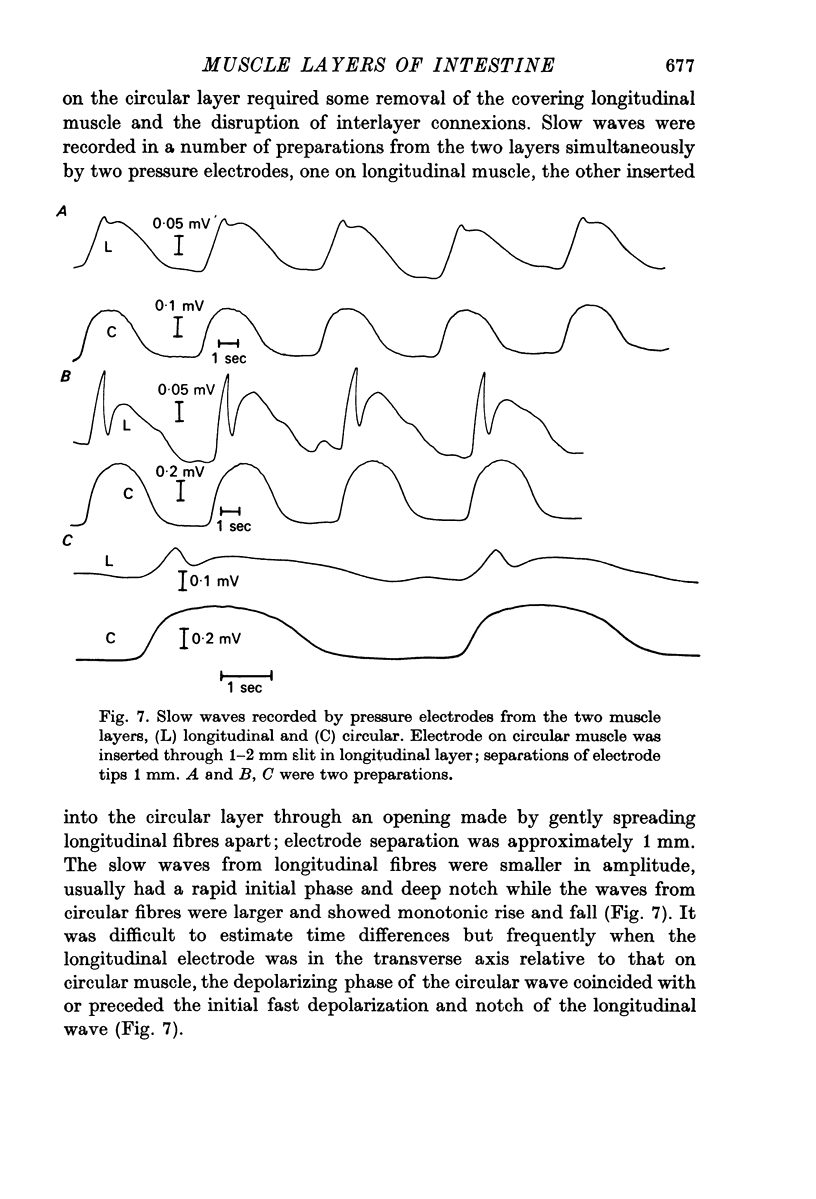
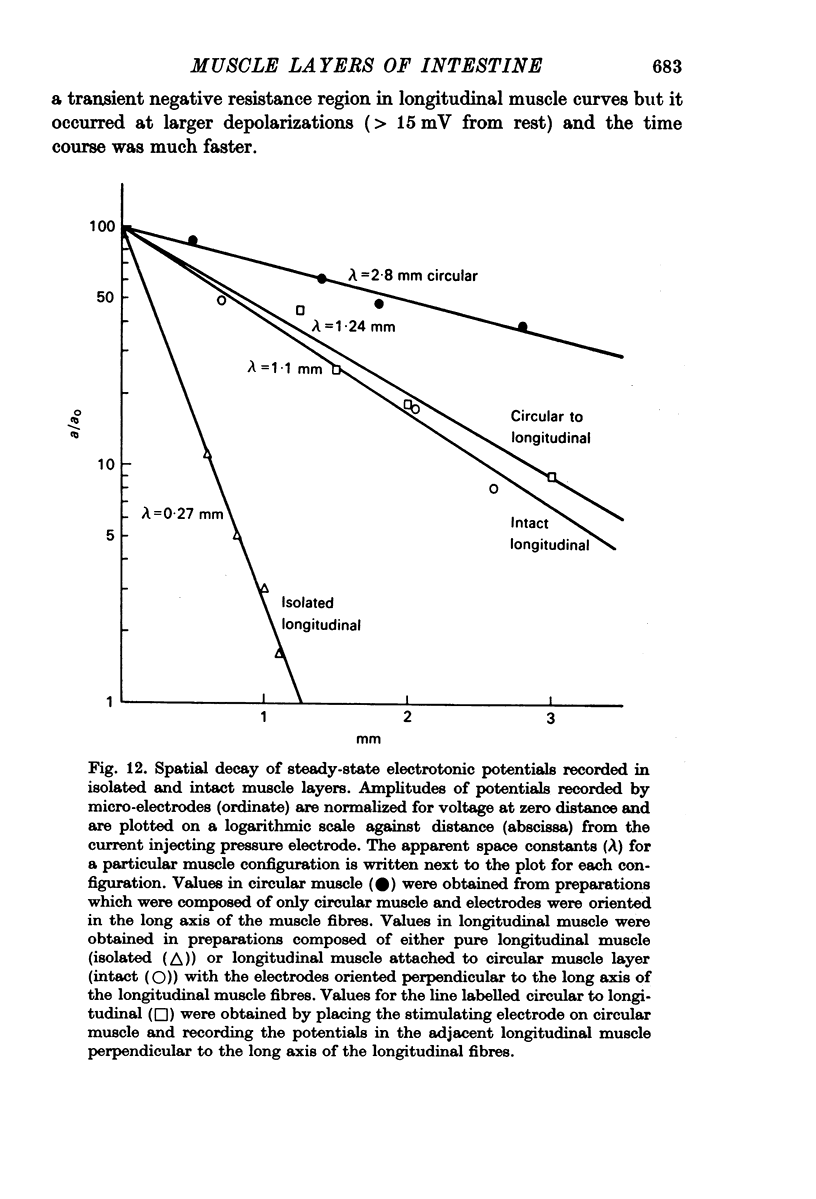
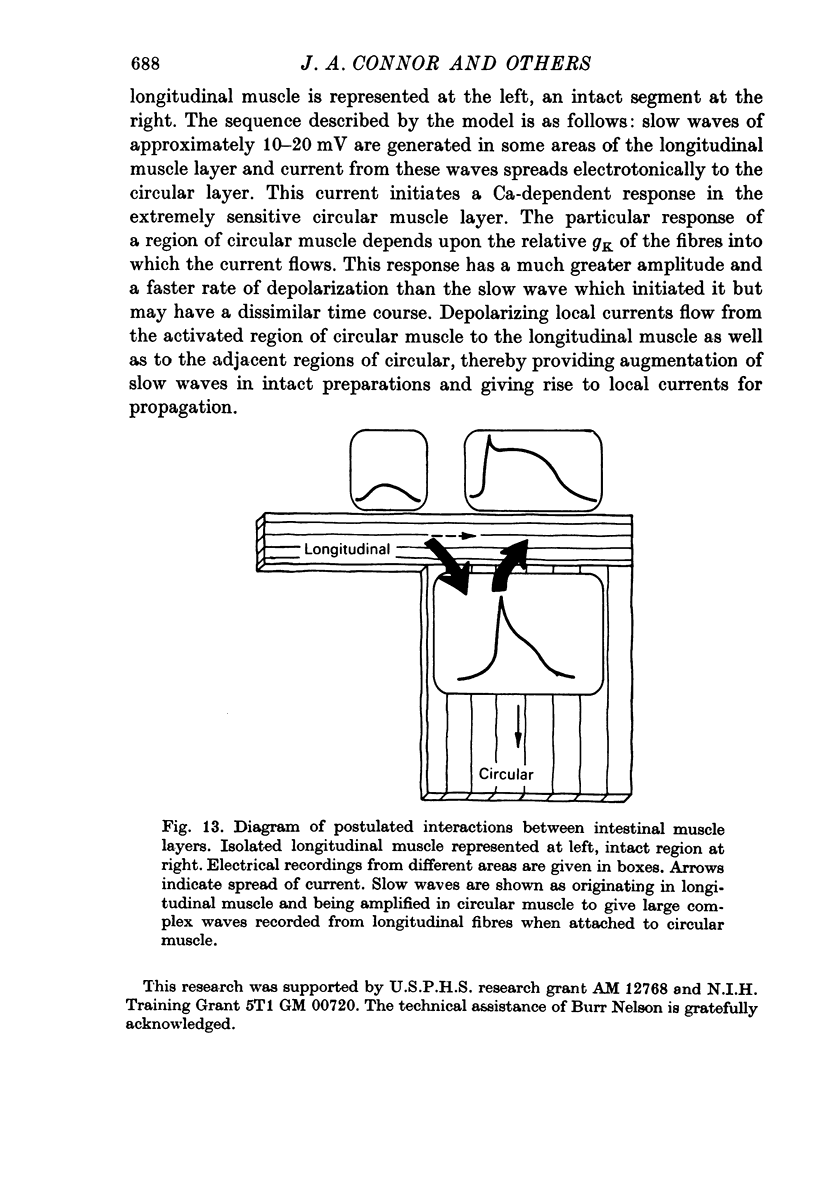
5. Non-polarized electrotonic coupling between longitudinal and circular layers indicates low-resistance pathways. Apparent space constants of longitudinal muscle are greater when attached to circular muscle than when isolated.
6. It is concluded that small slow potentials originate rhythmically in longitudinal muscle, that these spread passively to circular muscle where a regenerative amplification occurs which depends on Ca conductance and the amplified slow waves spread back to the longitudinal layer. In the intact intestine pacemaking is, therefore, separate from propagation and the circular muscle provides the bulk of depolarizing current for propagation.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bortoff A. Electrical transmission of slow waves from longitudinal to circular intestinal muscle. Am J Physiol. 1965 Dec;209(6):1254–1260. doi: 10.1152/ajplegacy.1965.209.6.1254. [DOI] [PubMed] [Google Scholar]
- Bortoff A., Sachs F. Electrotonic spread of slow waves in circular muscle of small intestine. Am J Physiol. 1970 Feb;218(2):576–581. doi: 10.1152/ajplegacy.1970.218.2.576. [DOI] [PubMed] [Google Scholar]
- Connor C., Prosser C. L. Comparison of ionic effects on longitudinal and circular muscle of cat jejunum. Am J Physiol. 1974 May;226(5):1212–1218. doi: 10.1152/ajplegacy.1974.226.5.1212. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Kreulen D. L., Prosser C. L. Relation between oxidative metabolism and slow rhythmic potentials in mammalian intestinal muscle. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4239–4243. doi: 10.1073/pnas.73.11.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Prosser C. L., Weems W. A. A study of pace-maker activity in intestinal smooth muscle. J Physiol. 1974 Aug;240(3):671–701. doi: 10.1113/jphysiol.1974.sp010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde A., Blondel B., Matter A., Cheneval J. P., Filloux B., Girardier L. Homo- and heterocellular junctions in cell cultures: an electrophysiological and morphological study. Prog Brain Res. 1969;31:283–311. doi: 10.1016/S0079-6123(08)63247-1. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H., Sakamoto Y. Effects of tetraethylammonium chloride on the membrane activity of guinea-pig stomach smooth muscle. J Physiol. 1970 Dec;211(2):445–460. doi: 10.1113/jphysiol.1970.sp009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D. D. Ionic basis of intestinal electrical activity. Am J Physiol. 1969 Nov;217(5):1534–1541. doi: 10.1152/ajplegacy.1969.217.5.1534. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Nagai T., Prosser C. L. Electrical interaction between muscle layers of cat intestine. Am J Physiol. 1966 Dec;211(6):1281–1291. doi: 10.1152/ajplegacy.1966.211.6.1281. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Prosser C. L., Nagai T. Electrical properties of intestinal muscle as measured intracellularly and extracellularly. Am J Physiol. 1967 Jul;213(1):275–286. doi: 10.1152/ajplegacy.1967.213.1.275. [DOI] [PubMed] [Google Scholar]
- Liu J., Prosser C. L., Job D. D. Ionic dependence of slow waves and spikes in intestinal muscle. Am J Physiol. 1969 Nov;217(5):1542–1547. doi: 10.1152/ajplegacy.1969.217.5.1542. [DOI] [PubMed] [Google Scholar]
- McGuigan J. A. Some limitations of the double sucrose gap, and its use in a study of the slow outward current in mammalian ventricular muscle. J Physiol. 1974 Aug;240(3):775–806. doi: 10.1113/jphysiol.1974.sp010634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier Y., Vassort G. Evidence for a transient potassium membrane current dependent on calcium influx in crab muscle fibre. J Physiol. 1975 Oct;251(3):609–625. doi: 10.1113/jphysiol.1975.sp011111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai T., Prosser C. L. Differentiation of slow potentials and spikes in longitudinal muscle of cat intestine. Am J Physiol. 1966 Mar;210(3):452–458. doi: 10.1152/ajplegacy.1966.210.3.452. [DOI] [PubMed] [Google Scholar]


