Abstract
The hybrid cluster protein (HCP; formerly termed the prismane protein) has been extensively studied due to its unique spectroscopic properties. Although the structural and spectroscopic characteristics are well defined, its enzymatic function, up to this point, has remained unidentified. While it was proposed that HCP acts in some step of nitrogen metabolism, a specific role for this enzyme remained unknown. Recent studies of HCP purified from Escherichia coli have identified a novel hydroxylamine reductase activity. These data reveal the ability of HCP to reduce hydroxylamine in vitro to form NH3 and H2O. Further biochemical analyses were completed in order to determine the effects of various electron donors, different pH levels, and the presence of CN− on in vitro hydroxylamine reduction.
Hybrid cluster protein (HCP), formerly known as prismane protein, has been studied extensively, mainly due to the unusual properties exhibited by one of its iron clusters. It was initially suggested that the protein contained a [6Fe-6S] cluster, thus the name prismane; more recently it was shown to contain two types of Fe clusters (11, 13). While recent crystallographic studies have determined a high-resolution structure of HCP (13), no classification of the enzymatic activity for this protein has yet been made.
Of the two clusters, one has been classified as a regular Fe4S4 cubane cluster, similar to those found in ferredoxins. While there are some unusual features to this cluster, it seems to fit within the parameters for clusters of other known species. However, this is not the case for the second cluster contained within the protein. The second cluster of the HCP contains four Fe atoms, two bridging sulfides, two bridging oxo groups, and an unidentified fifth bridging atom which links two of the Fe atoms of the cluster (13). One very interesting feature of the amino acid ligands to the hybrid cluster is their similarity, in terms of types of amino acids and positions in the primary sequence of the protein, to the ligands to the FeNiS active-site cluster of Rhodospirillum rubrum carbon monoxide dehyrogenase (CODH; the C cluster) (J. S. Garavelli, H. Z. Huang, and D. J. Miller, Abstr. Protein Sci. Meet., abstr. 131-M, 2000).
Recent studies on the alteration of ligands to the active site of CODH have uncovered interesting results (7). Upon substitution of some key residues that bind the CODH C cluster, it was found that the specificity of the CODH was altered, effectively converting it from an enzyme that catalyzes CO oxidation to one that has a good hydroxylamine reductase activity (H265V CODH). Interestingly, it has also been found that, by inserting Fe into the vacant position of Ni-deficient CODH, in a sense creating a Fe-CODH, a similar hydroxylamine reductase activity can be produced. These findings led to the investigation and discovery of a similar hydroxylamine reductase activity for the HCP from Escherichia coli, which is described here. Because HCP is optimally expressed under anaerobic conditions in the presence of either nitrate or nitrite, it has been suggested that HCP might play some role in nitrogen respiration (1, 5). In this paper we relate the discovery of new, oxygen-sensitive, hydroxylamine reductase activity for this enzyme and present some of the basic kinetic characteristics and spectroscopic data for this activity.
MATERIALS AND METHODS
Experimental conditions.
All experiments were performed anaerobically (<2 ppm O2) in a Vacuum Atmospheres Dri-Lab glove box (model HE-493) unless otherwise noted.
Preparation of chemicals.
Stock solutions of hydroxylamine (NH2OH; Sigma) and methylhydroxylamine (CH3NHOH; Aldrich) were prepared in CHES (2-[N-cyclohexylamino]ethanesulfonic acid; Sigma; 1 M, pH 9.0) anaerobically. Cyanide stock solution (1 mM) was freshly prepared in 0.01 M NaOH.
Cell growth.
Anaerobic cultures of E. coli cells were grown in sealed carboys, which had been sparged and kept under a nitrogen atmosphere. E. coli cells, transformed with the pWB208 plasmid (13), were grown in a modified tryptone-yeast extract medium, similar to that which was previously reported by van der Berg et al. (13). As one addition, Fe citrate (∼0.01 g/liter, final concentration) was added to the medium to insure sufficient Fe for proper formation of all [Fe-S] clusters in the target enzyme.
Purification of HCP.
The protocol used to purify HCP is similar to previously published procedures (13). All procedures were performed anaerobically unless otherwise noted; 100 mM MOPS (3-[N-morpholino]propanesulfonic acid), pH 7.5, was the buffer used for all chromatographic steps. Cells were resuspended in anaerobic 100 mM MOPS buffer (pH 8.0), which contained sodium dithionite (DTH), DNase, RNase, and protease inhibitors (2 mM leupeptin and 0.5 mM phenylmethylsulfonyl fluoride), and were disrupted in a French pressure cell (∼1,500 lb/in2). The solution was then separated by ultracentrifugation (4 h at 44,000 × g, 4°C). HCP was found in the soluble fraction and was loaded on an anaerobic Whatman DE-52 anion-exchange column equilibrated with buffer and 50 mM NaCl. The column was washed with a 0 to 0.5 M gradient of NaCl in buffer; HCP was eluted at ∼200 mM NaCl. Pooled fractions were then loaded onto a small column of Calbiochem fast-flow hydroxyapatite in 10 mM potassium phosphate, and the HCP was eluted from the column with a similar solution of 10 mM potassium phosphate, as described by van der Berg et al. (13). Fractions were then pooled and loaded onto a Mono-Q anion-exchange column equilibrated with 50 mM NaCl in buffer, and the column was washed with a 0 to 0.5 mM gradient of NaCl in buffer. Fractions with high activity were pooled, concentrated, and desalted. Preparative gel electrophoresis was used as the final step to completely remove all impurities (4). The purity of HCP was greater than 99% as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis.
Assay for hydroxylamine reductase activity.
Assays were performed anaerobically under an N2 atmosphere in a 1.0-ml assay mixture containing metal-free CHES (100 mM, pH 9.0), EDTA (10 μM), and methyl viologen (MV; 10 mM). For the assay, a specific amount of NH2OH was added to the anaerobically prepared assay mixture in cuvettes (1.5 ml), and this solution was then poised with DTH to reduce MV (in most assays, MV in the assay solution was reduced to give an absorption at 578 nm [A578] near 1). Once the assay solution was properly poised, the purified HCP sample was added to the vial and the vial was immediately placed into the spectrophotometer. All spectra presented in this paper were recorded on a Shimadzu 1605 dual-beam spectrophotometer. The overall decrease in A578 (oxidation of reduced MV) was monitored for 20 s. This slope was then used to calculate the rate of NH2OH reduction performed by the enzyme. The optimal activity for NH2OH reduction by HCP was observed near pH 9. Therefore, most assays for NH2OH reduction were performed at pH 9.0 unless otherwise stated. A control assay without enzyme was also performed, and there was no significant decrease in A578. Activities are expressed as micromoles of NH2OH reduced per minute per milligram of protein. For comparison with the hydroxylamine reductase activity observed in CODH, the assays were also performed at pH 7.5.
Determination of ammonia production from hydroxylamine reduction by HCP.
To determine the production of ammonia (NH3) from the reduction of hydroxylamine by HCP, assays were conducted as previously described (12). An NH2OH reduction reaction mixture was allowed to incubate for ∼10 min to insure full NH2OH reduction. Then 1 ml of saturated sodium carbonate (Na2CO3) was added to the solution. Immediately following the addition of Na2CO3, a microdiffusion stopper, upon which a drop of 1 N sulfuric acid (H2SO4) had been placed, was used to seal the vial without coming in contact with the reaction mixture. NH3 was then allowed to diffuse from the Na2CO3-treated reaction mixture to the 1 N H2SO4 on the tip of the microdiffusion stopper for 3 h. The stopper was then removed, and the microdiffusion rod was stirred in 3.75 ml of phenol reagent (100 ml of 0.14 M phenol, 26 μl of nitroprusside). Once this was complete, 1 ml of alkaline hypochlorite (10 ml NaOH, 0.2 ml hypochlorite) was added to the mixture. The A625 for this assay was measured after 20 min and compared to a standard curve in order to determine to amount of NH3 produced by the reaction. A control reaction mixture lacking HCP was tested, and no significant NH3 production was detected by this assay. NH2OH assays were performed using the method established by Korpela and Makela (8).
Determination of Michaelis constant.
To determine the Michaelis constant (Km) of NH2OH for HCP, the concentration of NH2OH was varied (0 to 100 mM). The determination of Km was conducted at two distinct pHs, 7.5 and 9.0. MOPS (50 mM, pH 7.5) and CHES (50 mM, pH 9.0) were used to obtain desired pH. The Kms for viologens were determined by varying the concentrations of viologens (0 to 10 mM) in the assay solution.
Determination of optimal pH for hydroxylamine reductase activity of HCP.
The pH-dependent rate of NH2OH reduction was determined by assaying the NH2OH reduction rate at various pHs (pH 6 to 10). A mixed buffer (MES [2-{N-morpholino}ethanesulfonic acid], MOPS, HEPES, Tris-HCl, and CHES; 10 mM each) was used to maintain the desired pHs. To maintain the desired pH, NH2OH stock solution in the mixed buffer was also adjusted to the desired pHs.
Protein assays.
Protein concentrations were determined colorimetrically by using bovine serum albumin (BSA; Sigma) as a standard (9). The BSA solution was standardized against carbonic anhydrase prior to use.
Studies of the effects of CN−, CO, and O2 on hydroxylamine reductase activity.
For the study of the effects of cyanide (CN−) on hydroxylamine reductase activity, HCP was assayed in a manner similar to that previously described; with the exception of having either CO-saturated buffer or various concentrations of CN− (0 to 10 mM) present in the reaction mixture during the assay. The combined effect of having both CN− and CO present in the assay solution was also tested by saturating solutions containing various concentrations of CN− (0 to 10 mM) with CO prior to assaying for hydroxylamine reductase activity. HCP samples were also incubated for 1 h with various concentrations of CN− (0 to 10 mM) or CO-saturated buffer in the presence of 4 mM DTH and tested for activity. For the study of the combination effect of CN− and CO incubation, HCP was preincubated with CO prior to treatment with CN−. The rates of NH2OH reduction by such effector-incubated HCP samples were determined as described above. Exposing HCP to air tested the effect of O2 on the hydroxylamine reductase activity of HCP. Activity measurements were taken at set times after exposure, and the resulting loss of activity was recorded. To determine if activity could be recovered after O2 exposure, samples were made anaerobic after air incubation by pumping and flushing serum-stoppered vials containing the air-oxidized samples with N2.
UV-visible absorption spectra.
DTH-reduced HCP samples were placed in anaerobically sealed serum-stoppered quartz cuvettes. Spectra were recorded prior to the addition of either slightly excess oxidized thionin (Eo′ [standard half-cell potential] = +64 mV) or NH2OH (20 mM, final concentration). The spectra of thionin- and NH2OH-treated HCP were recorded within 1 min.
RESULTS
Hydroxylamine reductase activity of HCP.
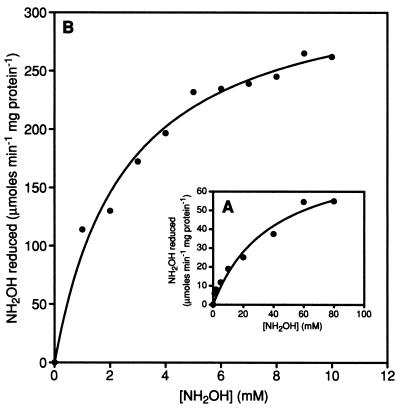
Table 1 shows that NH3 is produced in a 1:1 ratio with NH2OH loss when HCP is incubated with NH2OH. Therefore, it is proposed that HCP catalyzes the reduction of NH2OH to NH3 and H2O effectively. Figure 1 shows the rate of NH2OH reduction by HCP versus the concentration of NH2OH at pH 7.5 and 9.0. Vmax for the NH2OH reduction was estimated to be ∼92 μmol of NH2OH reduced min−1 mg of protein−1 at pH 7.5 (Fig. 1A). Figure 1B shows that the Vmax is increased at higher pH. At the optimal pH (9.0), the NH2OH reduction activity of HCP is approximately 458 μmol of NH2OH reduced min−1 mg of protein−1. The Km for NH2OH of HCP is also pH dependent; therefore, increasing pH decreases Km for NH2OH significantly. The lowest value of Km occurred at pH 9.0 and was determined to be 2.5 mM for NH2OH (calculated from data in Fig. 1B).
TABLE 1.
Reduction of hydroxylamine to ammonia by HCPa
| Sample | NH2OH added (μmol) | NH3 produced (μmol) | Final level of NH2OH (μmol) |
|---|---|---|---|
| Assay solution | 1.4 ± 0.2 | ND | 1.3 ± 0.3 |
| Assay solution + HCP | 1.4 ± 0.2 | 1.1 ± 0.4 | <0.3 ± 0.2 |
FIG. 1.
Determination of Km for NH2OH. The assay procedure and assay solution for these experiments are detailed in Materials and Methods. Assays were performed at pH 7.5 (A) and 9.0 (B). The estimated Km values for NH2OH are 38.9 ± 3.53 mM at pH 7.5 and 2.5 ± 0.36 mM at pH 9.0.
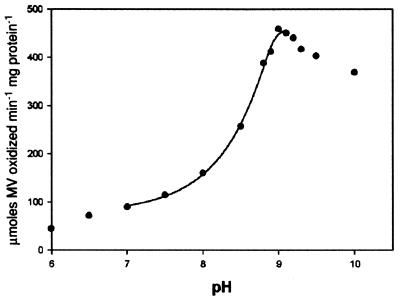
As was mentioned previously, HCP exhibited an unusually high Km value when Km was measured in solutions at pH 7.5. Because of this, experiments to test the possible effect that varying pH would have on the NH2OH reductase activity of the enzyme were performed. From the results of these experiments, shown in Fig. 2, it was apparent that changing the pH of the assay solution had a dramatic effect on the Vmax of the enzyme. The increase in activity was found to follow a curve indicative of a single protonation/deprotonation event within the range of pH 7 to 9. As shown in Fig. 2, the highest recorded activity occurred at pH 9.0, with the Vmax at this pH reaching 458 ± 19 μmol of NH2OH reduced min−1 mg of protein−1. It is also important that Fig. 2 indicates that, at pH values outside of this “physiological” range (pH 7 to 9), the NH2OH reductase activity does not follow this single-protonation/deprotonation trend.
FIG. 2.
Effect of pH on the hydroxylamine reductase activity of HCP. It is shown that the hydroxylamine reductase activity varies dramatically at various pHs. Measured activities ranged from 92 ± 19 (pH 7.0) to 458 ± 19 μmol of NH2OH reduced min−1 mg of protein−1 (pH 9.0).
UV-visible absorption spectra of HCP.
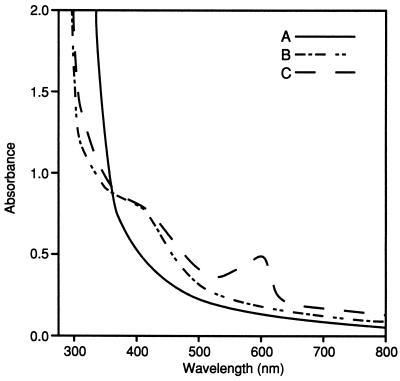
Figure 3 shows the UV-visible spectra of the enzyme under both oxidized and reduced conditions. HCP in the thionin-oxidized state shows a UV-visible spectrum characteristic of those of FeS cluster-containing enzymes (3). The FeS signal at 420 nm was diminished by reduction of the enzyme upon addition of DTH.
FIG. 3.
UV-visible absorption spectra of oxidized and reduced forms of the HCP. Experimental procedures are described in Materials and Methods. Line A, DTH-reduced form of the enzyme; line B, enzyme oxidized by addition of NH2OH; line C, thionin-oxidized form of the enzyme. The peak in the 600-nm region is caused by the presence of oxidized thionin in the sample.
Upon addition of NH2OH to the fully reduced HCP, an increase in the absorbance at 420 nm is observed, indicating that the FeS cluster in HCP is oxidized (Fig. 3). The coupling of hydroxylamine reduction with the oxidation of the Fe clusters present in HCP shows strong evidence that in fact HCP, and not some minor contaminate, is responsible for hydroxylamine reduction in vitro. The A420 values of NH2OH-oxidized and thionin-oxidized HCP are nearly identical, suggesting that the UV-visible-observable FeS cluster of HCP is fully oxidized upon the addition and subsequent reduction of NH2OH (6). The sample spectrum taken in the presence of thionin reflects full oxidation due to the appearance of the oxidized-thionin peak near the 600-nm region of the spectrum.
Effect of cyanide, carbon monoxide, and oxygen on the hydroxylamine reductase activity of HCP.
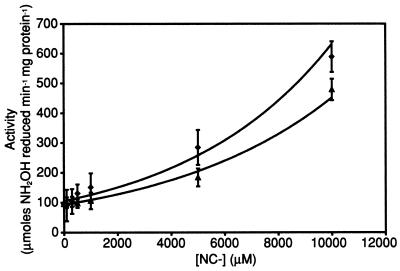
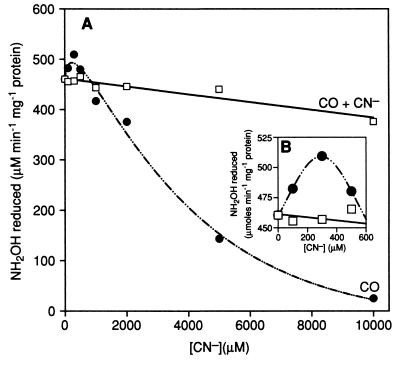
Figure 4 shows that cyanide (CN−) present in the assay solution stimulates the rate of NH2OH reduction by HCP at concentrations greater than 5 mM. However, at lower concentrations, less than 1 mM, the stimulation effect of CN− in solution is very minimal. Figure 4 also shows that the stimulatory effect of CN− is diminished when solutions are saturated with CO, whereas assay solutions containing only CO show no increase in activity compared to standard assay mixtures (data not shown).
FIG. 4.
Effect of hydroxylamine reductase activity of HCP in the presence of cyanide and CO. The assay of hydroxylamine reductase activity in the presence of effectors (CN− and CO) is described in Materials and Methods. Assays were performed in the presence of CN− only (⧫) and CO plus CN− (▴).
When HCP is incubated with various concentrations of CN− for 1 h before assaying, a slight stimulation of the rate of NH2OH reduction by HCP is observed at concentrations of CN− less than 500 μM (Fig. 5B). The maximum stimulation (approximately 10%) was observed at the concentration of 300 μM CN−. However, at CN− concentrations higher than 500 μM, the rate of NH2OH reduction is significantly inhibited (Fig. 5A). While carbon monoxide (CO) alone has no significant effect on the hydroxylamine reductase activity of HCP, CO appears to block both the CN−-dependent stimulation and inhibition effects on HCP (Fig. 5A). It is also important that, in samples that had been incubated with higher concentrations of CN− (>500 μM), activity could not be recovered either by removing CN− or by incubating with CO. Further, it is significant that assays carried out with CN− alone added as a possible substrate showed no activity, and therefore it is possible to rule out the theory that CN− might be a secondary substrate for this enzyme.
FIG. 5.
Effect of incubation with cyanide and CO on the hydroxylamine reductase activity of the HCP. The treatment of effectors CN− and CO is described in Materials and Methods. (A) Effect of CN− treatment both in the presence (□) and absence (•) of incubation with CO prior to CN− treatment. (B) Enhancement the region of panel A in which CN− activation occurs, in order to properly demonstrate the CN− activation effect on activity.
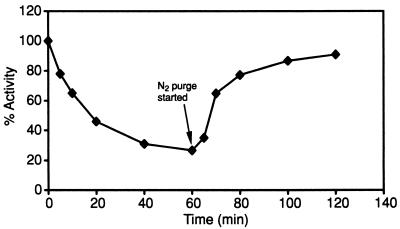
The effect of exposing HCP to O2 on hydroxylamine reductase activity can be seen in Fig. 6. After 5 min of exposure to air, the enzyme still retained ∼80% of its overall activity. However, this level decreased dramatically over time, to a final activity of only ∼30% of anaerobic activity 60 min after exposure to O2. Figure 6 also shows the effect on hydroxylamine reductase activity of removing the O2. As can be seen, by effectively making the air-exposed HCP sample anaerobic after O2 exposure, over 90% of activity can be recovered.
FIG. 6.
Effect of O2 exposure on hydroxylamine reductase activity of HCP. The exposure of HCP to O2 and subsequent assays for hydroxylamine reductase activity are described in Materials and Methods. Shown is the effect, over time, of exposure to O2 on the hydroxylamine reductase activity of HCP.
DISCUSSION
Novel hydroxylamine reductase activity of HCP.
Although the spectroscopic characteristics and crystal structure of this enzyme have been very well defined (2, 13), neither the enzymatic activity nor the physiological role of this enzyme has been discovered. While the fact that HCP was present in cells grown anaerobically in the presence of either nitrate or nitrite suggested that it was somehow involved in nitrogen metabolism (1, 5), no concrete evidence suggesting its specific role in this pathway has previously been reported.
This study provides evidence that HCP may function as a hydroxylamine reductase in vivo; however, other functions of HCP cannot be completely ruled out at this time. Optimal Vmax and Km values for the reduction of NH2OH by HCP are obtained at pH 9.0. The pH-dependent values of Vmax and Km for NH2OH reduction are consistent with NH2OH as the substrate for the enzyme (i.e., NH2OH pKa 6.03). These data also suggest that pH affects the reaction mechanism of the enzyme due to ionization of key residues.
In addition, HCP reduces NH2OH analogs CH3NHOH and hydroxyquinone (data not shown), as seen in H265V CODH (7). The rates of reduction of CH3NHOH and hydroxyquinone are nearly identical compared to those for H265V CODH. Further, the product (NH3) and product analog (NH2NH2) showed only minor effects on the hydroxylamine reductase activity of HCP (data not shown). Similar phenomena are also observed in H265V CODH (7). Based on these parallels between HCP and H265V CODH, it is reasonable to suggest that the modes of NH2OH reduction by HCP and H265V CODH are the same.
Effect of hydroxylamine on the redox state of UV-observable FeS clusters of HCP.
Upon the addition of NH2OH to the fully reduced HCP, the UV-visible-observable FeS clusters were oxidized. This result suggests that the reduction of NH2OH is coupled with the oxidation of the FeS clusters. It is therefore concluded that the FeS clusters of HCP are involved in the reduction of NH2OH.
Effect of cyanide and CO on the hydroxylamine reductase activity of HCP.
The activation of hydroxylamine reductase activity of HCP when CN− is present in the assay solution suggests that ligation of CN− to the active site, presumably the hybrid cluster, of the HCP provides a more favorable environment for the reduction of NH2OH. If this is the case, the coordinated low-molecular-weight entity on the hybrid cluster, inferred from the diffraction pattern (13), could in fact be CN−. The fact that CO prevents the effect of CN− (stimulation and inactivation) on the hydroxylamine reductase activity of HCP suggests that CO might coordinate to the same site to which CN− binds. CO slightly inhibits the hydroxylamine reductase activity of HCP. The same effect of CO is also observed in the hydroxylamine reductase activity of H265V CODH (7). These results suggest that the CO ligation on the active site is unfavorable for NH2OH reduction.
The inactivation in the prolonged presence of excess CN− could be a result of degradation of the metal cluster of HCP. This hypothesis is strengthened by the fact that activity could not be recovered upon the removal of CN− or upon incubation of CO. Degradation of metal clusters in the presence of excess CN− was observed in R. rubrum CODH (10).
In conclusion, results herein presented demonstrate a novel NH2OH reductase activity for the previously uncharacterized HCP. While a physiological role for this function cannot at this time be assured, it is possible to imagine the role of this enzyme as a scavenger of potentially toxic by-products of nitrate metabolism. If at some point in the metabolism of nitrate and nitrite small amounts of NH2OH are produced, it would be necessary to have present an enzyme that could quickly metabolize this compound before it could act on the cell. Therefore it is feasible that HCP could provide this detoxification, which would be necessary for organisms to safely carry out nitrate metabolism. These biochemical results not only provide an important insight into the activity of HCP but also strengthen the previously proposed relationship between HCP and CODH. While these studies demonstrate a catalytic function of HCP as an effective NH2OH reductase, other enzymatic roles for this enzyme cannot be dismissed at this time.
Acknowledgments
We thank Robert Kerby of the Department of Bacteriology for his work on preparing the transformed E. coli cells used in this study. We thank W. M. A. M. van Dongen at Wageningen University for supplying us with the overexpressing HCP plasmid utilized in these experiments and for helpful suggestions regarding the purification of the HCP.
Work described here was supported by grant DE-FG02-87ER13691 (DOE Basic Energy Sciences) to P.W.L.
REFERENCES
- 1.Burris, R. H., and G. P. Roberts. 1993. Biological nitrogen fixation. Annu. Rev. Nutr. 13:317-335. [DOI] [PubMed] [Google Scholar]
- 2.Cooper, S. J., C. D. Garner, W. R. Hagen, P. F. Lindley, and S. Bailey. 2000. Hybrid-cluster protein (HCP) from Desulfovibrio vulgaris (Hildenborough) at 1.6 Å resolution. Biochemistry 39:15044-15054. [DOI] [PubMed] [Google Scholar]
- 3.Ensign, S. A., D. Bonam, and P. W. Ludden. 1989. Nickel is required for the transfer of electrons from carbon monoxide to the iron-sulfur centers of carbon monoxide dehydrogenase from Rhodospirillum rubrum. Biochemistry 28:4968-4973. [DOI] [PubMed] [Google Scholar]
- 4.Ensign, S. A., and P. W. Ludden. 1991. Characterization of the CO oxidation/H2 evolution system of Rhodospirillum rubrum. Role of a 22-kDA iron-sulfur protein in mediating electron transfer between carbon monoxide dehydrogenase and hydrogenase. J. Biol. Chem. 266:18395-18403. [PubMed] [Google Scholar]
- 5.Halbleib, C. M., and P. W. Ludden. 2000. Regulation of biological nitrogen fixation. J. Nutr. 130:1081-1084. [DOI] [PubMed] [Google Scholar]
- 6.Heo, J., C. R. Staples, and P. W. Ludden. 2001. Redox dependence of CO2 reduction to CO by the [FeNi] cluster of CO-dehydrogenase from Rhodospirillum rubrum. Biochemistry 40:7604-7611. [DOI] [PubMed] [Google Scholar]
- 7.Heo, J., M. T. Wolfe, C. R. Staples, and P. W. Ludden. 2002. Converting the NiFeS carbon monoxide dehydrogenase to a hydrogenase and a hydroxylamine reductase. J. Bacteriol. 184:5894-5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korpela, T. K., and M. J. Makela. 1981. Spectrophotometric measurement of hydroxylamine and its O-alkyl derivatives. Anal. Biochem. 110:251-257. [DOI] [PubMed] [Google Scholar]
- 9.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 10.Staples, C. R., J. Heo, N. J. Spangler, R. L. Kerby, G. P. Roberts, and P. W. Ludden. 1999. Rhodospirillum rubrum CO-dehydrogenase. Part 1. Spectroscopic studies of CODH variant C531A indicate the presence of a binuclear [FeNi] cluster. J. Am. Chem. Soc. 121:11034-11044. [Google Scholar]
- 11.Stokkermans, J. P. W. G., W. A. M. van der Berg, W. M. A. M. van Dongen, and C. Veeger. 1992. The primary structure of a protein containing a putative [6Fe-6S] prismane cluster from Desulfovibrio desulfuricans. Biochim. Biophys. Acta 1132:83-87. [DOI] [PubMed] [Google Scholar]
- 12.Umbreit, W. W., R. H. Burris, and J. F. Stauffer. 1972. Manometric and biochemical techniques, 5th ed. Burgess Publishing Company, Minneapolis, Minn.
- 13.van der Berg, W. A. M., W. R. Hagen, and W. M. A. M. van Dongen. 2000. The hybrid-cluster protein (′prismane protein') from Escherichia coli: characterization of the hybrid-cluster protein, redox properties of the [2Fe-2S] and [4Fe-2S-2O] clusters and identification of an associated NADPH oxidoreductase containing FAD and [2Fe-2S]. Eur. J. Biochem. 267:666-676. [DOI] [PubMed] [Google Scholar]