Abstract
An ATP-dependent glucokinase of the hyperthermophilic aerobic crenarchaeon Aeropyrum pernix was purified 230-fold to homogeneity. The enzyme is a monomeric protein with an apparent molecular mass of about 36 kDa. The apparent Km values for ATP and glucose (at 90°C and pH 6.2) were 0.42 and 0.044 mM, respectively; the apparent Vmax was about 35 U/mg. The enzyme was specific for ATP as a phosphoryl donor, but showed a broad spectrum for phosphoryl acceptors: in addition to glucose, which showed the highest catalytic efficiency (kcat/Km), the enzyme also phosphorylates glucosamin, fructose, mannose, and 2-deoxyglucose. Divalent cations were required for maximal activity: Mg2+, which was most effective, could partially be replaced with Co2+, Mn2+, and Ni2+. The enzyme had a temperature optimum of at least 100°C and showed significant thermostability up to 100°C. The coding function of open reading frame (ORF) APE2091 (Y. Kawarabayasi, Y. Hino, H. Horikawa, S. Yamazaki, Y. Haikawa, K. Jin-no, M. Takahashi, M. Sekine, S. Baba, A. Ankai, H. Kosugi, A. Hosoyama, S. Fukui, Y. Nagai, K. Nishijima, H. Nakazawa, M. Takamiya, S. Masuda, T. Funahashi, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, and H. Kikuchi, DNA Res. 6:83-101, 145-152, 1999), previously annotated as gene glk, coding for ATP-glucokinase of A. pernix, was proved by functional expression in Escherichia coli. The purified recombinant ATP-dependent glucokinase showed a 5-kDa higher molecular mass on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, but almost identical kinetic and thermostability properties in comparison to the native enzyme purified from A. pernix. N-terminal amino acid sequence of the native enzyme revealed that the translation start codon is a GTG 171 bp downstream of the annotated start codon of ORF APE2091. The amino acid sequence deduced from the truncated ORF APE2091 revealed sequence similarity to members of the ROK family, which comprise bacterial sugar kinases and transcriptional repressors. This is the first report of the characterization of an ATP-dependent glucokinase from the domain of Archaea, which differs from its bacterial counterparts by its monomeric structure and its broad specificity for hexoses.
Hyperthermophilic prokaryotes with an optimal growth temperature higher than 80°C are considered to represent the phylogenetically most ancestral organisms (52-54). Various hyperthermophiles, including the euryarchaota Pyrococcus, Thermococcus, and Archaeoglobus; the crenarchaeota Thermoproteus, Desulfurococcus, and Sulfolobus; and the bacterium Thermotoga, have been shown to grow on glucose or glucose polymers (maltose and cellobiose starch) (12, 29, 44, 52). Comparative analyses of the sugar degradation pathways in hyperthermophiles indicate that the classical Embden-Meyerhof (EM) or Entner-Doudoroff (ED) pathway is operative only in the bacterium Thermotoga. In contrast, all archaea use modified versions of these pathways. With exception of the aerobic Sulfolobus, which uses a nonphosphorylated version of ED pathways (11, 46), all other anaerobic species degrade sugars predominantly via modified EM pathways. These pathways differ from the classical EM pathway by the presence of several unusual enzymes, such as ADP-dependent glucokinase (ADP-GLK), ADP-dependent 6-phosphofructokinase, nonregulatory ATP- and PPi-dependent 6-phosphofructokinases, glyceraldehyde-3-phosphate:ferredoxin oxidoreductase, nonphosphorylating NAD+-reducing glyceraldehyde-3-phosphate dehydrogenase, a novel type of glucose-6-phosphate (G6P) isomerase, and an archaeal class I type fructose 1,6,bisphosphate aldolase (for literature, see references 15-17, 24, 29, 39, 44, 46, and 47). Recently, all enzymes of a modified EM pathway, including ATP-dependent glucokinase, nonregulatory ATP-dependent 6-phosphofructokinase of the PFK-B family, and nonphosphorylative glyceraldehyde-3-phosphate dehydrogenase, were also detected in cell extracts of the aerobic crenarchaeon Aeropyrum pernix (P. Schönheit, unpublished results).
The first step of the modified EM pathways in Archaea, the phosphorylation of glucose to G6P, is catalyzed either by ADP-dependent or ATP-dependent kinases.
ADP-GLKs were only found in the euryarchaeotal branch of the archaea in the order Thermococcales, Pyrococcus furiousus, Thermococcus celer, and Thermococcus litoralis; in the hyperthermophilic sulfate reducer Archaeoglobus fulgidus strain 7324; and, recently, in the methanogenic archaeon Methanococcus jannaschii (25, 27, 29, 42). In the latter organism, the enzyme is bifunctional, showing both ADP-GLK and ADP-phosphofructokinase activities. The ADP-GLKs from P. furiosus, T. litoralis, and M. jannaschii and their encoding genes have been characterized. These extremely thermophilic GLKs represent homodimeric (P. furiosus and M. jannaschii) or monomeric (T. litoralis) proteins composed of subunits of about 50 kDa. Comparison of the primary structures indicates that ADP-GLKs from Pyrococcus, Thermococcus, and Methanococcus belong to a novel family of sugar kinases (27, 38, 42, 57, 59). Recently, the crystal structure of ADP-GLK from T. litoralis was solved, indicating some structural similarity to members of the PFK-B sugar kinase family (20).
Besides the euryarchaeotal ADP-GLKs, the crenarchaeota Thermoproteus tenax, Desulfurococcus amylolyticus, and A. pernix have been shown to contain ATP-dependent GLKs (ATP-GLKs) as the first enzymes of their modified EM pathways (46, 48). None of these archaeal ATP-GLKs have been purified and characterized. Several ATP-dependent glucose-phosphorylating enzymes from bacteria and eukarya were characterized in detail (for example, see references 7 and 9). ATP-GLKs from bacteria are usually dimers and show a high specificity for glucose. Sequence comparison of the primary structure group them either into the ROK family, comprising repressors, open reading frames (ORFs) of unknown function, and sugar kinases (56), or into the glucose kinase family, including, e.g., the GLKs from Escherichia coli, Brucella abortus, and Zymomonas mobilis (4). ATP-dependent glucose-phosphorylating enzymes from Eukarya belong to the hexokinase family, characterized by its specific conserved signature pattern (19). Generally, these enzymes show a broad specificity for hexoses: in addition to glucose, fructose, mannose, and galactose were effectively phosphorylated (for review, see reference 9). It has been postulated that the present hexokinases from Eukarya and the specific bacterial GLKs have evolved from a common ancestral hexokinase (9).
Here we report the purification, characterization, and expression of the encoding gene of the first archaeal ATP-GLK, from the hyperthermophilic crenarchaeon Aeropyrum pernix. The extremely thermophilic enzyme represents the first archaeal member of the ROK family of sugar kinases, which differs from bacterial GLKs by its monomeric structure and its broad specificity for hexoses.
MATERIALS AND METHODS
Sources of materials.
All commercially available chemicals used were of reagent grade and were obtained from Merck (Darmstadt, Germany), Fluka (Buchs, Switzerland), or Sigma (Deisenhofen, Germany). Yeast extract and peptone were obtained from Difco (Stuttgart, Germany), and enzymes, T4 DNA ligase, and coenzymes were obtained from Roche Diagnostics (Mannheim, Germany), New England Biolabs (Beverly Mass.), and PEQLAB (Erlangen, Germany). Substrates were obtained from Roche Diagnostics, Merck, and Sigma. Gases were obtained from Linde (Hamburg, Germany). A. pernix K1 (DSM 11879) was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). Linearized pBAD vector activated with topoisomerase I and the E. coli BL21(DH10B) expression host strain were purchased from Invitrogen (Groningen, The Netherlands). Genomic DNA, plasmid DNA, and DNA in gels were purified with kits from Qiagen (Hilden, Germany) and PEQLAB. All fast protein liquid chromatography (FPLC) material and columns (DEAE-Sepharose, ATP-agarose, and the Superdex 200 HiLoad gel filtration column) used were obtained from Pharmacia (Freiburg, Germany), Sigma, and Bio-Rad (Munich, Germany).
Growth of the organism.
A. pernix K1 (DSM 11879) was grown aerobically at 90°C in a 100-liter Biostat fermentor on a complex medium as described previously (41), except that artificial seawater was used instead of Biomaris water, and 1 g of starch was added per liter. Cells were grown and harvested (after 17 h) at the late-exponential growth phase. About 70 to 80 g (wet weight) was obtained from the 100-liter Biostat fermentor.
Preparation of cell extracts and purification of GLK.
Since the enzyme was not sensitive to oxygen, all steps of the purification procedure were carried out under oxic conditions at 4°C. Cell extracts were prepared from 214 g (wet weight) of frozen cells, which were suspended in 100 mM Tris-HCl (pH 7.5) containing 2 mM dithioerythritol (DTE) (buffer A). Cells were disrupted by passing through a French pressure cell at 1.3 × 108 Pa. Cell debris and unbroken cells were removed by centrifugation for 90 min at 100,000 × g at 4°C. The 100,000 × g supernatant was applied at a flow rate of 3 ml/min to DEAE-Sepharose (100 ml) that had been equilibrated with buffer A. All GLK activity was found in the flowthrough fraction, which was adjusted to pH 7.0 and applied to an ATP-agarose column (5 by 1 cm) at a flow rate of 1 ml/min equilibrated with buffer B (50 mM Tris-HCl [pH 7.0], 2 mM DTE). After the column had been washed with 20 ml of buffer B, protein was desorbed at a flow rate of 0.2 ml/min with linear gradients of both glucose and ADP (at equimolar concentrations) in buffer B: 0 to 5 (10 ml), 5 to 20 (20 ml), and 20 to 50 (8 ml) mM. GLK activity was recovered from 8 to 35 mM glucose-ADP; activities obtained from 12 to 18 mM glucose-ADP (8 ml) were essentially pure. The eluate was dialyzed with buffer B and stored at −20°C. Under these conditions, the activity remained about constant.
Analytical assays.
The purity of the preparations was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 14% polyacrylamide gels followed by staining with Coomassie brilliant blue R-250 according to standard procedures (30). Protein concentrations were determined by the method of Bradford (8) with bovine serum albumin as a standard. Gel filtration chromatography was carried out at ambient temperature with Bio-Prep SE1000/17 and Bio-Silect SEC 250-5 (with 50 mM Tris-HCl and 150 mM NaCl [pH 7.0] at 0.5 ml/min).
Enzyme assays and determination of kinetic parameters.
Since the enzyme activity was not sensitive to oxygen, all assays were performed under oxic conditions. The ATP-GLK activity (glucose + ATP → G6P + ADP) was measured by coupling the ATP-dependent formation of G6P to the reduction of NADP+ via G6P dehydrogenase (GPD) from yeast (maximum operating temperature, ∼50 to 55°C). GLK activity was measured at 40 to 90°C in discontinuous assays. In all assays, initial velocities were investigated in five to six parallel assays stopped at different time intervals. The velocity remained linear for a ΔA at 365 nm of 1.0 to 1.5 (except for those at low substrate concentrations) at all temperatures tested, indicating that the enzyme does not undergo thermal inactivation during the assay. The standard assay mixture (500 μl) contained 100 mM Tris-HCl (pH 6.2, 90°C), 5 mM glucose, 2 mM ATP, and 4 mM MgCl. (The ATP/Mg ratio was kept at the optimized ratio 1:2 for all kinetic measurements.) After preincubation at 90°C, the reaction was started with an aliquot of GLK (1 μg of enzyme). This mixture was then incubated at 0.25 to 20 min, and the reaction was stopped by rapid addition of EDTA to a final concentration of 10 mM and cooling on ice for 2 min. The G6P concentration was quantified by addition of 0.6 mM NADP+ and 0.5 U of GPD in 100 mM Tris (pH 7.5) to a final volume of 1 ml and incubation at 40°C. The reduction of NADP+ at 365 nm was detected exactly 5 min later. This assay was used as the standard test system for all enzymatic and kinetic measurements, except as otherwise stated. Alternatively, as a control of the above test system, GLK activity was also detetected by coupling the ADP formation from ATP to the oxidation of NADH via pyruvate kinase (PK) and lactate dehydrogenase (LDH). After incubation, the reaction was stopped by rapid cooling on ice for 2 min. Ice-cold detection solution (0.6 mM NADH, 5 mM phosphoenolpyruvate, 0.5 U of PK, 1 U of LDH in 100 mM Tris [pH 7.0]) was added to a final volume of 1 ml, and the oxidation of NADH at 365 nm was measured exactly 5 min later. With both assay systems, almost identical results were obtained. The latter system was used for the determination of the substrate specificity for sugars. The auxiliary enzymes in all assays were routinely tested to ensure that they were not rate limiting.
pH dependence, substrate specificity, cation specificity, and effectors.
The pH dependence of the enzyme was measured between 4.0 and 9.0 at 90°C with either piperazine, phosphate, morpholineethanesulfonic acid (MES), Tris-HCl, triethanolamine, or ethanolamine at a concentration of 100 mM each. The cation and nucleotide specificities were examined by using the standard discontinuous test system at 90°C by exchanging either Mg2+ (4 mM) for alternative divalent cations (Ni2+, Mn2+, Co2+, Ca2+, Zn2+, and Fe2+) or ATP (2 mM) alternative phosphoryl donors (ITP, GTP, UTP, CTP, UDP, GDP, ADP, CDP, acetyl phosphate, and PPi) at equivalent concentrations. For the test of substrate specificity for sugars, glucose was exchanged for adenosine, 2-deoxyglucose, fructose, fructose-1-phosphate, fructose-6-phosphate, galactose, G6P, glucosamine, N-acetylglucosamine, mannose, N-acetylmannosamine, ribose, and xylose.
Temperature dependence and thermal stability.
The temperature dependence of enzyme was measured between 40 and 99°C in 100 mM Tris-HCl or 100 mM triethanolamine (pH 6.2) (at the respective temperature). The activity was measured in the direction of G6P formation by using 1 μg of GLK as well as standard concentrations of G6P (5 mM), ATP (2 mM), and MgCl2 (4 mM), which ensured specific activities close to the Vmax. The thermostability of the purified enzyme (1 μg in 40 μl of 50 mM phosphate buffer [pH 7.0]) was tested in sealed vials, which were incubated at temperatures of between 70 and 100°C for 2 to 120 min. The vials were then cooled on ice for 10 min, and the remaining enzyme activity was tested at 90°C and compared to that of the controls (unheated sample).
Cloning of the gene encoding ATP-GLK from A. pernix.
In the complete sequenced genome of Aeropyrum, ORF APE2091 has previously been annotated as putative GLK gene (22). To prove its coding function, the ORF APE2091 was cloned and functionally expressed in Escherichia coli as follows. The coding region of the GLK was amplified from genomic DNA of A. pernix K1 by PCR with Pwo polymerase with Q-solution (Qiagen) added. The following primers were used: 5′CCTAACATGCATAGGAGCCCATTTAACAC3′ (forward APE2091) and 5′CTACTCCTA GGCTGCTAGAAGATTGGGA3′ (reverse APE2091). For the addition of 5′ T overhangs, the PCR product, designated glk, was incubated with Taq polymerase for 5 min at 72°C and then cloned into pBAD via a linearized vector activated with topoisomerase I. The vector pBAD-glk contained an additional 16-amino-acid N-terminal leader sequence (MGSGSGNNNNKLALPN), which, according to the manufacturer, increases the solubility of recombinant proteins in E. coli.
Transformation.
The vector pBAD-glk was transformed into E. coli BL21(DH10B) cells. The inserted gene sequence and the orientation were confirmed by sequencing each strand by standard methods (43).
Functional expression of the glk gene in E. coli and purification of recombinant A. pernix glucokinase.
Transformed E. coli BL21(DH10B) cells were grown in 400 ml of Luria-Bertani medium containing 100 μg of carbenicillin per ml at 37°C to an optical density at 600 nm of 0.8, and expression of the glk gene was initiated by induction of the araC promoter following the addition of 0.2% l-arabinose. After 4 h of further growth, the cells were harvested by centrifugation at 4°C and washed in 50 mM Tris-HCl (pH 7.0) containing 50 mM NaCl. The pellet was frozen at −20°C. Cell extracts were prepared by French press treatment of cell suspensions in buffer A. After centrifugation (100,000 × g for 60 min), the solution was heat precipitated at 80°C for 45 min and centrifuged again. A homogeneous enzyme preparation was achieved by extensive dialysis against buffer A and chromatography on DEAE-Sepharose (as described above for the purification of native enzyme) as well as an additional heat treatment at 90°C for 30 min. After centrifugation (5,000 × g for 15 min), the supernatant was concentrated by ultrafiltration (YM 10 membrane; Millipore, Eschborn), and protein was applied to Superdex 200 HiLoad gel filtration column equilibrated with 150 mM NaCl in buffer B. Protein was eluted at a flow rate of 1 ml/min. The eluate was stored at −20°C. Under these conditions, activity remained about constant.
Determination of N-terminal amino acid sequence.
N-terminal amino acid sequence determination was performed as previously described (15).
Sequence handling.
Following verification of APE2091 as a GLK, the deduced amino acid sequences of APE2091 were used in a BLASTP search (1, 2) to identify additional putative archaeal GLKs. Additional sequences of the GLK were retrieved from the Pfam database (4). Sequence alignments and phylogenetic trees were constructed by the neighbor-joining method of Clustal X (55) with default parameters. Confidence limits were estimated by 1,000 bootstrapping replicates.
RESULTS
Purification of ATP-GLK from A. pernix.
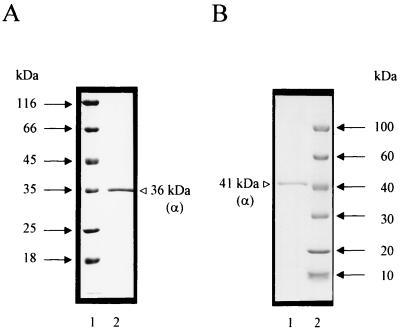
ATP-GLK was purified aerobically from cell extracts of A. pernix by only two purification steps involving anion-exchange chromatography on DEAE-Sepharose and affinity chromatography on ATP-agarose. By this procedure, the enzyme was purified about 230-fold to a specific activity of 35 U/mg (at 90°C) with a yield of 12%. (Table 1). The purified protein was electrophoretically homogeneous, as judged by denaturing SDS-PAGE (Fig. 1A). Thus, ATP-GLK represents about 0.4% of the soluble protein of A. pernix.
TABLE 1.
Purification of ATP-GLK from A. pernix
| Purification step | Amt of protein (mg) | Activity (U)a | Sp act (U/mg) | Yield (%) | Fold purification |
|---|---|---|---|---|---|
| Cell extract | 750 | 112 | 0.15 | 100 | 1 |
| DEAE-Sepharose | 100 | 60 | 0.6 | 53.6 | 4 |
| ATP-agarose | 0.37 | 13 | 35 | 11.6 | 233 |
Enzyme activity was measured at 90°C in the direction of G6P formation in a discontinuous assay with GPD as described in Material and Methods (1 U = 1 μmol of NADP+ reduced/min).
FIG. 1.
Purified ATP-GLK from A. pernix (A) and recombinant ATP-GLK from transformed E. coli (B) as analyzed by SDS-PAGE (30). Protein was denatured in SDS and separated in 12% polyacrylamide slab gels (8 by 7 cm), which were stained with Coomassie brilliant blue R-250. (A) Lane 1, molecular mass standards; lane 2, native enzyme purified from A. pernix. (B) Lane 1, recombinant enzyme purified from E. coli (see Materials and Methods); lane 2, molecular mass standards.
Molecular and catalytic properties.
The apparent molecular mass of native ATP-GLK was determined by gel filtration and was 33 ± 3 kDa on Bio-Prep SE1000/17 (semipreparative grade; Bio-Rad) and 30 ± 3 kDa on Bio-Silect SEC 250-5 (analytical grade; Bio-Rad). SDS-PAGE revealed only one subunit with an apparent molecular mass of 36 kDa (Fig. 1A), indicating that ATP-GLK is a monomeric protein.
Kinetic constants of purified ATP-GLK were determined at 90°C. The rate dependence on glucose and ATP followed Michaelis-Menten kinetics, with apparent Km values of 0.044 mM and 0.42 mM, respectively; apparent Vmaxs were about 35 U/mg. The pH optimum of ATP-GLK measured at 90°C was 6.2; 50% of activity was found at pHs of 5.8 and 7.9. The classical effectors of eukaryotic hexokinases, G6P and phosphate, were each tested at concentrations of 0.1, 1, and 10 mM. In addition, several substances were tested for potential effect on the catalytic activity: ADP, AMP, fructose-1,6-bisphosphate, phosphoenolpyruvate, citrate (10 mM, 1 mM, and 0.1 mM each) and NAD+, NADP+, pyruvate, oxalacetate, and glyceraldehyde-3-phosphate (1 mM each). None of the substances mentioned above showed any specific or regulatory effect on ATP-GLK activity.
Substrate specificities.
Besides ATP, ITP served as an effective phosphoryl donor for G6P phosphorylation. GTP and UTP were less effective, and ADP, GDP, UDP, and acetyl phosphate were not used (<1%) (Table 2). Thus, the enzyme is defined as ATP-GLK. The enzyme showed a broad spectrum for hexoses—in addition to glucose, which showed the highest catalytic efficiency (kcat/Km). The enzyme also phosphorylates fructose, mannose, glucosamine, N-acetylglucosamine, N-acetylmannosamine, and 2-deoxyglucose at significant rates (Table 3). Other sugars, such as adenosine, fructose-1-phosphate, fructose-6-phosphate, galactose, G6P, ribose, and xylose, were not phosphorylated. Divalent cations were required for maximal activity; Mg2+, which was most effective, could partially be replaced with Co2+, Mn2+, Ni2+, and Cu2+ (Table 2).
TABLE 2.
Biochemical and kinetic properties of ATP-GLK from A. pernixa
| Parameter | Result for enzyme from:
|
|
|---|---|---|
| A. pernix | E. coli (recombinant) APE2091 | |
| Apparent mol mass of enzyme (kDa) | ||
| Native | 30-33 | 30-33 |
| Subunit | 36 | 41 |
| Calculated | 33.843 | 42.119b |
| Oligomeric structure | α | α |
| pH optimum | 6.2 | 6.2 |
| Temp optimum (°C) | >100 | >100 |
| Arrhenius activation energy (kJ mol−1) | 66 | NDd |
| Apparent Km (mM) with ATP | 0.42 | 0.47 |
| Phosphoryl donor specificity (%)c | ||
| ATP | 100 | 100 |
| ITP | 40 | 41 |
| GTP | 9 | 7 |
| UTP | 3 | ND |
| CTP | 0 | ND |
| ADP | 0 | 0 |
| GDP | 0 | 0 |
| UDP | 0 | 0 |
| Acetyl phosphate | 0 | 0 |
| PPi | 1 | 1 |
| Cation specificity (% at 90°C)c | ||
| Mg2+ | 100 | ND |
| Co2+ | 83 | ND |
| Mn2+ | 64 | ND |
| Ni2+ | 23 | ND |
| Cu2+ | 6 | ND |
The molecular mass of native enzyme was determined by gel filtration of subunits by SDS-PAGE Enzyme activity was measured at 90°C in the direction of G6P formation in a discontinuous assay with GPD.
This value includes the 16 amino acids of the leader peptide.
The direction of G6P formation is represented as the percentage of Vmax, where 100% = 35 U/mg. Each value represents the specific substrate shown.
ND, not determined.
TABLE 3.
Substrate specificity of the GIK from A. pernix with alternate substratesa
| Substrate | Apparent Vmax (U/mg) | Apparent Km (μM) | kcat (s−1) | kcat/Km (μM−1 s−1) |
|---|---|---|---|---|
| Glucose | 35 | 44 | 23 | 0.53 |
| 2-Deoxyglucose | 45 | 330 | 30 | 0.09 |
| Glucosamine | 27 | 60 | 18 | 0.30 |
| Fructose | 15 | 150 | 10 | 0.07 |
| Mannose | 19 | 110 | 13 | 0.12 |
| N-Acetylglucosamine | 2 | ND | 1 | |
| N-Acetylmannosamine | 1.5 | ND | 0.8 |
ATP (2 mM) was used as a phosphoryl donor. Enzyme activity was measured at 90°C in a discontinuous assay with LDH and PK as described in Materials and Methods.
Temperature optimum and stability.
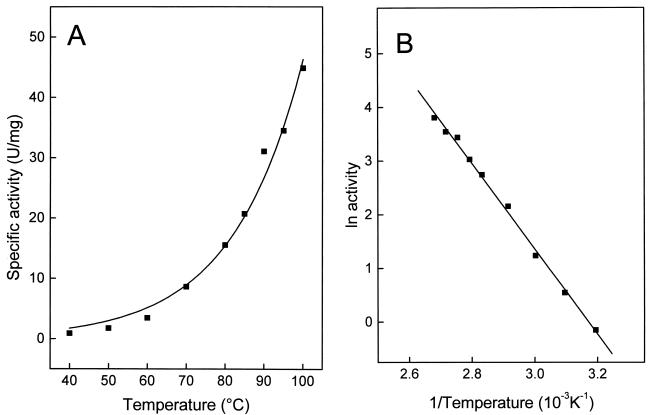
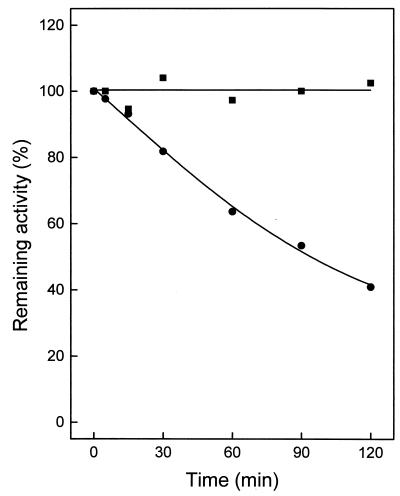
The temperature dependence of ATP-GLK is shown in Fig. 2A and B. At 40°C, the enzyme showed little activity, which, however, increased exponentially up to 100°C, the highest temperature tested. The Arrhenius plot was linear over the whole temperature range (up to 100°C), with a calculated activation energy of 66 kJ/mol. The temperature stability of ATP-GLK was tested between 95 and 100°C in 50 mM sodium phosphate buffer by incubating the enzyme for up to 120 min. The enzyme showed an extremely high stability against thermal inactivation (Fig. 3). After 120 min of incubation, the enzyme did not lose activity at 95°C, but it did lose about 60% of its activity at 100°C.
FIG. 2.
Effect of temperature on the specific activity of the ATP-GLK from A. pernix. (A) Temperature dependence of specific activity. (B) Arrhenius plot of the same data from panel A. The assay mixture contained 1 μg of enzyme purified from A. pernix, 100 mM triethanolamine or Tris-HCl (pH 6.2) at the indicated temperature, 2 mM ATP, 4 mM MgCl2, and 5 mM glucose.
FIG. 3.
Thermostability of ATP-GLK from A. pernix. Enzyme (1 μg purified from A. pernix) was incubated in 40 μl of 50 mM potassium phosphate buffer (pH 7.0) at 95°C (▪) and at 100°C (•). At the times indicated, samples were cooled on ice for 10 min and assayed for the remaining activity at 90°C in the direction of G6P formation. One hundred percent activity corresponded to a specific activity of 35 U/mg.
Cloning and expression of ORF APE2091 in E. coli and characterization of the recombinant protein.
In the genome of A. pernix, the ORF APE2091 was annotated as a putative glk gene encoding ATP-GLK (22). The ORF starts with a GTG and contains 1,131 bp coding for a polypeptide of 376 amino acids with a calculated molecular mass of 40.549 kDa. To prove the coding function of ORF APE2091, ATP-GLK was expressed in E. coli. The ORF was amplified by PCR, cloned into vector pBAD-glk, and transformed into E. coli BL21(DH10B). After induction with 0.2% l-arabinose, a polypeptide of 41 kDa was expressed (Fig. 1B) that showed thermoactive ATP-GLK activity. The recombinant ATP-GLK was purified from transformed E. coli about 400-fold by heat treatments, anion-exchange chromatography on DEAE-Sepharose, and gel filtration on Superdex 200. As shown in Table 2, ATP-GLK purified from A. pernix and recombinant protein from transformed E. coli showed almost identical kinetic and thermostability properties, including the unusual broad specificity for hexoses.
ORF APE2091 codes for ATP-GLK and an additional 57-amino-acid unstructured N-terminal extension.
The ORF APE2091-encoded recombinant protein, showing ATP-GLK activity, was about 5 kDa larger (on SDS-PAGE) than the native ATP-GLK isolated from A. pernix, suggesting that the ORF codes for a larger protein. Comparison of the deduced amino acid sequence with hypothetical homologous proteins from the closely related crenarchaeon Pyrobaculum aerophilum and other archaea (Fig. 4) (see Discussion) revealed that the A. pernix sequence contained an additional 57-amino-acid N-terminal extension. Assuming a size of the A. pernix enzyme similar to those of the hypothetical archaeal homologues, an alternative start codon (APE2091; 171 to 173 bp) could be used. To clarify this question, we determined the N-terminal amino acid sequence of the native ATP-GLK purified from A. pernix. In contrast to the annotation of ORF APE2091, the N-terminal amino acid sequence was VAEVVAVDVGA. Therefore, the coding gene of A. pernix glk comprises 963 bp, encoding a protein of 320 amino acids. However, the recombinant protein contains ATP-GLK and an additional 57-amino-acid N-terminal extension (plus a 16-amino-acid leader sequence), explaining the difference in apparent molecular mass on denaturing SDS-PAGE compared to the native ATP-GLK. However, gel filtration on Bio-Prep SE1000/17 and on Bio-Silect SEC 250-5 revealed almost identical molecular masses for both the native and the recombinant enzymes (30 to 33 kDa). This indicates that the additional 73-amino-acid extension (57-amino-acid extension plus 16-amino-acid leader sequence) obviously is unstructured and does not affect the globular size of the recombinant protein. Also, as shown in the previous paragraph, the N-terminal extension did not affect the kinetic and thermostability properties of the ATP-GLK.
FIG.4.
Multiple sequence alignment generated by Clustal X (55) of deduced amino acid sequences of ATP-GLK from A. pernix and putative archaeal GLKs with selected members of the ROK family, which comprises sugar kinases and repressors (6, 56). Deduced amino acid sequences are shown for proteins from the following organisms (accession numbers given in parentheses): GLK-Ap, GLK from Aeropyrum pernix, with the N-terminal extension of ORF APE2091 given in italic letters and brackets and with the N terminus according to N-terminal sequencing underlined; GLK-Pa, putative GLK from Pyrobaculum aerophilum (PAE3437); GLK-H, putative GLK from Halobacterium sp. strain NRC1 (Q9HMA7); GLK-Tv, putative GLK from Thermoplasma volcanicum (Q97AS0); GLK-Ta, putative GLK from Thermoplasma acidophilum (Q9HJY6); GLK-Bs, GLK from Bacillus subtilis (P54495); GLK-Sc, GLK from Streptomyces coelicolor (P40184); GLK-Rs, GLK from Renibacterium salmoninarum (Q53165); YHCI-Ec, product of E. coli yhcI (P45425); NAMK-h, N-acetylmannosamine kinase domain of the bifunctional enzyme UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (residues 413 to 722) (Q9Y223); ALSK-Ec, allokinase from E. coli (P32718); SCRK-Sm, fructokinase from Streptococcus mutans (Q07211); XYLR-Cs, xylose repressor from Caldicellulosiruptor sp. (P40981); XYLR-Sx, xylose repressor from Staphylococcus xylosus (P27159); and NAGC-Ec, N-acetylglucosamine repressor from E. coli (P15301). The helix-turn-helix-motif found in the repressors (56) is marked in boldface; the ATP-binding (3, 14) site found in the kinases is shaded gray; and the two consensus sequences proposed, [LIVM]-x(2)-G-[LIVMFCT]-G-X-[GA]-X-G-X(3-5)-[GATP]-X(2)-G-[RKH] (19) and C-X-C-G-X(2)-G-X-[WILV]-E-X-[YFVIN]-X-[STAG] (10), are shaded dark gray.
DISCUSSION
In this communication, we describe the purification and characterization of the first archaeal ATP-GLK from the crenarchaeon A. pernix. We examined both the native enzyme purified from A. pernix and the recombinant enzyme isolated from transformed E. coli after expression of ORF APE2091, which has been annotated as a putative glk gene. However, by using the N-terminal amino acid sequence of the native enzyme for A. pernix, it was demonstrated that the assumed translation start codon of ORF APE2091, GTG, was incorrect. Instead, a GTG 171 bp downstream was identified as the true translation start of the ATP-GLK. Thus, ORF APE2091 codes for a protein containing ATP-GLK and an additional unstructured 57-amino-acid terminal extension.
BLASTP searches of the nonredundant database with the amino acid sequence of the GLK from A. pernix revealed more than 60 hits (February 2002); almost all of them were members of the ROK family, including putative GLKs from archaea (PAU3437, VNG2629G, TA0825, and TV3591). The two best scores were obtained with the putative GLKs from P. aerophilum (PAU3437) and Halobacterium sp. (VNG2629G). The deduced amino acid sequence of ATP-GLK from A. pernix showed moderate degrees of similarity (24 to 38%) and identity (15 to 24%) to members of the ROK family, which comprise some bacterial transcriptional repressors (xylose repressor [XYLR] and N-acetylglucosamine repressors [NAGC]), some yet-uncharacterized ORFs, and several sugar kinases (GLKs, some fructokinases [SCRK], the yhcI gene products of E. coli and Haemophilus influenzae, allokinase from E. coli, and the N-acetylmannosamine kinase domain of the bifunctional enzyme UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase [NAMK] found in mammals) (10, 56). A multiple sequence alignment of A. pernix ATP-GLK and putative archaeal GLKs with selected characterized proteins from the ROK family is given in Fig. 4. The ROK repressors contain an N-terminal extension of about 80 amino acid residues, which includes a conserved DNA-binding (helix-turn-helix) motif (56), whereas an ATP-binding site (3, 14) is conserved in the N-terminal region of the ROK kinases, including the sequences of the archaeal glucose kinases.
The domain common to all proteins of the ROK family comprises about 180 amino acid residues (5) (residues 26 to 209 of the A. pernix sequence). Probably due to the diverse functions of the ROK proteins, only a few residues are conserved. These are found in the glycine-rich consensus pattern in the central part. Two alternative patterns for the ROK family have been proposed: [LIVM]-x(2)-G-[LIVMFCT]-G-X-[GA]-X-G-X (3-5)-[GATP]-X(2)-G-[RKH] (19) and C-X-C-G-X(2)-G-X-[WILV]-E-X-[YFVIN]-X-[STAG] (10) (Fig. 4). The latter was claimed to be more specific. It allowed the detection of the first eukaryotic member of the ROK family as well as differentiation between members of the ROK family and the GLK family (GLKs from E coli, Zymomonas mobilis, and Brucella abortus [Swiss-Prot accession no. P46880, P21908, and Q59171, respectively]). The latter lack the second consensus pattern. With few deviations, both consensus sequence patterns are also present in the archaeal sequences.
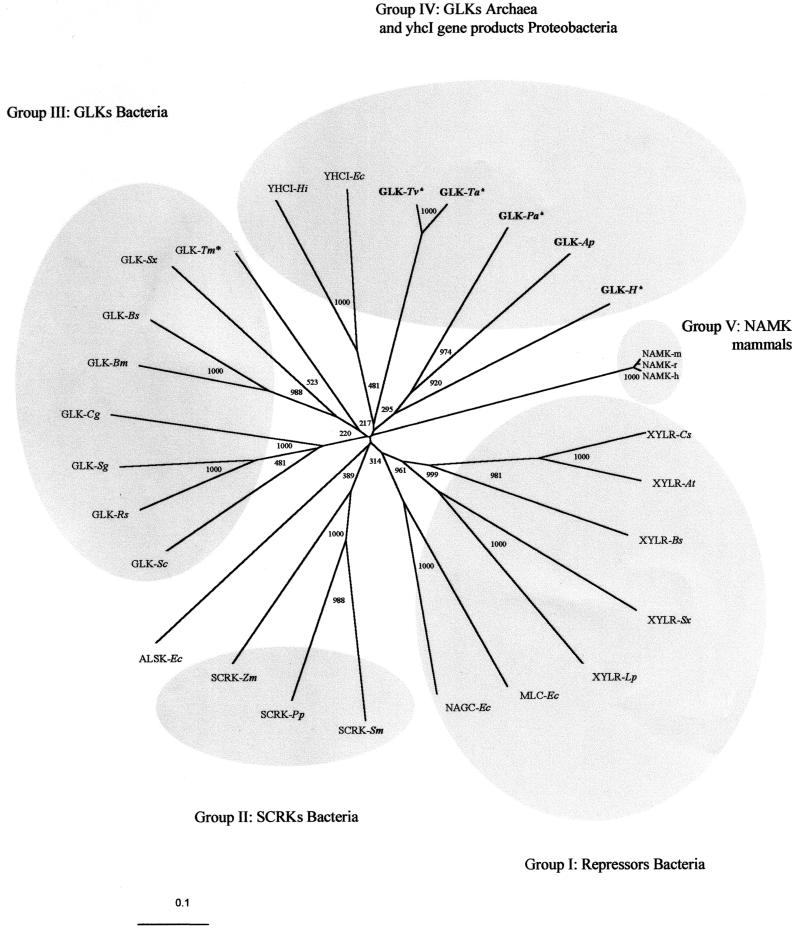
The GLK from A. pernix is the first characterized archaeal member of the ROK family, thus allowing phylogenetic studies. As demonstrated in the phylogram in Fig. 5, selected members of the ROK family including both functionally characterized proteins and hypothetical proteins clustered in at least five groups: (i) bacterial repressors, (ii) bacterial fructokinases, (iii) bacterial GLKs, (iv) archaeal GLKs, and (v) the yhcI gene products, representing the N-acetylmannosamine kinase domain found in mammals. The low bootstrap values of the basal nodes are probably due to the influence of both phylogenetic distance as well as functional evolutionary pressure. The bacterial ROK proteins cluster according to their function as glucokinase, fructokinase, and repressors: e.g., for Staphylococcus xylosus, Bacillus subtilis, and E. coli, two or more ROK homologues have been functionally described (26, 28, 32, 35, 49, 50, 60); and for the majority of the sequenced bacterial genomes, several ROK homologues have been annotated (Pfam database; http://www.sanger.ac.uk/cgi-bin/Pfam). These indicate gene duplication events of an ancestral ROK sequence followed by functional diversification in the early evolution of Bacteria. For the ROK repressors, gene duplication and domain shuffling have been proposed (56). The separate clustering of the archaeal GLK sequences might be due to both phylogenetic distance and/or difference in specificity. Interestingly, the yhcI gene product clusters with the archaeal sequences, which might be due to convergence. Genetic experiments suggest that the preferred substrate for YHCI is N-acetylmannosamine (35); however, the substrate specificity remains to be demonstrated.
FIG. 5.
Phylogenetic relationships among ROK proteins. The tree was generated by the neighbor-joining method of Clustal X (55). Bootstrap values are based on 1,000 replicates and are given at each node. Archaeal sequences are marked in boldface, and putative sequences are marked with an asterisk. Sequence accession numbers are shown in parentheses. Group I abbreviations: XYLRs, xylose repressors; Cs, Caldicellulosiruptor sp. (P40981); At, Anaerocellum thermophilum (Q44406); Bs, Bacillus subtilis (P16557); Sx, Staphylococcus xylosus (P27159); Lp, Lactobacillus pentosus (P21940); NAGC-Ec, N-acetylglucosamine repressor from E. coli (P15301); and MLC-Ec, product of E. coli mlc (P50456). Group II abbreviations: SCRKs, fructokinases; Sm, Streptococcus mutans (Q07211); Pp, Pediococcus pentosaceus (P43468); Zm; Zymomonas mobilis (Q03417); ALSK-Ec, allokinase from E. coli (P32718). Group III abbreviations: Sc, Streptomyces coelicolor (P40184); Rs, Renibacterium salmoni-narum (Q53165); Sg, Streptomyces griseus (Q9F1W1); Cg, Corynebacterium glutamicum (Q9KKE7); Bm, Bacillus megaterium (O31392); Bs, Bacillus subtilis (P54495); Sx, Staphylococcus xylosus (Q56198); Tm, putative Thermotoga maritima (Q9X1I0). Group IV abbreviations: YHCI, gene product of yhcI; Hi, Haemophilus influenzae (P44541); Ec, E. coli (P45425); Tv, putative Thermoplasma volcanicum (Q97AS0); Ta, putative Thermoplasma acidophilum (Q9HJY6); Pa, putative Pyrobaculum aerophilum (PAE3437); H, GLK-H, putative Halobacterium sp. strain NRC1 (Q9HMA7). Group V abbreviations: NAMK-h, N-acetylmannosamine kinase domain of the bifunctional enzyme UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (residues 413 to 722); h, human (Q9Y223); r, rat (O35826); m, mouse (Q9Z0P6).
The archaeal ATP-GLK from A. pernix differed from all of the characterized bacterial GLKs of the ROK family and the GLK family in various aspects. First, the A. pernix enzyme constitutes a monomer of 33 kDa, whereas all bacterial GLKs described so far have been dimeric proteins. Second, the archaeal enzyme showed broad specificity for hexoses. In addition to glucose, fructose, mannose, glucosamine, N-acetylglucosamine, and N-mannosamine were phosphorylated at significant rates.
A comparison of the substrate specificities of the A. pernix ATP-GLK with other selected bacterial ATP-GLKs and eukaryal hexokinases, as well as with the archaeal ADP-GLKs is given in Table 4. In contrast to the A. pernix enzyme, the bacterial ATP-GLKs from the ROK family (10, 36, 50) and the GLK family (E. coli and Z. mobilis) (32, 45) are highly specific for glucose as a phosphoryl acceptor. These enzymes do not phosphorylate other hexoses, such as fructose, mannose, and galactose, with significant catalytic efficiency. The broad specificity for hexoses of A. pernix GLK, in particular its ability to phosphorylate fructose and mannose in addition to glucose, indicates a functional similarity to most eukaryal hexokinases. The latter show a broad specificity for hexoses, catalyzing the ATP-dependent phosphorylation of various keto- and aldohexoses (for example, see references 31, 33, 37, and 51). The specificity of the other putative archaeal ROK GLKs remains to be described. However, due to the high sequence similarity to the A pernix enzyme, we expect a similar broad substrate specificity as well for these putative enzymes in particular, because only one ROK sequence is annotated for the genomes of Thermoplasma (23, 40), Halobacterium (34), and Pyrobaculum (13). The substrate specificities for the archaeal ADP-GLKs, which constitute a distinct GLK family (25, 42), were reported for the enzymes from P. furiosus, T. litoralis, and M. jannaschii (25, 27, 42) (Table 4). The Pyrococcus enzyme, when tested at 50°C with a coupled enzyme assay, was highly specific for glucose and used 2-deoxyglucose to some extent (25, 58). A broader specificity for hexoses, (e.g., for mannose, galactose, and glucosamine) was reported for the ADP-GLKs from P. furiosus and T. litoralis (27), as well as for the bifunctional ADP-GLK/ADP-phosphofructokinase from M. jannaschii (42), when assayed at 37°C by a high-performance liquid chromatography method for product determination. However, it has to be considered that the assay temperature of 37°C is 40 to 60°C below the growth temperature of these archaea. The substrate specificity of these hyperhtermophilic ADP-GLKs at physiologically more relevant temperatures (i.e., above 80°C) remains to be shown. The broad substrate specificity of the A. pernix ATP-GLK has been demonstrated at 90°C.
TABLE 4.
Substrate specificity of GLKs and hexokinases
| Source | Nucleotide specificity | Family | % Activity versus glucose under Vmax conditionsa
|
Reference | Method for detection of productb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| glc | 2dglc | frc | man | gal | glcN | manNac | glcNac | |||||
| Aeropyrum pernix | ATP | ROK | 100 | 129 | 43 | 54 | − | 77 | 4 | 6 | This work | CEA, 90°C |
| Renibacterium salminarium | ATP | ROK | 100 | − | ND | ND | ND | − | − | − | 10 | CEA |
| Bacillus subtilis | ATP | ROK | 100 | − | ND | ND | ND | − | − | − | 50 | TLC |
| Streptococcus mutans | ATP | ROKc | 100 | ND | ND | ND | ND | ND | − | ND | 36 | CEA |
| Aerobacter aerogenes | ATP | 100 | ND | ND | ND | ND | 26 | ND | ND | 21 | CEA | |
| Bacillus stearothermophilus | ATP | 100 | ND | ND | ND | ND | ND | ND | 50 (pH 7.6) | 18 | CEA | |
| Escherichia coli | ATP | Glucokinase | 100 | − | ND | ND | ND | − | − | − | 32 | CEA |
| Zymomonas mobilis | ATP | Glucokinase | 100 | ND | ND | ND | ND | ND | − | − | 45 | CEA |
| Aspergillus niger | ATP | Hexokinase | 100 | + | 86 | + | ND | − | − | − | 51 | CEA |
| Dog liver (GLK) | ATP | Hexokinase | 100 | 31 | 38 | 60 | ND | ND | − | − | 31 | CEA |
| Drosophila melanogaster | ||||||||||||
| Isoenzyme A | ATP | Hexokinase | 100 | 98 | 50 | 100 | − | 19 | − | − | ||
| Isoenzyme B | 100 | 51 | 41 | 98 | − | 0 | − | − | 33 | CEA | ||
| Isoenzyme C | 100 | 48 | 134 | 44 | − | 44 | − | |||||
| Human (glucokinase) | ATP | Hexokinase | 100 | 40 | 20 | 80 | − | − | − | − | 37 | CEA |
| Pyrococcus furiosus | ADP | ADP-GLK | 100 | 9.2 | ND | ND | ND | − | − | − | 25 | CEA, 50°C |
| 100 | 8 | <0.3 | 2 | <0.3 | − | − | − | 58 | CEA, 50°C | |||
| 100 | 3 | ND | 13 | 7 | 72 | − | − | 27 | HPLC, 37°C | |||
| Thermococcus litoralis | ADP | ADP-GLK | 100 | 4 | 2 | 13 | 9 | 67 | − | − | 27 | HPLC, 37°C |
| Methanococcus janaschii (bifunctional GLK/PFK) | ADP | ADP-GLK | 100 | 19 | 14 | ND | ND | ND | − | − | 42 | HPLC, 37°C |
Substrate abbreviations: glc, glucose; 2dglc, 2-deoxyglucose; frc, fructose; man, mannose; gal, galactose; glcN, glucosamine; manNac, N-acetylmannosamine; glcNac, N-acetylglucosamine; ND, not detected; +, no value given; − not tested.
CEA, coupled enzymatic assay; TLC, thin-layer chromatography; HPLC, high-performance liquid chromatography. Assay temperatures are shown.
Classification due to homology of 72-amino-acid N-terminal sequence (BAA96474).
The reason for the broad hexose specificity of A. pernix ATP-GLK cannot be explained so far. Usually prokaryotes contain a series of hexose-phosphorylating enzymes, each acting specifically on one particular hexose: fructose, mannose, or galactose (for review, see reference 9). One might speculate that functional diversity of hexose phophorylation in the archaeon A. pernix is achieved by a single multifunctional enzyme with low hexose specificity rather than by highly specific separate enzymes. In this context, it is interesting to note that the second characterized sugar kinase in A. pernix, the ATP-dependent phosphofructokinase, which belongs to the PFK-B family, also showed a broad substrate specificity for sugars, which is a characteristic feature of the PFK B family (17). Finally, ATP-GLK from A. pernix is the most thermoactive and thermostable of all ATP-dependent GLKs analyzed so far. It showed a temperature optimum of at least 100°C and exerts a high thermostability up to 100°C, which is in accordance with its function under the hyperthermophilic growth conditions of the organism. The enzyme does not show heat inactivation upon incubation at 95°C for 2 h. Similar thermoactivity and thermostability have been demonstrated for the ADP-dependent GLK from P. furiosus (25). However, the most thermophilic bacterial ATP-GLK known so far, from B. stearothermophilus, had a temperature optimum at about 60°C and was inactivated by 50% upon incubation at 70°C for 10 or 60 min, in the absence or presence, respectively, of DTE (18).
In summary, the first archaeal ATP-GLK, from A. pernix, belongs to the ROK family. The enzyme differs from all other ROK GLKs in its subunit size, monomeric structure, outstanding thermostability, and broad specificity for hexoses.
Acknowledgments
We thank H. Preidel for mass culturing A. pernix. The expert technical assistance of K. Lutter-Mohr and A. Brandenburger is also gratefully acknowledged.
This work was supported by grants from the Fonds der Chemischen Industrie.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arora, K. K., P. Shenbagamurthi, M. Fanciulli, and P. L. Pedersen. 1990. Glucose phosphorylation. Interaction of a 50-amino acid peptide of yeast hexokinase with trinitrophenyl ATP. J. Biol. Chem. 265:5324-5328. [PubMed] [Google Scholar]
- 4.Bateman, A., E. Birney, R. Durbin, S. R. Eddy, R. D. Finn, and E. L. Sonnhammer. 1999. Pfam 3.1: 1,313 multiple alignments and profile HMMs match the majority of proteins. Nucleic Acids Res. 27:260-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman, A., E. Birney, R. Durbin, S. R. Eddy, K. L. Howe, and E. L. Sonnhammer. 2000. The Pfam protein families database. Nucleic Acids Res. 28:263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman, A., E. Birney, L. Cerruti, R. Durbin, L. Etwiller, S. R. Eddy, S. Griffiths-Jones, K. L. Howe, M. Marshall, and E. L. Sonnhammer. 2002. The Pfam protein families database. Nucleic Acids Res. 30:276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bork, P., C. Sander, and A. Valencia. 1993. Convergent evolution of similar enzymatic function on different protein folds: the hexokinase, ribokinase, and galactokinase families of sugar kinases. Protein Sci. 2:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 9.Cardenas, M. L., A. Cornish-Bowden, and T. Ureta. 1998. Evolution and regulatory role of the hexokinases. Biochim. Biophys. Acta 1401:242-264. [DOI] [PubMed] [Google Scholar]
- 10.Concha, M. I., and G. Leon. 2000. Cloning, functional expression and partial characterization of the glucose kinase from Renibacterium salmoninarum. FEMS Microbiol. Lett. 186:97-101. [DOI] [PubMed] [Google Scholar]
- 11.Danson, M. J. 1993. Central metabolism of the archaea, p. 1-24. In M. Kates (ed.), The biochemistry of Archaea (Archaebacteria). Elsevier Science Publishers B.V., Amsterdam, The Netherlands.
- 12.de Vos, W. M., S. W. Kengen, W. G. Voorhorst, and J. van der Oost. 1998. Sugar utilization and its control in hyperthermophiles. Extremophiles 2:201-205. [DOI] [PubMed] [Google Scholar]
- 13.Fitz-Gibbon, S. T., H. Ladner, U. J. Kim, K. O. Stetter, M. I. Simon, and J. H. Miller. 2002. Genome sequence of the hyperthermophilic crenarchaeon Pyrobaculum aerophilum. Proc. Natl. Acad. Sci. USA 99:984-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaherty, K. M., S. M. Wilbanks, C. DeLuca-Flaherty, and D. B. McKay. 1994. Structural basis of the 70-kilodalton heat shock cognate protein ATP hydrolytic activity. II. Structure of the active site with ADP or ATP bound to wild type and mutant ATPase fragment. J. Biol. Chem. 269:12899-12907. [PubMed] [Google Scholar]
- 15.Hansen, T., M. Oehlmann, and P. Schönheit. 2001. Novel type of glucose-6-phosphate isomerase in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 183:3428-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen, T., and P. Schönheit. 2000. Purification and properties of the first-identified, archaeal, ATP-dependent 6-phosphofructokinase, an extremely thermophilic non-allosteric enzyme, from the hyperthermophile Desulfurococcus amylolyticus. Arch. Microbiol. 173:103-109. [DOI] [PubMed] [Google Scholar]
- 17.Hansen, T., and P. Schönheit. 2001. Sequence, expression, and characterization of the first archaeal ATP-dependent 6-phosphofructokinase, a non-allosteric enzyme related to the phosphofructokinase-B sugar kinase family, from the hyperthermophilic crenarchaeote Aeropyrum pernix. Arch. Microbiol. 177:62-69. [DOI] [PubMed] [Google Scholar]
- 18.Hengartner, H., and H. Zuber. 1973. Isolation and characterization of a thermophilic glucokinase from Bacillus stearothermophilus. FEBS Lett. 37:212-216. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann, K., P. Bucher, L. Falquet, and A. Bairoch. 1999. The PROSITE database, its status in 1999. Nucleic Acids Res. 27:215-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito, S., S. Fushinobu, I. Yoshioka, S. Koga, H. Matsuzawa, and T. Wakagi. 2001. Structural basis for the ADP-specificity of a novel glucokinase from a hyperthermophilic archaeon. Structure 9:205-214. [DOI] [PubMed] [Google Scholar]
- 21.Kamel, M. Y., D. P. Allison, and R. L. Anderson. 1966. Stereospecific d-glucokinase of Aerobacter aerogenes. Purification and properties. J. Biol. Chem. 241:690-694. [PubMed] [Google Scholar]
- 22.Kawarabayasi, Y., Y. Hino, H. Horikawa, S. Yamazaki, Y. Haikawa, K. Jin-no, M. Takahashi, M. Sekine, S. Baba, A. Ankai, H. Kosugi, A. Hosoyama, S. Fukui, Y. Nagai, K. Nishijima, H. Nakazawa, M. Takamiya, S. Masuda, T. Funahashi, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, and H. Kikuchi. 1999. Complete genome sequence of an aerobic hyper-thermophilic crenarchaeon, Aeropyrum pernix K1. DNA Res. 6:83-101, 145-152. [DOI] [PubMed] [Google Scholar]
- 23.Kawashima, T., N. Amano, H. Koike, S. Makino, S. Higuchi, Y. Kawashima-Ohya, K. Watanabe, M. Yamazaki, K. Kanehori, T. Kawamoto, T. Nunoshiba, Y. Yamamoto, H. Aramaki, K. Makino, and M. Suzuki. 2000. Archaeal adaptation to higher temperatures revealed by genomic sequence of Thermoplasma volcanium. Proc. Natl. Acad. Sci. USA 97:14257-14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kengen, S. W., A. J. Stams, and W. M. de Vos. 1996. Sugar metabolism of hyperthermophiles. FEMS Microbiol. Rev. 18:119-137. [Google Scholar]
- 25.Kengen, S. W., J. E. Tuininga, F. A. de Bok, A. J. Stams, and W. M. de Vos. 1995. Purification and characterization of a novel ADP-dependent glucokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 270:30453-30457. [DOI] [PubMed] [Google Scholar]
- 26.Kim, C., S. Song, and C. Park. 1997. The d-allose operon of Escherichia coli K-12. J. Bacteriol. 179:7631-7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koga, S., I. Yoshioka, H. Sakuraba, M. Takahashi, S. Sakasegawa, S. Shimizu, and T. Ohshima. 2000. Biochemical characterization, cloning, and sequencing of ADP-dependent (AMP-forming) glucokinase from two hyperthermophilic archaea, Pyrococcus furiosus and Thermococcus litoralis. J. Biochem. (Tokyo) 128:1079-1085. [DOI] [PubMed] [Google Scholar]
- 28.Kreuzer, P., D. Gartner, R. Allmansberger, and W. Hillen. 1989. Identification and sequence analysis of the Bacillus subtilis W23 xylR gene and xyl operator. J. Bacteriol. 171:3840-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labes, A., and P. Schönheit. 2001. Sugar utilization in the hyperthermophilic, sulfate-reducing archaeon Archaeoglobus fulgidus strain 7324: starch degradation to acetate and CO2 via a modified Embden-Meyerhof pathway and acetyl-CoA synthetase (ADP-forming). Arch. Microbiol. 176:329-338. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 31.Maccioni, R., and J. Babul. 1980. Purification and characterization of dog liver glucokinase. Arch. Biol. Med. Exp. (Santiago) 13:271-286. [PubMed] [Google Scholar]
- 32.Meyer, D., C. Schneider-Fresenius, R. Horlacher, R. Peist, and W. Boos. 1997. Molecular characterization of glucokinase from Escherichia coli K-12. J. Bacteriol. 179:1298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moser, D., L. Johnson, and C. Y. Lee. 1980. Multiple forms of Drosophila hexokinase. Purification, biochemical and immunological characterization. J. Biol. Chem. 255:4673-4679. [PubMed] [Google Scholar]
- 34.Ng, W. V., S. P. Kennedy, G. G. Mahairas, B. Berquist, M. Pan, H. D. Shukla, S. R. Lasky, N. S. Baliga, V. Thorsson, J. Sbrogna, S. Swartzell, D. Weir, J. Hall, T. A. Dahl, R. Welti, Y. A. Goo, B. Leithauser, K. Keller, R. Cruz, M. J. Danson, D. W. Hough, D. G. Maddocks, P. E. Jablonski, M. P. Krebs, C. M. Angevine, H. Dale, T. A. Isenbarger, R. F. Peck, M. Pohlschroder, J. L. Spudich, K. W. Jung, M. Alam, T. Freitas, S. Hou, C. J. Daniels, P. P. Dennis, A. D. Omer, H. Ebhardt, T. M. Lowe, P. Liang, M. Riley, L. Hood, and S. DasSarma. 2000. Genome sequence of Halobacterium species NRC-1. Proc. Natl. Acad. Sci. USA 97:12176-12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plumbridge, J., and E. Vimr. 1999. Convergent pathways for utilization of the amino sugars N-acetylglucosamine, N-acetylmannosamine, and N-acetylneuraminic acid by Escherichia coli. J. Bacteriol. 181:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter, V., and B. M. Chassy. 1982. Glucokinase from Streptococcus mutans. Methods Enzymol. 90:25-30 [DOI] [PubMed] [Google Scholar]
- 37.Printz, R. L., M. A. Magnuson, and D. K. Granner. 1993. Mammalian glucokinases. Annu. Rev. Nutr. 13:463-496. [DOI] [PubMed] [Google Scholar]
- 38.Ronimus, R. S., E. de Heus, and H. W. Morgan. 2001. Sequencing, expression, characterisation and phylogeny of the ADP-dependent phosphofructokinase from the hyperthermophilic, euryarchaeal Thermococcus zilligii. Biochim. Biophys. Acta 1517:384-391. [DOI] [PubMed] [Google Scholar]
- 39.Ronimus, R. S., and H. W. Morgan. 2001. The biochemical properties and phylogenies of phosphofructokinases from extremophiles. Extremophiles 5:357-373. [DOI] [PubMed] [Google Scholar]
- 40.Ruepp, A., W. Graml, M. L. Santos-Martinez, K. K. Koretke, C. Volker, H. W. Mewes, D. Frishman, S. Stocker, A. N. Lupas, and W. Baumeister. 2000. The genome sequence of the thermoacidophilic scavenger Thermoplasma acidophilum. Nature 407:508-513. [DOI] [PubMed] [Google Scholar]
- 41.Sako, Y., N. Nomura, A. Uchida, Y. Ishida, H. Morii, Y. Koga, T. Hoaki, and T. Maruyama. 1996. Aeropyrum pernix gen. nov., sp. nov., a novel aerobic hyperthermophilic archaeon growing at temperatures up to 100°C. Int. J. Syst. Bacteriol. 46:1070-1077. [DOI] [PubMed] [Google Scholar]
- 42.Sakuraba, H., I. Yoshioka, S. Koga, M. Takahashi, Y. Kitahama, T. Satomura, R. Kawakami, and T. Ohshima. 2002. ADP-dependent glucokinase/phosphofructokinase, a novel bifunctional enzyme from the hyperthermophilic archaeon Methanococcus jannaschii. J. Biol. Chem. 277:12495-12498. [DOI] [PubMed] [Google Scholar]
- 43.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schönheit, P., and T. Schäfer. 1995. Metabolism of hyperthermophiles. World J. Microbiol. Biotechnol. 11:26-57. [DOI] [PubMed] [Google Scholar]
- 45.Scopes, R. K., V. Testolin, A. Stoter, K. Griffiths-Smith, and E. M. Algar. 1985. Simultaneous purification and characterization of glucokinase, fructokinase and glucose-6-phosphate dehydrogenase from Zymomonas mobilis. Biochem. J. 228:627-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selig, M., K. B. Xavier, H. Santos, and P. Schönheit. 1997. Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium Thermotoga. Arch. Microbiol. 167:217-232. [DOI] [PubMed] [Google Scholar]
- 47.Siebers, B., H. Brinkmann, C. Dorr, B. Tjaden, H. Lilie, J. van der Oost, and C. H. Verhees. 2001. Archaeal fructose-1,6-bisphosphate aldolases constitute a new family of archaeal type class I aldolase. J. Biol. Chem. 276:28710-28718. [DOI] [PubMed] [Google Scholar]
- 48.Siebers, B., and R. Hensel. 1993. Glucose metabolism of the hyperthermophilic archaeum Thermoproteus tenax, an archaeal descendant of an ancient line in phosphofructokinase evolution. FEMS Microbiol. Lett. 111:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sizemore, C., E. Buchner, T. Rygus, C. Witke, F. Gotz, and W. Hillen. 1991. Organization, promoter analysis and transcriptional regulation of the Staphylococcus xylosus xylose utilization operon. Mol. Gen. Genet. 227:377-384. [DOI] [PubMed] [Google Scholar]
- 50.Skarlatos, P., and M. K. Dahl. 1998. The glucose kinase of Bacillus subtilis. J. Bacteriol. 180:3222-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinböck, F., S. Choojun, I. Held, M. Roehr, and C. P. Kubicek. 1994. Characterization and regulatory properties of a single hexokinase from the citric acid accumulating fungus Aspergillus niger. Biochim. Biophys. Acta 1200:215-223. [DOI] [PubMed] [Google Scholar]
- 52.Stetter, K. O. 1996. Hyperthermophilic procaryotes. FEMS Microbiol. Rev. 18:149-158. [Google Scholar]
- 53.Stetter, K. O. 1999. Extremophiles and their adaptation to hot environments. FEBS Lett. 452:22-25. [DOI] [PubMed] [Google Scholar]
- 54.Stetter, K. O., G. Fiala, G. Huber, and A. Segerer. 1990. Hyperthermophilic microorganisms. FEMS Microbiol. Rev. 75:117-124. [Google Scholar]
- 55.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Titgemeyer, F., J. Reizer, A. Reizer, and M. H. J. Saier. 1994. Evolutionary relationships between sugar kinases and transcriptional repressors in bacteria. Microbiology 140:2349-2354. [DOI] [PubMed] [Google Scholar]
- 57.Tuininga, J. E., C. H. Verhees, J. van der Oost, S. W. Kengen, A. J. Stams, and W. M. de Vos. 1999. Molecular and biochemical characterization of the ADP-dependent phosphofructokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 274:21023-21028. [DOI] [PubMed] [Google Scholar]
- 58.Verhees, C. H., D. G. Koot, T. J. Ettema, C. Dijkema, W. M. de Vos, and J. van der Oost. 2002. Biochemical adaptations of two sugar kinases from the hyperthermophilic archaeon Pyrococcus furiosus. Biochem. J. 366:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verhees, C. H., J. E. Tuininga, S. W. M. Kengen, A. J. M. Stams, J. van der Oost, and W. M. de Vos. 2001. ADP-dependent phosphofructokinases in mesophilic and thermophilic methanogenic archaea. J. Bacteriol. 183:7145-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner, E., S. Marcandier, O. Egeter, J. Deutscher, F. Götz, and R. Brückner. 1995. Glucose kinase-dependent catabolite repression in Staphylococcus xylosus. J. Bacteriol. 177:6144-6152. [DOI] [PMC free article] [PubMed] [Google Scholar]