Abstract
GroEL protein and groEL mRNA transcript were up-regulated in gyrB mutants of Borrelia burgdorferi, a causative agent of Lyme disease. Furthermore, the protein and transcript levels in gyrB mutants were greater than those in experimentally heat-shocked cultures of wild-type B. burgdorferi. Circular DNA in the gyrB mutants was more relaxed than in wild-type cells, although groEL is on the linear chromosome of B. burgdorferi. To our knowledge, this is the first evidence, albeit indirect, for the effect of DNA topology on gene expression from a linear DNA molecule in a bacterium.
Lyme disease is a multisystem disorder caused by the spirochete Borrelia burgdorferi (4, 24). Pathology to host tissues may be due in part to an autoimmune response to B. burgdorferi heat shock proteins (HSPs) (13). HSPs are synthesized when cells are exposed to elevated temperatures or to a variety of other stresses (11). Some HSPs have been shown to act as chaperones for the assembly of complex and oligomeric proteins (2, 8). The major HSP of ∼72 kDa, the DnaK homolog (1, 25), is immunoreactive, and antibodies to DnaK are commonly seen in sera from Lyme disease patients (1). GroEL is a major HSP of ∼60 kDa. After heat treatment, DnaK and GroEL were synthesized continuously in gyrA mutants of Escherichia coli but only transiently in wild-type cells (16). Inhibitors of DNA gyrase also induce HSPs (11, 17, 26). These responses are due to relaxation of DNA supercoiling (12). We observed that coumermycin A1-resistant gyrB mutants of B. burgdorferi had increased levels of an ∼68-kDa protein, which was subsequently identified as GroEL (Fig. 1 and 2A).
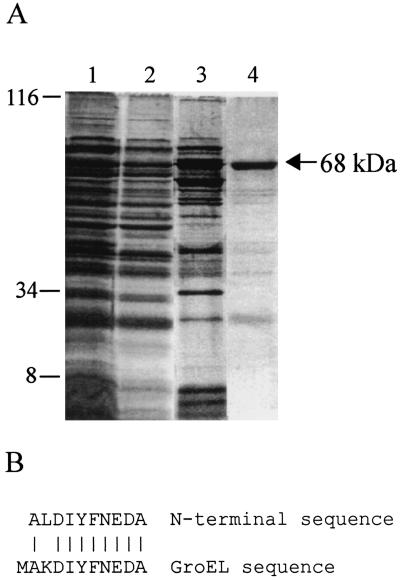
FIG. 1.
Purification of GroEL from a gyrB mutant of B. burgdorferi. (A) The 68-kDa protein (identified as GroEL) was purified by column chromatography. Lane 1, cell lysate; lane 2, flowthrough from a heparin column; lane 3, pooled fractions after elution from a phenyl Superose column; lane 4, pooled fractions after elution from a Mono-Q column. Molecular masses are in kilodaltons. (B) N-terminal sequence of the 68-kDa protein and alignment with GroEL (BB0649; GenBank accession no. AE001166) (6).
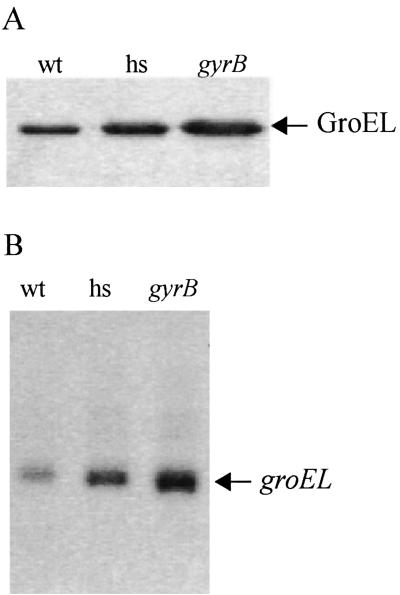
FIG. 2.
Increased GroEL synthesis and groEL expression in a gyrB mutant of B. burgdorferi. Immunoblot (A) and Northern blot (B) analyses of wild-type B31 (wt), experimentally heat-shocked wild-type B31 (hs), and the gyrB mutant X32 were carried out.
B. burgdorferi strain X32, a clone of strain B31 carrying a coumermycin A1-resistant gyrB mutation (Arg 133 → Leu) (22) (D. S. Samuels, B. J. Kimmel, D. C. Criswell, C. F. Garon, W. M. Huang, and C. H. Eggers, unpublished data), synthesizes the up-regulated 68-kDa protein. A crude lysate of X32 was prepared from a 1.5-liter culture grown in BSK-H medium (Sigma) at 32°C as previously described (15) with the following modifications. Cells from a 1.5-liter culture (in three 500-ml bottles) were collected at 10,500 × g for 20 min in a Sorvall GSA rotor. The cell pellet was washed twice in 30 ml of Dulbecco's phosphate-buffered saline (DPBS; 138 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4). Cells were collected in an SS-34 rotor at 7,500 × g for 10 min after the first wash and at 6,000 × g after the second wash. Cells were resuspended in 1.5 ml of 50 mM Tris-HCl (pH 8.0; the pH of Tris solutions was measured at 25°C)-15% sucrose and stored at −80°C. Four 1.5-ml aliquots were thawed at 37°C, and dithiothreitol (DTT; final concentration, 2 mM), EDTA (final concentration, 1 mM), and phenylmethylsulfonyl fluoride (final concentration, 0.5 mM) were added to each aliquot. The cells were then lysed by sonication (eight 15-s pulses at 3.5 in a Fisher Scientific Sonic Dismembrator 550 with a microtip probe for each of the four aliquots). Nucleic acid was precipitated by slowly adding 1/5 volume of 1 M KCl and 2/5 volume of 5% streptomycin sulfate (pH 7.2 with NH4HCO3) followed by rotation at 4°C for 10 min. The lysate was clarified first by centrifugation at 7,500 × g for 10 min in an SS-34 rotor and then by ultracentrifugation at 435,000 × g for 30 min in a TLA-100.2 (Beckman).
The 68-kDa protein was purified and identified (Fig. 1A) as previously described (10) with the following modifications. The clarified lysate was dialyzed overnight at 4°C against 50 mM Tris-HCl (pH 7.5)-10% glycerol-1 mM EDTA-5 mM DTT (A buffer) and loaded onto a 5-ml Econo-Pac heparin cartridge (Bio-Rad) at 2 ml min−1. The 68-kDa protein was in the flowthrough from the column, which was combined with an equal volume of 3.4 M (NH4)2SO4 in P buffer (50 mM Tris [pH 7.5], 1 mM EDTA, 5 mM DTT) and loaded onto a 1-ml phenyl Superose column (Pharmacia). The column was eluted with a 20-ml linear gradient from 1.7 to 0 M (NH4)2SO4 in P buffer at 0.3 ml min−1. Fractions containing the 68-kDa protein [which eluted at ∼0.85 M (NH4)2SO4] were dialyzed against A buffer overnight at 4°C and loaded onto a 1-ml Mono-Q column (Pharmacia). The column was eluted with a 20-ml linear gradient from 0 to 1 M NaCl in A buffer. The fractions containing the 68-kDa protein (which eluted at ∼0.5 M NaCl) were concentrated with a Centricon 10 concentrator (Amicon) in an SS-34 rotor for 60 min. An equal volume of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (125 mM Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, 1.4 M 2-mercaptoethanol, 0.2% bromphenol blue) was added to the concentrated fractions and boiled for 5 min. The sample was resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes (Immobilon P; Millipore), and stained with Coomassie brilliant blue. The 68-kDa bands were excised, stored in 1 ml of distilled H2O-2 mM DTT at 4°C, and subjected to Edman degradation. N-terminal sequencing and BLAST searching identified the up-regulated 68-kDa protein as GroEL (Fig. 1B).
Since GroEL is a HSP and its synthesis is induced by heat, we compared GroEL levels in cultures of experimentally heat-shocked wild-type B31 and the gyrB mutant. Experimentally heat-shocked cultures were treated by incubating cells at 42°C for 1 h before harvest. B. burgdorferi protein extracts were prepared by pelleting 10-ml cultures of wild-type B31, experimentally heat-shocked wild-type B31, and the gyrB mutant, washing twice with DPBS, obtaining the optical density at 600 nm in 1 ml of DPBS, resuspending in 200 μl of H2O per unit of optical density at 600 nm, adding an equal volume of 2× Laemmli buffer, and boiling for 5 min. Protein extracts from 3.5 × 107 cells were separated by electrophoresis through SDS-12.5% polyacrylamide gels and visualized by staining with Coomassie brilliant blue. Resolved proteins were also transferred to a polyvinylidene difluoride membrane by using a Trans-Blot cell (Bio-Rad) according to the instructions of the manufacturer. The antigenic proteins were detected with an Immun-Star chemiluminescent detection system according to the instructions of the manufacturer (Bio-Rad). Membranes were blocked for 30 min at room temperature with 0.2% nonfat dry milk-20 mM Tris (pH 7.5)-500 mM NaCl (TBS) and then washed for 10 min with TBS-0.1% Tween-20 (TTBS). The membranes were then incubated overnight at 4°C with monoclonal mouse anti-GroEL antibody 149 (13) (kindly provided by Barbara Johnson and Christian Rittner, Center for Disease Control, Fort Collins, Colo.), diluted 1:2,500 in 0.2% nonfat dry milk-TTBS. The membrane was rinsed twice for 10 min each time with TTBS. Alkaline phosphatase-linked goat anti-mouse immunoglobulin G (Bio-Rad) was diluted 1:3,000 in 0.2% nonfat dry milk-TTBS and incubated for 1.5 h at room temperature. The membrane was washed three times for 10 min each time with TTBS, developed in 5 ml of the substrate solution, and exposed to radiographic film at room temperature for 3 min.
As expected, the culture of experimentally heat-shocked wild-type B31 possessed higher levels of GroEL protein than the wild-type B31 (Fig. 2A). Interestingly, GroEL levels were even higher in the gyrB mutant (Fig. 2A).
We next examined groEL mRNA levels to determine the mechanism of increased protein levels. Total RNA was isolated from 100-ml cultures of wild-type B31, experimentally heat-shocked wild-type B31, and the gyrB mutant by using TRIzol reagent as described by the manufacturer (Gibco BRL). Fifteen micrograms of total RNA was fractionated on 1.2% formaldehyde-agarose gels and transferred to nylon membranes. Hybridization was performed as previously described (3, 14). Band intensity was determined by using an image acquisition and analysis system (Ambis); the mean of three independent experiments and the standard error of the mean (SEM) are reported. The hybridization probe for the groEL locus was generated by two rounds of PCR amplification from genomic DNA with oligonucleotides groEL 750F (5′-TGAGGATATTGAGGGGGATGC-3′) and groEL 1122R (5′-AACTCCGCCAACAAGTTTTGC-3′) (GenBank accession no. AE001166) (6) as described previously (21).
A 2.3-fold (SEM, 0.4-fold) increase in steady-state groEL mRNA levels occurred in the gyrB mutant strain relative to the wild-type B31 parental strain (Fig. 2B). This compares to a 1.6-fold (SEM, 0.2-fold) increase in mRNA in the gyrB mutant relative to the heat-shocked culture of B31 (Fig. 2B). The concomitant increase in groEL mRNA levels suggests that the higher level of GroEL protein represents increased transcription.
We wanted to determine why groEL gene expression was increased in the gyrB mutant. We speculated that the increase was due to DNA relaxation, since gyrB mutants of E. coli have more relaxed DNA than wild-type cells (5, 16) and groEL is regulated by DNA supercoiling (12, 16). A small (4-kb) reporter plasmid, pGOΔ15 (23), which is transiently maintained in B. burgdorferi, was transformed into wild-type B31 cells and gyrB mutant cells to compare DNA supercoiling. A small reporter plasmid was used because the circular plasmids of B. burgdorferi strain X32 are too large (≥26 kb) to resolve into individual topoisomers by gel electrophoresis (J. Alverson and D. S. Samuels, submitted for publication), and the remainder of the B. burgdorferi genome is linear. One-dimensional agarose gels with and without chloroquine readily differentiate plasmid DNA topology (7). Plasmids were transformed into B. burgdorferi cells by electroporation (20). Plasmid DNA was purified using an alkaline lysis plasmid miniprep kit according to the directions of the manufacturer (Wizard; Promega). The DNA was resuspended in 30 μl of H2O and stored at −20°C. Plasmid DNA was fractionated on a 0.8% agarose gel in TAE (40 mM Tris, 20 mM acetate, 1 mM EDTA) for 4 h at 50 V with 0 or 15 μM chloroquine, which intercalates into DNA and decreases negative DNA supercoiling, added to the gel and the running buffer. The gel was rinsed in three changes of water (1 h each) to remove the chloroquine before being stained with ethidium bromide for 45 min.
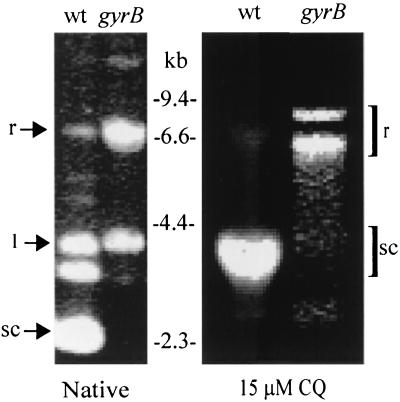
The plasmid DNA was negatively supercoiled in wild-type B31 and relaxed in the gyrB mutant (Fig. 3). The relaxation of DNA supercoiling has previously been shown to be associated with the induction of HSPs (16). Furthermore, DnaK plays a role in protecting negative DNA supercoiling in E. coli during heat shock (18). We observed increased synthesis of GroEL in a gyrB mutant of B. burgdorferi. DNA supercoiling is more relaxed in cells with coumermycin A1-resistant DNA gyrase (5; J. Alverson and D. S. Samuels, submitted for publication). We wondered whether the relaxation of DNA directly regulates groEL expression or whether the presence of a defective protein (DNA gyrase) indirectly induces GroEL synthesis. The GroEL in the latter case could stabilize DNA gyrase, resulting in the return of DNA supercoiling levels to those seen in the wild-type cells. The GroEL protein in this scenario would be acting similarly to DnaK in maintaining the negative supercoiling in the cell in response to a stress (18). This stress would be the mutation in the gyrB gene. However, our results suggest that DNA supercoiling is relaxed in the gyrB mutant. Therefore, the increase in GroEL is likely a response to DNA relaxation, which indicates that the groEL gene may be directly or indirectly regulated by DNA topology. DNA supercoiling is related to changes in topoisomerase levels (19). Reduced topoisomerase levels result in decreased DnaK and GroEL levels as well as attenuated thermotolerance in E. coli (19). DNA gyrase inhibitors relax DNA supercoiling and increase synthesis of DnaK, another HSP (9). In this study we observed decreased levels of DNA supercoiling in a gyrB mutant, as described previously (5). Notably, groEL maps to the chromosome (6), which is a linear DNA molecule in B. burgdorferi. The correlation between groEL regulation and DNA supercoiling suggests that the linear chromosome of B. burgdorferi may be subject to superhelical torsion. Therefore, the ends could be topologically constrained, perhaps by binding to the cell membrane or to a higher-order nucleoprotein complex. An alternative but more complicated explanation is that DNA supercoiling affects expression of a gene on a circular plasmid whose product directly regulates groEL.
FIG. 3.
Effect of a gyrB mutation on DNA topology. Plasmid pGOΔ15 was transiently introduced into wild-type B31 and the gyrB mutant X32. The topology of the plasmid was determined by one-dimensional 0.8% agarose gel electrophoresis in the absence (native) or presence of 15 μM chloroquine (CQ). The more negatively supercoiled molecules migrate faster through the gel; chloroquine intercalates into the plasmid DNA, introducing positive supercoiling that retards migration of negatively supercoiled DNA (sc) and expedites migration of relaxed DNA (r). The migration of the linearized (l) form of the plasmid is not affected in the mutant. Molecular size standards are in kilobase pairs.
Acknowledgments
We thank Costa Georgopoulos and Ralph Judd for useful discussions, Barbara Johnson, Christian Rittner, and Ben Luft for the antibody, Chuck Sohaskey for plasmid pGOΔ15, the late Joan Strange for protein sequencing, Bill Holben for use of the Ambis system, Sharyl Fyffe for technical assistance, and Doug Emlen for help with figure preparation. We appreciate the reviews of an earlier version of this paper by Shawn M. D. Bearson, USDA, ARS, Ames, Iowa, and Stephanie D. Collier, USDA, ARS, Mississippi State.
This work was supported by grants from the Arthritis Foundation, the National Science Foundation (MCB-9722408), the National Institutes of Health (AI39695), MONTS (NSF EPSCoR), and the University Grants Program to D.S.S. J.A. was the recipient of a Predoctoral Honors Fellowship from The University of Montana.
REFERENCES
- 1.Anzola, J., B. J. Luft, G. Gorgone, R. J. Dattwyler, C. Soderberg, R. Lahesmaa, and G. Peltz. 1992. Borrelia burgdorferi HSP70 homolog: characterization of an immunoreactive stress protein. Infect. Immun. 60:3704-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckmann, R. P., L. A. Mizzen, and W. J. Welch. 1990. Interaction of Hsp70 with newly synthesized proteins: implications for protein folding and assembly. Science 248:850-854. [DOI] [PubMed] [Google Scholar]
- 3.Brown, T. 1999. Preparation and analysis of RNA, p. 4.1-4.27. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Short protocols in molecular biology, 4th ed. John Wiley and Sons, New York, N.Y.
- 4.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 5.Contreras, A., and A. Maxwell. 1992. gyrB mutations which confer coumarin resistance also affect DNA supercoiling and ATP hydrolysis by Escherichia coli DNA gyrase. Mol. Microbiol. 6:1617-1624. [DOI] [PubMed] [Google Scholar]
- 6.Fraser, C. M., S. Casjens, W. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein, E., and K. Drlica. 1984. Regulation of bacterial DNA supercoiling: plasmid linking numbers vary with growth temperature. Proc. Natl. Acad. Sci. USA 81:4046-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmingsen, S. M., C. Woolford, S. M. van der Vies, K. Tilly, D. T. Dennis, C. P. Georgopoulos, R. W. Hendrix, and J. R. Ellis. 1988. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature (London) 333:330-334. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko, T., T. Mizushima, Y. Ohtsuka, K. Kurokawa, K. Kataoka, T. Miki, and K. Sekimizu. 1996. Coinduction of DNA relaxation and synthesis of DnaK and GroEL proteins in Escherichia coli by expression of LetD (CcdB) protein, an inhibitor of DNA gyrase encoded by the F factor. Mol. Gen. Genet. 250:593-600. [DOI] [PubMed] [Google Scholar]
- 10.Knight, S. W., and D. S. Samuels. 1999. Natural synthesis of a DNA-binding protein from the C-terminal domain of DNA gyrase A in Borrelia burgdorferi. EMBO J. 18:4875-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindquist, S. 1986. The heat-shock response. Annu. Rev. Biochem. 55:1151-1191. [DOI] [PubMed] [Google Scholar]
- 12.López-García, P., and P. Forterre. 2000. DNA topology and the thermal stress response, a tale from mesophiles and hyperthermophiles. BioEssays 22:738-746. [DOI] [PubMed] [Google Scholar]
- 13.Luft, B. J., P. D. Gorevic, W. Jiang, P. Munoz, and R. J. Dattwyler. 1991. Immunologic and structural characterization of the dominant 66- and 73-kDa antigens of Borrelia burgdorferi. J. Immunol. 146:2776-2782. [PubMed] [Google Scholar]
- 14.Marconi, R. T., D. S. Samuels, and C. F. Garon. 1993. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J. Bacteriol. 175:926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margolis, N., and D. S. Samuels. 1995. Proteins binding to the promoter region of the operon encoding the major outer surface proteins OspA and OspB of Borrelia burgdorferi. Mol. Biol. Rep. 21:159-164. [DOI] [PubMed] [Google Scholar]
- 16.Mizushima, T., S. Natori, and K. Sekimizu. 1993. Relaxation of supercoiled DNA associated with induction of heat shock proteins in Escherichia coli. Mol. Gen. Genet. 238:1-5. [DOI] [PubMed] [Google Scholar]
- 17.Mizushima, T., Y. Ohtsuka, H. Mori, T. Miki, and K. Sekimizu. 1996. Increase in synthesis and stability of σ32 on treatment with inhibitors of DNA gyrase in Escherichia coli. Mol. Gen. Genet. 253:297-302. [DOI] [PubMed] [Google Scholar]
- 18.Ogata, Y., T. Mizushima, K. Kataoka, K. Kita, T. Miki, and K. Sekimizu. 1996. DnaK heat shock protein of Escherichia coli maintains the negative supercoiling of DNA against thermal stress. J. Biol. Chem. 271:29407-29414. [DOI] [PubMed] [Google Scholar]
- 19.Qi, H., R. Menzel, and Y. C. Tse-Dinh. 1996. Effect of the deletion of the σ32-dependent promoter (P1) of the Escherichia coli topoisomerase I gene on thermotolerance. Mol. Microbiol. 21:703-711. [DOI] [PubMed] [Google Scholar]
- 20.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 47:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samuels, D. S., K. E. Mach, and C. F. Garon. 1994. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumermycin-resistant gyrB. J. Bacteriol. 176:6045-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samuels, D. S., R. T. Marconi, W. Huang, and C. F. Garon. 1994. gyrB mutations in coumermycin A1-resistant Borrelia burgdorferi. J. Bacteriol. 176:3072-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohaskey, C. D., C. Arnold, and A. G. Barbour. 1997. Analysis of promoters in Borrelia burgdorferi by use of a transiently expressed reporter gene. J. Bacteriol. 179:6837-6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steere, A. C. 2001. Medical progress: Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 25.Tilly, K., R. Hauser, J. Campbell, and G. J. Ostheimer. 1993. Isolation of dnaJ, dnaK, and grpE homologues from Borrelia burgdorferi and complementation of Escherichia coli mutants. Mol. Microbiol. 7:359-369. [DOI] [PubMed] [Google Scholar]
- 26.Van Bogelen, R. A., P. M. Kelley, and F. C. Neidhardt. 1987. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J. Bacteriol. 169:26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]