Abstract
β-Exotoxin I is a nonspecific insecticidal metabolite secreted by some Bacillus thuringiensis strains. Several studies of B. thuringiensis strains that have lost the capacity to produce β-exotoxin I have suggested that there is a strong correlation between high levels of β-exotoxin I production and the ability to synthesize crystal proteins. In this study, we showed that a mutant strain, B. thuringiensis 407-1(Cry−)(Pig+), with no crystal gene, produced considerable amounts of β-exotoxin I together with a soluble brown melanin pigment. Therefore, β-exotoxin I production can take place after a strain has lost the plasmids bearing the cry genes, which suggests that these curable plasmids probably contain determinants involved in the regulation of β-exotoxin I production. Using a mini-Tn10 transposon, we constructed a library of strain 407-1(Cry−)(Pig+) mutants. We screened for nonpigmented mutants with impaired β-exotoxin I production and identified a genetic locus harboring two genes (berA and berB) essential for β-exotoxin I production. The deduced amino acid sequence of the berA gene displayed significant similarity to the ATP-binding domains of the DRI (drug resistance and immunity) family of ATP-binding cassette (ABC) proteins involved in drug resistance and immunity to bacteriocins and lantibiotics. The berB gene encodes a protein with six putative transmembrane helices, which probably constitutes the integral membrane component of the transporter. The demonstration that berAB is required for β-exotoxin I production and/or resistance in B. thuringiensis adds an adenine nucleotide analog to the wide range of substrates of the superfamily of ABC proteins. We suggest that berAB confers β-exotoxin I immunity in B. thuringiensis, through active efflux of the molecule.
Bacillus thuringiensis is a sporogenic gram-positive insect pathogen that is extensively used in biological pest control (20). It produces parasporal crystals composed of proteins called δ-endotoxins or Cry proteins (26), which are toxic to various insects on ingestion. These toxins are highly diverse and display a wide range of activity spectra useful in pest management. Some strains also produce β-exotoxin I, a nonproteinaceous toxin that, unlike the Cry toxins, is secreted into the culture medium and is not specific (3, 9). This molecule is particularly active against dipteran, coleopteran, and lepidopteran species, but it is also active against beneficial species such as the honeybee Apis mellifera (7). β-Exotoxin I is an adenine nucleotide analog (10) that is thought to inhibit RNA polymerase (27), thereby affecting insect molting and pupation, in some cases having teratogenic effects (6). β-Exotoxin I is also toxic to mammalian cells (1, 19) and is very persistent in the environment (2). It has therefore been banned for public use in accordance with World Health Organization recommendations (32).
The synthesis and export of β-exotoxin I probably require the activation of various genes (13), which have yet to be identified. However, β-exotoxin I production has been linked with the presence of plasmids bearing cry genes. Indeed, several studies have reported that the abilities to secrete β-exotoxin I and to produce crystals were transferred together to B. thuringiensis and Bacillus cereus recipient strains by conjugation (22). Plasmids of various sizes, 55 and 60 MDa (16) and 65, 70, 75, and 110 MDa (18), encoding various Cry proteins have been identified as necessary for β-exotoxin I production in various B. thuringiensis strains. Nevertheless, little effort has focused on identification of the genetic determinants involved in β-exotoxin I production (regulation, synthesis, and/or export).
We report here a study of a B. thuringiensis acrystalliferous mutant strain, designated 407-1(Cry−)(Pig+), which produces a brown soluble pigment together with an insecticidal factor active against a wide range of insect species. Both the pigment and the insecticidal factor are directly secreted into the culture supernatant. The brown pigment was characterized and identified as melanin, and the insecticidal toxin was identified as β-exotoxin I. With the aim of identifying genes involved in β-exotoxin I production (synthesis, immunity, or secretion), we constructed a random mutant library, by using a mini-Tn10 transposon delivery system, and screened it for mutants with impaired production of both the pigment (white colonies) and β-exotoxin I (nontoxic culture filtrates). Sequence analysis of the insertion site of the transposon in a clone that produced neither β-exotoxin I nor pigment showed that the transposon had been inserted into a putative ATP-binding cassette (ABC) transporter gene system, similar to those of various export systems, including the systems involved in antibiotic resistance in various bacteria. The deletion of the genes in this cassette, designated berAB (β-exotoxin resistance), confirmed that these genes were essential for the production of β-exotoxin I.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. B. thuringiensis subsp. thuringiensis strain 407 (H1 serotype) is a natural isolate originating from South America that harbors two plasmid-borne cry genes encoding two Cry toxins, 130 and 140 kDa in size in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (17). This strain was cultured at 42°C for several generations until an acrystalliferous derivative, B. thuringiensis 407(Cry−), was obtained. This mutant had lost the plasmids encoding the Cry1A and Cry1B toxins. Ethyl methanesulfonate (EMS) treatment of the acrystalliferous 407(Cry−) strain resulted in the generation of a second mutant, which secreted a brown pigment; this mutant was designated 407-1(Cry−)(Pig+) (30). Escherichia coli K-12 strains TG1 [Δ(lac-proAB) supE thi hdsD5 (F′ traD36 pro+ proB+ lacIq lacZΔM15)] and strain SCS110 [rpsL (Strr) thr leu endA thi-1 lacY gal4 galT ara tonA Tsx dam dcm supE44 Δ(lac-proAB) (F′ traD36 proAB lacIqZΔM15)] (Stratagene, La Jolla, Calif.) were used as hosts for pRN5101 construction.
TABLE 1.
Strains and plasmids
| Strain or plasmid designation | Relevant genotype or plasmid characteristics | Reference |
|---|---|---|
| Strains | ||
| 407(Cry+) | Crystalliferous natural B. thuringiensis (H1 serotype) | 17 |
| 407(Cry−) | Acrystalliferous derivative of the wild-type B. thuringiensis strain 407(Cry+) isolated by O. Arantes | 17 |
| 407-1(Cry−)(Pig+) | Pigment- and exotoxin-producing mutant of strain 407(Cry−). This strain was obtained by O. Arantes, by EMS mutagenesis. | 30 |
| 407-1(Cry−)(Pig+)::mini-Tn10 | Mutant of strain 407(Cry−)(Pig+) resulting from a single mini-Tn10 transposition; low levels of pigment and β-exotoxin I secretion | This report |
| 407-1(Cry−)(Pig+)berAB::aphA3 | Strain 407-1(Cry−)(Pig+) with a disruption of the berAB locus encoding a putative ABC transporter | This report |
| Plasmids | ||
| pIC333 | Delivery vector used for mini-Tn10 transposition experiment | 28 |
| pRN5101 | Thermosensitive vector used in gene disruption experiments | 31 |
| pRN5101ΩberAB::aphA3 | Recombinant vector used for berAB gene replacement | This report |
Growth conditions.
B. thuringiensis strains were grown at 30°C with shaking at 170 rpm, in Luria-Bertani (LB) medium or brain heart infusion (Difco) supplemented with the following selective antibiotics: erythromycin (10 μg/ml), spectinomycin (300 μg/ml), and kanamycin (200 μg/ml). We assessed growth in liquid medium by monitoring optical density at 600 nm. The point T0, which corresponds to the end of exponential growth, was plotted by regression for each culture growth curve. E. coli K-12 strains TG1 and SCS110 were cultured in LB medium at 37°C, and selection was achieved with ampicillin (100 μg/ml), erythromycin (5 μg/ml), and kanamycin (20 μg/ml).
Bioassays of insecticidal activity.
We used a free ingestion technique to assess the toxicity to Spodoptera littoralis (Lepidoptera) of bacterial culture supernatant preparations. The strains were grown on LB agar plates at 30°C; we then inoculated 100 ml of LB medium with a single colony and incubated the culture at 30°C, with shaking, for 24 h. Cultures were harvested at midsporulation (before cell lysis) by centrifugation at 14,000 × g for 10 min at 4°C, and the supernatants were filtered twice through Nalgene filter units with 0.2-μm pores (Nalgene). The resulting extracts, to be used for toxicity assays and β-exotoxin I determination, were stored at −20°C until use. S. littoralis larvae were fed an artificial diet dispensed into 50-well plastic plates (each with an area of 1.65 cm2). Supernatant (25 μl) was applied uniformly over the surface of the food and allowed to dry. One neonatal larva was placed in each of the 35 wells, and the plate was incubated for 10 days at 25°C with a photoperiod of 16 h of light and 8 h of darkness and 70% relative humidity. Mortality was recorded on days 3, 6, and 10. In these conditions, the concentration of technical grade β-exotoxin I required to kill 50% of the S. littoralis neonates was 30 μg/ml. Typically, the larvae displayed impaired molting and developed into abnormal, white, puffy first instars.
Detection and quantification of β-exotoxin I.
β-Exotoxin I was isolated from the culture supernatant by solvent extraction and quantified by high-performance liquid chromatography (HPLC) (11). Briefly, for solvent extraction, acetone was added to 0.2 ml of the culture supernatant to a final concentration of 90%, and the mixture was centrifuged. The pellet was solubilized in 0.2 ml of double-distilled water. Acetonitrile was added to a final concentration of 40%, and the mixture was then centrifuged. The pellet was discarded, and the acetonitrile concentration of the supernatant was brought up to 90%. The precipitate was collected by centrifugation, and the pellet was solubilized in 100 μl of 50 mM potassium phosphate buffer, pH 2.5. For HPLC, we injected 25 μl of the sample into a Lichrospher (Merck) C18 end-capped 4- by 250-mm column. A gradient of 5 to 15% methanol in 50 mM potassium phosphate buffer, pH 2.5, was developed over 10 min. The flow rate was 1 ml/min, and UV absorption was monitored at 260 nm. β-Exotoxin I was eluted at 5.5 min. The detection limit of this method was 2 μg/ml. The recovery yield for β-exotoxin I in the supernatant of cultures grown in LB medium was 80% (11). A standard sample (70% purity) of β-exotoxin I was kindly provided by I. Thiery from the Laboratoire des Bactéries Entomopathogènes (Institut Pasteur, Paris, France).
Pigment purification.
The soluble dark brown pigment secreted into the medium was precipitated by adding 1 M HCl to a filtrate sample (10 ml) from a sporulating culture of strain 407-1(Cry−)(Pig+) in liquid LB medium (pH 9), after 24 h of culture. The resulting precipitate was pelleted by centrifugation (10 min, 5,000 × g), washed with 70% ethanol, and dried. At this stage, the pigment had a granular appearance, similar to that of the synthetic melanin obtained by tyrosine oxidation with hydrogen peroxide (Sigma Chemicals; M-8631). A synthetic melanin sample and the pigment sample were solubilized in 2 ml of 50 mM Tris-HCl (pH 8.5; 10-mg/ml final concentration) and analyzed by UV spectrophotometry, with the Tris buffer solution used as the blank, in a Beckman DU-series 70 spectrophotometer.
Random insertion mutagenesis.
The mini-Tn10 transposon used in this study is a derivative of the Tn10 transposon of Salmonella enterica serovar Typhimurium. It is delivered by the pIC333 vector and used for random insertion mutagenesis (28). The pIC333 plasmid includes a thermosensitive origin of replication and a gene that confers resistance to erythromycin. It is eliminated after transposition by a shift in temperature to 40°C. An insertion library was constructed in B. thuringiensis strain 407-1(Cry−)(Pig+), as described by Gominet et al. (12). Mutants with impaired pigment production were selected, and their supernatants were tested for insect toxicity. As the mini-Tn10 element contains an E. coli replicon, we digested the chromosomal DNA of insertion mutants with EcoRI (no restriction site for this enzyme is present in the mini-Tn10 element). The fragments were ligated and used to transform E. coli TG1 cells. The mini-Tn10 transposon conferred resistance to spectinomycin in E. coli (60 μg/ml), making it possible to select clones transformed with fragments containing the insertion locus. The chromosomal DNA flanking the insertion locus was sequenced, primarily with primers binding to the extremities of the transposon, and the sequence obtained was extended by chromosome walking, using the chromosome of strain B. thuringiensis 407(Cry−), as described in the work of Okstad et al. (21). Part of this extended sequence was determined by M. Rose (Institut für Mikrobiologie, J. W. Göethe Universität, Frankfurt, Germany).
Construction of a berAB deletion mutant.
We disrupted the berAB genes on the chromosome of B. thuringiensis strain 407-1(Cry−)(Pig+) with pRN5101, generated by inserting pE194ts (31) into the ClaI site of pBR322. The DNA sequence of the downstream and upstream regions of berAB was generated by PCR, by using 407-1(Cry−)(Pig+) total DNA as a template and the following oligonucleotides (restriction sites are indicated in parentheses): orf1-BamHI, 5′ (CGGATCC)GGTTCGGTCCTAAATAACC 3′; orf1-XbaI, 5′ (CGTCTAGA)TCAACCGTTACGAGTCGACC 3′; orf2-EcoRI, 5′ (CGGAATTC)GGTATGGAAAGTCCGATTCAAAACG 3′; and orf2-EagI, 5′ (CGCGGCCG)CGGTAAATCCCTCTTCTTAAACAC 3′.
The two PCR fragments were digested with BamHI and XbaI and with EcoRI and EagI, respectively, and were purified from agarose gels. The aphA3 gene of Enterococcus faecalis (Kmr cassette) (29), which confers resistance to kanamycin, was isolated as a 1.6-kb XbaI-EcoRI DNA fragment. The three DNA fragments were then ligated between the BamHI and EcoRI sites of pRN5101, to give pRN5101ΩberAB::aphA3. The intact berAB genes on the chromosome were replaced by their disrupted copy. This was achieved by double crossing-over, as described by Bravo et al. (4). The recombinant strains were selected at a nonpermissive temperature (40°C), with kanamycin. Strains carrying the disrupted copy of berAB and lacking pRN5101ΔberAB::aphA3 were kanamycin resistant but erythromycin sensitive. We checked that the locus was disrupted as planned by PCR, with primers binding to the chromosomal sequence and primers binding within the aphA3 gene.
Nucleotide sequence accession number.
The DNA sequence of the insertion sequence has been deposited in GenBank under accession no. AF499614.
RESULTS
Toxicity of the culture supernatants of strain 407(Cry+) and its acrystalliferous derivatives.
Strains 407(Cry+), 407(Cry−), and 407-1(Cry−)(Pig+) were cultured in LB medium, at 30°C for 24 h. The cultures were centrifuged, and the filtered supernatants were tested for toxicity to larvae of S. littoralis. The supernatants of strains 407(Cry+) and 407-1(Cry−)(Pig+) caused 80 to 100% mortality, whereas the supernatant of strain 407(Cry−) was not toxic. The toxic supernatants caused a slow death, most of the larvae dying within 15 days after passing through a long moribund stage. The effects were particularly obvious at molting, as poisoned larvae did not reach the second-instar stage. These symptoms are typical of β-exotoxin I poisoning.
Chemical characterization of the pigment and of the insecticidal compound secreted by the strain 407-1(Cry−)(Pig+) mutant.
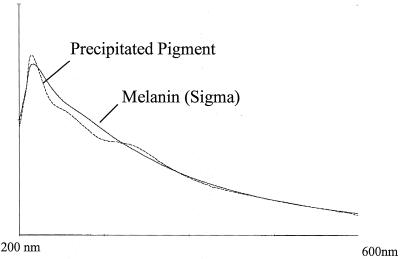
The pigment produced by strain 407-1(Cry−)(Pig+) took color only in alkaline conditions and in the presence of oxygen. The pigment was precipitated from the supernatant of a stationary-phase culture of strain 407-1(Cry−)(Pig+) by adding 1 M HCl. The precipitate was collected by centrifugation, washed with ethanol, solubilized in Tris (pH 8.5), and compared with a sample of synthetic melanin prepared by tyrosine oxidation. The UV spectrum of the pigment produced by strain 407-1(Cry−)(Pig+) was similar to that of melanin (Fig. 1). This solubilized fraction was not toxic to larvae of S. littoralis.
FIG. 1.
Comparison of the UV and visible spectra of a sample of the precipitated pigment of B. thuringiensis strain 407-1(Cry−)(Pig+) solubilized in Tris (pH 8.5) with those of commercial melanin.
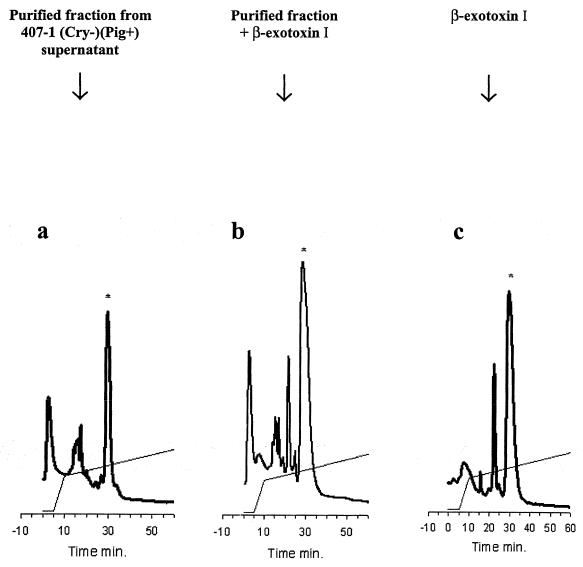
The insecticidal exotoxin that accumulated in the culture supernatant of strain 407-1(Cry−)(Pig+) was a low-molecular-weight molecule resistant to heating at 100°C for 10 min. Its UV absorption at 258 nm (pH 6) and overall absorption spectrum were identical to those of β-exotoxin I (data not shown). The toxin was purified by HPLC, on an anion-exchange column (MonoQ column) and in reverse phase (C18 column). The addition of standard β-exotoxin I to the purified toxin proportionally increased the peak of the unknown toxin in anion-exchange chromatography (Fig. 2). This demonstrated that the toxic compound was β-exotoxin I, excluding other insecticide metabolites such as β-exotoxin II (18). Quantification of β-exotoxin I by HPLC in strains 407(Cry+) and 407-1(Cry−)(Pig+) indicated that the supernatants of these strains yielded up to 90 and 172 μg of β-exotoxin I/ml, respectively, whereas strain 407(Cry−) produced 4.5 μg of β-exotoxin I/ml (Table 2). The amounts of β-exotoxin I produced by strains 407(Cry+) and 407-1(Cry−)(Pig+) are consistent with the observed pattern of mortality, suggesting that the toxicity of these strains was due to β-exotoxin I. By contrast, the amounts of β-exotoxin I produced by strain 407(Cry−) were well below 30 μg/ml, the threshold for detectable toxicity to S. littoralis in our test conditions. The pigmented and toxic phenotype of 407-1(Cry−)(Pig+) was very stable at high temperature (42°C), and we obtained no revertant clones in cultures over more than 50 generations (data not shown).
FIG. 2.
Ion-exchange chromatography of the insecticidal compound of strain 407-1(Cry−)(Pig+). (a) Purified compound; (b) coinjection of the purified compound with 1.5 mg of β-exotoxin I standard; (c) 1.5 mg of β-exotoxin I standard. The ion-exchange chromatography was performed on a MonoQ column (Amersham Biosciences) in Bis-Tris, pH 6.0, with a gradient of sodium chloride.
TABLE 2.
Toxicity and β-exotoxin I concentrations in the culture supernatants of the various B. thuringiensis strain 407 varients
| Strain of B. thuringiensis | Insecticidal activity of culture supernatanta | β-Exotoxin I concn (μg/ml; T0 + 10 h) |
|---|---|---|
| 407(Cry+) | 80-100% mortality | 90 |
| 40(Cry−) | Nontoxic | 4.5 |
| 407-1(Cry−)(Pig+) | 80-100% mortality | 172 |
| 407-1(Cry−)(Pig+) berA::Tn10 | Nontoxic | 2 |
| 407-1(Cry−)(Pig+) ΔberAB::aphA3 | Nontoxic | 2.2 |
The 50% lethal dose of β-exotoxin I for the first-instar larvae of S. littoralis is 30 μg/ml.
Time course of β-exotoxin I accumulation in strains 407-1(Cry−)(Pig+) and 407(Cry+).
We studied the accumulation over time of β-exotoxin I in strains 407-1(Cry−)(Pig+) and 407(Cry+), by precisely determining the concentration of β-exotoxin I in the culture supernatants of these strains by sampling 2 ml of culture at various times. We observed no production of β-exotoxin I during the exponential growth phase; this toxin was produced only during the stationary phase. We monitored β-exotoxin I between T0 (the onset of the stationary phase of the bacterial culture) and T10 (10 h after T0) and found that, although this toxin was continuously produced throughout the stationary phase, particularly large amounts were produced between T3 and T5 in strain 407-1(Cry−)(Pig+) (Fig. 3). Increases in β-exotoxin I levels was observed until T10, although spores had already formed in most of the bacterial cells. The pigment was first detected in the culture supernatant at about T8, coinciding with an increase in pH, conditions changing from acidic to basic. In strain 407(Cry+), β-exotoxin I accumulation was maximal between T6 and T10 (data not shown).
FIG. 3.
Time course of β-exotoxin I accumulation in the supernatant of B. thuringiensis strain 407-1(Cry−)(Pig+). Strain 407-1(Cry−)(Pig+) was cultured in LB medium at 30°C, with shaking at 170 rpm. Growth was monitored by monitoring optical density at 600 nm and was plotted on a logarithmic scale. T0, the onset of the stationary phase, is indicated on the growth curve. The supernatant was sampled at various times after T0 (T1, T3, T5, T7, and T10), plotted on the y axis. β-Exotoxin I concentration measured by HPLC at these time points is expressed in micrograms per milliliter and is represented by vertical bars.
Transposon mutagenesis of strain 407-1(Cry−)(Pig+).
Several B. thuringiensis mutants producing a melanin pigment and displaying higher-than-normal levels of toxicity have been described elsewhere (14, 23, 25) or for other pathogenic species of bacteria (5, 15). Assuming a possible common biosynthetic pathway (or regulation mechanism) for pigment and β-exotoxin I production in strain 407-1(Cry−)(Pig+), we performed Tn10 insertion mutagenesis, with the shuttle vector pIC333, and used pigment production as a genetic marker, for the screening of nonpigmented and possibly nontoxic mutant clones. We tested the toxicity to S. littoralis first-instar larvae of clones with impaired pigment production. One such nonpigmented mutant generated a nontoxic supernatant yielding only 2 μg of β-exotoxin I/ml after 24 h. We checked that the insertion of the mini-Tn10 transposon was unique by Southern blotting (data not shown), and the insertion locus was rescued by digesting chromosomal DNA with EcoRI. The insertion region was cloned and sequenced.
Analysis of the sequence of the insertion locus.
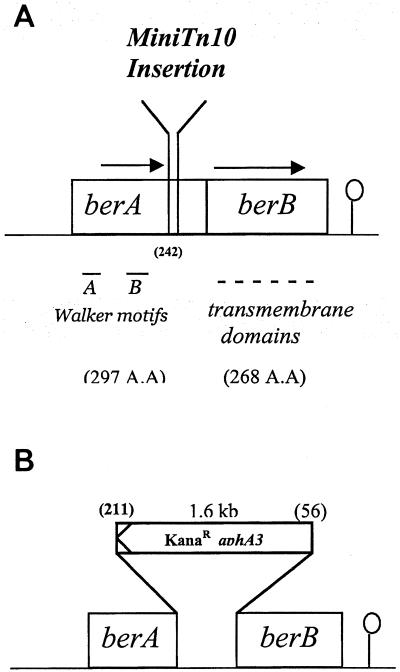
Sequence analysis revealed that the mini-Tn10 transposon had been inserted at the end of a putative 894-bp open reading frame (ORF) encoding a putative protein displaying Walker A and B ATP-binding motifs in its N-terminal region, as typically found in the members of a large family of proteins called the ABC transporters. This gene was designated berA, for β-exotoxin resistance (Fig. 4). The TAA termination codon of berA overlaps with the ATG initiation codon of a second putative 807-bp ORF that was designated berB, placing this ORF in the −1 reading frame with respect to berA. It is therefore likely that berA and berB are transcriptionally and translationally coupled. The berB gene encodes a putative protein with six hydrophobic transmembrane domains, as typically found in membrane-spanning proteins. BerA is 42% identical and 62% similar to the ATP-binding protein of a putative ABC transporter from Thermotoga maritima and 39% identical and 59% similar to YhaQ, a putative ABC ATPase from Bacillus subtilis, which is similar to BcrA, which is known to confer bacitracin resistance in Bacillus licheniformis (24). The putative BerB protein is also similar (21% identity and 43% similarity) to the putative ABC transporter permease from T. maritima, which is associated with the ABC ATP-binding protein similar to BerA. In the two species, the nucleotide overlap between the two genes is also conserved.
FIG. 4.
Schematic representation of the studied region of B. thuringiensis 407(Cry−) deduced from its nucleotide sequence. (A) Description of the mini-Tn10 locus of insertion. (B) berAB disruption by insertion of a kanamycin resistance cassette encoding aphA3. The different ORFs are boxed with their sizes (base pairs) and directions of transcription. The 8-nucleotide overlap between the two genes is not indicated in this figure. The loci of insertion and of deletion are indicated by the amino acid numbers in parentheses.
The berAB genes are involved in β-exotoxin I production and/or resistance.
To determine the role of berAB in β-exotoxin I production, we disrupted these genes in strains 407-1(Cry−)(Pig+) and 407(Cry+), by integrating a gene conferring resistance to kanamycin into their DNA sequence. The aphA3 gene was inserted in the opposite orientation, and much of the internal sequence of the berAB genes was removed. As for the 407-1(Cry−)(Pig+)berA::Tn10 mutant, the 407-1(Cry−)(Pig+)berAB::aphA3 mutant was not toxic to S. littoralis and produced only 2.2 μg of β-exotoxin I/ml in its culture supernatant (Table 2). This is a very small amount of β-exotoxin I compared with the 172 μg/ml produced by strain 407-1(Cry−)(Pig+) (Table 2). However, unlike mutant 407-1(Cry−)(Pig+)berA::Tn10, the 407-1(Cry−)(Pig+)berAB::aphA3 strain showed no impairment of pigment production. We were unable to obtain a similar deletion in the wild-type strain 407(Cry+), despite five attempts with the same pRN5101berAB::aphA3 plasmid. In each experiment, we obtained several deletion mutants that appeared to be acrystalliferous [i.e., similar in phenotype to B. thuringiensis 407(Cry−)] on microscopy. It is likely that, as for strain 407(Cry−), these mutants did not produce crystals due to loss of the cry-bearing plasmid. The culture supernatants of these 407(Cry−)ΔberAB mutants were not toxic to S. littoralis.
DISCUSSION
This study reports an analysis of the B. thuringiensis strain 407-1(Cry−)(Pig+), which overproduces β-exotoxin I, together with a brown pigment. A similar pigmented phenotype was previously described for two different B. thuringiensis strains exposed to a mutagenic agent (14, 23, 25). These two strains produced a brown pigment identical to synthetic melanin obtained by the persulfate oxidation of tyrosine and were more toxic to insects than were the parental strains from which they were derived. However, the genes conferring higher levels of insecticidal activity or the Pig+ phenotype were not characterized. We partially purified the pigment produced by strain 407-1(Cry−)(Pig+) and found that its UV spectrum was also similar to that of melanin. In Vibrio cholerae, pigment production is often associated with higher levels of virulence (15), suggesting that pigments and toxins may share common determinants. If we assume that the concomitant production of pigment and toxin in Pig+ mutants is not fortuitous, then the genetic study of these strains is promising for the identification of a possible cross-regulation network.
Purification of the exotoxin present in the supernatant of strain 407-1(Cry−)(Pig+) led to the identification of this compound as β-exotoxin I. The isolation of an acrystalliferous B. thuringiensis mutant that produced large amounts of β-exotoxin I was unexpected. Indeed, analysis of strain 407(Cry−), which has lost the capacity to produce high levels of β-exotoxin I, supported the generally held notion that β-exotoxin I production is linked to the presence of plasmids bearing cry genes (16, 18). Ozawa and Iwahana (22), by transferring a single 62-MDa plasmid from a B. thuringiensis subsp. darmstadiensis strain by conjugation, even managed to produce both β-exotoxin I and a crystal in a strain of B. cereus. The level of β-exotoxin I production was very low in strain 407(Cry−), and the protein crystal disappeared, but β-exotoxin I production was not entirely abolished. EMS treatment of this Cry− strain restored its capacity for high levels of β-exotoxin I production and led to the production of a melanin-like pigment. This suggests that β-exotoxin I production does not depend purely on cry plasmids and that these curable plasmids contain genetic determinants that are probably involved in the regulation of β-exotoxin I production but not directly responsible for the biosynthesis of this molecule. The genes for β-exotoxin I synthesis are probably chromosomal or located on other plasmids that are not easily eliminated.
Based on the assumption that the simultaneous appearance of the pigment and β-exotoxin I was not fortuitous in strain 407-1(Cry−)(Pig+), we performed transposition mutagenesis to select a nonpigmented mutant that was not toxic. In this mutant, the transposon was inserted into a chromosomal region that included two overlapping genes, designated berA and berB. The structural elements and similarity predicted for BerA and BerB strongly suggest that these proteins constitute a putative ABC transporter similar to the various systems involved in resistance to small molecules such as bacteriocins or that are responsible for lantibiotic immunity (8). These proteins belong to the DRI (drug resistance and immunity) subfamily of ABC systems. It is thought that such systems confer drug resistance by active efflux of the molecules through the membrane, but no direct experimental proof has yet been presented that this is indeed the case. It has been shown previously that β-exotoxin I, a small hydrophilic molecule (metabolite), is not released from the bacterial cell by free diffusion but is instead actively exported (13). However, no mechanism for this export has ever been proposed. The culture supernatant of strain 407-1(Cry−)(Pig+)berAB::aphA3 was not toxic and contained less than 2 μg of β-exotoxin I/ml. In contrast to our findings for strain 407-1(Cry−)(Pig+), we were unable to obtain a berAB deletion mutant in the wild-type strain 407(Cry+) unless it had a Cry− phenotype. The fact that we systematically obtained mutants of this type suggests that berAB disruption is not compatible with plasmid-dependent β-exotoxin I expression, which may be constitutive. It is therefore possible that, in the wild-type background, the accumulation of β-exotoxin I in the bacterial cell may be lethal, as this toxin has been reported previously to cause deleterious effects in various cellular models (1, 19). It is therefore possible that the berAB genes are directly involved in β-exotoxin I translocation through the bacterial membrane. In strain 407-1(Cry−)(Pig+), β-exotoxin I production may be subject to negative feedback control, and neither exotoxin production nor deleterious effects are observed in the 407(Cry−)(Pig+)berAB::aphA3 mutant. For unknown reasons, this mutant displayed no impairment in pigmentation, unlike the transposition mutant. This absence of pigmentation in the transposition mutant may be due to an indirect effect of mini-Tn10 on genes located upstream or downstream from the berAB operon. Therefore, although they may have common regulators, the production of pigment is probably independent of the production of β-exotoxin I.
In summary, this study of strain 407(Cry+), which produces high levels of β-exotoxin I, and of two of its acrystalliferous derivatives reveals that (i) the genetic determinants responsible for β-exotoxin I production found on Cry-dependent plasmids are likely to be regulatory elements; (ii) large amounts of β-exotoxin I can be produced in the absence of such plasmids; and (iii) a putative ABC transporter, encoded by berAB, is essential for β-exotoxin I production.
Acknowledgments
We thank Alex Edelman and Associates for editing the English manuscript. The B. thuringiensis mutant strain 407-1(Cry−)(Pig+) used in this study was kindly provided by O. Arantes (Universidade Estadual de Londrina, Brazil) and was isolated in her laboratory by G. Vilas-Boas.
This work was supported by the Institut Pasteur and the Institut National de la Recherche Agronomique (INRA). Funds for sequencing were supplied by the European Community (EC contract Bio-CT96-0655). Sylvain Espinasse was supported by a grant from Aventis Crop Science, Ghent, Belgium.
REFERENCES
- 1.Beebee, T., A. Korner, and R. P. Bond. 1972. Differential inhibition of mammalian ribonucleic acid polymerases by an exotoxin from Bacillus thuringiensis. The direct observation of nucleoplasmic ribonucleic acid polymerase activity in intact nuclei. Biochem. J. 127:619-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benz, G. 1966. On the chemical nature of the heat stable exotoxin of Bacillus thuringiensis. Experientia 22:81-82. [DOI] [PubMed] [Google Scholar]
- 3.Bond, R. P. M., C. B. C. Boyce, H. H. Rogoff, and T. R. Shieh. 1971. The thermostable exotoxin of Bacillus thuringiensis, p. 275-303. In H. D. Burges and N. W. Hussey (ed.), Microbial control of insects and mites. Academic Press, London, United Kingdom.
- 4.Bravo, A., H. Agaisse, S. Salamitou, and D. Lereclus. 1996. Analysis of cryIAa expression in sigE and sigK mutants of Bacillus thuringiensis. Mol. Gen. Genet. 250:734-741. [DOI] [PubMed] [Google Scholar]
- 5.Buchrieser, C., M. Prentice, and E. Carniel. 1998. The 102-kilobase unstable region of Yersinia pestis comprises a high-pathogenicity island linked to a pigmentation segment which undergoes internal rearrangement. J. Bacteriol. 180:2321-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgerjon, A., G. Biache, and P. Cals. 1969. Teratology of the Colorado potato beetle, Leptinotarsa decemlineata, as provoked by larval administration of the thermostable toxin of Bacillus thuringiensis. J. Invertebr. Pathol. 14:274-278. [Google Scholar]
- 7.Cantwell, G. E., D. A. Knox, and A. S. Michael. 1964. Mortality of honey bees, Apis mellifera Linnaeus, fed exotoxin of Bacillus thuringiensis var. thuringiensis Berliner. J. Insect Pathol. 6:532-536. [Google Scholar]
- 8.Dassa, E., and P. Bouige. 2001. The ABC of ABCs: a phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 152:211-229. [DOI] [PubMed] [Google Scholar]
- 9.De Barjac, H., and R. Dedonder. 1965. Isolement d'un nucléotide identifiable à la “toxine thermostable” de Bacillus thuringiensis var Berliner. C. R. Acad. Sci. Paris 260:7050-7053. [PubMed] [Google Scholar]
- 10.Farkas, J., K. Sebesta, K. Horska, Z. Samek, J. Dollijs, and F. Storm. 1969. The structure of exotoxin of Bacillus thuringiensis var. gelechiae. Collect. Czech. Chem. Commun. 34:1118-1120. [Google Scholar]
- 11.Gohar, M., and S. Perchat. 2001. Sample preparation for beta-exotoxin determination in Bacillus thuringiensis cultures by reversed-phase high performance liquid chromatography. Anal. Biochem. 298:112-117. [DOI] [PubMed] [Google Scholar]
- 12.Gominet, M., L. Slamti, N. Gilois, M. Rose, and D. Lereclus. 2001. Oligopeptide permease is required for expression of the Bacillus thuringiensis plcR regulon and for virulence. Mol. Microbiol. 40:963-975. [DOI] [PubMed] [Google Scholar]
- 13.Horska, K., J. Vankova, and K. Sebesta. 1975. Determination of exotoxin in Bacillus thuringiensis cells. Z. Naturforsch. 30c:120-123. [DOI] [PubMed] [Google Scholar]
- 14.Hoti, S. L., and K. Balaraman. 1993. Formation of melanin pigment by a mutant of Bacillus thuringiensis H-14. J. Gen. Microbiol. 139:2365-2369. [DOI] [PubMed] [Google Scholar]
- 15.Ivins, B. E., and R. K. Holmes. 1981. Factors affecting phaeomelanin production by a melanin-producing (mel) mutant of Vibrio cholerae. Infect. Immun. 34:895-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuzina, L. V., A. L. Andreeva, E. M. Aslanian, A. P. Dobritsa, E. B. Danilova, and E. A. Bocharov. 1993. Study of the nature of determinants for synthesis of delta-endotoxin and beta-exotoxin in Bacillus thuringiensis subsp. thuringiensis strains—bitoxybacillin producers. Mol. Gen. Mikrobiol. Virusol. 1: 20-24. (In Russian.) [PubMed] [Google Scholar]
- 17.Lereclus, D., O. Arantes, J. Chaufaux, and M. Lecadet. 1989. Transformation and expression of a cloned delta-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol. Lett. 51:211-217. [DOI] [PubMed] [Google Scholar]
- 18.Levinson, B. L., K. J. Kasyan, S. S. Chiu, T. C. Currier, and J. M. J. Gonzalez. 1990. Identification of beta-exotoxin production, plasmids encoding beta-exotoxin, and a new exotoxin in Bacillus thuringiensis by using high-performance liquid chromatography. J. Bacteriol. 172:3172-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackedonski, V. V., N. Nikolaev, K. Sebesta, and A. A. Hadjiolov. 1972. Inhibition of ribonucleic acid biosynthesis in mice liver by the exotoxin of Bacillus thuringiensis. Biochim. Biophys. Acta 272:56-66. [DOI] [PubMed] [Google Scholar]
- 20.Navon, A., and K. Van Frankenhuyzen. 2000. Field application and resistance management, p. 355-382. In J. F. Charles, A. Delecluse, and C. Nielsen-Leroux (ed.), Entomopathogenic bacteria: from laboratory to field application. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 21.Okstad, O. A., M. Gominet, B. Purnelle, M. Rose, D. Lereclus, and A. B. Kolsto. 1999. Sequence analysis of three Bacillus cereus loci carrying PIcR-regulated genes encoding degradative enzymes and enterotoxin. Microbiology 145:3129-3138. [DOI] [PubMed] [Google Scholar]
- 22.Ozawa, K., and H. Iwahana. 1986. Involvement of a transmissible plasmid in heat-stable exotoxin and delta-endotoxin production in Bacillus thuringiensis subspecies Darmstadiensis. Curr. Microbiol. 13:337-340. [Google Scholar]
- 23.Patel, K. R. 1999. A mutant of Bacillus thuringiensis producing a dark pigment with increased UV resistance and insecticidal activity. J. Invertebr. Pathol. 67:120-124. [Google Scholar]
- 24.Podlesek, Z., A. Comino, B. Herzog-Velikonja, D. Zgur-Bertok, R. Komel, and M. Grabnar. 1995. Bacillus licheniformis bacitracin-resistance ABC transporter: relationship to mammalian multidrug resistance. Mol. Microbiol. 16:969-976. [DOI] [PubMed] [Google Scholar]
- 25.Saxena, D., E. Ben-Dov, R. Manasherob, Z. Barak, S. Boussiba, and A. Zaritsky. 2002. A UV tolerant mutant of Bacillus thuringiensis subsp. kurstaki producing melanin. Curr. Microbiol. 44:25-30. [DOI] [PubMed] [Google Scholar]
- 26.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sebesta, K., and K. Horska. 1970. Mechanism of inhibition of DNA-dependent RNA polymerase by exotoxin of Bacillus thuringiensis. Biochim. Biophys. Acta 209:357-376. [DOI] [PubMed] [Google Scholar]
- 28.Steinmetz, M., and R. Richter. 1994. Easy cloning of mini-Tn10 insertions from the Bacillus subtilis chromosome. J. Bacteriol. 176:1761-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trieu-Cuot, P., and P. Courvalin. 1983. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3′5"-aminoglycoside phosphotransferase type III. Gene 23:331-341. [DOI] [PubMed] [Google Scholar]
- 30.Vilas-Boas, L. A., G. F. Vilas-Boas, H. O. Saridakis, M. V. Lemos, D. Lereclus, and O. M. Arantes. 2000. Survival and conjugation of Bacillus thuringiensis in a soil microcosm. FEMS Microbiol. Ecol. 31:255-259. [DOI] [PubMed] [Google Scholar]
- 31.Villafane, R., D. H. Bechhofer, C. S. Narayanan, and D. Dubnau. 1987. Replication control genes of plasmid pE194. J. Bacteriol. 169:4822-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. 1999. Guidelines specification for bacterial larvicides for public health use, WHO/CDS/CPC/WHOPES/99.2. Report of the WHO Informal Consultation, 28 to 30 April 1999. World Health Organization, Geneva, Switzerland.