Abstract
Aeromonas salmonicida subsp. salmonicida, the etiological agent of furunculosis, is an important fish pathogen. We have screened this bacterium with a broad-host-range probe directed against yscV, the gene that encodes the archetype of a highly conserved family of inner membrane proteins found in every known type III secretion system. This has led to the identification of seven open reading frames that encode homologues to proteins functioning within the type III secretion systems of Yersinia species. Six of these proteins are encoded by genes comprising a virA operon. The A. salmonicida subsp. salmonicida yscV homologue, ascV, was inactivated by marker replacement mutagenesis and used to generate an isogenic ascV mutant. Comparison of the extracellular protein profiles from the ascV mutant and the wild-type strain indicates that A. salmonicida subsp. salmonicida secretes proteins via a type III secretion system. The recently identified ADP-ribosylating toxin AexT was identified as one such protein. Finally, we have compared the toxicities of the wild-type A. salmonicida subsp. salmonicida strain and the ascV mutant against RTG-2 rainbow trout gonad cells. While infection with the wild-type strain results in significant morphological changes, including cell rounding, infection with the ascV mutant has no toxic effect, indicating that the type III secretion system we have identified plays an important role in the virulence of this pathogen.
Aeromonas salmonicida subsp. salmonicida, the causal agent of furunculosis in salmonids, causes large economic losses in the aquaculture of trout and salmon. The disease is characterized by the presence of hemorrhagic and necrotic lesions in the gills, gut, and muscle. Due to the high mortality and contagious nature of the disease, large amounts of antibiotics are often used in closed and open waters for therapy of furunculosis (19). Vaccination has become an important strategy in the control of furunculosis among farmed fish (10); however, current vaccines display considerable variability in efficacy, and epizootics commonly occur in fish farms. In order to develop more effective control measures, a better understanding of the virulence attributes of A. salmonicida is needed. To date, several potential virulence factors of A. salmonicida have been reported. These include the surface layer protein (9), salmolysin (25), the serine protease AspA (29), and the glycerolipid-cholesterol acyltransferase complexed with lipopolysaccharide (18). However, the roles these factors play in pathogenesis in vivo remain unclear. We have recently reported the identification of an ADP-ribosyltransferase toxin (AexT) in A. salmonicida subsp. salmonicida that was shown to play a direct role in virulence (6). This toxin has been shown to possess high sequence similarity to Pseudomonas aeruginosa exoenzyme S (ExoS), a protein that is secreted by a type III secretion system (TTSS) (31). This information, coupled with observations that secretion of AexT occurs only in contact with fish cells or, alternately, in low-calcium medium, prompted us to speculate that a TTSS is present in A. salmonicida subsp. salmonicida. To this end, we have screened this bacterium with a broad-host-range probe against yscV (formerly designated lcrD) of Yersinia entercolitica, a gene that encodes an inner membrane component of the type III secretion apparatus (21). The results of this study have led to the identification of several TTSS genes that together comprise an analogue of the virA locus, which is central to the TTSS of many gram-negative pathogens. In this communication, we present the findings of these studies and demonstrate that knockout mutagenesis of the ycsV homologue in A. salmonicida subsp. salmonicida prevents secretion of the AexT toxin. Furthermore, we utilize a fish cell infection model to show that inactivation of the yscV homologue significantly reduces the bacterium's pathogenicity.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and cloning vectors.
A summary of the bacterial strains and plasmids used in this study is provided in Table 1. A. salmonicida strains were cultured on Luria-Bertani (LB) agar plates at 18°C unless otherwise indicated. Escherichia coli strains were routinely grown in LB agar or broth at 37°C. Liquid cultures of A. salmonicida were grown in Trypticase soy broth (TSB; Becton Dickinson). The media used for selection included sucrose (15% [wt/vol]) in Trypticase soy agar (TSA; Becton Dickinson) and sucrose (15% [wt/vol]) in TSB. When indicated, antibiotics were added to the culture media at the following final concentrations: for Escherichia coli, ampicillin, 100 μg/ml; tetracycline, 20 μg/ml; and kanamycin, 50 μg/ml; for A. salmonicida, rifampin, 20 μg/ml; and kanamycin, 40 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Properties | Source or reference |
|---|---|---|
| Strains | ||
| A. salmonicida | ||
| JF2267 | Virulent isolate | 6 |
| JF2646 | Spontaneous Rifr derivative of JF2267 | This study |
| JF2678 | ΔascV::Km | This study |
| E. coli | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | 7 |
| S17-1 | thi pro hsdR hsdM+recA [RP4 2-Tc::Mu-Km::Tn7 (Tpr Smr) Tra+] | 23 |
| Y. enterocolitica NZ63-91 | yscV+ | SNRLa |
| Plasmids | ||
| pBSK | Bluescript II SK(−), cloning and sequencing | Stratagene |
| pSSVI186 | Kanamycin resistance determinant, located on a 1.3-kb PstI fragment from Tn903 | 28 |
| pSUP202sac | Mobilizable suicide vector, confers SucS (Mob+sacRB+ Tetr) | 27 |
SNRL, Swiss National Reference Laboratory for Foodborne Disease.
Screening for the presence of TTSS genes in A. salmonicida.
A. salmonicida subsp. salmonicida strain JF2267 was screened for the presence of a TTSS with a probe directed against yscV. The yscV gene was first amplified from Y. enterocolitica strain NZ63-91 with the primers LCRD-L (CCGGAATTCATCCCCATGATCTTGAGT) and LCRD-R (CCGGAATTCTATCGCTACCCAAGTCTG). The presence of EcoRI restriction sites in the two primers (underlined) allowed for the subsequent cloning of the PCR product into pBSK. In order to generate a digoxigenin (DIG)-labeled probe, the EcoRI fragment was excised from purified plasmid and used as a template for PCR (with primers LCRD-L and LCRD-R) carried out in the presence of 40 μM DIG-11-dUTP (Roche Diagnostics, Rotkreuz, Switzerland). Total DNA extracted from A. salmonicida subsp salmonicida strain JF2267 by the guanidium hydrochloride method (20) was screened by the dot blot technique. The DNA was denatured in a mixture containing 0.4 M NaOH and 10 mM EDTA and applied directly to positively charged nylon membranes. Processing of the membranes and subsequent hybridization with the yscV DIG-labeled probe were performed as described previously (17). Southern blot analysis was performed with the yscV DIG-labeled probe under low-stringency conditions (2), with total DNA isolated from strain JF2267 and digested with restriction endonucleases SacI and SalI (Roche Diagnostics).
DNA manipulation, cloning, and sequencing.
All cloning procedures and genetic methods were carried out according to standard protocols (2). A partial gene library of A. salmonicida subsp. salmonicida strain JF2267 was constructed from agarose gel-purified SacI-SalI-digested fragments 4 to 6 kb in size cloned into vector pBSK. Recombinant plasmids were transformed into E. coli XL1-Blue, and positive clones were screened by colony blotting. Plasmids were prepared from positive clones with the QIA Prep Spin Mini Prep kit (Qiagen) according to the instructions supplied.
For complete sequencing of positive clones, nested deletions were generated from the initial cloned DNA fragment with a double-stranded nested deletion kit (Pharmacia LKB). Primer walking was carried out on total DNA isolated from A. salmonicida subsp. salmonicida strain JF2267 by using the Vectorette system (Genosys) according to the manufacturer's instructions.
DNA sequencing was performed with the dRhodamine Terminator Cycle Sequencing kit (Applied Biosystems) according to the manufacturer's protocol with either the T3 and T7 primers or custom-synthesized internal primers (Microsynth). The details of all oligonucleotide sequences used are available upon request. All sequences were determined on both strands. Reaction products were analyzed on an ABI Prism 310 genetic analyzer (Applied Biosystems).
Sequence alignment and editing were performed with the software Sequencher (Gene Codes Corporation). Comparisons of DNA sequences and their corresponding amino acid sequences with sequences in the EMBL/GenBank and NBRF databases were performed with BLAST (1). The molecular mass and theoretical isoelectric pH (pI) of the TTSS proteins were calculated with ProtParam (13).
Marker replacment mutagenesis.
The A. salmonicida subsp. salmonicida gene ascV was inactivated by marker replacement mutagenesis. A 1,061-bp fragment from ascV was excised by using the restriction enzymes KpnI and SpeI (Roche Diagnostics) and replaced with the kanamycin (Km) cassette from pSSVI186 (28) that had been previously excised on a 1.3-kb KpnI-SpeI fragment. The inactivated ascV and flanking genes were then cloned into the mobilizable suicide vector pSUP202sac (27). The resulting plasmid was transformed into E. coli S17-1 (23) for subsequent conjugation into A. salmonicida subsp. salmonicida. In order to provide a means for selection against E. coli, spontaneous rifampin-resistant (Rifr) clones of A. salmonicida subsp. salmonicida strain JF2267 were isolated following growth of the organism on rifampin agar plates (40 μg/ml) for two passages. A single Rifr clone was selected (strain JF2646) and filter mated (23) with E. coli S17-1 carrying the pSUP202sac-ΔascV plasmid for 3 days at 15°C. Double-crossover mutants were selected directly by growth on TSA containing 15% sucrose (wt/vol), 40 μg of kanamycin per ml, and 20 μg of rifampin per ml at 15°C for 7 days. The absence of the wild-type ascV gene and insertion of the kanamycin cassette were verified by PCR with the primer pairs AcrD-fwd (GGGAATTCGATGAAGCCCGTTTTGCC) and AcrD-rev (GTGCGGCCGCACAGGCAGACCCTCCCGAG) and KMTN903-R (CCAATTCTGATTAGAAAAACTC) and KMTN903-L (AAGGGGTGTTATGAGCCATATT).
SDS-PAGE and immunoblot analyzes.
Proteins were separated on 12% acrylamide slabs by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli (26). Once separated, proteins were either stained with Coomassie brilliant blue or electroblotted onto nitrocellulose membranes (Bio-Rad Laboratories). Membranes were blocked for at least 1 h in 1% milk buffer. In order to detect AexT, the membranes were incubated with rabbit polyclonal anti-AexT immunoglobulin G (IgG) (6) diluted 1:1,000 in milk buffer, followed by incubation with a phosphate-labeled conjugate (goat anti-rabbit IgG heavy and light chains; Kirkegaard & Perry) diluted 1:2,000 in milk buffer. The proteins were then visualized with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (Sigma).
In vitro cell assay.
Rainbow trout (Oncorhynchus mykiss) gonad cells (RTG-2, ATCC CCL-55) were grown as described previously (6). RTG-2 cells grown in a monolayer (6 × 105 cells per 2-cm2 well in 1 ml of medium) were infected with A. salmonicida cells suspended in phosphate-buffered saline (PBS) (pH 7.4) at a multiplicity of infection of 20:1 (bacterium/fish cell ratio). The addition of PBS (pH 7.4) to fish cells was used as a negative control. After 6 h of infection at 18°C, the fish cells were photographed under a green-filtered phase-contrast microscope (Zeiss Aixovert 100).
Nucleotide sequence accession number.
The nucleotide sequences reported in this communication have been submitted to the EMBL Nucleotide Sequence Database under accession no. AJ458292.
RESULTS AND DISCUSSION
Cloning and sequence analysis of the virA locus.
YscV (formerly called LcrD) is the archetype for a family of inner membrane proteins found in every known TTSS. These proteins are highly conserved, and all members can be aligned over the entire length of their amino acid sequence (12, 21). We therefore chose to utilize the gene encoding this protein for screening a virulent isolate of A. salmonicida subsp. salmonicida. We utilized a probe directed against the yscV gene of Y. enterocolitica and screened total DNA from A. salmonicida subsp. salmonicida strain JF2267, a strain previously isolated from an arctic char (Savelinus alpinus) displaying typical furunculosis symptoms (6). The results of dot blot analysis revealed a strong signal with the yscV probe, suggesting the gene was present in strain JF2267. Subsequent Southern blot analysis of total DNA isolated from strain JF2267 and digested with restriction endonucleases SacI and SalI revealed a 4.8-kb fragment that hybridized with the yscV probe. This fragment was cloned on pBSK, and the nucleotide sequence was determined.
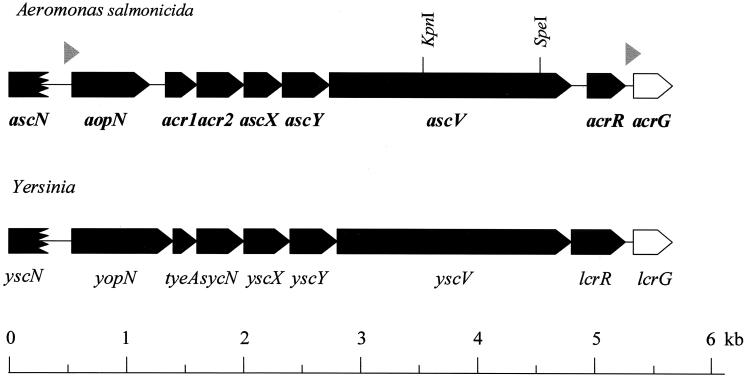
DNA sequence analysis revealed the presence of seven open reading frames (ORFs) encoding homologues to Yersinia proteins. In Yersinia species, six of these proteins are encoded by genes found within the virA locus, specifically tyeA, sycN, yscX, yscY, yscV, and lcrR (3, 15, 16, 21, 22, 26) (Fig. 1). The gene encoding the seventh protein, lcrG is found on a separate locus downstream of lcrR (5). Because the ORF of the tyeA homologue appeared to be incomplete on the initial cloned fragment, we utilized the primer-walking technique to obtain the complete sequence of this gene. In doing this, we also obtained the sequence of an ORF encoding a YopN homologue (11) and a partial ORF that appears to encode a YscN homologue (4, 30) (Fig. 1).
FIG. 1.
Genetic organization of the virA operon of A. salmonicida subsp. salmonicida strain JF2267 (and that of the Rifr derivative, JF2646). The virA locus of Yersinia species is shown for comparison. Genes that comprise the virA locus are represented by black boxes. Flanking genes are shown in white. The jagged box represents an incomplete gene sequence. Potential promoter sequences, represented by gray arrowheads, are found upstream of aopN and acrG. The KpnI and SpeI sites used for the inactivation of the ascV gene are indicated.
In keeping with the nomenclature currently used for the designation of TTSS genes in Yersinia species, the archetype for these systems, we have given the yscN, yscX, yscY, and yscV homologues the designations ascN, ascX, ascY, and ascV, respectively, with asc representing Aeromonas secretion). By the same token, the lcrR and lcrG homologues have been designated acrR and acrG; the yopN homologue has been designated aopN. For simplicity's sake, the tyeA homologue (for translocation of Yops into eukaryote cells) and the sycN homologue (for specific Yop chaperone) have been termed “acr1” and “acr2” in analogy to their P. aeruginosa counterparts (Fig. 1). All genes have been designated based solely on their sequence similarity to Yersinia and Pseudomonas TTSS genes. Relevant characteristics of the proteins encoded by these ORFs are summarized in Tables 2 and 3.
TABLE 2.
Comparison between predicted A. salmonicida subsp. salmonicida proteins and Yersinia homologues
| A. salmo- nicida protein | Yersinia homo- logue | Function (reference) | % Identity | % Sim- ilarity |
|---|---|---|---|---|
| AopN | YopN | Regulation of translocation (8, 11, 16) | 49 | 60 |
| Acr1 | TyeA | Translocation apparatus, selective translocation of Yops (16) | 55 | 71 |
| Acr2 | SycN | Chaperone for YopN (15) | 62 | 77 |
| AscX | YscX | Type III secretion apparatus (15) | 54 | 69 |
| AscY | YscY | Type III secretion apparatus (15) | 46 | 56 |
| AscV | YscV | Type III secretion apparatus (21, 22) | 73 | 79 |
| AcrR | LcrR | Unknown (3, 24) | 43 | 54 |
| AcrG | LcrG | Regulation of low-calcium response (24) | 43 | 64 |
TABLE 3.
Properties of A. salmonicida subsp. salmonicida proteins
| A. salmonicida protein | No. of residues | Theoretical pI | Molecular mass (kDa) |
|---|---|---|---|
| AopN | 229 | 5.6 | 25.0 |
| Acr1 | 93 | 4.3 | 10.6 |
| Acr2 | 123 | 5.5 | 13.7 |
| AscX | 121 | 5.4 | 13.6 |
| AscY | 116 | 5.2 | 12.9 |
| AscV | 721 | 6.0 | 79.3 |
| AcrR | 93 | 9.5 | 10.4 |
| AcrG | 94 | 5.5 | 10.5 |
Further analysis of the nucleotide sequence of the TTSS genes identified in A. salmonicida subsp. salmonicida has revealed a potential promoter region upstream of aopN (Fig. 1). In addition to −10 (TATAATG) and −35 (TTGGCA) consensus sequences, the promoter also possesses an ExsA consensus element (TAAAAATA) (14), which in P. aeruginosa is bound by the transcriptional activator ExsA (14). While we were unable to identify any potential transcription termination sites in the DNA fragment that we have cloned, we did identify another potential promoter region preceding the acrG gene (Fig. 1). This promoter also contains −10 (TAGAATA) and −35 (GTGACA) consensus sequences as well as a potential ExsA consensus element (ACAAAAGC). These data suggest that in A. salmonicida subsp. salmonicida, the genes comprising the virA locus may be transcribed by a single operon that in turn may be regulated by an ExsA homologue. We speculate that regulation of the virA operon occurs in a manner similar to that seen in P. aeruginosa and Yersinia species (5, 32).
Secretion of AexT.
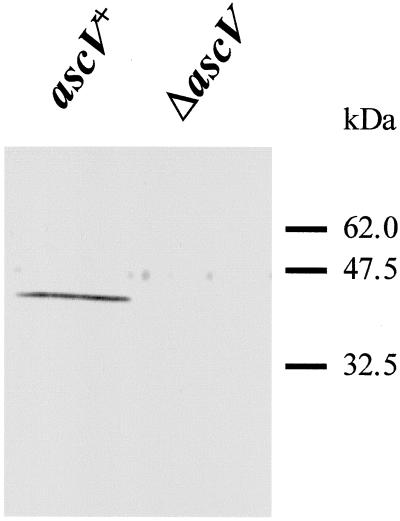
To determine whether the TTSS genes we have identified in A. salmonicida subsp. salmonicida are part of a functional secretion system, the ascV gene was inactivated by marker replacement mutagenesis with a Kmr cassette. We then examined the extracellular protein profiles of A. salmonicida subsp. salmonicida strain JF2646 (a rifampin-resistant derivative of strain JF2267 used in the construction of the ΔascV mutant [see Materials and Methods]) and the ΔascV mutant strain JF2678, under low-calcium conditions. The bacteria were grown overnight in TSB, and the culture supernatant was analyzed by SDS-PAGE. The results revealed a number of protein bands present in the culture supernatant of strain JF2646 that were not seen in that of strain JF2678 (results not shown), indicating that the type III secretion genes we have identified are part of a functional system. Because A. salmonicida subsp. salmonicida has previously been shown to secrete the ADP-ribosylating toxin AexT, when grown under low-calcium conditions, we speculated that AexT is secreted in a type III-dependent manner. If this were true, then AexT should be detected in the culture supernatant of strain JF2646, but not that of strain JF2678 (ΔascV). To confirm this hypothesis, we performed an immunoblot on culture supernatants from both strains by using anti-aexT antibodies. The results can be seen in Fig. 2. AexT is found in the culture supernatant of JF2646 (ascV+) cells, but not in the supernatant from JF2678 (ΔascV) cultures, indicating this toxin is secreted into the external environment via the TTSS we have identified.
FIG. 2.
Secretion of AexT under low-calcium conditions. Bacterial cultures were grown overnight in TSB medium. Left lane, A. salmonicida subsp. salmonicida strain JF2646 (ascV+); right lane, A. salmonicida subsp. salmonicida ΔascV mutant JF2678. Horizontal lines mark the positions of molecular mass standards.
Toxicity of the ascV mutant.
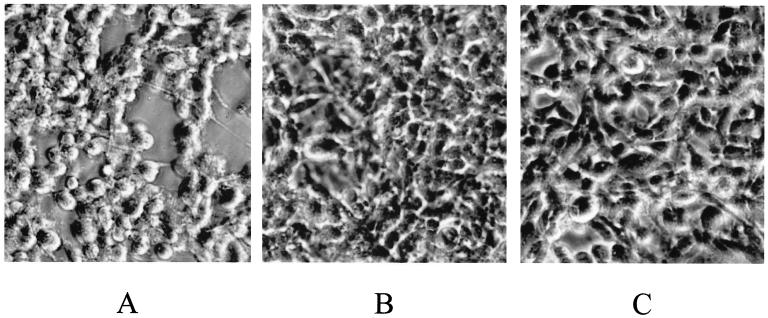
Finally, we were interested to determine whether the TTSS in A. salmonicida subsp. salmonicida plays a role in the virulence of this organism. We assayed the toxicity of A. salmonicida subsp. salmonicida strain JF2646 and that of its ΔascV mutant derivative, strain JF2678, by infection of cultured rainbow trout (Oncorhynchus mykiss) gonad cells (RTG-2 cells). Six hours following inoculation of RTG-2 cells with the bacteria, the cells that had been infected with the ascV+ cells (JF2646) displayed characteristic cell rounding and had become detached from the plastic support (Fig. 3A). In contrast, RTG-2 cells infected with the isogenic ascV deletion mutant displayed no marked morphological changes in spite of the high numbers of bacterial cells in the cultures (Fig. 3B). RTG-2 fish cells that were inoculated with PBS were used as a negative control (Fig. 3C), and as expected, they displayed no morphological changes.
FIG. 3.
Toxicity of A. salmonicida subsp. salmonicida to RTG-2 cells. (A) RTG-2 cells inoculated with A. salmonicida subsp. salmonicida strain JF2646 (ascV+). (B) RTG-2 cells inoculated with isogenic ΔascV mutant JF2678. (C) RTG-2 cells inoculated with PBS only.
The inability of the ascV mutant to cause damage to the RTG-2 cells indicates that this gene is required for the toxicity of A. salmonicida subsp. salmonicida against fish cells in vitro. Because AscV displays such high sequence homology to corresponding genes in other TTSSs (e.g., 73% identity and 80% similarity to YscV from Yersinia species and 72% identity and 78% similarity to PcrD from P. aeruginosa), we can expect it is an integral component of the TTSS apparatus. Therefore, AscV in itself is not likely to be directly responsible for the toxic effect of strain JF2646 toward RTG-2 cells. Rather, it can be expected to play a role in the secretion or translocation of toxins, including AexT, or other virulence factors into the external environment or into the cytosol of target cells.
While the TTSS of A. salmonicida subsp. salmonicida certainly appears to play a role in the pathogenesis of this organism in vitro, its role in the disease process in vivo has yet to be established. However, the identification of a TTSS in A. salmonicida subsp. salmonicida is clearly an important step toward a better understanding of the virulence mechanisms of this pathogen.
Acknowledgments
We are grateful to Shelia MacIntyre, University of Reading, for providing us with plasmid pSUP202sac and Lea Lagcher, University of Berne, for cultivation of the RTG-2 cells. We are also grateful to Guy Cornelis, University of Basel, for stimulating discussions and help with nomenclature.
This work was supported by the research fund of the Institute for Veterinary Bacteriology, University of Berne.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1999. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Barve, S. S., and S. C. Straley. 1990. lcrR, a low-Ca2+-response locus with dual Ca2+-dependent functions in Yersinia pestis. J. Bacteriol. 172:4661-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman, T., K. Erickson, E. Galyov, C. Persson, and H. Wolf-Watz. 1994. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J. Bacteriol. 176:2619-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman, T., S. Hakansson, Å. Forsberg, L. Norlander, A. Macellaro, A. Bäckman, I. Bölin, and H. Wolf-Watz. 1991. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J. Bacteriol. 173:1607-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun, M., K. Stuber, Y. Schlatter, T. Wahli, P. Kuhnert, and J. Frey. 2002. Characterization of an ADP-ribosyltransferase toxin (AexT) from Aeromonas salmonicida subsp. salmonicida. J. Bacteriol. 184:1851-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullock, W. O., J. M. Fernandez, and J. M. Short. 1987. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques 5:376-378. [Google Scholar]
- 8.Cheng, L. W., O. Kay, and O. Schneewind. 2001. Regulated secretion of YopN by the type III machinery of Yersinia enterocolitica. J. Bacteriol. 183:5293-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu, S., S. Cavaignac, J. Feutrier, B. M. Phipps, M. Kostrzynska, W. W. Kay, and T. J. Trust. 1991. Structure of the tetragonal surface virulence array protein and gene of Aeromonas salmonicida. J. Biol. Chem. 266:15258-15265. [PubMed] [Google Scholar]
- 10.Ellis, A. E. 1997. Immunization with bacterial antigens: furunculosis. Dev. Biol. Stand. 90:107-116. [PubMed] [Google Scholar]
- 11.Forsberg, A., A. M. Viitanen, M. Skurnik, and H. Wolf-Watz. 1991. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol. Microbiol. 5:977-986. [DOI] [PubMed] [Google Scholar]
- 12.Galán, J. E., C. Ginocchio, and P. Costeas. 1992. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J. Bacteriol. 174:4338-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182:319-326. (Erratum, 189:283, 1990.) [DOI] [PubMed] [Google Scholar]
- 14.Hovey, A. K., and D. W. Frank. 1995. Analyses of the DNA-binding and transcriptional activation properties of ExsA, the transcriptional activator of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 177:4427-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iriarte, M., and G. R. Cornelis. 1999. Identification of SycN, YscX, and YscY, three new elements of the Yersinia Yop virulon. J. Bacteriol. 181:675-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iriarte, M., M. P. Sory, A. Boland, A. P. Boyd, S. D. Mills, I. Lambermont, and G. R. Cornelis. 1998. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 17:1907-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhnert, P., J. Hacker, I. Muhldorfer, A. P. Burnens, J. Nicolet, and J. Frey. 1997. Detection system for Escherichia coli-specific virulence genes: absence of virulence determinants in B and C strains. Appl. Environ. Microbiol. 63:703-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, K. K., and A. E. Ellis. 1990. Glycerophospholipid:cholesterol acyltransferase complexed with lipopolysaccharide (LPS) is a major lethal exotoxin and cytolysin of Aeromonas salmonicida: LPS stabilizes and enhances toxicity of the enzyme. J. Bacteriol. 172:5382-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munro, A. L., and T. S. Hastings. 1993. Furunculosis, p. 122-142. In V. Inglis, R. J. Roberts, and N. R. Bromage (ed.), Bacterial diseases of fish. Blackwell Scientific, Oxford, United Kingdom.
- 20.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 21.Plano, G. V., S. S. Barve, and S. C. Straley. 1991. LcrD, a membrane-bound regulator of the Yersinia pestis low-calcium response. J. Bacteriol. 173:7293-7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plano, G. V., and S. C. Straley. 1993. Multiple effects of lcrD mutations in Yersinia pestis. J. Bacteriol. 175:3536-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 24.Skrzypek, E., and S. C. Straley. 1993. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J. Bacteriol. 175:3520-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Titball, R. W., and C. B. Munn. 1985. The purification and some properties of H-lysin from Aeromonas salmonicida. J. Gen. Microbiol. 131:1603-1609. [DOI] [PubMed] [Google Scholar]
- 26.Viitanen, A.-M., P. Toivanen, and M. Skurnik. 1990. The lcrE gene is part of an operon in the lcr region of Yersinia enterocolitica O:3. J. Bacteriol. 172:3152-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vipond, R., I. R. Bricknell, E. Durant, T. J. Bowden, A. E. Ellis, M. Smith, and S. MacIntyre. 1998. Defined deletion mutants demonstrate that the major secreted toxins are not essential for the virulence of Aeromonas salmonicida. Infect. Immun. 66:1990-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viret, J. F. 1993. Meganuclease I-SceI as a tool for the easy subcloning of large DNA fragments devoid of selection marker. BioTechniques 14:325-326. [PubMed] [Google Scholar]
- 29.Whitby, P. W., M. Landon, and G. Coleman. 1992. The cloning and nucleotide sequence of the serine protease gene (aspA) of Aeromonas salmonicida ssp. salmonicida. FEMS Microbiol. Lett. 78:65-71. [DOI] [PubMed] [Google Scholar]
- 30.Woestyn, S., A. Allaoui, P. Wattiau, and G. R. Cornelis. 1994. YscN, the putative energizer of the Yersinia Yop secretion machinery. J. Bacteriol. 176:1561-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yahr, T. L., J. Goranson, and D. W. Frank. 1996. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol. Microbiol. 22:991-1003. [DOI] [PubMed] [Google Scholar]
- 32.Yahr, T. L., L. M. Mende-Mueller, M. B. Friese, and D. W. Frank. 1997. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 179:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]