Abstract
Dehalococcoides ethenogenes is a member of the physiologically diverse division of green nonsulfur bacteria. Using a TBLASTN search, the D. ethenogenes lexA gene has been identified, cloned, and expressed and its protein has been purified. Mobility shift assays revealed that the D. ethenogenes LexA protein specifically binds to both its own promoter and that of the uvrA gene, but not to the recA promoter. Our results demonstrate that the D. ethenogenes LexA binding site is GAACN4GTTC, which is identical to that found in gram-positive bacteria. In agreement with this fact, the Bacillus subtilis DinR protein binds specifically to the D. ethenogenes LexA operator. This constitutes the first non-gram-positive bacterium exhibiting a LexA binding site identical to that of B. subtilis.
In Escherichia coli, the SOS regulon consists of a pool of at least 40 DNA damage-inducible genes whose functions are involved in DNA replication, DNA repair, mutagenesis, and control of the cell cycle (3, 5, 8, 11). This regulon is controlled by two key proteins, RecA and LexA, the positive and negative regulators, respectively (1, 12, 15, 22). Under normal conditions LexA binds specifically to an operator region located within the promoters of the SOS genes, repressing their expression (8, 22). When DNA is damaged or replication is stalled, RecA protein forms a nucleofilament with the resulting single-stranded DNA, achieving its activated conformation (RecA*). RecA* mediates the autocatalytic cleavage of LexA between residues Ala84 and Gly85, resulting in two parts, the N-terminal DNA binding domain and the C-terminal domain, which is involved in dimerization of the repressor (13, 14). In this process, the catalytic site formed by Ser119 and Lys156 is responsible for the hydrolysis of the Ala-Gly bond through a biochemical reaction characteristic of serine proteases (16). As a consequence of the cleavage, LexA no longer binds DNA, the SOS genes are derepressed, and their products participate in repairing the DNA lesions to guarantee cell survival. This process is known as the SOS response and was first suggested in the 1970s by Miroslav Radman (19). Once DNA is repaired, RecA* loses its active conformation, LexA autohydrolysis ceases, and the repressor accumulates again, inhibiting the expression of the SOS genes.
The lexA gene seems to be widespread in the Bacteria domain. However, lexA is not found in the fully sequenced genomes of several bacterial species, such as Aquifex aeolicus, Borrelia burgdorferi, Chlamydia pneumoniae, Mycoplasma pneumoniae, Campylobacter jejuni, Helicobacter pylori, and Porphyromonas gingivalis. Moreover, it appears that a lexA-like gene does not exist in the Archaea domain, as lexA homologues are absent in the 19 archaeal genomes currently available (4).
Recently, the three-dimensional structure of the E. coli LexA protein has been solved (16). In E. coli, LexA binds specifically to a DNA motif known as the SOS-box or LexA-box (22). Comparative analysis of the 31 E. coli LexA binding sites shows a 16-bp consensus sequence that responds to the motif CTGN10CAG (5). Besides the E. coli SOS-box, two more LexA-boxes have been identified so far in bacteria. The directed repeat GTTCN7GTTC is recognized by LexA protein of members of the alpha-class Proteobacteria, such as Rhodobacter sphaeroides and Rhizobium etli (6, 21). In gram-positive bacteria such as Bacillus subtilis and Mycobacterium tuberculosis, the consensus sequence CGAACRNRYGTTYG is the target for this repressor (2, 24).
Dehalococcoides ethenogenes is an anaerobic bacterium capable of dechlorinating tetrachloroethene, one of the most common groundwater contaminants, to ethene (17). D. ethenogenes is rather enigmatic taxonomically. Its cell wall composition is unlike that of gram-positive or gram-negative bacteria, instead more closely resembling the cell wall of archaea (17). However, on the basis of 16S rRNA gene sequence comparisons, D. ethenogenes has been assigned to the green nonsulfur bacteria, a division of physiologically diverse species with relatively few cultured representatives (10). The unusual phylogenetic position, structural properties, and metabolic capabilities of D. ethenogenes make it an interesting organism for further biological characterization. Here, we report the cloning of the key regulatory gene lexA from D. ethenogenes, purification of the protein, and characterization of its recognition site. Prior to this report, there were no data available pertaining to the motif recognized by LexA in this bacterial division. Finally, we report a genomic analysis of the composition of the D. ethenogenes LexA network.
Identification and cloning of D. ethenogenes lexA gene.
The D. ethenogenes strain 195 genome sequence is currently unassembled, but contigs are available for performing BLAST analysis at the Institute for Genomic Research and at the National Center of Biotechnology Information (http://www.ncbi.nlm.nih.gov/Microb_blast/unfinishedgenome.html).
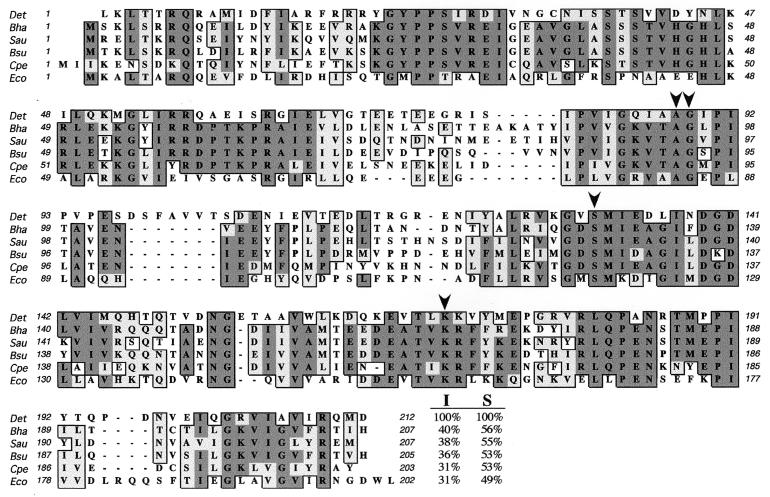
The Escherichia coli LexA amino acid sequence was used to query the D. ethenogenes genome with the TBLASTN program, revealing a protein homologue of 212 amino acids. Although D. ethenogenes is not a gram-positive organism, its LexA protein is highly homologous to the LexA proteins belonging to several members of this bacterial phylum, such as Bacillus halodurans, Staphylococcus aureus, and Clostridium perfringens. Specifically, D. ethenogenes LexA protein shows the highest level of identity with Bacillus halodurans LexA (40%), while the D. ethenogenes repressor is only 31% identical to E. coli LexA (Fig. 1). Furthermore, D. ethenogenes LexA contains all of the conserved residues involved in repressor autocleavage (Ala88, Gly89, Ser331, and Lys170) as deduced from CLUSTAL W alignment of different LexA proteins (Fig. 1). It is important to note that nine codons downstream of the proposed initiation site for the 212-amino-acid open reading frame there is a 203-amino-acid protein starting with methionine. However, this truncated version of D. ethenogenes LexA would lack several N-terminal residues likely to be essential to the LexA structure, since it would lack important residues belonging to the first α-helix characterized by nuclear magnetic resonance spectroscopy in E. coli LexA (7).
FIG. 1.
CLUSTAL W alignment performed with Mac Vector 6.5 (Oxford Molecular) with LexA proteins from Dehalococcoides ethenogenes (Det), Bacillus halodurans (Bha), Staphylococcus aureus (Sau), Bacillus subtilis (Bsu), Clostridium perfringens (Cpe), and Escherichia coli (Eco). Identities have a dark gray background, and similarities are light gray. The perfectly conserved residues Ala88 and Gly89 forming the LexA cleavage site and the amino acids Ser131 and Lys170 required for protein hydrolysis are indicated with arrowheads on the D. ethenogenes LexA sequence. At the end of each LexA sequence appears the percent identity (I) and percent similarity (S) that each protein shows with the D. ethenogenes LexA repressor. Accession numbers in the Entrez protein database at NCBI are as follows: B. halodurans, Q9KAD3; S. aureus, BAB57501; B. subtilis, P31080; C. perfringens, BAB80867; and E. coli, P03033.
The BLAST analysis returned 1 kb of DNA flanking the lexA coding sequence, which was used to design the primers AR31 and AR34 (Table 1). These primers were employed to PCR amplify the entire D. ethenogenes lexA and its promoter region with genomic DNA obtained from a 10-liter tetrachloroethene-methanol-fed anaerobic enrichment culture. The resulting 866-bp DNA fragment was then cloned into pGEM-T (Promega). To confirm that no mutation was introduced during the PCR, the sequence of the insert was determined by labeling DNA samples with the fmol DNA cycle sequencing system (Promega) and analyzed on an ALF sequencer (Amersham Pharmacia Biotech). This plasmid, called pUA969, was used as a template for further studies.
TABLE 1.
Oligonucleotide primers used in this worka
| Primer | Sequence | Position | Application |
|---|---|---|---|
| AR31 | 5′-CTCAACCAAGCCGCTAAATAACTCC-3′ | −121 | Upper primer for cloning D. ethenogenes lexA gene and LexA1 probe |
| AR33 | 5′-AGAGGTAGAGCTGATATTACAGCCG-3′ | +117 | Lower primer to amplify unlabeled specific DNA competitors |
| AR33DIG | 5′-DIG-AGAGGTAGAGCTGATATTACAGCCG-3′ | +117 | Lower primer to amplify digoxigenin-labeled probe fragments of lexA promoter |
| AR34 | 5′-GTCGACCTAATCCATCTGGCGGATTACAGC-3′ | +636 | Lower primer for cloning D. ethenogenes lexA gene |
| AR35 | 5′-TACCTGCCTGAATGACTTGACACC-3′ | −295 | Upper primer to amplify D. ethenogenes recA promoter |
| AR36 | 5′-CTTAGTTTCATTATTGAACCCTTGC-3′ | +85 | Lower primer to amplify D. ethenogenes recA promoter |
| AR38 | 5′-TATGCGGGGACAGAGTTGAC-3′ | −15 | Upper primer to amplify LexA4 probe |
| AR39 | 5′-TTATGTAGAACAAGTGTTCTTATGC-3′ | −35 | Upper primer to amplify LexA3 probe |
| AR40 | 5′-TTGTGTATTGACAGGGAAGG-3′ | −68 | Upper primer to amplify LexA2 probe |
| AR61 | 5′-GAATTCATGAAACTGACCACCAGACAGCG-3′ | +1 | Upper primer for directed cloning of D. ethenogenes lexA in pGEX4T1 |
| AR62 | 5′-GCCGCAGGCATGACCCAGCTGGGGC-3′ | −515 | Upper primer to amplify uvrA promoter region |
| AR63 | 5′-TTTTGAGGTTGTGTTCGCGGGCACC-3′ | +51 | Lower primer to amplify uvrA promoter region |
| AR64 | 5′-TAGTGGAGAAATACCACTCTCAGGC-3′ | −531 | Upper primer to amplify ruvA promoter region |
| AR65 | 5′-TTGCCGCTGGCTTCCAGAATA-3′ | +40 | Lower primer to amplify ruvA promoter region |
| AR66 | 5′-GGGTGAACTGGTAGACAAGTTCCGC-3′ | −525 | Upper primer to amplify ruvB promoter region |
| AR67 | 5′-GGCCAGTTTCCCGGATATAAGACGC-3′ | +42 | Lower primer to amplify ruvB promoter region |
| AR68 | 5′-AGCCGGTACCGCCGGATGCACCTGC-3′ | −532 | Upper primer to amplify recN promoter region |
| AR69 | 5′-CCAGGTGATATCCTCAATAATGCCG-3′ | +53 | Lower primer to amplify recN promoter region |
| AR81 | 5′-ACTCATGATCATAACCTCCAACAGG-3′ | −161 | Upper primer to amplify B. subtilis dinR promoter region |
| AR82 | 5′-GGACGCAAGCCCGACAGCCTCTCCG-3′ | +116 | Lower primer to amplify B. subtilis dinR promoter region |
Added restriction sites are shown in italics. The position of either the 5′ or 3′ end of the oligonucleotide with respect to the proposed translational starting point of each D. ethenogenes gene is shown except for AR81 and AR82, where the position of either the 5′ or 3′ end of the oligonucleotide with respect to the translational starting point of the B. subtilis dinR gene is shown. Primers AR41 to AR60 and AR76 are derivatives of AR39 but with the desired nucleotide changes.
Purification of D. ethenogenes LexA protein.
A glutathione S-transferase (GST)-LexA fusion protein was generated. The primers AR61 and AR34 (Table 1) were used to amplify the D. ethenogenes lexA gene from pUA969. The oligonucleotide AR61 incorporates an EcoRI restriction site upstream of the lexA translational start codon, permitting fusion of the protein in frame with the GST protein. The 650-bp PCR fragment was cloned into pGEM-T, generating plasmid pUA970. Afterwards, pUA970 was digested with EcoRI and SalI, and the resulting 0.65-kb fragment was cloned into pGEX4T1 (Amersham Pharmacia Biotech), creating the GST-LexA fusion.
This plasmid, designated pUA971, was transformed into Escherichia coli BL21 Codon Plus cells (Stratagene) to overproduce the protein. An overnight culture of the BL21 Codon Plus strain containing plasmid pUA971 was diluted in one-half liter of Luria-Bertani (LB) medium and incubated at 37°C until it reached an optical density at 600 nm of 0.8. At this time isopropylthiogalactopyranoside (IPTG) was added (1 mM final concentration), and the culture was incubated for 4 more hours. Afterwards, cells were collected by centrifugation for 15 min at 5,000 rpm.
The bacterial pellet was resuspended in phosphate-buffered saline (PBS) (10 mM Na2HPO4, 1.7 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl [pH 7.4]) containing complete Mini protease inhibitor cocktail (Roche) and sonicated to break the cells. The cell lysate was centrifuged at 15,000 rpm for 30 min, and the supernatant containing the soluble GST-LexA protein fusion was incubated for 2 h at 4°C with glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech), previously equilibrated in PBS. The beads were washed twice with PBS-0.1% Triton X-100 and three more times with PBS to eliminate contaminant proteins. The washed glutathione-Sepharose beads containing bound GST-LexA protein were equilibrated in 0.1 M Tris-HCl (pH 8)-0.1 M NaCl buffer, and GST-LexA was eluted in the same buffer containing 20 mM glutathione (Sigma), resulting in about 85% pure GST-LexA (Fig. 2).
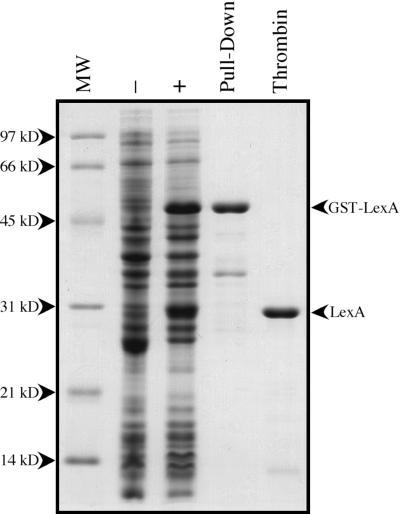
FIG. 2.
Purification of the D. ethenogenes LexA protein. D. ethenogenes LexA was purified to greater than 95%. Each line shown in the denaturing 13% polyacrylamide gel represents each of the different protein purification steps employed: crude extract of BL21 Codon Plus/pUA971 (−); crude extract of BL21 Codon Plus/pUA971 induced with 1 mM IPTG (+); D. ethenogenes GST-LexA affinity purified with glutathione-Sepharose 4B (pull-down); purified D. ethenogenes LexA protein after thrombin protease digestion of GST-LexA fusion (thrombin). Lane MW, molecular size markers.
The GST-LexA fusion protein has the amino acid sequence Leu-Val-Pro-Arg-Gly-Ser between GST and LexA. This motif is recognized by the thrombin protease, which cleaves the Arg-Gly peptide bond. In this case, proteolysis results in D. ethenogenes LexA protein with the peptide Gly-Ser-Pro-Glu-Phe attached to the N terminus. GST-LexA bound to glutathione-Sepharose was incubated at room temperature for 16 h with 50 U of thrombin protease (Amersham Pharmacia Biotech) in 1 ml of PBS. The liquid phase containing LexA was collected, and a portion was separated by electrophoresis on a denaturing 13% polyacrylamide gel. The purity of the D. ethenogenes LexA protein was greater than 95% (Fig. 2).
Determination of D. ethenogenes LexA binding motif.
Analysis of the DNA contig containing D. ethenogenes lexA revealed that it was located between the rpsT and fucA genes (Fig. 3A). From the TAA translational stop codon of rpsT to the putative TTG start codon of lexA, there are 104 bp that presumably contain the LexA binding site. Hence, a PCR fragment containing this sequence was amplified with primers AR31 and AR33DIG (Table 1). The resulting digoxigenin-labeled probe (LexA1) was used as a target to observe whether purified D. ethenogenes LexA was able to bind its own promoter in electrophoretic mobility shift assay experiments carried out as previously described (6).
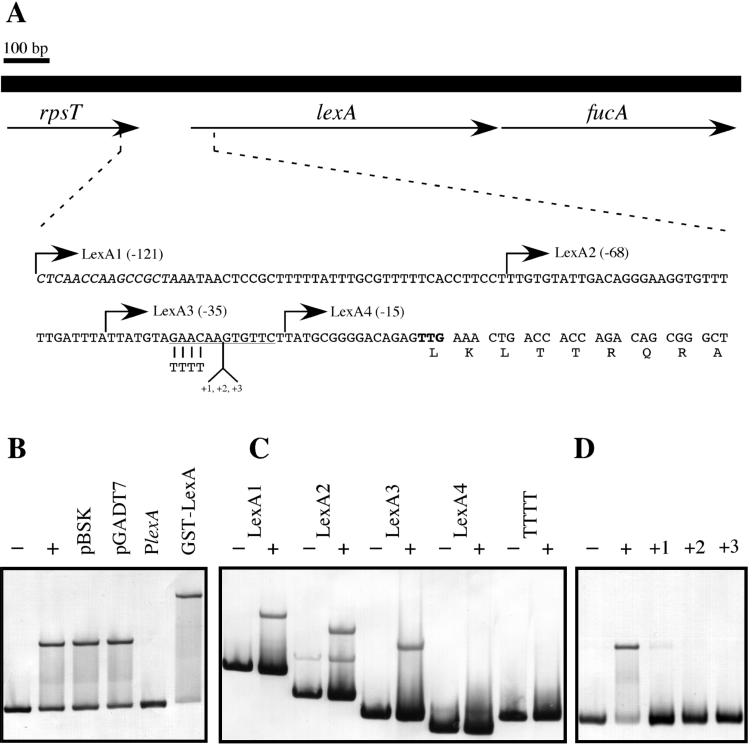
FIG. 3.
(A) Schematic representation of the D. ethenogenes chromosomal region containing lexA, which is located between the rpsT and fucA genes. The sequence of the lexA promoter region and the first nine amino acid residues of LexA are shown. The 3′ end of the rpsT gene is in italic type, the putative TTG translational start codon of lexA is in bold type, and the homologue of the DinR-box is underlined. The start points of each fragment of the lexA promoter (LexA1, LexA2, LexA3, and LexA4) used in electrophoretic mobility shift assay experiments are indicated by arrows, and the relative distances to the TTG are in parentheses. (B) Specific binding of D. ethenogenes LexA protein. Electrophoretic mobility shift assay experiments were performed with different DNAs as competitors. LexA1 fragment was incubated at 30°C for 30 min in the absence (−) and in the presence (+) of 25 ng of pure LexA protein (50 nM). To show the specificity of LexA binding, the LexA1 fragment was incubated in the presence of D. ethenogenes LexA protein and the following unlabeled DNA competitors: 2 μg of pBSK, 2 μg of pGADT7, or 1 μg of unlabeled LexA1 DNA fragment (PlexA). In the last lane, LexA1 probe was incubated in the presence of the D. ethenogenes GST-LexA fusion protein. (C) Establishment of the limits of the D. ethenogenes LexA binding site. LexA1, LexA2, LexA3, LexA4, and a derivative of the LexA3 fragment where GAAC was changed to TTTT (TTTT) were incubated in the absence (−) or presence (+) of purified LexA. (D) Electrophoretic mobility shift assays of LexA3 fragments where one (+1), two (+2), or three (+3) adenine residues were inserted at position −21 with respect to the initiation start codon. Different probes were incubated with LexA protein and loaded on a native Tris-glycine-5% polyacrylamide gel. The LexA3 probe alone (−) and the same fragment incubated in the presence of LexA (+) acted as negative and positive controls, respectively.
Basically, 20-μl reaction mixtures containing 10 ng of digoxigenin-labeled DNA probe and 25 ng (50 nM) of D. ethenogenes LexA were incubated in binding buffer: 10 mM HEPES NaOH (pH 8), 10 mM Tris-HCl (pH 8), 5% glycerol, 50 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 1 μg of bulk carrier DNA, and 50 μg of bovine serum albumin per ml. After 30 min of incubation at 30°C, the mixture was loaded onto a 5% nondenaturing Tris-glycine-polyacrylamide gel. Thus, D. ethenogenes LexA was able to shift the mobility of the LexA1 fragment (Fig. 3B). Furthermore, the retarded band was abolished when unlabeled LexA1 was used as a competitor (100-fold molar excess over the digoxigenin-labeled probe). However, the shifted band could not be eliminated when nonspecific DNA competitors were used (Fig. 3B), suggesting that the binding of the D. ethenogenes LexA protein to its promoter is specific. It is important to note that the GST-LexA fusion protein retained its DNA binding activity in electrophoretic mobility shift assay experiments (Fig. 3B).
To establish the bounds of the LexA binding site, serial deletion fragments of the upstream promoter region were analyzed in electrophoretic mobility shift assay experiments with purified LexA. The DNA probes LexA1, LexA2 and LexA3 exhibited retarded bands in the presence of LexA protein, but the mobility of the LexA4 DNA was not shifted (Fig. 3C). These results indicate that the LexA binding motif is located within the sequence 35 bp upstream of the lexA translation start codon. An examination of this region revealed the presence of the inverted repeat GAACAAGTGTTC 28 bp upstream of the putative TTG translational start codon (Fig. 3A). This sequence matches the DinR recognition motif (formerly Cheo-box), CGAACN4GTTCG (23). Changing the GAAC submotif to TTTT in the LexA3 fragment abolished LexA protein binding, demonstrating that this tetranucleotide is part of the D. ethenogenes LexA binding site (Fig. 3C).
The importance of the rotational orientation of the inverted repeats relative to one another was examined by introducing additional nucleotides into the center of the GAACN4GTTC motif, lengthening the linker region between the inverted repeats (Fig. 3A). With modified LexA3 PCR primers (AR58 to AR60, Table 1), one to three adenines were inserted at position −21 relative to the translational start codon (Fig. 3A). The introduction of a single adenine resulted in a sequence that retained some residual binding of the LexA protein (Fig. 3D). However, the introduction of two or three nucleotides into the linker region completely abolished LexA binding (Fig. 3D), consistent with studies of LexA binding sites in other microorganisms (6).
To gain insight into the importance of each of the 12 bases of this imperfect palindrome and the neighboring nucleotides in the LexA binding process, single nucleotide changes were introduced into the LexA3 probe by PCR amplification with primers AR41 to AR57 and AR76 (Table 1). The changes introduced were the least permissive based on the thorough characterization of the DinR binding site performed by Winterling et al. (23). Changes introduced in the main motifs GAAC and GTTC severely decreased or eliminated LexA binding (Fig. 4A). Also critical for binding were the flanking bases of the extended direct repeat, the adenine residues at positions −29 and −24, and the thymine residues at positions −16 and −21 (relative to the TTG translational start codon). These results indicated that the tetranucleotide GAAC and the inverted repeat GTTC are critical to LexA binding, consistent with previous studies of the DinR recognition site (23). Collectively, these results demonstrate that the sequence AGAACN4GTTCT is the specific recognition site for the D. ethenogenes LexA repressor.
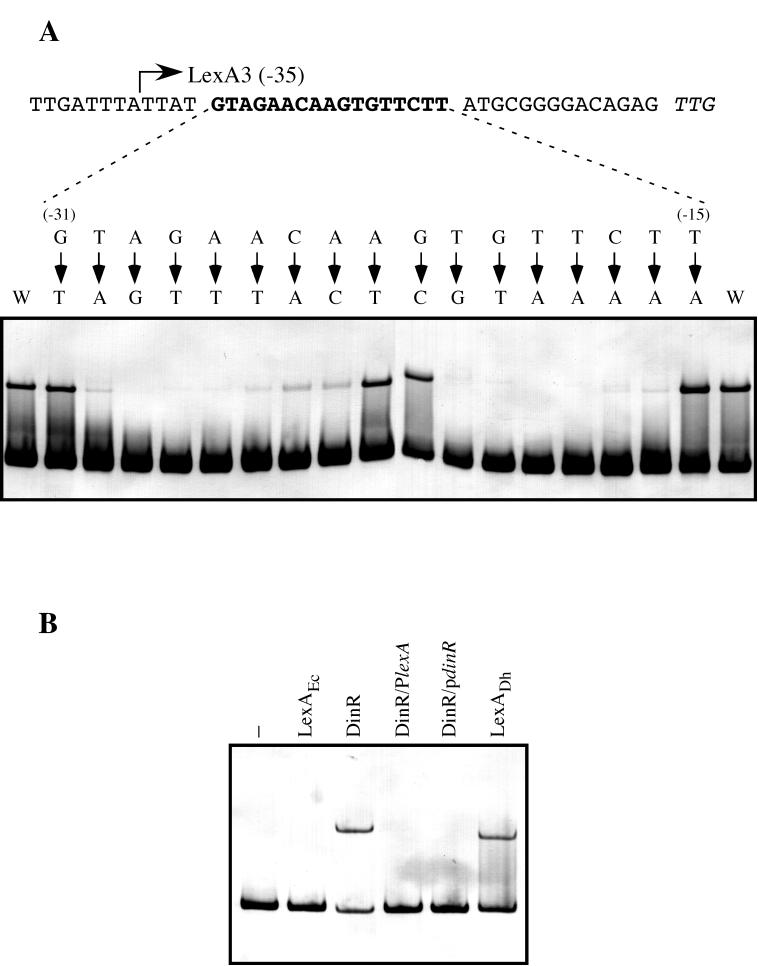
FIG. 4.
(A) Determination of the specific LexA binding site in the D. ethenogenes lexA promoter. Directed mutagenesis via PCR was used to change single nucleotides in the region from −31 to −15 (bold type) relative to the putative start codon of the D. ethenogenes lexA gene (italics). Electrophoretic mobility shift assays were used to determine the affinity of the purified LexA protein for the resulting digoxigenin-labeled DNA fragments. Arrows point to the nucleotide used to replace the native nucleotide. The relative positions of nucleotides with respect to the translation initiation codon are indicated in parentheses. The wild-type LexA3 fragment was used as a positive control (W). (B) Bacillus subtilis DinR recognizes the D. ethenogenes LexA binding site. Digoxigenin-labeled LexA1 fragment was incubated in the absence (−) or presence of 25 ng of either purified E. coli LexA (LexAEc), pure B. subtilis DinR (DinR), or D. ethenogenes LexA (LexADh). Furthermore, the LexA1 fragment and the DinR protein were incubated in the presence of a 100-fold molar excess of either the B. subtilis dinR (DinR/PdinR) or D. ethenogenes lexA (DinR/PlexA) promoter sequence.
The Bacillus subtilis DinR repressor recognizes the D. ethenogenes LexA binding site.
The electrophoretic mobility shift assay experiments performed with the wild-type and mutated variants of the D. ethenogenes lexA operator revealed that A and T at positions 1 and 14, respectively, of the AGAACN4GTTCT sequence are important bases for recognition by LexA. Nevertheless, the consensus LexA-box sequence established for B. subtilis and other gram-positive bacteria is CGAACN4GTTCG (23). Indeed, these two terminal nucleotides are part of only a few gram-positive LexA-boxes, especially the T at position 14, which is observed in only 1 of 20 boxes, the Streptomyces lividans recA operator (23).
In order to confirm that the LexA-box of D. ethenogenes is the same as that of gram-positive species, we analyzed the capacity of the DinR repressor to bind to the D. ethenogenes lexA operator. To examine binding, digoxigenin-labeled LexA1 DNA was incubated in the presence of purified B. subtilis DinR protein or D. ethenogenes LexA with or without DNA competitors containing the B. subtilis DinR- or D. ethenogenes LexA-box. The results (Fig. 4B) show that the DinR protein bound to the D. ethenogenes lexA operator and that binding was abolished by competition with unlabeled DNA containing either the D. ethenogenes LexA-box or the B. subtilis DinR-box. All these results clearly indicate that D. ethenogenes LexA protein specifically recognizes the motif GAACN4GTTC, as does DinR. Hence, D. ethenogenes is the first bacterium outside of the gram-positive phylum whose LexA repressor binds to the DinR-box.
The LexA regulon is different in B. subtilis than in D. ethenogenes.
In Escherichia coli, at least 40 genes are directly under the negative control of the LexA repressor (3, 5). The amino acid sequences of each of these E. coli SOS genes were used as queries in BLAST searches of the unfinished D. ethenogenes genome to seek homologous genes in this bacterial species. Homologues of recA, uvrA, ruvA, ruvB, uvrB, uvrD, ssb, dinB, and recN were identified. However, homologues to the rest of the E. coli LexA-regulated genes could not be identified, possibly because the D. ethenogenes genome has not been completely sequenced or because they simply do not exist in this microorganism. Subsequently, the promoters of the genes identified were analyzed to find potential LexA binding sites.
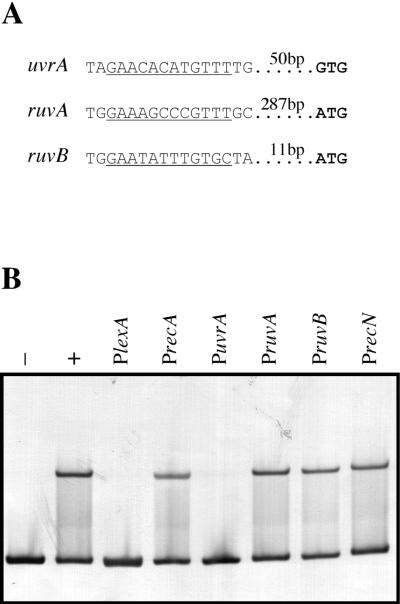
One clear motif, similar to the 12 bp found in the lexA promoter, was identified 64 bp upstream of the putative GTG translational start codon of the uvrA gene (Fig. 5A). Potential motifs were also found in the promoter regions of the ruvA and ruvB genes 301 and 25 bp upstream of their putative translation initiation codons, respectively (Fig. 5A). Examination of the remaining SOS gene homologues failed to reveal any potential LexA binding sites. Strikingly, at first glance, recA does not seem to be regulated by LexA because no LexA binding motif has been identified in its promoter region. To determine if these potential LexA binding sites were functional, PCR-amplified fragments of the promoter regions from uvrA, ruvA, ruvB, recN, and recA were used as competitors in band shift assays with the LexA1 DNA fragment as the labeled probe. While a 100-fold molar excess of DNA containing the uvrA promoter was able to abolish the band belonging to the DNA-LexA complex, the shifted band did not disappear when the same amount of DNA containing the recA, ruvA, ruvB, or recN promoter was used as the competitor (Fig. 5B). This result clearly indicates that LexA binds the uvrA promoter, suggesting that the motif AGAACACATGTTTT is indeed a D. ethenogenes LexA recognition site.
FIG. 5.
(A) Upstream sequences of D. ethenogenes uvrA, ruvA, and ruvB genes presenting putative LexA binding sites. Potential LexA binding sites are underlined, and the putative translation initiation codons are depicted in bold letters, with the distance between them indicated. (B). Electrophoretic mobility shift assay of the LexA1 fragment incubated with purified D. ethenogenes LexA in the presence of different DNA competitors. LexA1 fragment was incubated at 30°C for 30 min in the absence (−) or presence (+) of pure LexA protein. Simultaneous reactions contained the LexA1 fragment, D. ethenogenes LexA protein, and one of six unlabeled DNA competitors (100-fold molar excess) containing the promoter regions and potential LexA binding sites of genes lexA (PlexA,), recA (PrecA), uvrA (PuvrA), ruvA (PruvA), ruvB (PruvB), and recN (PrecN).
The lack of a visible LexA binding motif in the recA promoter sequence is consistent with the inability of the recA promoter fragment to abolish the retarded band. This confirms that no LexA binding motif is present in the recA promoter region and indicates that the recA gene in D. ethenogenes is not under the direct control of the LexA repressor. This has also been observed for Deinococcus radiodurans, where the recA gene is induced by gamma radiation in a LexA-independent manner. However, it has been reported that D. radiodurans possesses a second lexA gene that might be involved in the direct regulation of recA (18). Our results demonstrate that D. ethenogenes LexA does not bind to the recA promoter, thus constituting the first report of a recA gene that is not regulated directly by the LexA repressor.
On the basis of the signature sequences found in various proteins, it has been recently proposed that the green nonsulfur bacterial phylum, to which D. ethenogenes belongs, evolved from a universal ancestor later than the gram-positive bacteria but before the cyanobacteria (9). The Deinococcus-Thermus group seems to have appeared before the green nonsulfur bacteria, although this order has not been definitively fixed (9). Our results demonstrating that the LexA-box sequences of B. subtilis and D. ethenogenes are nearly identical and that the DinR repressor from a gram-positive organism binds to the D. ethenogenes LexA box give support to the hypothesis that a close phylogenetic relationship exists between the gram-positive and green nonsulfur bacteria. However, conclusions about the order of evolution of the green nonsulfur bacteria and the Deinococcus-Thermus group cannot be made on this basis due to the current lack of information on the LexA binding motif for members of the latter group.
Despite having similar LexA binding sequences, gram-positive bacteria and D. ethenogenes display significant differences in the composition of their LexA regulons. A BLAST search of GenBank indicates that genes with an upstream LexA binding sequence are more numerous in the gram-positive bacteria B. subtilis, Staphylococcus aureus, Mycobacterium tuberculosis, and Clostridium acetobutylicum than in D. ethenogenes (Table 2). The reasons for these differences are not clear but could be related to the environmental conditions in which each of these organisms has evolved.
TABLE 2.
Comparison of the presence of a LexA binding sequence upstream of different genes putatively belonging to the LexA regulon from several gram-positive bacteria, D. ethenogenes, and E. colia
| Gene | B. subtilis | S. aureus | M. tuber- culosis | C. aceto-butylicum | D. etheno- genes | E. coli |
|---|---|---|---|---|---|---|
| recA | + | + | + | + | − | + |
| lexA | + | + | + | + | + | + |
| ruvAB | + | − | + | − | −b | + |
| uvrA | − | − | − | − | + | + |
| uvrB | + | − | − | + | − | + |
| uvrD | + | − | − | − | − | + |
| ssb | − | + | − | − | − | + |
| recN | − | − | − | − | − | + |
The presence or absence of a LexA binding site is represented as + and −, respectively. Searches of the whole genomes of several bacteria were performed with the RCGScanner program, which uses a recursive method (20) to identify a given nucleotide sequence as well as its neighboring open reading frames (Erill et al., submitted).
The ruvA and ruvB genes do not form an operon in D. ethenogenes.
Finally, performing a TBLASTN analysis with D. ethenogenes LexA protein as a query at the DOE Joint Genome Institute, a LexA homologue was found in the unfinished genome of the photosynthetic bacterium Chloroflexus aurianticus, which is also a member of the green nonsulfur bacteria. The putative lexA gene is found at the 5′ end of a short contig (694), and thus the N terminus of the protein and the potential LexA-box could not be identified. However, the truncated LexA protein of C. aurantiacus contains the helix-turn-helix motif, which is involved in DNA binding (7), showing 87% similarity with the D. ethenogenes helix-turn-helix domain. This fact suggests that C. aurantiacus LexA might also recognize the GAACN4GTTC as in gram-positive bacteria. The availability of the assembled genome of this organism will be very useful for understanding the evolutionary and phylogenetic history of the green nonsulfur bacteria, for confirming the homogeneity of this phylum with respect to the LexA regulatory sequence, and for determining the composition of the LexA regulon. These data will also be useful for testing the hypothesis, cited above, that a relationship exists between environmental conditions and the genes which are under control of the LexA protein in bacteria.
Acknowledgments
This work was funded by grants BMC2001-2065 from the Ministerio de Ciencia y Tecnología (MCyT) de España and 2001SGR-206 from the Departament d'Universitats, Recerca i Societat de la Informació (DURSI) de la Generalitat de Catalunya. Antonio R. Fernández de Henestrosa is a recipient of a postdoctoral reincorporation contract from the MCyT.
We acknowledge Joan Ruiz, Susana Escribano, and Pilar Cortés for excellent technical assistance. Preliminary D. ethenogenes sequence data were obtained from the Institute for Genomic Research website at http://www.tigr.org. The Chlorefexus aurantiacus partial lexA sequence was provided free by the U.S.-DOE Joint Genome Institute (http://www.jgi.doe.gov/). We are deeply grateful to Roger Woodgate and Kevin Winterling for generously providing us with B. subtilis genomic DNA, B. subtilis DinR, and E. coli LexA protein.
REFERENCES
- 1.Brent, R., and M. Ptashne. 1981. Mechanism of action of the lexA gene product. Proc. Natl. Acad. Sci. USA 78:4204-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks, P. C., F. Movahedzadeh, and E. O. Davis. 2001. Identification of some DNA damage-inducible genes of Mycobacterium tuberculosis: apparent lack of correlation with LexA binding. J. Bacteriol. 183:4459-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courcelle, J., A. Khodursky, B. Peter, P. O. Brown, and P. C. Hanawalt. 2001. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics 158:41-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisen, J. A., and P. C. Hanawalt. 1999. A phylogenomic study of DNA repair genes, proteins, and processes. Mutat. Res. 435:171-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez de Henestrosa, A. R., T. Ogi, S. Aoyagi, D. Chafin, J. J. Hayes, H. Ohmori, and R. Woodgate. 2000. Identification of additional genes belonging to the LexA-regulon in Escherichia coli. Mol. Microbiol. 35:1560-1572. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez de Henestrosa, A. R., E. Rivera, A. Tapias, and J. Barbe. 1998. Identification of the Rhodobacter sphaeroides SOS box. Mol. Microbiol. 28:991-1003. [DOI] [PubMed] [Google Scholar]
- 7.Fogh, R. H., G. Ottleben, H. Ruterjans, M. Schnarr, R. Boelens, and R. Kaptein. 1994. Solution structure of the LexA repressor DNA binding domain determined by 1H NMR spectroscopy. EMBO J. 13:3936-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedberg, E. C., G. C. Walker, and W. Siede. 1995. DNA repair and mutagenesis. American Society for Microbiology, Washington, D.C.
- 9.Gupta, R. S. 2000. The natural evolutionary relationships among prokaryotes. Crit. Rev. Microbiol. 26:111-131. [DOI] [PubMed] [Google Scholar]
- 10.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khil, P. P., and R. D. Camerini-Otero. 2002. Over 1000 genes are involved in the DNA damage response of Escherichia coli. Mol. Microbiol. 44:89-105. [DOI] [PubMed] [Google Scholar]
- 12.Koch, W. H., and R. Woodgate. 1998. The SOS response, p. 107-134. In J. A. Nickoloff and M. F. Hoekstra (ed.), DNA damage and repair: DNA repair in prokaryotes and lower eukaryotes, 1st ed. Humana Press, Totowa, N.J.
- 13.Little, J. W. 1993. LexA cleavage and other self-processing reactions. J. Bacteriol. 175:4943-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little, J. W., S. H. Edmiston, L. Z. Pacelli, and D. W. Mount. 1980. Cleavage of the Escherichia coli LexA protein by the RecA protease. Proc. Natl. Acad. Sci. USA 77:3225-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little, J. W., D. W. Mount, and C. R. Yanisch-Perron. 1981. Purified LexA protein is a repressor of the recA and lexA genes. Proc. Natl. Acad. Sci. USA 78:4199-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo, Y., R. A. Pfuetzner, S. Mosimann, M. Paetzel, E. A. Frey, M. Cherney, B. Kim, J. W. Little, and N. C. Strynadka. 2001. Crystal structure of LexA: a conformational switch for regulation of self-cleavage. Cell 106:585-594. [DOI] [PubMed] [Google Scholar]
- 17.Maymó-Gatell, X., Y. Chien, J. M. Gossett, and S. H. Zinder. 1997. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568-1571. [DOI] [PubMed] [Google Scholar]
- 18.Narumi, I., K. Satoh, M. Kikuchi, T. Funayama, T. Yanagisawa, Y. Kobayashi, H. Watanabe, and K. Yamamoto. 2001. The LexA protein from Deinococcus radiodurans is not involved in RecA induction following gamma irradiation. J. Bacteriol. 183:6951-6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radman, M. 1974. Phenomenology of an inducible mutagenic DNA repair pathway in Escherichia coli: SOS repair hypothesis, p. 128-142. In L. Prakash, F. Sherman, M. Miller, C. W. Lawrence, and H. W. Tabor (ed.), Molecular and environmental aspects of mutagenesis. Charles C Thomas, Springfield, Ill.
- 20.Rodionov, D. A., A. A. Mironov, and M. S. Gelfand. 2001. Transcriptional regulation of pentose utilisation systems in the Bacillus/Clostridium group of bacteria. FEMS Microbiol. Lett. 205:305-314. [DOI] [PubMed] [Google Scholar]
- 21.Tapias, A., and J. Barbe. 1999. Regulation of divergent transcription from the uvrA-ssb promoters in Sinorhizobium meliloti. Mol. Gen. Genet. 262:121-130. [DOI] [PubMed] [Google Scholar]
- 22.Walker, G. C. 1984. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol. Rev. 48:60-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winterling, K. W., D. Chafin, J. J. Hayes, A. S. Levine, R. E. Yasbin, and R. Woodgate. 1998. The Bacillus subtilis DinR binding site: redefinition of the consensus sequence. J. Bacteriol. 180:2201-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winterling, K. W., A. S. Levine, R. E. Yasbin, and R. Woodgate. 1997. Characterization of DinR, the Bacillus subtilis SOS repressor. J. Bacteriol. 179:1698-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]