Abstract
Swarming is an adaptation of many bacteria to growth on surfaces. A search for genes controlling swarmer cell differentiation of Vibrio parahaemolyticus identified a novel three-gene operon that potentially encodes a pyridoxal-phosphate-dependent enzyme, an extracellular solute-binding protein, and a membrane-bound GGDEF- and EAL-motif sensory protein. The functions of these motifs, which are named after conserved amino acid sequences, are unknown, although the domains are found singly and in combination in a variety of bacterial signaling proteins. Studies with translational fusions supported the predicted localization of the gene products. When the operon was overexpressed, swarmer cell gene transcription was induced in liquid culture. Mutants with defects in any of the three genes exhibited decreased swarming and lateral flagellar (laf) gene expression. Complementation studies confirmed an operon organization and suggested that all three genes participated in laf regulation. The lesions that decreased swarming increased capsular polysaccharide (CPS) production, and overexpression of the operon inhibited transcription of the CPS gene cpsA. Thus, the scrABC locus appears to inversely regulate two gene systems that are pertinent to colonization of surface swarming and CPS.
Vibrio parahaemolyticus is a ubiquitous marine bacterium and human pathogen that exists in multiple cell types appropriate for life under different circumstances (reviewed in reference 17). One type, the swimmer cell, is adapted to life in liquid environments. It is rod shaped and ca. 2 μm in length and possesses a single polar flagellum that propels it through liquid. Another cell type, the swarmer, is adapted to life on surfaces. It is an elongated cell (∼30 μm) and possesses numerous peritrichously arranged flagella, called lateral flagella. These lateral flagella enable the bacterium to move over and colonize surfaces in a process called swarming.
V. parahaemolyticus controls differentiation from a swimmer to a swarmer cell in two known ways: surface recognition resulting from interference with rotation of the polar flagellum and nutritional limitation for iron (reviewed in reference 14). How these physical and chemical signals are transduced to regulate gene expression remains to be discovered. Prior work suggested that positive regulators might be involved (22). A mutant with a defect in a gene, lonS, encoding a homolog to the Escherichia coli Lon protease constitutively expressed lateral flagellar genes and produced long cells in liquid. Because of this constitutive swarmer cell phenotype and by analogy to the role of the Lon protease in E. coli, it was hypothesized that LonS targets a transcriptional activator of lateral flagellar genes and a cell division inhibitor. This work describes a search for additional genes positively controlling swarmer cell differentiation. Putative DNA-binding transcriptional activators of transcription were not identified; however, a novel operon was discovered and characterized.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in the present study are described in Table 1. All V. parahaemolyticus strains were derived from the wild-type strain BB22TR (3) and cultured at 30°C. HI broth contained 25 g of heart infusion broth (Difco) and 20 g of NaCl per liter. Swarm plates were prepared by adding 15 g of Bacto Agar (Difco) per liter to HI broth, and nonswarm plates contained 20 g of agar per liter. Antibiotics were used at final concentrations of 50 μg of kanamycin, 10 μg of tetracycline, 30 μg of gentamicin, and 10 μg of chloramphenicol/ml. The final IPTG (isopropyl-β-d-thiogalactopyranoside) concentration was 0.1 mM.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or descriptiona | Source or parent strain and/or reference |
|---|---|---|
| Strains | ||
| BB22TR | Wild type; translucent | R. Belas et al. (3) |
| LM1017 | lfgE313::lux; lux fusion in lateral flagellar hook gene | BB22TR (15) |
| LM4170 | motX118 lfgE313::lux | LM1017 (16) |
| LM4851 | LM1017/pLM1877 | This work |
| LM4897 | scrB1::TnphoAin | BB22TR (this work) |
| LM5126 | LM1017/pLM2032 | This work |
| LM5139 | LM1017/pLM2449 | This work |
| LM5140 | LM1017/pLM2502 | This work |
| LM5153 | LM1017/pLM2341 | This work |
| LM5200 | LM1017/pLM2102 | This work |
| LM5435 | LM1017/pLM2796 | This work |
| LM5545 | ΔscrA1::CamrlfgE313::lux | LM1017 (this work) |
| LM5547 | scrB1::TnphoAinlfgE313::lux | LM1017 (this work) |
| LM5549 | ΔscrC1::KanrlfgE313::lux | LM1017 (this work) |
| LM5719 | ΔscrC1::Kanr | BB22TR (this work) |
| LM5733 | ΔscrA1::Camr | BB22TR (this work) |
| LM5753 | LM1017/pLM2936 | This work |
| LM5789 | scrB1::TnphoAinmotX118 lfgE313::lux | LM 4170 (this work) |
| LM5792 | ΔscrA2::KanrlfgE313::lux | LM1017 (this work) |
| LM5793 | ΔscrA2::Kanr | BB22TR (this work) |
| LM5811 | LM5818/pLM1877 | This work |
| LM5812 | LM5818/pLM2449 | This work |
| LM5813 | LM5818/pLM2796 | This work |
| LM5818 | cpsA1::lacZ(Kanr) | BB22TR (this work) |
| LM5831 | cpsA1::lacZ(Kanr)ΔscrA1::Camr | LM5733 (this work) |
| Plasmids | ||
| pKD3 | Camr template plasmid | 6 |
| pKD11 | Kanr template plasmid | 6 |
| pBSL14 | Kanr cloning vector | 1 |
| pLM1877 | Genr expression vector | 5 |
| pLM2032 | Tetr cosmid containing scr locus | V. parahaemolyticus cosmid bank (13) |
| pLM2102 | Tetr pLAFRII control cosmid from bank | 7 |
| pLM2341 | Tetr Camr cosmid pLM2032 with scrB1::TnphoAin; fusion at aa 195 | pLM2032 (this work) |
| pLM2449 | Genr, IPTG-inducible scrC+ | pLM1877 (this work) |
| pLM2502 | Genr Camr pLM2449 carrying scrC309::TnlacZin; fusion at aa 309 | pLM2449 (this work) |
| pLM2796 | Genr, IPTG-inducible scrA+B+C+ | pLM1877 and pLM2032 (this work) |
| pLM2797 | Tetr Kanr cosmid pLM2032 with ΔscrC1::Kan | pLM2032 (this work) |
| pLM2811 | Tetr Camr cosmid pLM2032 with ΔscrA1::Cam | pLM2032 (this work) |
| pLM2876 | Genr, IPTG-inducible scrA+ | pLM1877 and pLM2796 (this work) |
| pLM2878 | Genr, IPTG-inducible scrB+ | pLM1877 and pLM2796 (this work) |
| pLM2879 | Genr, IPTG-inducible scrB+C+ | pLM1877 and pLM2796 (this work) |
| pLM2916 | Tetr Kanr cosmid pLM2032 with ΔscrA2::Kan | pLM2032 (this work) |
| pLM2936 | Genr Camr pLM2976 with scrA72::TnlacZin; fusion at aa 72 | pLM2796 (this work) |
Camr, chloramphenicol resistance; Kanr, kanamycin resistance; Genr, gentamicin resistance; Tetr, tetracycline resistance.
Genetic and molecular techniques.
General DNA manipulations were adapted from the methods of Sambrook et al. (20). Insertion/deletion mutations were made by using a λ Red recombinase system to introduce the mutations onto cosmids in E. coli (6). The procedures for conjugation and gene replacement in Vibrio parahaemolyticus have been previously described (21). All strain constructions were confirmed by Southern blot analysis of restricted chromosomal DNA. Chromosomal DNA was prepared according to the protocol of Woo et al. (26). A locus encoding extracellular polysaccharide (cps) was identified in a screen, which will be described elsewhere, for biofilm-defective mutants. The cps locus was retrieved on a cosmid by complementation of the biofilm-defective mutant and a promoterless lacZ fusion (with kanamycin resistance) was introduced into the cpsA gene, which encodes a potential capsular polysaccharide (CPS) biosynthesis glycosyl transferase that is most homologous to VC0934 of V. cholerae.
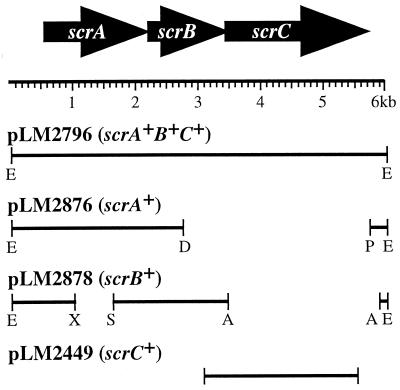
The 15.7-kb scrABC subclone, pLM2796, was generated by digesting cosmid pLM2032 with Eco47III and ligating a 6-kb band into pLM1877 (Fig. 2). The scrA subclone, pLM2876, was made by digesting pLM2796 with DrdI and PshAI, removing the 2.9-kb band containing scrBC and religating the 12.7-kb band containing scrA. To create the scrB subclone, pLM2878, pLM2896 was digested with XhoI and SmaI to remove a 0.73-kb fragment containing scrA creating an in-frame deletion (pLM2879). A 2.5-kb DNA fragment containing scrC was then removed by an AvrII digest of pLM2879. The remaining 12.5-kb band containing only an intact scrB was ligated, creating pLM2878. The scrC gene was amplified by high-fidelity PCR (Boehringer Mannheim) and cloned into pLM1877. Plasmids were mutagenized with λTnphoAin or λTnlacZin (11) or by restriction and insertion of a drug cassette. Active alkaline phosphatase or β-galactosidase fusion production was detected by screening for blue colonies on plates with the chromogenic substrates BCIP (5-bromo-4-chloro-3-indolylphosphate) p-toluidine salt or X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). The precise location of each transposon insertion was determined by DNA sequencing with a primer derived from one end of the transposon. Sequence determination was performed by the DNA Core Facility of the University of Iowa. The GenBank nucleotide sequence accession number for the scr locus is AYO26362. RNA was prepared as previously described (9), and reverse transcription-PCR (RT-PCR) performed according to Promega (Madison, Wis.).
FIG. 2.
Physical map of the scr locus and recombinant plasmids. Arrows point in the direction of transcription deduced from the open reading frames. The restriction sites relevant to constructs are indicated as follows: E, Eco47III; A, AvrII; X, XhoI; S, SmaI; D, DrdI; P, PshAI. Plasmids pLM2876 and pLM2878 were derived from pLM2796. PCR amplification was used to generate the scrC clone pLM2449.
Luminescence assays.
Bioluminescence was monitored by exposing colonies or microtiter dishes in a Fujifilm luminescent image analyzer dark box (LAS-1000) for 1 to 5 s. Luminescence was quantified in a TD20/22 luminometer (Turner Designs) by measuring 0.1-ml samples of culture appropriately diluted to yield a linear response. Luminescence is reported as specific light units (SLU), which are relative light units per minute per milliliter per unit of optical density at 600 nm (OD600). All light experiments were performed at least twice, with the activity of each sample measured in triplicate.
β-Galactosidase assays.
Strains were grown on HI plates at 30°C for 12 h, harvested from the plates, and diluted to an OD600 of 2. β-galactosidase enzyme activity was assayed by using the chloroform-sodium dodecyl sulfate (SDS) lysis procedure (18). Assays were performed at least twice, with the activity of each sample determined in triplicate.
Electron microscopy.
Bacterial colonies were grown on HI plates at 30°C for 2 days and fixed by flooding the plate with 2.5% glutaraldehyde for 1 h at 4°C, followed by washing with 0.2 M cacodylate buffer (pH 7.2). Colonies were cut out of the agar plates and stained with 1% osmium tetraoxide for 1 h, washed, and dehydrated in a series of acetone concentrations (25, 50, 75, 95, and 100%). Samples were then dried chemically with hexamethyldisilizane, coated with tungsten for surface conductivity, and examined with a Hitachi S4000 scanning electron microscope.
Cell fractionation and immunoblot analysis.
Cell fractions were prepared from 1-ml overnight cultures. To prepare whole membranes and cytoplasmic fractions, cells were pelleted and suspended in 0.5 ml of 50 mM Tris-HCl (pH 8)-50 mM EDTA-15% sucrose-0.3 mg of lysozyme per ml, incubated for 30 min on ice, and then centrifuged 5 min (13). The pellet was suspended in 0.25 ml of cold H2O. The sample was passed though a 22-gauge needle to reduce DNA viscosity. After a 15-min centrifugation, the pellet was suspended in 500 μl of Laemmli sample buffer (LSB) (10) and used as the whole membrane fraction. A 100-μl sample of the supernatant was taken, diluted 1:2 in 2× LSB and used as the cytoplasmic fraction. To prepare periplasmic fractions (2), 1 ml of overnight culture was centrifuged and suspended in 100 μl of 10 mM Tris-HCl (pH 8). To this, 20 μl of chloroform was added, followed by incubation on ice for 5 min, and then 150 μl of cold 10 mM Tris-HCl (pH 8) was added. The sample was centrifuged for 5 min, the supernatant was transferred to a new tube, and centrifuged of another 5 min. A 150-μl volume of supernatant was mixed with 150 μl of 2× LSB and used as the periplasmic fraction. Immunoblot analysis was performed as previously described (5) by using anti-alkaline phosphatase antiserum from 5-Prime 3-Prime (Boulder, Colo.) and anti-β-galactosidase antiserum from Chemicon International (Temecula, Calif.).
RESULTS
Identification of a swarmer cell regulatory operon.
To identify genes involved in the regulation of swarmer cell development, a luminescent reporter strain was used. This strain, LM1017, contains an insertion of the luxCDABE operon within the gene encoding the lateral flagellar hook protein, lfgE. The strain is unable to swarm or produce lateral flagella and makes light when grown on a surface but not when grown in liquid (15). Thus, strain LM1017 exhibits surface-regulated lux reporter gene expression. A cosmid library of V. parahaemolyticus DNA was introduced into LM1017 and cosmids were identified that increased lfgE::lux expression in liquid, i.e., constitutive lateral flagellar gene expression.
The cosmid library, which contained ∼25-kb inserts of V. parahaemolyticus DNA (13), was conjugated with LM1017 and ∼5,000 transconjugants were screened for the ability to produce light in liquid by monitoring light expression in microtiter wells. The cosmid backbone was pLAFRII, which contains a pLac promoter (7). Transconjugants appearing bright in microtiter wells were confirmed by measuring light of cultures grown in test tubes. Four classes of cosmids, based on DNA homology, were identified that caused constitutive lfgE::lux expression. One of these cosmids, pLM2032, which conferred the greatest lfgE::lux induction in strain LM5126, was selected for further studies. Induction of luminescence in this strain is shown in Fig. 1A.
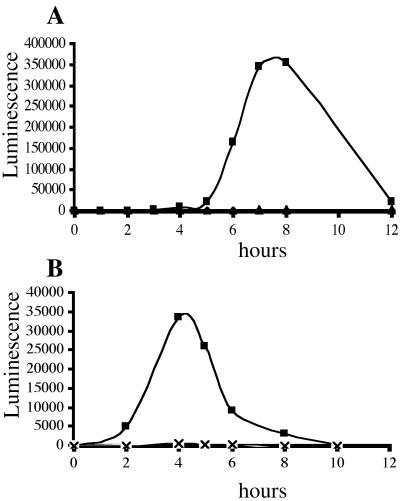
FIG. 1.
Overexpression of scrABC induces lfgE::lux expression. Luminescence (expressed as SLU) was monitored during growth of cultures in HI liquid medium. (A) Cosmid-induced luminescence. Lux reporter strain LM1017 contained pLM2032 (▪), pLM2032 carrying scrB::TnphoA (♦), and vector control (▴). (B) Subclone-induced luminescence. Lux reporter strain LM1017 contained pLM2796 (▪), pLM2796 carrying scrA:: TnlacZ (▴), pLM2796 carrying scrC::TnlacZ (×), and vector control (♦).
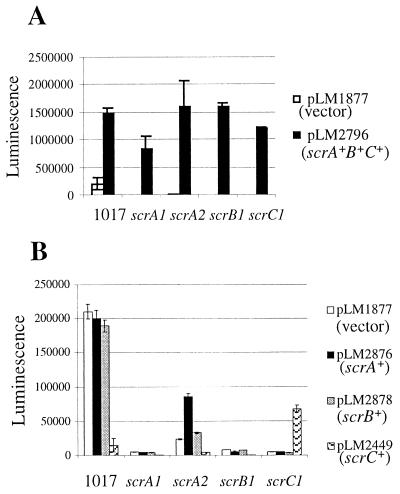
To identify genes responsible for the ability of pLM2032 to cause constitutive lfgE::lux expression, the cosmid was mutagenized with TnphoAin and TnlacZin, transferred to LM1017, and screened for mutations that abolished constitutive lfgE::lux expression. Strain LM5153, carrying a TnphoAin-mutated cosmid (pLM2341), was found to be unable to induce lfgE::lux in liquid (Fig. 1A). The point of transposon insertion on this cosmid, pLM2341, was sequenced and the insertion mapped in the second open reading frame (scrB) of a potential three-gene operon. To confirm whether this potential operon was responsible for lfgE::lux expression in liquid, an Eco47III fragment containing only the three open reading frames was subcloned from the cosmid into pLM1877 and named pLM2796. This clone contained sufficient genetic information to cause lfgE::lux expression in liquid (Fig. 1B). The potential operon it contained, scrABC, is depicted in Fig. 2. Although the maximal level of light produced by strain LM1017 containing pLM2796 (∼35,000 SLU) was lower than that produced by the cosmid-containing strain (∼350,000 SLU), it was significantly higher than strain LM1017 containing the vector (∼500 SLU). The difference between light induced by the cosmid and the subclone may be due to copy number or promoter differences between the vectors. Furthermore, introduction of insertion mutations in scrA or scrC genes carried by pLM2796 abolished the ability of the subclone to cause light production in liquid (Fig. 1B).
The first gene, scrA, encodes a 468-aa potential protein that shares a domain present in several pyridoxal-phosphate-dependent enzymes (conserved domain Pfam00266, class V aminotransferases). The second gene, scrB, encodes a product (321 aa) similar to many bacterial extracellular solute-binding proteins (family 3; ABC-type transporter components). For example, BLAST comparison with Salmonella enterica serovar Typhimurium FliY, which evidence suggests is an l-cystine binding protein (24), yielded a score of 70.1 bits, E = 4e − 11, with 25% identities and 42% similarities. The third gene, scrC, encodes a 774-aa potential sensory protein. The N terminus of ScrC has two predicted transmembrane domains flanking a potential periplasmic domain, which is ca. 300 aa in length. The C terminus contains two conserved domains of unknown function. The portion from aa 336 to 502 shows similarity to a number of proteins in the GGDEF family (Pfam00990; DUF9). The portion from aa 518 to 761 show similarity to an EAL domain (Pfam00563; DUF2). The GGDEF and EAL domains are often found in tandem in putative diguanylate cyclases and phosphodiesterases (8). A BLAST comparison of the C terminus of ScrC (300 to 774 aa) with diguanylate cyclase (from Gluconacetobacter xylinus; GenBank accession no. AF052518) yielded 28% identities and 47% positives (score of 177 bits, E = 2e − 43) and with phosphodiesterase A (from G. xylinus; GenBank accession no. AF052519) yielded 34% identities and 60% positives (score of 278 bits, E = 1e − 73).
The three potential open reading frames are transcribed in the same direction with very small intergenic regions, and scrB and scrC appear translationally coupled. RT-PCR analysis produced products with primers designed to reveal scrAB and scrBC transcription, whereas primers designed to show products upstream of scrA and downstream of scrC produced no products, although all primers could be shown to produce appropriately sized products with chromosomal DNA as a template (data not shown). Thus, the evidence suggests that these three genes comprise an operon.
Localization of Scr proteins.
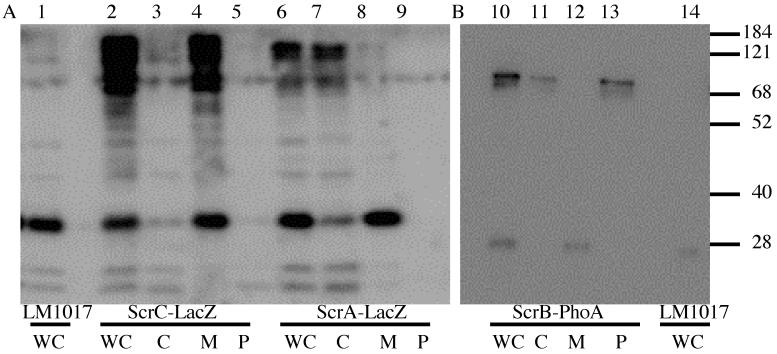
To probe protein topology and determine the subcellular localization of the scr gene products, the scrABC locus was mutagenized with the Tn5-derived transposons TnphoAin and TnlacZin. These transposons can be used to generate alkaline phosphatase and β-galactosidase gene fusions (11). Since active alkaline phosphatase fusions target exported protein sequences and active β-galactosidase fusions target cytoplasmic protein sequences they can be used to study protein localization and topology. Fusions producing active alkaline phosphatase or β-galactosidase were identified by screening on chromogenic substrates BCIP or X-Gal. Insertions in the scr region were mapped by restriction analysis and by sequencing. An alkaline phosphatase fusion with ScrB (pLM2341) and β-galactosidase fusions with ScrA (pLM2936) and ScrC (pLM2502) were identified. The fusion-containing plasmids were transferred to LM1017 and cytoplasmic, periplasmic, and whole membrane cell fractions were isolated. Immunoblots were performed and probed with anti-alkaline phosphatase or anti-β-galactosidase sera (Fig. 3). Although V. parahaemolyticus cannot utilize lactose as a carbon source and fails to hydrolyze X-Gal, there is a strong cross-reaction of the anti-β-galactosidase antibody with protein of low molecular mass (∼30 kDa). Samples prepared from LM1017 carrying pLM2502 (ScrC-LacZ, lanes 2 to 5) showed a high-molecular-mass product in the whole-cell (lane 2) and membrane (lane 4) fractions corresponding to the ScrC-LacZ fusion protein (predicted molecular mass of ∼204 kDa), which is not present in the parental strain (lane 1). Samples prepared from LM1017 carrying pLM2936 (ScrA-LacZ, lanes 6 to 9) showed a high-molecular-mass product in the whole-cell (lane 6) and cytoplasmic fractions corresponding to the ScrA-LacZ fusion protein (predicted molecular mass of ∼170 kDa) (lane 7). Samples prepared from LM1017 carrying pLM2341 (ScrB-PhoA, lanes 10 to 13) showed a high-molecular-mass product in the whole-cell (lane 10) and periplasmic (lane 13) fractions corresponding to the ScrB-PhoA fusion protein (predicted molecular mass of ∼80 kDa). A small amount of ScrB fusion product was also detected in the cytoplasm (lane 11), which may be due to inefficient osmotic lysis. High-molecular-mass immunoreactive products were not seen with the parental strain LM1017 (lanes 1 and 14). To summarize, the ScrA-LacZ hybrid was found in the cytoplasm, the ScrB-PhoA hybrid was primarily detected in the periplasm, and the ScrC-LacZ hybrid was associated with the membrane fraction. The specific localization of each of the hybrid products was consistent with the enzyme activity of the reporter fusion. The ScrC-LacZ fusion joint occurred after two predicted transmembrane domains in ScrC and was predicted to place β-galactosidase activity in the cytoplasm.
FIG. 3.
Cellular localization of Scr proteins. Cellular fractions were isolated from strains producing the fusion proteins: ScrA-LacZ (LM5753), ScrB-PhoA (LM5153), and ScrC-LacZ (LM5140). Immunoblots were performed by probing with anti-β-galactosidase (A) or anti-alkaline phosphatase (B). Sample fractions are denoted as whole cells (WC), cytoplasm (C), membrane (M), or periplasm (P). Lanes: 1, LM1017 (WC); 2, LM5140 (WC); 3, LM5140 (C); 4, LM5140 (M); 5, LM5140 (P); 6, LM5753 (WC); 7, LM5753 (C); 8, LM5753 (M); 9, LM5753 (P); 10, LM5153 (WC); 11, LM5153 (C); 12, LM5153 (M); 13, LM5153 (P); 14, LM1017 (WC). The molecular size standards are given in kilodaltons on the right.
scr mutants are defective in swarming.
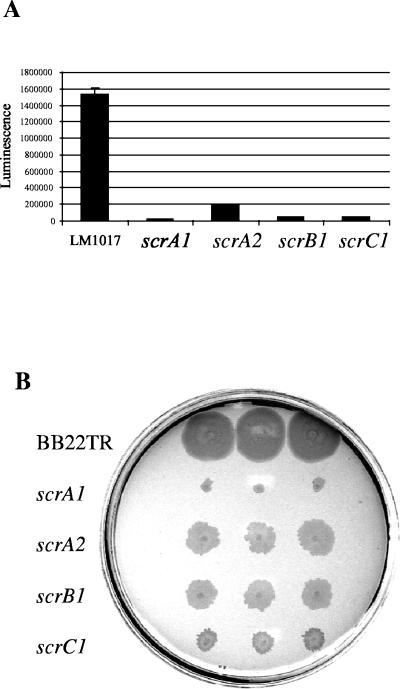
To initiate further studies on the scr operon, additional mutations in scr genes were constructed. Two antibiotic resistance cassettes were used to interrupt scrA, by deleting aa 108 to 385 and inserting a “polar” chloramphenicol cassette (from pKD3) whose transcriptional orientation was opposed to that of scrA to make pLM2811 (ΔscrA1) or inserting a “nonpolar” kanamycin cassette (from pKD11), whose transcription was in the same orientation as scrA and which possessed a ribosome-binding site and lacked a transcription terminator at its 3′ terminus, to make pLM2916 (ΔscrA2). In scrC, aa 142 to 515 were deleted by BglII digestion, and a kanamycin cassette (from pLM1871) was inserted to make pLM2797 (ΔscrC1). These mutations, along with scrB1::TnphoA (pLM2341) were introduced onto the chromosome of reporter strain LM1017 and wild-type strain BB22TR by homologous recombination, and lfgE::lux expression and swarming motility were monitored. Mutations in the scr operon greatly reduced lfgE::lux expression in the LM1017 background when grown on plates (Fig. 4A). When the drug cassette in the scrA2 allele was aligned with scr transcription, a direction that should not have been polar on downstream transcription, the phenotype was less severe than when transcription of the cassette was opposed to scr transcription (in scrA1). The scrA1 mutation in strain LM5545 had the most profound effect (19,800 SLU) of all scr alleles on reducing light production ∼70-fold compared to LM1017 (1,540,000 SLU), whereas the scrA2 mutation in strain LM5792 produced 195,000 SLU. This suggests that scrA is required for operon function. Strains containing the scrB1 mutation (LM5547) produced 47,300 SLU and the scrC mutation (LM5549) produced 47,500 SLU. Swarming motility was similarly perturbed in scr mutants in the BB22TR background (Fig. 4B). Mutants LM5793 (scrA2), LM4897 (scrB1), and LM5719 (scrC1) had much slower rates of swarming than the parent strain BB22TR. Mutant LM5733 (scrA1) had the most severe swarming defect with very little swarming movement. Western analysis confirmed that mutant strains produced significantly less lateral flagellin (data not shown). Swimming motility was unaffected in scr mutants.
FIG. 4.
scr mutants are defective in lateral flagellar gene expression and swarming. (A) Luminescence (reported as SLU) was measured from HI plate-grown samples after 9 h at 30°C. The values used for light production were as follows: LM1017 (lfgE::lux), 1,540,000 ± 75,000 SLU; LM5545 (scrA1), 19,800 ± 800 SLU; LM5792 (scrA2), 195,000 ± 9,000 SLU; LM5547 (scrB1), 47,300 ± 3,100 SLU; and LM5549 (scrC1), 47,500 ± 2,200 SLU. (B) Swarming motility of scr mutants after 9 h at 30°C. The strains included BB22TR (wild type), LM5733 (scrA1), LM5793 (scrA2), LM4897 (scrB1), and LM5719 (scrC1).
Complementation analysis was performed to confirm operon structure and gain insight into scr gene function (Fig. 5). Plasmid pLM2796, containing scrABC, restored lfgE::lux expression to all of the scr mutants and increased expression in LM1017 when grown on HI plates containing gentamicin to select maintenance of the plasmid (Fig. 5A). Plasmid pLM2876, containing scrA, was unable to restore lfgE::lux expression in LM5545 (scrA1), LM5547 (scrB1), and LM5549 (scrC1) but did increase lfgE::lux expression threefold in LM5792 (scrA2). This indicates that the scrA1 allele in LM5545 was more polar than the scrA2 mutation in LM5792. Plasmid pLM2878, containing scrB, did not restore lfgE::lux expression in any of the mutants. pLM2878 was derived from pLM2879 (scrB+C+), which was able to significantly increase lfgE::lux expression in liquid-grown LM1017 compared to liquid-grown LM1017 carrying a vector control or pLM2449 (scrC+) (data not shown), suggesting that the scrB gene can be expressed from pLM2879 (and consequently pLM2878). Therefore, scrB on a plasmid does not complement the scrB mutant. Plasmid pLM2449, containing scrC, increased lfgE::lux in LM5549 (scrC1) by 12-fold. To summarize the mutant phenotype and complementation results, scrA was found to be required for operon function. Expression of scrA from a plasmid resulted in partial complementation of lfgE::lux expression in the scrA mutant containing the aligned cassette but not with the opposed cassette. Expression of scrB resulted in no complementation of scr mutants. Expression of scrC restored lfgE::lux expression in an scrC but not an scrB mutant strain. The inability of scrC to complement the scrB mutant suggests scrB is required for normal functioning of the operon. These data are consistent with an operon organization for the scr genes. It also indicates that the product of each gene has an essential role in the functioning of this operon.
FIG. 5.
Complementation of lateral flagellar gene expression defects in scr mutants. (A) Plasmids pLM2796 (scrA+B+C+) and pLM1877 (vector) in LM1017 and scr-defective backgrounds. (B) Plasmids pLM1877 (vector), pLM2876 (scrA+), pLM2878 (scrB+), and pLM2449 (scrC+) in LM1017 and scr-defective backgrounds. Luminescence (reported in SLU) was measured from HI plate-grown samples after 10 h of growth at 30°C.
The scr operon is not required for polar flagellum-mediated surface sensing.
Surface sensing and induction of swarmer cell differentiation is hypothesized to occur upon restriction of polar flagellar function. Mutants with defects in the polar flagellar gene system constitutively express lateral flagellar genes (5). To determine whether the scr operon participated in surface sensing via the polar flagellar pathway, the mutant strain LM5789, containing motX and scrB alleles, was constructed in the LM1017 background. Strains were grown in HI broth with light measurements taken from late-logarithmic-phase cultures when maximal light was observed. LM1017 produced little light when grown in liquid (1,200 ± 55 SLU), as did LM5547 (scrB1) (1,500 ± 59 SLU). In contrast, LM4170 (motX118) produced 1,200,000 ± 40,000 SLU. Introduction of a scrB mutation into LM4170 (to make LM5789) caused little effect on light production (600,000 ± 22,000 SLU), indicating that the scr operon is not required for induction of the swarmer cell program via the polar flagellum-mediated surface sensing pathway.
The scr operon controls CPS production
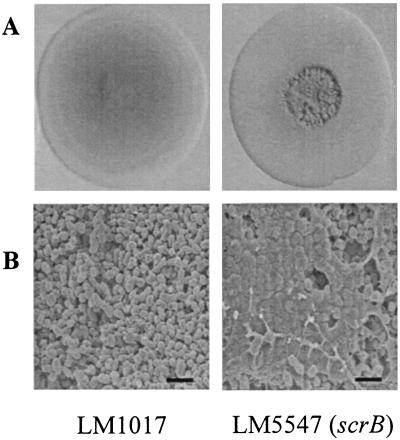
The scr mutants displayed abnormal colony morphologies that were more rugose than the smooth, translucent colony type of the parental strains (BB22TR or LM1017) (Fig. 6A). Colony roughness was not simply a result of lack of lateral flagella because mutants bearing lesions in laf genes, i.e., LM1017, do not form rugose colonies. To more closely examine the rough colony morphology, scanning electron microscopy was performed on rugose colonies of scrB mutant LM5547 and on smooth colonies of the parent LM1017 (Fig. 6B). In micrographs of the rough LM5547 colonies, cells appeared encased in an electron-dense extracellular matrix, whereas smooth LM1017 colonies contained cells with no extracellular matrixes.
FIG. 6.
scr mutants display a rugose colony morphology and produce an extracellular matrix. (A) Colony morphology of a smooth parent strain, LM1017, and a rough scrB mutant, LM5547. (B) Scanning electron microscopy of a smooth LM1017 colony and rugose LM5547 colony. Colonies were grown on HI plates at 30°C for 2 days. Bar, 2 μm.
CPS seemed a likely candidate for the extracellular matrix observed in the rugose colony by scanning electron microscopy. To test this idea, the expression of a CPS synthase gene, cpsA, fused to lacZ (cpsA::lacZ) was monitored in wild-type and scr mutant backgrounds. Strain LM5818, which contains the cpsA::lacZ mutation, produced 54 ± 1.1 Miller units when β-galactosidase assays were performed on cells harvested from HI plates grown for 12 h. In comparison, the double-mutant strain LM5831, which carries the cpsA::lacZ and ΔscrA::Cam mutations, produced 158 ± 2.1 Miller units. Thus, the scr mutations appear to have pleiotropic defects, inversely affecting lateral flagellar expression and CPS expression. Consistent with these results, strain LM5831 lacked the rugose colony phenotype normally seen in scr mutants. LM5831 (scrA1 cpsA1) formed smooth colonies that resembled the colonies of LM5818 (cpsA1) and the wild-type strain BB22TR. The reporter gene data and the colony morphology are consistent with the hypothesis that scr mutants have an increased level of CPS production that contributes to the rugose colony morphology. Furthermore, the cps lesion had no effect on swarming. Strain LM5818 swarmed as well as wild-type parental strain LM5674. In contrast, LM5831 showed a profound swarming defect that was equivalent to the defect seen for strain LM5733 (scrA1). Thus, the scr allele was dominant to the cps allele with respect to laf gene expression.
Overexpression of scrABC and scrC
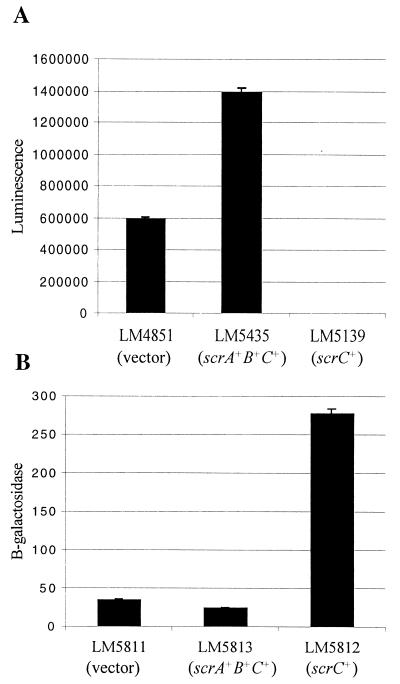
Surface-grown mutants with scr defects displayed reduced lfgE::lux levels but increased cpsA::lacZ levels. Overexpressing scrABC from a plasmid slightly increased lfgE::lux levels (1,400,000 SLU) compared to LM1017 carrying a vector control (600,000 SLU) (Fig. 7A). In contrast, expressing only scrC from a plasmid greatly decreased lfgE::lux levels (25 SLU) (Fig. 7A). The effect of ScrC overproduction on CPS was also examined (Fig. 7B). Strain LM5813 (cpsA::lacZ) expressing scrABC from a plasmid produced 25 Miller units, LM5812 expressing scrC from a plasmid produced 278 Miller units, and LM5811 containing the plasmid control produced 35 Miller units. Thus, overexpression of scrC significantly increased expression of a CPS gene (∼8-fold).
FIG. 7.
scrABC and scrC overexpression inversely affects lateral flagellar and CPS gene expression. (A) The amounts of luminescence produced were as follows: 600,000 ± 11,200 SLU in LM4851 (carrying the vector), 1,400,000 ± 25,500 SLU in LM5435 (carrying scrA+B+C+), and 25 ± 4 SLU in LM5139 (scrC+). (B) The amounts of β-galactosidase produced (expressed as Miller units) were as follows: 35 ± 1.2 in LM5811 (vector), 25 ± 1 in LM5813 (scrA+B+C+), and 278 ± 6 in LM5812 (scrC+). Samples were harvested from HI-gentamicin plates with IPTG after 12 h of growth.
DISCUSSION
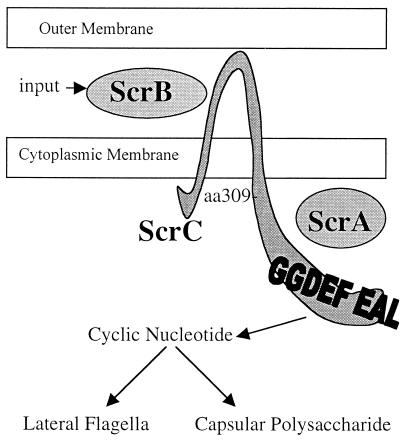
When grown on a surface V. parahaemolyticus differentiates from a swimmer cell to a swarmer cell. Differentiation allows efficient movement over and colonization of surfaces. One large gene set that is induced in response to growth on surfaces encodes the lateral flagellar motility system. In an attempt to discover regulators of lateral flagella gene expression, a cosmid library of V. parahaemolyticus DNA was screened for the ability to induce constitutive expression of the lateral flagellar system by monitoring lfgE::lux expression in liquid. One cosmid, which was chosen for these studies, contained a 6-kb, three-gene operon that was determined to be responsible for inducing constitutive lfgE::lux expression and repressing CPS production. This operon, which appears unique to V. parahaemolyticus, was named the “swarming and CPS regulatory” operon (scr), and the gene products are depicted in Fig. 8. None of the genes encode potential transcriptional activators, but instead the gene products appear to have the capacity to form a sensory signaling cascade. The first gene in the operon, scrA, encodes a polypeptide that contains a signature domain characteristic of pyridoxal-phosphate-dependent enzymes. ScrB appears to be an extracellular solute-binding protein whose closest homologs are bacterial extracellular amino acid solute-binding proteins. ScrC contains a potential periplasmic receptor domain and a cytoplasmic domain containing GGDEF and EAL domains. Localization studies revealed ScrA is found in the cytoplasm, ScrB is detected in the periplasm and ScrC is associated with the membrane fraction. The two potential transmembrane domains of ScrC in combination with membrane localization of an active β-galactosidase fusion positioned at aa 309 of ScrC suggest that the GGDEF and EAL domains are in the cytoplasm.
FIG. 8.
Model for the ScrABC sensory transduction system. ScrB has similarity to solute-binding proteins and is hypothesized to receive an input signal and interact with the periplasmic domain of ScrC. This interaction could modulate the activity of the cytoplasmic portion of ScrC, possibly controlling levels of a cyclic nucleotide signaling molecule by acting as a cyclic nucleotide phosphodiesterase or cyclase. ScrC aa 309 is the site of an active β-galactosidase fusion, suggesting that the GGDEF and EAL domains reside in the cytoplasm. ScrA contains a domain shared by pyridoxal-phosphate-dependent enzymes and is required for signal transduction, but its role is unclear. Levels of the small signaling molecule would ultimately determine output by affecting the expression of lateral flagella and CPS in an inverse manner.
To probe the function of these proteins, mutations in each gene were constructed and transferred to the chromosome. When grown on surfaces, the scr mutants displayed greatly reduced levels of lfgE::lux expression and were impaired in their ability to swarm. RT-PCR and complementation studies demonstrated that the scrABC genes are organized in an operon, and each gene appears to contribute to functioning of the operon. The scr mutants displayed a rugose colony phenotype. Examination by scanning electron microscopy revealed an extracellular matrix encasing the cells of the rough colonies. To determine whether this was a result of CPS production, a cpsA::lacZ and scrA double mutant was constructed. This double mutant had a fivefold increase in cpsA::lacZ expression compared to the strain containing only the cpsA::lacZ allele. Moreover, introduction of the cpsA::lacZ mutation into the rugose scrA strain caused loss of the rough colony phenotype.
Overexpression of scrABC increased lfgE::lux expression and decreased cpsA::lacZ expression. The overexpression of scrC had the opposite effect; decreasing lfgE::lux expression and increasing cpsA::lacZ expression. These results, along with mutant data, localization results, and sequence homology, lead us to propose the following model. ScrB may be a periplasmic binding protein involved in signal reception and interacts with the periplasmic domain of ScrC. The interaction could modulate the activity of the cytoplasmic GGDEF and EAL domains, which possibly control the intracellular concentration of a small signaling molecule. Although GGDEF and EAL domains are found widely in bacteria (8), little is known about their function. The two domains are found in tandem in the diguanylate cyclases and phosphodiesterases that regulate cellulose synthesis in G. xylinus (23). In this organism, it is thought that a cyclic nucleotide (cyclic di-GMP), whose level is controlled by numerous GGDEF and EAL domain-containing proteins, directly stimulates cellulose biosynthesis. By analogy to G. xylinus, Scr signaling may influence the level of a small regulatory molecule, e.g., interaction of ScrB with ScrC may activate a cyclic nucleotide phosphodiesterase (or cyclase) activity. The level of the small signaling molecule could then modulate the activity of a transcription factor (or factors) much as cyclic AMP activates CAP. The role of ScrA is less clear, although it does contain a domain found in many pyridoxal-phosphate-dependent enzymes. Final output of this signal transduction pathway influences both lateral gene expression and CPS expression, two gene systems important to life on a surface. Because an scr allele is dominant to the cps allele with respect to swarming and the cps allele is dominant to an scr allele with respect to CPS production, it does not seem likely that overproduction of one extracellular factor (e.g., CPS) negatively controls production of the second factor (e.g., lateral flagella or vice versa).
What is the role of the scr operon with respect to control of swarmer cell development?
Loss of function in scr genes greatly reduces but does not entirely eliminate swarming; therefore, the operon is not absolutely required for swarmer cell differentiation. Supporting this is the observation that when an scr mutation is introduced into a polar flagellum mutant, which constitutively expresses lateral flagella in liquid, lfgE::lux expression is not significantly reduced in liquid. Thus, the dominance of the polar motility allele also suggests that the scrABC operon is not required for the flagellum-sensing pathway. Epistasis of the polar motility allele to an scr allele could reflect participation in a common pathway initiating surface-induced gene expression and swarmer cell differentiation; however, because scr lesions do not completely abolish induction of swarmer cell differentiation, we favor the hypothesis that the scr operon plays a role at a later point of surface colonization. Swarmer cell differentiation is a transient phenomenon. The initial contact with a surface results in the restriction of polar flagellum rotation. Through the polar flagellum-mediated surface sensing pathway, this leads to cell elongation and production of hundreds of lateral flagella per cell. At some point the swarmer cells must dedifferentiate in order to divide and grow (22, 25). In addition, lateral flagellar production is a costly process that is not beneficial unless the cells are on the leading edge of a swarming colony. Studies on another swarming bacterium, Proteus mirabilis, indicate that swarming involves a highly regulated, complex sequence of multicellular events organized into distinct periodic cycles of differentiation, migration, and dedifferentiation (4, 12, 19). There are probably times during surface colonization when it is advantageous to be an elongated, hyperflagellated motile swarmer cell (e.g., when initially establishing the colonization of a surface). There are also probably instances on a surface when it is advantageous to be a less motile, more adhesive cell type producing CPS (e.g., during biofilm development). The scr operon, which has control over lateral gene expression and CPS expression, may play a role in mediating the switch between these cell types.
Acknowledgments
We thank Yun-Kyeong Kim for her excellent molecular biology support.
This work was supported by the National Science Foundation research grant MCB-0077327 to L.L.M. and National Science Foundation research training grant DBI9602247 to B.R.B.
REFERENCES
- 1.Alexyev, M. F. 1995. Three kanamycin resistant gene cassettes with different polylinkers. BioTechniques 18:52-55. [PubMed] [Google Scholar]
- 2.Ames, G. F., C. Prody, and S. Kustu. 1984. Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J. Bacteriol. 160:1181-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belas, R., M. Simon, and M. Silverman. 1986. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J. Bacteriol. 167:210-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belas, R. 1996. Proteus mirabilis and other swarming bacteria, p. 183-219. In J. A. Shapiro and M. Dworkin (ed.), Bacteria as multicellular organisms. Oxford University Press, Oxford, England.
- 5.Boles, B. R., and L. L. McCarter. 2000. Insertional inactivation of genes encoding components of the sodium-type flagellar motor and switch of Vibrio parahaemolyticus. J. Bacteriol. 182:1035-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman, A., S. R. Long, S. E. Brown, W. J. Buikema, and F. Ausubel. 1982. Construction of a broad-host-range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene 18:289-296. [DOI] [PubMed] [Google Scholar]
- 8.Galperin, M. Y., A. N. Nikolskaya, and E. V. Koonin. 2001. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol. Lett. 203:11-21. [DOI] [PubMed] [Google Scholar]
- 9.Kim, Y. K., and L. L. McCarter. 2000. Analysis of the polar flagellar gene system of Vibrio parahaemolyticus. J. Bacteriol. 182:3693-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 11.Manoil, C., and J. Bailey. 1997. A simple screen for permissive sites in proteins: analysis of Escherichia coli lac permease. J. Mol. Biol. 267:250-263. [DOI] [PubMed] [Google Scholar]
- 12.Matsuyama, T., Y. Takagi, Y. Nakagawa, H. Itoh, J. Wakita, and M. Matsushita. 2000. Dynamic aspects of the structured cell population in a swarming colony of Proteus mirabilis. J. Bacteriol. 182:385-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarter, L. L., and M. Silverman. 1987. Phosphate regulation of gene expression in Vibrio parahaemolyticus. J. Bacteriol. 169:3441-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarter, L. L., and M. Silverman. 1990. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol. Microbiol. 4:1057-1062. [DOI] [PubMed] [Google Scholar]
- 15.McCarter, L. L., and M. E. Wright. 1993. Identification of genes encoding components of the swarmer cell flagellar motor and propeller and a sigma factor controlling differentiation of Vibrio parahaemolyticus. J. Bacteriol. 175:3361-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarter, L. L. 1994. MotX, the channel component of the sodium-type flagellar motor. J. Bacteriol. 176:5988-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarter, L. L. 1999. The multiple identities of Vibrio parahaemolyticus. J. Mol. Microbiol. Biotechnol. 1:1-7. [PubMed] [Google Scholar]
- 18.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 19.Rauprich, O., M. Matsushita, C. J. Weijer, F. Siefert, S. E. Esipov, and J. A. Shapiro. 1996. Periodic phenomena in Proteus mirabilis swarm colony development. J. Bacteriol. 178:6525-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 21.Silverman, M., R. Showalter, and L. L. McCarter. 1991. Genetic analysis in Vibrio. Methods Enzymol. 204:515-536. [DOI] [PubMed] [Google Scholar]
- 22.Stewart, B. J., J. L. Enos-Berlage, and L. L. McCarter. 1997. The lonS gene regulates swarmer cell differentiation of Vibrio parahaemolyticus. J. Bacteriol. 179:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tal, R., H. C. Wong, R. Calhoon, D. Gelfand, A. L. Fear, G. Volman, R. Mayer, P. Ross, D. Amikam, H. Weinhouse, A. Cohen, S. Sapir, P. Ohana, and M. Benziman. 1998. Three cdg operons control cellular turnover of cyclic di-GMP in Acetobacter xylinum: genetic organization and occurrence of conserved domains in isoenzymes. J. Bacteriol. 180:4416-4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner, M. S., T. Woodberry, L. M. Hafner, and P. M. Giffard. 1999. The bspA locus of Lactobacillus fermentum BR11 encodes an l-cysteine uptake system. J. Bacteriol. 181:2192-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulitzur, S. 1974. Induction of swarming in Vibrio parahaemolyticus. Arch. Microbiol. 101:357-363. [DOI] [PubMed] [Google Scholar]
- 26.Woo, T. H. S., A. F. Cheng, and J. M. Ling. 1992. An application of a simple method for the preparation of bacterial DNA. BioTechniques 13:696-697. [PubMed] [Google Scholar]