Abstract
A 3,372-bp insertion sequence, ISPpu12, has been identified on the archetypal toluene-xylene TOL catabolic plasmid pWW0 from Pseudomonas putida mt-2. The insertion sequence element is located on the plasmid between bases 84397 and 87768 in a region which also contains the termini and transposase genes of the catabolic transposons Tn4651 and Tn4653 (A. Greated, L. Lambertson, P. A. Williams, and C. M. Thomas, Environ. Microbiol., in press). ISPpu12 has terminal inverted repeats of 24 bp with three mismatches and contains four open reading frames, a tnpA homologue and three open reading frames (lspA, orf1, and orf2) of undetermined function. After insertion in vitro of a Kmr cassette into ISPpu12 either in the intergenic region between orf1 and orf2 or directly into the orf1 gene and ligation into a suicide vector, the modified ISPpu12-Km transposes at high frequency, often in multiple copies, into the chromosome of a P. putida recipient. Inactivation of lspA, orf1, and orf2 by introducing a 7-bp deletion into the 5′ region of each gene had no major effect upon transposition, but a similar mutation of tnpA completely eliminated transposition. Analysis of the literature and of strains derived from the chlorobenzoate-degrading Pseudomonas sp. strain B13 suggests that the promiscuity of this element has played an important role in the history of plasmid pWW0. Database comparisons and the accompanying paper (A. J. Weightman, A. W. Topping, K. E. Hill, L. L. Lee, K. Sakai, J. H. Slater, and A. W. Thomas, J. Bacteriol. 184:6581-6591, 2002) show that ISPpu12 is a transposable element also found in other bacteria.
The 116,580-bp IncP9 plasmid pWW0 from Pseudomonas putida mt-2 (8) is an archetype of the large group of catabolic plasmids found widely in many genera of gram-negative soil bacteria. It has been much studied, mainly because of the four xyl regulons which encode the inducible catabolism of toluene and some substituted toluenes to central metabolites (2, 33). Studies on the two operons of structural genes and the regulation of their expression by the gene products of xylS and xylR have made this arguably one of the best understood long catabolic pathways for substrates of nonbiological origin. In addition to its catabolic function, the plasmid has a number of other interesting structural features. The complete xyl genes are located within a 39-kb span between two directly repeated copies of a 1,275-bp insertion sequence, IS1246 (23). The only reported phenotype resulting from this arrangement is that the entire catabolic region can be deleted from the plasmid by homologous recombination between the direct repeats. The result is a smaller, but still conjugative, 78-kb plasmid, pWW0-8, which is phenotypically cryptic (3, 7) and carries only a single copy of IS1246. There are two large nested transposons on pWW0. Tn4651 is 56 kb and encompasses the 39-kb catabolic region (27), and Tn4653 is 70 kb and spans Tn4651 (28). Both are class II (Tn3-like) transposons which share common tnpT, tnpS, and res genes but have individual tnpR and tnpA genes and are bounded by terminal inverted repeats of 46 and 38 bp, respectively (29). Although both carry the 39-kb catabolic region and both confer the ability to grow on the TOL substrates when they transpose, their transposition genes are not within the catabolic region and both transpose equally well when the 39-kb catabolic region has been deleted, when they are referred to as Tn4652 and Tn4654, respectively (27, 28).
In a number of early studies (15, 17, 22) pWW0 was found to carry insertions of 3 to 3.5 kb in various locations, usually within the catabolic regions, where their presence has been associated with a change in the catabolic phenotype. The only clue as to the origin of an insertion of this size was from Southern blots of several such plasmids, which suggested that they hybridized with a region of pWW0 well separated from the catabolic region (17). Analysis of the recently completed nucleotide sequence of pWW0 (accession number AJ344068) has suggested that the plasmid has had a complex evolution and that multiple transposition and recombination events have made a major contribution to its present structure (8).
The availability of the complete sequence has facilitated our reexamination of one ∼3-kb insertion in a plasmid derived from pWW0 (15) and its comparison with the wild-type pWW0 sequence. As a result, we report that pWW0 carries a functional 3,372-bp insertion sequence, ISPpu12, which is located between bases 84397 and 87768 in the annotated sequence.
MATERIALS AND METHODS
Bacterial plasmids and strains.
The bacterial plasmids used and constructed in this study are listed in Table 1. Escherichia coli DH5α [φ80dlacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17(rK− mK+) supE44 relA1 deoR Δ(lacZYA-argF)U169] (Invitrogen) was used routinely as a host in cloning experiments.
TABLE 1.
Strains and plasmids used in this study
| Bacterium or plasmid | Description | Reference(s) and/or source |
|---|---|---|
| Bacteria | ||
| P. putida mt-2 (ATCC 33015) | Wild-type strain carrying TOL plasmid pWW0 | 33, 34 |
| P. putida PaW82 | Plasmid-free derivative of P. putida mt-2 | 3 |
| P. putida PaW340 | Strr Trp− derivative of PaW85 | 14 |
| Pseudomonas sp. strain WR211 | Primary transconjugant of Pseudomonas sp. strain B13 derived from mating pWW0 from P. putida mt-2; carries plasmid pWW0-1211 | 15, 25 |
| Pseudomonas sp. strain WR216 | Carries plasmid pWW0-1216; derived from WR211 by selection for acquired ability to grow on 4-chlorobenzoate; strain appears no longer to exist. | 15, 25 |
| Pseudomonas sp. strain WR216-2 | Carries plasmid pWW0-1216/2; variant of WR216 obtained from old slope | 15, 25 |
| Pseudomonas sp. strain WR216-3 | Carries plasmid pWW0-1216/3; new variant of WR216 selected from WR211 for acquired ability to grow on 4-chlorobenzoate | This study |
| P. putida PRS2000 | Wild type (PRS1, A.3.12) | L. N. Ornston |
| P. putida PRS2000TK1 to -20 | Series of Kmr transconjugants with ISPpu12-Km integrated into chromosomes | This study |
| E. coli CC118λpir | Maintenance strain for pGP704 | 9 |
| E. coli S17-1λpir | Donor strain for pGP704 | 9 |
| E. coli DH5α | Invitrogen | |
| Plasmids | ||
| pUC18 | Cloning vector; Apr | 35 |
| pUC18K | pUC18 with KpnI site in MCS removed | R. M. Jones |
| pUC4K | Apr Kmr plasmid carrying Kmr cassette | 30; Promega |
| pGP704 | Suicide vector; Apr | 18 |
| p34SKm | Plasmid carrying Kmr cassette | 5 |
| pGEMTeasy | Vector for cloning PCR products; Apr | Promega |
| pXQ1 | 5.4-kb XhoI-Q fragment of pWW0-1216-2 cloned into SalI site of pUC18 | This study |
| pXQ2 | 4.58-kb SalI fragment from pXQ1 cloned into SalI site of pUC18 | This study |
| pXQ3 | 4.58-kb SalI fragment from pXQ2 cloned into SalI site of pUC18K | This study |
| pXQ3Km1 | Kmr cassette from p34SKm inserted into KpnI site of pXQ3 in same orientation as tnpA | This study |
| pXQ3Km2 | Kmr cassette from p34SKm inserted into KpnI site of pXQ3 in opposite orientation to tnpA | This study |
| pGEMM3Km1 | Kmr cassette from pUC4K inserted into BamHI site in orf1 of pGEMM3 in same orientation as orf1 | This study |
| pGEMM3Km2 | Kmr cassette from pUC4K inserted into BamHI site in orf1 of pGEMM3 in opposite orientation to orf1 | This study |
| pXQ3Km3 | KpnI-MfeI fragment of pGEMM3Km1 replacing KpnI-MfeI fragment of pXQ3 | This study |
| pXQ3Km4 | KpnI-MfeI fragment of pGEMM3Km2 replacing KpnI-MfeI fragment of pXQ3 | This study |
| pGPTKm1 | SalI fragment from pXQ3Km1 cloned into pGP704; suicide vector for delivery of ISPpu12-Km1 | This study |
| pGPTKm2 | SalI fragment from pXQ3Km2 cloned into pGP704; suicide vector for delivery of ISPpu12-Km2 | This study |
| pGPTKm3 | XbaI-SphI fragment from pXQKm3 cloned into pGP704; suicide vector for delivery of ISPpu12-Km3 | This study |
| pGPTKm4 | XbaI-SphI fragment from pXQKm4 cloned into pGP704; suicide vector for delivery of ISPpu12-Km4 | This study |
| pGEMM1 | 2.0-kb overlap extension mutagenesis PCR product containing 7-bp deletion and novel BamHI site in tnpA, cloned into pGEMTeasy | This study |
| pGPM1 | 2.0-kb XbaI-MfeI fragment from pGEMM1 replacing wild-type fragment in pGPTKm2 | This study |
| pGEMM2 | 1.6-kb overlap extension mutagenesis PCR product containing 7-bp deletion and novel BamHI site in lspA, cloned into pGEMTeasy | This study |
| pGPM2 | 1.6-kb MfeI-XhoI fragment from pGEMM2 replacing wild-type fragment in pGPTKm2 | This study |
| pGEMM3 | 1.6-kb overlap extension mutagenesis PCR product containing 7-bp deletion and novel BamHI site in orf1, cloned into pGEMTeasy | This study |
| pGPM3 | 1.6-kb MfeI-XhoI fragment from pGEMM3 replacing wild-type fragment in pGPTKm2 | This study |
| pGEMM4 | 1.3-kb overlap extension mutagenesis PCR product containing 7-bp deletion and novel BamHI site in orf2, cloned into pGEMTeasy | This study |
| pGPM4 | 1.3-kb PstI-XhoI fragment from pGEMM4 replacing wild-type fragment in pGPTKm1 | This study |
| pTK16S1 | ∼6-kb SalI fragment from genomic DNA of P. putida PRS2000TK16 cloned into pUC18 | This study |
| pTK16S2 | ∼6-kb SalI fragment from genomic DNA of P. putida PRS2000TK16 cloned into pUC18 | This study |
Media and bacterial culture.
Pseudomonas strains were grown on minimal medium supplemented with either 10 mM succinate, 5 mM 3-chlorobenzoate, or 4-chlorobenzoate as required. Liquid Luria-Bertani medium or Sensitivity Test agar (LabM, Bury, United Kingdom) was used for the cultivation of E. coli strains. Where appropriate, ampicillin (100 μg/ml) or kanamycin (15 μg/ml) was added.
DNA extraction and manipulations.
Plasmid DNA was prepared from Pseudomonas by the method of Wheatcroft and Williams (32). Plasmids in E. coli were prepared by using the Concert rapid plasmid miniprep system (Life Technologies). Genomic DNA was prepared from Pseudomonas by using the QIAamp tissue kit (Qiagen). Restriction endonuclease digestions and ligations with T4 ligase were done in accordance with the instructions of the manufacturer (Promega). DNA fragments were recovered from agarose gels by using the QIAquick gel extraction kit (Qiagen). Southern blotting was performed as described by Sambrook et al. (26). Hybridization of blots used enhanced chemiluminescence direct labeling (Amersham). All other DNA manipulations were by standard procedures. PCR-generated fragments were routinely cloned directly into pGEMTeasy vector (Promega).
DNA sequencing and analysis.
Nucleotide sequences were determined by MWG-Biotech Ltd. (Ebersburg, Germany). PCR primers (Table 2) were designed with the aid of the Lasergene software (DNASTAR, Madison, Wis.). Searches of databases were carried out with the BLAST programs (1).
TABLE 2.
Oligonucleotide primers used in this study
| Purpose | Directiona | 5′→3′ sequenceb | Equivalent position in pWW0 sequence (accession number AJ344068) |
|---|---|---|---|
| orf1 probe for blotting | Reverse | GGCGCCGGCGGTGATGAC | 85778-85761 |
| Forward | AGGATGCGGCAGCGACGAATAAAG | 84725-84748 | |
| External primer for OEMc of tnpA | Reverse | GATTACGAATTCGAGCTCGGTACCd | Derived from MCS of pGPTKm2 |
| Forward | TGATATTGCAATTGTCCTCGGTGCCT | 844157-86328 | |
| Internal mutagenesis of tnpA | Reverse | GCCCCAGGAtCc-Δ7-GGCGTTGAAGATGTCGCG | 86844-86808 |
| Forward | TCAACGCC-Δ7-gGaTCCTGGGGCGCTGGCACCG | 86818-86854 | |
| External primer for OEM of IspA and orf1 | Reverse | CCGAGGACAATTGCAATATCAGCCAGG | 86323-86297 |
| Forward | CGGCCTCGAGCAAGACGTTTCCCGT | Derived from Kmr cassette | |
| Internal mutagenesis of lspA | Reverse | CGATATGGAtcc-Δ7-TACGGCGAGAGCTTTTTGCCA | 85938-85898 |
| Forward | CTCTCGCCGTA-Δ7-ggaTCCATATCGGGCCTGCTG | 85947-85908 | |
| Internal mutagenesis of orf1 | Reverse | GCATAAACA-Δ7-ggaTCCCTGGCGCCTCGGAGGAGG | 85093-85054 |
| Forward | GCGCCAGGGAtcc-Δ7-TGTTTATGCCGTGCCGAAG | 85065-85103 | |
| External primer for OEM of orf2 | Reverse | GCGGGCCTCGAGCAAAGACGTTTCCCGTTGA | Derived from Kmr cassette |
| Forward | GCATGCCTGCAGGTCGACCAGTCCCT | Derived from MCS of pGPTKm1 | |
| Internal mutagenesis of orf2 | Reverse | TCGGCA-Δ7-GGaTcCCGTGTCTATACCGAGAAACAC | 84802-84763 |
| Forward | GGTATAGACACGGgAtCC-Δ7-TGCCGATCAGGCGGCG | 84772-84812 | |
| Sequencing out from IRR | Reverse | ACGATGACCGGGAAGTTGAGG | 84554-84534 |
| Sequencing out from IRL | Forward | GCCGGACTTCGCAGAGGA | 87659-87676 |
Forward primers operate from left to right relative to Fig. 1.
For the external primers for overlap extension mutagenesis, the relevant restriction sites (see Fig. 3) are in boldface. For the internal mutagenesis primers, the deletion of 7 bp is indicated by -Δ7-and the constructed BamHI site is underlined, with the bases which are changed from the wild-type sequence to form it indicated in lowercase letters. Forward primers function from left to right relative to Fig. 1.
OEM, overlap extension mutagenesis.
The XbaI site used for the overlap extension mutagenesis of tnpA (see Fig. 3) is within the PCR fragment and not on the primer.
Construction of Kmr derivatives of ISPpu12. (i) pGPTKm1 and pGPTKm2.
The XhoI fragment of pWW0-1216 containing ISPpu12 was cloned into the SalI site of pUC18 to form plasmid pXQ1. Using internal SalI sites, a smaller internal 4.6-kb fragment was excised and recloned into the SalI site of pUC18, forming pXQ2. From this, it was recloned into pUC18K, a derivative of pUC18 with the KpnI site deleted from the multiple cloning site (MCS) to form pXQ3. Into its single KpnI site, between orf3 and orf4, a cassette carrying the kanamycin resistance cassette from p34SKm (5) was inserted. Two plasmids were formed, with the Kmr gene oriented in the same direction as tnpA in pXQ3Km1 and in the opposite direction in pXQ3Km2 (Fig. 1). The SalI insert of each of these plasmids was recloned into the suicide vector pGP704 (18), forming pGPTKm1 and pGPTKm2, respectively. These were transformed into E. coli strains CC118λpir (for maintenance) and S17-1λpir (for mobilization).
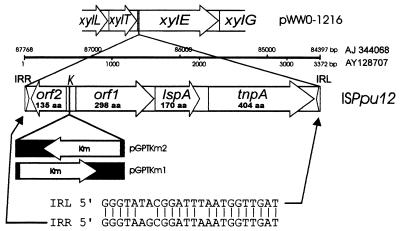
FIG. 1.
Genetic map of ISPpu12. The location and orientation of ISPpu12 relative to the xyl genes of the meta pathway operon on plasmid pWW0-1216, from which it was first isolated, are shown at the top. The bases are numbered according to GenBank accession numbers AJ344068 (complete pWW0 sequence) and AY128707 (ISPpu12). The sequences of the two terminal inverted repeats are given below the map. The location of the Kmr cassette in the unique KpnI site between orf1 and orf2 to form ISPpu12-Km on plasmids pGPTMKm1 and pGPTKm2 is indicated. aa, amino acids.
(ii) pGPTKm3 and pGPTKm4.
In order to insert a Kmr gene within orf1, the starting plasmid was pGEMM3, containing an engineered BamHI site within orf1 at its 5′ end (Table 1). The Kmr cassette from pUC4K (30) was cloned in both orientations into the BamHI site to form pGEMM3Km1 and pGEMM3Km2. Their KpnI-MfeI fragments spanning orf1 (Fig. 2, pGPM3) were removed by band extraction from a gel of digests and used to replace the corresponding KpnI-MfeI fragment of pXQ3, forming pXQ3Km3 and pXQ3Km4. The entire ISPpu12-Km insert of each was then excised with SphI and XbaI and cloned into the SphI and XbaI sites in the MCS of suicide vector pGP704, forming pGPTKm3 and pGPTKm4.
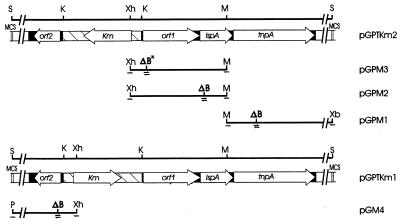
FIG. 2.
Maps of deletion mutants of plasmids carrying the Kmr derivatives of ISPpu12. Overlap extension PCR was used to generate fragments (shown as thin lines) with terminal restriction sites corresponding to unique sites within the two parental plasmids pPGPTKm2 and pPGPTKm1; within each fragment a 7-bp deletion adjacent to a unique BamHI site was engineered in the 5′ end of one of the ORFs to inactivate it (see Table 2). The PCR-generated fragment was spliced into pGP704 on the two terminal restriction sites to replace the wild-type DNA, forming plasmids pGPM1 to -4. The inserts were excised with the same restriction enzymes and inserted into one of two ISPpu12-Km constructs, pGPTKm2 or pGPTKm1, replacing the equivalent wild-type DNA. Black area, ISPpu12 DNA; hatched area, Kmr cassette inserted into the ISPpu12 KpnI site; shaded areas, MCS of the vector plasmid pGP704. The locations of the four primers used for each overlap extension PCR (see Table 2) are indicated by the half arrows, and ΔB shows the position of the constructed BamHI site deletion. The ΔB with an asterisk on pGPM3 is the site at which the Kmr cassette is inserted in pGPTKm3 and pGPTKm4. Abbreviations for restriction sites: S, SalI; K, KpnI; M, MfeI; Xb, XbaI; Xh, XhoI.
Mobilization and transposition
Plasmids pGPTKm1 to pGPTKm4 were transferred from S17-1 λpir into P. putida PRS2000 by filter mating, using a modification of the procedure described by Herrero et al. (9). Aliquots of stationary-phase cultures of donor and recipient were spotted together onto bacterial filters to give a cell/cell ratio of between 1:1 and 1:4. These were incubated at 30°C overnight on the surface of a nutrient agar plate. The mixed culture was resuspended in 100 mM phosphate (pH 7.5). For cell counts, the cell suspension and serial dilutions thereof were plated onto agar plates containing succinate plus kanamycin (for transconjugants), Isosensitest agar plus kanamycin (for donors plus transconjugants), or succinate alone (for recipients). The frequency of conjugation plus transposition was calculated as the ratio of transconjugants to donors.
Overlap extension mutagenesis.
Four primers were designed for each of the four open reading frames (ORFs) (Table 2; Fig. 2): (i) an upstream and a downstream primer, each of which spanned a unique restriction site on plasmid pGPTKm1 or pGPTKm2 on either side of the ORF, and (ii) two complementary primers for each ORF which were partially complementary to a location towards the 5′ end of each ORF but were designed to introduce a 7-bp deletion and a novel BamHI site in the gene (Table 2). Using a two-stage PCR (11), fragments spanning the gene between the native upstream and downstream restriction sites but including the frameshift 7-bp deletion and BamHI site were generated. These fragments were first cloned directly into pGEMTeasy vector. They were excised from this by using the upstream and downstream restriction sites, and these fragments were used to replace the corresponding wild-type fragments from pGPTKm1 and pGPTKm2 (Fig. 2).
Nucleotide sequence accession number.
The sequence of ISPpu12 as determined from the insert in pWW0-1216/2 has been deposited in GenBank with accession number AY128707.
RESULTS
The WR216 insert in xylE.
In order to extend the catabolic range of chlorobenzoate-degrading Pseudomonas sp. strain B13, strain WR216 was constructed by conjugative transfer of pWW0 into B13 followed by selection for acquisition of a novel phenotype, growth on 4-chlorobenzoate (24, 25). Previous comparison of the plasmid pWW0-1216 in WR216 with wild-type pWW0 had shown that, together with a deletion, it had acquired two novel insertions of about 3 to 3.5 kb, the critical one of which was in the xylE structural gene (for catechol 2,3-dioxygenase). This insert resulted in the polar inactivation of the downstream meta pathway operon (15), which was shown to be a biochemical prerequisite for the acquired 4-chlorobenzoate growth phenotype. The nature of either insert had never been explored previously.
We excised the insert in xylE from pWW0-1216, together with its flanking regions, on a 5.4-kb XhoI fragment and ligated it into pUC18, from which a 4.58-kb SalI subfragment was recloned to form plasmid pXQ2 (Table 1). The complete nucleotide sequence of the fragment was determined. Comparison of the flanking regions with the sequence of the pWW0 meta pathway operon showed that that the insertion had occurred after the 13th nucleotide of xylE (Fig. 1) and was sandwiched between an 8-bp direct repeat of bases 6 to 13 (CAAAGGTG) of xylE at both ends of the insertion.
The insertion in xylE, which is 3,372 bp in length and has been named ISPpu12, has several main features (Fig. 1). At either end is an almost perfect (21 of 24) inverted repeat,GGGTA(A/T)(G/A)CGGATT(A/T)AATGGTTGAT; wehave defined the two ends thus presented as IRR and IRL (Fig. 1), conforming with the nomenclature used by Weightman et al. (31). It also carries four ORFs (Table 3), one of which, from homology comparisons, clearly encodes a transposase (tnpA) and three of which are unrelated to known transposition genes. Transcribed in the same direction as tnpA and possibly forming a single transcriptional unit with it are lspA, encoding a lipoprotein signal peptidase homologue, and orf1, encoding a transporter or membrane protein homologue. Divergently transcribed from them is orf2, the putative product of which has similarities to regulator proteins.
TABLE 3.
Putative proteins encoded by ISPpu12
| Gene designation | Putative function of gene product | Position in sequencea (position, gene designation [in pWW0 sequence]b) | Product size (residues, kDa) | Most similar gene product (species) (% amino acid identity, % similarityc) (accession number)d |
|---|---|---|---|---|
| orf2 | Possible regulatory protein | 495-88 (87274-87681, pWW0164) | 135, 15.4 | PbrR protein (R. metallidurans) (44, 61) (CAC28872) |
| orf1 | Possible membrane protein or transporter | 591-1487 (87178-86282, pWW0163) | 298, 31.2 | Unknown protein (Actinobacillus actinomycemcomitans) (97, 98) (NP 067562) |
| IspA | Lipoprotein signal peptide homologue | 1491-2003 (86278-85766, IspA) | 170, 18.8 | Prelipoprotein signal peptidase (R. metallidurans) (42, 59) (CAC28875) |
| tnpA | Transposase | 2100-3314 (85669-84455, tnpA) | 404, 47.5 | Putative transposase (P. stutzeri) (90, 92) (CAB 42636) (4) |
The sequence is numbered from IRR through to IRL as shown in Fig. 1.
As annotated in GenBank (accession number AJ344068).
As calculated following alignment of the amino acid sequences with ClustalX.
The high degree of similarity between gene products and those of S. marcescens plasmid R471a is not included because they appear to be the same (see Discussion).
Comparison with pWW0 nucleotide sequence.
Alignment of the sequence of the 3,372-bp insert from pWW0-1216 with the complete nucleotide sequence of pWW0 showed the presence on the native plasmid of a single identical copy located between bases 84397 and 87768 within HindIII fragment E (6). This native copy, which is in the same orientation as the second copy in xylE of pWW0-1216, was not embedded between 8-bp directly repeated terminal sequences, the two flanking sequences being TCTTAAAT and AAAATTGA, respectively.
Demonstration of transposition.
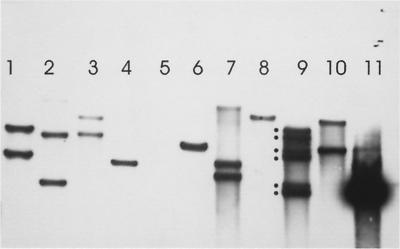
In order to demonstrate that ISPpu12 can transpose independently, derivatives which carried an inserted Kmr cassette as a phenotypic marker were constructed and then inserted into the suicide vector pGP704 (18). This plasmid is stable in λpir derivatives of E. coli and can be transferred from these into recipient strains but will not replicate within them. In pGPTKm1 and pGPTKm2 (Table 1) the Kmr cassette was inserted into the KpnI site in the intergenic region between the divergent orf1 and orf2 (Fig. 1) in the hope that, in this site, the cassette would not affect expression of any of the genes of ISPpu12 and thus disable any transposition functions. These two plasmids were transferred from E. coli S17-1 λpir into P. putida PRS2000 in four separate experiments selecting for Kmr transconjugants. In all of the matings a significant number were obtained (Table 4), and five were retained from each mating. Chromosomal digests of these were subject to electrophoresis and Southern blotted against a PCR probe generated from within the orf1 gene of ISPpu12. One of the Kmr transconjugants, PRS2000TK16, carried six distinct insertions, and at least 50% of those examined contained >1 insertion (Fig. 3). Fragments from SalI digests of the genomic DNA from PRS2000TK16 (Fig. 3, lane 9) were ligated into the SalI site of pUC18, and two Kmr recombinant plasmids (pTK16S1 and pTK16S2) were isolated, each of which contained a different ∼6-kb chromosomal fragment. These were subjected to single-strand sequencing outwards from each end of the ISPpu12-Km with primers complementary to regions about 100 bp inside the two terminal inverted repeats. The results clearly showed that the two termini of ISPpu12-Km were between coding sequences which showed similarities to other sequences in the databases but failed to show any significant length of nucleotide identity with any of the fragments from the partial sequence of P. putida PRS1 available in GenBank on 5 July 2002. In one transconjugant the two flanking sequences showed closest homology to the indoleacetamide hydrolase from Rhizobium rhizogenes (accession number QO9102) and the AMP nucleosidase from Pseudomonas aeruginosa (NP_252659), respectively, and in the second transconjugant they showed closest homology to a LysR-like regulator protein from Salmonella enterica serovar Typhimurium (NP_462508) and 1-cyclohexenyl carboxyl coenzyme A reductase from Streptomyces collinus (AAC44655). We were unable to confirm the exact chromosomal position from the limited data currently available from the partial sequence of P. putida PRS1 (PRS2000, A.3.12). In neither case was there an 8-bp direct repeat flanking the ends of the insertion sequence.
TABLE 4.
Transposition frequencies of ISPpu12-Km derivatives in P. putida
| Donor plasmid(genotype) | Range of transposition frequencya (Kmr transconjugants per donor) |
|---|---|
| pGPTKm1 | 10−4.2-10−5.0 |
| pGPTKm2 | 10−3.4-10−4.7 |
| pGPM1 (ΔtnpA) | No transconjugants |
| pGPM2 (ΔlspA) | 10−3.6-10−5.3 |
| pGPM3 (Δorf1) | 10−4.2-10−5.7 |
| pPGM4 (Δorf2) | 10−3.9-10−4.6 |
Each experiment was performed three or four times. The range is from the lowest to the highest in each series. Donor plasmids were in E. coli S-17 λpir, and the recipient was P. putida PRS2000.
FIG. 3.
Southern blots of SalI-digested genomic DNAs from P. putida PRS2000 transconjugants carrying copies of ISPpu12-Km. Lanes 1 to 4 and 6 to 10, nine different Kmr transconjugants; lane 5, PRS2000 control; lane 11, SalI-digested pXQ3Km2 control. Note that the strain in lane 9 (PRS2000K16) has at least six copies of the IS element (marked with dots). The probe was a PCR-generated fragment spanning orf1 (see Table 2).
When pGPTKm1 and pGPTKm2 were transferred into P. putida PaW82 and Pseudomonas sp. strain WR1, Kmr transconjugants were obtained at about the same frequencies (data not shown).
Two additional ISPpu12-Km constructs were made in which the Kmr cassette was located within the 5′ end of orf1 (Fig. 2), and these were also placed in pGP704 as pGPTKm3 and pGPTKm4 (Table 1). These plasmids transferred to Pseudomonas recipients PRS2000, PaW82, and WR1, producing Kmr transconjugants with similar frequencies (data not shown), but no Southern blot analysis of transformants was carried out.
Analysis of function of ORFs.
Using overlap extension PCR mutagenesis (10), we constructed four fragments, in each of which an internal 7-bp frameshift deletion coincident with a novel BamHI site (for recognition purposes) had been engineered into the 5′ end of one of the ORFs within ISPpu12. These were individually ligated into the plasmid pGPTKm1 or pGPTKm2 on restriction fragments replacing the wild-type fragments (Fig. 2), to produce plasmids pGPM1 (ΔtnpA), pGPM2 (ΔlspA), pGPM3 (Δorf1), and pGPM4 (Δorf2) (Table 1). After digestion to confirm the presence of the introduced BamHI site, the nucleotide sequence of each was determined on single strands to ensure that the expected mutations were incorporated. In the plasmids used, the PCR had introduced a few additional base changes as well as the designed deletion, but all were in the single gene being inactivated in each plasmid. S17-1 donors carrying each mutant plasmid were then mated with P. putida PRS2000 on filters under as near identical conditions (time and donor/recipient ratio) as was possible, and the frequency of transposition was determined as the ratio of Kmr transconjugants to donor cells in the mating mixture. Only in the case of the tnpA knockout was transposition totally abolished, whereas inactivation of the other ORFs did not significantly change the frequency (Table 4).
Analysis of pWW0-derived plasmids in Pseudomonas sp. strain B13 strains.
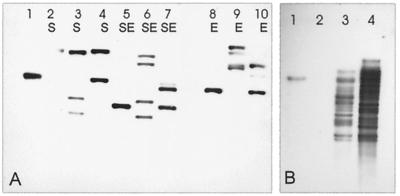
In the original analysis of pWW0-1216, the plasmid in Pseudomonas sp. strain WR216 (14, 15), comparison of its restriction digest with that of pWW0 showed that it contained two inserts of ∼3.4 kb, one in xylE and the other in HindIII fragment C (coordinates 98347 to 115800). We therefore blotted digests of the plasmid from WR216, a slope of which we had retained for >20 years, against the PCR-generated orf1 probe. We found that it contained four copies of ISPpu12 (Fig. 4A, lane 6): the two that we had originally identified (14); a copy in the native location in a fragment which contains part of HindIII fragment E, the existence of which we had been unaware at that time; and a fourth copy, which has proved difficult to locate precisely from digests but is probably in the region between pWW0 coordinates 16000 and 22000. This fourth copy appears to have been acquired since 1982 during maintenance in our laboratory, as the plasmid digests are distinguishable from the pictures of digests that we have from that period. Unfortunately, there are no independent cultures of WR216 still in existence, since those retained by our collaborators in Germany proved to be no longer viable. To distinguish the present strain and its plasmid from the original, we have renamed them WR216-2 and pWW0-1216/2 (Table 1). We have also obtained a phenotypic homologue, WR216-3. This was recently constructed, as WR216 was, by plating the original parental strain WR211 onto 4-chlorobenzoate plates. Analysis of digests of its plasmid is complicated by the existence of both deletions and insertions, but it is clearly different from the plasmid in WR216, and Southern blots show that it contains only two copies of ISPpu12 (Fig. 4A); the location of one of these is in the native position (as in pWW0), but the location of the second has not been determined.
FIG. 4.
Southern blots of digests of DNAs from P. putida mt-2 and Pseudomonas sp. strain B13 probed with the orf1-specific probe generated from ISPpu12. (A) Plasmid DNAs. Lane 1, plasmid pXQ3Km2 control; lanes 2, 5, and 8, pWW0; lanes 3, 6, and 9, pWW0-1216-2; lanes 4, 7, and 10, pWW0-1216-3. Lanes S, digestion with SalI; lanes SE, digestion with SalI and EcoRI; lanes E, digestion with EcoRI. Neither SalI nor EcoRI cuts within ISPpu12. (B) SalI digests of genomic DNAs. Lane 1, P. putida mt-2; lane 2, Pseudomonas sp. strain B13; lane 3, Pseudomonas sp. strain WR216-2; lane 4, Pseudomonas sp. strain WR216-3.
Chromosomal copies of ISPpu12 in Pseudomonas hosts.
In order to check whether the ability of ISPpu12-Km to transpose into the chromosome of PRS2000 was matched by the ability of the wild-type ISPpu12 to transpose into chromosomes, we blotted digests of genomic preparations of three derivatives of the pWW0 host P. putida mt-2 with the PCR-generated orf1 probe. Wild-type mt-2 contained only one hybridizing fragment (Fig. 4B, lane 1), consistent with the single copy on pWW0, and the plasmid-free strains PaW82 and PaW340 contained no hybridizing fragment (data not shown). Wild-type Pseudomonas sp. strain B13 also contained no hybridizing DNA (Fig. 4B, lane 2). However, in extreme contrast, we compared blots of genomic DNAs from the B13-derived strains WR216 and WR216-3. In each of these there was a multiplicity of hybridizing bands, considerably more than were found in their TOL-derived plasmids, and the two patterns of blotting showed differences (Fig. 4B, lanes 3 and 4). This indicates that ISPpu12 has transposed not only within the TOL plasmid in these strains but also from the plasmid into the chromosome on multiple occasions. Both WR216 and WR216-3 were derived from WR211, but >20 years separated their constructions. The difference in the banding patterns between the two strains suggests differences in the transposition events which have taken place in the two separately maintained lines.
DISCUSSION
Plasmids and transposons are tightly linked as important innovative elements in the evolution of bacterial genomes. The analysis of the nucleotide sequence of pWW0, one of only a limited number of catabolic plasmids to be sequenced, shows considerable evidence of a turbulent history of genetic rearrangements (8): a simple database comparison shows the presence of 13 transposase-like genes, gene fragments, or pseudogenes on the plasmid. In this paper we report a third active transposable element on the plasmid, the 3,372-bp insertion sequence ISPpu12, without apparent phenotype, in addition to the large transposons Tn4651 and Tn4653 described previously (27-29). On the native pWW0, ISPpu12 is located between the two large transposases specific to Tn4651 and Tn4653, thus being within Tn4653 and in a region of only about 10 kb which contains all three of the pWW0 transposase genes demonstrated to be functional (Table 5).
TABLE 5.
Genes surrounding ISPpu12 on pWW0
| Coordinatesa | Gene or gene product designation |
|---|---|
| 80113-79751 | TnpC for Tn4651 |
| 83127-80122 | TnpA for Tn4651 |
| 83233-83278 | Right IR for Tn4651 |
| 83532-83296 | Protein of unknown function, pWW0.154 |
| 83462-83785 | Putative multidrug exporter, pWW0.155 |
| 83838-84170 | Putative multidrug exporter, pWW0.156 |
| 84397-84420 | IRL of ISPpu12 |
| 84421-87744 | ISPpu12 |
| 87745-87768 | IRR of ISPpu12 |
| 88308-87763 | Terminated MerB protein |
| 88402-88836 | MerR homologue |
| 89101-89661 | TnpR for Tn4653 |
| 89665-92631 | TnpA for Tn4653 |
| 92627-92664 | Right IR for Tn4653 |
As designated in the GenBank sequence AJ344068, the direction of transcription is indicated by the coordinates.
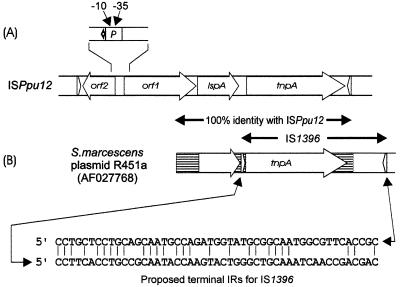
As is also shown in the accompanying paper for a virtually identical insertion sequence and derived composite transposon, DEH (31), ISPpu12 shows a remarkable ability to transpose in multiple copies. One pWW0-derived plasmid (pWW0-1216/2) carried four copies, only one of which (in xylE) appears to have been acquired in response to selective pressure. Multiple transposition of ISPpu12 with an added Kmr marker was also demonstrated from a suicide vector, where, in a single mating, more than half the transconjugants examined acquired between two and six chromosomal copies (Fig. 3). Remarkably, two strains derived from Pseudomonas sp. strain B13 which have been maintained with a TOL-derived plasmid for several decades contained a large number of chromosomal copies of the wild-type IS element (>10), whereas the wild-type B13 contained none (Fig. 4). Because of this property, it is surprising that P. putida mt-2, isolated and maintained as the natural host of pWW0 for an even longer period of time (>40 years), carries only the single plasmid-borne copy of ISPpu12, particularly since we have demonstrated that the Km-linked derivatives we have constructed in this work will transpose effectively within PaW82, a plasmid-free variant of strain mt-2. However, the facile transposability of ISPpu12 appears to have been a persistent feature of the history of pWW0 and strain mt-2. In the very earliest stages of the codiscovery of the plasmid-encoded nature of the toluene-xylene genes, our culture of P. putida mt-2 (ATCC 33105) grew well on m-toluate by the plasmid-encoded catabolic pathway (33), whereas the strain described by Nakazawa and Yokota (TN1100) grew very poorly (20). The reason for this was discovered after the construction of pTN1, an RP4-pWW0 cointegrate plasmid which also supported only poor growth on m-toluate (21). Selection for improved and inducible growth resulted in a deletion of ∼3 kb from pTN1 to form pTN2, the plasmid which subsequently served as the vehicle for studying the xyl catabolic genes in the Japanese laboratory (22). The nature of this deletion can be inferred from a second plasmid, pTN8, which was also selected from pTN1 for good growth on m-toluate but from which the meta pathway genes were expressed constitutively (12). The nucleotide sequence of a small, 355-bp region of pTN8 which determined the constitutivity was published (12) (accession number M12798). It contained a strong −10, −35 promoter, downstream of which was the 5′ end of a gene claimed to be xylE (and still in the databases as such). Examined retrospectively, the entire 355-bp sequence is identical to part of ISPpu12 (Fig. 5A) consisting of the intergenic region between orf3 and orf4, where the promoter is located, plus the 3′ end of orf1 (86 bp) and the 5′ end of orf2 (174 bp), incorrectly identified as xylE (12, 19). pTN1 can therefore confidently be deduced to have carried a copy of ISPpu12 located within the meta pathway operon upstream of xylE and blocking expression of the operon, causing the poor growth on m-toluate. Selection for spontaneous acquisition of faster growth on m-toluate resulted either in complete and precise excision to give pTN2 or in some smaller undetectable change to leave the orf2 promoter firing through to xylE downstream and to give the constitutive phenotype of pTN8. For this to happen, this insertion in pTN1 must have been present in the TOL plasmid in the culture of P. putida mt-2 maintained in Nakazawa's laboratory but not in the strain that we were using, and also its exact position must have been further upstream of xylE and in the opposite orientation to that we later found in pWW0-1216 (cf. Fig. 1 and 5).
FIG. 5.
Relationship between ISPpu12 and other reported sequences. (A) Location of the 355 bp containing the constitutive promoter, firing from right to left, on pTN8 reported by Inouye et al. (12). (B) Postulated IS1396 from S. marcescens plasmid R471a (16). The map of IS1396 is drawn from the sequence under accession number AF027768 and is proposed to carry a tnpA gene between two terminal inverted repeats (IRs) (27 of 49 bp), shown on the map as blunt arrows with their sequences below. The area of the AF027768 sequence corresponding to 100% homology with ISPpu12 is indicated by the hatched area spanning tnpA and running to the left to include an ORF identical to lspA. There is no similarity between ISPpu12 and the nucleotide sequence on R451a to the right of IRL.
During Jeenes' doctoral studies of pWW0 in Pseudomonas sp. strain B13 (13), he constructed many derivatives of B13 in addition to those constructed in Knackmuss' laboratory (24, 25), and the presence of inserts of ∼3 kb in novel locations within pWW0 were a frequent occurrence. Some of these plasmids, together with others generated in Broda's laboratory, were analyzed by restriction digestion and Southern blotting and, as we have shown here, were correctly deduced to be due to internal transposition of a region around HindIII fragment E (17).
The results presented here show that tnpA is the only one of the four genes on ISPpu12 which affects its ability to transpose. The genetic structure of ISPpu12 indicates that tnpA might be the third gene of an operon downstream of orf1 and lspA. If this is the case, then the expression of tnpA would not have been affected by our 7-bp deletion knockouts of the two upstream genes, which were deliberately designed not to have a polar effect. However, we also constructed two variants of ISPpu12 in which a Kmr cassette was located within orf1 in both orientations, which would certainly be expected to have a polar effect on expression of tnpA if it were part of the same transcription unit. The ease of transposability of these constructs suggests that tnpA is expressed independently of lspA and orf1.
The evidence from the work in Nakazawa's laboratory discussed above suggests that the promoter upstream of orf2 is both active and constitutive, and it is interesting to speculate whether orf2, a regulatory gene homologue, has any functional role in ISPpu12 biology. The Kmr cassette in two of the ISPpu12-Km constructs, pGPTKm1 and pGPTKm2 (Fig. 1), would certainly have disrupted this promoter, and therefore expression of Orf2, since the KpnI insertion site is directly between the −10 and −35 regions of the promoter (Fig. 5) and this does not affect the transposability. In addition, the similarity of transposition frequencies between the insertion sequence elements in which the Kmr insertion is in the promoter and those with it alternatively located within orf1 (pGPTKm3 and pGPTKm4), where the orf2 promoter is not disrupted, strongly indicates that Orf2 is not involved in a regulatory role which affects transposition.
Whether lspA, orf1, or orf2 confers a phenotype to ISPpu12+ cells remains a matter for speculation at this time. There are some possible links with genes involved in metal resistance. Two of the genes (orf2 and lspA) have as their closest neighbors in the data banks genes involved in the pbr (lead resistance) gene cluster of Ralstonia metallidurans (Table 3), but otherwise the pbr cluster bears no similarity to ISPpu12. Also, orf2 shows similarity with some merR genes, and some of the genes adjacent to ISPpu12 in the complete pWW0 sequence also appear to be related to mercury resistance genes (Table 5). It is possible that the ancestry of ISPpu12 is in some way associated with metal resistance functions but that the presence of the genes within the transposable element is a fortuitous result of its history and has no biological significance. It is, however, worth noting that we have on a number of occasions attempted to determine whether pWW0 encodes metal resistance, by comparing the resistance of wild-type P. putida mt-2 with those of a number of its plasmid-free derivatives, including PaW82 and PaW340 (Table 1), and have failed to show any differences (data not presented). It seems, therefore, that any genes with homologies to resistance functions on pWW0 and, by implication, ISPpu12, are no longer functional as such, if indeed they ever were.
Database searches show that the deduced amino acid sequence of the transposase is similar to those of other putative transposases in Pseudomonas strains described previously, such as ISPs1 from Pseudomonas stutzeri (4), but the overall structure of the complete insertion sequence is unlike any others, apart from two which appear to be virtually identical. One of these is described in the accompanying paper and was found associated with dehalogenase catabolic genes in Pseudomonas (31). The other is reported in the databases as IS1396 from Serratia marcescens plasmid R471a (11, 16) (GenBank accession number AF027768). No evidence is available to demonstrate that it can actually transpose, but from sequence analysis only, IS1396 is reported as a 1,771-bp element with inverted repeats of 45 bp containing 21 mismatches. Its first 447 bp bears no relationship to ISPpu12, but the remaining 1,325 bp is 100% identical and corresponds to IRL through the tnpA gene and lspA and into the 3′ end of orf1 (Fig. 5). However, the published sequence terminates at this point, and the postulated inverted repeat at this end of IS1396 corresponds to an internal part of orf1. In light of the sequences of ISPpu12 reported here and in the accompanying paper (31) and the experimental demonstration of its transposability, a more credible interpretation of the S. marcescens sequence is that its plasmid R471a carries a copy of ISPpu12 (or a very close relative) which has been incompletely sequenced and that the assigned terminal inverted repeats are the result of a fortuitous alignment of 24 of 45 bp, one within ISPpu12 and one upstream of it on plasmid R471a. If this is the correct explanation, it would indicate that ISPpu12 is a promiscuous element across a broad range of gram-negative genera and not exclusively confined to Pseudomonas.
Acknowledgments
We thank Chris M. Thomas and Alicia Greated for the collaboration which lead to their sequencing of pWW0, Hans Knackmuss and Walter Reineke for their continued collaboration on Pseudomonas sp. strain B13 and its derivatives, Teruko Nakazawa for exchanges on TOL plasmids over several decades, and Andrew Weightman for discussions and exchange of information on the relationships between the TOL plasmid copy of Ppu12 and the DEH copy.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Assinder, S. J., and P. A. Williams. 1990. The TOL plasmids: determinants of the catabolism of toluene and the xylenes. Adv. Microb. Physiol. 31:1-69. [DOI] [PubMed] [Google Scholar]
- 3.Bayley, S. A., C. J. Duggleby, M. J. Worsey, P. A. Williams, K. G. Hardy, and P. Broda. 1977. Two modes of loss of the Tol functions from Pseudomonas putida mt-2. Mol. Gen. Genet. 154:203-204. [DOI] [PubMed] [Google Scholar]
- 4.Bolognese, F., C. Di Lecce, E. Galli, and P. Barbieri. 1999. Activation and inactivation of Pseudomonas stutzeri methylbenzene catabolism pathways mediated by a transposable element. Appl. Environ. Microbiol. 65:1876-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dennis, J. J., and G. J. Zylstra. 1998. Plasposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl. Environ. Microbiol. 64:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Downing, R. G., and P. Broda. 1979. A cleavage map of the TOL plasmid of Pseudomonas putida mt-2. Mol. Gen. Genet. 177:189-191. [DOI] [PubMed] [Google Scholar]
- 7.Downing, R. G., C. J. Duggleby, R. Villems, and P. Broda. 1979. An endonuclease cleavage map of the plasmid pWW0-8, a derivative of the TOL plasmid of Pseudomonas putida mt-2. Mol. Gen. Genet. 168:97-99. [DOI] [PubMed] [Google Scholar]
- 8.Greated, A., L. Lambertson, P. A. Williams, and C. M. Thomas. Complete sequence analysis of the IncP-9 TOL plasmid pWW0 from Pseudomonas putida. Environ. Microbiol., in press. [DOI] [PubMed]
- 9.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho, C., O. L. Kulaeva, A. S. Levine, and R. Woodgate, R. 1993. A rapid method for cloning mutagenic DNA repair genes: isolation of umu-complementing genes from multidrug resistance plasmids R391, R446b, and R471a. J. Bacteriol. 175:5411-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 12.Inouye, S., Y. Asai, A. Nakazawa, and T. Nakazawa. 1986. Nucleotide sequence of a DNA segment promoting transcription in Pseudomonas putida. J. Bacteriol. 166:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeenes, D. J. 1982. The structure and properties of the TOL plasmid in Pseudomonas B13. Ph.D. thesis. University of Wales, United Kingdom.
- 14.Jeenes, D. J., and P. A. Williams. 1982. Excision and integration of degradative pathway genes from TOL plasmid pWW0. J. Bacteriol. 150:188-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeenes, D. J., W. Reineke, H.-J. Knackmuss, and P. A. Williams. 1982. TOL plasmid pWW0 in constructed halobenzoate-degrading Pseudomonas strains: enzyme regulation and DNA structure. J. Bacteriol. 150:180-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulaeva, O. I., E. V. Koonin, J. C. Wootton, A. S. Levine, and R. Woodgate. 1998. Unusual insertion element polymorphisms in the promoter and terminator regions of the mucAB-like genes of R471a and R446b. Mutat. Res. 397:247-262. [DOI] [PubMed] [Google Scholar]
- 17.Lehrbach, P. R., D. J. Jeenes, and P. Broda. 1983. Characterization by molecular cloning of insertion mutants in TOL catabolic functions. Plasmid 9:112-125. [DOI] [PubMed] [Google Scholar]
- 18.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutants: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakai, C., H. Kagiyama, M. Nozaki, T. Nakazawa, S. Inouye, Y. Ebina, and T. Nakazawa. 1983. Complete nucleotide sequence of the metapyrocatechase gene on the TOL plasmid of Pseudomonas putida mt-2. J. Biol. Chem. 258:2923-2928. [PubMed] [Google Scholar]
- 20.Nakazawa, T., and T. Yokota. 1977. Isolation of a mutant TOL plasmid with increased activity and transmissibility from Pseudomonas putida (arvilla) mt-2. J. Bacteriol. 129:39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakazawa, T., E. Hayashi, T. Yokota, Y. Ebina, and A. Nakazawa. 1978. Isolation of TOL and RP4 recombinants by integrative suppression. J. Bacteriol. 134:270-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakazawa, T., S. Inouye, and A. Nakazawa. 1980. Physical and functional mapping of RP4-TOL plasmid recombinants: analysis of insertion and deletion mutants. J. Bacteriol. 144:222-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy, B. R., L. E. Shaw, J. R. Sayers, and P. A. Williams. 1994. Two identical copies of IS1246, a 1275 base pair sequence related to other insertion sequences, enclose the xyl genes on TOL plasmid pWW0. Microbiology 140:2305-2307. [DOI] [PubMed] [Google Scholar]
- 24.Reineke, W., and H.-J. Knackmuss. 1979. Construction of haloaromatics utilising bacteria. Nature 277:385-386. [DOI] [PubMed] [Google Scholar]
- 25.Reineke, W., and H.-J. Knackmuss. 1980. Hybrid pathway for chlorobenzoate metabolism in Pseudomonas B13. J. Bacteriol. 142:467-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Tsuda, M., and T. Iino. 1987. Genetic analysis of a transposon carrying toluene degrading genes on a TOL plasmid pWW0. Mol. Gen. Genet. 210:270-276. [DOI] [PubMed] [Google Scholar]
- 28.Tsuda, M., and T. Iino. 1988. Identification and characterization of Tn4653, a transposon covering the toluene transposon Tn4651 on TOL plasmid pWW0. Mol. Gen. Genet. 213:72-77. [DOI] [PubMed] [Google Scholar]
- 29.Tsuda, M., K.-L. Minegishi, and T. Iino. 1989. Toluene transposons Tn4651 and Tn4653 are class II transposons. J. Bacteriol. 171:1386-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259-268. [DOI] [PubMed] [Google Scholar]
- 31.Weightman, A. J., A. W. Topping, K. E. Hill, L. L. Lee, K. Sakai, J. H. Slater, and A. W. Thomas. 2002. Transposition of DEH, a broad-host-range transposon flanked by ISPpu12, in Pseudomonas putida is associated with genomic rearrangements and dehalogenase gene silencing. J. Bacteriol. 184:6582-6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wheatcroft, R., and P. A. Williams. 1981. Rapid methods for the study of both stable and unstable plasmids in Pseudomonas. J. Gen. Microbiol. 124:433-437. [DOI] [PubMed] [Google Scholar]
- 33.Williams, P. A., and K. Murray. 1974. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J. Bacteriol. 120:416-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Worsey, M. J., and P. A. Williams. 1975. Metabolism of toluene and xylenes by Pseudomonas putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J. Bacteriol. 124:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]