Abstract
Pseudomonas putida strain PP3 produces two hydrolytic dehalogenases encoded by dehI and dehII, which are members of different deh gene families. The 9.74-kb DEH transposon containing dehI and its cognate regulatory gene, dehRI, was isolated from strain PP3 by using the TOL plasmid pWW0. DEH was fully sequenced and shown to have a composite transposon structure, within which dehI and dehRI were divergently transcribed and were flanked on either side by 3.73-kb identical direct repeats. The flanking repeat unit, designated ISPpu12, had the structure of an insertion sequence in that it was bordered by 24-bp near-perfect inverted repeats and contained four open reading frames (ORFs), one of which was identified as tnpA, putatively encoding an ISL3 family transposase. A putative lipoprotein signal peptidase was encoded by an adjacent ORF, lspA, and the others, ISPpu12 orf1 and orf2, were tentatively identified as a truncated cation efflux transporter gene and a PbrR family regulator gene, respectively. The orf1-orf2 intergenic region contained an exact match with a previously described active, outward-orientated promoter, Pout. Transposition of DEH-ISPpu12 was investigated by cloning the whole transposon into a suicide plasmid donor, pAWT34, and transferring the construct to various recipients. In this way DEH-ISPpu12 was shown to transpose in a broad range of Proteobacteria. Transposition of ISPpu12 independently from DEH, and inverse transposition, whereby the vector DNA and ISPpu12 inserted into the target genome without the deh genes, were also observed to occur at high frequencies in P. putida PaW340. Transposition of a second DEH-ISPpu12 derivative introduced exogenously into P. putida PP3 via the suicide donor pAWT50 resulted in silencing of resident dehI and dehII genes in about 10% of transposition transconjugants and provided a genetic link between transposition of ISPpu12 and dehalogenase gene silencing. Database searches identified ISPpu12-related sequences in several bacterial species, predominantly associated with plasmids and xenobiotic degradative genes. The potential role of ISPpu12 in gene silencing and activation, as well as the adaptation of bacteria to degrade xenobiotic compounds, is discussed.
A key step in the biodegradation of halo-organic compounds is cleavage of the carbon-halogen bond. Many different enzymes generally referred to as dehalogenases catalyze this reaction; for example, different classes include hydrolytic, oxygenolytic, and reductive enzymes (21, 22). Dehalogenation of α-halocarboxylic acids such as the herbicide Dalapon (2,2-dichloropropionic acid) is catalyzed by one of the classes of hydrolytic dehalogenases that is best characterized, in that protein structures and mechanisms have been reported (31, 32, 36, 37) for the two distinct evolutionary families of these enzymes, the group I and group II deh products (17). There is preliminary evidence to suggest that other families of halocarboxylic acid dehalogenases exist (17, 23).
The dehalogenase system of Pseudomonas putida strain PP3, a bacterium that was originally isolated from chemostat culture following selection on Dalapon (39), has been well studied. Strain PP3 produces two dehalogenases, DehI and DehII (55), which are encoded by genes of the group I and group II deh families, respectively (17). Thomas et al. (44, 45) showed that the dehI gene was carried on a mobile genetic element and gave it the general designation DEH, since it was found to vary in size following transposition into different plasmid targets. Thomas et al. (44) identified a hot spot for insertion of DEH into the TOL plasmid pWW0 (13, 57), and one such DEH element insertion was cloned and characterized (45) following its transposition from the PP3 genome to pWW0 and conjugal transfer of pWW0::DEH to another strain of P. putida. Thus, the DEH element was shown to carry the dehI gene immediately adjacent to dehRI, which encoded a σ54-dependent activator (45, 46).
Several plasmid and chromosomal genes involved in the degradation of xenobiotic compounds are carried on catabolic transposons (43). Indeed, the genes encoding enzymes of the toluene catabolic pathway on pWW0 are carried on two nested Tn3-like elements, Tn4651 and Tn4653 (49, 50). Such catabolic transposons and insertion sequence elements have been strongly implicated in the evolution of catabolic functions associated with adaptation of bacteria to degrade xenobiotic compounds (52). Evidence has also been reported to suggest that activation and/or silencing of such genes is associated with insertion sequence elements (11). Our previous studies have shown that spontaneous mutants of strain PP3 resistant to inhibition by dichloroacetic acid (DCA), a toxic α-halocarboxylic acid, were selected at high frequencies (41, 54) and that in most of these mutants either one or both of the dehalogenase genes were silenced or lost. In some mutants silenced dehII genes were reactivated, and it was suggested that silencing and reactivation of dehI and dehII genes, associated with DCA resistance, might involve movement of mobile DNA in strain PP3 (41); however, no genetic link between DEH transposition and dehalogenase gene switching was established. Mobile elements associated with other dehalogenase-producing bacteria have been reported (25, 26, 53), as has preliminary evidence for dehalogenase gene silencing in a Rhizobium species (29).
The aim of the present study was to investigate in more detail the structure and function of the DEH transposon from strain PP3, carrying dehI and dehRI. Here we report the full sequence of the DEH element, including ISPpu12, and present results from experiments investigating its transposition and associated effects.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Escherichia coli strain JM107 was used for routine plasmid maintenance (59). E. coli strain S17.1 (40) was used as the donor strain in mating experiments to transfer DEH-ISPpu12 on plasmids pAWT34 and pAWT50 and was kindly provided by Reinhard Simon. P. putida strains PaW1 and PaW340 (strain mt-2 derivatives) (3), UWC1 (also an mt-2 derivative) (34), PP3 (42), and PP3-KS1 (a spontaneous rifampin-resistant derivative of strain PP3) were used as recipients in mating experiments.
Plasmid pAWT6, a derivative of pHG327 containing the DEH element from a TOL plasmid pWW0 recombinant, was described previously (44, 45) and was ligated after partial digestion with EcoRI into pSUP202, a suicide plasmid donor constructed by Simon et al. (40) and carrying a tetracycline resistance (Tcr) marker, to produce pAWT34. Plasmid pAWT50 was a derivative of pAWT34 in which a kanamycin-resistant (Kmr) interposon (Ω-Km [10]) was inserted into the unique XhoI site in DEH (dehRI), producing a dehalogenase knockout and an alternative selective marker for transposition.
Media and growth conditions.
E. coli and P. putida cultures were routinely grown in Luria-Bertani medium (1% tryptone, 0.5% yeast extract, 1% NaCl) and on nutrient agar (Difco), with appropriate amino acid, vitamin, and antibiotic additions, at 37 and 30°C, respectively. SBS (42) minimal medium was also used, with the additional of filter-sterilized 14 mM 2-chloropropionic acid (2MCPA) (pH 7) or 10 mM sodium succinate for growth of prototrophic strains. Solid media were prepared by the addition of 1.5% agar (Difco).
Antibiotics and amino acids were sterilized by filtration through a 0.2-μm-pore-size cellulose acetate membrane (Whatman) and added to autoclaved media when cooled to 55°C.
Transposition of DEH suicide plasmid donation by filter matings.
E. coli strain S17.1 (40) was transformed with plasmids pAWT34 and pAWT50 by a rapid transformation protocol (14). E. coli S17.1 has the transfer functions of plasmid RP4 incorporated into the chromosome (40), allowing the mobilization of plasmids without transfer of a second conjugative plasmid. Conjugative transfer of pAWT34 into P. putida PaW340 and of pAWT50 into strain PP3 was achieved by filter mating. Overnight cultures of the donor and recipient organisms were concentrated 10-fold and mixed in equal proportions, and 50 μl of the mixture was placed on a sterile 0.45-μm-pore-size cellulose acetate membrane (Whatman) on a nonselective nutrient agar plate, together with the appropriate donor and recipient controls. Plates were incubated for 6 h at 30°C. Cells from each filter were then resuspended in 1 ml of sterile 1/10 Luria-Bertani medium and plated at an appropriate dilution onto selective agar. Any plasmids transferred into the P. putida recipient would not be maintained in this host (the host range is restricted to E. coli), and so by selecting for the phenotypes believed to be carried on the transposon, only those recipients in which transposition occurred prior to the loss of the plasmid were recovered. The matings to transfer pAWT34 to strain PaW340 were plated onto 2MCPA-SBS minimal medium supplemented with tryptophan (0.1 mM) and 500 μg of streptomycin (for selection of PaW340 strains containing DEH) per ml. Colonies were then screened by replica plating onto nutrient agar (NA) containing 50 μg of tetracycline per ml for absence of Tcr, to identify true transposition transconjugants that did not contain the pSUP202 vector. PaW340 transconjugants were also selected on NA with 50 μg of tetracycline per ml and 500 μg of streptomycin per ml (the Tcr of pAWT34 was derived from the pSUP202 vector [40]) and screened for inability to grow on 2MCPA (i.e., absence of dehI) to identify inverse (vector) transposition transconjugants. The matings to transfer pAWT50 to strain PP3 were plated onto Pseudomonas selective agar (PSA) (Oxoid) containing 50 μg of kanamycin per ml (DEH::Ω-Km transposition) and on PSA containing 40 μg of tetracycline per ml (inverse transposition), with screening to eliminate double-resistant (Kmr Tcr) colonies. Transconjugants from the latter matings were tested for resistance to DCA on SBS-succinate medium containing 50 mM DCA. The host range of DEH-ISPpu12 was investigated by using donor E. coli S17-1(pAWT34) in matings, as described above, with the following recipient proteobacterial strains: Agrobacterium tumefaciens A136, Burkholdaria cepacia K56-2, Comamonas terugina, Ralstonia eutropha JMP222 (9), Acinetobacter sp. strain ADP1, Pseudomonas aeruginosa PAO1, and P. putida UWC1 (34). All of these strains were prototrophic, and transposition transconjugants were selected on either 2MCPA-SBS minimal medium or NA with 50 μg of tetracycline per ml.
DNA isolation, purification, and manipulation.
Cultures used for isolation of total DNA were grown overnight in Luria-Bertani medium. A 1.5-ml portion of culture was harvested, and the cells were washed in 1 ml of TES buffer (50 mM Tris-HCl [pH 8], 5 mM EDTA, 50 mM NaCl). Cells were then resuspended in 500 μl of 25% sucrose in 50 mM Tris-HCl (pH 8), and 100 μl of lysozyme (50 mg/ml in 0.25 M EDTA [pH 8]) was added. Tubes were incubated on ice for 30 min. Lysis was completed by the addition of 25 μl of 20% SDS and 25 μl of proteinase K (25 mg/ml) (Roche). The lysates were transferred to dialysis tubing (Fisher) and dialyzed overnight against 50 mM EDTA (pH 8)-0.5% SDS at 56°C. At least 50 ml of dialysis solution was allowed for each cell lysate. After overnight incubation, the EDTA concentration of the lysates was reduced by replacing the dialysis solution with TE buffer (10 mM Tris-HCl, 1 mM EDTA) and gently stirring at room temperature for at least 2 h. Lysates were then removed from the dialysis tubing and gently extracted twice with phenol-chloroform (50% phenol, 48% chloroform, 2% isoamyl alcohol) in order to remove proteinase K. Phenol was removed by two extractions with water-saturated ether. DNA was precipitated by the addition of 2 volumes of absolute ethanol (−20°C) and gentle mixing. DNA was immediately harvested by centrifugation, washed in 1 ml of 70% ethanol, dried under vacuum, and dissolved in 200 μl of sterile deionized water.
Genomic DNA for pulsed-field gel electrophoresis (PFGE) was prepared in agarose blocks from overnight cultures of P. putida, as follows. Five hundred microliters of culture was harvested by centrifugation, washed in SE (75 mM NaCl, 25 mM EDTA, pH 8), and finally resuspended in 500 μl of agarose buffer (10 mM Tris-HCl, 10 mM MgCl2, 0.1 mM EDTA, pH 7.5). The cell suspension was warmed to 37°C and mixed with an equal volume of 2% low-melting-point agarose (Sigma type VII) in agarose buffer also at 37°C. The mix was transferred to an agarose block mold (Bio-Rad) and allowed to set at 4°C for 20 to 30 min. Blocks were removed and placed in a partitioned petri dish to separate them. Lysis solution (100 mM EDTA, 1% SDS, 1 M NaCl, and 1 mg of lysozyme per ml in 10 mM Tris-HCl, pH 8.0) was added at 2 ml per dish and incubated overnight at 37°C. The lysis solution was removed carefully, and the blocks were then washed in TE buffer. Each block was incubated in protein digestion solution (50 mM EDTA [pH 9.5], 1% SDS, 0.5 mg of proteinase K per ml) for 48 h at 56°C and washed again in TE buffer. Proteinase K was inactivated by incubating each block in 2 ml of TE buffer containing 1 mM phenylmethylsulfonyl fluoride overnight. Following a final wash for at least 1 h in TE buffer, the blocks were stored at 4°C in fresh TE buffer until required.
DNA digestion with restriction endonucleases was carried out for at least 3 h, using an excess of enzyme and the manufacturer's recommended buffer and temperature. Restriction endonucleases were purchased from Promega, Roche, and Amersham-Pharmacia.
Standard agarose gel electrophoresis of DNA fragments was carried out using 0.8% agarose (Ultrapure grade; Life Technologies) and Tris-borate-EDTA buffer as described by Sambrook et al. (38). For PFGE, slices of the agarose blocks (ca. 1/5 of the total) containing genomic DNA were equilibrated on ice for 1 h in SpeI restriction endonuclease buffer and then overnight at 37°C in 200 μl of fresh buffer containing 20 U of SpeI. Following digestion, gel slices were placed in the wells of a 1.5% agarose gel (in 0.5× TBE) and sealed with low-melting-point agarose. PFGE was carried out at 200 V at 18°C with the switch time set to ramp from 3 to 15 s over the first 5 h and from 1 to 33 s over the next 19 h. After staining with ethidium bromide and destaining in H2O for 30 min, DNA in the gels was depurinated by incubation at 37°C in 0.05 M HCl for 30 min prior to Southern hybridization.
Southern hybridizations.
Following electrophoresis and staining, DNA in agarose gels was denatured by gentle agitation of the gel in 0.5 M NaOH-1.5 M NaCl for 20 min. The denaturing solution was then replaced, and agitation was continued for another 20 min. The gel was then rinsed in distilled water and neutralized for 30 min in 0.5 M Tris-HCl (pH 7.5)-3 M NaCl, with a change of solution after 15 min. DNA was transferred overnight to a Hybond-N nylon membrane (Amersham-Pharmacia) by using the double squash blot technique (38). DNA was fixed to the damp membrane by 3 min of exposure on a 310-nm transilluminator. Membranes were air dried and stored at room temperature.
DNA hybridizations were performed with the digoxigenin (DIG) nonradioactive labeling system (Roche). As indicated in Fig. 1, a 2.56-kb SmaI fragment and a 1.23-kb BstEII fragment were used as probes to detect transposition of dehI-dehRI and ISPpu12, respectively. Inverse (vector) transposition was detected using EcoRI-linearized pSUP202 as a probe. Restriction fragments used as probes were gel purified with GeneClean II resin (Bio Inc.) according to the manufacturer's recommended protocol. Probes were separately labeled with DIG by using a random multiprime labeling system, following the protocol of the manufacturer (Roche). A 125-bp dehI gene probe was also used in some experiments and was DIG labeled using a PCR protocol (30) with primers dehIf (5′ TGT ACG CTG CAG GAT TCG AT) and dehIr (5′ CGC ATC TGC ATG CTT TCA CT). PCRs were carried out with pAWT6 as the template and standard concentrations of reagents under the following cycling conditions: denaturation, 95°C for 30 s; annealing, 55°C for 30 s; and extension, 72°C for 60 s (32 cycles). The probe was gel purified and stored at 4°C until required.
FIG. 1.
Genetic map of the 9.74-kb DEH-ISPpu12 composite transposon. The gene designations are as follows: dehI, dehalogenase I; dehRI, σ54-dependent activator; tnpA, putative ISL3 family transposase; lspA, putative lipoprotein signal peptidase; orf1, putative heavy-metal-associated cation efflux transporter (incomplete); orf2, putative PbrR family regulator. The positions of the following sequence elements and motifs are also indicated: IRR and IRL, 24-bp IRs bordering the right and left ends, respectively, of ISPpu12; PdehRI, putative −35/−10 promoter for dehRI identified by Topping et al. (46); PdehI, putative −24/−12 promoter for dehI. The positions of dehI-dehRI and ISPpu12 hybridization probes used in the detection of transposition products in Fig. 3 to 5 are shown, as is the unique XhoI restriction endonuclease site. DEH contained no site for EcoRI or SpeI.
Hybridizations were carried out at 42°C, using a hybridization solution containing 5× SSC (0.75 M NaCl plus 0.0825 M sodium citrate), 50% formamide, 2% skim milk powder (blocking agent), 0.1% N-lauryl sulfate, and 0.02% SDS. After hybridization, high-stringency washes of the membranes were carried out. Hybridizing bands were developed in accordance with the instructions of the manufacturer (Roche), using a 1:10,000 dilution of anti-DIG alkaline phosphatase conjugate and AMPPD as the enzyme substrate. X-ray film was exposed, at room temperature, for 1 to 12 h and developed using FX-40 developer (Kodak) and Hypam fixer (Ilford).
DNA sequencing and analysis.
The strategy for DEH transposon sequencing involved both shotgun cloning of purified restriction fragments from plasmid pAWT6 into pUC-based vectors (59) and primer walking on PCR fragments amplified and cloned from P. putida PP3. At least triple coverage was achieved on both strands. The following software was used for sequence assembly and analysis: DNAsis (Hitachi), Artemis (www.sanger.ac.uk/Software/Artemis/v4), and various other programs available from the Sanger Centre (www.sanger.ac.uk), the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov), and the European Bioinformatics Institute (www.ebi.ac.uk). Classification of ISPpu12 was done with reference to the insertion sequence database (www-is.biotoul.fr/is.html) (33) with the kind assistance of Jacques Mahillon. Motifs associated with the σ54 promoter were identified by using SEQSCAN (www.bmb.psu.edu/seqscan) with reference to the work of Barrios et al. (2).
Nucleotide accession number.
The EMBL accession number of the complete DEH sequence is AY138113.
RESULTS
Structure of the DEH element and its flanking insertion sequence, ISPpu12.
The structure of the 9.74-kb DEH element originally cloned from pWW0::DEH (44, 45) was determined by complete sequence analysis (Fig. 1). The full sequence of dehI adjacent to and transcribed divergently from dehRI (its cognate regulatory gene) is reported for the first time; however, the sequence of dehRI was previously reported (46), as were results showing that expression of dehI in the DEH element was not observed in an rpoN mutant of P. putida (45). A putative −10/−35 promoter for dehRI and a −12/−24 promoter for dehI were identified, the presence of the latter being consistent with this σ54 (RpoN) dependence. In addition, sequence analysis of the intergenic region between dehRI and dehI identified other motifs, specifically, a putative integration host factor-binding region and two upstream-activating sequences, which are commonly associated with σ54-dependent promoters (2, 58).
The dehI-dehRI region had one copy of a 3.37-kb element on either side, directly repeated (Fig. 1). This element, designated ISPpu12, contained four open reading frames (ORFs) and had the structure of a large insertion sequence, in that one of the ORFs encoded a putative transposase and it was flanked by 24-bp imperfect inverted repeats (IRs) (Fig. 2B). Highly significant nucleotide matches (>90% sequence identity) were uncovered between ISPpu12 and regions (0.8 to 3.37 kb) of the following plasmids: pWW0 from P. putida mt-2 (13), R471a from Serratia marcescens (28), pVT745 from Actinobacillus actinomycetemcomitans (12), and pLEM from Pasteurella multocida (35). The following unrelated xenobiotic degradative gene clusters in Pseudomonas species also contained flanking sequences that showed >90% sequence identity to regions of ISPpu12: ortho-halobenzoate, ohb (48); alkylbenzene, xyl (5); chlorocatechol, clc (16); and naphthalene, nah (7). The intergenic region between the hypothetically divergently transcribed orf1 and orf2 in ISPpu12 shared 97% sequence identity with a promoter-containing sequence derived from the TOL plasmid described by Inouye et al. (18).
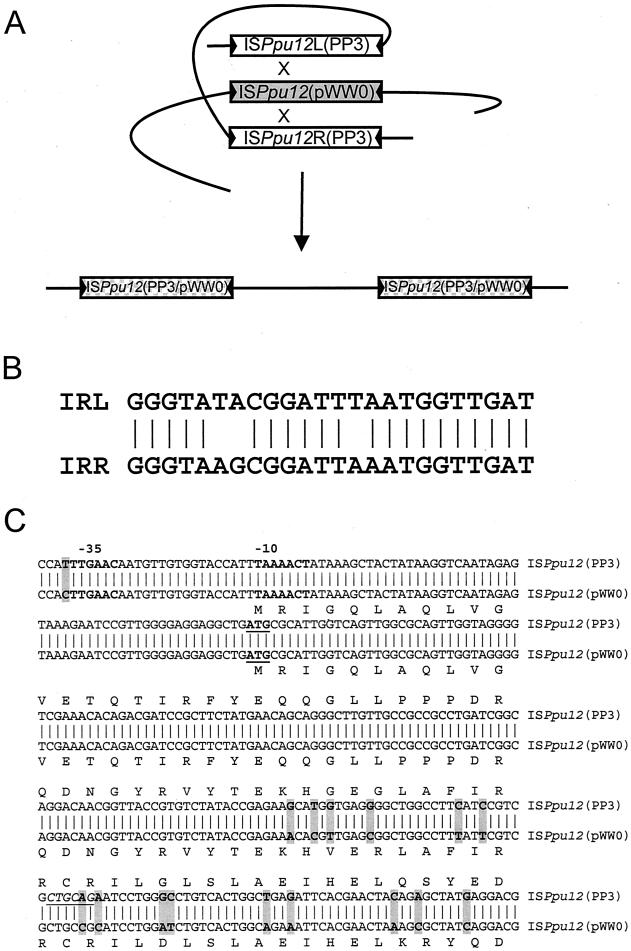
FIG. 2.
(A) Hypothetical schematic pathway by which DEH-ISPpu12 recombined with resident copy of ISPpu12 on pWW0 during plasmid rescue of DEH-ISPpu12 from P. putida strain PP3 (44). The precise sites of the proposed recombination between the different ISPpu12 elements, indicated by X, are not known. (B) Nucleotide alignment of ISPpu12 IRs IRL and IRR. (C) Nucleotide alignment of ISPpu12 from strain PP3 and ISPpu12 from pWW0 (56) in the intergenic orf1-orf2 region and within orf2, highlighting (in grey) the sequence differences around the −35/−10 Pout promoter (shown in boldface) (18). The position of the putative start to orf2 is indicated in boldface and underlined, and the derived amino acid sequences for ISPpu12 from PP3 and from pWW0 are given above and below their respective nucleotide sequences. The unique diagnostic PstI site in ISPpu12 from PP3 is in italics and underlined.
The putative transposase gene, tnpA, of ISPpu12 was identical to that reported by Kulaeva et al. (28) for a 1,771-bp element designated IS1396, but only one of the IRs proposed for IS1396 was found in the DEH sequence. On the basis of alignments of the TnpA-derived sequence with those of other transposases and further analysis (J. Mahillon, personal communication), ISPpu12 was tentatively assigned to the ISL3 family according to the classification of Mahillon and Chandler (33).
As indicated in Fig. 1, the three ORFs upstream of tnpA in ISPpu12 encoded putative products with high levels of predicted amino acid sequence similarity to the following gene products: a lipoprotein signal peptidase (44% similarity over the ORF with LspA from P. fluorescens [19, 20]), a putative heavy-metal-associated cation efflux transporter (truncated, Orf1 [1, 51]), and a heavy metal resistance regulator (Orf2, >95% similarity to PbrR family regulator helix-turn-helix N-terminal domain [15]). None of these three ORFs is commonly contained within insertion sequence elements, nor is any of them thought to be directly associated directly with transposition. It was noted that three ORFs each had homologues in Ralstonia metallidurans strain CH34 (6), as follows: PbrC (LspA), CzcD (Orf1), and PbrR (Orf2). All of these homologues are associated with other insertion sequence elements in that organism.
Origins of ISPpu12L and ISPpu12R elements in transposon DEH.
Comparison of ISPpu12L and ISPpu12R (Fig. 1) in the DEH element cloned from pWW0::DEH revealed some minor sequence differences in the intergenic region between orf1 and orf2 and within orf2 (Fig. 2C), comprising 15 nucleotide positions downstream and 1 upstream from promoter Pout. In order to establish whether the structure of DEH and the sequences of its flanking ISPpu12 elements were the same in P. putida PP3 as in the pWW0::DEH derivative, PCR cloning was used to isolate separately ISPpu12L and ISPpu12R directly from strain PP3. PCR restriction mapping and sequencing confirmed that the DEH element in strain PP3 was flanked by two identical copies of ISPpu12. Southern hybridization experiments showed that strain PP3 contained only one copy of the dehI gene and one copy of DEH (L. L. Lee and A. J. Weightman, unpublished results). However, restriction mapping results experimentally confirmed that one copy of ISPpu12 in pWW0::DEH was slightly different from the ISPpu12 elements in strain PP3, in that the latter contained an extra PstI site located within orf2 (Fig. 2C).
This was further investigated by high-stringency Southern hybridization experiments using the ISPpu12 probe (derived from pWW0::DEH), which showed that pWW0 contained a very similar element within the EcoRI-G restriction fragment of that plasmid (47), the region that Thomas et al. (44) had shown to be a hot spot for DEH insertion. Comparison of the ISPpu12R sequence derived from pWW0::DEH with the copy of ISPpu12 resident in pWW0 (13) confirmed the hybridization results and showed an exact match. Therefore, it is proposed that the DEH element originally described by Thomas et al. (44, 45) was formed by recombination between the ISPpu12 elements of DEH in strain PP3 and the ISPpu12 element resident in pWW0 (Fig. 2A). However the specific site of recombination could not be identified because of the extensive homology between ISPpu12 in PP3 and that in pWW0. Although there were these minor sequence differences between ISPpu12 in strain PP3 and ISPpu12 in pWW0, the elements appeared to be identical with respect to all of their major features (Fig. 1), including IRs, ORF sequences, and the presence of an active promoter sequence located upstream from orf2 (18, 56).
Transposition of DEH and ISPpu12.
The DEH element from plasmid pWW0::DEH was recloned from plasmid pAWT6 (45) by using the suicide donor plasmid pSUP202 (40) to form plasmid pAWT34, and E. coli strain S17-1(pAWT34) was used as a transposon donor in 6-h filter matings.
In the first series of filter mating experiments, plasmid pAWT34 was transferred to the recipient P. putida strain PaW340 and transconjugants were selected on the following media: (i) SBS minimal medium containing tryptophan, streptomycin, and 2MCPA to select for true (DEH) transposition, with screening for absence of Tcr associated with the pSUP202 vector; and (ii) NA containing streptomycin and tetracycline to select for inverse (vector) transposition, with screening for inability to utilize 2MCPA (i.e., absence of dehI). The use of a suicide plasmid donation system to introduce DEH into the recipients prevented the calculation of transposition frequencies per plasmid transfer; however, the combined transfer-transposition frequencies observed were remarkably high. For example, after a 6-h filter mating, approximately 6% of all potential strain PaW340 recipients were able to grow on 2MCPA-containing minimal medium, indicating that true transposition had occurred. Furthermore approximately 2.5% of all potential recipients were Tcr, indicating that inverse (vector) transposition had occurred at high frequency. Unexpectedly, it was found that a high proportion (43%) of unscreened transconjugants selected on 2MCPA were also Tcr and, conversely, 52% of unscreened transconjugants selected as Tcr were also able to grow on 2MCPA-containing minimal medium. It is possible that such transconjugants resulted from a single recombination and integration of pAWT34 into the recipient chromosome; however, hybridization results with probes to detect true and inverse transposition products (see below) indicated that multiple independent transposition events had occurred in these strains (47).
Figures 3 and 4 show the results from Southern hybridizations using three probes (the dehI-dehRI and ISPpu12 probes shown in Fig. 1 and a pSUP202 vector probe) against EcoRI- and XhoI-digested genomic DNAs from transconjugants selected from independent filter matings. None of the probes hybridized with DNA from the recipient strain PaW340, and the hybridization patterns in transconjugants confirmed that ISPpu12 was associated with all true (Fig. 3) and inverse (Fig. 4) transposition events. Figure 3A shows that the copy number of DEH in transconjugants selected for true transposition, estimated by halving the total number of hybridizing XhoI fragments (DEH contained one XhoI site located within the dehI-dehRI probe [Fig. 1]) varied from 1 (Fig. 3A, lane 8) to >7 (lanes 3, 4, 5, and 6). Figure 3B shows that independent transposition of ISPpu12 was observed in a high proportion (at least 6 of 10) of these transconjugants and that in half (3 of 6) of these >1 additional independent copy of ISPpu12 was evident. It should be noted that these transconjugants were selected as Tcs, and none hybridized with the pSUP202 vector probe (results not shown). Conversely, none of the transconjugants shown in Fig. 4, selected for inverse transposition and screened for their inability to grow on 2MCPA (i.e., lack of dehalogenase activity), hybridized with the dehI-dehRI probe (results not shown). The characteristic features of inverse transposition transconjugants were that multiple copies of the vector-containing transposition product were not observed but independent transposition of ISPpu12 still occurred (Fig. 4B).
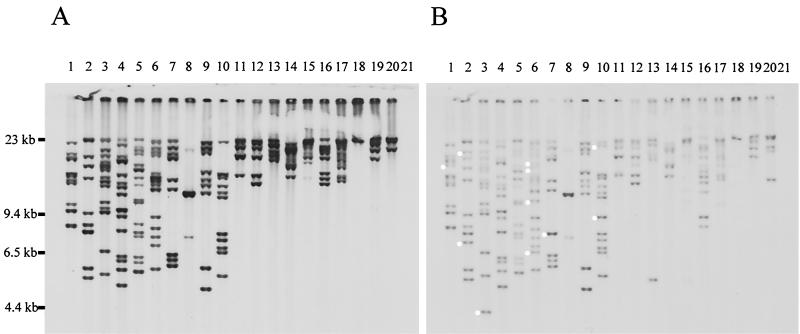
FIG. 3.
Southern hybridization showing true transposition of DEH-ISPpu12 from pAWT34 into the chromosome of P. putida PaW340. Ten independent transposition transconjugants were selected on 2MCPA-SBS medium from 6-h filter matings between the donor E. coli S17-1(pAWT34) and recipient PaW340. (A) Genomic DNAs from strains PaW340 T1 to T10 digested with XhoI (lanes 1 to 10, respectively) and EcoRI (lanes 11 to 20, respectively), blotted, and hybridized with the DIG-labeled dehI-dehRI probe (Fig. 1). Lane 21, control DNA from strain PaW340 digested with EcoRI. (B) Replicate Southern blot hybridized with the ISPpu12 probe (Fig. 1). Circles are placed alongside XhoI DNA fragments hybridizing only with DIG-labeled ISPpu12, indicating transposition of this element independently of DEH. All strains were Tcs, and none hybridized with the pSUP202 vector probe (results not shown).
FIG. 4.
Southern hybridization showing inverse transposition of DEH-ISPpu12 from pAWT34 into the chromosome of P. putida PaW340. Ten independent transposition transconjugants were selected on NA-tetracycline medium from 6-h filter matings between the donor E. coli S17-1(pAWT34) and recipient PaW340. (A) Genomic DNAs from strains PaW340 I1 to I10 digested with XhoI (lanes 1 to 10, respectively), blotted, and hybridized with the DIG-labeled pSUP202 probe. (B) Replicate Southern blot hybridized with the DIG-labeled ISPpu12 probe (Fig. 1). Circles are placed alongside DNA fragments hybridizing only with ISPpu12. Dehalogenase activity was not detected in any of these strains, nor did they hybridize with the dehI-dehRI probe (results not shown), and none was able to grow on 2MCPA.
Southern hybridizations of Tcr, 2MCPA-positive transconjugants (i.e., those originally selected on 2MCPA and subsequently found to be Tcr) showed a pattern of results similar to those illustrated in Fig. 3, with multiple insertions of DEH-ISPpu12 (with ISPpu12 also transposing independently from DEH) and single-copy insertions of the inverse product associated with Tcr. Thus, it was evident that these transconjugants had not simply arisen from whole plasmid pAWT34 integration into the recipient genome.
In all transposition transconjugants tested, DEH-ISPpu12 transposons could be rescued in P. putida UWC1 by mating-out experiments with exogenously introduced pWW0 and appropriate selection, i.e., growth on 2MCPA for true transposition products and Tcr for inverse transposition products. Frequencies of transposition in these rescue experiments ranged from 5 × 10−4 to 1.5 × 10−6 per plasmid pWW0 transfer but did not correlate with ISPpu12 copy number in the donor strain.
The host range of ISPpu12 was determined by using pAWT34 and selection for Tcr (inverse) transposition where DEH (true) transposition could not be selected directly. In this way, ISPpu12 transposition was observed in the following species, representing the α, β, and γ subdivisions of the Proteobacteria: A. tumefaciens, B. cepacia, C. terugina, R. eutropha, Acinetobacter sp., P. aeruginosa, and P. putida.
Gross genomic rearrangements in P. putida PaW340 associated with DEH transposition.
PFGE was used to investigate the distribution of multiple copies of DEH in transconjugants selected on 2MCPA. Figure 5B shows a Southern hybridization of SpeI-digested genomic DNA from selected true-transposition PaW340 transconjugants, using a PCR-generated dehI probe. Since DEH contained no SpeI site, the presence of multiple large hybridizing fragments evident in most transconjugants indicated that the element had transposed promiscuously into different loci on the PaW340 chromosome, and in most cases multiple transposition events had occurred during the selection process. Unexpectedly, the SpeI digest patterns of the transconjugants showed polymorphisms (Fig. 5A), to such an extent that no two had the same SpeI restriction fragment length polymorphism profile and all were different from that of the PaW340 recipient. The observation that several large (>100-kb) SpeI fragments were affected suggested that DEH transposition was associated with gross genomic rearrangements of the PaW340 chromosome. No dehI-hybridizing fragment was seen in one of the transconjugants (Fig. 5B, lane 3), because the DEH element had inserted into an SpeI fragment that was too small (i.e., <35 kb) to be visible on the gel after PFGE under the conditions used.
FIG. 5.
(A) PFGE of SpeI-digested genomic DNAs from 10 strain PaW340 true-transposition transconjugants, isolated independently as described in the legend to Fig. 3. Lanes 1 to 10, DNAs from strains PaW340 T1 to T10, respectively. (B) Southern blot of the gel shown in panel A hybridized with the DIG-labeled 125-bp dehI PCR probe (see Materials and Methods).
Transposition of DEH associated with mutagenesis and dehalogenase gene silencing in P. putida PP3.
A derivative of plasmid pAWT34 containing a Kmr Ω interposon (10), inserted to knock out dehRI and thereby prevent activation of dehI expression, was constructed and designated pAWT50. This suicide donor plasmid derivative was used to transfer DEH::Ω-Km to a rifampin-resistant derivative of P. putida strain PP3, designated PP3-KS1. Strain PP3-KS1 transconjugants in which true transposition (insertion of DEH::Ω-Km) had occurred were selected on PSA-kanamycin, and inverse (vector) transposition transconjugants were selected on PSA-tetracycline. The results summarized in Table 1 confirmed that ISPpu12 was associated with true and inverse transposition. The observation that ca. 3% of transconjugants were putative auxotrophs (i.e., unable to grow on succinate-containing minimal medium) suggests that ISPpu12 and DEH derivatives were mutagenic, as is frequently observed with insertion sequence elements and transposons. Five of these putative auxotrophs were further characterized by auxanography (8) and were shown to contain different mutations in one of the following independent amino acid biosynthetic pathways: methionine (strain PP3-KS2), cysteine (PP3-KS3), tryptophan (PP3-KS4 and PP3-KS6), and leucine (PP3-KS5).
TABLE 1.
Transposition of DEH-ISPpu12 transferred exogenously to P. putida strain PP3 by using suicide donor plasmid pAWT50
| Selected transposition transconjugant class (mode of detection) | % of total transposition transconjugantsa after selection for:
|
|
|---|---|---|
| True transposition | Inverse transposition | |
| True transposition (Kmr) | 100 | 17 |
| Inverse transposition (Tcr) | 54 | 100 |
| Auxotrophic (unable to grow on succinate-SBS)b | 3 | 3 |
| DCA resistant (growth on succinate-DCA-SBS medium)c | 22 | 18 |
| Silenced dehI and dehII (2MCPA negative, unable to grow on SBS-2MCPA medium) | 9 | 7 |
Filter matings (6 h) were carried out as described in Materials and Methods; the donor was E. coli S17-1(pAWT50), and the recipient was P. putida PP3. True- and inverse-transposition transconjugants were selected independently on PSA- kanamycin and PSA-tetracycline media, respectively. Five hundred transconjugants from three independent matings were isolated, purified, and characterized by patching onto the appropriate media to detect each of the specified classes of transconjugant.
Ten auxotrophs were further characterized by auxanography (see text).
More than 80% of DCA-resistant mutants of PP3 do not express dehI and/or dehII (54; L. L. Lee and A. J. Weightman, unpublished results).
Mutations causing DCA resistance (ca. 20%) and inability to utilize 2MCPA (2MCPA-negative mutants constituted ca. 5% of transconjugants, and all were DCA resistant as expected) were detected at high frequencies in true- and inverse-transposition transconjugants (Table 1). Both of these types of mutations are known to be associated with silencing of the dehalogenase genes in strain PP3, in that it was previously shown that mutants unable to utilize 2MCPA expressed neither dehI nor dehII, and most DCA-resistant mutants expressed either only one or neither of these genes (41, 54). Southern hybridizations of inverse (Kms Tcr)-transposition transconjugants of strain PP3 that had become 2MCPA negative (i.e., produced no dehalogenase) with a dehI probe showed that they all still contained this region. This indicated that the dehI gene was silenced rather than deleted.
DISCUSSION
The results reported here show that the DEH element in P. putida PP3 is a composite transposon on which the dehalogenase gene dehI and its cognate regulatory gene, dehRI, are located side by side and flanked by identical direct repeats of an insertion sequence, ISPpu12. Transposon Tn5 and interposon Ω-Km and Ω-Tc mutations in DEH, originally described by Thomas et al. (45) and mapped more accurately by Topping et al. (46), showed that insertional inactivation of dehRI abolished expression of dehI. It was previously shown that expression of dehI in the DEH element was rpoN dependent (45). In the present study the identification of putative integration host factor-binding and upstream activation sequence motifs associated with a −12/−24 promoter upstream from dehI is consistent with the previous proposal that DehRI regulates expression of dehI and is a σ54-dependent activator.
The main evidence suggesting that DEH-ISPpu12 is a composite transposon may be summarized as follows: (i) 24-bp imperfect IRs identified in the flanking insertion sequences, ISPpu12L and ISPpu12R; (ii) identification of an ORF in ISPpu12 encoding a putative transposase, TnpA; (iii) promiscuous and high-frequency insertion of the cloned DEH element from a suicide donor plasmid into the P. putida genome; (iv) true and inverse transposition from the same suicide donor plasmid, accompanied in all cases studied by movement of ISPpu12; and (v) transposition of ISPpu12 independently from the rest of DEH.
There are some additional features of DEH worth noting. First, the element cloned from the TOL plasmid pWW0 and described by Thomas et al. (44, 45) was a hybrid, presumably formed by recombination between ISPpu12R and ISPpu12 from pWW0 (56) during transposition of DEH from the original donor (i.e., the chromosome of strain PP3) to pWW0. The presence of an element essentially identical to ISPpu12 in pWW0 was a remarkable coincidence, explaining the hot spot for DEH in this region (44). The pWW0 sequence (13) and the further characterization of ISPpu12 of pWW0 described by Williams et al. (56) in the accompanying paper showed that the minor sequence differences between ISPpu12 in PP3 and pWW0 were trivial in functional terms. Thus, it may be assumed that the activities of these two elements are the same and that the same results would have been obtained from the transposition experiments described in this paper even if they had been carried out with DEH cloned directly from PP3 instead of with the hybrid ISPpu12 element produced by recombination between ISPpu12R from PP3 and ISPpu12 resident in pWW0. In addition, since Williams et al. (56) were able to conclude that only TnpA was absolutely required for transposition of ISPpu12 in pWW0, it is reasonable to suggest that the same would hold true for ISPpu12 in PP3.
Second, the tnpA-IRL end of ISPpu12 was found to be identical to a putative insertion sequence designated IS1396 in plasmid R471a originally described by Kulaeva et al. (28). Those authors did not demonstrate transposition of IS1396, and our further analysis of the region upstream of its tnpA gene (2,150 bp available from the sequence reported by Kulaeva et al. [28], including an ORF identified as lspA) showed 100% sequence identity with ISPpu12. This strongly suggests that the identification of IS1396 as an insertion sequence and the IRs proposed by Kulaeva et al. (28) were incorrect and that the region of the plasmid R471a sequence annotated as IS1396 is partially a copy of ISPpu12.
Third, the fact that DEH transposed to sites in pWW0 and other plasmid targets (e.g., RP4-5) that did not contain ISPpu12-like elements (44) indicated that ISPpu12 copies in DEH were actively involved in transposition. However, the structure of DEH elucidated here provided no obvious explanation as to the mechanism by which transposition of DEH into pWW0 and RP4-5 plasmid targets gave rise to insertions varying from 6 to 13 bp in size (44). DEH products of different sizes may result from the arrangements of ISPpu12 copies in the PP3 genome and/or complexities in the ISPpu12-mediated transposition mechanism. Southern hybridization experiments (L. L. Lee and A. J. Weightman, unpublished results) suggested that the PP3 genome contains only one copy of DEH (i.e., single copies of dehI, dehRI, ISPpu12L, and ISPpu12R) as delineated in Fig. 1, but a part(s) of ISPpu12 was present outside the region of DEH (47). To date ca. 10 kb of the region downstream from tnpA in ISPpu12L on the PP3 chromosome has been sequenced, and we have just started to sequence from ISPpu12R in the opposite direction (L. L. Lee, R. E. Dodds, K. E. Hill, and A. J. Weightman, unpublished results), but no ISPpu12 sequences or other insertion sequence elements have so far been located.
Fourth, the frequency of transposition of DEH-ISPpu12 and the effects of transposition on the target were extreme. The use of a suicide donation system prevented the calculation of transposition frequencies per plasmid transfer, but the fact that after a 6-h filter mating ca. 8% of all potential recipients had received a copy of DEH and/or the inverse transposition product indicated that the transposition frequency was very high indeed. Also, since ISPpu12 was able to transpose independently but there was no selection to detect such events, the proportion of recipients that received ISPpu12 may have been even higher than 8%. By comparison, van der Meer et al. (52) used the pSUP202 donation system and reported that the catabolic transposon Tn5280 was recovered in transconjugants at 1/10,000 the frequency of ISPpu12. The observed ratios of true- to inverse-transposition products in recipients suggested that TnpA showed no preference for IRR or IRL. With pAWT34 as a donor, a preference for IRR would have favored DEH (true) transposition, while a preference for IRL would have favored vector (inverse) transposition. This differs from the case for other composite transposons, such as the well-characterized Tn5 and Tn10, the transposases of which show preferences for the outer IRs of IS50 and IS10, respectively (4, 27). Also, although independent transposition of insertion sequence elements contained in composite transposons is rarely investigated, the fact that independent transposition of ISPpu12 was observed at high frequencies without selection makes it quite unusual.
The ability of DEH-ISPpu12 to produce multiple insertions is noteworthy and was also shown to be the case with transposition of ISPpu12::Kmr derivatives reported by Williams et al. (56). This might provide a potential explanation for the gross genomic rearrangements observed in recipients (Fig. 5), in that recombination between multiple copies of DEH-ISPpu12 distributed around the recipients' genomes could have resulted in extensive deletions and/or insertions. The results from the present study did not allow us to determine whether multiple transpositions of DEH-ISPpu12 took place from the same pAWT34 delivery or whether sequential transposition of the element followed insertion of a single copy into the target genome. Also, further work will be required to determine why multiple insertions of the composite transposon were only associated with true transposition and not with inverse transposition (cf. Fig. 3 and 4).
Mutagenesis associated with DEH-ISPpu12 transposition was evident. Indeed, it would seem quite possible that a proportion of recipients may have sustained lethal mutations and would not have been detected, as a result of the element's high transposition activity, promiscuity, and potential to cause multiple gene knockouts and target rearrangements. This raises interesting questions regarding the control of DEH-ISPpu12 activity in strain PP3. The transposition of DEH-ISPpu12 exogenously introduced into strain PP3 produced some of the most intriguing results, in that it was clearly associated with high-frequency silencing of dehI and dehII (Table 1), leading to the observed 2MCPA-negative and DCA-resistant phenotypes. Previously, dehalogenase silencing was observed phenotypically in response to environmental stress, for example, direct selection for resistance to the toxic dehalogenase substrates such as DCA (41, 54). The results in this paper implicate DEH-ISPpu12 in dehalogenase gene silencing and have provided a firm basis for our current investigations into the silencing mechanism. In particular, it will be interesting to discover whether the portable promoter, Pout, in ISPpu12 has any role in dehalogenase gene activation, as seen in the activation of phenol-degradative genes by an outward-directed promoter in IS1411, which was also assigned to the ISL3 family (24).
In addition to ISPpu12 copies in pWW0 (56) and R471a (28), several other ISPpu12-like elements have been identified. Our group (K. E. Hill and A. J. Weightman, submitted for publication) recently reported the isolation of a group of related IncP catabolic plasmids carrying dehalogenase genes, most of which also contained a region that hybridized with ISPpu12 probes. Tsoi et al. (48) described the cloning and characterization of a 6-kb DNA segment containing an ohb operon from P. aeruginosa strain 142, encoding enzymes catalyzing oxygenolytic ortho dehalogenation of halobenzoates. They identified an “IS1396-like sequence” just upstream from ohbB, and our analysis of their data showed that this sequence contained the IRL of ISPpu12 7 bp upstream from the ohbB terminator. Only part of this IS1396-like sequence, containing the TnpA ORF and part of the LspA ORF, is available, but again it seems likely that part or all of ISPpu12 is linked to this ohb operon. Bolognese et al. (5) reported that a 3-kb insertion sequence designated ISPs1, also related to the ISL3 family, mediated activation and inactivation of aromatic hydrocarbon catabolic pathway genes in P. stutzeri. This element is clearly related to ISPpu12 in that it contained the same tnpA gene (99% nucleotide sequence identity) and was bordered by almost identical IRs; however, ISPs1 and ISPpu12 differed with respect to their other ORFs. The involvement of this ISPs1 in xyl gene activation and inactivation is of considerable interest given the presence of ISPpu12 in the archetypal TOL plasmid pWW0 and the latter's association with xylE activation (18, 56).
Thus, there is emerging evidence regarding the distribution of ISPpu12-like elements that also points towards their general involvement in transposition and catabolic gene switching. Recent searches of currently available sequence data from bacterial genome projects, including those for P. putida strains KT2440 and PRS1, have identified only one copy of ISPpu12 (3,367 identical nucleotides of 3,372) and several shorter sequences almost identical to regions of ISPpu12 in Burkholdaria fungorum strain LB400 (L. L. Lee and A. J. Weightman, unpublished results). Significant matches were also found between the ORFs in ISPpu12 and several prokaryotic genome sequences, mainly from species of Proteobacteria. However, DEH remains the only ISPpu12-based transposon identified to date.
Acknowledgments
We thank Peter Williams, Chris Thomas, and Alicia Greated for kindly sharing their unpublished data and results with us and Jacques Mahillon for helpful advice on the classification of ISPpu12. Peter Williams' comments on this work were also very helpful and contributed to the writing of this paper. Some of this work was carried out by A.J.W. on a sabbatical in Nick Ornston's lab at Yale University, for which thanks are given. Thanks also go to our collaborators Dick Janssen, Mike Larkin, Charlie van Peé, Dave Harper, Gerrit Poelarends, Johan van Hylckama Vlieg, and Leonid Kulakov for fruitful discussions regarding this study. Julian Marchesi also participated enthusiastically in and made contributions to discussions on this study.
This work was supported by EC Research Contract ENV4-CT95-0086. Additional financial support from the following agencies is also gratefully acknowledged: the Natural Environment Research Council for studentships to Andrew W. Thomas and Andrew W. Topping, the British Council and Oita University for funds to support K.S.'s sabbatical at Cardiff University, the Overseas Research Students Awards Scheme (ORS) for funds to assist L.L.L., the Royal Society for travel funds to support A.J.W.'s sabbatical at Yale, and Steve Taylor and Avecia (formerly ICI Bioproducts) for funds to assist Andrew W. Topping.
REFERENCES
- 1.Anton, A., C. Groβe, J. Reiβmann, T. Pribyl, and D. H. Nies. 1999. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J. Bacteriol. 181:6876-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrios, H., B. Valderrama, and E. Morett. 1999. Compilation and analysis of σ54-dependent promoter sequences. Nucleic Acids Res. 27:4305-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayley, S. A., C. J. Duggleby, M. J. Worsey, P. A. Williams, K. G. Hardy, and P. Broda. 1977. Two modes of loss of the TOL functions from Pseudomonas putida mt-2. Mol. Gen. Genet. 154:203-204. [DOI] [PubMed] [Google Scholar]
- 4.Berg, D. E. 1989. Transposon Tn5, p. 185-201. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology, Washington, D.C.
- 5.Bolognese, F., C. Di Lecce, E. Galli, and P. Barbieri. 1999. Activation and inactivation of Pseudomonas stutzeri methylbenzene catabolism pathways mediated by a transposable element. Appl. Environ. Microbiol. 65:1876-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borremans, B., J. L. Hobman, A. Provoost, N. L. Brown, and D. van der Lelie. 2001. Cloning and functional analysis of the pbr lead resistance determinant of Ralstonia metallidurans CH34. J. Bacteriol. 183:5651-5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosch, R., E. R. Moore, E. Garcia-Valdes, and D. H. Pieper. 1999. NahW, a novel, inducible salicylate hydroxylase involved in mineralization of naphthalene by Pseudomonas stutzeri AN10. J. Bacteriol. 181:2315-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, R. W., D. Botsein, and J. R. Roth. 1980. A manual for genetic engineering: advanced bacterial genetics, p. 251. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 9.Don, R. H., and J. M. Pemberton. 1981. Properties of six pesticide degradation plasmids isolated from Alcaligenes paradoxus and Alcaligenes eutrophus. J. Bacteriol. 145:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellay, R., H. M. Krisch, P. Prentki, and J. Frey. 1987. Omegon-Km: a transposable element designed for in vivo insertional mutagenesis and cloning of genes in gram-negative bacteria. Gene 76:215-226. [DOI] [PubMed] [Google Scholar]
- 11.Galas, D. J., and M. Chandler. 1989. Bacterial insertion sequences, p. 109-162. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology, Washington, D.C.
- 12.Galli, D. M. J. Chen, K. F. Novak, and D. J. Leblanc. 2001. Nucleotide sequence and analysis of conjugative plasmid pVT745. J. Bacteriol. 183:1585-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greated, A., L. Lambertson, P. A. Williams, and C. M. Thomas. Complete sequence analysis of the IncP-9 TOL plasmid pWW0 from Pseudomonas putida. Environ. Microbiol., in press. [DOI] [PubMed]
- 14.Hanahan, D. 1985. Techniques for transformation of Escherichia coli, p. 109-135. In D. M. G. Glover (ed.), DNA cloning: a practical approach, vol. 1. IRL Press, Oxford, United Kingdom.
- 15.Helmann, J. D., B. T. Ballard, and C. T. Walsh. 1990. The MerR metalloregulatory protein binds mercuric ion as a tri-co-ordinate, metal-bridged dimmer. Science 247:946-948. [DOI] [PubMed] [Google Scholar]
- 16.Hickey, W. J., G. Sabat, A. S. Yuroff, A. R. Arment, and J. Pérez-Lesher. 2001. Cloning, nucleotide sequencing, and functional analysis of a novel, mobile cluster of biodegradation genes from Pseudomonas aeruginosa strain JB2. Appl. Environ. Microbiol. 67:4603-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill, K. E., J. R. Marchesi, and A. J. Weightman. 1999. Investigation of two evolutionarily unrelated halocarboxylic acid dehalogenase gene families. J. Bacteriol. 181:2535-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye, S., Y. Asai, A. Nakazawa, and T. Nakazawa. 1986. Nucleotide sequence of a DNA segment promoting transcription in Pseudomonas putida. J. Bacteriol. 166:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isaki, L., M. Kawasaki, R. Beers, R. Hom, and H. C. Wu. 1990. Cloning and nucleotide sequence of the Enterobacter aerogenes signal peptidase II (Isp) gene. J. Bacteriol. 172:469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaki, L., R. Beers, and H. C. Wu. 1990. Nucleotide sequence of the Pseudomonas fluorescens signal peptidase II gene (Isp) and flanking genes. J. Bacteriol. 172:6512-6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen, D. B., J. E. Oppentocht, and G. J. Poelarends. 2001. Microbial dehalogenation. Curr. Opin. Biotechnol. 12:254-258. [DOI] [PubMed] [Google Scholar]
- 22.Janssen, D. B., J. R. van der Ploeg, and F. Pries. 1994. Genetics and biochemistry of dehalogenating enzymes. Annu. Rev. Microbiol. 48:163-191. [DOI] [PubMed] [Google Scholar]
- 23.Janssen, D. B., T. Bosma, and G. J. Poelarends. 1997. Diversity and mechanisms of bacterial dehalogenation mechanisms, p. 119-128. In D. B Janssen, K. Soda, and R. Weaver (ed.), Mechanisms of biohalogenation and dehalogenation. Royal Netherlands Academy of Arts and Sciences, Amsterdam, The Netherlands.
- 24.Kallastu, A., R. Hõrak, and M. Kivisaar. 1998. Identification and characterization of IS1411, a new insertion sequence which causes transcriptional activation of the phenol degradation genes in Pseudomonas putida. J. Bacteriol. 180:5306-5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawasaki, H., K. Tsuda, I. Matsushita, and K. Tonomura. 1992. Lack of homology between two haloacetate dehalogenase genes encoded on a plasmid from Moraxella sp strain B. J. Gen. Microbiol. 138:1317-1323. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki, H., M. Takao, A. Koiso, and K. Tonomura. 1985. Genetic rearrangement of plasmids: in vivo recombination between a dehalogenation plasmid and multiple-resistance plasmid RP4 in Pseudomonas sp. Appl. Environ. Microbiol. 49:1544-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleckner, N. 1989. Transposon Tn10, p. 227-268. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology, Washington, D.C.
- 28.Kulaeva, O. I., E. V. Koonin, J. C. Wootton, A. S. Levine, and R. Woodgate. 1998. Unusual insertion element polymorphisms in the promoter and terminator regions of the mucAB-like genes of R471a and R446b. Mutat. Res. 397:247-262. [DOI] [PubMed] [Google Scholar]
- 29.Leigh, J. A., A. J. Skinner, and R. A. Cooper. 1986. Isolation and partial characterization of dehalogenase-deficient mutants of a Rhizobium sp. FEMS Microbiol. Lett. 36:163-166. [Google Scholar]
- 30.Lion, T., and O. A. Haas. 1990. Non-radioactive labelling of probe with digoxigenin using polymerase chain reaction. Anal. Biochem. 188:335-337. [DOI] [PubMed] [Google Scholar]
- 31.Liu, J.-Q., T. Kurihara, M. Miyagi, N. Esaki, and K. Soda. 1995. Reaction mechanism of L-2-haloacid dehalogenase of Pseudomonas sp. YL. J. Biol. Chem. 270:18309-18312. [PubMed] [Google Scholar]
- 32.Liu, J.-Q., T. Kurihara, S. Ichiyama, M. Miyagi, S. Tsunasawa, H. Kawasaki, K. Soda, and N. Esaki. 1998. Reaction mechanism of gluoroacetate dehalogenase from Moraxella sp. B. J. Biol. Chem. 273:30897-30902. [DOI] [PubMed] [Google Scholar]
- 33.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClure, N. C., A. J. Weightman, and J. C. Fry. 1989. Survival of Pseudomonas putida UWC1 containing cloned catabolic genes in a model activated-sludge unit. Appl. Environ. Microbiol. 55:2627-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGee, J. E., and B. E. Bejcek. 2001. A cryptic plasmid from Pasteurella multocida has a predicted protein nearly identical to a transport protein from Actinobacillus actinomycetemcomitans. Plasmid 46:60-64. [DOI] [PubMed] [Google Scholar]
- 36.Nardi-Dei, V., T. Kurihara, C. Park, M. Miyagi, S. Tsunasawa, K. Soda, and N. Esaki. 1999. DL-2-haloacid dehalogenase from Pseudomonas sp. 113 is a new class of dehalogenase catalyzing hydrolytic dehalogenation not involving enzyme-substrate ester intermediate. J. Biol. Chem. 274:20977-20981. [DOI] [PubMed] [Google Scholar]
- 37.Ridder, I. S., H. J. Rozeboom, K. H. Kalk, D. B. Janssen, and B. W. Dijkstra. 1997. Three-dimensional structure of L-2-haloacid dehalogenase from Xanthobacter autotrophicus GJ10 complexed with the substrate-analogue formate. J. Biol. Chem. 272:33015-33022. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Senior, E., A. T. Bull, and J. H. Slater. 1976. Enzyme evolution in a microbial community growing on the herbicide Dalapon. Nature 263:476-479. [DOI] [PubMed] [Google Scholar]
- 40.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 41.Slater, J. H., A. J. Weightman, and B. G. Hall. 1985. Dehalogenase genes of Pseudomonas putida PP3 on chromosomally located transposable elements. Mol. Biol. Evol. 2:557-567. [DOI] [PubMed] [Google Scholar]
- 42.Slater, J. H., D. Lovatt, A. J. Weightman, E. Senior, and A. T. Bull. 1979. The growth of Pseudomonas putida on chlorinated aliphatic acids and its dehalogenase activity. J. Gen. Microbiol. 114:125-136. [Google Scholar]
- 43.Tan, H.-M. 1999. Bacterial catabolic transposons. Appl. Microbiol. Biotechnol. 51:1-12. [DOI] [PubMed] [Google Scholar]
- 44.Thomas, A. W., J. H. Slater, and A. J. Weightman. 1992. The dehalogenase gene dehI from Pseudomonas putida strain PP3 is carried on an unusual mobile genetic element, designated DEH .J. Bacteriol. 174:1932-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas, A. W., A. W. Topping, J. H. Slater, and A. J. Weightman. 1992. Localization and functional analysis of structural and regulatory dehalogenase genes carried on DEH, a mobile genetic element from Pseudomonas putida strain PP3. J. Bacteriol. 174:1941-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topping, A. W., A. W. Thomas, J. H. Slater, and A. J. Weightman. 1995. The nucleotide sequence of a transposable haloalkanoic acid dehalogenase regulatory gene (dehRI) from Pseudomonas putida strain PP3 and its relationship with σ54-dependent activators. Biodegradation 6:247-255. [DOI] [PubMed] [Google Scholar]
- 47.Topping, A. W. 1992. An investigation into the transposition and dehalogenase functions of DEH, a mobile genetic element from Pseudomonas putida strain PP3. Ph.D. thesis. University of Wales, Cardiff, United Kingdom.
- 48.Tsoi, T. V., E. G. Plotnikova, J. R. Cole, W. F. Guerin, M. Bagdasarian, and J. M. Tiedje. 1999. Cloning, expression, and nucleotide sequence of the Pseudomonas aeruginosa 142 ohb genes coding for oxygenolytic ortho dehalogenation of halobenzoates. Appl. Environ. Microbiol. 65:2151-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuda, M., and T. Iino. 1987. Genetic analysis of a transposon carrying toluene degrading genes on a TOL plasmid pWW0. Mol. Gen. Genet. 201:270-276. [DOI] [PubMed] [Google Scholar]
- 50.Tsuda, M., and T. Iino. 1988. Identification and characterisation of Tn4653, a transposon covering the toluene transposon TN4651 on TOL plasmid pWW0. Mol. Gen. Genet. 210:72-77. [DOI] [PubMed] [Google Scholar]
- 51.van der Lelie, D., T. Schwuchow, U. Schwidetzky, S. Wuertz, W. Baeyens, M. Mergeay, and D. H. Nies. 1997. Two-component regulatory system involved in transcriptional control of heavy-metal homoeostasis in Alcaligenes eutrophus. Mol. Microbiol. 23:493-503. [DOI] [PubMed] [Google Scholar]
- 52.van der Meer, J. R. A. J. B. Zehnder, and W. M. de Vos. 1991. Identification of a novel composite transposable element, Tn5280, carrying chlorobenzene dioxygenase genes of Pseudomonas sp. strain P51. J. Bacteriol. 173:7077-7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Ploeg, J., M. Willemsen, G. Vanhall, and D. B. Janssen. 1995. Adaptation of Xanthobacter autotrophicus GJ10 to bromoacetate due to activation and mobilization of the haloacetate dehalogenase gene by insertion element IS1247. J. Bacteriol. 177:1348-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weightman, A. J., A. L. Weightman, and J. H. Slater. 1985. Toxic effects of chlorinated and brominated alkanoic acids on Pseudomonas putida PP3: selection at high frequencies of mutations in the genes encoding dehalogenases. Appl. Environ. Microbiol. 49:1494-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weightman, A. J., J. H. Slater, and A. T. Bull. 1979. The partial purification of two dehalogenases from Pseudomonas putida PP3. FEMS Microbiol. Lett. 6:231-234. [Google Scholar]
- 56.Williams, P. A., R. M. Jones, and L. E. Shaw. 2002. A third transposable element, ISPpu12, from the toluene-xylene catabolic plasmid pWW0 of Pseudomonas putida mt-2. J. Bacteriol. 184:6572-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Worsey, M. J., and P. A. Williams. 1975. Metabolism of toluene and xylenes by Pseudomonas putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J. Bacteriol. 124:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu, H., and T. R. Hoover. 2001. Transcriptional regulation at a distance in bacteria. Curr. Opin. Microbiol. 4:138-144. [DOI] [PubMed] [Google Scholar]
- 59.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequence of M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]