The bacterial strains and plasmids are described in Table 1. E. faecalis clinical isolate BM4405 is resistant to low levels of vancomycin (MIC = 16 μg/ml) (15). VanC-type E. gallinarum BM4174 (12) and VanA-type Enterococcus faecium BM4147 (20) were used as controls in pulsed-field gel electrophoresis experiments. E. faecalis JH2-2, used in electrotransformation experiments, is susceptible to glycopeptides and resistant to fusidic acid and rifampin (19). Escherichia coli JM83 (42) and Top10 (Invitrogen, Groningen, The Netherlands) were used as the hosts in cloning experiments. Strains were cultured in brain heart infusion broth or agar (Difco Laboratories, Detroit, Mich.) at 37°C. Susceptibility to glycopeptides was determined by agar dilution with 105 CFU per spot on Mueller-Hinton agar (Bio-Rad, Marnes-La-Coquette, France) after 24 h of incubation.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Strains | ||
| E. coli JM83 | F−ara Δ(lac-proAB) rpsL (Strr) [φ80dlacΔ(lacZ)M15] | 43 |
| E. coli Top10 | F− [lacIqTn10(Tetr)] mcrA Δ(mrr-hsdRMS mcrBC) φ80lacZΔM15ΔlacX74 recA araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen 15 |
| E. faecalis BM4405 | Vmr (VanE type) | 19 |
| E. faecalis JH2-2 | Fusr Rifr | |
| E. faecalis V583 | Vmr (VanB type) | 13 |
| E. gallinarum BM4174 | Vmr (VanC type) | 12 |
| E. faecium BM4147 | Vmr Ter (VanA type) | 20 |
| Plasmids | ||
| pCR2.1 | Apr Kmr; lacZα oriR from ColE1 | Invitrogen |
| pUC18 | Apr, lacZα vector | 41 |
| pAT29 | oriRpAMβ1 oriRpUC oriTRK2 SprlacZα | 39 |
| pAT663 | 0.5-kb PCR fragment (vanE) of BM4405 cloned in pCR2.1 | 15 |
| pAT664 | Sau3AI fragment (vanE′XYETE′) of BM4405 cloned in pUC18 | This work |
| pAT667 | 4.4-kb fragment (600 bp, vanEXYETE) of BM4405 cloned in pAT29 | This work |
| pAT668 | 3.8-kb fragment (vanEXYETE) of BM4405 cloned in pAT29 | This work |
Fusr, fusidic acid resistance; Rifr, rifampin resistance; Spr, spectinomycin resistance; Strr, streptomycin resistance; Ter, teicoplanin resistance; Tetr, tetracycline resistance; Vmr, vancomycin resistance.
Recombinant DNA techniques.
Cleavage of DNA with restriction endonucleases (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, England, and Gibco BRL-Life Technologies Inc.), purification of restriction fragments from agarose gel, and ligation with T4 DNA ligase (Amersham Pharmacia Biotech) were performed by standard methods (35).
Plasmid construction.
The plasmids were constructed as follows (Fig. 1).
FIG. 1.
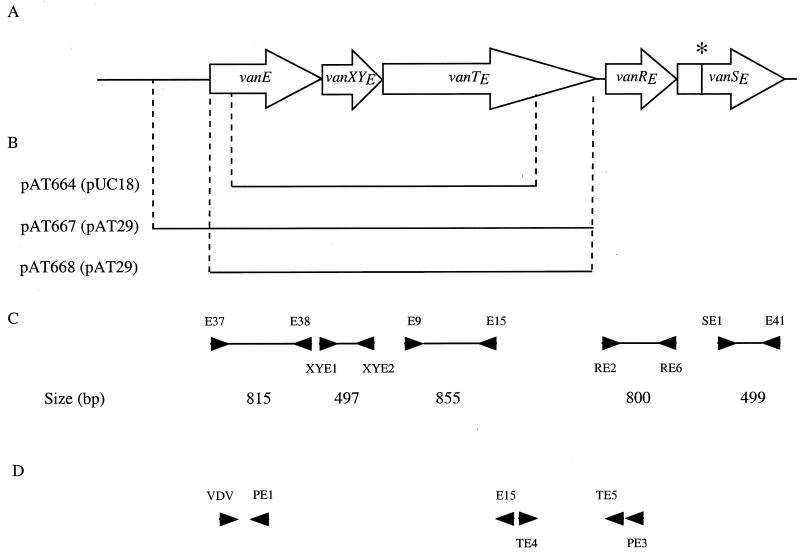
Schematic representation of the vanE gene cluster and of recombinant plasmids. (A) Open arrows represent coding sequences and the direction of transcription. The asterisk indicates the stop codon in vanSE. (B) The inserts in the recombinant plasmids are represented by solid lines, and the vectors are indicated in parentheses. (C) PCR fragments used as probes in Northern hybridization. (D) Oligonucleotides used in RT-PCR and in primer extension. Arrowheads indicate positions and orientations of primers.
(i) Plasmid pAT664.
Total DNA from BM4405 was partially digested with Sau3AI and ligated with pUC18 DNA cleaved by BamHI. Clones harboring recombinant plasmids were screened by colony hybridization (35) with the 513-bp fragment internal to vanE purified from pAT663 (15) as a probe.
(ii) Plasmids pAT667 and pAT668.
A fragment encompassing the vanE, vanXYE, and vanTE genes, with or without 600 bp upstream from vanE, was amplified by using primer pairs E35-TE5 and E43-TE5, respectively, and E. faecalis BM4405 DNA as a template. Oligodeoxynucleotides E35 and E43 contained a SacI site, and TE5 contained SphI site. The PCR products were digested with SacI and SphI and cloned in pAT29.
Plasmid pAT667 (600 bp upstream from vanE, vanEXYETE) and pAT668 (vanEXYETE) were introduced into E. faecalis JH2-2 by electrotransformation, and transformants were selected with spectinomycin (60 μg/ml).
Probes and hybridization.
DNA was transferred onto Hybond N+ membranes (Amersham Pharmacia Biotech) and fixed under UV illumination. Plasmid pAT663 DNA labeled with [α-32P]dCTP (Amersham Pharmacia Biotech) by nick translation was used as a probe for colony hybridization, and Southern experiments were carried out under stringent conditions (35).
PCR and nucleotide sequencing.
The PCR mixture consisted of reaction buffer (final concentrations of 1.5 mM MgCl2 and 10 mM Tris-HCl at pH 8.3); 500 μM (each) dATP, dCTP, dTTP, and dGTP; 40 pmol of each primer; 2 U of Taq DNA polymerase (Amersham Pharmacia Biotech); and 100 ng of enterococcal DNA in a total volume of 50 μl. DNA amplification was carried out in a GeneAmp PCR system 2400 thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.). DNA sequencing was performed by the dideoxynucleotide chain termination method (36) with α-35S-dATP (Amersham) and the T7 Sequenase version 2.0 DNA sequencing kit (Amersham). Plasmid DNA used as a template was extracted with the commercial Wizard Plus Minipreps DNA purification system (Promega, Madison, Wis.).
Computer analysis of sequence data.
Sequence data were analyzed with the Sequence Analysis Software Package (version 7; Genetics Computer Group, Madison, Wis.). Phylogenetic analysis was carried out with the PHYLIP program package (14).
Analysis of peptidoglycan precursors.
Extraction and analysis of peptidoglycan precursors were performed as described previously (26). Enterococci were grown in brain heart infusion broth overnight at 37°C in the presence (4 μg/ml) or absence of vancomycin with gentle agitation to an optical density at 600 nm of 1 (mid-exponential phase). Ramoplanin was added to a concentration of 3 μg/ml, and incubation was continued for 30 min. Bacteria were harvested, and the cytoplasmic precursors were extracted with 8% trichloroacetic acid (15 min at 4°C), desalted, and analyzed by high-performance liquid chromatography. Results were expressed as the percentages of total late peptidoglycan precursors represented by UDP-MurNAc-tetrapeptide, UDP-MurNAc-pentapeptide, and UDP-MurNAc-pentapeptide-d-Ser that were determined from the integrated peak areas.
Pulsed-field gel electrophoresis.
Genomic DNA embedded in agarose plugs (24) was digested for 3 h at 37°C with 0.01 U of I-CeuI, an intron-encoded endonuclease specific for rRNA genes. Fragments were separated on a 1.2% agarose gel with a contour-clamped homogeneous electric field DR III system (Bio-Rad Laboratories, Hercules, Calif.) under the following conditions: total migration, 24 h; initial pulse, 60s; final pulse, 120s; voltage, 6 V/cm; included angle, 120°; and temperature, 16°C. Fragments were blotted onto Hybond N+ membranes (Amersham Pharmacia Biotech) and hybridized (i) with an [α-32P]dCTP-labeled 16S rRNA (rrs) probe obtained by amplification of an internal portion of the rrs gene (17) and (ii) with a vanE-specific probe (15).
RNA techniques. (i) Extraction of total RNA.
E. faecalis BM4405 was grown to an optical density at 600 nm of 0.7, and bacteria were disrupted with a Mickle disintegrator by using 3.5-g (106-μm-diameter) glass beads (Sigma Chemical Co., St. Louis, Mo.) in the presence of 0.25 ml of 10% sodium dodecyl sulfate, 1 ml of 2% macaloid (National Lead Co., New York, N.Y.), and 3 ml of phenol (16). The mixture was shaken three times for 1 min each at 4°C and centrifuged for 15 min at 8,500 × g. The supernatant was extracted with phenol and chloroform. Total RNA was precipitated by addition of 0.1 volume of 3 M sodium acetate (pH 5.2) and 3 volumes of ice-cold 100% ethanol. RNA pellets were resuspended in diethyl pyrocarbonate-water.
(ii) Northern analysis.
Equal amounts of total RNA (20 μg) were separated under denaturing conditions in 1.2% agarose-formaldehyde-MOPS (morpholinepropanesulfonic acid) gel, stained with ethidium bromide, and blotted onto Hybond N+ membranes (Amersham Pharmacia Biotech) (35). DNA probes obtained by PCR with total DNA from BM4405 as a template and primers E37-E38 (vanE), XYE1-XYE2 (vanXYE), E9-E15 (vanTE), R2-R6 (vanRE), and S1-E41 (vanSE) (Fig. 1C; Table 2) were labeled with [α-32P]dCTP (3,000 Ci/mmol; Amersham Pharmacia Biotech) by using the Megaprime DNA labeling system (Amersham Pharmacia Biotech). Hybridizations were carried out under stringent conditions, and washes were performed as described previously (22). The size of the transcripts was determined according to RNA molecular weight marker I (Boehringer, Mannheim, Germany).
TABLE 2.
Oligonucleotides used in this study
| Primer | Sequence | Positiona |
|---|---|---|
| E9 | 5′ ACT GTG TTT CGG GTA GC | 1722-1738 |
| E12 | 5′ TAT GGG AGT TGT GAA | 2711-2725 |
| E15 | 5′ CAG AAG CTG AGC TAG T | 2576-2561 |
| E35 | 5′ GCG AGC TCA CAG ATC AGG AAA TCG GAb | NAc |
| E37 | 5′ GGA TCA CCG AAG AAG GT | 119-135 |
| E38 | 5′ CCA GGC ATT GTA TTG ATC T | 932-914 |
| E41 | 5′ GCA ATT GCT AAC CCT AGA CC | 5464-5445 |
| E43 | 5′ GCG AGC TCA ACA AAT ACT GGA GGT Ab | NA |
| XYE1 | 5′ GTT CAG GCT CCG TTT GCG C | 1103-1121 |
| XYE2 | 5′ GCA GTT CCT CTT ATT GAC TC | 1599-1580 |
| TE4 | 5′ GCA GCG GTT CAG GTG TTT | 2159-2174 |
| TE5 | 5′ GCG CAT GCA GCC ATT AAA CAT CCTd | 3785-3770 |
| RE1 | 5′ CCG AGA CAG CCA AAT | 4410-4424 |
| RE2 | 5′ TCG ACT GTC GAC AAA T | 4214-4199 |
| RE6 | 5′ AGA TCG ATT TAG CCA TAC | 3913-3930 |
| SE1 | 5′ AGC TAA CAG ATT AGA ACA T | 5027-5045 |
| SE2 | 5′ GGA GTT CTT AAG TCA TGT | 4921-4904 |
| VDV | 5′ GAT CGT TGG TTT TTA GAT | 178-195 |
| RDeg2 | 5′ CCN ACH CCS CRB ACV GTTe | NA |
| PE1 | 5′ CCA ATG ACC TTC TTC GGT GAT CC | 120-96 |
| PE3 | 5′ AAG CTT TCT TTT CCT GAC ATA GCC TC | 3877-3851 |
| SDEg1 | 5′ ATS GSM ARH CCM ARW CCf | NA |
Nucleotide numbering begins at the first base of the vanE gene.
The SacI site is underlined.
NA, not applicable.
The SphI site is underlined.
B = C, G, or T; H = A, C, or T; N = A, C, G, or T; R = A or G; S = G or T; V = A, C, or G.
H = A, C or T; M = A or C; R = A or G; S = G or T; W = A or T.
(iii) RT-PCR experiments.
Total RNA samples were digested with RNase-free DNase I (5 U/μg of RNA) (Amersham Pharmacia Biotech) in a final volume of 1 ml for 10 min at 37°C. Samples were treated with proteinase K (0.2 mg/ml) (Boehringer), extracted with phenol-chloroform, and precipitated with ethanol. Reverse transcription (RT) was carried out with 2 μg of purified RNA in a 20-μl final volume containing 1× enzyme buffer (Superscript II; Gibco), 50 mM magnesium chloride, 0.1 mg of bovine serum albumin (New England Biolabs Inc., Beverly, Mass.) per ml, 1 mM (each) of four deoxyribonucleoside triphosphates (Amersham Pharmacia Biotech), 50 pmol of the primer TE5 or PE3 (Fig. 1D; Table 2), 20 U of RNase inhibitor (RNAguard; Amersham Pharmacia Biotech), and 200 U of Moloney murine leukemia virus modified reverse transcriptase (Superscript II; Gibco). Samples were incubated for 30 min at 37°C, and the enzyme was inactivated at 95°C for 5 min. The DNA products were amplified by PCR in an 80-μl reaction volume containing the previous 20-μl samples, 50 pmol each of the VDV and E15 primers or TE4 and PE3 (Fig. 1D; Table 2), 1× enzyme buffer (Amersham Pharmacia Biotech), and 2 U of Taq DNA polymerase (Amersham Pharmacia Biotech). PCR (30 cycles) was performed in a GeneAmp PCR system 2400 (Perkin-Elmer Cetus, Norwalk, Conn.). PCR products were transferred from agarose gel to a Hybond N+ membrane (Amersham Pharmacia Biotech) and hybridized with specific probes (Fig. 1C).
(iv) Primer extension analysis.
The synthetic oligodeoxynucleotide PE1 (Fig. 1D; Table 2) was 5′ end labeled with [γ-32P]ATP (4,500 Ci/mmol; Amersham Pharmacia Biotech) and T4 polynucleotide kinase (Amersham Pharmacia Biotech). After phenol-chloroform extraction, the labeled primer was precipitated with ethanol and redissolved in sterile water to a final concentration of 1 pmol/μl. Labeled primer (1 pmol) was annealed to 50 μg of total RNA at 65°C for 3 min, and extension was performed in a 20-μl final volume with 40 U of Moloney murine leukemia virus modified reverse transcriptase (Superscript II; Gibco) for 45 min at 50°C. After addition of 5 μl of stop solution (Amersham Pharmacia Biotech) and heat denaturation, the sample was immediately loaded onto 6% polyacrylamide-urea sequencing gels for electrophoresis. Sequencing reactions using the same primer and appropriate plasmid DNA templates were run in parallel to allow determination of the endpoints of extension products.
RESULTS AND DISCUSSION
Cloning of the vanE gene cluster.
Fragments obtained after partial digestion of E. faecalis BM4405 total DNA with Sau3AI were cloned in pUC18 DNA cleaved with BamHI into E. coli, and transformants were screened by hybridization with a vanE internal probe (Fig. 1A). Plasmid pAT664 (vanE′XYETE′) carried an insert of 8 kb that was sequenced. Three open reading frames (ORFs), designated vanE, vanXYE, and vanTE, were found, but the two distal ones were truncated (Fig. 1). The 5′ portion of vanE and 1.5 kb upstream were obtained by successive inverted PCRs. We assumed that the vanE cluster had the same gene organization as the vanC operon, i.e., that vanTE should be followed by the vanRE and vanSE genes. We thus amplified BM4405 DNA by using oligodeoxynucleotide E12, specific for vanTE, and degenerate oligodeoxynucleotide RDeg2, complementary to the sequence encoding a conserved motif in the C-terminal part of VanR-type proteins (Table 2). The PCR product obtained, with the expected size of 1.7 kb, was sequenced, providing the 3′ end of vanTE and entire vanRE. To sequence further downstream from vanRE, specific primer RE1, deduced from the sequence obtained, and degenerate oligodeoxynuleotide SDeg1, complementary to the sequence encoding a conserved motif in the C-terminal part of VanS-type proteins, were used to amplify total DNA from BM4405 (Table 2). Determination of the sequence of the PCR product indicated the presence of the vanSE gene. A 1.5-kb fragment downstream from vanSE was obtained by inverted PCR and sequenced, but no ORF was found. The gene organization of the vanE cluster is shown in Fig. 1. Recently, the sequence of the vanE gene cluster of E. faecalis N00-410 (40) was released, and it exhibits 96 to 98% identity with that of BM4405 (unpublished data; accession number AF 430807).
Analysis of the proteins encoded by the vanE gene cluster.
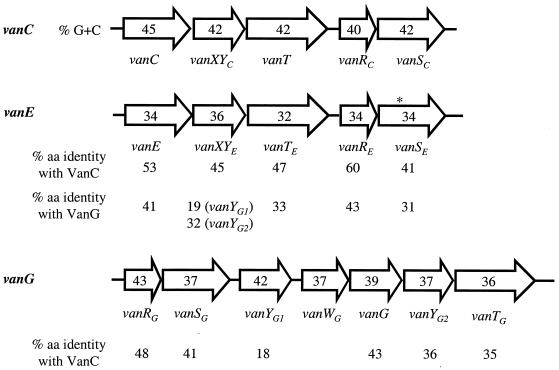
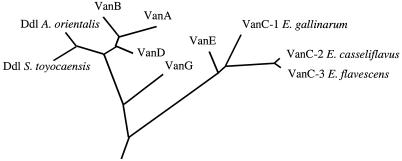
The deduced amino acid sequence of vanE exhibited 53 and 41% identity with the VanC (2) and VanG (25) d-Ala:d-Ser ligases, respectively (Fig. 2). The EKYQ motif conserved in the VanC-type resistance ligases (13) was found in VanE (EKYN) at positions 198 to 201. The phylogenetic tree based on the alignment of the d-Ala:d-Lac and d-Ala:d-Ser ligases confirmed that VanE was related to VanC (Fig. 3).
FIG. 2.
Comparison of the d-Ala-d-Ser gene clusters. Arrows represent coding sequences and indicate the direction of transcription. The asterisk indicates the stop codon in vanSE. The guanosine-plus-cytosine content (% G+C) is indicated in the arrows. The percentages of amino acid (aa) identity between the deduced proteins are indicated under the arrows.
FIG. 3.
Phylogenetic tree derived from the alignment of d-Ala:Lac and d-Ala:d-Ser ligases. The tree was constructed by the neighbor-joining method, taking into account the results of maximum-parsimony and bootstrapping analysis.
The second putative protein, VanXYE, was 45% identical with the VanXYC bifunctional enzyme (1) (Fig. 2). VanXYE displayed higher identity with VanY and VanYB dd-carboxypeptidases (23 and 16%, respectively) than with VanX and VanXB dd-dipeptidases (13 and 16%, respectively). The identity was lower with VanYG1 than with VanYG2 (19 and 32%, respectively). The consensus sequences found in VanX dd-dipeptidases (23), VanY dd-carboxypeptidases (5), and VanXYC dd-peptidases (34) were also present in VanXYE. An SxHxxGxAxD motif, in which the histidine and aspartate are zinc ligands, was found in VanXYE (S95EHEIGLAVD104). Furthermore, another histidine ligand to zinc, conserved in the zinc binding domain of dd-peptidases (33), was found in VanXYE (H157). A conserved glutamate/aspartate residue functioning as a catalytic base (1) was present in VanXYE (E154). The hydrophobicity profile of VanXYE suggested a cytoplasmic localization for the protein (data not shown). Previous study of vancomycin-induced E. faecalis BM4405 indicated weak dd-dipeptidase and dd-carboxypeptidase activities in cytoplasmic extracts (15). Both activities are also found in the cytoplasm of VanC-type E. gallinarum BM4174 (1).
The third ORF, vanTE, encoded a putative protein with 47 and 33% identity with VanTC (2) and VanTG (25) serine racemases, respectively (Fig. 2). The N-terminal half of VanTE contained 11 clusters of hydrophobic amino acids, suggesting that, like VanTC, it may be a membrane-associated protein. The serine racemase activity present in the membrane fractions of BM4405 is ca. 10-fold higher than that of E. gallinarum BM4174 (15). The C-terminal domain of VanTE had substantial sequence identity (28%) with that of alanine racemase Alr1 from E. coli. The putative pyridoxal 5′-phosphate attachment motif, which is highly conserved in alanine racemases and in VanT (33), was found in VanTE (V373VKANAYGCG382). Furthermore, the residues implicated in the hydrogen-bonding interactions with the phosphate group of pyridoxal 5′-phosphate in VanT (Y379, S540, and N688) (2) were present in VanTE. Finally, residues which putatively play a structural role and maintain the geometry of the active site of alanine racemases and VanT (2) were identified in VanTE: A377, A379, Y380, R410, G619, D622, R626, and E688.
The two genes downstream from the three resistance determinants are likely to encode a two-component regulatory system (Fig. 1A). The putative VanRE protein exhibited 61 and 43% identity with VanRC (1) and VanRG (25), respectively (Fig. 2). The conserved aspartate and lysine residues typical of response regulators in two-component systems from gram-positive bacteria (28) were present in VanRE (D10, D53, and K102). VanRE displayed 44% identity with the CheY-like response regulator of Clostridium acetobutylicum (29).
The deduced amino acid sequence of vanSE showed 41 and 31% identity with VanSC (1) and VanSG (25), respectively (Fig. 2). The N-terminal part of VanSE contained transmembrane segments characteristic of the sensor proteins of two-component systems (6). The carboxyl-terminal part of VanSE had four of the five conserved amino acid motifs (H, N, F, and G2) characteristic of transmitter modules of histidine protein kinases (30, 38). However, a stop codon at position 78 of vanSE will result in the production of a truncated protein, suggesting that VanSE is nonfunctional. The level of phosphorylation of VanR-type proteins is controlled by the kinase and phosphatase activities of VanS-type sensors (3, 7, 42). However, kinases encoded by the host chromosome are able to activate the VanR response regulator (3, 8, 37), and it has been demonstrated that both PhoR and acetylphosphate are capable of activating VanR (18). In the absence of a functional VanSE, inducibility of vancomycin resistance expression in BM4405 (15) could be due to cross talk either with another two-component system or of VanRE with an heterologous histidine kinase.
Location of the vanE gene cluster.
Fragments of E. faecalis BM4405 total DNA digested with I-CeuI, an intron-encoded endonuclease specific for rRNA genes (21), were separated by pulsed-field gel electrophoresis and transferred onto a nylon membrane which was hybridized successively to 16S rRNA (rrs)- and vanE-specific probes. The probes cohybridized with a ca. 350-kb fragment from BM4405, indicating a chromosomal location for the vanE cluster (data not shown).
Genes necessary for vancomycin resistance in E. faecalis BM4405.
To test if the vanE, vanXYE, and vanTE genes were sufficient to confer vancomycin resistance to the host, a 3.8-kb fragment encompassing the three structural genes but devoid of any 5′ upstream sequence was cloned in pAT29, leading to plasmid pAT668 (vanEXYETE). The plasmid was introduced into E. faecalis JH2-2 by electrotransformation, and, irrespective of the absence or presence of a low concentration of vancomycin in the culture medium (1 or 2 μg/ml), the transformants remained susceptible to vancomycin (MIC = 2 μg/ml) (Table 3). A 4.4-kb fragment containing the structural genes together with 600 bp upstream from vanE was then cloned in pAT29, generating plasmid pAT667 (600 bp, vanEXYETE) (Fig. 1B). In the absence of induction, strain JH2-2 harboring pAT667 was susceptible to vancomycin. However, a reproducible threefold increase in the vancomycin MIC was observed after growth in the presence of vancomycin (Table 3). Taken together, these results indicate that the vanE, vanXYE, and vanTE genes are sufficient to confer vancomycin resistance and that the region upstream from the vanE cluster may act as a promoter for activation of transcription of the resistance genes, as in the vanC operon (1). When JH2-2/pAT667 was grown in the absence of vancomycin, a very high proportion (89%) of precursors ending in d-Ala-d-Ala was found in the cells. In contrast, precursors ending in d-Ala-d-Ser represented 53% of total peptidoglycan precursors in cells grown in the presence of vancomycin (Table 3), confirming that the resistance genes were inducibly expressed. In vancomycin-induced JH2-2/pAT667 cells, nearly half of the peptidoglycan precursors were of the susceptible type. This might indicate that, possibly due to inefficient cross talk, expression of the vanE operon was lower in induced JH2-2/pAT667 than in BM4405. This finding could account for the fact that the transformant was inhibited by a vancomycin concentration lower than that for strain BM4405 (Table 3).
TABLE 3.
Glycopeptide MICs and nature of peptidoglycan precursors in E. faecalis strains
| E. faecalis | MIC (μg/ml) of vancomycin | Precursorsa (%)
|
||
|---|---|---|---|---|
| Tetra | Penta-d-Ser | Penta-d-Ala | ||
| BM4405 | 16 | 2 | 8 | 90 |
| BM4405 (Vm4)b | 16 | 10 | 90 | 0 |
| JH2-2 | 2 | NDc | ND | ND |
| JH2-2/pAT667 | 2 | 8 | 3 | 89 |
| JH2-2/pAT667 (Vm4) | 6 | 13 | 53 | 34 |
| JH2-2/pAT668 | 2 | ND | ND | ND |
Tetra, UDP-MurNAc-l-Ala-γ-d-Glu-l-Lys-d-Ala; penta-d-Ser, UDP-MurNAc-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ser; penta-d-Ala, UDP-MurNAc-l-Ala-γ-d-Glu-l-Lys-d-Ala-d-Ala.
BM4405 induced with 4 μg of vancomycin per ml.
ND, not determined.
Transcription analysis of the vanE gene cluster.
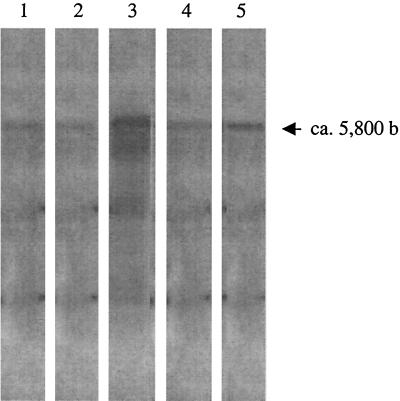
The vanA, vanB, and vanD operons are cotranscribed from their respective PH (6), PYB (37), and PYD (10) promoters. The start codons of the vanXYE and vanTE genes overlap the termination codons of vanE and vanXYE, respectively, suggesting that the vanE, vanXYE, and vanTE genes are cotranscribed. The vanSE start codon also overlaps the vanRE stop codon, suggesting the existence of a second transcription unit. Total RNA from BM4405 was extracted and analyzed by Northern hybridization with probes internal to every gene in the vanE operon (Fig. 1C). A single transcript of ca. 5800 nucleotides was observed, which hybridized with all of the probes, including those internal to vanRE and vanSE (Fig. 4). The size of the transcript and the absence of a smaller mRNA encompassing the last two van genes are consistent with the production of a single mRNA corresponding to the five genes and originating from a promoter upstream from vanE. Cotranscription of the entire vanE gene cluster was tested by RT of total RNA from BM4405 with primer TE5, internal to vanTE (Fig. 1D; Table 2). The cDNA was amplified by PCR with primers VDV and E15, internal to vanE and vanTE, respectively (Fig. 1D; Table 2). A PCR product of the expected size of 2.4 kb that cohybridized with probes specific for vanE, vanXYE, and vanTE (Fig. 1D and Fig. 5) was obtained. To confirm that a single transcript corresponded to the five genes, RT of total RNA from BM4405 with primer PE3, internal to vanRE (Fig. 1D; Table 2), was performed. The cDNA was then amplified using primers internal to vanTE (TE4) and vanRE (PE3) (Fig. 1D; Table 2). A PCR product of ca. 1.7 kb, which cohybridized with the vanTE and vanRE probes, was obtained (data not shown), indicating that the genes for the two-component system were cotranscribed with the resistance genes. Based on these observations, primer extension was performed to locate the transcriptional start site for vanE by using primer PE1, complementary to the 5′ end of that gene (Fig. 1D; Table 2) (Fig. 6). The proposed initiation codon for vanE was preceded by a putative ribosome binding site (5′ ATACTGGAGGN8ATG) (Fig. 6) that displayed high complementarity to the 3′ extremity of Bacillus subtilis 16S rRNA (3′-OH-UCUUUCCUCC) (27). The PE promoter region contained two overlapping putative −10 regions, TTTCAA and TTCAAT, similar to the −10 σ70 recognition consensus. Both regions were at a correct distance, 10 and 11 bp, respectively, from the transcription start. At bp 22 or 23 upstream from the proposed −10 sequences lies a TTGAGG putative −35 sequence. However, due to spacing, it remains open whether this sequence plays a role in the recognition of the promoter region by the σ70 RNA polymerase complex. Furthermore, expression of the vanE operon is likely to depend on the VanRE transcriptional activator, which is known to render the −35 sequence dispensable for expression (11).
FIG. 4.
Analysis of vanE gene cluster transcription by Northern hybridization. Total RNA from BM4405 was hybridized with the vanE (lane 1), vanXYE (lane 2), vanTE (lane 3), vanRE (lane 4), and vanSE (lane 5) probes. The sizes of the transcripts were determined according to RNA molecular weight marker I (Boehringer) (not shown). b, bases.
FIG. 5.
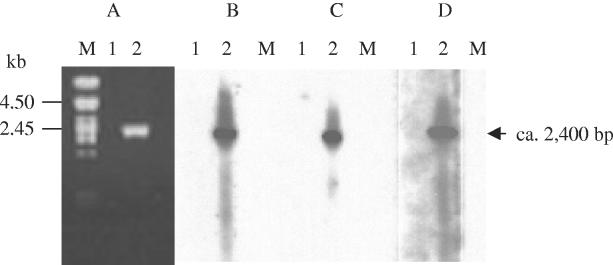
Analysis of the transcription of the vanE, vanXYE, and vanTE genes. Electrophoresis of the product obtained by RT-PCR with primers VDV and E15 (Fig. 1D and Table 2) (A) and corresponding Southern hybridizations with vanE (B), vanXYE (C), and vanTE (D) probes (Fig. 1C) are shown. Incubations were carried out in the absence (lanes 1) or presence (lanes 2) of reverse transcriptase. Lanes M, DNA from bacteriophage lambda digested by PstI as a marker.
FIG. 6.
Identification of the transcriptional start site for the vanE, vanXYE, vanTE, vanRE, and vanSE genes in BM4405 by primer extension analysis. (Left panel) Lane 1, primer elongation product obtained with oligodeoxynucleotide PE1 and 50 μg of total RNA from BM4405 (arrowhead); lanes T, G, C, and A, results of sequencing reactions performed with the same primer. Right panel, sequence from nucleotide positions −353 to +141 (numbering from the A of the ATG start codon of vanE, negative in the 3′-to-5′ direction and positive in the 5′-to-3′ direction). The +1 transcriptional start site for the vanE, vanXYE, vanTE, vanRE, and vanSE mRNA in BM4405 and the −35 and −10 promoter sequences located upstream are in boldface. The ATG start codon of vanE is indicated by an arrow, and the ribosome binding site (RBS) is in boldface and underlined.
In conclusion, the vanE operon comprises fives genes, with three of them being sufficient to confer vancomycin resistance whereas the last two encode a two-component system postulated to regulate expression of the operon. However, since VanSE appears not to be functional, inducibility of resistance by vancomycin is likely to be due to cross talk reactions with another two-component regulatory system of the host. Comparative analysis of the vanE operon indicated that VanE-type resistance in E. faecalis BM4405 was due to the presence of a chromosomal operon related to vanC. It has been demonstrated that transfer of vancomycin resistance among enterococci can be associated with the movement of large genetic elements from chromosome to chromosome (31). Our results suggest acquisition by E. faecalis of a cluster of genes from an intrinsically resistant species such as E. gallinarum or E. casseliflavus-flavescens. To find a clue as to the mechanism of acquisition of the resistance operon, we are determining the sequence of the flanking regions.
Acknowledgments
We thank T. Msadek and P. Reynolds for technical advice on RNA preparation and peptidoglycan precursor determination, respectively, and M. Chippaux, F. Depardieu, and I. Marchand for helpful discussions.
This work was supported in part by a Bristol-Myers Squibb Unrestricted Biomedical Research Grant in Infectious Diseases. L.A.P. was a recipient of a grant from the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICIT) of the Venezuelan government.
REFERENCES
- 1.Arias, C., P. Courvalin, and P. Reynolds. 1999. The vanC gene cluster of vancomycin-resistant Enterococcus gallinarum BM4174. Antimicrob. Agents Chemother. 44:1660-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias, C., M. Martín-Martinez, T. Blundell, M. Arthur, P. Courvalin, and P. Reynolds. 1999. Characterization and modelling of VanT: a novel membrane-bound serine racemase from vancomycin resistant Enterococcus gallinarum BM4174. Mol. Microbiol. 31:1653-1654. [DOI] [PubMed] [Google Scholar]
- 3.Arthur, M., F. Depardieu, G. Gerbaud, M. Galimand, R. Leclercq, and P. Courvalin. 1997. The VanS sensor negatively controls VanR-mediated transcriptional activation of glycopeptide resistance genes of Tn1546 and related elements in the absence of induction. J. Bacteriol. 179:97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur, M., C. Molinas, T. Bugg, G. Wright, C. Walsh, and P. Courvalin. 1992. Evidence for in vivo incorporation of d-lactate into peptidoglycan precursors of vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 36:867-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthur, M., C. Molinas, and P. Courvalin. 1992. Sequence of the vanY gene required for production of a vancomycin-inducible d,d-carboxypeptidase in Enterococcus faecium BM4147. Gene 120:11-114. [DOI] [PubMed] [Google Scholar]
- 6.Arthur, M., C. Molinas, and P. Courvalin. 1992. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 173:2582-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baptista, M., F. Depardieu, P. Reynolds, P. Courvalin, and M. Arthur. 1997. Mutations leading to increased levels of resistance to glycopeptide antibiotics in VanB-type enterococci. Mol. Microbiol. 25:93-105. [DOI] [PubMed] [Google Scholar]
- 8.Baptista, M., P. Rodriguez, F. Depardieu, P. Courvalin, and M. Arthur. 1999. Single-cell analysis of glycopeptide resistance gene phenotype in teicoplanine-resistant mutants of VanB-type Enterococcus faecalis. Mol. Microbiol. 32:17-28. [DOI] [PubMed] [Google Scholar]
- 9.Billot-Klein, D., L. Gutmann, S. Sable, E. Guittet, and J. van Heijenoort. 1994. Modification of peptidoglycan precursors is a common feature of the low-level vancomycin-resistance VANB-type Enterococcus sp. strain D366 and of the naturally glycopeptide-resistant species Lactobacillus casei, Pedicoccus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. J. Bacteriol. 176:2398-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadewall, B., P. Reynolds, and P. Courvalin. 2001. Regulation of expression of the vanD glycopeptide resistance gene cluster from Enterococcus faecium BM4339. J. Bacteriol. 183:3436-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeHaseth, P., M. Zupancic, and M. Record, Jr. 1998. RNA polymerase-promoter interactions: the comings and goings of RNA polymerase. J. Bacteriol. 180:3019-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutka-Malen, S., C. Molinas, M. Arthur, and P. Courvalin. 1992. Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a d-alanine:d-alanine ligase-related protein necessary for vancomycin resistance. Gene 112:53-58. [DOI] [PubMed] [Google Scholar]
- 13.Evers, S., B. Casadewall, M. Charles, S. Dutka-Malen, M. Galimand, and P. Courvalin. 1996. Evolution of structure and substrate specificity in d-alanine:d-alanine ligases and related enzymes. J. Mol. Evol. 42:706-712. [DOI] [PubMed] [Google Scholar]
- 14.Felsenstein, J. 1993. PHYLIP version 3.5c. University of Washington, Seattle.
- 15.Fines, M., B. Périchon, P. Reynolds, D. Sahm, and P. Courvalin. 1999. VanE, a new type of acquired glycopeptide resistance in Enterococcus faecalis BM4405. Antimicrob. Agents Chemother. 43:2161-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glatron, M. F., and G. Rapoport. 1972. Biosynthesis of the parasporal inclusion of Bacillus thuringiensis: half-life of its corresponding messenger RNA. Biochimie 54:1291-1301. [DOI] [PubMed] [Google Scholar]
- 17.Greisen, K., M. Loeffelholz, A. Purohit, and D. Leong. 1994. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J. Clin. Microbiol. 32:335-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haldimann, A., S. Fisher, L. Daniels, C. Walsh, and B. Wanner. 1997. Transcriptional regulation of the Enterococcus faecium BM4147 vancomycin resistance gene cluster by the VanS-VanR two-component regulatory system in Escherichia coli K-12. J. Bacteriol. 179:5903-5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob, A., and S. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclercq, R., E. Derlot, J. Duval, and P. Courvalin. 1988. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N. Engl. J. Med. 319:157-161. [DOI] [PubMed] [Google Scholar]
- 21.Liu, S., A. Hessel, and K. Sanderson. 1993. Genomic mapping with I-CeuI, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc. Natl. Acad. Sci. USA 90:6874-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnet, S., P. Courvalin, and T. Lambert. 1999. Activation of the cryptic aac(6′)-Iy aminoglycoside resistance gene of Salmonella by a chromosomal deletion generating a transcriptional fusion. J. Bacteriol. 181:6650-6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCafferty, D., I. Lessard, and C. Walsh. 1997. Mutational analysis of potential zinc-binding residues in the active site of the enterococcal d-Ala-d-Ala dipeptidase VanX. Biochemistry 36:10498-10505. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy, A., G. Victor, K. Ramotor, and B. Toye. 1994. Risk factors for acquiring ampicillin-resistant enterococci and clinical outcomes at a Canadian tertiary-care hospital. J. Clin. Microbiol. 32:2671-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKessar, S., A. Berry, J. Bell, J. Turnidge, and J. Paton. 2000. Genetic characterization of vanG, a novel vancomycin resistance locus of Enterococcus faecalis. Antimicrob. Agents Chemother. 44:3224-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messer, J., and P. Reynolds. 1992. Modified peptidoglycan precursors produced by glycopeptide-resistant enterococci. FEMS Microbiol. Lett. 94:195-200. [DOI] [PubMed] [Google Scholar]
- 27.Moran, C., N. Lang, S. LeGrice, G. Lee, M. Stephens, A. Sonenshein, J. Pero, and R. Losick. 1982. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol. Gen. Genet. 186:339-346. [DOI] [PubMed] [Google Scholar]
- 28.Msadek, T., F. Kunst, and G. Rapopport. 1993. Two-component regulatory systems, p. 729-745. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 29.Nölling, J., G. Breton, M. V. Omelchenko, K. S. Makarova, Q. Zeng, R. Gibson, H. M. Lee, J. Dubois, D. Qiu, J. Hitti, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkinson, J. S., and E. C. Kofoid. 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26:71-112. [DOI] [PubMed] [Google Scholar]
- 31.Quintiliani, J. R., and P. Courvalin. 1994. Conjugal transfer of the vancomycin resistance determinant vanB between enterococci involves the movement of large genetic elements from chromosome to chromosome. FEMS Microbiol. Lett. 119:359-364. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds, P. 1985. Inhibitors of bacterial cell wall synthesis. Symp. Soc. Gen. Microbiol. 38:13-40. [Google Scholar]
- 33.Reynolds, P., C. Arias, and P. Courvalin. 1999. Gene vanXYC encodes d,d-dipeptidase (VanX) and d,d-carboxypeptidase (VanY) activities in vancomycin-resistant Enterococcus gallinarum BM4174. Mol. Microbiol. 34:341-349. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds, P., H. Snaith, A. Maguire, S. Dutka-Malen, and P. Courvalin. 1994. Analysis of peptidoglycan precursors in vancomycin-resistant Enterococcus gallinarum BM4174. Biochem. J. 301:5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Sanger, F., S. Nicklen, and A. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva, J. A. Haldimann, M. Prahalad, C. Walsh, and W. Banner. 1998. In vivo characterization of the type A and B vancomycin-resistant enterococci (VRE) VanRS two-component systems in Escherichia coli: a nonpathogenic model for studying the VRE signal transduction pathways. Proc. Natl. Acad. Sci. USA 95:11951-11956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stock, J. B., A. J. Ninfa, and A. M. Stock. 1989. Protein phosphorylation and regulation of adaptative responses in bacteria. Microbiol. Rev. 53:450-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trieu-Cuot, P., C. Carlier, C. Poyart-Salmeron, and P. Courvalin. 1990. A pair of mobilizable shuttle vectors conferring resistance to spectinomycin for molecular cloning in Escherichia coli and Gram-positive bacteria. Nucleic Acids Res. 18:4296.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Caeseele, P., S. Giercke, J. Wylie, D. Boyd, M. Mulvey, S. Amin, and M. Ofner-Agostini. 2001. Identification of the first vancomycin-resistant Enterococcus faecalis harbouring vanE in Canada. Can. Commun. Dis. Rep. 27:101-104. [PubMed] [Google Scholar]
- 41.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259-268. [DOI] [PubMed] [Google Scholar]
- 42.Wright, G. D., T. R. Holman, and C. T. Walsh. 1993. Purification and characterization of VanR and the cytosolic domain of VanS: a two-component regulatory system required for vancomycin resistance in Enterococcus faecium BM4147. Biochemistry 32:5057-5063. [DOI] [PubMed] [Google Scholar]
- 43.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]