Abstract
AmpG was originally identified as a gene required for induction of β-lactamase. Subsequently, we found AmpG to be a permease required for recycling of murein tripeptide and uptake of anhydro-muropeptides. We have now studied the specificity of the AmpG permease. The principal requirement is for the presence of the disaccharide, N-acetylglucosaminyl-β-1,4-anhydro-N-acetylmuramic acid (GlcNAc-anhMurNAc). These unique substrates for AmpG, which contain murein peptides linked to GlcNAc-anhMurNAc, are produced by turnover of the cell wall during logarithmic growth. AmpG permease is sensitive to carbonylcyanide m-chlorophenylhydrazone, demonstrating that AmpG permease is a single-component permease and that transport is dependent on the proton motive force.
AmpG is a cytoplasmic membrane protein required for recycling of murein tripeptide as well as induction of Citrobacter freundii β-lactamase (4, 6, 7). AmpG has been presumed to be the permease for N-acetylglucosaminyl-β-1,4-anhydro-N-acetylmuramic acid (GlcNAc-anhMurNAc)-peptides, since anhydro-N-acetylmuramyl-l-alanyl-γ-d-glutamyl-meso-diaminopimelic acid (anhMurNAc-tripeptide) accumulates in the cytoplasm of ampD cells but not in ampG, ampD cells (4) and since the only β-N-acetylglucosaminidase in Escherichia coli is cytoplasmic (12, 13). GlcNAc-anhMurNAc-peptides are the products of breakdown of the murein sacculus of E. coli by multiple lytic transglycosylases (11). During active growth, well over half of the side wall of the sacculus is broken down each generation (1). To determine the specificity of the AmpG permease, a number of radioactive ligands were prepared from E. coli cells labeled with d-[6-3H(N)]glucosamine (except for three ligands labeled with 3H-diaminopimelic acid [3H-Dap] as noted). These ligands were used to compare uptake by freeze-thawed cells of E. coli ampG+ with those of ampG freeze-thawed cells.
MATERIALS AND METHODS
Bacterial strain, plasmids, and growth conditions.
The E. coli K-12 strains and plasmids used in this study are listed in Table 1. Cells were grown aerobically at 37°C in L broth, which is Luria-Bertani broth (10) modified to contain only 5 g of NaCl per liter. Ampicillin (100 μg/ml) and chloramphenicol (10 μg/ml) were used as required.
TABLE 1.
E. coli K-12 strains and plasmids used in this work
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| TP71 | F−lysA opp araD139 rpsL150 relA1 deoC1 ptsF25 ftbB5301 rbsR Δ(argF-lac) | 4 |
| TP72 | TP71 ampG::Kan | 4 |
| TP72/pGKS273-3 | This work | |
| TP73 | TP71 ΔampDE | 4 |
| TP74 | TP71 ΔampDE, ampG::Kan | 4 |
| TP74/pGKS273-3 | This work | |
| TP77B | TP71 nagZ::Cm nagB::Kan | 1 |
| TP78B | TP71 ΔampDE nagZ::Cm nagB::Kan | 1 |
| Plasmids | ||
| pGKS273-3 | ampG+ Cmr | 4 |
| pKM1 | pGem- carrying nagZ+ | 1 |
| pEtSlt70 | Plasmid expressing slt70, Apr | A. Dijkstra |
Preparation of freeze-thawed cells.
Cells from 40 ml of overnight culture were harvested, washed with 0.1 M phosphate buffer (pH 7.0) in the cold, and resuspended in 1.3 ml of the same buffer to give a suspension usually containing 3 to 5 mg of protein/ml. Stationary-phase cells were used because they were found to be more active than mid-log-phase cells. The cell suspension was adjusted to contain 1 mg of protein/ml. Two-hundred-microliter aliquots were rapidly frozen in a dry ice alcohol bath and were then thawed in a water bath and held at room temperature for 15 to 30 min. After this period, radioactive substrate was added and the incubation was continued for 50 min. An incubation time of 50 min at room temperature was chosen since significant uptake continued for that length of time. Thereafter, the sample was diluted with 1.4 ml of stop solution (0.1 M LiCl in 0.1 M KPO4 [pH 5.5]), filtered immediately through a 24-mm, 0.7-μm-pore-size fiberglass filter (Whatman International Ltd., Maidstone, England), and washed once with another 1.5 ml of stop solution. The dilution, filtration, and washing procedures were conducted in less than 30 s. The filter was removed immediately, dried, and counted.
HPLC analysis.
High-performance liquid chromatography (HPLC) was performed with Rainin Rabbit HP pumps and mixer equipment (Rainin Instrument Co., Woburn, Mass.) by two different methods (1). In method 1, the column used was a LiChrosphere RP-18 column (250 by 4.6 mm, 3-μm particle size; E. Merck). Isocratic elution with 50 mM sodium phosphate (pH 4.31) at a flow rate of 0.5 ml/min for 20 min was followed by a linear gradient of 0 to 35% 75 mM sodium phosphate (pH 4.95) in 15% methanol over 40 min and then by isocratic treatment for 60 min. The samples were desalted by method 2. In method 2, the sample was adjusted to a pH of ∼2.5 with trifluoroacetic acid, adsorbed on a 150- by 4.6-mm X-Terra RP-18 column (Waters Corp., Milford, Mass.) and was eluted at 0.5 ml/min with 0.05% trifluoroacetic acid for 35 min, followed by a gradient from 0.05% trifluoroacetic acid to 50% acetonitrile-containing 0.035% trifluoroacetic acid over a period of 15 min and then by isocratic treatment for 40 min.
Preparation of radioactive substrates.
In general, a strain of E. coli was labeled for about five generations during growth in L broth supplemented with 1 μCi of d-[6-3H(N)]glucosamine (21.6 Ci/mmol; NEN Life Science Products, Boston, Mass.)/ml. Some substrates were derived from well-washed sacculi recovered after the cells had been boiled in 4% sodium dodecyl sulfate for 30 min. Radioactive muropeptide monomers and dimers were obtained by digestion of murein sacculi with Chalaropsis muramidase. These muropeptides contained the native muramic acid present in murein. Muropeptide monomers and dimers containing anhydromuramic acid in the disaccharide (GlcNAc-anhMurNAc) were obtained by digestion of radiolabeled sacculi with a partially purified preparation of E. coli soluble lytic transglycosylase (1). Radioactive N-acetylglucosaminyl-β-1,4-anhydro-N-acetylmuramyl-l-alanyl-γ-d-glutamyl-meso-diaminopimelyl-d-alanine (GlcNAc-anhMurNAc-tetrapeptide) was then isolated from the digest by HPLC. The retention time was 85 min. Radioactive GlcNAc-anhMurNAc-tripeptide was isolated from hot-water extracts of E. coli TP78B labeled as described earlier (1). This compound accumulates in large amounts in TP78B, which lacks nagZ, the structural gene for β-N-acetylglucosaminidase, as well as the ampD anhMurNAc-l-alanine amidase (1). Radioactive N-acetylglucosaminyl-β-1,4-anhydro-N-acetylmuramyl-l-alanyl-γ-d-glutamyl-meso-diaminopimelyl-d-alanyl-d-alanine (GlcNAc-anhMurNAc-pentapeptide) was obtained from late-log-phase cells of E. coli TP78B treated with 0.02 μg (MIC/3) of imipenem/ml for 80 min. The washed cells were extracted with water at 95°C for 5 min. The extract was concentrated, and GlcNAc-anhMurNAc-pentapeptide was recovered by HPLC. The retention time was 100 min. The identities of GlcNAc-anhMurNAc-tetrapeptide and GlcNAc-anhMurNAc-pentapeptide were confirmed by mass spectrometry. Radioactive anhMurNAc-tripeptide labeled with either 3H-Dap or 3H-GlcNH2 was obtained by HPLC fractionation of hot-water extracts of TP73(ΔampDE) labeled as described above. Radioactive anhMurNAc and free murein tripeptide (l-alanyl-γ-d-glutamyl-meso-diaminopimelic acid) were isolated by HPLC following treatment of the anhMurNAc-tripeptide with AmpD amidase (3, 5) in 10 mM phosphate buffer (pH 7.0) as described earlier (9). The radioactive disaccharide GlcNAc-anhMurNAc was obtained as described earlier (1) from hot-water extracts of TP77B(nagZ::Cm).
Other methods.
The protein content of cell suspensions was determined by the Bradford Protein Assay (Bio-Rad, Hercules, Calif.) with bovine serum albumin as a standard. Transformations were performed as described elsewhere (8, 10). Mass spectrometry was performed at the Tufts Protein Chemistry Facility utilizing a PE Biosystems Voyager Maldi Mass Spectrometer. The concentration of GlcNAc-anhMurNAc-tripeptide was determined by an amino acid analysis performed with a Waters Picotag System at the Tufts Protein Chemistry Facility.
RESULTS
Specificity of the AmpG permease.
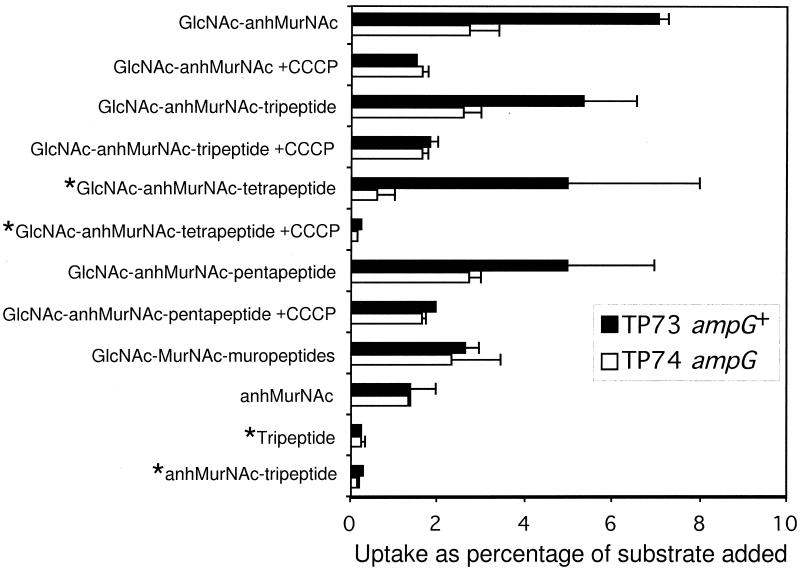
The data in Fig. 1 compare the uptake by strain TP73 with the amount taken up by the isogenic ampG strain TP74. The concentration of ligand used in these experiments was approximately 10 μM. This is well below an apparent Km of 100 μM as determined for GlcNAc-anhMurNAc-tripeptide under non-steady-state conditions. In terms of radioactivity, usually 5,000 to 20,000 cpm of ligand was added. In cells lacking AmpG, 1 to 3% of the added ligand was retained by the cells. As can be seen from examination of Fig. 1, at the low concentrations employed, AmpG permease only transports anhydro-muropeptides. The principal requirement for uptake is the presence of the disaccharide GlcNAc-anhMurNAc. Muropeptides lacking either GlcNAc or anhMurNAc are not transported. A parallel set of experiments comparing uptake by TP74/pGKS273-3(ampG+) with TP74 confirmed these results (data not shown). TP72(ampG::kan) also converted to active uptake upon introduction of pGKS273-3(ampG+). Although duplicate samples gave reproducible results, the uptake by individual batches of freeze-thawed cells was variable. This is reflected in the rather wide range of values shown in Fig. 1. Uptake of GlcNAc-anhMurNAc by intact whole cells of TP78(ΔampDE, nagZ) (3.5%) was much greater than by the isogenic strain TP78G(ΔampDE, nagZ, ampG) (0.4%). This compares favorably with the results from using freeze-thawed cells.
FIG. 1.
Uptake of various ligands by freeze-thawed cells of wild-type and ampG strains. *, labeled with 3H-Dap; all other ligands labeled with 3H-GlcNH2.
Figure 1 also shows that 20 μM carbonylcyanide m-chlorophenylhydrazone (CCCP) prevented the uptake of GlcNAc-anhMurNAc and GlcNAc-anhMurNAc-peptides.
DISCUSSION
The method for measuring uptake requires some explanation. We developed this method because of difficulty demonstrating measurable uptake by membrane vesicles. In contrast, each batch of freeze-thawed cells gave significant uptake though there was significant variation from batch to batch. Freeze-thawed cells have been used in the past to make cells permeable to substrates that normally cannot cross the cytoplasmic membrane. Since we consistently observed more uptake by freeze-thawed wild-type cells than by freeze-thawed ampG cells, its evident that some freeze-thawed cells must reseal their cytoplasmic membrane. It follows also that a fraction of the population resealed their cytoplasmic membrane but not their outer membrane, since several muropeptides studied here are too large to pass through an intact outer membrane. Although the amount was quite variable, from 1 to 3% of the added substrate appeared to be taken up by the ampG cells that presumably should be impermeable to the ligands. Because E. coli possesses only a cytoplasmic β-N-acetylglucosaminidase, we believe that this apparent uptake is the result of utilization of GlcNAc that is released from the muropeptides by that fraction of the freeze-thawed cells whose cytoplasmic membrane remained defective. Consistent with this interpretation, note that ampG cells retained a much lower percentage of 3H-Dap-labeled ligands than of 3H-GlcNH2-labeled ligands (Fig. 1).
Since we found that the permease was sensitive to 20 μM CCCP, this suggests that AmpG permease is a single-component permease dependent on the proton motive force.
Our results indicate that, for a muropeptide to be imported by the AmpG permease, it must contain the disaccharide GlcNAc-anhMurNAc. Muropeptides lacking either GlcNAc or anhMurNAc were not imported under the conditions employed here. For example, anhMurNAc-tripeptide and GlcNAc-MurNAc-muropeptides were not taken up via AmpG. Uptake was independent of the length of the peptide side chain (Fig. 1). Dietz et al., based on the observation that various anhydro-muropeptides accumulated in ampD cells, also concluded that peptide chain length was not critical (2). Interestingly, our results demonstrate that the disaccharide itself was readily transported and that the N-acetylmuramic acid moiety must be present in the 1,6-anhydro form. Thus, gram-negative bacteria have evolved to produce a permease exquisitely specific for high- molecular-weight degradation products from their own cell wall. Remarkably, these products are further degraded and efficiently recycled (1, 4).
Acknowledgments
This work was supported in part by Public Health Service grant GM51610 from the National Institute of General Medical Sciences.
We thank the Digestive Disease Center (NIDDK, P30 DK34928) for production of E. coli cells.
REFERENCES
- 1.Cheng, Q., H. Li, K. Merdek, and J. T. Park. 2000. Molecular characterization of the β-N-acetylglucosaminidase of Escherichia coli and its role in cell wall recycling. J. Bacteriol. 182:4836-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietz, H., D. Pfeifle, and B. Wiedemann. 1997. The signal molecule for β-lactamase induction in Enterobacter cloacae is the anhydromuramyl-pentapeptide. Antimicrob. Agents Chemother. 41:2113-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Höltje, J.-V., U. Kopp, A. Ursinus, and B. Wiedemann. 1994. The negative regulator of β-lactamase induction AmpD is a N-acetyl-anhydromuramyl-L-alanine amidase. FEMS Microbiol. Lett. 122:159-164. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs, C., L.-J. Huang, E. Bartowsky, S. Normark, and J. T. Park. 1994. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 13:4684-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs, C., B. Joris, M. Jamin, K. Klarsov, J. van Beemen, D. Mengin-Lecreulx, J. van Heijenoort, J. T. Park, S. Normark, and J.-M. Frere. 1995. AmpD, essential for both β-lactamase regulation and cell wall recycling, is a novel cytosolic N-acetylmuramyl-L-alanine amidase. Mol. Microbiol. 15:553-559. [DOI] [PubMed] [Google Scholar]
- 6.Korfmann, G., and C. C. Sanders. 1989. AmpG is essential for high-level expression of AmpC β-lactamase in Enterobacter cloacae. Antimicrob. Agents Chemother. 33:1946-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindquist, S., K. Weston-Hafer, H. Schmidt, C. Pul, G. Korfmann, J. Erickson, C. Sanders, H. H. Martin, and S. Normark. 1993. AmpG, a signal transducer in chromosomal β-lactamase induction. Mol. Microbiol. 9:703-715. [DOI] [PubMed] [Google Scholar]
- 8.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria, p. 268-274. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 9.Park, J. T., D. Raychaudhuri, H. Li, S. Normark, and D. Mengin-Lecreulx. 1998. MppA, a periplasmic binding protein essential for import of the bacterial cell wall peptide l-alanyl-γ-d-glutamyl-meso-diaminopimelate. J. Bacteriol. 180:1215-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 11.Shockman, G. D., and J.-V. Holtje. 1994. Microbial peptidoglycan (murein) hydrolases, p. 131-166. In J.-M. Ghuysen and R. Hakenbeck (ed.), Bacterial cell wall. Elsevier, Amsterdam, The Netherlands.
- 12.Yem, D. W., and H. C. Wu. 1976. Isolation of Escherichia coli K-12 mutants with altered levels of β-N-acetylglucosaminidase. J. Bacteriol. 125:372-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yem, D. W., and H. C. Wu. 1976. Purification and properties of β-N-acetylglucosaminidase from Escherichia coli. J. Bacteriol. 125:324-331. [DOI] [PMC free article] [PubMed] [Google Scholar]