Abstract
In Staphylococcus carnosus, the nreABC (for nitrogen regulation) genes were identified and shown to link the nitrate reductase operon (narGHJI) and the putative nitrate transporter gene narT. An nreABC deletion mutant, m1, was dramatically affected in nitrate and nitrite reduction and growth. Transcription of narT, narGHJI, and the nitrite reductase (nir) operon was severely reduced even when cells were cultivated anaerobically without nitrate or nitrite. nreABC transcripts were detected when cells were grown aerobically or anaerobically with or without nitrate or nitrite. NreA is a GAF domain-containing protein of unknown function. In vivo and in vitro studies showed that NreC is phosphorylated by NreB and that phospho-NreC specifically binds to a GC-rich palindromic sequence to enhance transcription initiation. This binding motif was found at the narGHJI, nir, and narT promoters but not at the moeB promoter. NreB is a cytosolic protein with four N-terminal cysteine residues. The second cysteine residue was shown to be important for NreB function. In vitro autophosphorylation of NreB was not affected by nitrate, nitrite, or molybdate. The nir promoter activity was iron dependent. The data provide evidence for a global regulatory system important for aerobic and anaerobic metabolism, with NreB and NreC forming a classical two-component system and NreB acting as a sensor protein with oxygen as the effector molecule.
Staphylococcus carnosus, traditionally used as a starter culture in the production of raw fermented sausages, reduces nitrate to ammonia in two steps: (i) nitrate is taken up and reduced by a dissimilatory nitrate reductase to nitrite, which is subsequently excreted, and (ii) after depletion of nitrate, the accumulated nitrite is imported and reduced by an NADH-dependent nitrite reductase to ammonia, which then accumulates in the medium. Nitrate reductase is a membrane-bound enzyme involved in energy conservation, whereas nitrite reductase is a cytosolic enzyme involved in NADH reoxidation. The absence of oxygen and the presence of nitrate and/or nitrite induce nitrate reductase and nitrite reductase activities. Nitrite reduction is inhibited by nitrate and by high concentrations of nitrite (≥10 mM), whereas nitrate reduction is not influenced by nitrite and ammonia (19).
Although the amino acid sequences of the S. carnosus nitrate reductase and nitrite reductase enzymes are similar to those of the corresponding Escherichia coli proteins, we have found evidence that the regulatory proteins and operator sequences differ (21, 24).
In E. coli, expression from the nir promoter (Pnir) is dependent both on FNR (fumarate and nitrate reductase regulation) and on NarL or NarP (36, 37). Recently, Browning et al. (7) have shown that Pnir is repressed by three DNA binding proteins, i.e., Fis (factor for inversion stimulation), integration host factor (IHF), and H-NS (histone-like nucleoid structuring protein), and that NarL and NarP can relieve IHF- and Fis-mediated repression but are unable to counteract H-NS-mediated repression.
FNR, NarL, and IHF are also required for transcription of the E. coli nitrate reductase operon narGHJI (11). The molybdate sensor ModE serves as a secondary transcriptional activator of the narXL operon and therefore indirectly influences transcription of the nitrate reductase operon, which encodes a molybdoenzyme (30).
While the NarX-NarL and NarQ-NarP two-component regulatory systems coordinate transcriptional responses to nitrate and nitrite availability, respectively (11, 14), the global regulator FNR controls gene transcription in response to anaerobiosis by acting as a sensor and a regulator. Under anoxic conditions, FNR specifically binds to target DNA sites as a dimer containing one [4Fe-4S]2+ cluster per monomer. The iron-sulfur cluster is coordinated by four cysteine residues. Upon exposure to air, FNR is inactivated; the [4Fe-4S]2+ cluster is disassembled, resulting in FNR monomers that do not bind target DNA (16, 38).
In the narGHJI promoter region of S. carnosus, a putative binding site for NarL, but not for FNR and IHF, has been identified (24). Studies to determine whether the E. coli proteins can regulate the S. carnosus narGHJI genes have indicated that the promoter is not functional in E. coli (24). Furthermore, in S. carnosus, no FNR homolog has been identified by Southern hybridization using the E. coli or the Bacillus subtilis fnr gene as a probe (G. Unden [Mainz, Germany], personal communication). Staphylococcus aureus also seems to lack an FNR homolog (35). Thus, a protein that senses oxygen per se in staphylococci still awaits identification.
In this study, we constructed an nreABC deletion mutant, m1, and showed that this mutant not only is severely impaired in nitrate and nitrite reduction but also exhibits a general defect in growth. Results are presented that identify NreB-NreC as a typical two-component regulatory system in which NreB represents the histidine sensor kinase and NreC represents the response regulator. We demonstrate that NreC specifically binds to GC-rich palindromic sequences at the promoters of the nir and narGHJI operons and of the narT gene, which encodes a putative nitrate transporter (12). Preliminary results point to oxygen being the effector molecule of NreB.
MATERIALS AND METHODS
Bacterial strains, growth conditions, plasmids, and oligonucleotides.
The bacterial strains and plasmids used in this work are listed in Table 1. E. coli strains were grown in Luria-Bertani medium (18) at 37°C. S. carnosus strains were grown in basic medium (BM) (13) or modified BM (mBM) (19) unless otherwise stated. Media were supplemented with ampicillin (100 μg ml−1), chloramphenicol (10 μg ml−1), or erythromycin (2.5 μg ml−1) as appropriate. Aerobic cultures were incubated on a rotary shaker at 160 rpm. Anaerobic cultures were incubated in screw-cap bottles with stirring at 100 rpm. mBM was supplemented with Oxyrase (20 ml/liter of medium; Oxyrase Inc., Mansfield, Ohio) to create anoxic conditions. The oligonucleotides used were obtained from MWG Biotech (Ebersberg, Germany). Primer sequences will be provided upon request.
TABLE 1.
S. carnosus and E. coli strains and plasmids used
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| S. carnosus | ||
| TM300 | Wild-type strain | 27 |
| SC2 | pPT2 | This study |
| SC3 | pBT2-HOM1 | This study |
| m1 | nreABC::ermB | This study |
| SC4 | pRB474 | This study |
| SC5 | pRB474nreABC | This study |
| SC6 | pRB474nreAB*C | This study |
| m2 | ΔnreABC::ermB ΔlacRH::nreABC | This study |
| m3 | ΔnreABC::ermB ΔlacRH::nreAB*C | This study |
| m4 | ΔlacRH | This study |
| SC7 | pCQE1 | This study |
| SC8 | pCQE1nreB | This study |
| SC9 | pPT1 | This study |
| SC10 | pPT1nreABC | This study |
| SC11 | pPT1nreAB*C | This study |
| SC12 | pPT1nir | This study |
| m5 | ΔlacR, PnirlacH | This study |
| E. coli | ||
| SURE | Stratagene | |
| SURE | pMAL-c2X | This study |
| EC2 | pMalNreB | This study |
| EC3 | pMalNreC | This study |
| Plasmids | ||
| pBT2 | Shuttle vector with temperature-sensitive replication origin for staphylococci | 8 |
| pEC2 | pUC18 derivative containing the ermB fragment of Tn551 | 8 |
| pBT2-HOM1 | pBT2 derivative with flanking regions of approximately 1.87 kb upstream and 0.75 kb downstream of nreABC and an ermB cassette replacing major parts of nreABC | This study |
| pRB474 | pRB374 derivative, shuttle vector carrying the B. subtilis vegII promoter, chloramphenicol resistance | 9 |
| pRB474nreABC | pRB474 derivative containing the nreABC genes under the control of the vegII promoter | This study |
| pRB474nreAB*C | pRB474nreABC derivative, point mutation in nreB* (NreB* with a C62S exchange) | This study |
| pCQE1 | pCX15 derivative, staphylococcal His tag expression vector with xylose-inducible and glucose-repressible xylA promoter | This study |
| pCQE1nreB | pCQE1 derivative carrying nreB on an HpaI/BglII fragment | This study |
| pMAL-c2X | MBPa expression vector for the production of MBP fusion proteins in E. coli | New England Biolabs |
| pMalNreB | pMAL-c2X derivative carrying nreB on an XmnI/BamHI fragment | This study |
| pMalNreC | pMAL-c2X derivative carrying nreC on an XmnI/BamHI fragment | This study |
| pPT2 | lac deletion plasmid | This study |
| pPT1 | Promoter probe plasmid | This study |
| pPT1nreABC | pPT1 derivative used for the insertion of nreABC into the lac locus | This study |
| pPT1nreAB*C | pPT1 derivative used for the insertion of nreAB*C into the lac locus | This study |
| pPT1nir | pPT1 derivative carrying the nir promoter on a KpnI/BamHI fragment | This study |
MBP, maltose binding protein.
Construction of the S. carnosus nreABC deletion mutant m1.
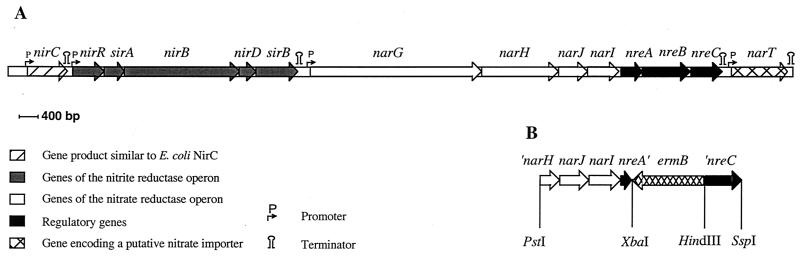
The nreABC genes were replaced by an erythromycin resistance cassette (ermB) on a 1.5-kb HindIII/XbaI fragment from pEC2 (8). Upstream and downstream regions of the genes to be replaced were obtained as follows. First, the 3.25-kb PstI fragment of pACYC-NaR101 (24) was subcloned into pUC18. The resultant plasmid, pUC-A4, was cleaved with PstI and XbaI to obtain the required 1.87-kb fragment (Fig. 1B). The 0.75-kb HindIII/SspI fragment encoding the 3′ end of nreC was isolated after successive digestion of plasmid pRBnitIII-4 (12) with HpaI/HindIII and SspI. These three fragments were combined with the PstI/SmaI-digested vector pBT2 to yield pBT2-HOM1. Allelic replacement of wild-type nreABC by nreA′ ermB ′nreC (truncated at the 3′ or 5′ end) was carried out as described by Brückner (8). The sequence of the altered area of the resulting strain, mutant m1, was confirmed by Southern blot and PCR analyses.
FIG. 1.
(A) Genetic map of the genes involved in nitrite and nitrate reduction in S. carnosus. The promoters of the nir (21) and nar (24) operons and of the narT gene were mapped by primer extension analysis; the promoter upstream of nirC is postulated. Putative transcription terminators are indicated. (B) Fragments that were ligated into shuttle vector pBT2 to obtain plasmid pBT2-HOM1, used for homologous recombination to construct mutant m1. Restriction sites indicated occur naturally in the depicted genes. ′, truncation at the 3′ or 5′ end.
Construction of nreABC and nreAB*C expression plasmids.
Primers P9 and P10 were used to amplify the nreABC genes. The PCR product was digested with SphI and BamHI and ligated into SphI- and BamHI-digested vector pRB474. In the resultant plasmid, pRB474nreABC, the nreABC genes were constitutively expressed from the vegII promoter of B. subtilis. For complementation studies, mutant m1 was transformed with plasmid pRB474nreABC.
Plasmid pRB474nreAB*C is identical to pRB474nreABC except for a point mutation introduced in nreB by using two PCR products generated with primers P9-P11 and P10-P12. After digestion with SphI-BglII and BglII-BamHI, respectively, the fragments were ligated into the SphI-BamHI-digested vector pRB474. In NreB*, cysteine residue C62 is replaced by serine.
Construction of the staphylococcal His tag expression vector pCQE1 and of pCQE1nreB.
Vector pCQE1 (3.96 kb) is a derivative of pCX15 (39) in which the Staphylococcus hyicus lipase gene is replaced by a His tag-coding sequence. HpaI, PvuI, BamHI, and BglII can be used for the construction of C-terminal His tag fusions. Expression is under the control of the xylA promoter (39), i.e., xylose inducible and glucose repressible. The ribosome binding site is optimized, and the codon usages of S. aureus and S. carnosus have been taken into account; the His tag is encoded by five CAT codons and one CAC codon in position 5. Additionally, the phage λ t0 terminator, which mediates only weak transcriptional termination in staphylococci (R. Brückner, personal communication), was replaced by the transcription terminator of the S. carnosus sceD gene (accession number U96108).
The nreB gene was amplified by PCR using primers P7 and P8. The BglII and HpaI recognition sites were used for cloning into pCQE1.
Construction of the pMalNreB and pMalNreC expression vectors.
nreB and nreC were amplified by using primers P16-P7 and P17-P10, respectively. Each of the obtained PCR products was cleaved with BamHI and ligated into the XmnI- and BamHI-digested vector pMAL-c2X. For expression of the MalE-NreB and MalE-NreC fusion proteins, the resulting plasmids, pMalNreB and pMalNreC, were each introduced into E. coli.
Construction of the lac deletion mutant m4.
To use the endogenous β-galactosidase gene lacH as a reporter gene, the chromosomal copy of the gene was inactivated by using the lac deletion plasmid pPT2. On this plasmid, the lac locus of S. carnosus has a deletion comprising the intergenic region (97 bp) between lacR and lacH, the first 100 bp of the coding region of lacR, and one-third of the 5′ end of lacH. Primers P24 and P2 were used to amplify the 3′-terminal portion of lacH. The resultant 1.8-kb fragment was digested with BamHI and SacI. A second fragment of 0.9 kb comprising the 3′ ends of lacP and lacR was amplified by using primers P3 and P4 and cleaved with XbaI and BamHI. These two fragments were introduced into the XbaI- and SacI-digested vector pBT2, yielding pPT2. After homologous recombination, truncation of the wild-type lacR and lacH genes in S. carnosus mutant m4 was confirmed by Southern blot and PCR analyses.
Construction of S. carnosus strain m5.
To analyze the effect of iron availability on Pnir activity, strain m5, in which the nir promoter is fused to lacH, was constructed. First, the promoter probe vector pPT1 was constructed in analogy to pPT2, except that pPT1 contains the entire lacH gene (2.8 kb), which was amplified by using primers P1 and P2. Nucleotides −195 to +10 of Pnir (21) were amplified by using primers P5 and P6 and inserted into the KpnI and BamHI sites of pPT1 upstream of the promoterless lacH gene. The resulting plasmid, pPTnir, was introduced into S. carnosus strain m4. Double-crossover replacement of the truncated lac locus with Pnir transcriptionally fused to lacH resulted in strain m5; the insertion was verified by sequencing.
Construction of S. carnosus strains m2 and m3.
Introduction of plasmid pRB474nreABC in strain m1 did not restore the wild-type phenotype. Therefore, a copy of the nreABC genes was integrated into the lac locus of strain m1. To analyze the role of the cysteine residues in NreB, nreAB*C was integrated into the lac locus of strain m1. The plasmids used for allelic gene replacement were constructed as follows. To obtain pPT1nreAB∗C, three different fragments were ligated into the KpnI- and SacI-digested vector pPT1: (i) a 2-kb fragment comprising the nreB* and nreC genes and the majority of nreA, obtained after cleavage of plasmid pRB474AB*C with XbaI and BamHI; (ii) a 0.6-kb fragment comprising the remainder of nreA, the 5′ end of nreA, and approximately 370 bp upstream of nreA as a potential promoter region, obtained via PCR using primers P13 and P14 and digestion with XbaI and KpnI; and (iii) a 1.1-kb fragment containing a 5′-truncated lacH gene (lacking the first 1,700 bp), amplified with primers P1 and P2, and cleaved with BamHI and SacI. For pPT1nreABC, pRB474nreABC instead of pRB474nreAB*C served as the template for the amplification of the 2-kb XbaI/BamHI fragment encoding nreBC and the majority of nreA, and the construction was otherwise the same as that described for pPT1nreAB*C.
DNA preparation, transformation, and molecular techniques.
Staphylococcal chromosomal DNA was isolated according to the method of Marmur (17). E. coli plasmid DNA was prepared with a QIAfilter plasmid midi kit 100 (Qiagen, Hilden, Germany) according to the manufacturer's protocol. S. carnosus plasmid DNA was prepared as described for E. coli, except that lysostaphin (Sigma) was added to the lysis buffer (final concentration, 12.5 μg/ml). S. carnosus was transformed by protoplast transformation (13) or by electroporation (3). Other molecular techniques followed established protocols (26).
PCR, DNA sequencing, and sequence analysis.
Vent polymerase (New England Biolabs, Schwalbach, Germany) was used for PCRs. Constructs with PCR-amplified fragments were checked by sequencing. Double-stranded plasmid and chromosomal DNAs were sequenced by using the dideoxy procedure, a Thermo Sequenase fluorescence-labeled primer cycle sequencing kit (Amersham), and a DNA sequencer from LI-COR Inc. (Lincoln, Nebr.). The programs Gapped BLAST and PSI-BLAST (1) were used for protein similarity searches. The programs SMART (28, 29) and TopPredII (version 1.3) (10) were used to predict protein structures.
RNA preparation, primer extension, and Northern blot analyses.
Total RNA from S. carnosus cells was isolated as described by Sizemore et al. (32) or by using the RNeasy midi kit (Qiagen). Primer extension experiments and Northern blot analyses were carried out as described earlier (21).
Protein purification.
S. carnosus m1(pCQE1nreB) was grown aerobically in BM without glucose. Cells were disrupted with glass beads in lysis buffer (50 mM potassium phosphate, 300 mM NaCl, 10% [wt/vol] glycerol, and 5 mM β-mercaptoethanol, pH 6.8) 1.5 h after induction with 0.5% xylose. Protein was further purified by using QIAexpressionist (Qiagen) as recommended by the manufacturer. Protein was eluted in lysis buffer containing 150 mM imidazole. Protein concentrations were determined by the method of Bradford (6).
NreB, NreC, and LacZα were purified from E. coli as MalE fusion proteins by using the pMal protein fusion and purification system as recommended by the manufacturer (New England Biolabs). Fusion proteins were eluted in buffer (20 mM Tris-HCl, 200 mM NaCl, 1 mM EDTA, pH 7.4) containing 10 mM maltose. Contaminating proteins were removed from the MalE-NreC preparation by anion-exchange chromatography (Resource Q; Pharmacia). The elution buffer contained 20 mM Tris-HCl and 364 mM NaCl, pH 8.
Determination of β-galactosidase activity in cell extracts.
The preparation of crude extracts and the assay of β-galactosidase activity have been described previously (4). Specific β-galactosidase activity was expressed as nanomoles of nitrophenol released minute−1 milligram of protein−1. Protein concentrations were determined by the method of Bradford (6).
Measurement of nitrate reductase and nitrite reductase activities.
Nitrate reductase and nitrite reductase activities were measured as described elsewhere (19). The nitrite concentration was determined colorimetrically (23, 31). The concentration of nitrate was determined by using the conversion of nitrate to nitrite catalyzed by nitrate reductase of Aspergillus sp. (Roche).
Phosphorylation assays.
For autophosphorylation, MalE-NreB or NreB-His was diluted in reaction buffer (50 mM HEPES, 50 mM KCl, 5 mM MgCl2, 0.5 mM EDTA, 2 mM dithiothreitol, pH 8) to a final concentration of 2.9 or 2.4 μM, respectively. The phosphorylation reaction at 24°C was initiated by addition of 0.22 μM [γ-32P]ATP (0.37 MBq; Amersham Pharmacia Biotech). Reactions were stopped by the addition of gel loading buffer (180 mM Tris-HCl, 30% [vol/vol] glycerol, 3% [vol/vol] β-mercaptoethanol, 3% [wt/vol] sodium dodecyl sulfate [SDS], and 0.03% [wt/vol] bromophenol blue, pH 6.8). Where indicated, NaNO3 (20 mM), NaNO2 (2 mM), NaMoO4 (0.5 mM), or ADP (250 μM) was added. It was verified that MalE-LacZα (2.7 μM) did not autophosphorylate in the presence of [γ-32P]ATP.
MalE-NreB autophosphorylation was started by the addition of [γ-32P]ATP. A 4-μl aliquot was sampled after 14.5 min of incubation at 24°C. After 15 min, the phosphoryl transfer reaction was initiated by the addition of MalE-NreC (final concentration, 11.6 μM). Samples (4 μl) were taken after 0.5, 1, 2, 4, 10, and 15 min of incubation. Reactions were stopped by the addition of gel loading buffer. Phosphorylated proteins were separated by SDS-polyacrylamide gel electrophoresis (10% polyacrylamide) and visualized using a phosphorimager screen (Fuji). As a control, MalE-LacZα (8.3 μM) was used instead of MalE-NreC.
Gel retardation assays.
Band shift assays with digoxigenin-labeled nir, nar, narT, and moeB promoter fragments (40 pg each) essentially followed the protocol of the DIG gel shift kit supplied by Roche Molecular Biochemicals. Competition experiments were carried out with unlabeled promoter fragments in excess (125-fold for narT, 25-fold for nir, and 300-fold for nar).
For amplification of the various promoter fragments, the following primers were used: P18 and P19 for the nir promoter (after digestion with KpnI and HindIII, a 217-bp fragment comprising nucleotides 986 to 1202 was obtained [accession no. AF029224]), P20 and P21 for the nar promoter (yielding a 208-bp fragment comprising nucleotides 6052 to 6259 [accession no. AF029224]), P22 and P23 for the narT promoter (a 181-bp fragment comprising nucleotides 14995 to 15175 was obtained [accession no. AF029224]), and P25 and P26 for the moeB promoter (yielding a 129-bp fragment comprising nucleotides 7319 to 7447 [accession no. AF029224]).
To delineate the NreC binding site, fragments of 18 to 32 bp were employed as competitive DNA. Each fragment was composed of a pair of single-stranded, complementary oligonucleotides at a concentration of 1.9 pmol per μl of reaction mixture.
In a total volume of 15 μl, MalE-NreC (90 ng for the narT or nir promoter, 100 ng for the moeB promoter, and 120 ng for the nar promoter) was incubated with the DNA of interest and unspecific or competitive DNA at room temperature for 15 min. Protein-DNA complexes were separated on a nondenaturing 7% polyacrylamide gel. After electroblotting onto a positively charged nylon membrane (Roche Molecular Biochemicals), the digoxigenin-labeled probes were detected by using an enzyme immunoassay with antidigoxigenin-alkaline phosphatase Fab fragments and the chemiluminescent substrate CDP-Star (Roche Molecular Biochemicals). The chemiluminescent signals were recorded by exposure to Lumi-Film (Roche Molecular Biochemicals) for 15 to 40 min.
Nucleotide sequence accession numbers.
The nucleotide sequences of the lac locus and the locus shown in Fig. 1A are available in GenBank under accession no. AY099473 and AF029224, respectively.
RESULTS
The nreABC genes.
We identified three open reading frames downstream of the narGHJI operon that link the narGHJI operon (24) and the narT gene (12). The open reading frames were designated nreABC (for nitrogen regulation) (Fig. 1A).
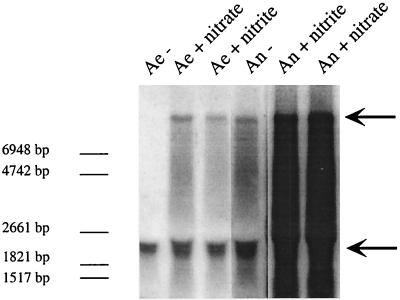
nreA starts 12 bp downstream of the last stop codon of narI. nreA and nreB overlap, and the start codon of nreC follows 17 bp downstream of nreB. The only transcription terminator sequence was identified downstream of nreC (Fig. 1A). Northern blot analyses with nreC as an antisense RNA probe (covering nucleotide positions 14428 to 14739 [accession no. AF029224]) revealed that transcripts of nreABC were present irrespective of whether cells were grown aerobically or anaerobically with or without nitrate or nitrite (Fig. 2). Furthermore, a transcript comprising narGHJI and nreABC was found in cells grown aerobically with nitrate or nitrite, grown anaerobically, or grown anaerobically with nitrate or nitrite (Fig. 2). This became evident because these large transcripts also hybridized when narI (covering nucleotide positions 12189 to 12718 [accession no. AF029224]) was used as an antisense RNA probe (data not shown). The different amounts of the narGHJI-nreABC transcription unit (theoretically 8.8 kb) under the various conditions reflected the regulation of the narGHJI operon promoter in response to oxygen, nitrate, and nitrite (24). These results suggest that the nreABC operon is both cotranscribed with narGHJI and transcribed via its own promoter.
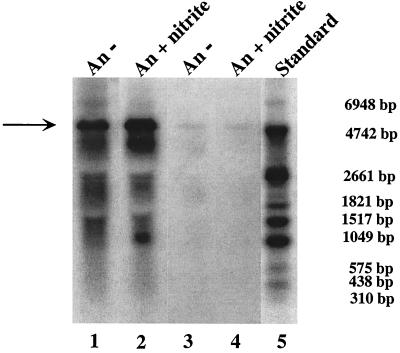
FIG. 2.
Northern blot analyses of the nreABC operon. Total RNA (15 μg) isolated from wild-type cells grown aerobically (Ae) or anaerobically (An) with (+) or without (−) nitrate (20 mM) or nitrite (2 mM), separated by gel electrophoresis, was transferred to a positively charged nylon membrane and hybridized with a digoxigenin-labeled antisense RNA fragment comprising the nreC gene. For chemiluminescent detection, CSPD (Roche) was used. The full-length transcript of the nreABC operon (approximately 2.2 kb) and the narGHJI-nreABC transcription unit (approximately 8.8 kb) are indicated by arrows. As a standard, 90 ng of digoxigenin-labeled RNA molecular weight marker I (Roche Molecular Biochemicals) was used. The lengths of the standard RNAs are shown in the left margin.
NreA (154 amino acids, 17.3 kDa) showed no significant similarity to any protein of known function, but a GAF domain (SMART accession number SM0065) was identified. In addition, predictions of secondary structure revealed a putative membrane-spanning segment (amino acids 92 to 112), but NreA is clearly not an integral membrane protein.
NreB (347 amino acids, 40.0 kDa) and NreC (217 amino acids, 24.4 kDa) have many features in common with the superfamily of bacterial sensor-transmitter response regulators. NreB possesses a histidine kinase domain with conserved histidine residue (H159) that serves as the phosphoryl acceptor. NreC has a conserved aspartate residue (D53) that serves as a phosphoryl acceptor in the receiver domain and a helix-turn-helix motif of the LuxR family in the transmitter domain, identified with the aid of the PROSITE database (European Molecular Biology Laboratory outstation Hinxton, Cambridge, United Kingdom). NreB is probably a cytoplasmic protein, since no transmembrane regions were predicted by computer analysis (TopPredII). The first 120 N-terminal amino acids show no similarity to any other protein in the database; this region contains four cysteine residues (Cys-X2-Cys-X11-Cys-X2-Cys), with spacing different from that of regions that serve as ligands for conventional ferredoxin-type [4Fe-4S] clusters (Cys-X2-Cys-X2-Cys-X3-Cys) (38).
Phenotypic characterization of the nreABC deletion mutant.
To verify our hypothesis that the nreABC genes are involved in the regulation of nitrate and nitrite reduction, mutant strain m1, in which the nreABC genes were replaced by an erythromycin resistance cassette, ermB, was constructed. During exponential growth, the wild type and mutant had significantly different generation times under various growth conditions; in all cases, mutant m1 had a generation time longer than that of the wild type (Table 2). In both strains, neither nitrate nor nitrite exerted a stimulating effect on aerobic growth. In anaerobically grown wild-type cells, nitrate and nitrite decreased the doubling time by 16 and 9 min, respectively; this stimulating effect was less pronounced in the mutant (Table 2).
TABLE 2.
Doubling time of S. carnosus TM300 and S. carnosus m1 grown aerobically or anaerobically in mBM in the presence or absence of nitrate (1 mM) or nitrite (0.5 mM)
| Growth | Doubling time (min)a
|
|
|---|---|---|
| S. carnosus TM300 | S. carnosus m1 | |
| Aerobic | 44 | 52 |
| Aerobic + nitrate | 44 | 52 |
| Aerobic + nitrite | 44 | 52 |
| Anaerobic | 64 | 74 |
| Anaerobic + nitrate | 48 | 68 |
| Anaerobic + nitrite | 55 | 68 |
Mean values from three independent cultures are presented. The standard deviations did not exceed 4%.
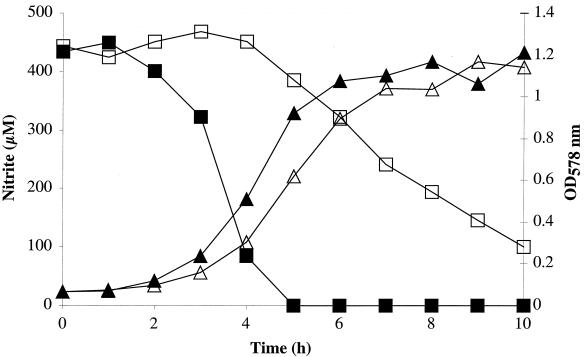
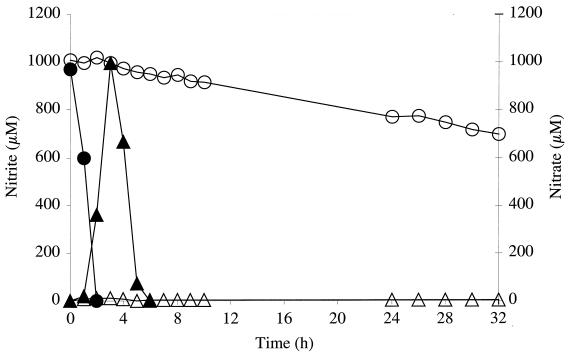
Since no nitrate reductase or nitrite reductase activities were detectable in mutant m1 cells (clearly under the detection limit), we decided to investigate nitrate and nitrite reduction by monitoring nitrate and nitrite concentrations in the growth medium. First, nitrite reduction by the wild type and mutant m1 during anaerobic growth in mBM supplemented with nitrite (500 μM) was analyzed. The wild type reduced all of the nitrite in approximately 5 h, whereas the mutant required approximately 10 h to reduce 400 μM nitrite (Fig. 3). When wild-type cells were grown anaerobically in the presence of 1 mM nitrate, the typical two-step mechanism was observed (Fig. 4). First, nitrate was reduced to nitrite, and then the excreted nitrite was taken up and reduced to ammonia. Nitrite accumulation in the medium by mutant m1 was marginal; the maximum of 13 μM was measured after 3 h of growth. After 32 h of anaerobic growth in presence of nitrate, however, the nitrate concentration was reduced from 1,000 to 700 μM (Fig. 4). The phenotype of mutant m1 supports the idea that the nreABC operon is involved in mediating the presence of nitrate, nitrite, and/or oxygen.
FIG. 3.
Nitrite reduction by S. carnosus (closed symbols) and mutant m1 (open symbols) during anaerobic growth in mBM with 500 μM nitrite. During growth, the optical density at 578 nm (OD578 nm) (triangles) and nitrite reduction (monitored as the decrease in nitrite in the growth medium) (squares) were measured.
FIG. 4.
Nitrate reduction (monitored as the decrease in nitrate in the growth medium) (squares) and nitrite accumulation in the growth medium (triangles) by wild-type S. carnosus (closed symbols) and mutant m1 (open symbols) during anaerobic growth in mBM with 1 mM nitrate.
Complementation studies.
To prove that the deletion of the nreABC genes is responsible for the observed impact on growth of and nitrate and nitrite reduction by mutant m1, complementation studies were carried out. Mutant m1 was transformed with plasmid pRB474nreABC, from which the nreABC genes are constitutively expressed from the vegII promoter. The wild-type phenotype was only marginally restored (data not shown). Apparently the constitutive expression of regulatory genes in trans from a multicopy plasmid resulted in a somewhat deregulated system. This is not uncommon and has also been observed with the NarXL two-component regulatory system in E. coli (15). We therefore inserted the nreABC genes with a potential promoter region into the lac locus on the chromosome to circumvent the gene dosage effect. The resultant strain, m2, exhibited the wild-type phenotype regarding growth and nitrate and nitrite reduction (data not shown); this also indicated the presence of a promoter.
Effect of C62S substitution in NreB.
The importance of the cysteine residues of NreB for protein function was tested with strain m3, in which the nre genes were replaced by ermB (as in strain m1) and the nreAB*C genes were introduced in the lac locus under the control of their own promoter. The nreB* gene carries a point mutation in the codon of the second cysteine of NreB (C62S). Strain m3 showed the same phenotype as mutant m1 regarding growth and nitrate and nitrite reduction (data not shown), thereby emphasizing the importance of C62 in NreB.
Phosphorylation of NreB and NreC.
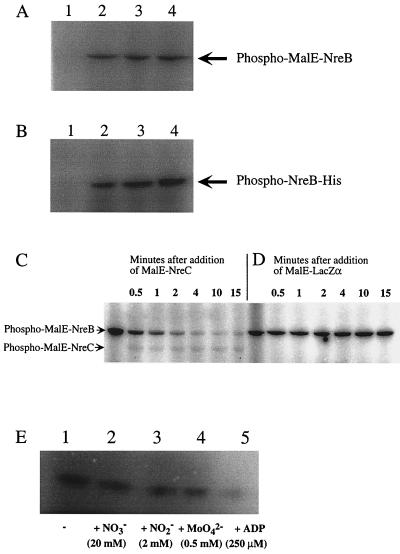
Phosphorylation assays were used to determine whether NreB and NreC form a typical two-component system. NreB was purified as a MalE fusion protein and as a His tag fusion protein. The His tag was fused to the C-terminal end of NreB to avoid affecting the four cysteine residues at the N terminus. Since a His tag-NreC fusion protein was insoluble, NreC was purified as a MalE fusion protein only. Unfortunately, neither MalE-NreB nor MalE-NreC could be cleaved with factor Xa; therefore, the fusion proteins were used in the assays, and MalE-LacZα was used as a control. Since MalE-NreC was not autophosphorylated in the presence of [γ-32P]ATP (data not shown), there was no need to remove [γ-32P]ATP when analyzing whether phospho-NreB is a substrate of NreC. NreB was autophosphorylated when incubated with [γ-32P]ATP (Fig. 5A and B), and the radioactively labeled phosphoryl group was transferred to NreC (Fig. 5C and D), although phospho-MalE-NreC was quite unstable under the conditions used.
FIG. 5.
Phosphorylation of NreB and NreC. (A and B) MalE-NreB (2.9 μM, 82.5 kDa) (A) and NreB-His (2.4 μM, 41 kDa) (B) were incubated with 0.22 μM [γ-32P]ATP for 15 min (lanes 2), 30 min (lanes 3), and 45 min (lanes 4) in reaction buffer at 24°C. As a control, protein was omitted (lanes 1). (C and D) Prior to the addition of MalE-NreC (11.6 μM, 67 kDa), MalE-NreB (2.9 μM) was incubated with 20 μCi of [γ-32P]ATP in reaction buffer at 24°C for 14.5 min (first lanes). Samples (4 μl) were taken 0.5, 1, 2, 4, 10, and 15 min after addition of MalE-NreC (C) or MalE-LacZα (8.3 μM, 51 kDa) (D). (E) NreB-His (2.4 μM) was incubated in the presence of 10 μCi of [γ-32P]ATP without additions (lane 1), or with 20 mM nitrate (lane 2), 2 mM nitrite (lane 3), 0.5 mM molybdate (lane 4), or 250 μM ADP (lane 5) in reaction buffer (15-μl reaction mixture) at 24°C for 15 min. All reactions were stopped by adding gel loading buffer. Phosphorylated proteins were separated by SDS-polyacrylamide gel electrophoresis (10% polyacrylamide) and visualized using a phosphorimager screen.
Effect of nitrate, nitrite, molybdate, and ADP on NreB autophosphorylation.
The effect of nitrate and nitrite on NreB autophosphorylation was tested by adding each anion separately to the reaction mixture at a final concentration of 2 and 20 mM, respectively. No stimulating effect was detected in any case (Fig. 5E). Whether molybdate (0.5 mM), an essential trace element for the molybdenum cofactor of the nitrate reductase (22), influences autophosphorylation of NreB was also tested. No effect was observed (Fig. 5E); however, the addition of ADP (250 μM) had an inhibitory affect on autophosphorylation of NreB (Fig. 5E), as expected for a kinase reaction.
NreC is a sequence-specific DNA binding protein.
The amino acid sequence of NreC as well as the phenotype of mutant m1 suggested that NreC acts as an activator of transcription of genes involved in nitrate and nitrite reduction, including narT, which encodes a putative transport protein required for nitrate uptake under anoxic conditions (12). Thus, we predicted that the promoters of the nir and narGHJI operons and of narT are targets of NreC. Regulation of transcription initiation at the nir (21) and narGHJI (24) promoters has already been analyzed. Primer extension experiments (Fig. 6) revealed that anaerobiosis and nitrate induce narT transcription. Nitrite also led to induction, but only in combination with oxygen deprivation. Anaerobiosis plus nitrate, however, resulted in the highest level of initiation of transcription. No transcription initiation of narT was detected with RNA isolated from cells grown aerobically without nitrate or nitrite.
FIG. 6.
Transcriptional regulation of the narT gene. The autoradiograph shows the reverse transcripts obtained with 0.5 pmol of IRD800-labeled narT-specific primer and RNA isolated from wild-type (20 μg) and mutant m1 (40 μg) cells grown aerobically (Ae) or anaerobically (An) in the presence or absence of nitrate (20 mM) or nitrite (2 mM). Lanes A, C, G, and T contained the respective sequencing reaction mixture.
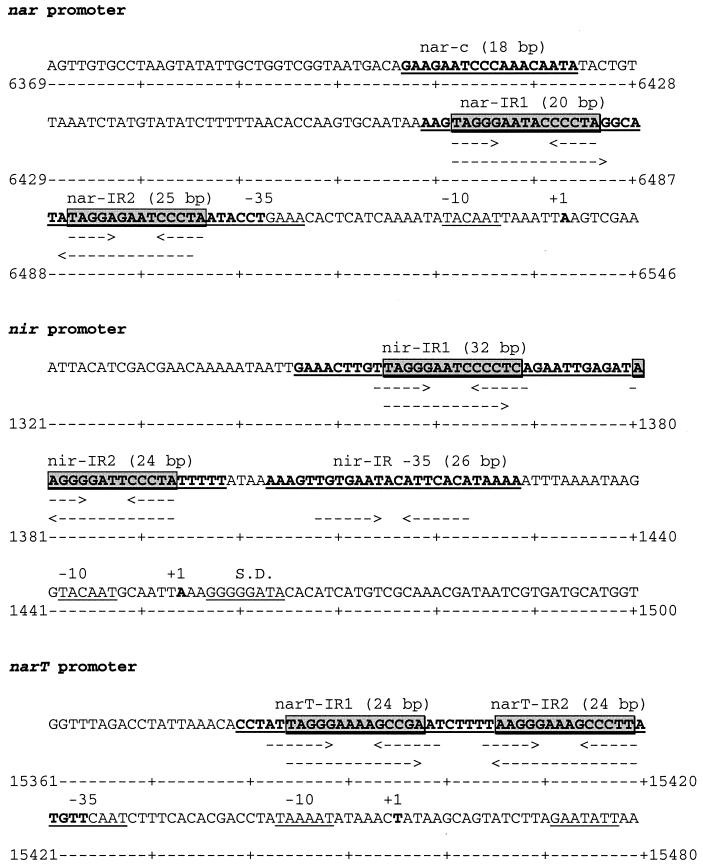
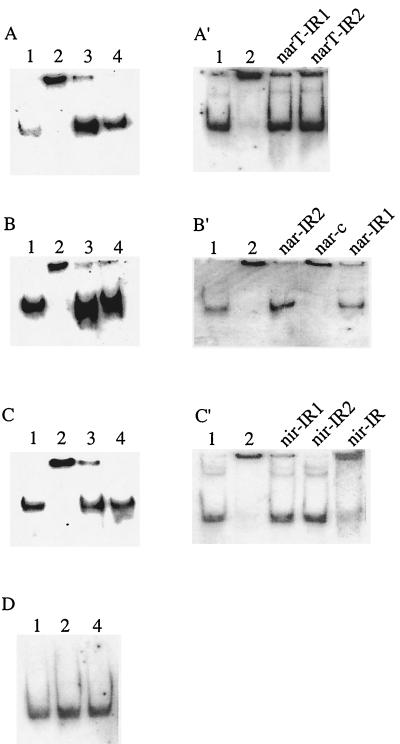
Subsequently, inspection of the nir, narGHJI, and narT promoter sequences revealed that each contains two GC-rich palindromes (Fig. 7), and a consensus sequence (TAGGGN4CCCTA) was predicted. Gel mobility shift assays were used to determine whether NreC binds to these sites. Large DNA fragments comprising the nir (217 bp), nar (208 bp), or narT (181 bp) promoter were first tested. As shown in Fig. 8A to C′ (lanes 2), NreC retarded the mobility of all of these fragments. The specificity of the mobility shift was demonstrated by using unlabeled DNA fragments (lanes 3). As a control, the promoter fragments were incubated with MalE-LacZα to show that the MalE portion of the MalE-NreC fusion protein did not bind the promoter fragments (lanes 4).
FIG. 7.
Nucleotide sequence of the narGHJI, nir, and narT promoter regions. The transcriptional start sites (+1) were determined by primer extension analysis. The predicted −10 and −35 regions are underlined. Arrows indicate inverted repeats, and shaded boxes mark NreC recognition sites. The sequences of the oligonucleotides used for the band shift assays are underlined and shown in boldface. The lengths of oligonucleotides are indicated. Numbering refers to the sequence of the complete nir-nar locus available from GenBank under accession no. AF029224.
FIG. 8.
Gel mobility shift assays with purified MalE-NreC and digoxigenin-labeled DNA fragments comprising the narT (A and A′), narGHJI (B and B′), nir (C and C′), and moeB (D) promoters. Lanes: 1, promoter fragment; 2, promoter fragment plus MalE-NreC; 3, promoter fragment plus MalE-NreC plus unlabeled promoter fragment; 4, promoter fragment plus MalE-LacZα. Unlabeled oligonucleotides (A′, narT-IR1 and narT-IR2 of the narT promoter; B′, nar-IR2, nar-c, and nar-IR1 of the narGHJI promoter; and C′, nir-IR1, nir-IR2, and nir-IR of the nir promoter [locations are shown in Fig. 7]) were employed as competitive DNA. (For details, see Materials and Methods.)
Unlabeled oligonucleotides (18 to 32 bp in length) (Fig. 7) were then used as competitive DNAs in the assays to verify the GC-rich palindromic sequence as the NreC binding site. All of these short DNA fragments containing the putative NreC binding motif (narIR-1, narIR-2, nir-IR1, nir-IR2, narT-IR1, and narT-IR2, each annealed to their respective complementary sequence) competed for NreC binding (Fig. 8A′, B′, and C′). Oligonucleotides with an unrelated sequence (nar-c) or containing an inverted-repeat-like structure that did not include the deduced binding motif (oligonucleotide nirIR-35 of the nir promoter annealed to its complementary sequence) did not compete for NreC binding (Fig. 8B′ and C′). Thus, the results clearly showed that NreC specifically binds to the deduced consensus sequence found in the nir, nar, and narT promoters.
Recently, we showed that transcription of the moeB gene, which is part of the molybdenum cofactor biosynthesis gene cluster in S. carnosus, is also enhanced by anaerobiosis and nitrate (20). The moeB promoter region, however, does not contain a conspicuous NreC binding motif, and no band shift was obtained by using the same conditions applied to the other promoter fragments (Fig. 8D).
In order to find further indications that NreC enhances transcription initiation of the narT gene and of the nir and narGHJI operons, RNA was isolated from the wild type and the nreABC deletion mutant m1 grown aerobically or anaerobically with or without nitrate or nitrite. Even though twice as much RNA from the mutant as from the wild type was used, no or only very faint signals were visible (Fig. 6 and data not shown). This indicates that transcription of these genes in the mutant was severely reduced compared to that in the wild type.
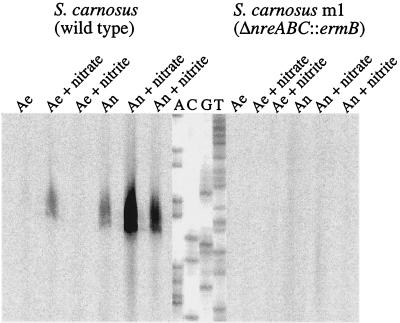
Northern blot analyses (Fig. 9) using RNA isolated from wild-type (15 μg) and mutant m1 (30 μg) cells grown anaerobically in the presence or absence of nitrite (2 mM) and using a nir operon RNA probe (covering nucleotide positions 1801 to 2675 [accession no. AF029224]) showed that the amount of the approximately 5-kb nir transcript was significantly smaller in mutant m1 grown with or without nitrite (Fig. 9). The stimulatory effect of nitrite on nir operon transcription during anaerobic growth of the wild type has been described recently (21) and was reproduced here. The results showed that at least one of the NreABC proteins, possibly NreB, senses the absence of oxygen, directly or indirectly.
FIG. 9.
Northern blot analyses of the nir operon. Total RNA isolated from wild-type (lanes 1 and 2) and mutant m1 (lanes 3 and 4) (30 μg each) cells grown anaerobically (An) with (+ nitrite) or without (−) 2 mM nitrite, separated by gel electrophoresis, was transferred to a positively charged nylon membrane and probed with a digoxigenin-labeled antisense RNA fragment comprising the entire sirA gene, approximately 300 bp of the 5′ end of nirB, and approximately 100 bp of the 3′ end of nirR. For chemiluminescent detection, CSPD (Roche) was used. The full-length transcript of the nir operon (approximately 5 kb) is indicated by an arrow. As a standard (lane 5), 90 ng of digoxigenin-labeled RNA molecular weight marker I (Roche Molecular Biochemicals) was used.
Transcription from Pnir depends on iron availability.
In many redox-sensing processes the activity of the corresponding proteins depends on iron availability (5). Since nitrate reductase and nitrite reductase contain FeS centers, their activities in the presence of an iron chelator could not be measured directly. To measure NreB activity indirectly, strain m5, in which Pnir was fused to the lacH gene on the chromosome, was constructed. First, we verified that the wild type and mutant m1 exhibited the same β-galactosidase activities when cultivated with or without the iron chelator dipyridyl (data not shown), indicating that the system is uninfluenced by NreABC and dipyridyl. Strain m5 cells grown anaerobically in the presence of nitrite (2 mM) exhibited β-galactosidase activity (16,800 nmol · min−1 · mg−1). However, when dipyridyl (final concentration of 2 or 0.2 mM) was added, no activity was detected. This illustrates that transcription from Pnir depends on iron availability and is consistent with the idea that iron in the form of an FeS cluster might be the redox-sensitive compound liganded by the four cysteine residues of the NreB protein.
DISCUSSION
In S. carnosus, the nreABC genes were identified directly downstream of the narGHJI operon. In this study we showed that the nreABC deletion mutant m1 was severely impaired in nitrate and nitrite reduction due to the reduced transcription of narT and of the nir and narGHJI operons. In addition, however, this mutant had a growth defect irrespective of the presence of nitrate or nitrite. This indicates that the nreABC genes are not related exclusively to the nitrate- and nitrite-reducing system. Apparently, at least one of the genes is important for aerobic and anaerobic metabolism. Since transcription of narT and of the nir and narGHJI operons was also reduced when mutant m1 was cultivated anaerobically without nitrate or nitrite, we conclude that a system that mediates the absence of oxygen at the transcriptional level was affected.
NreB and NreC are the first proteins characterized that regulate the nitrate- and nitrite-reducing system of S. carnosus. Phosphorylation assays demonstrated that NreB and NreC form a two-component system. Sequence similarities to related two-component systems point to autophosphorylation of NreB on H159 and to D53 of NreC serving as the phosphoryl group acceptor. The loss of radiolabel during phosphoryl group transfer (Fig. 5C) might hint that NreB is not only a kinase but also a phosphatase or that NreC also has autophosphatase activity. Both phenomena have been described for other two-component systems (33).
A GC-rich palindromic sequence (that was conspicuous in an AT-rich organism) was identified as the NreC binding sequence. This motif is present twice in the narGHJI, nir, and narT promoters. Interestingly, in the narGHJI promoter and the narT promoter, the NreC recognition sites are located at similar positions (near the −35 region and around −60 to −70). In the nir promoter, the motif close to −35 is missing; instead, the motif is found further downstream around −90. Moreover, a palindromic sequence including the −35 region of the nir promoter that differs from the narGHJI and narT promoter sequences gives rise to the speculation that another protein is additionally involved in the expression of the nir operon. Irrespective of this, it is very unlikely that NreC is the only protein involved in transcriptional regulation of the nitrate- and nitrite-reducing system in S. carnosus.
The function of NreA is unknown. Protein structure predictions suggest that NreA is not an integral membrane protein but that it contains one possible membrane-spanning segment. Another feature of NreA is the presence of a GAF domain, which occurs in phytochromes, in cyclic GMP-specific phosphodiesterases, and in NifA, a transcriptional activator of σ54-dependent promoters (34). Since it is assumed that GAF domains regulate catalytic activities allosterically via the binding of a ligand such as nucleotides and small molecules (2), one can imagine NreA as the nitrate and nitrite sensor. GAF domains also have regulatory roles in redox transduction pathways (2); however, a mutant defective only in nreA is needed for a functional analysis of NreA.
The FNR protein is a global transcription regulator that controls genes in response to anaerobiosis. Oxygen is sensed by oxygen-labile [4Fe-4S] clusters that promote dimerization, DNA binding, and productive interaction with RNA polymerase (16, 38). S. aureus (35) and S. carnosus (G. Unden, personal communication) seem to lack an FNR homolog. Thus, a protein in staphylococci that senses oxygen has awaited identification. The results presented here are consistent with NreB being an oxygen-sensing protein in staphylococci and with oxygen availability being transduced via NreBC: (i) homologs were found in other staphylococcal species for which sequences are available (data not shown); (ii) like FNR, NreB has four cysteine residues at the N terminus, and the importance of one of the four residues for the function of NreB was shown by mutation; (iii) nir promoter activity is iron dependent, in keeping with the hypothesis that NreB senses oxygen via a redox reaction at an Fe cofactor (however, other modes of action cannot be excluded); (iv) NreB, which lacks a membrane-spanning segment, is apparently a cytosolic protein, and oxygen, which can be supplied to cell layers for aerobic respiration simply by diffusion (25), is probably the direct effector molecule; (v) NreB and NreC form a classical two-component system, and promoters that were identified as targets of NreC in vitro had reduced transcription levels in vivo in the nreABC deletion mutant m1 irrespective of the presence of nitrate or nitrite; (vi) mutant m1 showed a general defect in growth and was affected not only in nitrate and nitrite reduction; (vii) autophosphorylation of NreB was not affected by nitrate, nitrite, or molybdate in vitro, and although one cannot draw conclusions about the in vivo situation, this finding does not conflict with oxygen being the effector of NreB; and (viii) nreABC transcripts (and most likely the gene products) are present during aerobic and anaerobic growth, a requirement expected for an oxygen-sensing system.
PAS domains have received widespread attention as signaling modules that perceive diverse stimuli, such as molecular oxygen, light, and redox potential. They are found predominantly in signal transduction pathways. Most PAS domains in prokaryotes are in histidine kinase sensor proteins (34). No conspicuous PAS domain (determined using the PSI-BLAST program) was found in NreB, which suggests a PAS domain-independent oxygen-sensing mechanism. Further research is needed to elucidate the mode of action of NreB, including the signals received and their modulation.
Acknowledgments
We are indebted to Reinhold Brückner, University of Kaiserslautern, for providing plasmids pRB474, pBT2, and pEC2 and for many fruitful discussions. The technical assistance of Vera Augsburger and Detlinde Futter-Bryniok is gratefully acknowledged. We thank Ralf Rosenstein for help with computer analysis, Michael Otto for constructive help with the KTA purifier, and Karen A. Brune and Sarah E. Cramton for critically reading the manuscript.
This study was supported by grants (GO371/4-2) from the DFG (Deutsche Forschungsgemeinschaft).
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravind, L., and C. P. Ponting. 1997. The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem. Sci. 22:458-459. [DOI] [PubMed] [Google Scholar]
- 3.Augustin, J., and F. Götz. 1990. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol. Lett. 66:203-208. [DOI] [PubMed] [Google Scholar]
- 4.Bassias, J., and R. Brückner. 1998. Regulation of the lactose utilization genes in Staphylococcus xylosus. J. Bacteriol. 180:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer, C. E., S. Elsen, and T. H. Bird. 1999. Mechanisms for redox control of gene expression. Annu. Rev. Microbiol. 53:495-523. [DOI] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Browning, D. F., J. A. Cole, and S. J. Busby. 2000. Suppression of FNR-dependent transcription activation at the Escherichia coli nir promoter by Fis, IHF and H-NS: modulation of transcription initiation by a complex nucleo-protein assembly. Mol. Microbiol. 37:1258-1269. [DOI] [PubMed] [Google Scholar]
- 8.Brückner, R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 151:1-8. [DOI] [PubMed] [Google Scholar]
- 9.Brückner, R. 1992. A series of shuttle vectors for Bacillus subtilis and Escherichia coli. Gene 122:187-192. [DOI] [PubMed] [Google Scholar]
- 10.Claros, M. G., and G. von Heijne. 1994. TopPredII: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10:685-686. [DOI] [PubMed] [Google Scholar]
- 11.Darwin, A. J., and V. Stewart. 1996. The NAR modulon systems: nitrate and nitrite regulation of anaerobic gene expression, p. 343-359. In E. C. C. Lin and A. S. Lynch (ed.), Regulation of anaerobic gene expression in Escherichia coli. R.G. Landes Company, Austin, Tex.
- 12.Fast, B., P.-E. Lindgren, and F. Götz. 1997. Cloning, sequencing, and characterization of a gene (narT) encoding a transport protein involved in dissimilatory nitrate reduction in Staphylococcus carnosus. Arch. Microbiol. 166:361-367. [DOI] [PubMed] [Google Scholar]
- 13.Götz, F., and B. Schumacher. 1987. Improvements of protoplast transformation in Staphylococcus carnosus. FEMS Microbiol. Lett. 40:285-288. [Google Scholar]
- 14.Gunsalus, R. P. 1992. Control of electron flow in Escherichia coli: coordinated transcription of respiratory pathway genes. J. Bacteriol. 174:7069-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalman, L. V., and R. P. Gunsalus. 1989. Identification of a second gene involved in global regulation of fumarate reductase and other nitrate-controlled genes for anaerobic respiration in Escherichia coli. J. Bacteriol. 171:3810-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiley, P. J., and H. Beinert. 1999. Oxygen sensing by the global regulator, FNR: the role of the iron-sulfur cluster. FEMS Microbiol. Rev. 22:341-352. [DOI] [PubMed] [Google Scholar]
- 17.Marmur, J. 1961. A procedure for isolation of deoxyribonucleic acid from microorganisms. J. Mol. Biol. 3:208-218. [Google Scholar]
- 18.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 19.Neubauer, H., and F. Götz. 1996. Physiology and interaction of nitrate and nitrite reduction in Staphylococcus carnosus. J. Bacteriol. 178:2005-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neubauer, H., I. Pantel, and F. Götz. 1998. Characterization of moeB—part of the molybdenum cofactor biosynthesis gene cluster in Staphylococcus carnosus. FEMS Microbiol. Lett. 164:55-62. [DOI] [PubMed] [Google Scholar]
- 21.Neubauer, H., I. Pantel, and F. Götz. 1999. Molecular characterization of the nitrite-reducing system of Staphylococcus carnosus. J. Bacteriol. 181:1481-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neubauer, H., I. Pantel, P. E. Lindgren, and F. Götz. 1999. Characterization of the molybdate transport system ModABC of Staphylococcus carnosus. Arch. Microbiol. 172:109-115. [DOI] [PubMed] [Google Scholar]
- 23.Nicholas, D. J. D., and A. Nason. 1957. Determination of nitrate and nitrite. Methods Enzymol. 3:981-984. [Google Scholar]
- 24.Pantel, I., P.-E. Lindgren, H. Neubauer, and F. Götz. 1998. Identification and characterization of the Staphylococcus carnosus nitrate reductase operon. Mol. Gen. Genet. 259:105-114. [DOI] [PubMed] [Google Scholar]
- 25.Piiper, J., and P. Scheid. 1881. Oxygen exchange in the metazoa, p. 150-176. In D. L. Gilbert (ed.), Oxygen and living processes. Springer, Berlin, Germany.
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Schleifer, K. H., and U. Fischer. 1982. Description of a new species of the genus Staphylococcus: Staphylococcus carnosus. Int. J. Syst. Bacteriol. 32:153-156. [Google Scholar]
- 28.Schultz, J., R. R. Copley, T. Doerks, C. P. Ponting, and P. Bork. 2000. SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 28:231-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signalling domains. Proc. Natl. Acad. Sci. USA 95:5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Self, W. T., A. M. Grunden, A. Hasona, and K. T. Shanmugam. 1999. Transcriptional regulation of molybdoenzyme synthesis in Escherichia coli in response to molybdenum: ModE-molybdate, a repressor of the modABCD (molybdate transport) operon is a secondary transcriptional activator for the hyc and nar operons. Microbiology 145:41-55. [DOI] [PubMed] [Google Scholar]
- 31.Showe, M. K., and J. A. DeMoss. 1968. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J. Bacteriol. 95:1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sizemore, C., E. Buchner, T. Rygus, C. Witke, F. Götz, and W. Hillen. 1991. Organization, promoter analysis and transcriptional regulation of the Staphylococcus xylosus xylose utilization operon. Mol. Gen. Genet. 227:377-384. [DOI] [PubMed] [Google Scholar]
- 33.Stock, J. B., A. J. Ninfa, and A. M. Stock. 1989. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 53:450-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor, B. L., and I. B. Zhulin. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63:479-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Throup, J. P., F. Zappacosta, R. D. Lunsford, R. S. Annan, S. A. Carr, J. T. Lonsdale, A. P. Bryant, D. McDevitt, M. Rosenberg, and M. K. Burnham. 2001. The srhSR gene pair from Staphylococcus aureus: genomic and proteomic approaches to the identification and characterization of gene function. Biochemistry 40:10392-10401. [DOI] [PubMed] [Google Scholar]
- 36.Tyson, K. L., A. I. Bell, J. A. Cole, and S. J. W. Busby. 1993. Definition of nitrite and nitrate responsive elements at the anaerobically inducible Escherichia coli nirB promoter: interactions between FNR and NarL. Mol. Microbiol. 7:151-157. [DOI] [PubMed] [Google Scholar]
- 37.Tyson, K. L., J. A. Cole, and S. J. W. Busby. 1994. Nitrite and nitrate regulation at the promoters of two Escherichia coli operons encoding nitrite reductase: identification of common target heptamers for both NarP- and NarL-dependent regulation. Mol. Microbiol. 13:1045-1055. [DOI] [PubMed] [Google Scholar]
- 38.Unden, G., and J. Schirawski. 1997. The oxygen-responsive transcriptional regulator FNR of Escherichia coli: the search for signal and reactions. Mol. Microbiol. 25:205-210. [DOI] [PubMed] [Google Scholar]
- 39.Wieland, K.-P., B. Wieland, and F. Götz. 1995. A promoter-screening plasmid and xylose-inducible, glucose-repressible expression vectors for Staphylococcus carnosus. Gene 158:91-96. [DOI] [PubMed] [Google Scholar]