Abstract
The Bacillus subtilis zinc uptake repressor (Zur) regulates genes involved in zinc uptake. We have used DNA microarrays to identify genes that are derepressed in a zur mutant. In addition to members of the two previously identified Zur-regulated operons (yciC and ycdHI-yceA), we identified two other genes, yciA and yciB, as targets of Zur regulation. Electrophoretic mobility shift experiments demonstrated that all three operons are direct targets of Zur regulation. Zur binds to an ∼28-bp operator upstream of the yciA gene, as judged by DNase I footprinting, and similar operator sites are found preceding each of the previously described target operons, yciC and ycdHI-yceA. Analysis of a yciA-lacZ fusion indicates that this operon is induced under zinc starvation conditions and derepressed in the zur mutant. Phenotypic analyses suggest that the YciA, YciB, and YciC proteins may function as part of the same Zn(II) transport pathway. Mutation of yciA or yciC, singly or in combination, had little effect on growth of the wild-type strain but significantly impaired the growth of the ycdH mutant under conditions of zinc limitation. Since the YciA, YciB, and YciC proteins are not obviously related to any known transporter family, they may define a new class of metal ion uptake system. Mutant strains lacking all three identified zinc uptake systems (yciABC, ycdHI-yceA, and zosA) are dependent on micromolar levels of added zinc for optimal growth.
Zinc is an essential element for living organisms. It plays a vital role as a cofactor for numerous enzymes and DNA-binding proteins and serves as a structural scaffold for several proteins (32). Consequently, living cells have developed systems for high-affinity zinc uptake (14). Several zinc uptake systems in bacteria have been shown to be under the control of a zinc-sensing Fur homolog, the zinc uptake repressor (Zur) (8, 10, 20, 25).
Bacillus subtilis Zur regulates the expression of two putative zinc transport systems, an ABC transporter encoded by the ycdHI-yceA operon and a conserved membrane protein encoded by yciC (10). In addition, B. subtilis was recently shown to express a third zinc uptake system, ZosA, under conditions of oxidative stress (11). The zosA gene is not repressed by zinc and is expressed under the regulation of the peroxide-sensing repressor PerR rather than Zur (11). Increased zinc uptake under oxidative stress conditions is postulated to help protect cells against metal-catalyzed oxidation reactions by displacing redox-active metals from adventitious binding sites (11). B. subtilis also expresses a fourth transporter that contributes to zinc homeostasis, CadA. CadA is a CPx-type ATPase efflux pump that is induced by high zinc concentrations and plays a major role in zinc resistance (A.G. and J.D.H., unpublished results).
Microarray analysis is a powerful tool for the study of metal ion metabolism (9). For example, the iron starvation stimulon has been characterized in several organisms (2, 26, 29, 30, 38). Zinc deprivation in Saccharomyces cerevisiae alters the expression of about 15% of its genes, several of which are regulated by the Zap1 transcriptional activator (21). Comparable studies of zinc regulons in bacterial systems have not been reported.
Here, we used DNA microarray analysis, lacZ reporter fusions, and DNase I footprinting to characterize the B. subtilis Zur regulon. In addition to the previously reported genes, we identified two more genes, yciA and yciB, as targets of Zur regulation. Mutational analyses suggest that the YciABC proteins may function together as a new type of metal uptake system.
MATERIALS AND METHODS
Media and growth conditions.
B. subtilis CU1065 (Table 1) was grown on Luria-Bertani (LB) medium or minimal medium (MM) containing 40 mM potassium morpholinepropanesulfonate (MOPS) (adjusted to pH 7.4 with KOH), 2 mM potassium phosphate buffer (pH 7.0), glucose (2%, wt/vol), (NH4)2SO4 (2 g/liter), MgSO4 · 7H2O (0.2 g/liter), trisodium citrate.2H2O (1 g/liter), potassium glutamate (1 g/liter), tryptophan (10 mg/liter), 3 nM (NH4)6Mo7O24, 400 nM H3BO3, 30 nM CoCl2, 10 nM CuSO4, 10 nM ZnSO4, and 80 nM MnCl2 (5). Low-zinc MM (LZMM) was prepared by omitting zinc from the standard MM. Metals from filter-sterilized stocks were added before inoculation. Escherichia coli DH5α was used for routine DNA cloning (27). Unless indicated otherwise, liquid media were inoculated from an overnight preculture and incubated at 37°C with shaking at 200 rpm. Erythromycin (1 μg/ml) and lincomycin (25 μg/ml) (for testing of macrolide-lincosamide-streptogramin B resistance), spectinomycin (100 μg/ml), kanamycin (10 μg/ml), neomycin (10 μg/ml), and chloramphenicol (5 μg/ml) were used for the selection of various B. subtilis strains.
TABLE 1.
Bacterial strains used in this study
| Strain | Characteristics | Source or reference |
|---|---|---|
| B. subtilis | ||
| CU1065 | W168 att SPβ trpC2 | 33 |
| HB1000 | ZB307A att SPβ | 6 |
| ZB307A | W168 SP βc2 Δ2::Tn917::pSK10Δ6 (MLSr) | 40 |
| HB8132 | CU1065 zur::spc | 4 |
| HB8072 | HB1000 yciC::kan | 10 |
| HB8073 | HB1000 ycdH::cat | 10 |
| HB8074 | HB1000 yciC::kan ycdH::cat | 10 |
| HB8182 | CU1065 SPβc2 Δ2::Tn917::φ(yciA′-cat-lacZ) (MLSr Neor) | This work |
| HB8183 | HB8132 SPβc2 Δ2::Tn917::φ(yciA′-cat-lacZ) (MLSr Neor) | This work |
| HB8184 | CU1065 SPβc2 Δ2::Tn917::φ(sboA′-cat-lacZ) (MLSr Neor) | This work |
| HB8185 | HB8132 SPβc2 Δ2::Tn917::φ(sboA′-cat-lacZ) (MLSr Neor) | This work |
| HB8190 | HB1000 yciA::spc | This work |
| HB8191 | HB1000 zosA::kan | 11 |
| HB8192 | HB1000 yciC::kan yciA::spc | This work |
| HB8193 | HB1000 yciC::kan yciA::spc ycdH::cat | This work |
| HB8194 | HB1000 zosA::kan ycdH::cat | This work |
| HB8195 | HB1000 ycdH::cat zosA::kan | This work |
| HB8196 | HB1000 yciA::spc zosA::kan | This work |
| HB8197 | HB1000 yciA::spc zosA::kan ycdH::cat | This work |
| E. coli DH5α | φ80 Δ(lacZ)M15 Δ (argF-lac)U169 endA1 recA1 hsdR17 (rK− mK+) deoR thi-1 supE44 gyrA96 relA1 | 27 |
DNA manipulations.
Routine molecular biology procedures were done as described by Sambrook et al. (27). Isolation of B. subtilis chromosomal DNA, transformation, and specialized SPβ transduction were done as described by Cutting and Vander Horn (7). Restriction enzymes, DNA ligase, Klenow fragment, and DNA polymerase were used in accordance with the manufacturer's (New England Biolabs) instructions.
Microarray analysis: RNA isolation, cDNA synthesis, and slide hybridization.
For RNA isolation, 100 μl of an overnight cell culture of the wild type or the isogenic zur mutant was diluted into 6 ml of LB medium (in tubes measuring 18 by 150 mm) and grown at 37°C with shaking at 250 rpm. When the cells reached an optical density at 600 nm of 0.6, the tubes were transferred to an ice-water bath and the cells were collected by centrifugation for 1 min at 5,000 rpm. The pellets were frozen in a dry-ice-methanol bath and resuspended in 10 mM Tris HCl (pH 8.0)-1 mM EDTA buffer containing 15 mg of lysozyme per ml and incubated for 5 min at room temperature. Total RNA was extracted with RNeasy Minikits (Qiagen), contaminating DNA was removed with a DNA-free kit (Ambion), and the resulting RNA preparation was frozen on dry ice and stored at −80°C until further use. Microarrays contained 4,020 PCR products spotted in duplicate on each glass slide and were prepared as described previously (36). The protocol for labeling utilized random hexamers and was performed as described previously (36).
Two different RNA preparations (from cells grown on different days) were prepared for both the wild-type and zur mutant strains. Each RNA preparation was used to make both Cy3- and Cy5-labeled cDNAs, and all competitive hybridizations were done twice, once with each cDNA preparation, to control for any differences in labeling between the two fluorophores. Since all PCR products are spotted twice on each slide, all signal intensities and calculated ratios are the averages of four values.
Microarray data analysis.
Signal intensities were detected and quantified with ArrayVision software (Molecular Dynamics) and assembled into EXCEL spreadsheets. Expression ratios were calculated as zur mutant/wild type (zur/WT) for each experiment, and the mean and standard deviation were calculated. The data were filtered to remove measurements with high variability (those where the standard deviation was equal to or larger than the mean) or those where valid ratios were found for only one of the two replicates.
Protein purification and DNA-binding assays.
B. subtilis Zur, overexpressed in E. coli and purified as described previously (10), was used in electrophoretic mobility shift assays (EMSA) and DNase I footprinting experiments performed essentially as previously described (10, 11). For EMSA experiments, the gel was prerun for 30 min and samples were electrophoresed at 4°C.
Construction of yciA and sboA transcriptional fusions.
Promoter regions were amplified from the B. subtilis genome by PCR with primers 5′-GCGAAGCTTGCCGAACATCTTAGGATCT-3′ and 5′-TGACGGATCCAAAGTATTGAAGAC-3′ for yciA and primers 5′-GCGAAGCTTTTCATCATTCCACTTTGACT-3′ and 5′-GCGGGATCCAAGAGGTAGATTTTAGTTA-3′ for sboA. The resulting PCR products were cloned as HindIII-BamHI fragments (sites are underlined) into pJPM122 (28) to generate the corresponding lacZ operon fusions. The resulting plasmids were linearized with ScaI and introduced by transformation into ZB307A (Table 1) with selection for neomycin resistance. SPβ transducing lysates were prepared by heat induction and used to lysogenize B. subtilis CU1065.
Construction of a yciA mutant.
The yciA region was amplified from chromosomal DNA with the primers 5′-GCGAAGCTTGCCGAACATCTTAGGATCT-3′ and 5′-GCGGAATTCTTCTGCATGATCGGAGCA-3′ and digested with HindIII (site underlined) and EcoRI (at a site internal to yciA), and the resulting fragment was cloned in pGEM3Zf(+)cat-1 (37). A gene cassette coding for spectinomycin resistance from pDG1726 (13) was isolated as a PstI fragment and cloned into an internal PstI site in the yciA gene. The resulting construct was linearized with ScaI and introduced by transformation into B. subtilis CU1065 with selection for spectinomycin resistance, and the transformants were screened for loss of the plasmid-borne chloramphenicol resistance to ensure the double-crossover event. Genomic DNA was isolated from selected transformants, and the mutation was confirmed by PCR.
Primer extension assays.
Total RNA was isolated from wild-type or zur mutant cells with the RNeasy RNA isolation kit (Qiagen). For primer extension analysis, 100 μg of total RNA was precipitated with 4 pmol of 5′-end 32P-labeled reverse primer and the reverse transcripts were generated as previously described (18, 19). Reverse transcripts were analyzed by 8 M urea-6% polyacrylamide gel electrophoresis. The PCR product was sequenced with the same primer, and the resulting sequence ladder was used to identify the 3′ end of the reverse transcript (corresponding to the 5′ end of the RNA).
β-Galactosidase assays.
Overnight cultures were diluted 1:100 in LZMM medium containing different concentrations of metal ions (as indicated) and grown to mid-logarithmic phase. Cells were collected and assayed for β-galactosidase as previously described (4, 22).
RESULTS AND DISCUSSION
The zinc uptake repressor Zur was originally discovered in B. subtilis (10) and E. coli (25), and homologs have been identified in Listeria monocytogenes (8) and Staphylococcus aureus (20). B. subtilis Zur regulates the expression of two proposed zinc uptake systems, YcdHI-YceA and YciC, and purified Zur binds, in a zinc-dependent manner, to the promoter region of both yciC and ycdH in vitro (10).
Transcriptome analyses.
To identify other genes regulated by Zur, we used DNA microarray analysis of RNA isolated from both wild-type and zur mutant cells grown under zinc-replete conditions [in LB medium estimated to contain ca. 15 μM Zn(II)] (24). The zur/WT expression ratios in two independent experiments were compared. Overall, 97% of the expressed genes varied less than twofold in expression level between the wild-type and zur mutant cells.
To identify those genes whose expression was most significantly (and reproducibly) altered in the zur mutant, we used a two-dimensional graphical display of the two sets of measurements (Fig. 1A). Ten genes were found to reproducibly differ more than threefold between the two samples (Fig. 1B). By far, the most dramatic change is the >300-fold derepression of the yciC gene in the zur mutant strain. This is consistent with the original identification of the corresponding gene product as an abundant membrane protein in the zur mutant but not in the isogenic wild type (10). Three other strongly derepressed genes correspond to the ycdHI-yceA operon, which was previously shown to be under Zur control (10). Unexpectedly, we also identified the yciA and yciB genes as strongly derepressed in the zur mutant. These genes are immediately upstream of and codirectional with yciC. Since the yciC promoter region has already been identified as a target of Zur-mediated regulation (10), this suggests that the yciC gene can be transcribed independently from the yciA and yciB genes. Indeed, the yciC mRNA is present at much higher levels than an overlapping yciABC transcript, as judged by Northern blot analyses (data not shown), consistent with the finding that YciC is an abundant protein in zur mutant membranes, whereas YciB was not observed.
FIG. 1.
Identification of the Zur regulon by microarray analysis. (A) zur/WT expression ratios of B. subtilis genes are compared for two independent measurements made with RNAs from separate cultures. As an arbitrary cutoff value, genes showing expression ratios below threefold were considered unchanged (gray diamonds). Genes that constitute the Zur regulon are represented by closed squares, genes in the sbo operon are represented by closed circles, and genes with lowered expression in the zur mutant are represented by open squares. The apparent up-regulation of yckA (closed triangle) is likely an artifact due to convergent transcription from the strongly expressed yciC gene. (B) The zur/WT expression ratios of selected genes are illustrated. The results shown are averages of two measurements, and the error bars represent the ranges.
The yckA gene was also apparently derepressed in the zur mutant, but this is likely to be due to readthrough transcription from the convergently transcribed yciC gene. The yckA gene is likely to be part of an operon with yckB. However, expression of yckB was not altered by the zur mutation nor is there a candidate Zur box in the promoter region of the yckBA operon.
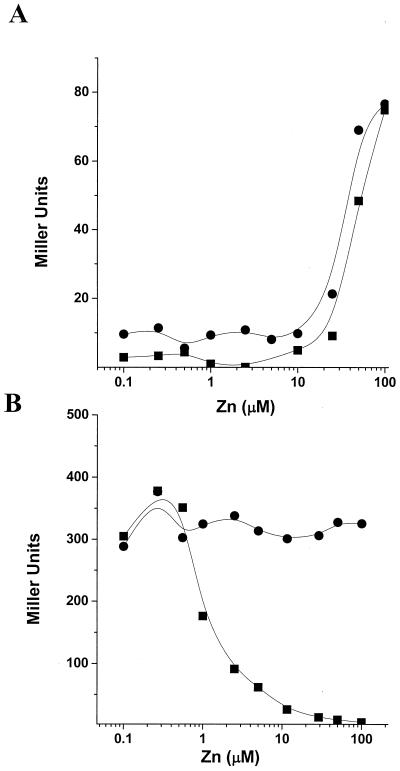
The remaining genes up-regulated by the zur mutation (increased 2.3- to 3.7-fold) all belong to the sbo operon encoding subtilosin production and immunity (23, 39). To determine if Zur regulates the sbo operon directly, we constructed a PsboA-cat-lacZ fusion. At zinc levels above 10 μM, expression of the sbo operon was induced independently of zur. In addition, the basal level of sbo expression was slightly increased in the zur mutant (Fig. 2A). This is in contrast to genes known to be directly regulated by Zur which are repressed by Zn(II). The induction of the PsboA-cat-lacZ fusion was Zn(II) specific since there was no significant induction with Cu(II), Cd(II), or Ni(II) (data not shown). As previously reported (23), PsboA'-cat-lacZ was strongly induced under anaerobic conditions (∼2,250 Miller units) and under these conditions, no effect of Zn(II) or the zur mutation could be detected (data not shown). We speculate that the increased basal expression of the sbo operon in the zur mutant may be due to elevated zinc levels, which can inhibit enzymes in the respiratory chain and may thereby mimic anaerobiosis (3). Indeed, in the experiment in which sbo induction was most pronounced, other anaerobically induced, ResDE-regulated genes (e.g., hmp and nasDE) were also up-regulated.
FIG. 2.
Effects of zinc ions on the expression of sbo and yciA were determined with cat-lacZ transcriptional fusions. Wild type (closed squares) or zur mutant (closed circles) cells containing an sbo′-cat-lacZ (A) or a yciA′-cat-lacZ (B) transcriptional fusion were grown to mid-logarithmic phase in LZMM with different concentrations of zinc, and β-galactosidase activity was determined.
Only two genes, rocE and rocD, were repressed more than threefold in the zur mutant (Fig. 1). These genes encode an arginine and ornithine amino acid permease (12). The σ54-dependent rocDE operon is under the control of rocR and is induced by the presence of arginine, ornithine, or proline in the growth medium (12). The relationship between rocDE expression and zur is not clear. There are no obvious candidates for Zur box-like elements in the rocDE regulatory region, and we favor the idea that the decreased expression in the zur mutant is an indirect effect of altered zinc homeostasis.
Regulation of yciA by zinc and Zur.
To investigate the role of Zur in the regulation of yciA and yciB, we constructed a PyciA-cat-lacZ fusion. Expression from the yciA promoter region was repressed by zinc at concentrations above 1 μM and completely derepressed in the zur mutant background (Fig. 2B). This regulation precisely parallels that shown previously for the Zur-regulated yciC and ycdH promoter regions (10).
To identify the promoter element for yciA, we used primer extension analysis with RNA extracted from wild-type and zur mutant cells grown in LB medium. Transcription of the yciA gene starts at or near a G residue 37 bases upstream of the translation start site (Fig. 3A). The yciA transcript could only be detected with RNA from the zur mutant, consistent with the microarray and lacZ fusion data (Fig. 3A).
FIG. 3.
Primer extension mapping of the transcription start sites of yciA (A) and ycdH (B). The primer extension product was generated with RNA isolated from either wild type (WT) or zur mutant cells grown in LB medium. The sequence ladders were generated by PCR cycle sequencing with the same primer as in the primer extension reaction.
Comparison of Zur-regulated promoter regions.
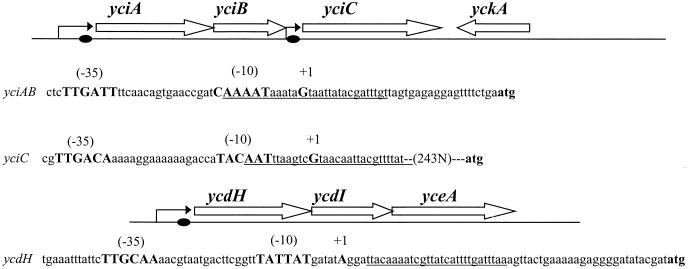
In parallel with the above studies, we have also determined the transcription start site for the ycdHI-yceA operon (Fig. 3B) and the yciC gene (F. Miyagi, A. Gaballa, and J. D. Helmann, unpublished data). The ycdH transcript originates from an A residue 60 bases upstream of the translation start site (Fig. 3B) and could only be detected in the RNA extracted from zur mutant cells. The yciC regulatory region is especially complex and includes a long untranslated leader region and two Zur boxes. The promoter-proximal Zur box (Fig. 4) is primarily responsible for Zn-dependent repression (Miyagi et al., unpublished). Note that this site differs from that previously shown to bind Zur (10). All three promoters conform to the consensus sequences expected for recognition by σA holoenzyme (15). On the basis of these DNA microarray analysis results, we propose that these three transcription units constitute the zur regulon (Fig. 4).
FIG. 4.
The yciABC and ycdHI-yceA regions of B. subtilis. Open reading frames are indicated by open arrows, promoter sites are indicated by bent arrows, and Zur boxes are indicated by closed circles. Sequences of the three Zur-controlled promoters are shown with σA-dependent recognition sites (bold), and the residue corresponding to the predominant transcription start site is indicated by +1. Note that the yciC transcript initiates more than 200 bases upstream of the yciC start codon. Regions of Zur protection in the operators of yciA, yciC, and ycdH are underlined (see Fig. 5; data not shown).
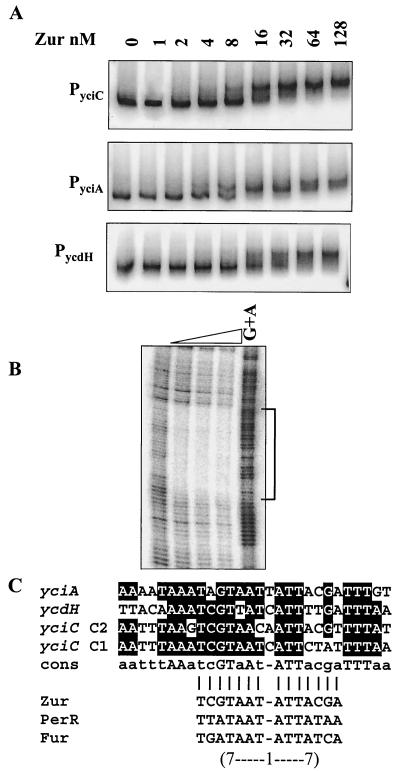
Zur interaction with Zur boxes.
EMSA showed that purified Zur protein binds to the promoter regions of yciC, yciA, and ycdH with high affinity (Fig. 5A). By DNase I footprinting, we demonstrated that purified Zur protein bound to an extended region of ∼28 bases overlapping the yciA promoter (Fig. 5B). This region contains an imperfect inverted repeat similar to those noted previously for the yciC and ycdH operators (10). Alignment of the four known Zur boxes with CLUSTAL W (31) reveals a conserved sequence of aatttAAatcGTaAT-ATTacgTTTTaa (Fig. 5C and data not shown). This sequence contains a core element of a 7-1-7 inverted repeat similar to the known binding sites for other Fur homologs in B. subtilis (1, 2, 16).
FIG. 5.
Interaction of Zur with its target operons. (A) EMSA of Zur binding to the promoter regions of yciC, yciA, and ycdH. Zur protein (concentrations indicated in nanomolar monomer) was incubated with 32P-end-labeled fragments and analyzed by 6% polyacrylamide gel electrophoresis. (B) DNase I footprinting analysis of Zur binding to the yciA promoter region. Zur protein was incubated with a 32P-end-labeled DNA fragment, and a G+A chemical cleavage ladder was included for localization of the binding sites. The concentration of Zur (monomer) in each reaction was (from left to right) 0, 20, 40, or 100 nM. (C) Alignment of the Zur-protected DNA regions in the promoters of yciA, yciC, and ycdH. The bottom part of panel C compares the core region protected by Zur (a 7-1-7 inverted repeat motif designated a Zur box) with similar operator sites recognized by the Fur homolog PerR and by Fur (1, 17).
A yciA mutation affects zinc-limited growth.
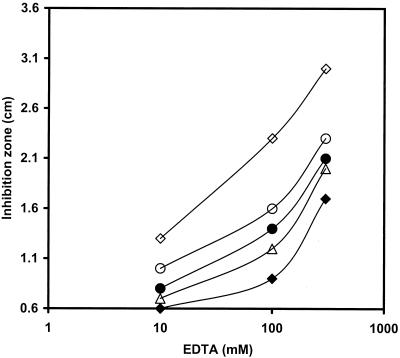
It has been shown previously that a mutation in ycdH affected the ability of the cell to grow in a zinc-deficient medium (LZMM) (10). While a yciC mutation had no effect on cell growth in LZMM, it clearly exacerbated the growth defect of the ycdH single mutant in LZMM and this defect could be suppressed by added zinc (10). To determine the role of YciA in zinc uptake, we constructed a yciA::spc mutant by allelic replacement. While this mutation is likely to be polar on yciB, yciC is expressed from its own promoter and should not be affected. As noted for the yciC mutant, a yciA mutant is unaffected in growth under conditions of zinc limitation induced by EDTA addition to MM (Fig. 6). However, the yciA mutation increased the growth defect of the ycdH single mutant. This is indistinguishable from the phenotype of a yciC mutant studied previously (10). A yciA yciC double mutant is phenotypically identical to either single mutant (Fig. 6 and data not shown). Both YciC and YciB are predicted membrane proteins with at least one membrane-spanning segment (and YciC fractionates with the membrane; 10). Together, these results suggest that YciAB and YciC may function in the same pathway for zinc uptake, presumably as subunits of a low-affinity Zn(II) uptake system or permease. The lack of similarity to known zinc uptake or metal transport systems suggests that these conserved proteins may define a new family of transporter.
FIG. 6.
Effects of EDTA on the growth of the wild type and zinc uptake mutants. Inhibition of the growth of the wild type (♦) and the ycdH (▵), ycdH yciA (○), ycdH yciC (•), and yciA ycdH zosA (⋄) mutants by EDTA is shown. The zosA, yciA, and yciC single mutants and the yciA-yciC double mutant were indistinguishable from the wild type. For each strain, the diameter of the zone of growth inhibition was determined on MM plates overlaid with a 0.6-cm-diameter filter paper disk containing 10 μl of EDTA at either 10, 100, or 500 mM. Values represent the average of three independent measurements with an average error of ± 0.1 cm.
Characterization of strains deficient in three zinc uptake systems.
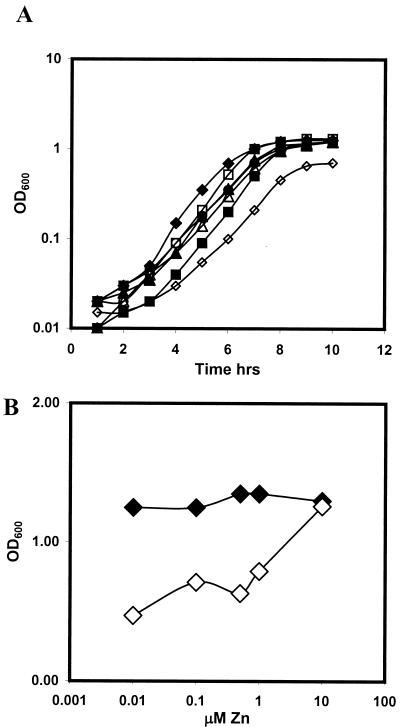
B. subtilis encodes three known zinc uptake systems: an ABC transporter encoded by the Zur-regulated ycdHI-yceA operon, a postulated low-affinity transport system encoded by the yciABC operon (10), and the P-type ATPase ZosA regulated by the PerR protein (11). We constructed single, double, and triple mutant strains affected in these systems and tested their abilities to grow under conditions of zinc limitation. As expected, the triple mutant was sensitive to zinc limitation (induced by EDTA) in comparison to the single and double mutants (Fig. 6). In experiments with LZMM supplemented with 500 nM Zn(II), no dramatic differences were found between the single and double yciA, yciC, ycdH, and zosA mutants (Fig. 7A). However, the yciA ycdH zosA triple mutant grew poorly under these same conditions (a doubling time of 68 min versus 49 min for the wild type) and showed an extended lag phase (Fig. 7A). A direct comparison of the growth curves of the wild type and the isogenic triple mutant strain as a function of the zinc concentration revealed that the growth rate and yield of the triple mutant are highly sensitive to the amount of zinc added to the LZMM, with a concentration of greater than 1 μM zinc required to achieve an optical density at 600 nm of greater than 1.0 (Fig. 7B). These data indicate that YciABC, YcdHI-YceA, and ZosA constitute the major zinc uptake pathways in B. subtilis. Indeed, under zinc-replete conditions, the yciABC and ycdHI-yceA systems are repressed and ZosA provides a major conduit for zinc influx (11).
FIG. 7.
Effect of zinc limitation on the growth of the wild type and zinc uptake mutants. (A) Growth curves of the wild type (♦) and the yciA (▪), ycdH (▴), ycdH yciA (○), yciA zosA (□), ycdH zosA (▵), and yciA ycdH zosA (⋄) mutants in LZMM with 500 nM Zn(II). (B) Effect of zinc concentration on the growth yield (10 h after 1:100 dilution into LZMM supplemented as indicated) of the wild type (♦) and the yciA ycdH zosA mutant (⋄). OD600, optical density at 600 nm.
The ability of B. subtilis to grow, albeit at a reduced rate and with a reduced yield, even in the absence of these three systems indicates that there are other low-affinity pathways that allow the import of zinc. It has been suggested that the CitM transporter may help supply zinc in a medium that contains citrate (14). While LZMM does contain citrate, it also contains a high glucose concentration, which completely represses the expression of citM (34, 35). More work is needed to identify the transporter(s) that allows zinc uptake in the triple-mutant strain.
Summary.
We have used DNA microarray analyses to identify genes whose expression is affected by a zur mutation. Remarkably, the most strongly derepressed gene was yciC, corresponding to an abundant membrane protein that was first identified as a target for Zur-mediated repression by sodium dodecyl sulfate-polyacrylamide gel analysis of protein profiles (10). We also identified the upstream genes yciA and yciB as targets of Zur-mediated repression. This regulation is mediated by Zur binding to a high-affinity binding site overlapping a σA-type promoter element for the yciABC operon. Sequence comparisons of known Zur-regulated genes identified a conserved motif, the Zur box, that is associated with Zur binding. Genetic and physiological studies suggest that yciA, yciB, and yciC likely function in the same pathway for zinc uptake. Since these genes encode proteins conserved in many bacterial species, and at least YciC is membrane localized (10), we suggest that the YciABC system may define a new family of metal ion transporters.
Acknowledgments
We thank Mayuree Fuangthong for assistance with the analysis of Zur box sequences and Faith Miyagi for helpful comments on the manuscript.
This work was supported by a grant from the National Science Foundation (MCB-9905682).
REFERENCES
- 1.Baichoo, N., and J. D. Helmann. 2002. Recognition of DNA by Fur: a reinterpretation of the Fur box consensus sequence. J. Bacteriol. 184:5826-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baichoo, N., T. Wang, Y. Rick, and J. D. Helmann. 2002. Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Mol. Microbiol. 45:1613-1629. [DOI] [PubMed] [Google Scholar]
- 3.Beard, S. J., M. N. Hughes, and R. K. Poole. 1995. Inhibition of the cytochrome bd-terminated NADH oxidase system in Escherichia coli K-12 by divalent metal cations. FEMS Microbiol. Lett. 131:205-210. [DOI] [PubMed] [Google Scholar]
- 4.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 5.Chen, L., L. P. James, and J. D. Helmann. 1993. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J. Bacteriol. 175:5428-5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, L., L. Keramati, and J. D. Helmann. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. USA 92:8190-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutting, S. M., and P. B. Vander Horn. 1990. Genetic analysis, p. 27-74. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom.
- 8.Dalet, K., E. Gouin, Y. Cenatiempo, P. Cossart, and Y. Hechard. 1999. Characterisation of a new operon encoding a Zur-like protein and an associated ABC zinc permease in Listeria monocytogenes. FEMS Microbiol. Lett. 174:111-116. [DOI] [PubMed] [Google Scholar]
- 9.Eide, D. J. 2001. Functional genomics and metal metabolism. Genome Biol. 2:1028.1-1028.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaballa, A., and J. D. Helmann. 1998. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J. Bacteriol. 180:5815-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaballa, A., and J. D. Helmann. 2002. A peroxide-induced zinc uptake system plays an important role in protection against oxidative stress in Bacillus subtilis. Mol. Microbiol. 45:997-1005. [DOI] [PubMed] [Google Scholar]
- 12.Gardan, R., G. Rapoport, and M. Debarbouille. 1995. Expression of the rocDEF operon involved in arginine catabolism in Bacillus subtilis. J. Mol. Biol. 249:843-856. [DOI] [PubMed] [Google Scholar]
- 13.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 14.Hantke, K. 2001. Bacterial zinc transporters and regulators. Biometals 14:239-249. [DOI] [PubMed] [Google Scholar]
- 15.Helmann, J. D. 1995. Compilation and analysis of Bacillus subtilis sigma A-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res. 23:2351-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbig, A., and J. D. Helmann. 2002. Metal ion uptake and oxidative stress, p. 405-414. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives, 2nd ed. ASM Press, Washington, D.C.
- 17.Herbig, A. F., and J. D. Helmann. 2001. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol. Microbiol. 41:849-859. [DOI] [PubMed] [Google Scholar]
- 18.Huang, X., A. Decatur, A. Sorokin, and J. D. Helmann. 1997. The Bacillus subtilis σX protein is an extracytoplasmic function sigma factor contributing to survival at high temperature. J. Bacteriol. 179:2915-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, X., and J. D. Helmann. 1998. Identification of target promoters for the Bacillus subtilis sigma X factor using a consensus-directed search. J. Mol. Biol. 279:165-173. [DOI] [PubMed] [Google Scholar]
- 20.Lindsay, J. A., and S. J. Foster. 2001. Zur: a Zn2+-responsive regulatory element of Staphylococcus aureus. Microbiology 147:1259-1266. [DOI] [PubMed] [Google Scholar]
- 21.Lyons, T. J., A. P. Gasch, L. A. Gaither, D. Botstein, P. O. Brown, and D. J. Eide. 2000. Genome-wide characterization of the Zap1p zinc-responsive regulon in yeast. Proc. Natl. Acad. Sci. USA 97:7957-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Nakano, M. M., G. Zheng, and P. Zuber. 2000. Dual control of sbo-alb operon expression by the Spo0 and ResDE systems of signal transduction under anaerobic conditions in Bacillus subtilis. J. Bacteriol. 182:3274-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Outten, C. E., and T. V. O'Halloran. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488-2492. [DOI] [PubMed] [Google Scholar]
- 25.Patzer, S. I., and K. Hantke. 1998. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 28:1199-1210. [DOI] [PubMed] [Google Scholar]
- 26.Paustian, M. L., B. J. May, and V. Kapur. 2001. Pasteurella multocida gene expression in response to iron limitation. Infect. Immun. 69:4109-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Slack, F. J., J. P. Mueller, and A. L. Sonenshein. 1993. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J. Bacteriol. 175:4605-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thimm, O., B. Essigmann, S. Kloska, T. Altmann, and T. J. Buckhout. 2001. Response of Arabidopsis to iron deficiency stress as revealed by microarray analysis. Plant Physiol. 127:1030-1043. [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson, D. K., A. S. Beliaev, C. S. Giometti, S. L. Tollaksen, T. Khare, D. P. Lies, K. H. Nealson, H. Lim, J. Yates III, C. C. Brandt, J. M. Tiedje, and J. Zhou. 2002. Transcriptional and proteomic analysis of a ferric uptake regulator (Fur) mutant of Shewanella oneidensis: possible involvement of Fur in energy metabolism, transcriptional regulation, and oxidative stress. Appl. Environ. Microbiol. 68:881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallee, B. L., and K. H. Falchuk. 1993. The biochemical basis of zinc physiology. Physiol. Rev. 73:79-118. [DOI] [PubMed] [Google Scholar]
- 33.Vander Horn, P. B., and S. A. Zahler. 1992. Cloning and nucleotide sequence of the leucyl-tRNA synthetase gene of Bacillus subtilis. J. Bacteriol. 174:3928-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warner, J. B., B. P. Krom, C. Magni, W. N. Konings, and J. S. Lolkema. 2000. Catabolite repression and induction of the Mg2+-citrate transporter CitM of Bacillus subtilis. J. Bacteriol. 182:6099-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto, H., M. Murata, and J. Sekiguchi. 2000. The CitST two-component system regulates the expression of the Mg-citrate transporter in Bacillus subtilis. Mol. Microbiol. 37:898-912. [DOI] [PubMed] [Google Scholar]
- 36.Ye, R. W., W. Tao, L. Bedzyk, T. Young, M. Chen, and L. Li. 2000. Global gene expression profiles of Bacillus subtilis grown under anaerobic conditions. J. Bacteriol. 182:4458-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youngman, P. 1990. Use of transposons and integrational vectors for mutagenesis and construction of gene fusions in Bacillus species, p. 221-266. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom.
- 38.Yun, C. W., T. Ferea, J. Rashford, O. Ardon, P. O. Brown, D. Botstein, J. Kaplan, and C. C. Philpott. 2000. Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. Evidence for two pathways of iron uptake. J. Biol. Chem. 275:10709-10715. [DOI] [PubMed] [Google Scholar]
- 39.Zheng, G., R. Hehn, and P. Zuber. 2000. Mutational analysis of the sbo-alb locus of Bacillus subtilis: identification of genes required for subtilosin production and immunity. J. Bacteriol. 182:3266-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuber, P., and R. Losick. 1987. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J. Bacteriol. 169:2223-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]