Abstract
A Streptomyces clavuligerus ccaR::aph strain, which has a disruption in the regulatory gene ccaR, does not produce cephamycin C or clavulanic acid, but does produce a bioactive compound that was identified as holomycin by high-performance liquid chromatography (HPLC) and infrared and mass spectrometry. S. clavuligerus strains with disruptions in different genes of the clavulanic acid pathway fall into three groups with respect to holomycin biosynthesis. (i) Mutants with mutations in the early steps of the pathway blocked in the gene ceaS (pyc) (encoding carboxyethylarginine synthase), bls (encoding a β-lactam synthetase), or open reading frame 6 (ORF6; coding for an acetyltransferase of unknown function) are holomycin nonproducers. (ii) Mutants blocked in the regulatory gene ccaR or claR or blocked in the last gene of the pathway encoding clavulanic acid reductase (car) produce holomycin at higher levels than the wild-type strain. (iii) Mutants with disruption in cyp (coding for cytochrome P450), ORF12, and ORF15, genes that appear to be involved in the conversion of clavaminic acid into clavaldehyde or in secretion steps, produce up to 250-fold as much holomycin as the wild-type strain. An assay for holomycin synthetase was developed. This enzyme forms holomycin from holothin by using acetyl coenzyme A as an acetyl group donor. The holomycin synthase activities in the different clavulanic acid mutants correlate well with their production of holomycin.
Streptomyces clavuligerus produces several secondary metabolites with interesting pharmacological activities. It synthesizes the β-lactam antibiotic cephamycin C, the β-lactamase inhibitor clavulanic acid, and several antifungal compounds with a clavam structure (for reviews, see references 3 and 15). The clavulanic acid biosynthesis pathway has several steps in common with the pathway for clavam biosynthesis (18, 19). In addition to the compounds indicated above, S. clavuligerus produces the antibiotics holomycin and tunicamycin (10). Holomycin is a compound with pyrrothine structure, while tunicamycin is a glucosamine-containing antibiotic. This wealth of genetic information for the biosynthesis of secondary metabolites is characteristic of some Streptomyces species (4, 22).
S. clavuligerus is an excellent model for the study of the relationships between the regulatory mechanisms controlling the biosynthesis of the different secondary metabolites produced by these microorganisms. Formation of clavulanic acid is controlled by a LysR-type regulatory protein encoded by the claR gene. Formation of both clavulanic acid and cephamycin C in S. clavuligerus is controlled by the positive autoregulatory protein CcaR (25, 32). Mutant strains with disruption in ccaR do not express the claR gene (26), although this control is not exerted directly by the CcaR regulatory protein and appears to involve a cascade mechanism (32). The control of the formation of cephamycin C and/or clavulanic acid by CcaR or ClaR is exerted at the transcription level (1, 25).
However, the ccaR::aph S. clavuligerus mutant, with disruption in ccaR, was found to produce traces of antibiotic activity in some media, and we decided to characterize and purify this antibiotic compound in order to understand its response to the CcaR regulator. The compound produced was found to be holomycin. The availability of a complete set of S. clavuligerus mutants blocked in the different steps of the clavulanic acid pathway allowed us to establish that whereas mutants blocked in the early steps of the clavulanic pathway are holomycin nonproducers, mutants blocked in the late steps of the clavulanic acid pathway synthesize very large amounts of holomycin. No enzymes or genes for holomycin biosynthesis have been described yet, but from the structure of holomycin, a putative enzyme activity in the holomycin pathway would be the formation of holomycin from deacetyl-holomycin (holothin). In this paper, we describe the formation of holomycin and holomycin synthase activities in several mutants of S. clavuligerus with disruptions in different genes involved in clavulanic acid production.
MATERIALS AND METHODS
Strains and culture conditions.
The strains used in this work are described in Table 1. To study antibiotic production, the cells were pregrown in TSB (Trypticasein soy broth) medium for 48 h, 5% of the culture was used to inoculate SA (starch-asparagine) medium (24), and the cultures were then incubated at 28°C with shaking at 220 rpm. For specific purposes, defined GSPG (glycerol-sucrose-proline-glutamate) medium (29) was used. To grow S. clavuligerus transformants carrying plasmid pVK99, pVK99-ORF15, or pVK99-cyp, the cultures were supplemented with thiostrepton (4 μg/ml). All fermentation experiments were repeated two to three times with triplicate flasks. To purify holomycin, TSB medium was inoculated to 5% (vol/vol) with a seed culture in the same medium, and the culture was incubated for 48 h under the conditions indicated above.
TABLE 1.
Strains used in this work
| Strains | Characteristics or use | Origin or reference |
|---|---|---|
| S. clavuligerus | ||
| ATCC 27064 | Wild type | ATCCa |
| ccaR::aph | Cephamycin C, clavulanic acid-nonproducer (CCA−), disruption in ccaR, kanamycin resistant (Kmr) | 25 |
| claR::aph | Clavulanic acid nonproducer (CA−), disruption in claR | 26 |
| ORF6::apr | Disruption in ORF6, apramycin resistant (Aprr) | 7 |
| ORF6::apr ccaR::aph | CCA−, disruption in ccaR and ORF6, Kmr Aprr | 7 |
| ceaS::aph | CA−, disruption in ceaS, Kmr | 27 |
| RLF3 | CA−, disruption in bls, Kmr | 2 |
| car::aph | CA−, disruption in car, Kmr | 26 |
| cyp::aph | CA−, disruption in cyp, Kmr | 17 |
| ORF12::aph | CA−, disruption in ORF12, Kmr | 17 |
| ORF15::aph | CA−, disruption in ORF15, Kmr | 17 |
| ORF15::aph (pIJ699) | CA−, disruption in ORF15, Kmr Thior | 16 |
| ORF15::aph (pIJ699-ORF15) | CA−, disruption in ORF15, complemented, Kmr Thior | 16 |
| cyp::aph (pVK99) | CA−, disruption in cyp, Kmr Thior | 16 |
| cyp::aph (pVK99-cyp) | Disruption in cyp, complemented, Kmr Thior | 16 |
| Micrococcus luteus ATCC 9341 | Sensitive strain in bioassay | ATCC |
| Bacillus sp. strain 27860 | Sensitive strain in bioassay | ATCC |
| Escherichia coli Ess22-31 | Sensitive strain in bioassay | 26 |
| Klebsiella pneumoniae 29665 | Sensitive strain in bioassay | ATCC |
| Alcaligenes faecalis | Sensitive strain in bioassay | Laboratory stock |
| Enterobacter cloacae P99 | Wide-spectrum cephalosporinase producer | Laboratory stock |
ATCC, American Type Culture Collection.
The bifunctional Streptomyces-Escherichia coli plasmids pULVK99 and pIJ699 (6) were used to express the cyp and open reading frame 15 (ORF15) genes of S. clavuligerus, respectively. The tipAp promoter was placed upstream of ORF15 in plasmid pIJ699-ORF15, while the cyp gene was expressed from its own promoter in plasmid pULVK-cyp. DNA manipulation was performed by standard methods (31). Transformation of S. clavuligerus protoplasts was carried out as described by Kieser et al. (13).
Purification of holomycin and holothin.
Holomycin was purified initially from a 2-liter culture of an S. clavuligerus ccaR::aph strain grown for 48 h in TSB medium by the procedure described by Kenig and Reading (10). The purified compound (8 mg) was used for chemical characterization. Additional batches of holomycin were purified from cultures of an S. clavuligerus cyp::aph strain. Holothin (11 mg) was obtained by hydrolysis of holomycin (20 mg) in 2N HCl by the method described by Gaeumann et al. (9). Chemical hydrolysis of pure, ninhydrin-negative, holomycin (Rf, 0.5) was followed by the detection of the ninhydrin-positive holothin (Rf, 0.45) when the hydrolysis mixture was applied to a Silica Gel 60 thin-layer chromatography plate and developed in chloroform-methanol (9:1). The compound with an Rf of 0.45 was converted by treatment with acetic anhydride (9) into a compound chromatographically identical to pure holomycin, confirming that the substance was holothin. Holothin was further purified by preparative high-performance liquid chromatography (HPLC) with a μBondapack C18 (30 by 7.8 mm) column (Waters).
Holomycin synthase assay.
Holomycin synthase activity was assayed in a final volume of 50 μl of 50 mM Tris-HCl buffer (pH 8.0). The reaction mixture contained dialyzed cell extracts (2 to 50 μg), acetyl coenzyme A (acetyl-CoA) (0.5 mM), and holothin (0.5 mM) and was incubated at 30°C for 10 min. The reaction was stopped by adding 30 μl of methanol. Formation of holomycin was quantified by HPLC with a Nucleosil C18 (30 by 4 mm) column (Scharlau) with methanol (40%) at a flow rate of 1 ml/min. Both holothin (retardation time [Rt], 4.7 min) and holomycin (Rt, 6.7 min) were detected at 360 nm. The pyrrothin compounds give peaks that are concentration dependent, with a standard plot for holothin of y = 0.293x + 0.163 and with a standard plot for holomycin of y = 0.261x − 0.182, where x corresponds to peak area and y corresponds to the pyrrothin compound concentration. The plots have correlation coefficients of 0.9982 and 0.9987 for holothin and holomycin, respectively.
A unit of enzyme is defined as the enzyme activity producing 1 μmol of holomycin per min.
Chemical characterization of holomycin.
The mass spectrum of holomycin was determined by A. Rumbero (Universidad Autónoma, Madrid, Spain). The solid-phase infrared (IR) spectrum was obtained with a Perkin-Elmer 2000 FTIR. The UV spectrum was obtained with a Hitachi-2000 spectrophotometer.
RESULTS
Characterization of the antibiotic compound produced by the ccaR::aph strain of S. clavuligerus.
The ccaR::aph strain of S. clavuligerus does not produce cephamycin C, clavulanic acid, or clavams, as measured by bioassay or HPLC. However, depending on the culture medium or the culture conditions used, a small amount of a compound with antibiotic activity against E. coli Ess22-31 and other bacteria was found in the supernatant cultures. To clarify whether this bioactive compound could be cephamycin that was still being produced even in the absence of CcaR, we decided to characterize the antibiotic substance.
The concentrated supernatant of a 48-h culture of the ccaR::aph S. clavuligerus strain grown in TSB medium was treated for 15 min at 22°C with a narrow-spectrum penicillinase (Difco) or with the broad-spectrum cephalosporinase from Enterobacter cloacae P99. The bioactivity of the sample remained unaffected by the penicillinase or cephalosporinase treatment, indicating that the compound responsible is not cephamycin C or a β-lactam antibiotic. Samples of pure penicillin G and cephamycin C treated in parallel under the same conditions completely lost their bioactivities. No β-lactamase inhibitory activity characteristic of clavulanic acid was detected in the sample.
The bioactive compound produced by the S. clavuligerus ccaR::aph strain was found to be extractable with n-butanol and was active against Micrococcus luteus, Bacillus sp. strain ATCC 27860, E. coli E22-31, Klebsiella pneumoniae 29665, or Alcaligenes faecalis. In addition, the broth from cultures of the S. clavuligerus ccaR::aph strain grown in defined SA medium or GSPG medium was found to be slightly yellow, suggesting that the compound produced by this mutant might be holomycin. When butanol extracts were chromatographed on Silica Gel 60 plates with chloroform-methanol (9:1) as the solvent system, a large bioactive spot was found at an Rf of 0.5, which agrees with the Rf of pure holomycin in this solvent system (20). Therefore, we proceeded to purify the bioactive compound from an S. clavuligerus ccaR::aph culture supernatant.
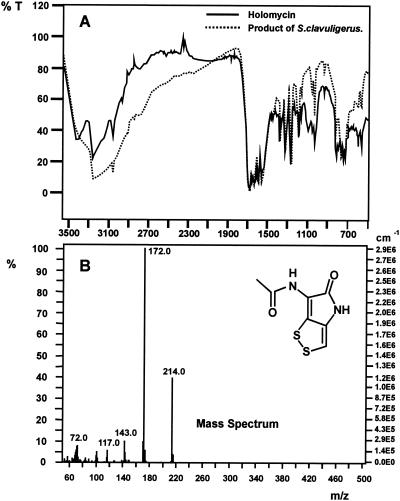
The UV spectrum of the purified compound in methanol showed peaks at 246, 300, and 384 nm, which are characteristic of the pyrroline ring. Moreover the IR spectrum was very similar to that of a pure preparation of holomycin provided by M. Okanishi (Fig. 1A) (21). The mass spectrum of the purified compound gave m/e peaks of 214 (M+) and 172 (M+-CH2CO), as well as peaks of 72 (C2H2SN) and 143 (C4H5SNO2), which correspond to fragmentation of the S-S linkage and the peptide linkage internal to the holomycin ring (Fig. 1B), indicating a compound with a mass of 213.98, which corresponds to the holomycin structure (Fig. 1B, inset).
FIG. 1.
(A) IR spectra of a pure sample of holomycin (solid line) and the compound purified from cultures in TSB medium of the S. clavuligerus ccaR::aph strain (dotted lane). (B) Mass spectra of the purified compound and chemical structure of holomycin.
Formation of holomycin by different strains of S. clavuligerus with disruption in genes of the clavulanic acid pathway.
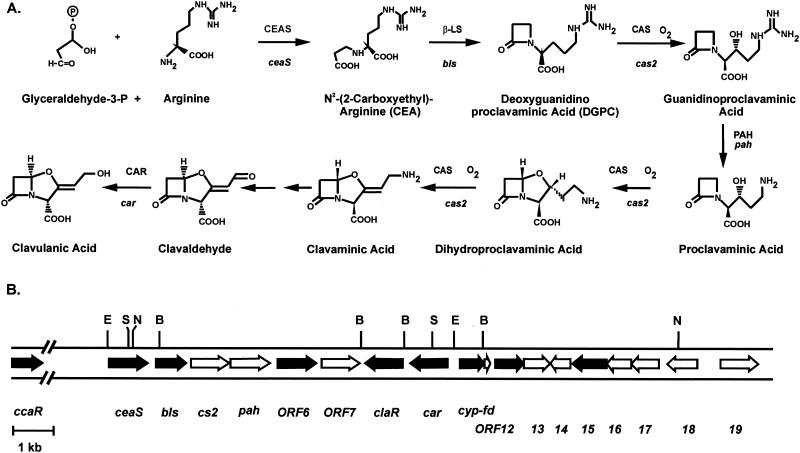
HPLC analysis showed that the wild-type strain S. clavuligerus 27064 produces negligible amounts of holomycin in both of the media (TSB and SA) and under all of the conditions used to grow this strain. Therefore, we were interested in knowing whether the increased formation of holomycin was related to the lack of production of any of the antibiotics produced by the strain. Ten clavulanic acid mutants with disruptions in different genes of the clavulanic biosynthesis pathway (Fig. 2 and Table 1) obtained by replacement with either the aph gene (conferring kanamycin resistance) or the apr gene (conferring apramycin resistance) were used for this study. They include mutants with deletions of the ceaS (pyc) gene, which encodes the carboxyethylarginine synthase (27); the bls gene, which encodes the β-lactam synthetase (2); the cla-ORF6 gene, which encodes a protein with ornithine acetyltransferase activity (7, 11, 28); the car gene, which encodes clavaldehyde reductase (26); the cyp gene, which encodes the cytochrome P450 involved in clavulanic acid formation (14, 17); the two genes cla-ORF12 and cla-ORF15, which are located downstream of cyp (16, 17); as well as mutants blocked in genes coding for the two β-lactam biosynthesis regulatory proteins, ccaR (25) and claR (26). In addition, the holomycin production of double mutants with disruptions in the ccaR and ORF6 genes was also studied.
FIG. 2.
(A) Pathway of clavulanic acid biosynthesis indicating the known intermediates and the genes encoding the different enzymes. (B) Organization of the clavulanic acid gene cluster. The disrupted genes in the mutant strains used in this work are indicated by black arrows.
The wild type strain and all of the mutant strains were grown in SA medium as indicated previously, and the aliquots were assayed as follows: (i) by immediate derivatization with imidazole to analyze the presence of compounds with clavam structure as indicated by Bird et al. (5) and quantification by HPLC (18); (ii) by using bioassays to test cephamycin C production; and (iii) by HPLC to analyze holomycin production. All of the disrupted mutants tested gave negative production of clavulanic acid, except for the S. clavuligerus ORF6::apr mutant, which produced clavulanic acid at levels 40% lower than those of the wild-type strain when grown in SA medium and up to 80% of those of the wild type when grown in TSB medium, indicating that ORF6 is not essential for clavulanic acid production.
The wild-type S. clavuligerus strain produces only traces of holomycin.
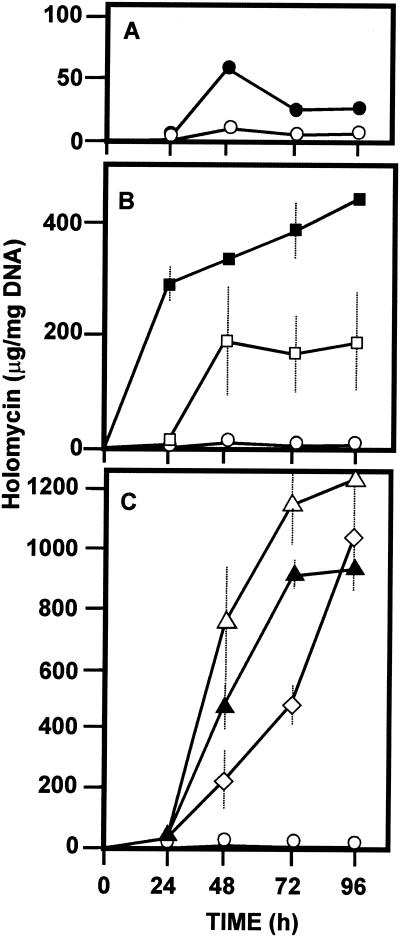
Culture broth from the S. clavuligerus wild-type strain 27064, grown as indicated above, showed an uniform production of holomycin of about 11 μg per mg of DNA, close to its detection limits, which is less than 20% of the holomycin produced by ccaR::aph S. clavuligerus at 48 h of culture (Fig. 3A).
FIG. 3.
Production of holomycin in SA medium by the wild-type S. clavuligerus strain ATCC 27064 (○) and the following clavulanic acid-nonproducing strains: (A) S. clavuligerus ccaR::aph (•); (B) S. clavuligerus claR::aph (▪) and S. clavuligerus car::aph (□); (C) S. clavuligerus cyp::aph (▵), S. clavuligerus ORF12::aph (▴), and S. clavuligerus ORF15::aph (⋄).
Clavulanic acid mutants blocked in the early steps of the pathway showed a holomycin-negative phenotype.
Two strains defective in early steps of the clavulanic acid pathway were totally unable to produce holomycin at any time during the fermentation. They were the S. clavuligerus ceaS::aph strain and strain RLF3, which is blocked in the bls gene. The lack of holomycin production by these strains is interesting. The ceaS gene encodes the first enzyme of the clavulanic acid pathway, condensing arginine with glyceraldehyde-3-phosphate (12); the bls gene is essential for cyclization of the β-lactam ring of clavulanic acid. Therefore, these mutants do not accumulate clavaminic acid or any other intermediate of the clavulanic acid pathway that might be related to holomycin formation
Also the S. clavuligerus ORF6::apr strain and the S. clavuligerus ccaR::aph ORF6::apr double mutant strain showed a holomycin-negative phenotype. ORF6 encodes an ornithine acetyltransferase of unknown function in clavulanic acid biosynthesis, and the S. clavuligerus ORF6::apr strain is able to produce clavulanic acid, as well as probably the intermediates that trigger holomycin biosynthesis, although at levels lower than those of the wild-type strain. Therefore, holomycin production in this strain might be below the limits of detection. Moreover, in the S. clavuligerus ccaR::aph ORF6::apr double mutant strain, the mutations in ORF6 reverse the positive effect exerted by the ccaR mutation on holomycin formation
High levels of holomycin are produced by mutants blocked in the late steps of the clavulanic acid pathway.
The S. clavuligerus car::aph and claR::aph strains showed a steady pattern of production, reaching holomycin levels of around 200 and 450 μg per mg of DNA at 96 h (Fig. 3B). Consistently, holomycin formation by the S. clavuligerus claR::aph strain was detected earlier than in the parental strain in several experiments. Both S. clavuligerus car::aph and claR::aph strains produced holomycin at levels 7- and 16-fold higher, respectively, than those of the S. clavuligerus ccaR::aph strain, in which holomycin was first detected and purified. A third group of clavulanic acid-nonproducing mutants, with the genotypes cyp::aph, ORF12::aph, and ORF15::aph (14, 16), showed very high levels of holomycin production, which reached up to 1,200 μg of holomycin per mg of DNA, or about 112-fold in relation to the wild-type strain and 45-fold in relation to the S. clavuligerus ccaR::aph strain at 96 h. The production of holomycin by the S. clavuligerus ORF15::aph strain reached levels similar to those of the S. clavuligerus cyp::aph strain at late times during the fermentation.
Effect of arginine addition on holomycin formation by the wild type and the clavulanic acid-nonproducing mutants.
In early studies designed to examine the effect of arginine addition on clavulanic acid formation, we observed a slightly yellow color in the culture broths of S. clavuligerus ccaR::aph cells. Therefore, a systematic study on the effect of addition of arginine (10 mM) to SA medium on the formation of holomycin by the clavulanic acid-nonproducing mutants was performed. The S. clavuligerus ccaR::aph, claR::aph, and cyp::aph strains produced consistently more holomycin in the presence of arginine, with increases ranging from 2- to 10-fold, depending on the strain and time of the culture. The arginine stimulatory effect was stronger at early times during the fermentation (24 to 48 h), and 24 to 48 h was the only time in which any effect was observed in the ORF15::aph strain. This result is probably due to an arginine precursor effect that favors accumulation of clavulanic acid intermediates (see Discussion).
Complementation of the cyp and ORF15 mutants reverses production of high levels of holomycin.
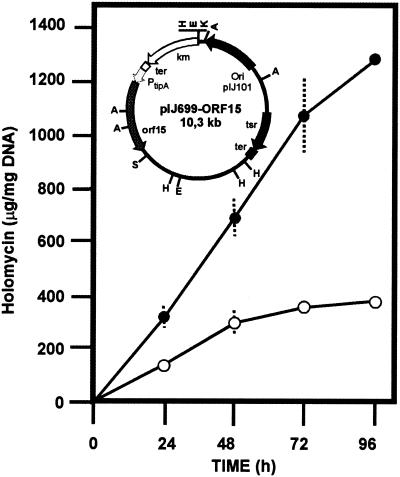
In order to determine whether the mutations in the clavulanic acid genes were responsible for the increasing production of holomycin, two disrupted mutants, S. clavuligerus cyp::aph and ORF15::aph strains, were transformed with control plasmids pULVK99 and pIJ699, and the mutations were complemented with plasmids containing ORF15 (pIJ699-ORF15) or the cyp gene (pVK99-cyp), in which those genes are expressed from the tipAp promoter (Fig. 4, inset) or their own promoter, respectively. These results showed (Fig. 4) that holomycin overproduction was clearly reduced (to 29% at 96 h) in the complemented S. clavuligerus ORF::15(pIJ699-ORF15) transformant. Similar results were observed in the S. clavuligerus cyp::aph(pVK99-cyp) transformant (not shown). The incomplete lack of reversion to nonproduction observed in the complemented ORF::15(pIJ699-ORF15) strain may be due to the presence of thiostrepton (4 μg/ml) in the cultures, which positively affects the biosynthesis of holomycin in both the control strain and the complemented strain (compare with Fig. 3).
FIG. 4.
Formation of holomycin by the disrupted S. clavuligerus mutant ORF15::aph(pIJ699) (•) strain and the S. clavuligerus ORF15::aph(pIJ699-ORF15) (○) complemented strain. (Inset) Physical map of plasmid pIJ699-ORF15 used in the complementation studies. tsr and aph correspond to the thiostrepton and kanamycin resistance genes, respectively. Ter, terminator (from pIJ699). PtipA, tipA gene promoter. The restriction sites for HindIII (H), ApaI (A), SalI (S), and KpnI (K) are shown.
Holomycin synthase activity in wild-type S. clavuligerus and mutants blocked in clavulanic acid biosynthesis.
Holothin was prepared by hydrolysis of holomycin by the method described by Gaeumann et al. (9).
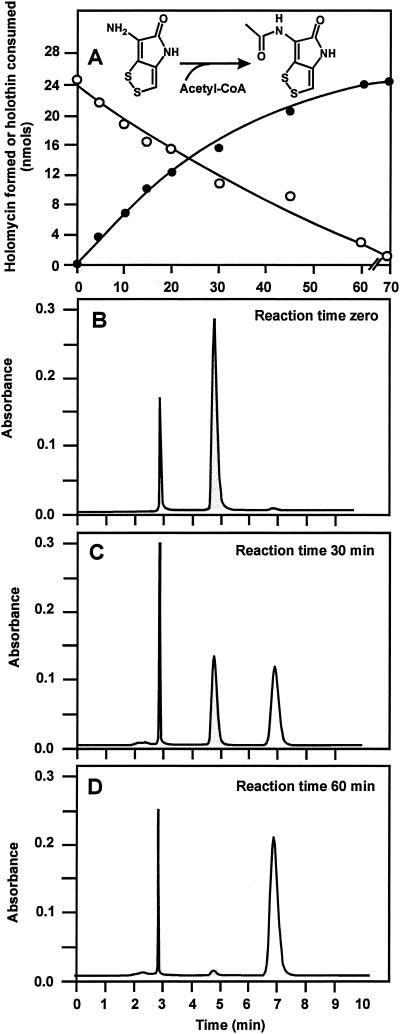
Holomycin synthase activity was assayed with holothin as the acceptor substrate and either acetylglutamic acid, acetylornithine, acetylarginine, or acetyl-CoA at a concentration of 0.5 or 1 mM as the acetyl group donor. Formation of holomycin from holothin was monitored by HPLC under the conditions indicated above (Fig. 5). Only acetyl-CoA was used as acetyl donor in the reaction, and no holomycin was formed with other acetylated compounds as acetyl donors. The reaction was linear for up to 20 min for enzyme activities in the order of 0.1 to 0.8 mU/μg of protein under the standard assay conditions; therefore, all of the enzyme preparations were diluted appropriately before holomycin synthase activity was measured. As shown in Fig. 5, the reaction is irreversible, and the entire amount of holothin in the sample is converted to holomycin if enough reaction time is allowed (Fig. 5D).
FIG. 5.
Conversion of holothin into holomycin. (A) Holothin consumed (○) and holomycin formed (•) during the reaction. (B to D) Holomycin (Rt, 6.7 min) and holothin (Rt, 4.7 min) present in the reaction at 0 min (B), 30 min (C), and 60 min (D), as measured by HPLC. The holothin peak is shaded. The level of holomycin synthase activity in the reaction was 0.7 mU/μg of protein.
The wild-type S. clavuligerus strain and the mutant strains described above were analyzed for holomycin synthase activity throughout the fermentation. Holomycin synthase activities in the different strains correlate well with their holomycin production, but not in a strictly proportional manner. In general, the level of holomycin synthase activity was higher at 72 h of culture. The holomycin-nonproducing S. clavuligerus single mutant ceaS::aph, RLF3, and ORF6::apr strains or the double mutant ccaR::aph ORF6::apr strain generally showed levels of holomycin synthase activity below those of the wild-type strain, except for the 72-h data for the ORF6::apr and ceaS::aph strains (Table 2). The S. clavuligerus ccaR::aph, car::aph, and claR::aph strains contained 4- to 17-fold more activity than the parental strain in 72-h samples, which correlates well with their higher levels of holomycin production. Finally, the high-holomycin-producing strains blocked in the cyp, ORF12, and ORF15 genes showed holomycin synthase activities 25- to 60-fold higher than that of the wild-type strain at 72 h of culture.
TABLE 2.
Holomycin synthase activity in different strains of S. clavuligerusa
| Type of S. clavuligerus strain | Holomycin synthase activity (mU) atb:
|
|
|---|---|---|
| 48 h | 72 h | |
| 27064 | 3.3 (1.0) | 2.4 (1.0) |
| ORF6::apr | 2.7 (0.81) | 3.9 (1.6) |
| ORF6::apr ccaR::aph | 0.8 (0.24) | 1.7 (0.7) |
| ceaS::aph | 1.5 (0.45) | 3.7 (1.5) |
| RF3 (bls::aph) | 1.2 (0.36) | 1.8 (0.7) |
| ccaR::aph | 6.7 (2.0) | 9.7 (4.0) |
| claR::aph | 1.9 (0.6) | 42.2 (17.5) |
| car::aph | 2.9 (0.9) | 9.3 (3.8) |
| cyp::aph | 45.8 (13.9) | 141.0 (58.7) |
| ORF12::aph | 38.3 (11.6) | 62.8 (26.2) |
| ORF15::aph | 136.2 (41.3) | 147.5 (61.5) |
Fermentations to assay holomycin synthase were performed in duplicate. Assays for holomycin synthase were performed in duplicate for each fermentation.
Values in parentheses represent fold activity relative to that of the wild type.
DISCUSSION
Early work by Kenig and Reading (10) indicated that an uncharacterized S. clavuligerus mutant produced large amounts of holomycin. This strain might correspond to any of the mutants shown in Fig. 3B and C. The role of some genes of the clavulanic acid cluster is well established (3, 15), but the function of other genes of the cluster is far from clear. Very little is known about the cyp, ORF12, or ORF15 genes, disruption of which results in a large accumulation of holomycin. It has been suggested that the cyp-fd gene and probably the protein encoded by ORF12 are involved in the poorly characterized oxidation steps between clavaminic and clavulanic acid (14, 23). The ClaR regulatory protein appears to control the expression of late genes of the pathway: i.e., car and cyp (23). In fact both the claR mutant and therefore the cyp- and car-disrupted mutants accumulate clavaminic acid in the culture supernatant (23), which may act as an inducer of the holomycin pathway.
There are no studies on the holomycin biosynthetic pathway, but tentatively, the compound is formed from two cysteine molecules (8) or perhaps from the condensation of cysteine and 2-amino-ethanethiol, compounds clearly unrelated to clavulanic acid, clavulanic biosynthesis intermediates, or clavulanic acid precursors.
The high level of production of holomycin by the mutant strains studied in this work suggests a role, probably regulatory, of an intermediate of the clavulanic acid (e.g., clavaminic acid or other intermediates between proclavaminic acid and clavaminic acid) in the formation of holomycin. Indeed, mutants blocked in early genes of the pathway, such as the mutants blocked in the ceaS or bls genes, showed a holomycin-negative phenotype (i.e., are unable to produce holomycin). The wild-type S. clavuligerus strain 27064, which produces low levels of clavulanic acid and does not accumulate a high enough concentration of the putative intermediate to trigger holomycin biosynthesis, is also a low holomycin producer. The stimulatory effect of arginine on holomycin production by all of the strains tested might be interpreted in the same sense. Arginine stimulates the biosynthesis of clavulanic acid in S. clavuligerus (30) as a limiting precursor of the C5 fragment of the β-lactamase inhibitor, but in mutants with mutations in the genes car, cyp, and claR blocked in late steps of the pathway, this stimulation by arginine of the first part of the pathway results in even higher accumulation of clavaminic acid or other intermediates of the middle steps of the pathway.
The role of cla-ORF6 is especially intriguing. This gene encodes a relatively unspecific N-acetyltransferase, homologous to the argJ gene, and the proteins encoded by both genes possess ornithine-n-acetyltransferase activity (7, 11, 28). Despite producing clavulanic acid at 40% of the wild-type level, this mutant is totally unable to form holomycin under any of the conditions tested, thus excluding that clavulanic acid itself is an inducer of the holomycin pathway. There is a good correlation between the holomycin synthase activity and the level of holomycin produced by the different mutants blocked in clavulanic acid biosynthesis. The acetylation catalyzed by this enzyme is essential for antibiotic activity, since the deacetylated compound holothin is 100-fold less active than holomycin against Bacillus and Micrococcus strains (9). Dithiopyrrolone compounds, which include holomycin, have been described as having potential antitumor activity (33). The use of the mutant strains described in this work and the higher level of production of holomycin in the presence of arginine are extremely useful tools to increase the yield of this antibiotic.
Acknowledgments
This work was supported by grants 1FD97-1419-CO2-O2 and BIO2000-0272 from the Spanish Ministry of Education and Culture. A. de la Fuente was supported by a Fellowship of the Spanish Ministry of Science and Technology (Madrid, Spain).
We acknowledge M. Okanishi for providing a sample of pure holomycin and R. Pérez-Redondo and C. A. Townsend for the gifts of the S. clavuligerus ceaS::aph strain and Streptomyces sp. strain RLF3, respectively. We thank A. Rumbero for help with interpretation of the IR and MS spectra of holomycin.
REFERENCES
- 1.Alexander, D. C., M. J. Brumlik, L. Lee, and S. E. Jensen. 2000. Early cephamycin biosynthetic genes are expressed from a polycistronic transcript in Streptomyces clavuligerus. J. Bacteriol. 182:348-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann, B. O., R. Li, and C. A. Townsend. 1998. Beta-lactam synthetase: a new biosynthetic enzyme. Proc. Natl. Acad. Sci. USA 95:9082-9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggaley, K. H., A. G. Brown, and C. J. Schofield. 1997. Chemistry and biosynthesis of clavulanic acid and other clavams. Nat. Prod. Rep. 140:309-333. [DOI] [PubMed] [Google Scholar]
- 4.Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 5.Bird, A. E., J. M. Bellis, and B. C. Gasson. 1982. Spectrophotometric assay of clavulanic acid by reaction with imidazole. Analyst 107:1241-1245. [Google Scholar]
- 6.Chary, V. K., J. L. de la Fuente, P. Liras, and J. F. Martín. 1997. amy as a reporter gene for promoter activity in Nocardia lactamdurans: comparison of promoters of the cephamycin cluster. Appl. Environ. Microbiol. 63:2977-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Fuente, A. 2002. Caracterización de la agrupación de genes de biosíntesis de arginina y su relación con la producción de ácido clavulánico en S. clavuligerus. Ph.D. thesis. University of León, León, Spain.
- 8.Ellis, J. E., J. H. Fried, I. T. Harrison, E. Rapp, and C. H. Ross. 1977. Synthesis of holomycin and derivatives. J. Org. Chem. 42:2891-2893. [DOI] [PubMed] [Google Scholar]
- 9.Gaeumann, E., V. Prelog, and E. Vischer. December1961. Holothin and derivatives thereof. U.S. patent 3.014.922.
- 10.Kenig, M., and C. Reading. 1979. Holomycin and an antibiotic (MM19290) related to tunicamycin, metabolites of Streptomyces clavuligerus. J. Antibiot. 32:549-554. [DOI] [PubMed] [Google Scholar]
- 11.Kershaw, N. J., H. J. McNaughton, K. S. Hewitson, H. Hernández, J. Griffin, C. Hughes, P. Greaves, B. Barton, C. V. Robinson, and C. J. Schofield. 2002. ORF6 from the clavulanic acid gene cluster of Streptomyces clavuligerus has ornithine acetyltransferase activity. Eur. J. Biochem. 269:2052-2059. [DOI] [PubMed] [Google Scholar]
- 12.Khaleeli, N., R. Li, and C. A. Townsend. 1999. Origin of the β-lactam carbons in clavulanic acid from an unusual thiamine pyrophosphate-mediated reaction. J. Am. Chem. Soc. 121:9223-9224. [Google Scholar]
- 13.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, U.K.
- 14.Li, R., N. Khaleeli, and C. A. Townsend. 2000. Expansion of the clavulanic acid gene cluster: identification and in vivo functional analysis of three new genes required for biosynthesis of clavulanic acid by Streptomyces clavuligerus. J. Bacteriol. 182:4087-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liras, P., and A. Rodríguez-García. 2000. Clavulanic acid, a β-lactamase inhibitor: biosynthesis and molecular genetics. Appl. Microbiol. Biotechnol. 54:467-475. [DOI] [PubMed] [Google Scholar]
- 16.Lorenzana, L. M. 2002. Agrupacion de genes de biosíintesis de ácido clavulánico en Streptomyces clavuligerus. Análisis de la región corriente arriba del gen car. Ph.D. thesis. University of León, León, Spain.
- 17.Mellado, E., L. M. Lorenzana, M. Rodríguez-Sáiz, B. Díez, P. Liras, and J. L. Barredo. 2002. The clavulanic acid biosynthesis cluster of Streptomyces clavuligerus: analysis of the DNA region upstream of the car gene. Microbiology 148:1427-1438. [DOI] [PubMed] [Google Scholar]
- 18.Mosher, R. H., A. S. Paradkar, C. Anders, B. Barton, and S. E. Jensen. 1999. Genes specific for the biosynthesis of clavam metabolites antipodal to clavulanic acid are clustered with the gene for clavaminate synthase 1 in Streptomyces clavuligerus. Antimicrob. Agents Chemother. 43:1215-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholson, N. H., K. H. Baggaley, R. Cassels, M. Davison, S. W. Elson, M. Fulston, J. W. Tyler, and S. R. Woroniecki. 1994. Evidence that the immediate biosynthetic precursor of clavulanic acid is its N-aldehyde analogue. J. Chem. Soc. Chem. Commun. 1994:1281-1282. [Google Scholar]
- 20.Okamura, K., K. Soga, Y. Shimauchi, T. Ishikura, and J. Lein. 1977. Holomycin and N-propionylholothin, antibiotics produced by a cephamycin C producer. J. Antibiot. 30:334-336. [DOI] [PubMed] [Google Scholar]
- 21.Okanishi, M. 1979. Plasmids and antibiotic synthesis in streptomycetes, p. 134-140. In O. K. Sebek and A. I. Laskin (ed.), Genetics of industrial microorganisms. American Society for Microbiology, Washington, D.C.
- 22.Omura, S., H. Ikeda, J. Ishikawa, A. Hanamoto, C. Takahashi, M. Shinose, Y. Takahashi, H. Horikawa, H. Nakazawa, T. Osonoe, H. Kikuchi, T. Shiba, Y. Sakaki, and M. Hattori. 2001. Genome sequence of an industrial microorganism, Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. USA 98:12215-12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paradkar, A. S., K. A. Aidoo, and S. E. Jensen. 1998. A pathway-specific transcriptional activator regulates late steps of clavulanic acid biosynthesis in Streptomyces clavuligerus. Mol. Microbiol. 27:831-843. [DOI] [PubMed] [Google Scholar]
- 24.Paradkar, A. S., and S. E. Jensen. 1995. Functional analysis of the gene encoding the clavaminate synthase 2 isoenzyme involved in clavulanic acid biosynthesis in Streptomyces clavuligerus. J. Bacteriol. 177:1307-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez-Llarena, F., P. Liras, A. Rodríguez-García, and J. F. Martín. 1997. A regulatory gene (ccaR) required for cephamycin and clavulanic acid production in Streptomyces clavuligerus: amplification results in overproduction of both β-lactam compounds. J. Bacteriol. 179:2053-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Redondo, R., A. Rodríguez-García, J. F. Martín, and P. Liras. 1998. The claR gene of Streptomyces clavuligerus, encoding a LysR-type regulatory protein controlling clavulanic acid biosynthesis, is linked to the clavulanate-9-aldehyde reductase (car) gene. Gene 211:311-321. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Redondo, R., A. Rodríguez-García, J. F. Martín, and P. Liras. 1999. Deletion of the pyc gene blocks clavulanic acid biosynthesis except in glycerol-containing medium: evidence for two different genes in formation of the C3 unit. J. Bacteriol. 181:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez-García, A., A. de la Fuente, R. Pérez-Redondo, and P. Liras. 2000. Characterization and expression of the arginine biosynthesis gene cluster of Streptomyces clavuligerus. Mol. Microbiol. Biotechnol. 2:543-550. [PubMed] [Google Scholar]
- 29.Romero, J., P. Liras, and J. F. Martín. 1984. Dissociation of cephamycin and clavulanic acid biosynthesis in Streptomyces clavuligerus. Appl. Microbiol. Biotechnol. 20:318-325. [Google Scholar]
- 30.Romero, J., P. Liras, and J. F. Martín. 1986. Utilization of ornithine and arginine as specific precursors of clavulanic acid. Appl. Environ. Microbiol. 52:892-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and J. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Santamarta, I., A. Rodríguez-García, R. Pérez-Redondo, J. F. Martín, and P. Liras. 2002. CcaR is an autoregulatory protein that binds to the ccaR and cefD-cmcI promoters of the cephamycin C-clavulanic acid cluster in Streptomyces clavuligerus. J. Bacteriol. 184:3106-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webster, J. M., J. Li, and G. Chen. February 2000. Anticancer properties of dithiopyrrolones. U.S. patent 6020360.