Abstract
Sinorhizobium meliloti growth inside infection threads was monitored after inoculation of alfalfa with red- or green-tagged bacteria. Most threads were populated with single bacterial types. Mixed infections were present but gave mixed nodules less often than expected. These patterns are explained by a model describing bacterial growth during infection.
Sinorhizobium meliloti cells grow as free-living organisms but also as nitrogen-fixing symbionts inside root nodule cells of alfalfa and related species (2, 4, 10-13). Bacteria must get from the root surface to inner root tissue, where they will populate nodule cells. Thus, they grow and divide inside a plant-derived tubule, called an infection thread (7-9). Infection threads grow down host root hairs, and S. meliloti grows and divides inside the thread, keeping it filled. The thread branches during growth through root tissue, and bacteria eventually exit the resulting network and enter the cytoplasm of nodule cells.
Studies of mixed infections during symbiosis can lead to insights into the infection and invasion process because they allow investigators to discern spatially distinct subpopulations of bacteria, and thus, something about growth and behavior of the whole population can be inferred. Earlier studies showed that mixing fluorescent and nonfluorescent S. meliloti gave infection threads that contained both types of bacteria. Both strains entered the infection thread but did not randomly mix. Instead, the growing bacteria formed sectors that apparently originated from the clonal expansion of a few founder cells (8).
In this study, I inoculated alfalfa with mixtures of S. meliloti tagged with red or green fluorescent proteins. This allowed observation of all bacteria inside infection threads and showed that infection was more complicated that previously thought.
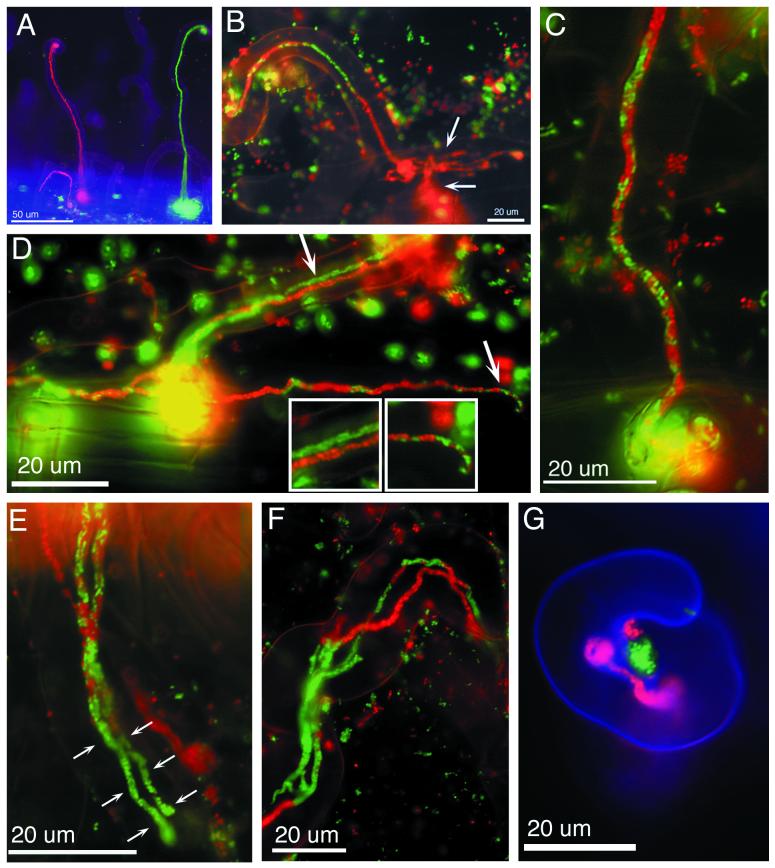
Alfalfa seedlings placed on microscope slides covered with BNM agar (8) were inoculated with a 1:1 mixture of two S. meliloti strains. Strain L5-30/pDG71(ptrp-Gfpmut3) constitutively produced Gfp(mut3) under the control of a Salmonella enterica serovar Typhimurium trp promoter (3, 6), and strain L5-30/pDG77(ptrp-DsRed) constitutively produced DsRed. Five days after inoculation, green infection threads were seen along the length of the root, but red threads were seen only near the top of the root. This was likely because DsRed matures slowly compared to Gfp(mut3) (1). After 7 to 10 days, red and green infection threads were visible all along the root (Fig. 1A). Sectored infection threads containing red and green bacteria were readily observed (Fig. 1B).
FIG. 1.
Examples of mixed infection threads following coinoculation of alfalfa with red and green fluorescent bacteria. (A) Infection threads containing only Gfp-expressing or DsRed-expressing S. meliloti. (B) Sectored infection thread in which the mixed population gave rise to a series of sectors which increase in length along the thread. The tipmost sector advanced into the epidermal cell body, branched (top arrow), and penetrated the underlying cell (bottom arrow), leaving the other sectors behind. (C) Jumbled type of mixed infection thread. Gfp-expressing and DsRed-expressing cells appear randomly mixed throughout the infection thread. (D) Dual-type mixed infection thread. The infected root hair contained two infection threads, one filled with Gfp-expressing bacteria and the other filled with DsRed-expressing bacteria (top arrow and left inset). These two infection threads fused together at the base of the root hair (yellow sphere), and new infection threads grew from the fusion point. These new threads contained green and red bacteria mixed together in a jumbled fashion (bottom arrow and right inset). (E) Dual infection threads inside a root hair. The threads began as jumbled-type threads but later gave rise to green sectors at their tips (arrows show the extent of the sectors in each thread). (F) Root hair infected with dual infection threads. Each thread contains sectors of Gfp-expressing and DsRed-expressing bacteria. (G) Curled root hair with red and green microcolonies of S. meliloti in the interior bend of the curl. The red microcolony has given rise to an infection thread containing only DsRed-expressing bacteria.
In addition, other types of mixed infection threads were seen. Threads with a jumbled population of red and green bacteria and root hairs containing dual infection threads where one was red and one was green were observed (Fig. 1C and D). Even this classification is a simplification because jumbled threads often gave rise to threads with sectors (Fig. 1E). Root hairs sometimes contained dual threads, each of which was sectored (Fig. 1F). Dual threads containing bacteria of a single type could fuse and then form threads that contained red and green bacteria jumbled together (Fig. 1D).
Not all infection threads invade nodules; most terminate, and accompanying nodules never develop (14). An unanswered question is whether mixed infection threads that do deliver bacteria to nodules give rise to nodules that contain mixed populations of bacteria. If so, then the portion of threads that contain mixed populations should equal the portion of nodules that contain mixed populations. To test this, alfalfa seedlings were inoculated with a 1:1 mixture of L5-30/pDG71(ptrp-Gfpmut3) and L5-30/pDG77(ptrp-DsRed) at densities of 105, 106, 107, and 108 cells per slide. Slides were observed 7 to 10 days later, and threads were classified as red, green, or mixed. Nodules were allowed to develop for a total of 20 to 30 days. Selected nodules were surface sterilized, crushed, and plated on selective agar. Colonies were observed to determine if the population inside each nodule was mixed. I excluded lobed nodules and nodules from tight clusters from analysis because these may have arisen from closely spaced infection threads of different colors.
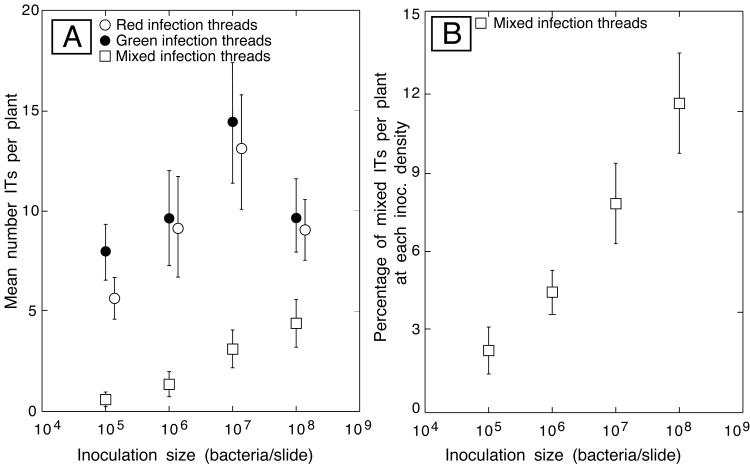
Figure 2 shows results from experiments in which 2,546 infection threads were classified. At the time I had not yet classified dual threads as a type of mixed infection; therefore, the mixed category included only sectored and jumbled types. Infection threads per plant increased with increasing inoculum size, except on plants inoculated with the highest number of bacteria. Note that these experiments revealed only mixed infections that started when red and green cells populated an infection thread. Cases where two independent green, or red, cells populated a thread could not be discerned and counted as mixed. In another set of experiments, 1,109 threads were observed and classified according to type—unmixed, dually infected, jumbled, or sectored. These experiments showed that sectored infection threads were the most prevalent type of mixed thread and accounted for slightly less than half of all mixed threads (Table 1).
FIG. 2.
Quantification of infection thread (IT) types following coinoculation of alfalfa seedlings with Gfp-expressing and DsRed-expressing S. meliloti. (A) Plant roots were examined by epifluorescence 7 to 10 days after inoculation with the indicated number of bacteria, and the number of red, green, and mixed (sectored and jumbled) infection threads per plant was determined. (B) Data for mixed infection threads replotted as the percentage of total infection threads per plant at each inoculation density.
TABLE 1.
Infection thread types following coinoculation with red- and green-tagged S. melilotia
| Thread type | No. (%) of type |
|---|---|
| Total | 1,109 |
| Mixedb | 169 (15.2) |
| Dual | 34 (3.1) |
| Jumbled | 57 (5.1) |
| Sectored | 78 (7.0) |
Results of two experiments in which the three types of mixed infection threads were categorized.
Total of dual, jumbled, and sectored types.
Of 170 nodules, 146 contained bacteria that grew on selective plates; 10 of the 146 contained both red and green strains. Thus, the portion of nodules that contained mixed populations (7.4%) was less than the portion of threads that contained mixed infections (15.2%) (Table 1). This indicated that at most, half of mixed infection threads gave rise to nodules populated with both types of bacteria. Half is a maximum because some mixed nodules may have arisen from closely spaced infection threads containing bacteria of different colors.
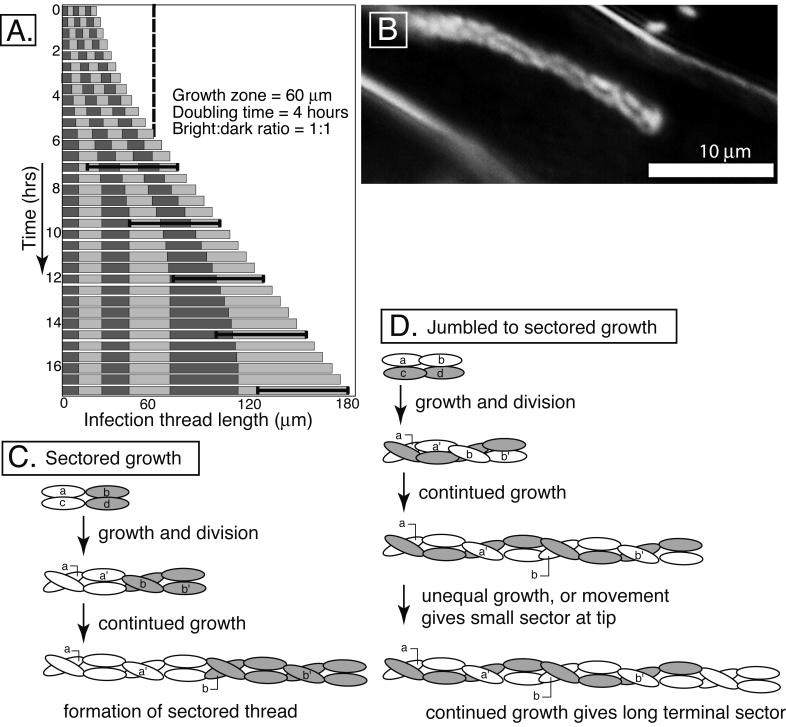
A model describing the dynamics of bacterial growth inside of infection threads was developed. In the model, bacterial growth rate, growth zone size, number of founder cells, and the ratio of marked strains can be specified. Sizes and placement of sectors are calculated and presented numerically and graphically. The modeling program is at http://www.sp.uconn.edu/∼gage/Pages/research.html. For Fig. 3A, the model was started with a small thread containing six alternating equal-sized sectors. The growth zone was set at 60 μm; bacteria which were that distance, or less, from the tip of the thread grew and divided with a doubling time of 4 h. Modeling indicates that the size of the penultimate sector should be equal to the size of the growth zone divided by ln2. Based on the observed sizes of these sectors, the 60-μm growth zone is likely typical of real growth zone sizes.
FIG. 3.
Models of infection thread growth. (A). A computer model was used to simulate sectored infection thread growth. For the case shown, it was assumed that the thread started with six alternating bright and dark sectors of equal size. The doubling time for the growing bacteria (those 60 μm or less from the growing tip of the thread) was 4 h. The black brackets indicate the 60-μm growth zone. Each row is a representation of the length of the thread and its six sectors at each time point. Growth of the thread is exponential until it reaches 60 μm in length (dashed line) and becomes linear thereafter, when only cells in the tipmost 60 μm contribute to subsequent growth. Once the tipmost sector is more than 60 μm in length, it will be the only sector which continues to increase in size (see the last column for an example). (B) Micrograph of Gfp-labeled S. meliloti inside an infection thread. Note the braided appearance of the population, which is typical for growing infection threads. Threads that have terminated growth often lose this braided appearance. (C) Diagram of a small, two-column infection thread giving rise to a sectored infection thread. (D) Diagram of a small, two-column infection thread giving rise to a jumbled infection thread and later conversion into a sectored thread. The braiding of columns populated with single bacterial clones of different colors (Fig. 2D) would give rise to the appearance of a random arrangement of bacteria, especially in cases where three or more columns are involved. Lowercase letters indicate the original cells; lowercase letters with primes indicate first-generation daughter cells.
The model predicts that sector size will increase along the length of the thread. This occurs because sectors at the rear stop growing earlier than those near the advancing tip. Such increases in sector length are commonly seen in sectored threads (Fig. 1B and F) (8). It also predicts that sectored threads would result in nodules populated with only one of the two strains. This occurs because the only subpopulation that continues to multiply indefinitely are descendants of the bacteria which were at the tip of the thread when it initiated. All founder cells which were not at the very tip will eventually fall behind the growth zone and cease to increase in number. A concrete example of this is shown in Fig. 1B.
The quantitative data presented above also support the model. The percentage of nodules containing both red- and green-tagged bacteria (7.4%) was about half of what was expected based on the percentage of mixed threads (15.2%). Therefore, at most, half the mixed infections gave rise to nodules populated with both bacterial types. In addition, the percentage of mixed but nonsectored threads was 8.2%. This agrees well with the percentage of nodules containing red and green bacteria. Thus, the data are consistent with the idea that jumbled and dual-type threads are responsible for populating the mixed nodules.
The random distribution of bacteria in jumbled-type infection threads appears to conflict with the growth model. However, the fact that jumbled threads often become sectored suggests that the growth patterns of these types are not too different. An addition to the model can explain jumbled growth (Fig. 3). Actively growing threads contain two or three braided columns of bacteria (8) (Fig. 3B). Sectored growth should occur when bacteria of a single type load into the thread side by side (Fig. 3C). When similar types of bacteria initially load end to end, threads which appear jumbled should result (Fig. 3D). If, through faster growth, or random movement, the tipmost bacteria of a jumbled thread become a single type, then continued growth should eventually result in the appearance of a large terminal sector (Fig. 3D and 1E).
Theoretical work suggests that the frequency at which mixed nodules occur has important implications for the evolution of rhizobium-host symbioses (5). My work suggests that bacterial growth patterns inside infection threads can influence whether nodules contain mixed or single strains of bacteria. Thus, such growth patterns may ultimately influence the ecology and evolution of this rhizobium-plant symbiosis.
Acknowledgments
This work was supported by a grant from the National Science Foundation (IBN9974483) and by the University of Connecticut Research Foundation.
REFERENCES
- 1.Baird, G. S., D. A. Zacharias, and R. Y. Tsien. 2000. Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc. Natl. Acad. Sci. USA 97:11984-11989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewin, N. J. 1991. Development of the legume root nodule. Annu. Rev. Cell Biol. 7:191-226. [DOI] [PubMed] [Google Scholar]
- 3.Bringhurst, R. M., Z. G. Cardon, and D. J. Gage. 2001. Galactosides in the rhizosphere: utilization by Sinorhizobium meliloti and development of a biosensor. Proc. Natl. Acad. Sci. USA 98:4540-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denarie, J., F. Debelle, and J. C. Prome. 1996. Rhizobium lipo-chitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 65:503-535. [DOI] [PubMed] [Google Scholar]
- 5.Denison, R. F. 2000. Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am. Nat. 156:567-576. [DOI] [PubMed] [Google Scholar]
- 6.Egelhoff, T. T., and S. R. Long. 1985. Rhizobium meliloti nodulation genes: identification of nodDABC gene products, purification of nodA protein, and expression of nodA in Rhizobium meliloti. J. Bacteriol. 164:591-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahraeus, G. 1957. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J. Gen. Microbiol. 16:374-381. [DOI] [PubMed] [Google Scholar]
- 8.Gage, D. J., T. Bobo, and S. R. Long. 1996. Use of green fluorescent protein to visualize the early events of symbiosis between Rhizobium meliloti and alfalfa, Medicago sativa. J. Bacteriol. 178:7159-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gage, D. J., and W. Margolin. 2000. Hanging by a thread: invasion of legume plants by rhizobia. Curr. Opin. Microbiol. 3:613-617. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch, A. M. 1992. Developmental biology of legume nodulation. New Phytol. 122:211-237. [DOI] [PubMed] [Google Scholar]
- 11.Long, S. R. 1996. Rhizobium symbiosis: Nod factors in perspective. Plant Cell 8:1885-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mylona, P., K. Pawlowski, and T. Bisseling. 1995. Symbiotic nitrogen fixation. Plant Cell 7:869-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spaink, H. P. 1995. The molecular basis of infection and nodulation by rhizobia: the ins and outs of sympathogenesis. Annu. Rev. Phytopathol. 33:345-368. [DOI] [PubMed] [Google Scholar]
- 14.Vasse, J., F. de Billy, and G. Truchet. 1993. Abortion of infection during the Rhizobium meliloti-alfalfa symbiotic interaction is accompanied by a hypersensitive reaction. Plant J. 4:555-566. [Google Scholar]