Abstract
The developmentally regulated gene dofA, identified from pulse-labeling experiments by two-dimensional gel electrophoresis, and its homologue, dofB, were cloned and characterized in Myxococcus xanthus. Deletion of dofA and dofB did not affect the vegetative growth and development of M. xanthus. dofA was specifically expressed during development, while dofB expression was observed during vegetative growth and development. The dofA-lacZ fusion was introduced into a fruA mutant and A, B, C, D, and E extracellular signal mutants. The pattern of dofA expression in the C signal mutant was similar to that of the wild-type strain, while dofA expression was not detected in the fruA mutant. These results are consistent with those of the pulse-labeling experiments. dofA expression was reduced in A and E signal mutants, whereas dofA expression was delayed in B and D signal mutants. The patterns of expression of the dofA gene in the fruA mutant and the five signal mutants are strikingly similar to that of the tps gene, which encodes protein S, a major component of the outer surface of the myxospore; this result suggests that the dofA and tps genes are similarly regulated. The involvement of a highly GC-rich inverted repeat sequence (underlined), CGGCCCCCGATTCGTCGGGGGCCG, in developmentally regulated dofA expression is suggested.
Myxococcus xanthus, a gram-negative soil bacterium, undergoes multicellular development (14, 36). Upon nutrient starvation at a high cell density on a solid surface, cells move by gliding into aggregation centers, where they form mounds consisting of approximately 105 cells. Within the mounds, the motile, rod-shaped cells differentiate into nonmotile, spherical myxospores. The mounds of myxospores are referred to as fruiting bodies.
Cell-to-cell communications via the exchange of five extracellular signals, A, B, C, D, and E signals, are essential for the expression of a large number of developmental genes. These genes are required for the execution of the normal process of fruiting body formation in M. xanthus (11, 18, 26, 37). The requirement for each signal in the expression of many developmental genes was established from an analysis of developmental Tn5 lac marker expression and fruiting body morphogenesis in signal mutants (12, 28, 29, 31). The fruA gene encodes a putative transcription factor belonging to a family of DNA-binding response regulator proteins in the His-Asp phosphorelay system (15, 32). FruA was found to be essential for fruiting body formation and sporulation. Based on these results, a model of the roles of FruA in the C signaling cascade was proposed (15, 20, 38).
The tps gene, encoding protein S, a major component of the outer surface of the myxospore, and its developmentally regulated expression have been well characterized (22, 23, 26). The tps gene is expressed at low levels during vegetative growth, and its expression is highly induced at 5 h poststarvation (7, 10, 13). Developmentally regulated tps expression is absolutely dependent on the A and E signals and FruA, partially dependent on the B and D signals, and independent of the C signal (6, 11, 17, 28, 30, 32).
To investigate the roles of FruA and CsgA (C signal) in developmental gene expression, M. xanthus wild-type, fruA::Tc, and csgA731 cells were pulse-labeled with [35S]methionine during vegetative growth and development (20). An analysis of whole-cell proteins by two-dimensional immobilized pH gradient-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) (2D gel analysis) revealed that the rates of synthesis of more than 150 proteins in wild-type cells were down-regulated in comparison to those in vegetative cells at 12 h poststarvation, while the rates of synthesis of more than 100 proteins were up-regulated. The expression of approximately half of the up-regulated proteins was dependent on both FruA and CsgA, while the regulation of the other half was independent of FruA and CsgA. Several proteins, including DofA and protein S (Tps), were expressed in a fruA-dependent but csgA-independent manner. A model was proposed in which the two types of FruA-dependent expression may be regulated by different levels of FruA phosphorylation. The dofA gene was previously cloned by using degenerate oligonucleotides based on the amino acid sequences of two tryptic peptides analyzed by tandem mass spectrometry, and the nucleotide sequence of the gene was subsequently determined (20).
In this study, the expression of the dofA and dofB genes was characterized during the M. xanthus life cycle. dofA expression was specific to development, while dofB expression was observed during vegetative growth and development. The upstream promoter region required for the developmental expression of the dofA gene was investigated. The construction and characterization of M. xanthus ΔdofA, ΔdofB, and ΔdofAB mutants revealed that the two genes are dispensable for vegetative growth and development under the conditions tested.
MATERIALS AND METHODS
Bacterial strains and plasmids.
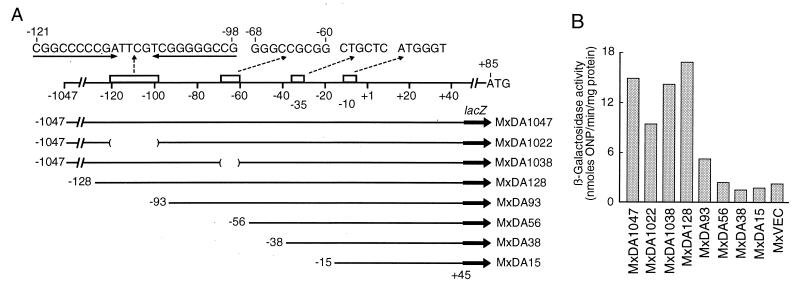
Bacterial strains and plasmids used in this work are listed in Table 1. See Fig. 6 for a description of MxDA1047 to MxDA15 and MxVEC.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| E. coli DH5α | supEA4 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-I relA1 | 19 |
| M. xanthus | ||
| DZF1 | sglA1 | 23 |
| MO1 | fruA::Tc Ω5 | 32 |
| DK731 | csgA731 | 37 |
| DK3068 | dsgA439 TcrsglA1 | 11 |
| JD275 | esg::Tn5 Ω258 Kmr | 11 |
| JD276 | asgA476 Kmr | 11 |
| JD278 | bsgA330 Tcr | 11 |
| TH1 | sglA1 ΔdofA::Km | This work |
| TH2 | sglA1 ΔdofB::Km | This work |
| TH3 | sglA1 ΔdofA::Km ΔdofB::Tc | This work |
| MxDAL | sglA1 dofA::pDAL-Km | This work |
| MxDAL-A | asgA476 KmrdofA::pDAL-Tc | This work |
| MxDAL-B | bsgA330 TcrdofA::pDAL-Km | This work |
| MxDAL-C | csgA731 dofA::pDAL-Km | This work |
| MxDAL-D | dsgA439 TcrsglA1 dofA::pDAL-Km | This work |
| MxDAL-E | esg::Tn5 Ω258 KmrdofA::pDAL-Tc | This work |
| MxDAL-F | fruA::Tc Ω5 dofA::pDAL-Km | This work |
| MxDAL-2 | sglA1 dofA::pDAL-2 | This work |
| MxDAL-2F | sglA1 fruA::Tc Ω5 dofA::pDAL-2 | This work |
| MxDAL-2C | sglA1 csgA731 dofA::pDAL-2 | This work |
| MxDBL | sglA1 dofB::pDBL | This work |
| Plasmids | ||
| pGEM-T easy | AprlacZ cloning vector | Promega |
| pMC1403 | AprlacZ translational fusion vector | 5 |
| pMC1403Km | KmrlacZ translational fusion vector; derivative of pMC1403 | This work |
| pMC1403Tc | TcrlacZ translational fusion vector; derivative of pMC1403 | This work |
| pSI1403 | KmrlacZ transcriptional fusion vector; derivative of pMC1403 | 20 |
| pSI1403attP | Kmrint attP lacZ transcriptional fusion vector; derivative of pSI1403 | 20 |
| pSP11 | 1.1-kb HincII fragment containing dofA of DZF1 in pUC19 | 20 |
| pGEM-dofB | 3,032-bp dofB fragmenta in pGEM-T easy | This work |
| pDAL-Km | 1,135-bp dofA upstream fragmenta in pMC1403Km | This work |
| pDAL-Tc | 1,135-bp dofA upstream fragmenta in pMC1403Tc | This work |
| pDAL-2 | 1,135-bp dofA upstream fragmenta in pSI1403 | This work |
| pDBL | 1,255-bp dofB upstream fragmenta in pMC1403Km | This work |
DNA fragments were synthesized by PCR with M. xanthus DZF1 chromosomal DNA.
FIG. 6.
Upstream elements required for dofA expression. (A) Schematic diagram of the dofA regulatory region (upper part of diagram) and structures of various dofA-lacZ fusions. The transcription initiation site is indicated as +1. Putative −35 and −10 sequences and putative regulatory sequences are indicated by open bars with nucleotide sequences. The indicated DNA segments were amplified by PCR and inserted into pSI1403attP. The resultant plasmids were introduced into the attB site of the M. xanthus chromosome to give MxDA series strains. MxDA1022 and MxDA1038 lacked the DNA segments indicated by parentheses. (B) lacZ expression under the control of various segments of the dofA regulatory region. The specific activities of β-galactosidase (averages of four independent experiments) in developing cells (20 h poststarvation) of the indicated M. xanthus strains were measured. MxVEC carried pSI1403attP. ONP, o-nitrophenol.
Conditions for vegetative growth and development.
M. xanthus cells were grown at 30°C in CYE (4) and CTT (3) media or on their agar plates containing 1.5% agar. Kanamycin sulfate (80 μg/ml) or oxytetracycline (6.25 μg/ml) was used when required. Development of M. xanthus was induced on CF (18) or TM (10 mM Tris-HCl [pH 7.6], 8 mM MgSO4) agar plates as previously described (22).
Escherichia coli cells were grown in Luria-Bertani medium at 37°C (33). Ampicillin (100 μg/ml), chloramphenicol (25 μg/ml), tetracycline (12.5 μg/ml), or kanamycin sulfate (50 μg/ml) was used when required.
DNA manipulation and sequencing.
Preparation of plasmid DNA, transformation, and other methods of DNA manipulation were described previously (2, 33). PCR was performed with LA Taq DNA polymerase (Takara, Kyoto, Japan). The DNA sequence was determined by the dideoxy chain termination method (34). DNA fragments used as probes were end labeled by using a 5′-end-labeling kit (Amersham Pharmacia) with [γ-32P]ATP (7,000 Ci/mmol; ICN) or labeled by using a Megaprime DNA labeling system with dCTP (Amersham Pharmacia) and [α-32P]dCTP (800 Ci/mmol; ICN).
To clone the M. xanthus dofB region, a 3,032-bp dofB fragment (1,229 bp upstream from the 5′ end and 1,353 bp downstream from the 3′ end of the dofB coding region) was synthesized by PCR with appropriate primers and the M. xanthus DZF1 chromosome as a template; the fragment was inserted into pGEM-T easy vector DNA to give pGEM-dofB. DNA sequences were obtained from the M. xanthus genome sequence database (Cereon Microbial Sequence Database).
Transformation of M. xanthus cells was performed by electroporation (27). Total RNAs were prepared from vegetative and developing cells of M. xanthus by the hot-phenol method (1).
Construction of deletion mutations in the dofA and dofB genes.
Deletion mutations were first constructed in pSP11 and pGEM-dofB. The dofA coding sequence (amino acid residues 16 to 135) was replaced by a DNA fragment that carries a Kmr determinant to give pSP11 ΔdofA::Km. For that purpose, the dofA upstream and downstream sequences were synthesized by PCR with appropriate primers. EcoRI-digested pSP11 ΔdofA::Km DNA was introduced into M. xanthus DZF1 cells by electroporation to give M. xanthus TH1 ΔdofA::Km. To construct TH2 ΔdofB::Km, the dofB coding sequence (residues 16 to 137) in pGEM-dofB was replaced by a DNA fragment carrying a Kmr determinant. To construct TH3 ΔdofA::Km ΔdofB::Tc, the dofB coding sequence (residues 16 to 137) was replaced by a DNA fragment carrying a Tcr determinant. Insertion of the ΔdofA and ΔdofB alleles into the appropriate sites of the M. xanthus chromosome by double homologous crossover was confirmed by PCR and Southern blot analysis.
Sporulation and germination.
The measurement of spores was adapted from the method of Jain and Inouye (25). Fruiting bodies of M. xanthus strains DZF1, TH1, TH2, and TH3 were scraped from the surface of CF agar plates, suspended in TM buffer, and sonicated to disrupt the fruiting bodies. Sonication-resistant refractile spores were counted under a microscope. For germination, the sonication-resistant refractile spores were spread on CYE agar plates. After incubation at 30°C for 5 days, colonies of vegetative cells were counted.
RT-PCR.
DNA primers, CGAGGACGAGCGGGTGCTGAAGC for dofA and TCACCGACCCCACGTAGATGAGG for dofB, were hybridized with 3 μg of RNA prepared from M. xanthus DZF1 cells and treated with RAV-2 reverse transcriptase (RT) (Takara). PCR was performed with Taq DNA polymerase (Takara), 5′ gene-specific primers (ATGGCCACATCCGTCTACAAG for dofA and TGAACGGCCCTACCTACAAAG for dofB), and 3′ gene-specific primers (same primers as those listed above). The PCR products were analyzed by 6% PAGE followed by ethidium bromide staining.
Primer extension.
Primer extension was carried out as described previously (16). 32P-labeled primers, GACGGTGTGGATTTCTTCCTGACTGGGTG for dofA and AGCACCAGCGTCACGTCCTCCTTGGTCGG for dofB, were hybridized with 80 μg of RNA prepared from M. xanthus cells and treated with RAV-2 RT. The extended products and the sequencing reaction products generated with the same primers and with pSP11 or pGEM-dofB DNA as a template were analyzed by 6% PAGE containing 7 M urea.
Construction of dofA-lacZ and dofB-lacZ fusion genes.
To construct the dofA-lacZ translational fusion, a 1,135-bp dofA upstream fragment containing the 57-bp dofA coding region was synthesized by PCR with appropriate primers containing EcoRI and BamHI sites at each 5′ end and M. xanthus DZF1 chromosomal DNA as a template; the fragment was inserted into the EcoRI-BamHI site of pMC1403Km and pMC1403Tc in the proper orientation to generate pDAL-Km and pDAL-Tc, respectively. Plasmid pDAL-Km or pDAL-Tc was introduced into M. xanthus DZF1, MO1, JD276, JD278, DK731, DK3068, and JD275 cells by electroporation to generate MxDAL, MxDAL-F, MxDAL-A, MxDAL-B, MxDAL-C, MxDAL-D, and MxDAL-E, respectively.
To construct the dofA-lacZ transcriptional fusion, the 1,135-bp dofA upstream fragment described above was inserted into the EcoRI-BamHI site of pSI1403 in the proper orientation to generate pDAL-2. Plasmid pDAL-2 was introduced into M. xanthus DZF1, MO1, and DK731 cells by electroporation to generate MxDAL-2, MxDAL-2F, and MxDAL-2C, respectively.
To construct the dofB-lacZ translational fusion, a 1,255-bp dofB upstream fragment containing the 24-bp dofB coding region was synthesized by PCR with appropriate primers containing EcoRI and BamHI sites at each 5′ end; the fragment was inserted into the EcoRI-BamHI site of pMC1403Km in the proper orientation to generate pDBL. Plasmid pDBL was introduced into M. xanthus DZF1 cells by electroporation to generate MxDBL.
To construct pDA1047, pDA128, pDA93, pDA56, pDA38, and pDA15, each DNA segment of the dofA regulatory region was synthesized by PCR with appropriate primers having a BamHI site at the 5′ end and then inserted into the BamHI site of pSI1403attP. The orientation and sequence of inserted fragments were confirmed by DNA sequencing. Deletion derivatives of pDA1047 were constructed by PCR followed by ligation. pDA series plasmids and pSI1403attP were introduced into M. xanthus DZF1 cells by electroporation to generate MxDA series strains.
Insertion of a single copy of every plasmid into the desired site of the M. xanthus chromosome was confirmed by Southern blot analysis.
β-Galactosidase assay during vegetative growth and development of M. xanthus.
The β-galactosidase activity of M. xanthus cells was determined by using o-nitrophenyl-β-galactoside as a substrate (29), and the protein concentration was measured with Bio-Rad protein assay reagent. β-Galactosidase specific activity (units) was expressed as nanomoles of o-nitrophenol produced per minute per milligram of protein. To determine the β-galactosidase activity inside spores, spores were disrupted by sonication with acid-washed glass beads (0.15 to 0.21 mm) as described previously (39).
Nucleotide sequence accession numbers.
The nucleotide sequence data for dofA and dofB reported in this study will appear in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession numbers AB073987 and AB084416, respectively.
RESULTS
Sequence analysis of M. xanthus dofA and dofB regions.
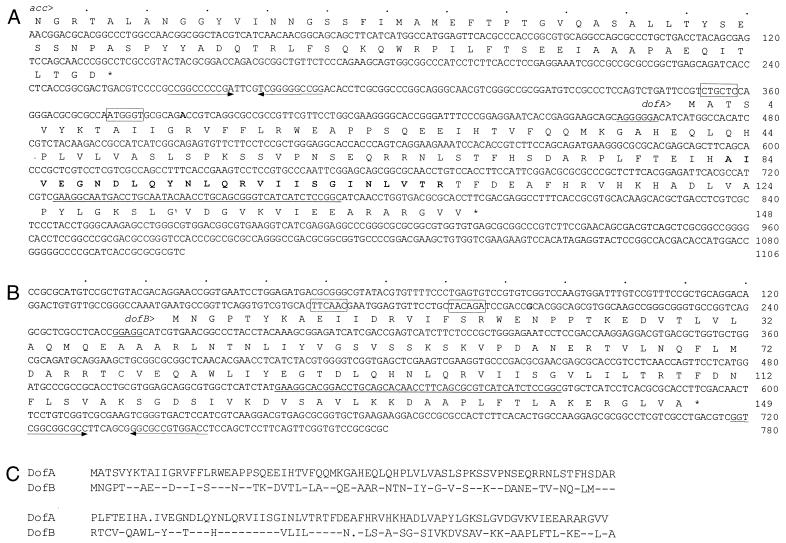
Previously (20), the DNA fragment encompassing the dofA gene, a fruA-dependent developmental gene, was cloned and its DNA sequence was determined (Fig. 1A). The dofA initiation codon and coding region were determined as follows. Based on nucleotide sequence analysis, an open reading frame (ORF) extending from the ATG codon at position 374 to the TGA codon at position 914 was determined to be the longest ORF, encoding the amino acid sequences of the two DofA tryptic peptides (boldface type in Fig. 1A). When the E. coli lacZ gene was translationally fused to the third codon of this ORF, no significant β-galactosidase activity was observed during vegetative growth and development (data not shown). The second ATG codon of the ORF was located at position 470. When the 19th codon from this site was translationally fused to the lacZ gene, significant β-galactosidase activity was observed during the development of M. xanthus (see Fig. 3). Thus, the dofA coding region was determined to extend from the ATG codon at position 470 to the TGA codon at position 914. The dofA product from the putative initiation codon consists of 148 amino acid residues and has a calculated molecular mass of 16,606 Da and a calculated isoelectric point of 9.17, data which agreed well with those estimated from 2D gel analysis (20). This assignment was verified by determination of the transcription initiation site as described below. The ATG initiation codon was preceded by a Shine-Dalgarno sequence consisting of seven consecutive purine residues (underlined in Fig. 1A).
FIG. 1.
Nucleotide and amino acid sequences of dofA and dofB regions in M. xanthus and amino acid sequence alignment of DofA and DofB proteins. (A) The nucleotide sequence of a 1,106-bp HincII fragment and the deduced amino acid sequences of the acc and dofA products are shown. Amino acid sequences determined by mass spectrometry (20) are indicated in boldface type. (B) The nucleotide sequence of the dofB region obtained from the M. xanthus genome sequence database and the deduced amino acid sequence of the dofB product are shown. Asterisks indicate stop codons. The transcription initiation sites of the dofA and dofB genes are indicated in boldface type. The −35 and −10 sequences of the putative promoters are boxed. The putative Shine-Dalgarno sequences are underlined. The similar 48-bp sequences of dofA and dofB are also underlined. The inverted repeat sequences are indicated by arrows. (C) The amino acid sequence of DofA is shown in its entirety. For DofB, hyphens indicate amino acid residues identical to those of DofA. A dot indicates a gap in the sequence.
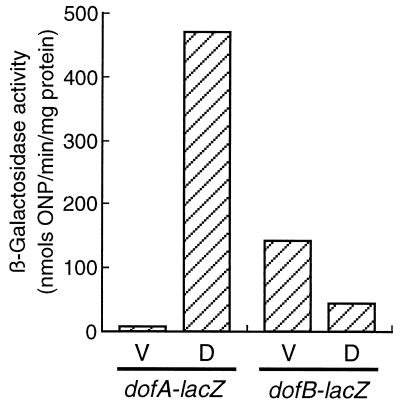
FIG. 3.
Expression of dofA-lacZ and dofB-lacZ translational fusions in M. xanthus. The β-galactosidase activity of M. xanthus MxDAL or MxDBL was determined during vegetative growth (V) and development at 20 h poststarvation (D). The β-galactosidase activities shown represent the averages of three independent experiments. ONP, o-nitrophenol.
A portion of an additional ORF oriented in the same direction as the dofA gene was identified in this region (Fig. 1A). The amino acid sequence encoded by the second ORF displayed 39% identity with the C-terminal 85-amino-acid sequence of the acc product, aculeacin A acylase in Actinoplanes utahensis (21).
Although database searches have not revealed any proteins exhibiting significant similarity to DofA, a nucleotide sequence sharing 88% identity with a 48-bp dofA sequence (from positions 725 to 772) (underlined in Fig. 1A) was identified in the M. xanthus genome sequence database (Cereon Microbial Sequence Database). A 780-bp sequence containing the 48-bp sequence was obtained from the database and is shown in Fig. 1B. Sequence analysis revealed an ORF of 149 codons initiating at GTG at position 264 and extending to TGA at position 711; this ORF has tentatively been designated the dofB gene. The molecular mass of the dofB product was calculated to be 16,579 Da, and the isoelectric point was calculated to be 8.57. The deduced amino acid sequence of the DofB protein shared 43% identity with that of the DofA protein (Fig. 1C).
The M. xanthus genes were reported to have high G+C contents at the third positions of their codons (35). The G+C contents at the third-codon positions of three genes, acc, dofA, and dofB, were 96, 87, and 91%, respectively.
Construction and characterization of ΔdofA, ΔdofB, and ΔdofAB mutants of M. xanthus.
To examine the requirement of the dofA and dofB genes for the vegetative growth and development of M. xanthus, dofA and dofB deletion mutants were constructed. First, DNA segments of the dofA and dofB genes encoding internal 120 and 122 amino acids were replaced by DNA fragments conferring kanamycin or tetracycline resistance to generate ΔdofA and ΔdofB alleles, respectively, in E. coli plasmids. Then, these constructs were introduced into the M. xanthus DZF1 chromosome by double homologous crossover to generate TH1 ΔdofA, TH2 ΔdofB, and TH3 ΔdofA ΔdofB.
M. xanthus ΔdofA, ΔdofB, and ΔdofA ΔdofB cells grew in CYE medium at the same rate as wild-type DZF1 cells (data not shown), indicating that the dofA and dofB genes are dispensable for vegetative growth. When concentrated cells of the three deletion mutants were spotted on CF or TM agar plates, they underwent processes of development indistinguishable from those of the wild-type strain (data not shown). On CF agar plates, 11 to 14% of input cells of the four strains were converted into sonication-resistant refractile myxospores at 72 h poststarvation. Approximately 70% of the myxospores produced on CF agar plates from the four strains germinated and formed colonies after incubation at 30°C for 5 days on CYE agar plates. These results indicate that neither the dofA nor the dofB gene is required for development.
Expression of dofA and dofB genes during vegetative growth and development of M. xanthus.
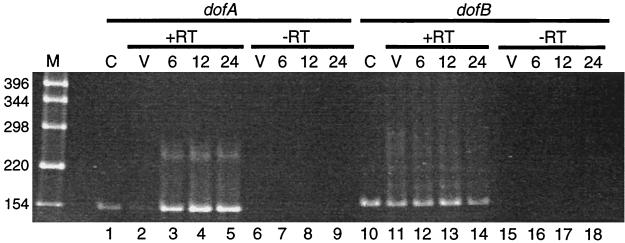
The synthesis of dofA and dofB mRNAs during the vegetative growth and development of M. xanthus strain DZF1 was analyzed by RT-PCR (Fig. 2). Total RNAs were prepared from vegetative cells and developing cells at 6, 12, and 24 h poststarvation and treated with RT to synthesize cDNAs that were used as templates for PCR amplification with appropriate primers. The 151-bp PCR products synthesized from dofA mRNA-derived cDNA of developing cells at 6, 12, and 24 h poststarvation are shown in Fig. 2, lanes 3 to 5. These results indicate that dofA is expressed primarily during development. The development-specific synthesis of DofA protein has been observed in pulse-labeling experiments (20). On the other hand, the 163-bp PCR products synthesized from dofB mRNA-derived cDNA were detected in RNAs prepared from vegetative and developing cells (Fig. 2, lanes 11 to 14), indicating that dofB is expressed during both vegetative growth and development.
FIG. 2.
RT-PCR analysis of the dofA and dofB mRNAs in M. xanthus. Total RNAs prepared from M. xanthus vegetative cells (V, lanes 2, 6, 11, and 15) and developing cells at 6 h (lanes 3, 7, 12, and 16), 12 h (lanes 4, 8, 13, and 17), and 24 h (lanes 5, 9, 14, and 18) poststarvation were treated with RT (+RT, lanes 2 to 5 and 11 to 14) or not treated with RT (−RT, lanes 6 to 9 and 15 to 18) and subjected to PCR by using the appropriate primers for cDNAs of dofA (lanes 1 to 9) and dofB (lanes 10 to 18). The PCR products were analyzed by 6% PAGE followed by ethidium bromide staining. Lanes C (lanes 1 and 10), PCR product amplified from the M. xanthus chromosome. Lane M, molecular weight markers. The molecular sizes of DNA fragments are indicated at the left in base pairs.
To examine the levels of dofA and dofB expression, pDAL and pDBL plasmids carrying the dofA-lacZ and dofB-lacZ translational fusions, respectively, were introduced into the M. xanthus DZF1 chromosome by single-crossover recombination. The β-galactosidase activities of M. xanthus cells carrying the dofA-lacZ or dofB-lacZ fusion were measured during vegetative growth and development at 20 h poststarvation (Fig. 3). More than 400 U of β-galactosidase activity was produced from the dofA-lacZ fusion in developing cells at 20 h poststarvation, while less than 10 U of β-galactosidase activity was produced in vegetative cells. Developmentally regulated DofA synthesis has been observed in pulse-labeling experiments during development (20). On the other hand, β-galactosidase activity from the dofB-lacZ fusion was observed during both vegetative growth and development. The β-galactosidase activity in developing cells was threefold lower than that in vegetative cells.
Determination of transcription initiation sites.
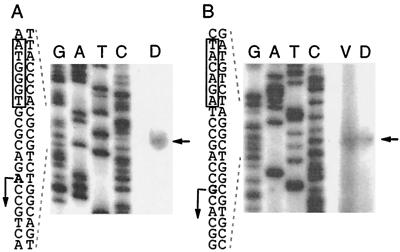
To determine the transcription initiation sites of the dofA and dofB genes, primer extension experiments were carried out with total RNAs prepared from developing cells at 24 h poststarvation for dofA and from vegetative cells and developing cells at 24 h poststarvation for dofB. The 5′-end-labeled primers located 93 and 95 bp downstream of the dofA and dofB translation initiation sites, respectively, were annealed with approximately 100 μg of RNA and extended with RT. As shown in Fig. 4A, an extension product for dofA mRNA was detected. The most probable dofA transcription initiation site was the adenine residue at position 386 (Fig. 1A). The putative dofA promoter regions were assigned as CTGCTC (positions 353 to 358) for the −35 sequence and as ATGGGT (positions 374 to 379) for the −10 sequence (Fig. 1A, boxes).
FIG. 4.
Transcription initiation sites of dofA and dofB mRNAs. Total RNAs (100 μg) prepared from M. xanthus vegetative cells (V) and developing cells at 24 h poststarvation (D) were subjected to primer extension analysis. The products of primer extension with appropriate primers are marked by arrows to the right of each panel. (A) dofA mRNA. (B) dofB mRNA. Lanes G, A, T, and C, sequence ladders generated by the dideoxy chain termination method with the same primers and with pSP11 or pGEM-dofB DNA as a template. The DNA sequences around the transcription initiation sites (bent arrows) are indicated to the left of each panel. The −10 sequences of the putative promoters are boxed.
For dofB mRNA, the same extension products were detected for both vegetative and developing cells (Fig. 4B). The transcription initiation site of dofB was the guanine residue at position 204 (Fig. 1B), and TTCAAC (positions 168 to 173) and TACAGA (positions 191 to 196) were assigned for the −35 and −10 sequences of the putative dofB promoter, respectively (Fig. 1B). The signal for vegetative cells was more intense than that for developmental cells, consistent with the results for dofB-lacZ expression (Fig. 3).
Effects of fruA and signal mutations on dofA expression.
To examine the effects of fruA and csgA mutations on the transcription of the dofA gene, plasmid pDAL-2, carrying the dofA-lacZ transcriptional fusion, was introduced into the dofA locus of the chromosome of M. xanthus wild-type, fruA::Tc, and csgA731 strains by single-crossover recombination. β-Galactosidase activities were measured at 20 h poststarvation during the development of the three tested strains, MxDAL-2, MxDAL-2F, and MxDAL-2C. Thirty-two and 23 U of β-galactosidase activity were detected in the wild-type strain and the csgA mutant, respectively, whereas only 3.2 U of β-galactosidase activity was detected in the fruA mutant.
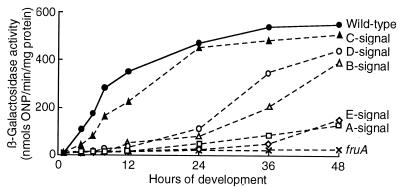
To further examine the effects of fruA and signal mutations on dofA expression, a pDAL plasmid carrying the dofA-lacZ translational fusion or its tetracycline-resistant derivative was introduced into the dofA locus of the chromosome of M. xanthus wild-type, fruA::Tc, asgA476, bsgA330, csgA731, dsgA439, and esg::Tn5 strains, resulting in strains MxDAL, MxDAL-F, MxDAL-A, MxDAL-B, MxDAL-C, MxDAL-D, and MxDAL-E, respectively. The β-galactosidase activities of these strains were measured during development (Fig. 5). β-Galactosidase activities in the wild-type strain and the csgA mutant were first detectable after 4 to 6 h poststarvation and increased gradually until 48 h. In the fruA mutant, less than 10 U of β-galactosidase activity, a value comparable to vector-level background expression, was detected throughout development. A low level of dofA-lacZ expression was observed during late stages of development in the asgA mutant and the esg mutant. In the bsgA mutant and the dsgA mutant, dofA-lacZ expression was delayed until 24 h poststarvation and then increased slowly.
FIG. 5.
Effects of fruA and signal mutations on dofA expression. The β-galactosidase specific activities of dofA-lacZ translational fusions in M. xanthus MxDAL (filled circles), MxDAL-F fruA::Tc (multiplication signs), MxDAL-A asgA476 (open squares), MxDAL-B bsgA330 (open triangles), MxDAL-C csgA731 (filled triangles), MxDAL-D dsgA439 (open circles), and MxDAL-E esg::Tn5 (open diamonds) were measured during development. The activities presented are the averages of three independent experiments. ONP, o-nitrophenol.
The results obtained with dofA-lacZ transcriptional and translational fusions indicate that the expression of dofA is dependent on FruA and on A and E signals, partially dependent on B and D signals, and independent of the C signal.
Role of the upstream regulatory region in dofA expression.
To investigate the regulation of dofA expression during development, the promoter activities of various segments of the dofA regulatory region were assessed by using the lacZ reporter gene in vivo. Figure 6A shows the structure of the dofA regulatory region, including the putative −35 and −10 sequences of the promoter.
Six DNA segments, DA1047, DA128, DA93, DA56, DA38, and DA15 (Fig. 6A), were synthesized by PCR and inserted into lacZ transcriptional fusion vector pSI1403attP in the proper orientations. Recombinant and vector plasmids were integrated into the phage Mx8 attB site of the DZF1 chromosome to give MxDA series and MxVEC strains, respectively. The promoter activities of the six DNA fragments transcriptionally fused to the lacZ gene were monitored by assaying β-galactosidase activity at 20 h poststarvation (Fig. 6B).
The DA128 segment,extending 128 bp upstream from the transcription initiation site, is most likely sufficient for developmentally regulated dofA expression, since MxDA128 and MxDA1047 exhibited similar levels of β-galactosidase activity (Fig. 6B). Two strains, MxDAL-2 and MxDA1047, carrying similar dofA-lacZ transcriptional fusions at the dofA locus and at the Mx8 attB site, exhibited 32 and 15 U, respectively, of β-galactosidase activity. The differences in the respective locations of dofA-lacZ on the chromosomes of these two strains may affect their β-galactosidase activities.
MxDA93 exhibited significant β-galactosidase activity during development, while the β-galactosidase activities of MxDA56, MxDA38, and MxDA15 were similar to that of MxVEC (Fig. 6B). These results suggest that the 128-bp sequence upstream from the transcription initiation site is required for full expression of the dofA gene.
Two characteristic sequences were found within the region from positions −128 to −56 (see Discussion): (i) a highly GC-rich inverted repeat sequence (underlined), CGGCCCCCGATTCGTCGGGGGCCG (positions −121 to −98), and (ii) a 9-bp sequence, GGGCCGCGG (positions −68 to −60), for which a similar sequence was found at positions −109 to −101 of the tps gene (Fig. 6A). Hence, dofA-lacZ fusions lacking these sequences were constructed and integrated into the DZF1 chromosome to give MxDA1022 and MxDA1038, respectively. MxDA1022 exhibited less β-galactosidase activity than MxDA1047, whereas MxDA1038 exhibited activity similar to that of MxDA1047 (Fig. 6B). These results suggest an important role of the highly GC-rich inverted repeat sequence in dofA expression.
DISCUSSION
In the present study, an M. xanthus developmental gene, dofA, and its homologue, dofB, were cloned and characterized. The dofA gene was previously identified as a fruA-dependent developmental gene by 2D gel analysis (20). The dofB gene was identified by a sequence homology search of the M. xanthus genome sequence database with the dofA DNA sequence. DofA and DofB are comprised of 148 and 149 amino acid residues, respectively, and share 43% identity. However, the expression of dofA and dofB was differently regulated in the M. xanthus life cycle. The dofA gene was expressed during development only, while the dofB gene was expressed during both vegetative growth and development. Two other homologous genes, ops and tps, encoding protein S1 and protein S, respectively, showed a similar pattern of regulation. Protein S1 and protein S consist of 173 and 174 residues, respectively, and share 88% identity (24). The ops and tps genes are located in the same orientation and are separated by a 1.4-kb spacer sequence on the M. xanthus chromosome. The tps gene is expressed at a very low level during vegetative growth, and its expression is highly induced at 5 h poststarvation during development (10), while the ops gene is expressed at a late stage of development within spores (40). Both the dofA and the dofB genes were found to be dispensable for vegetative growth, fruiting body formation, sporulation, and germination of myxospores, similar to the situation with the ops and tps genes. In the M. xanthus genome sequence database, the dofA and dofB genes were found on separate contigs, suggesting that they are located at least 2.8 kb apart in the M. xanthus chromosome.
In the course of 2D gel analysis of developmental protein synthesis in the wild-type, fruA::Tc, and csgA731 strains, dofA expression and tps expression were found to be FruA dependent but CsgA independent (20). The present results obtained with dofA-lacZ fusions revealed that the expression of dofA is dependent on FruA and A and E signals, partially dependent on B and D signals, and independent of the C signal. The production of protein S was previously shown to be absolutely dependent on FruA and A and E signals, partially dependent on B and D signals, and independent of the C signal (6, 11, 17, 20, 28, 30, 32). These results suggest that dofA expression and tps expression are similarly regulated. In A or E signal mutants, the expression of the fruA gene was delayed until 9 to 12 h poststarvation, and the amount of FruA produced was much smaller than that produced by the wild-type strain during development (15). It is possible that the A, B, D, and E signals affect dofA expression via FruA synthesis. The possible direct interaction of purified FruA with the dofA and tps promoter region sequences was investigated by a gel shift assay. However, no band shift was detected in such experiments (data not shown). The phosphorylation of FruA may be required for binding to the dofA and tps promoter regions.
Analysis of the dofA promoter region with lacZ fusions indicated that the sequence 93 bp upstream from the transcription initiation site was necessary for the developmental expression of the dofA gene (Fig. 6). Developmentally regulated tps expression has been well characterized and requires the region extending 110 bp upstream from the transcription initiation site (8). A comparison of the upstream regions of the dofA and tps genes reveals the presence of two highly conserved 9-bp sequences, GGGCCGCGG and GGGCCTCGG, at positions −68 to −60 in the dofA gene and −109 to −101 in the tps gene, respectively. However, deletion of the 9-bp sequence did not affect dofA expression, while the involvement of the sequence from positions −110 to −82, containing GGGCCTCGG, in tps expression has been suggested (8, 9).
The construction of deletion mutants suggested the involvement of a highly GC-rich inverted repeat sequence (underlined), CGGCCCCCGATTCGTCGGGGGCCG (positions −121 to −98), in dofA expression. Since the deletion mutant lacking this sequence exhibited two-thirds β-galactosidase activity, some additional element may be required for the full activation of developmentally regulated dofA expression.
Although the development of dofA and dofB mutants was normal under the laboratory conditions used, dofA and dofB products may play some important roles in the natural environment. Further investigations of regulatory elements of the dofA and tps genes will elucidate the functions of FruA, which plays crucial roles in M. xanthus development and sporulation.
Acknowledgments
We are grateful to Monsanto's Cereon Microbial Sequence Database for allowing us to access the database. The nucleotide sequence of the dofB gene was downloaded from Monsanto's Cereon Microbial Sequence Database. Monsanto has granted permission to deposit this sequence in GenBank as part of the publication process. We thank K. Takayama for critical reading of the manuscript.
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Aiba, H., S. Adhya, and B. de Crombrugghe. 1981. Evidence for two functional gal promoters in intact Escherichia coli. J. Biol. Chem. 256:11905-11910. [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1988. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Bretscher, A. P., and D. Kaiser. 1978. Nutrition of Myxococcus xanthus, a fruiting myxobacterium. J. Bacteriol. 133:763-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campos, J. M., J. Geisselsoder, and D. R. Zusman. 1978. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J. Mol. Biol. 119:167-178. [DOI] [PubMed] [Google Scholar]
- 5.Casadaban, M. J., J. Chou, and S. N. Cohen. 1980. In vitro gene fusions that join an enzymatically active β-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J. Bacteriol. 143:971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, Y., and D. Kaiser. 1989. dsg, a gene required for cell-cell interaction early in Myxococcus development. J. Bacteriol. 171:3719-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Downard, J. S. 1987. Identification of the RNA products of the ops gene of Myxococcus xanthus and mapping of the ops and tps RNAs. J. Bacteriol. 169:1522-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downard, J. S., S. Kim, and K. Kil. 1988. Localization of the cis-acting regulatory DNA sequences of the Myxococcus xanthus tps and ops genes. J. Bacteriol. 170:4931-4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downard, J. S., and L. Kroos. 1993. Transcriptional regulation of developmental gene expression in Myxococcus xanthus, p. 183-199. In M. Dworkin and D. Kaiser (ed.), Myxobacteria II. American Society for Microbiology, Washington, D.C.
- 10.Downard, J. S., D. Kupfer, and D. Zusman. 1984. Gene expression during development of Myxococcus xanthus. Analysis of the genes for protein S. J. Mol. Biol. 175:469-492. [DOI] [PubMed] [Google Scholar]
- 11.Downard, J. S., S. V. Ramaswamy, and K. S. Kil. 1993. Identification of esg, a genetic locus involved in cell-cell signaling during Myxococcus xanthus development. J. Bacteriol. 175:7762-7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downard, J. S., and D. Toal. 1995. Branched-chain fatty acids—the case for a novel form of cell-cell signaling during Myxococcus xanthus development. Mol. Microbiol. 16:171-175. [DOI] [PubMed] [Google Scholar]
- 13.Downard, J. S., and D. R. Zusman. 1985. Differential expression of protein S genes during Myxococcus xanthus development. J. Bacteriol. 161:1146-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dworkin, J. 1996. Recent advances in the social and developmental biology of the myxobacteria. Microbiol. Rev. 60:70-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellehauge, E., M. Nørregaard-Madsen, and L. Søgaard-Andersen. 1998. The FruA signal transduction protein provides a checkpoint for the temporal co-ordination of intercellular signals in Myxococcus xanthus development. Mol. Microbiol. 30:807-817. [DOI] [PubMed] [Google Scholar]
- 16.Geliebter, J., R. A. Zeff, R. W. Melvold, and S. G. Nathenson. 1986. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc. Natl. Acad. Sci. USA 83:3371-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill, R., and M. G. Cull. 1986. Control of developmental gene expression by cell-to-cell interactions of Myxococcus xanthus. J. Bacteriol. 168:341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagen, D. C., A. P. Bretscher, and D. Kaiser. 1978. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev. Biol. 64:284-296. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 20.Horiuchi, T., M. Taoka, T. Isobe, T. Komano, and S. Inouye. 2002. Role of fruA and csgA genes in gene expression during development of Myxococcus xanthus: analysis by two-dimensional gel electrophoresis. J. Biol. Chem. 277:26753-26760. [DOI] [PubMed] [Google Scholar]
- 21.Inokoshi, J., H. Takeshima, H. Ikeda, and S. Omura. 1992. Cloning and sequencing of the aculeacin A acylase-encoding gene from Actinoplanes utahensis and expression in Streptomyces lividans. Gene 119:29-35. [DOI] [PubMed] [Google Scholar]
- 22.Inouye, M., S. Inouye, and D. R. Zusman. 1979. Gene expression during development of Myxococcus xanthus: pattern of protein synthesis. Dev. Biol. 68:579-591. [DOI] [PubMed] [Google Scholar]
- 23.Inouye, M., S. Inouye, and D. R. Zusman. 1979. Biosynthesis and self-assembly of protein S, a development specific protein of Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 76: 209-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inouye, S., T. Franceschini, and M. Inouye. 1983. Structural similarities between the development-specific protein S from a gram-negative bacterium, Myxococcus xanthus, and calmodulin. Proc. Natl. Acad. Sci. USA 80:6829-6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain, R., and S. Inouye. 1998. Inhibition of development of Myxococcus xanthus by eukaryotic protein kinase inhibitors. J. Bacteriol. 180:6544-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser, D., and L. Kroos. 1993. Intercellular signaling, p. 257-284. In M. Dworkin and D. Kaiser (ed.), Myxobacteria II. American Society for Microbiology, Washington, D.C.
- 27.Kashefi, K., and P. L. Hartzell. 1995. Genetics suppression and phenotypic masking of a Myxococcus xanthus frzF defect. Mol. Microbiol. 15:483-494. [DOI] [PubMed] [Google Scholar]
- 28.Kroos, L., and D. Kaiser. 1987. Expression of many developmentally regulated genes in Myxococcus depends on a sequence of cell interactions. Genes Dev. 1:840-854. [DOI] [PubMed] [Google Scholar]
- 29.Kroos, L., A. Kuspa, and D. Kaiser. 1986. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev. Biol. 117:252-266. [DOI] [PubMed] [Google Scholar]
- 30.Kuspa, A., L. Kroos, and D. Kaiser. 1986. Intercellular signaling is required for developmental gene expression in Myxococcus xanthus. Dev. Biol. 117:267-276. [DOI] [PubMed] [Google Scholar]
- 31.Kuspa, A., L. Plamann, and D. Kaiser. 1992. A-signaling and the cell density requirement for Myxococcus xanthus development. J. Bacteriol. 174:7360-7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa, M., S. Fujitani, X. Mao, S. Inouye, and T. Komano. 1996. FruA, a putative transcription factor essential for the development of Myxococcus xanthus. Mol. Microbiol. 22:757-767. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimkets, L. J. 1993. The myxobacterial genome, p. 85-107. In M. Dworkin and D. Kaiser (ed.), Myxobacteria II. American Society for Microbiology, Washington, D.C.
- 36.Shimkets, L. J. 1999. Intercellular signaling during fruiting-body development of Myxococcus xanthus. Annu. Rev. Microbiol. 53:525-549. [DOI] [PubMed] [Google Scholar]
- 37.Shimkets, L. J., and S. J. Asher. 1988. Use of recombination techniques to examine the structure of the csg locus of Myxococcus xanthus. Mol. Gen. Genet. 211:451-461. [DOI] [PubMed] [Google Scholar]
- 38.Søgaard-Andersen, L., F. J. Slack, H. Kimsey, and D. Kaiser. 1996. Intercellular C-signaling in Myxococcus xanthus involves a branched signal transduction pathway. Genes Dev. 10:740-754. [DOI] [PubMed] [Google Scholar]
- 39.Teintze, M., M. Inouye, and S. Inouye. 1988. Characterization of calcium-binding sites in development-specific protein S of Myxococcus xanthus using site-specific mutagenesis. J. Biol. Chem. 263:1199-1203. [PubMed] [Google Scholar]
- 40.Teintze, M., R. Thomas, T. Furuichi, M. Inouye, and S. Inouye. 1985. Two homologous genes coding for spore-specific proteins are expressed at different times during development of Myxococcus xanthus. J. Bacteriol. 163:121-125. [DOI] [PMC free article] [PubMed] [Google Scholar]