Abstract
Stationary-phase mutations occur in populations of stressed, nongrowing, and slowly growing cells and allow mutant bacteria to overcome growth barriers. Mutational processes in starving cells are different from those occurring in growing bacteria. Here, we present evidence that changes in mutational processes also take place during starvation of bacteria. Our test system for selection of mutants based on creation of functional promoters for the transcriptional activation of the phenol degradation genes pheBA in starving Pseudomonas putida enables us to study base substitutions (C-to-A or G-to-T transversions), deletions, and insertions. We observed changes in the spectrum of promoter-creating mutations during prolonged starvation of Pseudomonas putida on phenol minimal plates. One particular C-to-A transversion was the prevailing mutation in starving cells. However, with increasing time of starvation, the importance of this mutation decreased but the percentage of other types of mutations, such as 2- to 3-bp deletions, increased. The rate of transversions was markedly elevated in the P. putida MutY-defective strain. The occurrence of 2- to 3-bp deletions required the stationary-phase sigma factor RpoS, which indicates that some mutagenic pathway is positively controlled by RpoS in P. putida.
Natural microbial populations spend most of their life under nutrient deprivation due to intense competition for available resources. Under these limiting conditions, there is selective pressure for any mutation that confers a competitive advantage. Differences in the spectra of mutations have been observed between sets of mutants appearing in starving bacterial populations under selective conditions and in actively growing bacterial cultures (22, 32, 52, 55). The starvation conditions encountered during stationary-phase incubation may permit a transient increase in the mutation rate due to a variety of factors, including decreased fidelity during replication and reduction of DNA repair activity (9, 18, 58, 65; see also references in reference 21). Another mechanism that may increase genetic diversity is the movement of transposable elements (14, 56). Also, some data (51) indicate that different mutagenic pathways might be involved in mutation processes creating either early- or late-arising mutants in the stationary-phase cell population.
It has been suggested that the methyl-directed mismatch repair system might be limiting in stationary phase and nutritionally deprived cells, giving rise to stationary-phase mutations (19, 22, 54, 55). However, the role of mismatch repair in stationary-phase mutations is controversial (20, 26). Bridges et al. (12) proposed that in nongrowing bacteria, oxidized guanine residues, including 7,8-dihydro-8-oxoguanine, constitute an important component of spontaneous mutation. Pairing of adenine with 7,8-dihydro-8-oxoguanine, an oxidatively damaged form of guanine, is known to cause G:C to T:A transversions during DNA synthesis (41). The DNA repair enzymes MutY and MutM are part of a multiple line of defenses against oxidative damage to DNA (42). Cells that lack active MutY protein have elevated rates of G:C to T:A transversions (45). In Escherichia coli, accumulation of prototrophic mutants during amino acid starvation was caused by 7,8-dihydro-8-oxoguanine, and the rate of reversions enabling a prototrophic phenotype in starved cells was remarkably elevated in MutY-defective strains (12).
Knowledge about mechanisms of mutational processes in starving bacteria is mostly based on investigations of E. coli (21). The genus Pseudomonas is a diverse and ecologically significant group of bacteria (59), but there are only a few examples of studies on mutagenesis in Pseudomonas spp. (32, 35, 38, 48, 49; see also references in reference 21). Our test system employing a promoterless pheBA cluster in plasmid pEST1414 as a reporter enables us to isolate and characterize mutations that create functional promoters for the transcription of the pheBA genes in Pseudomonas putida (32). The pheBA genes encode catechol 1,2-dioxygenase and phenol monooxygenase, respectively (33). When the pheBA-expressing plasmid is introduced into phenol-nondegrading P. putida strain PaW85, bacteria gain the ability to utilize phenol as a sole carbon source. We have shown that promoters for the transcription of the initially promoterless phenol degradation genes pheBA were created as a result of base substitutions, deletions, or transposition of the mobile DNA elements Tn4652 and IS1411 (31, 32).
Previous results (32) indicated that different mechanisms are responsible for the appearance of mutations in exponentially growing and stationary-phase cells of P. putida. The accumulation rate of the Phe+ mutants on selective plates was found to be dependent on the physiological state of the bacteria before plating: the accumulation was much higher for bacteria plated from stationary-phase culture than for those plated from exponentially growing cells. Also, we found that stationary-phase mutants appeared mainly due to one particular C-to-A base substitution, whereas different deletions (mostly in the range of 6 to 40 bp) prevailed in cultures growing exponentially.
In this report, we focused on studies of the effect of time of starvation on the spectrum of stationary-phase mutations. It appeared that the spectrum of stationary-phase mutations among early-arising mutants differed from that of the later-arising ones. We observed that one particular C-to-A transversion was the dominant mutation type in the cell population at the beginning of starvation, but the proportion of the other type of mutations, 2- to 3-bp deletions, increased remarkably with time of starvation. The formation of 2- to 3-bp deletions required stationary-phase sigma factor RpoS, whereas the frequency of occurrence of transversions was affected by the functionality of the 7,8-dihydro-8-oxoguanine repair enzyme MutY. The balance between various processes involved in mutagenesis in starving cells will be discussed.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are described in Table 1. Complete medium was Luria-Bertani (LB) medium (44), and minimal medium was M9 (1). Phenol minimal plates with 1.5% Difco agar contained 2.5 mM phenol as the sole carbon and energy source. Antibiotics were added at the following final concentrations: for E. coli, ampicillin at 100 μg/ml; for P. putida, carbenicillin at 1,000 to 3,000 μg/ml; for both organisms, kanamycin at 50 μg/ml, tetracycline at 10 μg/ml, and rifampin at 100 μg/ml. E. coli was incubated at 37°C, and P. putida was incubated at 30°C. E. coli was transformed with plasmid DNA as described by Hanahan (25). P. putida was electrotransformed as described by Sharma and Schimke (57). E. coli TG1 (15) was used for the DNA cloning procedures.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or construction | Source or reference |
|---|---|---|
| E. coli | ||
| TG1 | supE hsdΔ5 thi Δ(lac-proAB) F′ (traD36 proAB+laclqlacZΔM15) | 15 |
| CC118 λpir | Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE(Am) recA1 λpir phage lysogen | 29 |
| P. putida | ||
| PaW85 | Tn4652 | 5 |
| PKS54 | Tn4652 rpoS::km | 47 |
| PaW85 mutY::tet | Tn4652 mutY::tet | This work |
| Plasmids | ||
| pEST1414 | Plasmid pAYC32 carrying promoterless pheBA operon | 32 |
| pBluescript SK(+) | Cloning vector (Apr) | Stratagene |
| pBR322 | Cloning vector (Apr Tetr) | 8 |
| pBlscrMutY | pBluescript SK(+) containing PCR-amplified P. putida mutY gene cloned into EcoRV- and SmaI-cleaved vector | This work |
| pBLscrMutY-Tetr | tet gene from pBR322 inserted into EcoRI-Eco47III-cleaved mutY gene in pBLscrMutY | This work |
| pGP704 L | Delivery plasmid for homologous recombination | 50 |
| pGPMutY-Tetr | mutY::tet-sequence-containing SacI-KpnI fragment from pBlscrMutY-Tet cloned into pGP704L | This work |
| pRK2013 | Helper plasmid for conjugal transfer of pGP704L | 16 |
Isolation of Phe+ mutants.
Independent cultures of P. putida strains carrying plasmid pEST1414 were generated by growing cells to saturation in LB medium, diluting this culture by 106 into fresh LB medium, dispensing 2-ml aliquots into test tubes, and allowing cells again to reach saturation by growing cells for 16 to 18 h. Then 0.5-ml samples (approximately 5 × 108 cells) were harvested by centrifugation, washed with M9 solution, and plated on phenol minimal plates. When different amounts of pEST1414-carrying cells of the same cultures were plated on selective plates, they were plated with equal amounts of scavenger cells (approximately 109 cells). Scavenger cells (P. putida PaW85 carrying a pEST1414 derivative lacking the pheA coding sequence) were grown to saturation in LB medium and concentrated by centrifugation and resuspension in 0.1 volume of M9 solution.
To analyze the Phe+ mutants from the growing cultures, independent Phe+ colonies appearing on selective plates on day 2 were picked from separate plates and used for DNA sequence analysis of the plasmids conferring constitutive expression of the pheBA genes. To characterize mutations occurring in starving cultures, the Phe+ mutants accumulating on selective plates on day 3 and later were analyzed. To exclude the copies of original, wild-type (Phe−) plasmids present in the Phe+ colonies, we isolated plasmid DNA from the mutants and transformed E. coli TG1, selecting for resistance to ampicillin. Transformants were assayed for expression of the pheB gene by testing expression of catechol 1,2-dioxygenase as described before (27) except that the measurements were carried out in cell suspensions.
Analysis of insertions of Tn4652 and IS1411 into pEST1414.
Constitutively expressed fusion promoters are created as a result of transposition of transposon Tn4652 upstream of the pheA coding sequence (46). Kasak et al. have previously shown that transposon insertions account for one-third of all stationary-phase mutations in bacteria carrying plasmid pEST1414 (32). Two primers, pheA (5′-TGCTCAAGATTATCATTACGCT-3′), complementary to the pheA coding sequence at nucleotides 11 to 32, and TnR (5′-ATCAGCATAGACGGCTAGCCAG-3′), complementary to the right end of Tn4652 at nucleotides 101 to 122, were used to amplify the Tn4652 insertion regions in Phe+ cells containing hybrid plasmids. Nurk et al. have previously shown (46) that fusion promoters are preferentially created by the right-end sequence of the transposon. Therefore, the detection scheme used here was designed to reveal promoters generated by fusion of the right end of the element with the upstream sequences of the pheA gene. IS1411 can activate the promoterless pheBA genes due to outward-directed promoters on its left end (31). The oligonucleotide ORF2 (5′-CGAGGTTATTCAGTT-3′), complementary to nucleotides 47 to 61 relative to the start codon of the tnpA gene of IS1411, and oligonucleotide pheA were used for PCR analysis of Phe+ mutants for insertions of IS1411 upstream of the pheA gene.
DNA sequence analysis.
The ∼250-bp DNA region of the Phe+ mutants upstream of the pheBA genes in plasmid pEST1414 was analyzed by DNA sequencing. The DNA segment containing this region was amplified by PCR with the oligonucleotides PAYC32 (5′-CTCGACCTTTGAGCCAAATG-3′) and CAT2,1 (5′-TTTTAACAGTCATAATTACTCTCTC-3′), complementary to the sequences of vector plasmid pAYC32 upstream of the SacI site and to the sequence −12 upstream of the pheB initiator codon, respectively. The nucleotide sequences were determined with the DYEnamic ET terminator cycle sequencing kit (Amersham Pharmacia Biotech Inc.). The oligonucleotide used in the sequencing of the mutant DNA region upstream of the pheBA genes in plasmid pEST1414 was either CAT2,1 or AB0 (5′-GGAAGTATGCTTGGC-3′), complementary to the sequences −136 nucleotides upstream of the pheB initiator codon. The DNA sequencing reactions were analyzed with an ABI Prism 377 DNA sequencer (Perkin Elmer).
Construction of P. putida PaW85 mutY knockout mutant.
The mutY gene sequence of P. putida was obtained by searching for mutY homologs in the unfinished P. putida KT2440 Genome Project website (http://www.tigr.org). The mutY gene was amplified by PCR from genomic DNA of P. putida PaW85 which is isogenic to P. putida strain KT2440. Two primers, KTYFw (5′-GCGCTCAAGGGGCTGTTCAAC-3′) and KTYRev (5′-CGGTGCGGGTCATCGGGCGT-3′), complementary to the sequences −23 nucleotides upstream of the ATG initiator codon and 9 nucleotides downstream of the TAG stop codon of the P. putida mutY gene, respectively, were used for DNA amplification in a standard PCR (96°C for 1 min, 57°C for 1 min, and 72°C for 1.5 min; 25 cycles). The amplified DNA fragment containing the mutY gene was subcloned into pBluescript SK(+) cleaved with EcoRV and SmaI to obtain pBlscrMutY. The EcoRI-Van9I DNA fragment containing the tet gene from pBR322 was inserted into the EcoRI- and Eco47III-cleaved mutY gene. The resulting mutY-tet sequence from pBlscrMutY-Tet was inserted into plasmid pGP704L (50) by using the SacI and KpnI sites. pGPMutY-Tet was selected in E. coli strain CC118 λpir (29).
The interrupted mutY gene was inserted into the chromosome of P. putida PaW85 by homologous recombination. Plasmid pGPMutY-Tet, which does not replicate in hosts other than E. coli CC118 λpir, was conjugatively transferred into P. putida PaW85 by using helper plasmid RK2013 (16). The PaW85 mutY::tet knockout was verified by PCR analysis. In addition, the mutator phenotype of the clones containing the interrupted mutY sequence but lacking the original sequence was examined by measuring the spontaneous frequency of mutation to rifampin resistance.
Measurement of mutation frequency.
The spontaneous mutation frequency of P. putida wild-type and MutY-defective strains was measured by calculating the average number of mutants (±standard deviation) per 109 cells. At least 20 independent cultures grown in LB as described above were plated on either rifampin or phenol minimal plates. Phe+ colonies appearing on phenol minimal plates on day 2 were counted to estimate Phe+ mutation frequency in growing cells.
Measurement of viability of MutY-defective P. putida on phenol minimal plates.
The growth conditions for bacteria were the same as described for the isolation of Phe+ mutants. About 5 × 108 M9-washed cells of P. putida wild-type strain PaW85 and its MutY-defective derivative were plated onto four phenol minimal plates, and small plugs were cut from the agar on each day of starvation. Bacteria from the plugs were suspended in M9 solution, and the number of CFU was determined on 0.1% glucose minimal plates.
RESULTS
Changes in spectrum of mutations during starvation.
Previous experiments demonstrated that promoters for the transcriptional activation of the pheBA genes were created as a result of base substitutions, deletions, and transpositions of transposon Tn4652 (32). In the article by Kasak et al. (32), we focused on differences between mutations occurring in growing cells and in stationary-phase cells but did not pay attention to whether the spectrum of stationary-phase mutations might be influenced by the duration of starvation. In the current report, mutant colonies that appeared on the phenol selective plates on days 3 to 7 after plating were analyzed on each separate day.
The accumulation curve of the Phe+ mutants in starving cultures of P. putida wild-type strain PaW85 carrying the promoterless reporter plasmid pEST1414 is shown in Fig. 1. Approximately 60 to 70 Phe+ mutants per day were subjected to sequence analysis. Mutants that appeared due to insertions of Tn4652 (they accounted for one-third of all stationary-phase mutations) were excluded from this analysis (see Materials and Methods). The 250-bp sequence upstream of the pheBA genes containing raw material for promoter creation (Fig. 2) was analyzed in mutant plasmids. Kasak et al. have previously shown that Phe+ mutants that appeared on selective plates on day 2 contained mutations that occurred before plating in growing cultures, whereas colonies that emerged on phenol minimal plates on day 3 and later contained mutations that occurred after the cells were plated (32).
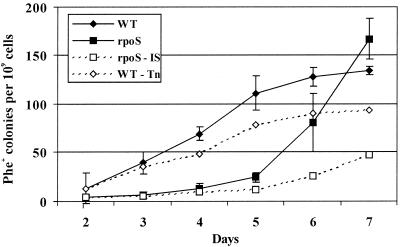
FIG. 1.
Accumulation of Phe+ mutants on phenol minimal plates in P. putida wild-type strain PaW85 (WT) and its RpoS-defective derivative PKS54 (rpoS). Dashed lines indicate the theoretical appearance of mutants (deduced from the results of PCR analysis of Tn4652 and IS1411 insertions in Phe+ mutants) in the wild-type strain if Tn4652-linked mutants are subtracted (WT − Tn) and in PKS54 if IS1411-linked mutants are subtracted (rpoS − IS). Each point represents the mean of at least five independent determinations, and error bars represent standard deviations. Ilves et al. have previously shown that survival of the wild-type P. putida strain and its RpoS-defective mutant is not decreased during the first 5 days of starvation of bacteria on phenol minimal plates (30).
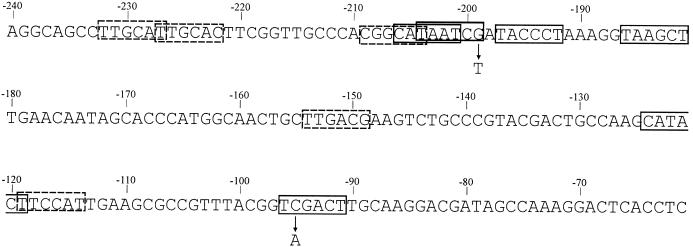
FIG. 2.
Sequence upstream of pheB in plasmid pEST1414 contains raw material for promoter creation. The potential −35 and −10 hexamers of promoters are framed, −35 hexamers by dashed lines and −10 hexamers by continuous lines. The G-to-T and C-to-A transversions are marked by arrows. As shown in previous studies (32), base substitutions, deletions, and insertions can create promoter sequences similar to that of the E. coli RNA polymerase σ70 promoter consensus sequence TTGACAN16-18TATAAT.
The results shown in Table 2 reveal changes in the spectrum of promoter-creating mutations during starvation. We observed a significant decline in the percentage of C-to-A transversion when the spectrum of the mutations of the Phe+ colonies isolated on either day 3 or day 4 was compared to that of the mutants that appeared on day 7 (χ2 = 22 and 5.5, respectively; P < 0.01). Comparison of the spectra of early-arising and late-arising Phe+ mutants revealed remarkable changes in the nature of the deletions occurring at different stages of starvation (Table 2). Deletions in the range of 6 to 45 bp were rarely represented among the mutants that appeared on days 3 to 5 and were not observed later. At the same time, the amount of smaller, 2- to 3-bp deletions increased among the promoter-creating mutations with time of the starvation. The proportion of 2- to 3-bp deletions was significantly different when the spectrum of the Phe+ mutants that emerged on days 3 and 4 was compared to that of the mutants that appeared on day 7 (χ2 = 11, P < 0.001). On each day except day 5, we also detected another type of changes, formation of 2- to 3-bp insertions (Table 2). However, as shown in Table 2, in comparison with the deletions, the insertions occurred quite rarely. Every day, approximately 20 to 30% of the mutations remained undefined (Table 2) because they occurred outside the sequencing window.
TABLE 2.
Effect of duration of starvation of P. putida cells on spectrum of Phe+ mutations
| Day of appearance of mutants | No. of mutants analyzed | No. (%) of mutants with:
|
|||||
|---|---|---|---|---|---|---|---|
| C-to-A transversion | G-to-T transversion | Deletions
|
Insertions (2 to 3 nt) | Undefined mutations | |||
| 6 to 45 ntb | 2 to 3 nt | ||||||
| 2a | 77 | 40 (52) | 14 (18) | 16 (21) | 2 (2.5) | 3 (4) | 2 (2.5) |
| 3 | 69 | 47 (68) | 4 (6) | 4 (6) | 0 | 2 (3) | 12 (17) |
| 4 | 62 | 30 (48) | 10 (16) | 2 (3) | 5 (8) | 3 (5) | 12 (20) |
| 5 | 70 | 30 (43) | 9 (13) | 3 (4) | 6 (9) | 0 | 22 (31) |
| 6 | 60 | 18 (30) | 12 (20) | 0 | 13 (22) | 1 (2) | 16 (26) |
| 7 | 61 | 16 (26) | 11 (18) | 0 | 12 (20) | 5 (8) | 17 (28) |
Phe+ mutants appearing on selective plates on day 2 contained mutations that occurred in growing cultures before plating.
nt, nucleotides.
Kasak et al. have previously shown that deletions in the range of 6 to 45 bp were characteristic of the Phe+ mutations occurring in the growing cultures of P. putida PaW85 (32). A similar pattern of deletions was also revealed in the current study (Table 2). Only a few Phe+ mutants appearing on day 2 were created by the 2- to 3-bp deletions, but the number of these deletions increased significantly among the mutations characterized on days 3 to 7 (χ2 = 4, P = 0.04). Thus, the occurrence of 2- to 3-bp deletions seems to be induced during long-term starvation.
Deficiency of MutY leads to increased frequency of C-to-A and G-to-T transversions in P. putida.
There are several reports demonstrating that the lack of DNA repair enzyme MutY leads to an increased frequency of G:C to T:A transversions (41, 45). Moreover, in the case of the E. coli mutY trpA23 strain, prototrophic mutants containing either G-to-T transversions at the trpA23 site or small in-frame deletions in the trpA gene arise at an elevated rate when the bacteria are incubated under starvation conditions (11).
In order to study whether the mutY background would affect the nature and frequency of the appearance of Phe+ mutants, we constructed a MutY-defective P. putida strain. The mutY gene sequence of P. putida was obtained from the unfinished P. putida KT2440 Genome Project website. The 1,065-nucleotide open reading frame of P. putida mutY encodes 355 amino acids. The deduced amino acid sequence of P. putida MutY revealed 78% identity with the putative MutY of Pseudomonas aeruginosa and 57% identity with E. coli and Salmonella enterica serovar Typhimurium MutY sequences (data not shown).
The mutY gene of P. putida was disrupted by the tetracycline resistance-encoding gene, and the mutY::tet sequence was used to replace the wild-type mutY by homologous recombination. The frequency of rifampin resistance mutations in wild-type and MutY-defective P. putida strains was examined by plating independent cultures on rifampin plates (Materials and Methods). The mutation frequency increased approximately 60-fold in the MutY-defective strain compared to the wild-type strain: the average number of Rifr colonies per 109 cells was 1.9 ± 1.5 in the case of the wild-type strain and 120 ± 92 in the case of PaW85 mutY::tet. This demonstrates that lack of MutY leads to the mutator phenotype of P. putida.
We studied the frequency and spectrum of Phe+ mutations in growing cells of P. putida PaW85 mutY::tet. Approximately 5 × 108 cells carrying the reporter plasmid pEST1414 were plated onto phenol minimal plates. Comparison of the wild-type and MutY-defective strains revealed about a 50-fold increase in the frequency of promoter-creating mutations in PaW85 mutY::tet when growing cultures were examined; the average number of Phe+ colonies per 109 cells was 7.5 ± 5 in the case of the wild-type strain and 380 ± 220 in the case of the MutY-defective strain.
The Phe+ mutants collected from separate selective plates in two parallel experiments (experiments I and II) were subjected to further characterization. The spectra of mutants obtained from two studies (Table 3) were statistically similar (χ2 = 1.7, P = 0.2). The results shown in Table 3 demonstrated that all mutants characterized in the mutY::tet background contained either a C-to-A or G-to-T transversion at the same sites as in the mutants characterized by us before (Fig. 2). No other type of mutations (deletions, insertions) or Tn4652 insertions could be detected. We observed a difference in the mutation frequency at the two different sites: the promoter-creating C-to-A mutation was represented at a higher frequency than the G-to-T mutation in both experiments.
TABLE 3.
Spectrum of Phe+ mutations occurring in the MutY-defective P. putida strain
| Day | No. of mutants analyzed | No. (%) of mutants with:
|
||
|---|---|---|---|---|
| C-to-A transversion | G-to-T transversion | Undefined mutation | ||
| 2a | 45 | 30 (67) | 15 (33) | 0 |
| 2b | 72 | 57 (80) | 15 (20) | 0 |
| 3 | 44 | 27 (61) | 15 (34) | 2 (5) |
| 6 | 40 | 12 (30) | 10 (25) | 18 (45) |
Experiment I; one mutant from each of 45 separate cultures was analyzed.
Experiment II; four mutants from each of 18 separate cultures were analyzed.
The average number of Phe+ mutants accumulating on phenol minimal plates per day per 5 × 108 plated P. putida MutY-defective cells decreased rapidly during starvation (Table 4). The lack of MutY causes the mutator phenotype of P. putida, and this could increase the frequency of lethal mutations in the cell population. Therefore, we studied survival of MutY-defective cells on phenol minimal plates (Materials and Methods). There were no differences in viability between the wild-type and mutY::tetr strain during the first 6 days of starvation (data not shown). Instantly, survival of the MutY-defective strain decreased 100-fold for day 7 (5 × 106 cells per plate) and thereafter remained constant over the next week of starvation. Thus, the rapid decline in the rate of accumulation of Phe+ mutants on selective plates was not caused by the death of bacteria. Rather, this could be explained by inhibition of accumulation of the mutants due to the high number of mutant colonies already present on selective plates.
TABLE 4.
Effect of number of Phe+ colonies on selective plates on further accumulation of mutants in wild-type and MutY-defective P. putida strainsa
| Day | Mean no. of Phe+ colonies ± SD at initial inocolum:
|
|||||
|---|---|---|---|---|---|---|
| 5 × 108 cells
|
5 × 107 cells
|
5 × 106 cells
|
||||
| Wild type | mutY | Wild type | mutY | Wild type | mutY | |
| 2 | 2.0 ± 1.7 | 260 ± 108 | 0 | 30 ± 13 | 0 | 3.0 ± 1.8 |
| 3 | 2.3 ± 1.7 | 423 ± 33 | 0 | 92 ± 13 | 0 | 10 ± 2.6 |
| 4 | 9.3 ± 4.5 | 108 ± 30 | 1.8 ± 0.5 | 183 ± 29 | 0 | 23 ± 2.6 |
| 5 | 19 ± 9.8 | 37 ± 15 | 0.8 ± 0.9 | 113 ± 43 | 0 | 25 ± 5.1 |
| 6 | 41 ± 20 | 8.3 ± 2.5 | 1.8 ± 2.4 | 22 ± 11 | 0 | 60 ± 6.5 |
| 7 | 39 ± 21 | 5.2 ± 1.9 | 2.8 ± 2.0 | 15 ± 6.7 | 0 | 72 ± 9.6 |
Different amounts of pEST1414-carrying cells (5 × 108, 5 × 107, or 5 × 106) were plated with a constant number (109) of scavenger cells onto phenol minimal plates. Results of four separate platings are shown.
To control whether the high number of the Phe+ mutants per plate would inhibit subsequent accumulation of mutants in the MutY-deficient background, we repeated the experiment by plating different amounts (5 × 108, 5 × 107, and 5 × 106) of cells on selective plates. Cells of the wild-type P. putida strain and PaW85 mutY::tet were plated onto phenol minimal plates with approximately 109 scavenger cells (Materials and Methods). The data shown in Table 4 clearly demonstrate that the large number of Phe+ colonies on selective plates inhibited further accumulation of the mutants. The accumulation of Phe+ mutants was not inhibited if we plated 100 times fewer MutY-defective cells. The inhibition effect became apparent even in this case when 5 × 107 MutY-defective cells were plated (Table 4, MutY results, compare days 4 and 5 with day 6).
At the same time, the data presented in Table 4 show that the frequency of appearance of Phe+ mutants was remarkably higher in the MutY-defective strain compared to that in the wild-type strain during all 7 days studied. The Phe+ mutants that accumulated when fewer cells (5 × 106) of the MutY-defective strain were plated were subjected to further characterization. The spectrum of mutations that appeared on selective plates on days 3 and day 6 is shown in Table 3. Most of the Phe+ mutants analyzed contained either C-to-A or G-to-T transversions. We did not detect deletions among the mutants studied. Some mutants, however, contained changes outside the sequencing window. The proportion of this type of mutant increased during the starvation period from 5% among colonies that appeared in day 3 on selective plates to 45% among those that arose on day 6.
Lack of stationary-phase sigma factor RpoS affects the spectrum of promoter-creating mutations in starving P. putida cells.
As described above, the spectrum of the Phe+ mutations changed during prolonged starvation: the proportion of 2- to 3-bp deletions among the promoter-creating mutations increased and the number of transversions declined (Table 2). This indicated changes in expression of some mutagenic pathway in P. putida cells during starvation. Stationary-phase sigma factor σS (RpoS) is the main sigma factor activating gene expression in stationary phase or otherwise stressed bacterial cells (reviewed in reference 28).
To study the possible effect of RpoS on stationary-phase mutations in P. putida, we characterized the spectrum of Phe+ mutations in starving cultures of the P. putida rpoS-defective mutant PKS54, which is a derivative of P. putida wild-type strain PaW85 (47). Ilves et al. have previously shown that transposition of Tn4652 into the reporter plasmid pEST1332 is suppressed in the P. putida RpoS-defective strain (30). Therefore, we expected to see some decrease in the frequency of Phe+ mutations in this background.
Indeed, in comparison with the wild-type strain, the frequency of appearance of Phe+ mutants was lower in the RpoS-defective strain (Fig. 1). During the first 5 days of starvation, this was even lower than the theoretical frequency of appearance of mutants in the wild-type strain if Tn4652 insertions were subtracted (Fig. 1). Because, during the first 5 days of starvation, the RpoS-defective strain survives as well as the wild-type strain (30), the lower rate of accumulation of Phe+ mutants in strain PKS54 than in PaW85 cannot be explained by lower viability of the RpoS mutant. Surprisingly, on days 6 and 7 we observed an increase in the number of Phe+ mutants that emerged per plate in PKS54 compared to that in the wild-type strain.
Analysis of the mutants revealed that the higher rate of accumulation of Phe+ mutants in the RpoS-defective strain was caused by activation of transposition of IS1411 (Fig. 1). The proportion of IS1411-linked Phe+ mutants increased during starvation from 10% on day 3 to 75% on day 7. IS1411 is located just downstream of the pheBA genes in reporter plasmid pEST1414 (31). IS1411 can insert upstream of the promoterless pheBA operon and activate transcription of these genes due to the presence of outward-directed promoters at the left end of IS1411. However, intramolecular transposition of IS1411 in the wild-type strain PaW85 is rare, being lower than 1% of the events that activate the reporter genes in pEST1414 (31). We did not detect transposition of Tn4652 into the reporter plasmid pEST1414 in the RpoS-defective P. putida strain PKS54.
As the next step of our investigations, we concentrated on characterization of the mutants that emerged independently of IS1411 insertions. The Phe+ colonies that appeared on selective plates on day 4 and days 6 to 7 were subjected to sequence analysis. The data presented in Table 5 show that the occurrence of 2- to 3-bp deletions was positively controlled by RpoS. Only a few deletions in range of 7 to 80 bp were observed in starving cells of the RpoS mutant, but no 2- to 3-bp deletions were recorded. In addition to small deletions, 2- to 3-bp insertions were also absent in the RpoS mutant.
TABLE 5.
Spectrum of Phe+ mutations occurring in starving cultures of the RpoS-defective P. putida strain
| Days of starvation | No. of mutants analyzed | No. (%) of mutants with:
|
|||
|---|---|---|---|---|---|
| C-to-A transversion | G-to-T transversion | Deletions (7 to 80 nt) | Undefined mutations | ||
| 4 | 74 | 46 (62) | 23 (31) | 3 (4) | 2 (3) |
| 6 and 7 | 71 | 26 (36) | 22 (31) | 2 (3) | 21 (30) |
We controlled whether the promoters created by the 2- to 3-bp deletions could express the pheBA genes in P. putida RpoS-defective strain at the level enabling growth of bacteria on phenol minimal plates. We transformed the RpoS-defective strain PKS54 with deletant plasmids that were isolated from Phe+ mutants of the wild-type cells. In all cases studied, as in the wild-type strain, the Phe+ transformants of the RpoS-defective strain emerged on phenol minimal plates on day 2 after plating.
DISCUSSION
The test system for the selection of mutants based on creation of functional promoter sequences for the transcriptional activation of the pheBA phenol degradation genes in P. putida enables us to study different types of mutations and transposition of mobile DNA elements. Based on the analysis of the mutants characterized in this study and previously (32), the sequence upstream of the pheBA genes contains many potential −35 and −10 hexamers for promoter creation (Fig. 2). Most of these −35 and −10 elements are located too far from each other, but the distance between the promoter elements can be optimized by deletions.
The length of the deletions between six different −35 hexamers and four different −10 hexamers that created a functional promoter varied from 2 to 80 bp. In some cases, the length of the spacer between the −35 sequence TTGCAC and −10 sequence CATAAT was extended from a nonoptimal 15 bp to the optimal distance (17 or 18 bp) due to 2-bp or 3-bp insertions. Additionally, two point mutations changed two originally nonfunctional −10 hexamers located at the right distance from the −35 promoter elements to functional −10 sequences. One particular C-to-A transversion 96 nucleotides upstream from the ATG codon of the pheB gene changed the original sequence TCGACT to TAGACT, which is more similar to the −10 hexamer consensus sequence TATAAT. Another G-to-T transversion 200 nucleotides upstream from the pheB gene converted the potential −10 sequence TAATCG to TAATCT.
The nature of the mutations creating promoters for the transcriptional activation of the pheBA genes in P. putida is influenced by physiological state of the bacteria. Data presented previously (32) and herein indicate that the mutation generation process in starving cells is different from that in growing bacteria. Various deletions, mostly larger than 2 to 3 bp, were highly represented in mutants emerging among growing bacteria, but they rarely appeared in starving cultures. Moreover, differences were also revealed between early-arising and late-arising stationary-phase mutations. The most dominant mutation type in starving cells, the C-to-A transversion, was characteristic of the mutants that appeared on selective plates on days 3 to 5 after plating. Later (days 6 and 7) we observed an increase in the frequency of 2- to 3-bp deletions and a decrease in the frequency of the C-to-A transversion (Table 2).
The specific spectrum of mutations depends on a complex array of factors, including replication errors, lesions in DNA, and changes due to repair. There are several hints about DNA repair insufficiency in starving cells of E. coli (reviewed in reference 21). The oxidation of guanine to 7,8-dihydro-8-oxoguanine is one of the most common types of DNA damage in cells. Oxidative DNA damage accumulation within bacteria is a major contributor to the generation of stationary-phase mutations in bacteria (10). MutY is part of a complex DNA repair network that reduces the mutagenic effects of 7,8-dihydro-8-oxoguanine. MutY is a glycosylase which removes adenine from A:7,8-dihydro-8-oxoguanine mispairs in duplex DNA (42), and failure to perform this function in mutY strains leads to a significant increase in G:C to T:A transversions (45).
Recently, it was found that artificial overexpression of methyl-directed DNA mismatch repair enzyme MutS in the E. coli CC104 tester strain also significantly decreased G:C to T:A transversions (68). We have constructed a MutY-defective strain of P. putida. Study of the spectrum of promoter-creating mutations in the P. putida MutY-defective strain revealed a tight connection between expression of MutY and the frequency of occurrence of two-base substitutions and C-to-A and G-to-T transversions. The lack of 7,8-dihydro-8-oxoguanine repair enzyme MutY in P. putida cells gave rise to this type of mutation in both growing and starving cells. The frequency of promoter-creating mutations in mismatch repair enzyme MutS-defective P. putida also constructed in our laboratory increased only in starving cultures, but most of these mutations occurred outside the sequencing window (data not shown).
Based on these data, we suppose that the lower efficiency of the 7,8-dihydro-8-oxoguanine repair pathway in starving P. putida might be the reason for the high frequency of occurrence of C-to-A transversion at one particular site upstream of the pheB gene in early-arising stationary-phase mutants. However, some other types of Phe+ mutations (located outside of the sequencing window) occurred in starving populations of P. putida PaW85 mutY::tet cells in addition to C-to-A and G-to-T transversions, and the frequency of these undefined mutations increased during starvation.
Bridges and Timms (11) reported that when trpA mutY cells of E. coli were held under tryptophan starvation conditions, the tryptophan-independent mutations that arose included small in-frame deletions in addition to transversions. Such deletions were also found in starved bacteria defective in the mismatch repair system. The deletant mutants appeared later, and the delayed appearance was caused by slower growth of these mutants compared to the transversion mutants (11). In the case of the appearance of the Phe+ mutants during starvation, the shift from transversions to 2- to 3-bp deletions cannot be accounted for by slower growth of the deletant mutants on phenol minimal plates. Also, we did not observe an increase in the rate of occurrence of the 2- to 3-bp deletions in either the P. putida mismatch repair- or 7,8-dihydro-8-oxoguanine repair pathway-defective strain.
We have found that the formation of small deletions is a regulated process that needs stationary-phase sigma factor RpoS. These deletions (and also small 2- to 3-bp insertions) were absent in the RpoS-defective P. putida strain. The results obtained by us indicate that some mutagenic pathway responsible for the creation of small deletions and insertions must be positively controlled by the stationary-phase sigma factor in P. putida. The effect of this pathway becomes more evident in cells that have been starving for some days. It is obvious that there should be a balance among the various processes involved in mutagenesis, DNA damage, various types of DNA replication and DNA repair, metabolic activation, detoxification, and, perhaps, also in the environment of cells.
The effect of environment on mutation spectra has recently been demonstrated by studying compensatory mutations to antibiotic resistance (7). One could speculate that during prolonged starvation, the physiological parameters of the bacterial cell and also the environment where bacteria are starving might differ from those at the beginning of starvation. There are several reports indicating that changes in gene expression take place even in late stationary phase (23, 24, 43, 63; reviewed in reference 36). Therefore, these changes might affect DNA metabolism functions in starving cells.
Evidence for the involvement of error-prone polymerases in stationary-phase mutations has been demonstrated in several studies (6, 13, 38, 40, 64). Recent studies indicate that E. coli DNA polymerases act coordinately with their associated accessory factors in the context of DNA replication, repair, and recombination (60). Most prokaryotes carry homologues of the error-prone DinB/UmuC/Rad30/Rev1 superfamily polymerases (39). Thus, drawing parallels with mutagenesis in E. coli, the DNA polymerase switch is one possible mechanism for changes in the spectrum of promoter-creating mutations during starvation of P. putida. A gene homologous to E. coli dinB is also present in the genome of P. putida, but regulation of the expression of the mutagenic polymerases in this organism has not been unexplored.
It seems very likely that increased activity of mutagenic DNA polymerase in cells that have already been starved for some days could explain changes in the spectrum of Phe+ mutations. However, one could also argue that different extents of stress-induced DNA protection at different stages of starvation would affect the outcome of mutagenesis. Under conditions of either oxidative or nutritional stress, E. coli produces high level of the nonspecific DNA-binding protein Dps (DNA-binding protein from starved cells), which efficiently protects DNA against oxidative agents (2, 3, 37). dps mutants exhibit an increased level of G:C to T:A transversions (37). Dps was discovered as a protein synthesized at a high level after E. coli had been in stationary phase for over 3 days (2). An increase in Dps level after prolonged cultivation of E. coli cells was also observed by Azam et al. (4).
Close Dps homologs have been identified in distantly related bacteria (see the references in reference 66), which implies that this mode of DNA protection might be general and crucial. DNA sequence database analysis indicates that a dps homolog is also present in Pseudomonas spp. (data not shown). Thus, drawing parallels with Dps function in E. coli, we can speculate that the effect of Dps on protecting DNA against oxidative damage should become obvious only after prolonged starvation of P. putida. Phe+ cells of P. putida mixed with Phe− cells need 2 days to form visible colonies on phenol minimal plate (32). Therefore, the mutants which were picked from selective plates earlier (on days 3 to 5; they appeared mostly due to transversions) represent events that were happening at the beginning of starvation, when DNA is probably less protected. Later, on days 6 to 7, when DNA is becoming more resistant to 7,8-dihydro-8-oxoguanine mutagenesis, the proportion of the other type of mutations, not dependent on the 7,8-dihydro-8-oxoguanine repair system (e.g., small deletions), increases. This possible change, in addition to hypothetical induction of mutagenic DNA polymerase, would also explain why small deletions become more abundant during prolonged starvation.
Both transposon Tn4652 and IS1411 can activate transcription of promoterless pheBA genes in P. putida (30, 31, 32). The movement of transposable DNA elements is mediated by functions encoded by the element and by its host (34). Transposons can usually insert into many different sites of the genome, which renders the movement of a transposon potentially deleterious for host gene expression. Therefore, the movement of mobile DNA elements is usually strongly downregulated. The advantageousness of transposition sometimes appears only in extreme situations, when transposition permits new traits to evolve (56).
Our studies of transposition of Tn4652 and IS1411 also indicate that DNA rearrangement activities under stress are controlled by many cellular regulatory functions which might be affected by how long bacteria have been starved. Ilves et al. found that the insertion of transposon Tn4652 is an exclusively stationary-phase-specific event regulated by stationary-phase sigma factor σS (30). Control of the timing of genetic changes became evident in transposition of Tn4652; Kasak et al. have demonstrated that the maximum rate of transposition frequency of this DNA element is achieved after a few days of starvation of P. putida cells (32). The results presented in the current report demonstrate that activation of the transposition of IS1411 (in contrast to the movement of Tn4652) seems to be negatively controlled by RpoS, which means that expression of some factor(s) downregulating IS1411 transposition activity requires RpoS. Interestingly, the transposition of IS1411 increases remarkably with time of starvation. This hints that activation of IS1411 needs some late-starvation signal.
To summarize the results of studies on stationary-phase mutations presented in this report, we suggest that mutation processes in cells that have been starved for short periods are not entirely compatible with prolonged starvation, at least in P. putida. In nature, survival is the normal mode for most microorganisms, and growth occurs only occasionally. Prolonged starvation is a condition in which bacteria undergo rapid evolution by natural selection (61, 62, 67; see also the references in references 17 and 53). Thus, to understand the mechanisms of molecular evolution in microorganisms, more attention should be paid to stationary-phase mutations occurring during prolonged starvation.
Acknowledgments
We thank T. Alamäe, R. Hõrak, and other coworkers for critically reading the manuscript. We are also grateful to J. Truu for help in statistical analysis of the data.
This work was supported by grants 4481 and 4482 from the Estonian Science Foundation and by grant HHMI 55000316 from the Howard Hughes Medical Institute International Research Scholars Program.
REFERENCES
- 1.Adams, M. H. 1959. Bacteriophages, p. 445-447. Interscience Publishers Inc., New York, N.Y.
- 2.Almiron, M., A. J. Link, D. Furlong, and R. Kolter. 1992. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 6:2646-2654. [DOI] [PubMed] [Google Scholar]
- 3.Altuvia, S., M. Almiron, G. Huisman, R. Kolter, and G. Storz. 1994. The dps promoter is activated by OxyR during growth by IHF and σS in stationary-phase. Mol. Microbiol. 13:265-272. [DOI] [PubMed] [Google Scholar]
- 4.Azam, T. A., A. Iwata, A. Nishimura, S. Ueda, and A. Ishihama. 1999. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J. Bacteriol. 181:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayley, S. A., C. J. Duggleby, M. J. Worsey, P. A. Williams, K. G. Hardy, and P. Broda. 1977. Two modes of loss of the TOL function from Pseudomonas putida mt-2. Mol. Gen. Genet. 154:203-204. [DOI] [PubMed] [Google Scholar]
- 6.Bhamre, S., B. B. Gadea, C. A. Koyama, S. J. White, and R. G. Fowler. 2001. An aerobic recA-, umuC-dependent pathway of spontaneous base-pair substitution mutagenesis in Escherichia coli. Mutat. Res. 473:229-247. [DOI] [PubMed]
- 7.Björkman, J., I. Nagaev, O. G. Berg, D. Hughes, and D. I. Andersson. 2000. Effects of environment on compensatory mutations to ameliorate costs of antibiotic resistance. Science 287:1479-1482. [DOI] [PubMed] [Google Scholar]
- 8.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95-113. [PubMed] [Google Scholar]
- 9.Bridges, B. A. 1997. DNA turnover and mutation in resting cells. BioEssays 19:347-352. [DOI] [PubMed] [Google Scholar]
- 10.Bridges, B. A. 1998. The role of DNA damage in stationary-phase (“adaptive”) mutation. Mutat. Res. 408:1-9. [DOI] [PubMed] [Google Scholar]
- 11.Bridges, B. A., and A. R. Timms. 1997. Mutation in Escherichia coli under starvation conditions: a new pathway leading to small deletions in strains defective in mismatch correction. EMBO J. 16:3349-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bridges, B. A., M. Sekiguchi, and T. Tajiri. 1996. Effect of mutY and mutM/fpg-1 mutations on starvation-associated mutation in Escherichia coli: implications for the role of 7,8-dihydro-8-oxoguanine. Mol. Gen. Genet. 251:352-357. [DOI] [PubMed] [Google Scholar]
- 13.Bull, H. J., M.-J. Lombardo, and S. M. Rosenberg. 2001. Stationary-phase mutation in the bacterial chromosome: recombination protein and DNA polymerase IV dependence. Proc. Natl. Acad. Sci. USA 98:8334-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capy, P., G. Gasperi, C. Biemont, and C. Bazin. 2000. Stress and transposable elements: coevolution or useful parasites? Heredity 85:101-106. [DOI] [PubMed] [Google Scholar]
- 15.Carter, P., H. Bedouelle, and G. Winter. 1985. Improved oligonucleotide site-directed mutagenesis with M13 vectors. Nucleic Acids Res. 13:4431-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkel, S. E., E. R. Zinser, and R. Kolter. 2000. Long-term survival and evolution in the stationary phase, p. 231-238. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. American Society for Microbiology, Washington, D.C.
- 18.Foster, P. L. 1997. Nonadaptive mutations occur on the F′ episome during adaptive mutation conditions in Escherichia coli. J. Bacteriol. 179:1550-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster, P. L. 1998. Adaptive mutation: has the unicorn landed? Genetics 148:1453-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster, P. L. 1999. Are adaptive mutations due to a decline in mismatch repair? The evidence is lacking. Mutat. Res. 436:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster, P. L. 1999. Mechanisms of stationary-phase mutation: a decade of adaptive mutation. Annu. Rev. Genet. 33:57-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster, P. L., and J. M. Trimarchi. 1994. Adaptive reversion of a frameshift mutation in Escherichia coli by simple base deletions in homopolymeric runs. Science 265:407-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giskov, M., L. Eberl, and S. Molin. 1994. Responses to nutrient starvation in Pseudomonas putida KT2442: two-dimensional electrophoretic analysis of starvation- and stress-induced proteins. J. Bacteriol. 176:4816-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giskov, M., L. Eberl, S. Møller, L. K. Poulsen, and S. Molin. 1994. Responses to nutrient starvation in Pseudomonas putida KT2442: analysis of general cross-protection, cell shape, and macromolecular content. J. Bacteriol. 176:7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanahan, D. 1983. Studies on the transformation of E. coli with plasmids. J. Mol. Biol. 166:577-580. [DOI] [PubMed] [Google Scholar]
- 26.Harris, R. S., G. Feng, K. J. Ross, R. Sidhu, C. Thulin, S. Longerich, S. K. Szigety, P. J. Hastings, M. E. Winkler, and S. M. Rosenberg. 1999. Mismatch repair is diminished during stationary-phase mutation. Mutat. Res. 437:51-60. [PubMed] [Google Scholar]
- 27.Hegeman, G. D. 1966. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of the enzymes by wild type. J. Bacteriol. 91:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hengge-Aronis, R. 2000. The general stress response in E. coli, p. 161-177. In G. Storz, and R. Hengge-Aronis (ed.), Bacterial stress responses. American Society for Microbiology, Washington, D.C.
- 29.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilves, H., R. Hõrak, and M. Kivisaar. 2001. Involvement of σS in starvation-induced transposition of Pseudomonas putida transposon Tn4652. J. Bacteriol. 183:5445-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kallastu, A., R. Hõrak, and M. Kivisaar. 1998. Identification and characterization of IS1411, a new insertion sequence which causes transcriptional activation of the phenol degradation genes in Pseudomonas putida. J. Bacteriol. 180:5306-5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasak, L., R. Hõrak, and M. Kivisaar. 1997. Promoter-creating mutations in Pseudomonas putida: a model system for the study of mutation in starving bacteria. Proc. Natl. Acad. Sci. USA 94:3134-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kivisaar, M., L. Kasak, and A. Nurk. 1991. Sequence of the plasmid-encoded catechol 1,2-dioxygenase-expressing gene, pheB, of phenol-degrading Pseudomonas sp. strain EST 1001. Gene 98:15-20. [DOI] [PubMed] [Google Scholar]
- 34.Kleckner, N. 1990. Regulation of transposition in bacteria. Annu. Rev. Cell Biol. 6:297-327. [DOI] [PubMed] [Google Scholar]
- 35.Kurusu, Y., T. Narita, M. Suzuki, and T. Watanabe. 2000. Genetic analysis of an incomplete mutS gene from Pseudomonas putida. J. Bacteriol. 182:5278-5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loewen, P. C., and R. Hengge-Aronis. 1994. The role of the sigma factor σS (KatF) in bacterial global regulation. Annu. Rev. Microbiol. 48:53-80. [DOI] [PubMed] [Google Scholar]
- 37.Martinez, A., and R. Kolter. 1999. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J. Bacteriol. 179:5188-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McBeth, D. I., and B. Hauer. 1996. Increased mutagenesis mediated by cloned plasmid CAM-OCT genes: potential for expanding substrate ranges of Pseudomonas spp. Appl Environ. Microbiol. 62:3538-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenzie, G. J., and S. M. Rosenberg. 2001. Adaptive mutations, mutator DNA polymerases and genetic change strategies of pathogens. Curr. Opin. Microbiol. 4:586-594. [DOI] [PubMed] [Google Scholar]
- 40.McKenzie, G. J., P. L. Lee, M.-J. Lombardo, P. J. Hastings, and S. M. Rosenberg. 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol. Cell 7:571-579. [DOI] [PubMed] [Google Scholar]
- 41.Michaels, M. L., and J. H. Miller. 1992. The 7,8-dihydro-8-oxoguanine system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine). J. Bacteriol. 174:6321-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michaels, M. L., C. Cruz, A. P. Grollman, and J. H. Miller. 1992. Evidence that MutY and MutM combine to prevent mutations by an oxidatively damaged form of guanine in DNA. Proc. Natl. Acad. Sci. USA 89:7022-7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miksch, G., and P. Dobrowolski. 1995. Growth phase-dependent induction of stationary-phase promoters of Escherichia coli in different gram-negative bacteria. J. Bacteriol. 177:5374-5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Nghiem, Y., M. Cabrera, C. G. Cupples, and J. H. Miller. 1988. The mutY gene: a mutator locus in Escherichia coli that generates G:C-T:A transversions. Proc. Natl. Acad. Sci. USA 85:2709-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nurk, A., A. Tamm, R. Hõrak, and M. Kivisaar. 1993. In-vivo-generated fusion promoters in Pseudomonas putida. Gene 127:23-29. [DOI] [PubMed] [Google Scholar]
- 47.Ojangu, E.-L., A. Tover, R. Teras, and M. Kivisaar. 2000. Effects of combination of different −10 hexamers and downstream sequences on stationary-phase-specific sigma factor σS-dependent transcription in Pseudomonas putida. J. Bacteriol. 182:6707-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliver, A., F. Baquero, and J. Blázquez. 2002. The mismatch repair system (mutS, mutL and uvrD genes) in Pseudomonas aeruginosa: molecular characterization of naturally occurring mutants. Mol. Microbiol. 43:1641-1650. [DOI] [PubMed] [Google Scholar]
- 49.Oliver, A., R. Cantón, P. Campo, F. Baquero, and J. Blázquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251-1253. [DOI] [PubMed] [Google Scholar]
- 50.Pavel, H., M. Forsman, and V. Shingler. 1994. An aromatic effector specificity mutant of the transcriptional regulator DmpR overcomes the growth constraints of Pseudomonas sp. strain CF600 on para-substituted methylphenols. J. Bacteriol. 176:7550-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powell, S. C., and R. M. Wartell. 2001. Different characteristics distinguish early versus late arising adaptive mutations in Escherichia coli FC40. Mutat. Res. 473:219-228. [DOI] [PubMed] [Google Scholar]
- 52.Prival, M. J., and T. A. Cebula. 1992. Sequence analysis of mutations arising during prolonged starvation of Salmonella typhimurium. Genetics 132:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosche, W. A., and P. L. Foster. 2000. Mutation under stress: adaptive mutation in Escherichia coli, p. 239-248. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. American Society for Microbiology, Washington, D.C.
- 54.Rosenberg, S. M., C. Thulin, and R. S. Harris. 1998. Transient and heritable mutators in adaptive evolution in the lab and in nature. Genetics 148:1559-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenberg, S. M., S. Longerich, P. Gee, and R. S. Harris. 1994. Adaptive mutation by deletions in small mononucleotide repeats. Science 265:405-407. [DOI] [PubMed] [Google Scholar]
- 56.Shapiro, J. A. 1997. Genome organization, natural genetic engineering and adaptive mutation. Trends Genet. 13:98-104. [DOI] [PubMed] [Google Scholar]
- 57.Sharma, R. C., and R. T. Schimke. 1996. Preparation of electro-competent E. coli with salt-free growth medium. BioTechniques 20:42-44. [DOI] [PubMed] [Google Scholar]
- 58.Sniegowski, P. D., P. J. Gerrish, and R. E. Lenski. 1997. Evolution of high mutation rates in experimental populations of E. coli. Nature 387:703-705. [DOI] [PubMed] [Google Scholar]
- 59.Spiers, A. J., A. Buckling, and P. B. Rainey. 2000. The causes of Pseudomonas diversity. Microbiology 146:2345-2350. [DOI] [PubMed] [Google Scholar]
- 60.Sutton, M. D., and G. C. Walker. 2001. Managing DNA polymerases: coordinating DNA replication, DNA repair, and DNA recombination. Proc. Natl. Acad. Sci. USA 98:8342-8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taddei, F., I. Matic, and M. Radman. 1995. cAMP-dependent SOS induction and mutagenesis in resting bacterial populations. Proc. Natl. Acad. Sci. USA 92:11736-11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taddei, F., J. A. Halliday, I. Matic, and M. Radman. 1997. Genetic analysis of mutagenesis in aging Escherichia coli colonies. Mol. Gen. Genet. 256:277-281. [DOI] [PubMed] [Google Scholar]
- 63.Talukder, A. A., S. Yanai, T. Nitta, A. Kato, and M. Yamada. 1996. RpoS-dependent regulation of genes expressed at late stationary-phase in Escherichia coli. FEBS Lett. 386:177-180. [DOI] [PubMed] [Google Scholar]
- 64.Timms, A. R., W. Muriel, and B. A. Bridges. 1999. UmuD,C-dependent pathway for spontaneous G:C to C:G transversions in stationary-phase Escherichia coli mutY. Mutat. Res. 434:77-80. [DOI] [PubMed] [Google Scholar]
- 65.Torkelson, J., R. S. Harris, M.-J. Lombardo, J. Nagendran, C. Thulin, and S. M. Rosenberg. 1997. Genome-wide hypermutation in a subpopulation of stationary-phase cells underlies recombination-dependent adaptive mutation. EMBO J. 16:3303-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolf, S. G., D. Frenkiel, T. Arad, S. E. Finkel, R. Kolter, and A. Minsky. 1999. DNA protection by stress-induced biocrystallization. Nature 400:83-85. [DOI] [PubMed] [Google Scholar]
- 67.Zambrano, M. M., D. A. Siegele, M. Almiron, A. Tormo, and R. Kolter. 1993. Microbial competition: Escherichia coli mutants that take over stationary-phase cultures. Science 259:1757-1760. [DOI] [PubMed] [Google Scholar]
- 68.Zhao, J., and M. E. Winkler. 2000. Reduction of GC → TA transversion mutation by overexpression of MutS in Escherichia coli K-12. J. Bacteriol. 182:5025-5028. [DOI] [PMC free article] [PubMed] [Google Scholar]