Abstract
The present study was performed to identify stress-induced putative virulence proteins of Streptococcus suis. For this, protein expression patterns of streptococci grown at 32, 37, and 42°C were compared by one- and two-dimensional gel electrophoresis. Temperature shifts from 32 and 37 to 42°C induced expression of two cell wall-associated proteins with apparent molecular masses of approximately 47 and 53 kDa. Amino-terminal sequence analysis of the two proteins indicated homologies of the 47-kDa protein with an ornithine carbamoyltransferase (OCT) from Streptococcus pyogenes and of the 53-kDa protein with the streptococcal acid glycoprotein (SAGP) from S. pyogenes, an arginine deiminase (AD) recently proposed as a putative virulence factor. Cloning and sequencing the genes encoding the putative OCT and AD of S. suis, octS and adiS, respectively, revealed that they had 81.2 (octS) and 80.2% (adiS) identity with the respective genes of S. pyogenes. Both genes belong to the AD system, also found in other bacteria. Southern hybridization analysis demonstrated the presence of the adiS gene in all 42 serotype 2 and 9 S. suis strains tested. In 9 of these 42 strains, selected randomly, we confirmed expression of the AdiS protein, homologous to SAGP, by immunoblot analysis using a specific antiserum against the SAGP of S. pyogenes. In all strains AD activity was detected. Furthermore, by immunoelectron microscopy using the anti-S. pyogenes SAGP antiserum we were able to demonstrate that the AdiS protein is expressed on the streptococcal surface in association with the capsular polysaccharides but is not coexpressed with them.
Streptococcus suis is a major cause of meningitis, septicemia, arthritis, and bronchopneumonia in young pigs and can also cause meningitis in humans (2, 10, 19). Despite increasing research on S. suis in recent years, little is known about pathogenesis and virulence factors. Studies on the pathogenicity of S. suis are complicated by the presence of multiple serotypes (of which currently 35 are known) based on the capsular polysaccharides (CPS) and by the high diversity in levels of virulence among different strains and serotypes (1, 19, 25). CPS type 2 is considered the most dominant one among the highly virulent strains, but frequently disease is caused by strains of other serotypes (1, 19, 40). This suggests that serotype-independent virulence factors exist. Putative virulence factors identified so far include the muramidase-released protein (38), the extracellular protein factor (38), hemolysins such as suilysin (20, 21), and adhesins (37). In summary, these factors have been shown to be associated with virulence depending on the serotype and geographic origin of the strains. However, absence of these factors cannot necessarily be associated with nonvirulence, and, vice versa, virulent strains that lack these factors have been isolated (19).
The major ecological niche harbored by S. suis is the epithelium of the upper respiratory tract in pigs (12, 19). Critical events in the development of disease are bacterial invasion from the mucosal surface into deeper tissues and the blood circulation, survival in blood, and invasion from blood to the central nervous system (19). Therefore, as a successful pathogen S. suis has to display rapid responses when encountering an array of adverse environmental conditions such as changes of temperature, oxygen pressure, and pH, as well as limitations in nutrients or iron. Presumably, these responses involve the expression of proteins induced when the respective signals are sensed. It is quite likely that this capacity is a serotype-independent feature of highly virulent strains. Based on this assumption we attempted to identify proteins of S. suis which are expressed or up-regulated in response to a shift in temperature, a stress signal to which the pathogen is exposed when entering deeper tissues and the bloodstream. Our findings presented here demonstrate that S. suis responds to changes in growth temperature from 32 or 37 to 42°C with a change in the expression of several proteins among which one, designated the AdiS protein, is a novel serotype-independent temperature-induced surface protein which might represent a novel type of virulence factor in this species.
MATERIALS AND METHODS
If not stated otherwise, all chemicals were purchased from Sigma (Munich, Germany).
Bacterial strains, media, and antisera.
The S. suis serotype 2 strain I9841/1 used in this study originated from the brain of a diseased piglet and expressed muramidase-released protein, the extracellular protein factor, and suilysin as previously described (1). S. suis strain 10 and its isogenic capsule-deficient mutant strain 10ΔEF have been described previously (31). In addition, 40 S. suis strains belonging to serotypes 2 and 9 were used for Southern analyses (Table 1). All strains originated from clinically infected pigs and have been described in an earlier study (1). A Streptococcus pyogenes strain (Kiel 4875) was used as positive control for expression of streptococcal acid glycoprotein (SAGP) and arginine deiminase (AD) activity. All streptococci were maintained on blood agar and cultured in Todd-Hewitt broth medium (Oxoid, Wesel, Germany) at 37°C under aerobic conditions. Chemically competent Escherichia coli cells (TOPO PCR cloning kit; Invitrogen, Groningen, The Netherlands) were grown on Luria-Bertani (LB) agar plates or in LB medium containing 100 μg of ampicillin/ml. The TOPO PCR cloning kit was used as recommended by the manufacturer (Invitrogen). Rabbit antiserum against SAGP from S. pyogenes strain Su was a kind gift from J. Yoshida (Department of Pharmacology, Kanazawa Medical University, Uchinada, Ishikawa, Japan).
TABLE 1.
Origins and serotypes of 42 S. suis strains found to carry the adiS gene by Southern analysis
| Straina | Origin | Serotype |
|---|---|---|
| P24-330 | Meningitis | 2 |
| 10∗ | Meningitis | 2 |
| D282∗ | Meningitis | 2 |
| Sc. suis II∗ | Meningitis | 2 |
| A6/3/97 | Pneumonia | 2 |
| A6169/1/96 | Meningitis | 2 |
| B2647/96 | Pneumonia | 9 |
| A2062/2/97 | Pneumonia | 9 |
| A3973/4798 | Arthritis | 9 |
| Vecht 4005 | Meningitis | 2 |
| P1-R79344 | Meningitis | 2 |
| I4627 E | Meningitis | 9 |
| A78/94 | Septicemia | 2 |
| B2441/96 | Pneumonia | 2 |
| P204 | Meningitis | 2 |
| P202 | Meningitis | 2 |
| DSM 9684 | Meningitis | 2 |
| A3863/6/97 | Meningitis | 9 |
| B631/97 | Pneumonia | 9 |
| B422/97 | Pneumonia | 9 |
| I4627 F∗ | Meningitis | 9 |
| B418/97 | Pneumonia | 9 |
| A3313/1/98 | Arthritis | 9 |
| B398/97 | Pneumonia | 9 |
| A2409/98 | Arthritis | 2 |
| P1/7 | Meningitis | 2 |
| P203 | Meningitis | 2 |
| DSM9682 | Meningitis | 2 |
| B2681/96 | Meningitis | 9 |
| A5455/93 | Carriage state | 9 |
| A1147/94 | Meningitis | 9 |
| A5683/94 | Septicemia | 9 |
| A2195/1/97 | Meningitis | 2 |
| I9841/1* | Meningitis | 2 |
| A3286/94 | Meningitis | 9 |
| A2321/1/97 | Meningitis | 2 |
| T15 | Pneumonia | 2 |
| B2663/96 | Meningitis | 9 |
| P25-348 | Meningitis | 2 |
| I4627 C | Meningitis | 2 |
| I4627 D∗ | Meningitis | 9 |
| B2628/96 | Meningitis | 9 |
∗, strain that was also tested for AD activity and expression of the AdiS protein.
Stress treatment of S. suis.
The ability of S. suis to respond to a temperature stress was assessed by growing the organism at 37°C in Todd-Hewitt broth medium to the mid-log growth phase. Then the culture was divided into two equal parts. One part was kept at the same (noninducing) condition; the other one was immediately transferred to 42°C (inducing condition). Both cultures were further incubated for 1 h. In some experiments the initial noninducing growth condition was at 32 instead of 37°C.
Fractionation of S. suis proteins.
S. suis cultures (50 ml) were harvested and centrifuged for 10 min at 10,000 × g and 4°C. The culture supernatant was concentrated 100-fold by precipitation with trichloroacetic acid (TCA; final dilution, 10% [vol/vol]). The bacterial pellets were dissolved in 30 mM Tris-HCl (pH 7.5)-3 mM MgCl2-25% sucrose-mutanolysin (125 U/ml) and incubated for 90 min at 37°C. The resulting protoplast fraction was separated by centrifugation for 10 min at 10,000 × g and 4°C and then suspended in 500 μl of deionized water. The remaining supernatant containing the murein-associated proteins (MAP) was precipitated by TCA (final dilution, 10% [vol/vol]). Precipitated MAP were collected by centrifugation for 10 min (10,000 × g, 4°C) and dissolved in 500 μl of phosphate-buffered saline (PBS). Preparation of bacterial membranes was done by a lysozyme-EDTA method as described by Kaback (22).
1-D and 2-D gel electrophoresis and immunoblot analysis.
Protein concentrations were determined by the Bio-Rad (Munich, Germany) Dc protein assay. Equal amounts of proteins from noninduced and induced cultures were loaded on the gels. For one-dimensional (1-D) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), proteins were separated as described by Laemmli (24) with 4% stacking and 10% separating gels. Two-dimensional (2-D) gel electrophoresis was done with the IPGphor system (Amersham Pharmacia, Freiburg, Germany). Isoelectric focusing was performed on Immobiline strips (18 cm long; Amersham Pharmacia) with an immobilized linear pH gradient ranging from 3 to 10. One hundred micrograms of protein in 350 μl of rehydration buffer (8 M urea, 2% [vol/vol] Triton X-100, 0.2% [wt/vol] bromophenol blue, 0.11 mM dithiothreitol, 0.5% [vol/vol] IPG buffer [Amersham]; prepared according to the manufacturer's instructions) was loaded, and rehydration was carried out for 13 h at 20°C. After a preliminary run for 2 h at 150 V, IPGphor was run for 1 h at 500 V, followed by 1 h at 1,000 V and 4 h at 8,000 V. For second-dimension SDS-PAGE, strips were equilibrated in 50 mM Tris-HCl, pH 8.8-6 M urea-30% (vol/vol) glycerol-2% (wt/vol) SDS, placed on SDS-10% PAGE gel, and run for 15 min at 20 mA followed by 4 h at 40 mA. Proteins separated by 1-D and 2-D electrophoresis were visualized by silver staining according to the method of Blum et al. (7). For immunoblotting, electrophoretically separated proteins were transferred to a nitrocellulose membrane (Protran; Schleicher and Schuell, Dassel, Germany) as described by Burnett (8) with a semidry-transfer blotting system (Bio-Rad). Nonspecific binding was blocked by incubation in TBST (30 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.005% [vol/vol] Tween 20) containing 0.5% gelatin for 30 min at room temperature. All washing steps were done with TBST. After three washings blots were incubated with anti-SAGP antiserum (diluted 1:100 in TBST) for 1 h. After three 5-min washes in TBST, blots were incubated with alkaline phosphatase-labeled goat anti-rabbit conjugate (DIANOVA, Hamburg, Germany). The blots were developed with nitroblue tetrazolium (final concentration, 100 μg/ml) and 5-bromo-4-chloro-3-indolylphosphate (final concentration, 50 μg/ml) in 100 mM Tris-HCl, pH 9.5-50 mM NaCl-5 mM MgCl2.
Amino-terminal protein sequencing.
After separation by 1-D SDS-PAGE, proteins were blotted on a Fluorobind transfer polyvinylidene difluoride-like membrane (pore size, 0.2 μm; Serva, Heidelberg, Germany). Protein bands were visualized by first staining the membranes for 15 min in 0.25% (wt/vol) Coomassie blue-45% (vol/vol) methanol-10% (vol/vol) glacial acetic acid and subsequently destaining the background with 30% (vol/vol) methanol-10% (vol/vol) glacial acetic acid. Then, the differentially expressed protein bands were cut out, and the amino-terminal protein sequencing was performed with a Procise pulsed liquid-phase sequencer (model 494A; Applied Biosystems, Goettingen, Germany) and a model 190 on-line PTH amino acid analyzer (Applied Biosystems) according to the manufacturer's instructions.
DNA preparation, PCR, and Southern analysis.
For PCR and Southern analysis S. suis chromosomal DNA from strain I9841/1 was prepared according to standard procedures (3). For PCR, 50 ng of chromosomal DNA of S. suis was subjected to 40 cycles of denaturation for 3 min at 91°C, annealing for 1 min at 57°C, and extension for 2 min at 72°C. The oligonucleotide primer sequences for cloning octS fragments were taken from the published sequences of S. pyogenes (accession no. AE006587; website, http://dna1.chem.ou.edu/strep/html). The sense primer represented nucleotide sequence positions 1876 to 1895 of the oct gene (5′-AAAAGATTTTACACGCGCTGA-3′), and the antisense primer represented nucleotide sequence positions 2061 to 2080 of the oct gene of S. pyogenes (5′-CATTGGCACCGAGGTATTCT-3′). PCR products were fractionated on a 1% agarose gel at 65 V for 1 h. After being stained with ethidium bromide, the expected DNA band of 204 bp was excised and eluted with the Geneclean II Kit 101 (Q-Biogene, Hamburg, Germany). The resulting 204-bp PCR product was cloned with the TOPO cloning kit (Invitrogen) and sequenced (Seqlab, Göttingen, Germany) to give the template sequence for further cloning of the adjacent up- and downstream regions (see below). For Southern analyses, S. suis chromosomal DNA was prepared and digested with HindIII overnight at 37°C. The DNA fragments were then separated by 1% agarose gel electrophoresis for 4 h at 120 V, transferred overnight by Southern blotting to a nylon membrane (Q-Biogene) (34), and immobilized by baking the membrane at 80°C for 2 h. The oligonucleotide probe used spanned 1,077 bp; it extended from 150 bp from the 3′ end of the sagp gene to bp 300 of the oct gene (see Fig. 2). The probe was labeled with [α-32P]dCTP (3,000 Ci/mmol) (NEN, Cologne, Germany) by using a nick translation kit (GIBCO-Invitrogen, Karlsruhe, Germany). The blots were prehybridized, hybridized, washed according to the method described by Church and Gilbert (11), and exposed to Kodak Bio Max films with intensifying screens at −80°C.
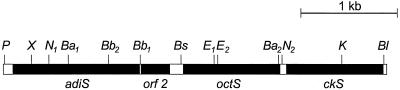
FIG. 2.
Model of the genetic organization of the octS and adiS genes of S. suis strain I9841/1, including the adjacent regions. Restriction sites: P, PsiI; X, XbaI; Ba, BamHI; Bb, BbsI; N, NciI; Bl, BlpI; Bs, BsmAI; E, EcoRI; K, KpnI.
Cloning and sequencing of the putative ADS operon.
For cloning purposes, oligonucleotide primers were designed from the internal sequence of the 204-bp fragment (see above). Cloning the upstream and downstream regions of this fragment was done by primer walking PCR (18). A random primer only was used for downstream cloning; upstream cloning was started with the same random primer and was completed with specific primers, which were designed according to the genomic sequence of the 5′ end of the SAGP gene of S. pyogenes (accession no. AE006587; website, http://dna1.chem.ou.edu/strep/html).
Assay of AD activity.
The assay of AD activity was done by measuring the rate of conversion of l-arginine to citrulline by the method of Oginsky (27) and Degnan et al. (16) using whole-cell lysates and culture supernatants of the streptococci. Results were expressed as nanomoles of citrulline produced per hour per milligram of streptococcal protein. S. pyogenes strain Kiel 4875 grown under conditions similar to those for S. suis served as a positive control.
IEM.
Immunoelectron microscopy (IEM) was done essentially as described previously (36) with some modifications. Streptococci were fixed with 1% formaldehyde for 1 h on ice. After being washed with PBS containing 10 mM glycine to quench free aldehyde groups, samples were dehydrated with a graded series of ethanol (10, 30, 50, 70, 90, and 100%) on ice and infiltrated with the acrylic resin LRWhite (1 part ethanol/1 part resin overnight, 1 part ethanol/2 parts resin for 8 h, and pure resin for 1 day with several changes). Polymerization of the resin was carried out at 60°C for 24 h. Ultrathin sections were cut with a diamond knife, and the sections were collected with Formvar-coated grids. Grids were placed onto drops of the 1:5-diluted stock solution of the anti-SAGP antiserum and incubated at 4°C overnight. A normal rabbit serum was used as a negative control. After the grids were washed with PBS, the bound antibodies were visualized by floating the grids on drops of a 1:100 dilution of the stock solution of 10 nm protein A-gold complexes (Biocell, Cardiff, United Kingdom). After several washing steps with PBS containing 1% Tween 20 grids were rinsed in water and air dried. Counterstaining was performed with aqueous uranyl acetate for 5 min. Samples were examined in a Zeiss transmission electron microscope (EM910) at an acceleration voltage of 80 kV and at calibrated magnifications.
Nucleotide sequence accession number.
The DNA sequence of the AD system (ADS) operon has been deposited in the GenBank nucleotide sequence database under accession no. AF546864.
RESULTS
Stress-responsive protein expression pattern and identification of two novel stress-induced proteins.
S. suis strain I9841/1, a serotype 2 strain isolated from the central nervous system of a diseased pig (1), was cultured under noninducing conditions (at 32 or 37°C) to mid-log growth phase, and the culture was then divided into two equal parts. One part was further incubated at noninducing conditions (control); the other was shifted to a growth temperature of 42°C (inducing condition). Growth was monitored by measuring optical densities (OD) at 600 nm. No difference in the growth kinetics of the different cultures was observed under these conditions (data not shown). After further growth for 1 h to late log phase (OD of approximately 0.8), cultures were fractionated, resulting in culture supernatant proteins (CSP), a protoplast fraction representing the cytosolic and plasma membrane-bound proteins (protoplast proteins [PP]), and a protoplast supernatant fraction representing the MAP. All fractions (PP, MAP, and CSP) of induced and noninduced control cultures were separated by SDS-PAGE using equal amounts of proteins from noninduced and induced cultures. The protein expression patterns were visualized by silver staining the gels. In the fractions of the induced (42°C) culture three up-regulated proteins were detected, one of approximately 42 kDa in the PP fraction and two of approximately 47 and 53 kDa in the MAP fraction (Fig. 1). The patterns of the CSP fraction in induced and noninduced cultures were similar (Fig. 1). Since many streptococcal virulence factors are surface proteins, the induced 47- and 53-kDa proteins were selected for further characterization because they were the most significant in the MAP fraction and, thus, were most likely associated with the streptococcal surface.
FIG. 1.
Protein expression pattern of S. suis in response to temperature stress as analyzed by SDS-PAGE. S. suis strain I9481/1 was grown at noninducing conditions (aerobically at 32 or 37C°, as indicated) until early logarithmic phase, and then one-half of the culture was shifted to inducing conditions (42°C). Noninduced and induced cultures were fractionated into a PP fraction representing the cytosolic and membrane-bound proteins, the MAP, and the CSP. Fractions were separated by SDS-PAGE, with equal amounts of proteins from noninduced and induced cultures loaded, and protein bands were visualized by silver staining. Three up-regulated proteins are indicated (arrows), one of approximately 42 kDa in the PP fraction and two of approximately 47 and 53 kDa in the MAP fraction. No difference in protein expression patterns of the CSP fractions of standard and stressed cultures could be detected. The molecular sizes are given on the left.
After separation by SDS-PAGE both proteins were transferred to a polyvinylidene difluoride-like membrane and subjected to amino-terminal sequencing. The obtained sequences were TNVFKGRSFLAEKDFTRAELEYLID for the 47-kDa protein and PIDVFSEIGKLKKVMLSEPGKE for the 53-kDa protein. Comparison of the sequence with sequences in the Swissprot database (4) revealed a strong homology (92%) of the 47-kDa protein to the ornithine carbamoyltransferase (OCT) of S. pyogenes (accession no. AE006587) (15) and a strong homology (77%) of the 53-kDa protein to SAGP, representing an AD of S. pyogenes (accession no. AE006587) (15). Both proteins belong to the ADS; ADSs are found in other bacteria including streptococci and are genetically organized as operons (13, 14).
Molecular characterization of the stress-induced 47- and 53-kDa proteins.
Based on the published nucleotide sequences of the S. pyogenes oct gene (accession no. AE006587; website http://dna1.chem.ou.edu/strep/html) we designed the respective primer sequences and amplified a DNA fragment homologous to oct from S. suis by PCR using chromosomal DNA from S. suis strain I9841/1 as a template. Subsequently, upstream and downstream regions of this fragment were cloned by primer walking using an in vitro cloning system and sequenced. The assembly of the sequences of the obtained fragments revealed a total sequence of 4,020 bp with four open reading frames (ORF) with high homologies to those of the corresponding genes in S. pyogenes. The first ORF showed 80.2% homology to that of the sagp gene, the second ORF (orf 2) showed 59.8% homology to that of a gene encoding a hypothetical cytosolic protein, the third ORF showed 81.2% homology to that of the oct gene, and the fourth ORF showed 70.1% homology to that of the ck gene, encoding a carbamate kinase. The nucleotide sequence homologies corresponded well to the homologies of the amino-terminal sequences obtained for the 53- and 47-kDa proteins (see above). During the preparation of this paper most of the genome sequence of S. suis became available (http://www.sanger.ac.uk/Projects/S_suis/). A comparison of the published sequence with the 4,020-bp total sequence from strain I9841/1 revealed an overall homology of 99.8%. As indicated in the model shown in Fig. 2, the genes identified in S. suis were designated adiS, octS, and ckS (for adi gene, oct gene, and ck gene of S. suis, respectively) and orf 2 (Fig. 2). The adiS gene encodes a polypeptide of 432 amino acids with a predicted molecular mass of 47,600 Da; the orf 2 gene encodes a polypeptide of 101 amino acids with a predicted molecular mass of 1,110 Da, the octS gene encodes a polypeptide of 337 amino acids with a predicted molecular mass of 37,140 Da, and the ckS gene encodes a polypeptide of similar size, i.e., 337 amino acids with a predicted molecular mass of 37,140 Da. The deduced amino acid sequences of the AdiS and OctS proteins lacked typical signal sequence cleavage sites of gram-positive bacteria (39) and contained neither the hexameric LPXTGX motif typical for anchoring proteins of gram-positive bacteria (17) nor uncharged repeats of 20 amino acids each that mediate the noncovalent anchoring of choline-binding proteins to the cell surface (42). Since the corresponding genes in S. pyogenes are located on a putative operon (15), a similar organization can be hypothesized for S. suis.
Further characterization of the SAGP homologue of S. suis (AdiS protein).
A very interesting feature of the AdiS and OctS proteins was their up-regulation in response to a shift in growth temperature to 42°C. This has not been reported yet for the ADSs in other bacteria but is very important with respect to a possible association with virulence since many virulence genes are regulated by host environmental signals such as a change in temperature. Another important feature is the location of the proteins. Based on our finding that both proteins were up-regulated mainly in the MAP fraction, we assumed that they are associated with the streptococcal cell wall. To study this in more detail, we selected the AdiS protein because it has been suggested that the homologue SAGP of S. pyogenes is a novel virulence factor (15). We first confirmed that the band of the amino-terminally sequenced temperature-induced 53-kDa protein corresponded to the putative AdiS protein. The MAP fractions of standard and stressed S. suis cultures were compared by 2-D analysis and subsequent immunoblot analysis using a specific antiserum against the SAGP from S. pyogenes (kindly provided by J. Yoshida). Results of 2-D analysis showed that a single protein spot of approximately 53 kDa was clearly up-regulated under temperature stress. This spot reacted specifically with the anti-S. pyogenes SAGP antiserum (Fig. 3), confirming that the 53-kDa protein is a SAGP homologue protein in S. suis. Furthermore, results confirmed that the AdiS protein was induced by a temperature shift to 42°C (Fig. 3). Elucidation of the presence of the encoding adiS gene by Southern hybridization analysis of 42 S. suis strains of serotypes 2 and 9 (the most common serotypes in Germany and Europe [1, 40]) revealed that the gene was present in all strains tested (Table 1). By immunoblot analysis using an anti-SAGP antiserum we could show that all 9 strains randomly selected from the 42 strains expressed a protein homologous to SAGP since a protein similar in size (approximately 53 kDa) to the SAGP of the S. pyogenes control strain was recognized by the antiserum (Fig. 4). We also tested the AD activity of these S. suis strains by determining the production of citrulline. Results showed that all strains expressed cell-associated and extracellular AD activity (Table 2). Activities were generally relatively low compared to that for S. pyogenes strain Kiel 4875. Most interestingly, cell-associated AD activity was up-regulated in response to a temperature shift to 42°C only in the serotype 2 strains, not in the two serotype 9 strains and the S. pyogenes control strain (Table 2). On the other hand, AD activity in the CSP of the serotype 9 strains was significantly higher than that in the CSP of the serotype 2 strains (Table 2). Since the adiS gene and the AdiS protein appeared to be widely distributed and independent of the serotype, we analyzed the exact localization of the AdiS protein. We prepared protoplast membranes (cellular membrane protein [CMP] fraction) as well as PP, MAP, and CSP fractions from induced and noninduced control cultures of S. suis strain I9841/1 and analyzed them by immunoblotting using the anti-S. pyogenes SAGP antiserum. Results clearly showed that the AdiS protein was present not only in the PP and MAP fractions but also in the membrane fraction and the culture supernatant (Fig. 5). Up-regulation of the protein most clearly occurred in the MAP and CMP fractions. The AdiS protein showed similar molecular sizes in the different fractions, indicating that there is no (visible) maturation (e.g., cleavage of a signal sequence) during translocation. Furthermore, IEM analysis was done using the anti-S. pyogenes SAGP antiserum and protein A coupled to colloidal gold. Significant labeling (gold particles) was seen only in cultures grown at 42°C, not in noninduced cultures. A representative picture is shown in Fig. 6A and B. In control experiments using normal rabbit serum almost no label was found, indicating that there was no interference by possible immunoglobulin G-binding proteins, which have been found in S. suis (5). Since gold particles were indicative of the presence of the AdiS protein, these results clearly demonstrate that the AdiS protein is located on the bacterial surface and confirm its up-regulation by a shift of the growth temperature to 42°C. Gold particles were also detected in the streptococcal cytosol and plasma membrane. This corresponded well to our results from SDS-PAGE analysis of different protein fractions, in which we detected the protein in all fractions (PP, MAP, and CMP) and observed up-regulation in the MAP and CMP fractions (Fig. 5). Another interesting finding of IEM analysis was that surface-located gold particles indicative of the presence of the AdiS protein were often seen at some distance from the cell wall. This raised the question of whether the protein might be linked to the bacterial CPS. Therefore, similar experiments were done using capsule-deficient S. suis strain 10ΔEF and its isogenic wild-type strain, 10. Both strains expressed the protein in response to stress stimuli, as shown by SDS-PAGE analysis (Fig. 5). Results of IEM demonstrated that both strains expressed the protein on the surface only when cultured under temperature stress conditions. This indicated that the expression of the AdiS protein was independent of CPS expression (Fig. 6C to F).
FIG. 3.
Identification of the AdiS protein homologous to SAGP in S. suis by 2-D gel electrophoresis (A) and subsequent immunoblot analysis (B). S. suis strain I9481/1 was grown as described for Fig. 1. The MAP fractions of noninduced (37°C) and temperature-induced (42°C) cultures were separated by 2-D gel electrophoresis and then transferred to a membrane by Western blotting. Blots were probed with a specific antiserum against the SAGP protein from S. pyogenes. The silver-stained 2-D gels show several differentially expressed proteins in the temperature-induced culture (A). Arrow (A), temperature-induced 53-kDa protein. This protein is specifically detected by the anti-SAGP antiserum (B). Up-regulation is confirmed by a significantly stronger reaction in the 42°C culture (B, right). The pH scales are given at the top, and the molecular sizes are given at the right.
FIG. 4.
Detection of the AdiS protein in different S. suis strains by immunoblot analysis. Whole-cell lysates of S. suis strains I9841/1 (lane 2), 10 (lane 3), 10ΔEF (lane 4), Sc. suis II (lane 5), D282 (lane 6), I4627 D (lane 7), and I4627 F (lane 8) and S. pyogenes strain Kiel 4875 (lane 1; positive control) were prepared from temperature-induced cultures as described in Materials and Methods and separated by SDS-PAGE. Proteins were transferred to membranes by Western blotting and then probed with a specific antiserum against the SAGP protein from S. pyogenes. In all strains a specific reaction was seen with a single band at approximately 53 kDa. Lane M, molecular size markers.
TABLE 2.
AD activities of different S. suis serotype 2 and 9 strains grown at noninducing (37°C) or inducing (42°C) conditions
| Straina | Serotype | AD activity (nmol of citrulline/h/mg of protein) in:
|
||
|---|---|---|---|---|
| Whole-cell lysates at:
|
CSP at 37°C | |||
| 37°C | 42°C | |||
| I9841/1 | 2 | 827 | 1,374 | 660 |
| 10 | 2 | 528 | 873 | 801 |
| 10ΔEFb | 2 | 292 | 501 | 892 |
| D282 | 2 | 463 | 691 | 638 |
| Sc. suis II | 2 | 676 | 880 | 770 |
| I4627 D | 9 | 793 | 770 | 2,246 |
| I4627 F | 9 | 1,226 | 1,248 | 1,852 |
| Kiel 4875 | 6,806 | 6,906 | 3,745 | |
All strains are S. suis strains except Kiel 4875, which is an S. pyogenes strain.
Nonencapsulated isogenic mutant version of strain 10.
FIG. 5.
Detection of the AdiS protein in the membrane fraction of S. suis by immunoblot analysis. S. suis strain I9481/1 was grown as described in Fig. 1 under noninducing conditions (37°C) until early logarithmic phase, and then one-half of the culture was shifted to a temperature stress at 42°C. Noninduced and temperature-induced cultures were fractionated and separated by SDS-PAGE as described for Fig. 1. In addition, CMPs were included in the analysis. After separation by SDS-PAGE proteins were transferred to membranes by Western blotting and then probed with a specific antiserum against the SAGP protein from S. pyogenes. A specific reaction was seen with a single band at approximately 53 kDa, which was significantly stronger in the MAP and CMP fractions of the 42°C culture than in those of the 37°C culture. This difference was almost not seen in the PP fraction and was only moderate in the CSP fraction. Molecular sizes are given on the left.
FIG. 6.
Localization of the AdiS protein on the streptococcal surface by IEM. S. suis strains I9481/1 (A and B) and 10 (C and D) and its isogenic capsule-deficient mutant, 10ΔEF (E and F), were grown under noninducing conditions (at 32C°) until early logarithmic growth, and then one-half of each of the cultures was shifted to a higher temperature (42°C). Noninduced and temperature-induced cultures were then fixed and subjected to IEM by using a specific antiserum against SAGP from S. pyogenes and gold particles (10 nm) coupled to protein A. Several gold particles, indicative of the presence of the AdiS protein, homologues to SAGP, are clearly visible at the surfaces of all streptococcal cells grown at 42°C (B, D, and F) but are absent in those grown at 32°C (A, C, and E), irrespective of the strain used, including the nonencapsulated mutant. Scale bars, 0.25 μm.
DISCUSSION
S. suis is an important swine pathogen which colonizes the surface of the upper respiratory tract and which can cause severe infections such as meningitis and septicemia. Thus, virulent S. suis strains have to invade deeper tissues and reach the blood circulation. Consequently, they have to adapt to different environments during infection. In the present study we analyzed protein expression patterns of S. suis in response to temperature shifts from 32 and 37 to 42°C in order to mimic the environmental temperature encountered by the bacteria during infection, i.e., colonization of the upper respiratory tract, invasion into deeper tissues, and fever. Two up-regulated proteins with apparent molecular sizes of 47 and 53 kDa were further characterized. We selected these proteins since both appeared to be associated with the cell wall (they were detected in the MAP fraction) and, as such, were putative surface proteins. Furthermore, it seemed that neither protein represented one of the common heat shock proteins, which have been found in many bacteria including S. suis (5), because they did not react with antisera to heat shock proteins in immunoblot analysis (data not shown). Characterization of both proteins revealed a high amino-terminal sequence homology with the OCT and SAGP from S. pyogenes. The SAGP represents an AD and, together with the OCT and the carbamate kinase, is a member of the ADS, found in many bacteria including streptococci and enterococci (14). After cloning and sequencing the S. suis genes homologous to sagp and oct (designated adiS and octS, respectively) and adjacent regions we also identified two ORFs corresponding to the ck gene and a gene of unknown function in S. pyogenes. Organization of the four genes (Fig. 2) was similar to that described for other bacteria and suggests that they are localized on an operon-like structure.
The functions proposed for the ADS are (i) provision of ATP by metabolizing l-arginine, (ii) biosynthesis of citrulline or pyrimidines by providing carbamoyl phosphatase, and (iii) protection against acidic damage by producing NH3 (9, 13, 14). The SAGP of S. pyogenes was described as an acidic glycoprotein purified from a cell extract of S. pyogenes strain Su which could inhibit the growth of several tumor cell lines (reviewed by Yoshida et al. [41]). The respective gene was cloned and expressed in E. coli by Kanaoka and coworkers (23). The SAGP was found in other S. pyogenes strains and its AD activity was related to an inhibitory effect on proliferation of human T lymphocytes (16). In addition, Degnan et al. demonstrated by mutational analysis that the SAGP of S. pyogenes might enable S. pyogenes to better survive acidic conditions such as those present in phagolysosomes (15).
Two striking features of the AdiS protein of S. suis which have not been described for the SAGP (and the ADS) are its association with the cell wall and its up-regulation by temperature stress. The SAGP of S. pyogenes has been characterized as a cytosolic enzyme based on its possible function(s) and the fact that it was purified from a cell extract (16, 41). In addition, the functions proposed for the SAGP, i.e., its AD activity, favor a cytosolic location. In this study we could demonstrate expression of the AdiS protein, homologous to SAGP, in the MAP fraction and the culture supernatant of S. suis. Furthermore, AD activity was detected also in the culture supernatant, and IEM clearly showed that the AdiS protein is present on the surface. Surprisingly, the amino acid sequence of the AdiS protein predicted from the nucleotide sequence data did not reveal typical signal sequences or motifs characteristic of streptococcal surface or secreted proteins. This raises the question of how this protein might be translocated. In other streptococcal species a number of surface-bound proteins with enzymatic activities which seem to have important roles in pathogenesis have been identified. Examples include the glyceraldehyde-3-phosphatate dehydrogenase of S. pyogenes (29) and the plasmin(ogen) binding alpha-enolases of S. pyogenes (30) and Streptococcus pneumoniae (6). Interestingly, some of these proteins, e.g., the alpha-enolases, also lack typical structural features found in surface proteins of gram-positive bacteria. Thus, it might be speculated that these proteins, including the AdiS protein, require alternative translocation routes.
The second very interesting feature of the AdiS (and OctS) protein of S. suis is its up-regulation by temperature stress. In recent years a number of other stress-regulated genes have been identified in streptococci including S. suis, and some of these might be associated with virulence. Examples include genes involved in the transcriptional control of the antiphagocytic M proteins in S. pyogenes by environmental signals such as CO2, osmolarity, temperature, gas exchange, and iron limitation (26, 28); several genes in S. suis environmentally regulated (induced by iron restriction) by a promoter selection system (32), and genes involved in the induction of general and stress-specific proteins in Streptococcus mutans (35). In a recent comprehensive approach by Smoot et al. (33) a combination of microarray and TaqMan analyses has been used with S. pyogenes to show that temperature significantly alters the streptococcal proteome and that several putative virulence factors are differentially regulated by signals that alter the global gene expression. In S. suis we observed that expression of the AdiS protein was differentially influenced by stress signals other than temperature. A reduction in oxygen pressure increased expression, whereas a decrease in pH did not affect its expression (data not shown). The latter is notable because, as mentioned above, in other bacteria the ADS seems to play a role in survival under acidic conditions (see above). Therefore, it remains to be elucidated whether or not the AdiS protein plays a similar role in S. suis.
This study also showed that up-regulation of the AdiS protein in response to a temperature stress resulted in a higher AD activity. Interestingly, this was only found in the serotype 2 strains tested, whereas AD activities of the serotype 9 strains in noninduced and induced cultures were almost the same. Correspondingly, in earlier studies we and others have found significant differences between the two serotypes, indicating that the serotype 2 and 9 strains express different virulence-associated properties (1, 40). Therefore, it will be very interesting in the future to analyze whether or not there is a correlation between regulation of cell-associated AD activity, serotype, and virulence.
In conclusion, based on the features of the AdiS protein of S. suis described in this study (i.e., presence on the bacterial surface, up-regulation by temperature stress, and wide distribution among S. suis strains independent of the CPS serotype) and the virulence-associated features of the highly homologous S. pyogenes SAGP, it is very plausible to speculate that this protein might represent a novel stress-regulated virulence factor in S. suis, even though the importance of AdiS (and OctS) will have to be elucidated in future studies.
Acknowledgments
We gratefully acknowledge J. Yoshida for generously supplying us with an antiserum against SAGP from S. pyogenes. We thank Sandra Sommer for her help with the AD activity experiments as well as Sabine Jeckstadt and Ellruth Müller for excellent technical assistance.
This study was supported by the Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany (Va 81/5-1), and the Impfstoffwerk Dessau-Tornau (IDT), Rosslau, Germany.
N. Winterhoff, R. Goethe, and P. Gruening contributed equally to this work.
REFERENCES
- 1.Allgaier, A., R. Goethe, H. J. Wisselink, H. E. Smith, and P. Valentin-Weigand. 2001. Relatedness of Streptococcus suis isolates of various serotypes and clinical backgrounds as evaluated by macrorestriction analysis and expression of potential virulence traits. J. Clin. Microbiol. 39:445-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arends, J. P., and H. C. Zanen. 1988. Meningitis caused by Streptococcus suis in humans. Rev. Infect. Dis. 10:131-137. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, F. A. Smith, and K. Struhl (ed.). 1987. Current protocols in molecular biology, vol. 1. Wiley & Sons, Hoboken, N.J.
- 4.Bairoch, A., and R. Apweiler. 1996. The SWISS-PROT protein sequence data bank and its new supplement tREMBL. Nucleic Acids Res. 24:21-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benkirane, R., M. G. Gottschalk, and J. D. Dubreuil. 1997. Identification of Streptococcus suis 60-kDa heat-shock protein using Western blotting. FEMS Microbiol. Lett. 153:379-385. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2001. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 40:1-16. [DOI] [PubMed] [Google Scholar]
- 7.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 8.Burnett, W. N. 1981. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112:195-203. [DOI] [PubMed] [Google Scholar]
- 9.Casiano-Colon, A., and R. E. Marquis. 1988. Role of arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl. Environ. Microbiol. 54:1318-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chanter, N., P. W. Jones, and T. J. L. Alexander. 1993. Meningitis in pigs caused by Streptococcus suis—a speculative review. Vet. Microbiol. 36:39-55. [DOI] [PubMed] [Google Scholar]
- 11.Church, G. M., and W. Gilbert. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clifton-Hadley, F. A., and T. J. L. Alexander. 1980. The carrier site and carrier rate of Streptococcus suis type II in pigs. Vet. Rec. 107:40-41. [DOI] [PubMed] [Google Scholar]
- 13.Cunin, R., N. Glansdorff, A. Pierard, and V. Stalon. 1986. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50:314-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curran, T. M., Y. Ma, G. C. Rutherford, and R. E. Marquis. 1998. Turning on and off the arginine deiminase system in oral streptococci. Can. J. Microbiol. 44:1078-1085. [DOI] [PubMed] [Google Scholar]
- 15.Degnan, B. A., M. C. Fontaine, A. H. Doebereiner, J. J. Lee, P. Matsroeni, G. Dougan, J. A. Goodacre, and M. A. Kehoe. 2000. Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect. Immun. 68:2441-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degnan, B. A., J. M. Palmer, T. Robson, C. E. D. Jones, M. Fischer, M. Glanville, G. D. Mellor, A. G. Diamond, M. A. Kehoe, and J. A. Goodacre. 1998. Inhibition of human peripheral blood mononuclear cell proliferation by Streptococcus pyogenes cell extract is associated with arginine deiminase activity. Infect. Immun. 66:3050-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischetti, V. A., V. Pancholi, and O. Schneewind. 1990. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol. Microbiol. 4:1603-1605. [DOI] [PubMed] [Google Scholar]
- 18.Froussard, P. 1992. A random-PCR method (rPCR) to construct whole cDNA library from low amounts of RNA. Nucleic Acids Res. 20:2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottschalk, M., and M. Segura. 2000. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet. Microbiol. 76:259-272. [DOI] [PubMed] [Google Scholar]
- 20.Gottschalk, M., S. Lacouture, and J. D. Dubreuil. 1995. Characterization of Streptococcus suis capsular type 2 haemolysin. Microbiology 141:189-195. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs, A. A. C., P. L. W. Loeffen, A. J. G. van den Berg, and P. K. Storm. 1994. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect. Immun. 62:1742-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaback, H. R. 1971. Bacterial membranes. Methods Enzymol. 22:99-120. [Google Scholar]
- 23.Kanaoka, M., C. Kawanaka, T. Negoro, Y. Fukita, K. Taya, and H. Agui. 1987. Cloning and expression of the antitumor glycoprotein gene of Streptococcus pyogenes Su in Escherichia coli. Agric. Biol. Chem. 51:2641-2648. [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 25.MacInnes, J. I., and R. Desrosiers. 1999. Agents of the “suis-ide diseases” of swine: Actinobacillus suis, Haemophilus suis, and Streptococcus suis. Can. J. Vet. Res. 63:83-89. [PMC free article] [PubMed] [Google Scholar]
- 26.McIver, K. S., A. S. Heath, and J. R. Scott. 1995. Regulation of virulence by environmental signals in group A streptococci: influence of osmolarity, temperature, gas exchange, and iron limitation on emm transcription. Infect. Immun. 63:4540-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oginsky, E. L. 1957. Isolation and determination of arginine and citrulline. Methods Enzymol. 3:639-643. [Google Scholar]
- 28.Okada, N., R. T. Geist, and M. G. Caparon. 1993. Positive transcriptional control of mry regulates virulence in the group A Streptococcus. Mol. Microbiol. 7:893-903. [DOI] [PubMed] [Google Scholar]
- 29.Pancholi, V., and V. A. Fischetti. 1992. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J. Exp. Med. 176:415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pancholi, V., and V. A. Fischetti. 1998. Alpha-enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J. Biol. Chem. 273:14503-14515. [DOI] [PubMed] [Google Scholar]
- 31.Smith, H. E., M. Damman, J. van der Velde, V. Veenburgen, F. Wagenaar, N. Stockhofe-Zurwieden, and M. A. Smits. 1999. Identification and characterization of the complete CPS locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 67:1750-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, H. E., H. Buijs, R. de Vries, H. E. Wisselink, N. Stockhofe-Zurwieden, and M. A. Smits. 2001. Environmentally regulated genes of Streptococcus suis: identification by the use of iron-restricted conditions in vitro and by experimental infection of pigs. Microbiology 147:271-280. [DOI] [PubMed] [Google Scholar]
- 33.Smoot, L. M., J. C. Smoot, M. R. Graham, G. A. Somerville, D. E. Sturdevant, C. A. L. Migliaccio, G. L. Sylva, and J. M. Musser. 2001. Global differential gene expression in response to growth temperature alteration in group A Streptococcus. Proc. Natl. Acad. Sci. USA 98:10416-10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 35.Svensäter, G., B. Sjögreen, and I. R. Hamilton. 2000. Multiple stress responses in Streptocccus mutans and the induction of general and stress-specific proteins. Microbiology 146:107-117. [DOI] [PubMed] [Google Scholar]
- 36.Talay, S. R., P. Valentin-Weigand, K. N. Timmis, and G. S. Chhatwal. 1994. Domain structure and conserved epitopes of Sfb protein, the fibronectin-binding adhesin of Streptococcus pyogenes. Mol. Microbiol. 13:531-539. [DOI] [PubMed] [Google Scholar]
- 37.Tikkanen, K., S. Haataja, and J. Finne. 1996. The galactosyl-(1-4)-galactose-binding adhesin of Streptococcus suis: occurrence in strains of different hemagglutination activities and induction of opsonic antibodies. Infect. Immun. 64:3659-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vecht, U., H. J. Wisselink, M. L. Jellema, and H. E. Smith. 1991. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect. Immun. 59:3156-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Heijne, G. 1986. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 14:4683-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wisselink, H. J., H. E. Smith, N. Stockhofe-Zurwieden, K. Peperkamp, and U. Vecht. 2000. Distribution of capsular types and production of muramidase-released protein (MRP) and extracellular factor (EF) of Streptococcus suis isolated from diseased pigs in seven European countries. Vet. Microbiol. 74:237-248. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida, J., S. Takamura, and M. Nishio. 1998. Characterization of a streptococcal antitumor glycoprotein. Life Sci. 62:1043-1053. [DOI] [PubMed] [Google Scholar]
- 42.Yother, J., and J. M. White. 1994. Novel surface attachment mechanisms of the Streptococcus pneumoniae protein PspA. J. Bacteriol. 176:2976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]