Abstract
Transketolase (TK) catalyzes reactions in the Calvin cycle and the oxidative pentose phosphate pathway (OPPP) and produces erythrose-4-phosphate, which is a precursor for the shikimate pathway leading to phenylpropanoid metabolism. To investigate the consequences of decreased TK expression for primary and secondary metabolism, we transformed tobacco with a construct containing an antisense TK sequence. The results were as follows: (1) a 20 to 40% reduction of TK activity inhibited ribulose-1,5-bisphosphate regeneration and photosynthesis. The inhibition of photosynthesis became greater as irradiance increased across the range experienced in growth conditions (170 to 700 μmol m−2 sec−1). TK almost completely limited the maximum rate of photosynthesis in saturating light and saturating CO2. (2) Decreased expression of TK led to a preferential decrease of sugars, whereas starch remained high until photosynthesis was strongly inhibited. One of the substrates of TK (fructose-6-phosphate) is the starting point for starch synthesis, and one of the products (erythrose-4-phosphate) inhibits phosphoglucose isomerase, which catalyzes the first reaction leading to starch. (3) A 20 to 50% decrease of TK activity led to decreased levels of aromatic amino acids and decreased levels of the intermediates (caffeic acid and hydroxycinnamic acids) and products (chlorogenic acid, tocopherol, and lignin) of phenylpropanoid metabolism. (4) There was local loss of chlorophyll and carotene on the midrib when TK activity was inhibited by >50%, spreading onto minor veins and lamina in severely affected transformants. (5) OPPP activity was not strongly inhibited by decreased TK activity. These results identify TK activity as an important determinant of photosynthetic and phenylpropanoid metabolism and show that the provision of precursors by primary metabolism colimits flux into the shikimate pathway and phenylpropanoid metabolism.
INTRODUCTION
The interaction between primary and secondary pathways is an important but poorly understood aspect of plant metabolism. Phenylpropanoids represent an important class of secondary metabolites, with roles in plant structure, defense, and signaling (Dixon and Paiva, 1995). They are derived from aromatic amino acids, which are synthesized via the shikimate pathway in the plastid. Up to 20% of the total carbon in a plant passes through this pathway (Jensen, 1985). Even though there have been numerous studies of transformants with altered expression of genes that encode enzymes in the Calvin cycle, glycolysis, the tricarboxylic acid cycle, and the oxidative pentose phosphate pathway (OPPP) (Stitt and Sonnewald, 1995; Stitt, 1999), the consequences for phenylpropanoid metabolism or other secondary pathways have not been explored. The presence of redundant enzymes and pathways for carbohydrate breakdown and glycolysis (Dennis, 1987; Dennis et al., 1997; Stitt, 1998) and the absence of marked phenotypic changes after alterations in the expression of key enzymes, including phosphofructokinase (Burrell et al., 1994), pyrophosphate: fructose-6-phosphate phosphotransferse (PFP) (Hajirezaei et al., 1994; Paul et al., 1995), pyruvate kinase (Gottlob-McHugh et al., 1992), and citrate synthase (Landschütze et al., 1995), indicate that primary carbon metabolism is highly flexible and able to respond to changing demands in the biosynthetic and secondary pathways.
Phenylpropanoid metabolism is regulated by coordinate changes of gene expression (Glassgen et al., 1998; Grotewald et al., 1998; Tamagnone et al., 1998). Evidence is emerging that these are accompanied by changes in the expression of genes that encode enzymes in primary metabolism; for example, elicitors or UV light leads to an increased level of transcripts for the OPPP enzyme glucose-6-phosphate dehydrogenase and enzymes of the shikimate pathway (Batz et al., 1998; Logemann et al., 2000). The immediate carbon substrates for the shikimate path are phosphenolpyruvate (PEP) and erythrose-4-phosphate (Ery4P). Because chloroplasts lack glycolysis downstream of glycerate-3-phosphate (3PGA) (Stitt, 1997), in leaves PEP is provided via an envelope membrane PEP:Pi translocator that exchanges Pi and PEP between the cytosol and the plastids (Fischer et al., 1997). Insertion mutants in the gene encoding this transporter are severely deficient in phenylpropanoids (Streatfield et al., 1999). Ery4P is provided by transketolase (TK).
TK catalyzes the reversible transfer of a two-carbon glycoaldehyde fragment from keto-sugars to the C-1 aldehyde group of aldo-sugars (Schenk et al., 1998) and facilitates the reversible interconversion of hexose-, pentose-, tetrose-, and triose-phosphates. The immediate substrates and products of this reaction act as precursors for phenylpropanoid metabolism (Ery4P), nucleotide synthesis (pentose phosphates), carbohydrate synthesis (fructose-6-phosphate [Fru6P]), and the lower part of glycolysis (glyceraldehyde-3-phosphate) leading to respiratory metabolism and amino acid and lipid synthesis. TK is also universally required for the OPPP, which produces NADPH. In bacteria and yeast, null genotypes demonstrate the role of TK in oxidative stress resistance (Juhnke et al., 1996; Slekar et al., 1996) and aromatic amino acid and phenylpropanoid metabolism (Patnaik and Liao, 1994; Flores et al., 1996; Gosset et al., 1996). In plants, TK is additionally required in the Calvin cycle, in which it converts glyceraldehyde-3-phosphate and Fru6P to xylulose-5-phosphate and Ery4P, and glyceraldehyde-3-phosphate and sedoheptulase-7-phosphate to ribose-5-phosphate and xylulose-5-phosphate. Plants contain one major isoform of TK (Schnarrenberger et al., 1995), which is located in the chloroplast (Schnarrenberger et al., 1995; Debnam and Emes, 1999). In spinach, TK encodes a protein with a plastid-targeting sequence (Teige et al., 1995; Flechner et al., 1996) and is expressed in photosynthetic and nonphotosynthetic tissues (Bernacchia et al., 1995; Teige et al., 1995), indicating that an amphibolic TK participates in the Calvin cycle and the plastid OPPP. In view of the high degree of redundancy in many processes in primary carbon metabolism (see above), it is surprising that this critical reaction is apparently restricted to a single compartment.
Despite this strategic location at the interface between primary and secondary metabolism, the consequences of altered expression of the gene encoding plastid TK have not been studied. TK catalyzes a readily reversible reaction and is not susceptible to “fine” regulation. It is often assumed that such enzymes are expressed in excess (Rolleston, 1972; Newsholme and Start, 1973), and most investigations have focused on enzymes that catalyze irreversible reactions and are subject to fine regulation by low molecular weight effectors and/or post-translational regulation. Nevertheless, theoretical analyses predict that all of the enzymes in a pathway can contribute to the control of flux (Kacser and Burns, 1973; Fell and Thomas, 1995), and studies of mutants and transformants with decreased activity of enzymes in many pathways, including the Calvin cycle and sucrose and starch synthesis (Stitt and Sonnewald, 1995; Haake et al., 1998, 1999), show that small changes in the activity of “nonregulated” enzymes do sometimes affect metabolic fluxes. The experiments described here investigated the consequences of decreased activity of plastid TK on photosynthetic carbon metabolism and phenylpropanoid metabolism.
RESULTS
Generation of Transformants
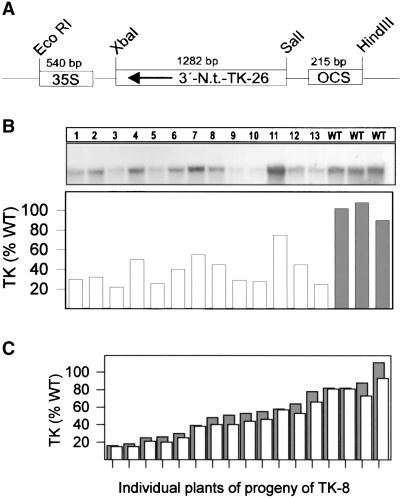
Using a heterologous sequence from resurrection plant (Bernacchia et al., 1995), we isolated a full-length tobacco cDNA (TK-23; EMBL accession number A52295) with homology to TK from resurrection plant (69%), potato (89%), and yeast (44%). The N terminus contained a 77–amino acid plastid transit peptide. A 1300-bp fragment (TK-26) encoding amino acids 425 to 745 of the mature TK polypeptide was placed in the antisense orientation behind the constitutive 35S promotor (Figure 1A) and introduced into tobacco by Agrobacterium-mediated transformation. After selection for kanamycin resistance, 100 primary transformants were transferred to soil and screened for decreased TK protein (data not shown), and four lines with a strong reduction (TK-4, TK-7, TK-8, and TK-12) were identified and self-fertilized.
Figure 1.
Production and Screening of Antisense Transformants with Decreased Expression of TK.
(A) Tobacco (N.t.) was transformed with a construct containing the partial length cDNA TK-26 under the control of the constitutive 35S promotor. OCS, octapine synthase.
(B) T2 progeny were grown on sand on 12 mM nitrate in a greenhouse with 170 μmol m−2 sec−1 supplementary light, and the youngest fully expanded leaves of 8-week-old plants were harvested and analyzed for TK transcript and TK activity. Segregation of TK activity in the T2 generation is shown for TK-8. WT, wild type. Each RNA gel blot and the corresponding activity are for an individual segregant.
(C) TK activity in each transformant was related to the activity in wild-type plants of the same age (white bars) and in younger wild-type plants of the same size as the transformant (dark bars) to ensure that plant size–dependent changes in expression did not complicate the quantification of the inhibition of TK expression. WT, wild type.
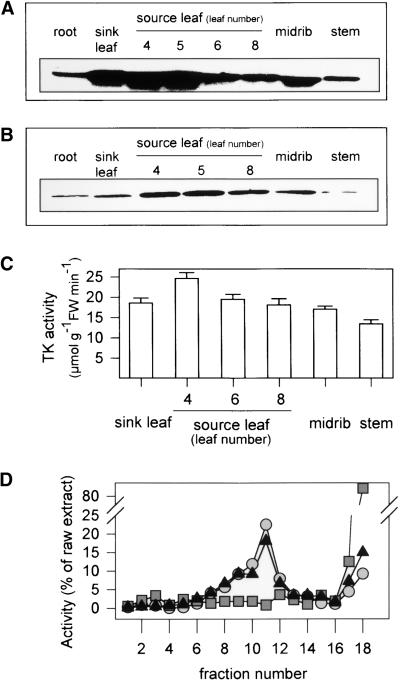
Reliable quantification of antisense-mediated inhibition requires background information about expression in wild-type plants (Haake et al., 1998). TK transcript (Figure 2A), TK protein (Figure 2B), and TK activity (Figure 2C) were low in the roots and stems, higher in sink leaves and the midrib, and highest in source leaves. There were only small differences between young and old source leaves (Figures 2A to 2C) and between the leaf apex and base (data not shown). TK activity in the youngest fully expanded leaf increased slightly with plant age (27.9 ± 0.2, 27.9 ± 1.2, 30.4 ± 5.6, and 36 ± 6.2 μmol m−2 sec−1 in 35-, 42-, 49-, and 56-day-old plants, respectively). There were no significant diurnal changes of activity (data not shown).
Figure 2.
Expression and Subcellular Compartmentation of TK in Wild-Type Tobacco.
Different organs and successive source leaves were harvested from 8-week-old plants growing as described for Figure 1.
(A) TK transcript levels. Transcript levels were analyzed using TK-23 as a probe.
(B) TK protein. Protein was analyzed by immunoblotting with TK antibody raised against overexpressed TK-26.
(C) TK activity. Activity is given as means ±se (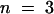 separate plants). FW, fresh weight.
separate plants). FW, fresh weight.
(D) Subcellular location of TK. Protoplasts were prepared from leaves, disrupted by passage through a 14-μm-mesh nylon net, and fractionated on a nonequilibrium density gradient; the individual fractions were assayed for TK (triangles), a plastid marker (NADP-GAPDH; circles), and a cytosolic marker (PFP; squares).
A recent study (Debnam and Emes, 1999) reported an unusually large proportion of TK activity in the cytosol of tobacco leaves, so we fractionated tobacco leaf mesophyll protoplasts on sucrose density gradients to reinvestigate its subcellular localization (Figure 2D). On the basis of the distribution of the plastid enzyme NADP glyceraldehyde-3-phosphate dehydrogenase (NADP-GAPDH) and the cytosolic enzyme PFP, 95% of the plastids were recovered intact with negligible cytosolic contamination. TK had an almost identical distribution to NADP-GAPDH, showing that all or almost all of the transketolase activity is located in the plastids. Debnam and Emes (1999) used differential centrifugation of tobacco leaf extracts, which provides only a low yield of chloroplasts and introduces possible errors because of the selective loss of enzymes.
TK expression segregated in the T2 progeny of each transformed line (shown in Figure 1B for 13 individuals segregating from TK-8). Whereas some progeny had TK activity only slightly less than that of wild-type plants, others showed a progressive decrease down to ∼15% of the wild-type value. The decrease in TK activity correlated with the decrease of TK transcript (Figure 1B). Transformants with very low TK activity did not produce seed (data not shown); thus, for the next set of experiments, >100 T2 progeny from each of the three lines (TK-7, TK-8, and TK-12) were grown and individually screened for TK activity in the leaf material used for the physiological analyses, and transformants with similar TK activity were grouped. Each data point represents progeny from at least two transformant lines. The plants were grown in a greenhouse with supplementary irradiance and automatic shading on days with high sunlight, so that they received minimum and maximum light intensities of ∼170 and 700 μmol m−2 sec−1.
Decreased TK expression led to decreased shoot fresh weight (Figure 3A) and dry weight (data not shown), a slight decrease in leaf number (Figure 3B), and a marked decrease in shoot length (data not shown). To ensure that age- or size-dependent changes were not interfering with quantification of the inhibition of TK expression in the transformants, we related TK activity in the youngest fully expanded leaf of each T2 transformant to the average TK activity in the youngest fully expanded leaf of wild-type plants of the same age or to the average TK activity in the youngest fully expanded leaf of wild-type plants of the same size (Figure 1C). A similar decrease in TK activity was obtained in both cases.
Figure 3.

Whole Plant Growth and Visual Appearance of the Transformants.
(A) Shoot fresh weight (FW). WT, wild type.
(B) Leaf number. WT, wild type.
(C) Leaf of a transformant with a small decrease of TK expression.
(D) Leaf of a transformant with a large decrease of TK expression.
Wild-type and T2 progeny of TK-7, TK-8, and TK-12 were grown as described for Figure 1 and harvested after 9 weeks. The results are means ±se. Each individual data point in (A) and (B) corresponds to eight separate wild-type plants (closed circles) or at least four separate transformants (open circles). The plants shown in (C) and (D) had 40 and 60% decreases, respectively, of TK activity in the first fully expanded leaf.
Calvin Cycle Enzymes, Chlorophyll, and Carotenoids
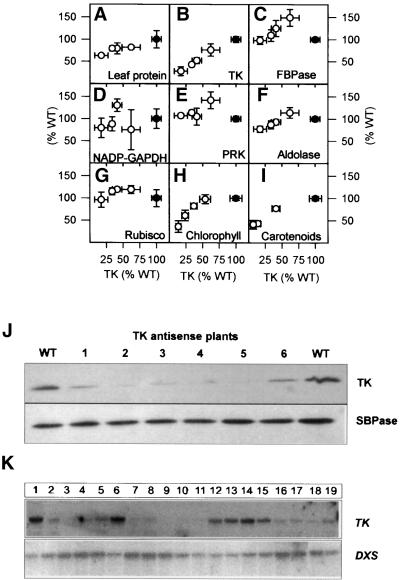
We next investigated whether there were pleiotropic changes in the activity of other Calvin cycle enzymes or in the levels of photosynthetic pigments. Total leaf protein (Figure 4A) was 19 and 40% lower in transformants with 40 and 80% reductions of TK activity, respectively. When enzyme activities were related to protein, there was a specific decrease of TK activity in the transformants (Figure 4B). Plastid fructose-1,6-bisphosphatase (FBPase; Figure 4C), NADP-GAPDH (Figure 4D), phosphoribulokinase (PRK; Figure 4E), aldolase (Figure 4F), and ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco; Figure 4G) activities were unaltered or increased in transformants with moderate inhibition of TK expression. Sedoheptulose-1,7-bisphosphatase (SBPase) expression, monitored by immunoblotting, was unaltered (Figure 4J). The activities of two cytosolic enzymes, PFP and UDP-glucose pyrophosphorylase, also remained high (data not shown).
Figure 4.
Leaf Protein, Calvin Cycle Enzyme Activities, Chlorophyll and Carotenoid Content, and DXS Transcript.
(A) Leaf protein.
(B) TK activity.
(C) Plastid FBPase activity.
(D) NADP-GAPDH activity.
(E) PRK activity.
(F) Aldolase activity.
(G) Rubisco activity.
(H) Chlorophyll.
(I) Carotenoids.
(J) SBPase protein.
(K) DXS transcript.
Plants were grown and the youngest fully expanded leaves harvested as described for Figure 1. Because total leaf protein decreased on a fresh weight basis (A), individual enzyme activities ([B] to [G]) were related to total leaf protein. Each data point corresponds to seven separate wild-type (WT) plants (closed circles) or at least four separate transformants (open circles). Chlorophyll (H) was determined in seven wild-type plants (closed circles) or at least four transformants (open circles) for each data point, and carotenoids (I) were determined in four wild-type plants (closed circles) or at least four transformants (open circles) per data point and are expressed on a fresh weight basis. All enzyme activities and pigment contents are given as means ±se. In (J), equal amounts of leaf protein from wild-type plants (lanes WT), TK-7 (lanes 1, 3, and 5), and TK-8 (lanes 2, 4, and 6) were separated and probed with antibody against SBPase protein. To determine DXS transcript levels (K), RNA was probed with a full-length tobacco DXS cDNA. TK expression was determined by probing with TK-26. To exclude artifacts due to plant age– or plant size–dependent changes in DXS transcript, wild-type plants were harvested after 8 weeks (lanes 12 to 15, corresponding to shoot fresh weight of 16 to 18 g) and earlier (lanes 1 and 6, corresponding to shoot fresh weight of 5 and 8 g, respectively) and compared with transformants (TK-8 and TK-12) sampled after 8 weeks. Lanes 2 to 5 show transformants with a fresh weight similar to that of the wild-type plant represented in lane 1, lanes 7 to 11 show transformants with a fresh weight similar to that of the wild-type plant represented in lane 6, and lanes 16 to 19 show transformants with a fresh weight similar to those of the wild-type plants represented in lanes 12 to 15.
Chlorophyll (Figure 4H) was unaltered in transformants with a 50% decrease in TK activity. Greater inhibition of TK expression led to localized loss of chlorophyll along the midrib and the largest side veins, sometimes spreading along smaller veins and onto sectors of the lamina (Figures 3C and 3D). TK activity in bleached sectors was ∼50% less than in adjacent green areas of the same leaf (data not shown).
Total carotenoids also decreased (Figure 4I) when TK activity decreased to <50% of the wild-type value. The first step in plastid carotenoid synthesis, conversion of pyruvate and glyceraldehyde-3-phosphate to d-1-deoxyxylulose-phosphate, is catalyzed by deoxyxylulose-7-phosphate synthase (DXS) (Lois et al., 1998; Lichtenthaler, 1999). Because Arabidopsis TK shares 23% identity with DXS at the nucleotide level (Mandel et al., 1996), we investigated DXS expression in a large set of transformants with varying losses of chlorophyll and carotenoids. The transformants contained similar or greater DXS transcript levels than did wild-type plants, irrespective of whether plants of similar size or similar age were compared (Figure 4K).
Photosynthesis
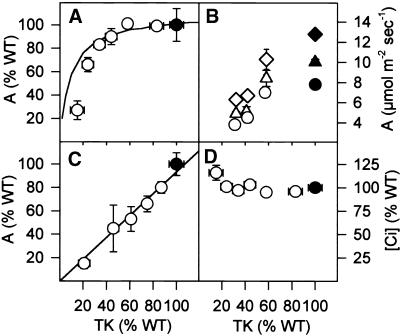
Photosynthesis on an overcast day (light intensity in the gas exchange cuvette of 170 μmol m−2 sec−1) was inhibited markedly when TK activity decreased to <40% of the wild-type value (Figure 5A). Photosynthesis in ambient CO2 became increasingly sensitive to a reduction of TK activity as the irradiance was increased to 220, 350, and 700 μmol m−2 sec−1 (Figure 5B), representative of the range experienced in growth conditions. The progressive increase of the rate of photosynthesis in wild-type plants that occurred as the measuring light intensity was increased represents the typical response to an increase of the light intensity across the growth range. There was a linear relation between TK activity and photosynthesis in saturating light and saturating CO2 (Figure 5C).
Figure 5.
Photosynthesis.
(A) Photosynthesis at 170 μmol m−2 sec−1 and ambient CO2.
(B) Photosynthesis at 220, 350, or 700 μmol m−2 sec−1 and ambient CO2.
(C) Photosynthesis in saturating light and saturating CO2.
(D) CO2 concentration inside the leaf ([Ci]).
Plants were grown in a greenhouse in natural irradiance supplemented with 170 μmol m−2 sec−1, with light intensities increasing to ∼600 μmol m−2 sec−1 on sunny days. TK activity was measured in each leaf area after the measurement of photosynthesis was completed. The results are given as means ±se, and each individual data point corresponds to five to seven separate wild-type (WT) plants (closed symbols) or three to seven transformants (open symbols). Photosynthesis was measured at 350 ppm CO2 on an overcast day (A) without additional light or (B) with supplementary light to bring the intensity in the cuvette to 220 (circles), 350 (triangles), or 700 (diamonds) μmol m−2 sec−1, and (C) at ∼5000 ppm CO2 and 700 μmol m−2 sec−1. [Ci] (D) was calculated from the rate of photosynthesis and stomatal conductance, as determined in parallel with photosynthesis.
The effect of decreased TK activity on the rate of photosynthesis was quantified by calculating the flux control coefficient, CTK (Kacser and Burns, 1973). After normalizing each data set in Figures 5A to 5C to make the wild-type values for TK activity and photosynthetic rate geometrically equivalent (plot not shown), we estimated CTK from the initial slope of a fitted curve (Small and Kacser, 1993; Haake et al., 1998). CTK increased from 0.07 at 170 μmol m−2 sec−1 to 0.14, 0.28, and 0.32 at 220, 350, and 700 μmol m−2 sec−1, respectively, and ambient CO2, and it increased to almost 1 in saturating light and saturating CO2.
Stomatal Conductance
Stomatal conductance was unaltered or increased slightly in transformants with a small inhibition of TK expression, and decreased slightly in transformants with a larger inhibition of TK expression (data not shown). The estimated CO2 concentration inside the leaf ([Ci]) therefore increased as photosynthesis was inhibited (shown at an irradiance of 170 μmol m−2 sec−1 in Figure 5D). The strong inhibition of CO2-saturated photosynthesis in response to decreased TK activity (Figure 5C) emphasizes that photosynthesis is not inhibited as a result of secondary effects on stomatal aperture.
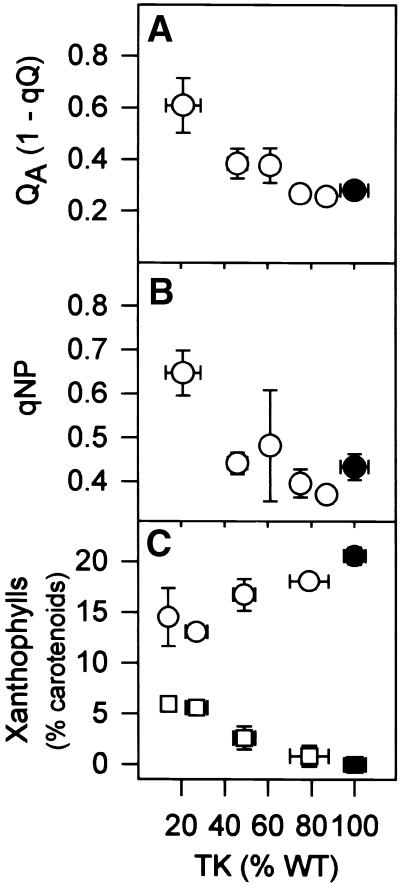
Chlorophyll Fluorescence and Xanthophyll Cycle Intermediates
The inhibition of photosynthesis at the lowest light intensity (Figure 5A) resembled the change in leaf chlorophyll (Figure 4H). At higher light intensities or in saturating CO2 (Figures 5B and 5C), photosynthesis was inhibited in transformant lines with a smaller decrease in TK activity, in which chlorophyll was unaltered. Decreased TK activity led to increased reduction of the primary acceptor for photosystem 2 (QA; Figure 6A), increased nonphotochemical quenching (qNP; Figure 6B), and a reciprocal decrease of violaxanthin and increase of zeaxanthin (Figure 6C). These changes occurred in transformants with a 50% decrease of TK activity in which chlorophyll was unaltered. The Fv/Fm ratio (Fv is variable fluorescence, Fm is the maxium fluorescence level after a saturating light flash, and the ratio is an indicator of functional energy transfer to the active center; Björkman and Demmig, 1987) remained high until TK activity was very low (data not shown).
Figure 6.
Reduction State of Thylakoid Membranes and Energy Dissipation.
(A) Reduction of the primary receptor for photosystem 2 (QA), determined as the inverse of the photochemical quenching (1-qQ).
(B) Nonphotochemical chlorophyll fluorescence quenching (qNP).
(C) Violaxanthin (circles) and zeaxanthin (squares) expressed as a percentage of total carotenoids.
Plants were grown as described for Figure 1, leaf discs were harvested from the youngest fully expanded leaves and predarkened for at least 30 min, and chlorophyll fluorescence was determined in saturating light and CO2. The results are means ±se, and each data point corresponds to five separate wild-type (WT) plants (closed symbols) or more than three separate transformants (open symbols).
These results show that the inhibition of photosynthesis is accompanied by an increased reduction of the electron transport chain, an increased transthylakoid pH gradient, and increased energy dissipation (Horton et al., 1996), and they reveal that the primary site of inhibition is farther downstream in carbon metabolism.
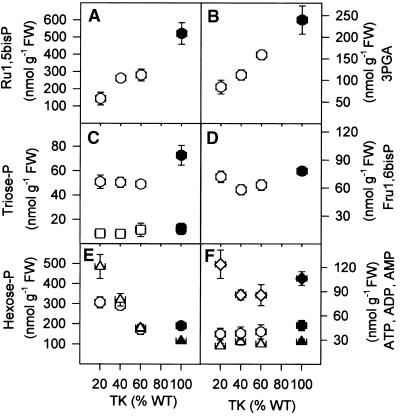
Phosphorylated Intermediates
Phosphorylated intermediates were investigated in ambient light. Decreased TK activity led to a dramatic decrease of ribulose-1,5-bisphosphate (Ru1,5bisP; Figure 7A) and 3PGA (Figure 7B), a small decrease of triose phosphates (Figure 7C) and fructose-1,6-bisphosphate (Fru1,6bisP; Figure 7D), an increase of glucose-6-phosphate (Glc6P; Figure 7E), and a dramatic increase of Fru6P (Figure 7E). These changes occurred in transformants with a 40% decrease in TK activity in which chlorophyll was unaltered. Ery4P was below the detection limit (∼5 nmol/g fresh weight; data not shown). PEP and pyruvate decreased slightly (data not shown). The ATP/ADP ratio did not change significantly (Figure 7F). Overall levels of adenine (Figure 7F) and uridine nucleotides (data not shown) in source leaves were unaltered.
Figure 7.
Phosphorylated Intermediates in Leaves.
(A) Ru1,5bisP.
(B) 3PGA.
(C) Glyceraldehyde-3-phosphate (squares) and dihydroxyacetone phosphate (circles).
(D) Fru1,6bisP.
(E) Glc6P (circles) and Fru6P (triangles).
(F) ATP (diamonds), ADP (circles), and AMP (triangles).
Plants were grown as described for Figure 1 and sampled from the youngest fully expanded leaves. The results are means ±se, and each data point corresponds to nine separate wild-type (WT) plants (closed symbols) or more than five separate transformants (open symbols). FW, fresh weight.
The response of the ATP/ADP ratio (Figure 7F) and the 3PGA:triose phosphate ratio (cf. Figures 7B and 7C) confirms that ATP and NADPH delivery does not limit photosynthesis in the transformants and confirms that the primary inhibition is in the “dark” reactions. Interpretation of the changes in the levels of Calvin cycle intermediate requires background information about their subcellular distribution (Stitt et al., 1980; Gerhardt et al., 1987). Ru1,5bisP and most of the 3PGA and Fru1,6bisP are located in the plastid, triose phosphates are equilibrated between the plastid and the cytosol, and there are separate pools of hexose phosphates in the plastid and the cytosol. The Fru6P:Glc6P ratio is close to equilibrium in the cytosol (0.25 to 0.3) and higher in the plastid (∼1), because the phosphoglucoisomerase reaction is close to equilibrium in the former but displaced from equilibrium in the latter (Dietz and Heber, 1986; Gerhardt et al., 1987). The preferential accumulation of Fru6P compared with Glc6P (Figure 7E) reveals that hexose phosphates increase in the chloroplast in the transformants, as expected if Ru1,5bisP regeneration is inhibited at the first reaction catalyzed by TK in the Calvin cycle.
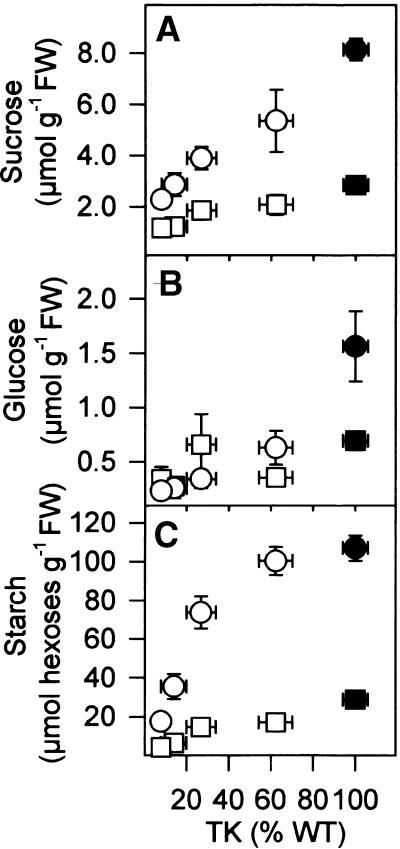
Carbohydrates
Decreased TK activity led to lower levels of sucrose (Figure 8A), glucose (Figure 8B), and fructose (data not shown) in the leaves. This decrease was found in transformants with a small decrease of TK activity, providing independent evidence that TK colimits photosynthesis in growth conditions. Sugars also decreased in the roots (data not shown). Starch (Figure 8C) did not decrease in transformants with a small decrease of TK activity. Comparison of starch at the end of the day and night (Figure 8C) revealed that the diurnal turnover of starch was not strongly inhibited until TK activity decreased to <20% of the wild-type value. Decreased TK activity therefore alters photosynthate allocation, to favor starch rather than sucrose synthesis.
Figure 8.
Carbohydrate Levels in Leaves at the End of the Day and the End of the Night.
(A) Sucrose.
(B) Glucose.
(C) Starch.
Plants were grown as described for Figure 1. Samples were harvested in growth conditions from the youngest fully expanded leaves at the end of the night (squares) and 13 hr into the light period (circles). The results are means ±se, and each data point corresponds to seven separate wild-type (WT) plants (closed symbols) or more than four separate transformants (open symbols). FW, fresh weight.
Aromatic Amino Acids
The shikimate pathway converts Ery4P and PEP into aromatic amino acids. Phe (Figure 9A), Trp (Figure 9B), and Tyr (Figure 9C) decreased significantly in transformants with a 20 to 50% reduction of TK activity and decreased to negligible levels when TK activity was decreased further. Similar results were obtained for plants growing in soil with slow-release fertilizer (data not shown). Total amino acid content did not decrease (Figure 9D).
Figure 9.
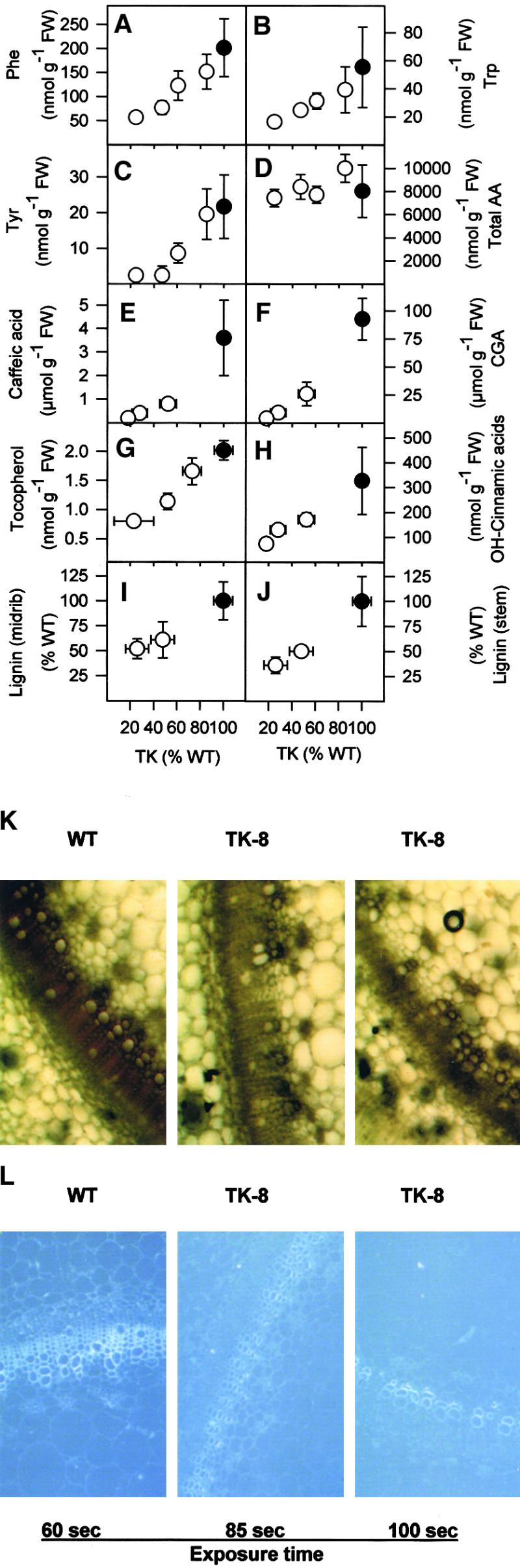
Aromatic Amino Acids and Intermediates and Products of Phenylpropanoid Metabolism.
(A) Phenylalanine.
(B) Tryptophan.
(C) Tyrosine.
(D) Total amino acids (AA).
(E) Caffeic acid.
(F) CGA.
(G) Tocopherol.
(H) Hydroxycinnamic acids.
(I) Lignin determined in midrib extracts using thioglycollate.
(J) Lignin determined in stem extracts using thioglycollate.
(K) Lignin visualized in stem sections by phloroglucinol-HCl staining.
(L) Lignin visualized in stem sections by autofluorescence.
Plants were grown as described for Figure 1. Samples were harvested from the youngest fully expanded leaves 8 to 9 hr into the light period in growth conditions from source leaves for (A) to (H). The results are means ±se, and each data point corresponds to separate wild-type (WT) plants (closed circles) or more than seven separate transformants (open circles). For thioglycollate determination of lignin ([I] and [J]), samples were prepared from the stem between the eighth and eleventh internodes. The results are means ±se, and each individual data point corresponds to three separate wild-type plants (closed circles) or at least three separate transformants (open circles). For phloroglucinol-HCl staining (K), stem sections from the fifth to seventh internodes of wild-type plants and two TK-8 transformants were sectioned, stained, and photographed at ×40 magnification and standardized illumination intensity. For immunofluorescence (L), stem sections from the third to fourth internodes of wild-type plants and two TK-8 transformants were embedded in plastic, 6-μm sections were prepared in a microtome, and photographs were taken at ×30 magnification and exposure times of 60 sec for wild-type material and 85 and 100 sec for transformants. FW, fresh weight.
Phenylpropanoids and Lignin
Aromatic amino acids are the starting point for phenylpropanoid metabolism. The major soluble phenylpropanoids in tobacco are caffeic acid (Figure 9E) and chlorogenic acid (CGA; Figure 9F). Both decreased dramatically in leaves of transformants with a 50% reduction in TK activity and were almost undetectable in transformants with an 80% reduction of wild-type TK activity. A similar decrease was found in the midrib and stem (data not shown).
Tocopherols consist of an isoprenoid side chain and an aromatic component derived from the shikimic acid pathway (Hirschberg, 1999). Decreased TK activity led to a nearly linear decrease of overall tocopherol content (Figure 9G). Because α-tocopherol represents the main isomer (Hirschberg, 1999), these results indicate that vitamin E decreases in response to a small decrease of TK activity.
Hydroxycinnamic acids (coumaric acid, sinapic acid, and ferulic acid) are precursors for lignin synthesis and contribute to cross-linking in the cell wall. They decreased in the leaves (Figure 9H), midribs, and stems (data not shown) of the transformants. The decrease was already significant in transformants with a 50% decrease in TK activity.
Because individual techniques can lead to errors (Lewis and Yamamoto, 1990), lignin was investigated in midrib (Figure 9I) and stem (Figure 9J) extracts by using thioglycollate and in stem sections by using phloroglucinol staining (Figure 9K) or autofluorescence (Figure 9L). Decreased TK activity led to a marked decrease of lignin. Whereas the large xylem elements and the smaller xylem elements lying between them were lignified in wild-type tobacco, lignin was detected only in the large vessels in the transformants.
Cell wall properties were investigated by incubating leaf discs with cell wall–digesting enzymes. Whereas discs from wild-type leaves remained physically intact for 20 hr, discs from transformants with a 50% decrease in TK activity were digested more rapidly, and discs from transformants with very low TK disintegrated within 3 hr (data not shown).
OPPP Turnover
To investigate OPPP activity, researchers incubated leaf discs with specifically labeled glucose for 4 hr in the dark (ap Rees, 1980). After C-1 and C-6, C-2 and C-5, and C-3 and C-4 are equilibrated in glycolysis, 14C-CO2 is released from C-3/4 by pyruvate dehydrogenase and from C-2/5 and C-1/6 during the second and third pass, respectively, through the tricarboxylic acid cycle. The OPPP preferentially releases 14C-CO2 from 1-14C-glucose. Although 6-14C-glucose is usually used as a reference, 2-14C-glucose is more reliable in plants because 14C-CO2 is released from 6-14C-glucose during the synthesis of pentoses for cell wall polysaccharides (Stitt and ap Rees, 1978; ap Rees, 1980). The ratio between 14C-CO2 release from 1-14C-glucose and 2-14C-glucose was higher in transformant than in wild-type discs (Table 1), showing that OPPP flux is not inhibited. In a second treatment, leaves were wounded 18 hr before the start of the incubation. Wounding stimulated 14C-CO2 release from 1-14C-glucose compared with its release from 2-14C-glucose in wild-type but not in transformant discs, indicating that a 50 to 70% decrease in TK activity may compromise the wounding-induced stimulation of the OPPP.
Table 1.
Release of 14C-CO2 after Incubating Leaf Discs with 1-14C- and 2-14C-Glucosea
| TK Activity (as % Wild Type) | Fresh Leaf Discsb | Wounded Leaf Discsc |
|---|---|---|
| 100 | 1.67 | 3.22 |
| 50 | 4.25 | 2.03 |
| 30 | 2.29 | 1.29 |
14C-CO2 release was expressed as a percentage of the label supplied, and the ratio of release from 1-14C- and 2-14C-glucose was calculated. Each result is the mean of four separate sets of discs from different plants. The se was 5 to 7% of the mean.
Plants were left in the dark overnight, and leaf discs removed and incubated for 4 hr in the dark with 1 mM glucose containing 1 mM 1-14C-, 2-14C-, and 6-14C-glucose (final specific activity 1 μCi/mmol) and the evolved 14C-CO2 trapped in KOH.
Alternately, leaves were wounded with a needle-cushion and placed in the dark overnight; leaf discs were then incubated for 4 hr with specifically labeled glucose.
DISCUSSION
A Small Inhibition of TK Expression Has a Marked Effect on Photosynthesis, Secondary Metabolism, and Plant Growth
A 20 to 40% decrease of plastid TK activity leads to a stoichiometric inhibition of the maximum rate of photosynthesis, a slight inhibition of ambient photosynthesis, decreased levels of sugars but not starch, and decreased levels of aromatic amino acids. A 50% decrease of plastid TK activity leads to a marked inhibition of ambient photosynthesis, much lower levels of sugars, a dramatic decrease of aromatic amino acids, phenylpropanoid intermediates, CGA, and lignin, and decreased growth. These results demonstrate that there are no major alternative pathways that are able to substitute for plastid TK, an important finding in view of the strategic location of plastid TK in metabolism (see Introduction). Further, and most unexpectedly, metabolism and growth are inhibited in response to an unexpectedly small decrease of activity, demonstrating that TK is colimiting or near-limiting for several important metabolic pathways.
The “Unregulated” Enzyme Plastid TK Partially Limits Ambient Photosynthesis and Strongly Limits the Maximum Rate of Photosynthesis in Saturating CO2 and Light
Photosynthesis is decreased in the antisense TK transformants as a result of an inhibition of Ru1,5bisP regeneration. This is a direct consequence of the decreased TK activity, and it is associated with a dramatic accumulation of Fru6P, one of the substrates for the first reaction catalyzed by TK in the Calvin cycle. The other substrate (glyceraldehyde-3-phosphate) does not accumulate, presumbly because aldolase and plastid FBPase continue to convert triose phosphates to Fru6P. Our results provide evidence against other, more indirect explanations for the inhibition of photosynthesis. In contrast to transformants with decreased expression of aldolase in which the activity of other Calvin cycle enzymes, especially plastid FBPase, decreased (Haake et al., 1998, 1999), in transformants with a small decrease of TK expression, the activities of the other Calvin cycle enzymes remained high or even increased slightly. Although there was a decrease of chlorophyll when TK activity was decreased to <50% of the wild-type value, this is not the reason for the inhibition of photosynthesis. Photosynthesis was inhibited in transformants with a smaller decrease of TK expression in which chlorophyll was unaltered, and the accompanying decrease of the 3PGA:triose phosphate ratio and increase in QA reduction and energy dissipation show that the primary block is in the dark reactions. Chlorophyll loss was localized along the veins, occasionally spreading onto the lamella when TK activity was very low. This response resembles that seen in transformants with decreased expression of SBPase (Harrison et al., 1998) and glutamate-1-semialdehyde aminotransferase (Höfgen et al., 1994), whereas decreased expression of Rubisco (Stitt and Schulze, 1994) led to loss of chlorophyll over the whole leaf surface. The decrease of chlorophyll is probably an indirect consequence of low TK activity, because it did not occur until expression was strongly inhibited and many aspects of metabolism were altered.
Photosynthesis in ambient CO2 and the light intensities experienced in growth conditions was partly limited by TK, with the contribution rising as the light intensity was increased. The flux control coefficient of TK for photosynthesis (0.07 to 0.32) resembles that of Rubisco (0.1 to 0.3; Stitt and Schulze, 1994) and aldolase (0.15 to 0.24; Haake et al., 1998, 1999) in ambient conditions. Although SBPase has a higher flux control coefficient (>0.7; Harrison et al., 1998), the inhibition of photosynthesis in antisense SBPase transformants was mainly because of an inhibition of stomatal conductance and the resulting decrease of [Ci]. If photosynthesis is normalized on [Ci], it is inhibited only marginally by a 30 to 40% decrease of SBPase activity (Harrison et al., 1998), indicating that SBPase has a fairly small flux control coefficient for the metabolic processes in the mesophyll. Ambient photosynthesis was not strongly inhibited until >60% of plastid FBPase (Kossmann et al., 1994) and NADP-GAPDH (Price et al., 1994) activity and 90% of PRK (Gray et al., 1995; Paul et al., 1995) activity were removed.
Photosynthesis in saturating light and saturating CO2 was directly proportional to TK activity. It is remarkable that the maximum rate of photosynthesis is almost completely limited by an enzyme previously considered irrelevant to regulation. Another nonregulated enzyme, plastid aldolase, also exerts some control on the maximum rate of photosynthesis (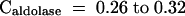 ; Haake et al., 1998), whereas Rubisco, which contributes to the control of photosynthesis in ambient CO2, does not exert control in saturating light and CO2 (Stitt and Schulze, 1994).
; Haake et al., 1998), whereas Rubisco, which contributes to the control of photosynthesis in ambient CO2, does not exert control in saturating light and CO2 (Stitt and Schulze, 1994).
Photosynthesis is therefore colimited by several enzymes, including some that catalyze irreversible reactions and are subject to fine regulation (Rubisco and SBPase) and some that are not regulated and catalyze readily reversible reactions (aldolase and TK). Regulatory enzymes are often present in excess to allow feedback control to regulate their momentary activity, and decreased activity can often be compensated for by changing the levels of metabolites in these feedback loops. Compensation for decreased activity of a nonregulated enzyme requires changes in the levels of the immediate substrates and products, and fluxes are inhibited if these changes impair the operation of other enzymes in the pathway (Stitt and Sonnewald, 1995; Stitt, 1999). The levels of the substrates and products of TK are clearly critical for the operation of other enzymes in the Calvin cycle.
Decreased Expression of TK Leads to a Preferential Inhibition of Sucrose Synthesis Compared with Starch Synthesis
Photosynthate is converted to sucrose and exported from the leaf or stored as starch and remobilized during the subsequent night. Decreased expression of TK leads to a shift of allocation in favor of starch. Changes of TK activity (20 to 50%) that lead to a small inhibition of photosynthesis resulted in a marked decrease of leaf sugar levels, whereas starch did not decrease until TK activity was much lower and photosynthesis was strongly inhibited. This response differs from that in transformants with decreased activity of other Calvin cycle enzymes. Decreased activity of Rubisco led to a parallel decrease of sugars and starch (Stitt and Schulze, 1994). Decreased activity of plastid aldolase (Haake et al., 1998), plastid FBPase (Kossmann et al., 1994), and SBPase (Harrison et al., 1998) led to a very marked decrease of starch, whereas sugar levels remained high or even increased in transformants in which photosynthesis was affected only slightly.
This shift from sucrose to starch synthesis is linked to the function of TK in the interconversion of sugar phosphate pools. Carbon is withdrawn at different sites during the Calvin cycle for sucrose and starch synthesis (Stitt, 1997). Whereas triose phosphates are exported to the cytosol for conversion to sucrose, Fru6P represents the starting point for starch synthesis. The strong inhibition of starch synthesis in transformants with decreased activity of aldolase and plastid FBPase was explained (Kossmann et al., 1994; Haake et al., 1998) because these enzymes lie downstream of the site at which carbon is removed for sucrose synthesis and upstream of the site at which carbon is withdrawn for starch synthesis. As TK catalyzes the first step in the Calvin cycle downstream of Fru6P, the relatively high rates of starch synthesis in the antisense TK lines may be due partly to the accumulation of Fru6P. This cannot be the full explanation, however, because starch synthesis was preferentially inhibited in transformants with decreased SBPase activity (Harrison et al., 1998), even though SBPase lies downstream from TK. The first step in photosynthetic starch synthesis, conversion of Fru6P to Glc6P, is catalyzed by phosphoglucose isomerase. Ery4P is a structure homolog for carbons 3 to 6 of Fru6P, and it is a universal and potent competitive inhibitor of phosphoglucose isomerases, the Ki(Ery4P) for the plastid isoform being in the range of 0.4 to 4 μM (Kelly and Latzko, 1980; Backhausen et al., 1997). Because Ery4P is removed by the sequential action of aldolase and SBPase, an increase of Ery4P might contribute to the preferential inhibition of starch synthesis in transformants in which their expression is decreased. Decreased expression of TK presumably leads to a lower Ery4P concentration, which will favor starch synthesis.
A Small Decrease in TK Expression Leads to Lower Levels of Aromatic Amino Acids and Phenylpropanoids
A 20 to 40% reduction of TK activity led to decreased levels of aromatic amino acids, decreased levels of intermediates in phenylpropanoid metabolism such as caffeic acid and hydroxycinnamic acids, a marked decrease of CGA and lignin, which are the major soluble and insoluble products of phenylpropanoid metabolism, and increased susceptibility of the cell wall to digestion by hydrolytic enzymes. The latter might be related to the reduced lignin content or to decreased cross-linking of polysaccharide components by hydroxycinnamic acids (Chapple and Carpita, 1998). Similar changes in cell wall digestibility occur in transformants with decreased activity of phenylalanine ammonia-lyase, caffeic acid:3-O-methyltransferase (Sewalt et al., 1997), and cinnamyl alcohol dehydrogenase (Bernard-Vailhe et al., 1998; Baucher et al., 1999).
The simplest explanation for these changes is that flux into the shikimate pathway and phenylpropanoid metabolism is restricted by the supply of Ery4P and aromatic amino acids, respectively. Independent support for the latter is provided by comparison with a study by Yao et al. (1995) in which a similar decrease of Trp and Phe due to ectopic overexpression of tryptophan decarboxylase led to a marked decrease of CGA and lignin. Our results show that the decrease of aromatic amino acid and phenylpropanoid levels in the antisense TK transformants is not an indirect effect that is due to lower rates of photosynthesis, decreased levels of carbohydrates, or nonspecific changes in nitrogen metabolism. Decreased TK activity did not lead to a general decrease of amino acids, and aromatic amino acids do not show a selective decrease when photosynthesis is inhibited by decreased activity of Rubisco (P. Matt and M. Stitt, unpublished results); in fact, they even increase when aldolase activity is decreased (V. Haake, S. Henkes, and M. Stitt, unpublished results).
The flux control coefficient of TK for the shikimate pathway cannot be estimated directly, because we did not monitor export or the use of aromatic amino acids for protein synthesis. On the basis of the reasonable assumption that export will decrease when the pools decrease and the observation that total protein decreases slightly and the levels of intermediates and products of phenylpropanoid metabolism decrease markedly in the transformants, it is likely that flux into the shikimic acid pathway is inhibited appreciably. Flux into phenylpropanoid metabolism can be estimated by summing the major products, lignin and CGA (Figure 9). Our results indicate that TK has a remarkably high flux control coefficient (>0.7) for phenylpropanoid metabolism. This is similar to or larger than the effects of the decreased expression of phenylalanine ammonia-lyase (Bate et al., 1994; Howles et al., 1996; Sewalt et al., 1997), 4-coumarate:CoA lyase (Lee et al., 1997), and cinnamate-4-hydroxylase (Sewalt et al., 1997), three enzymes that build a regulatory unit that responds to a range of developmental and environmental factors (Chapple and Carpita, 1998; Koopman et al., 1999).
These results highlight two important features of the interaction between primary and secondary metabolism. First, fluxes in secondary metabolism can be limited by the precursor supply in primary metabolism, showing that major changes in secondary metabolism will require appropriate reprogramming of primary metabolism. Second, coordination will require changes in the expression of suites of genes, including “nonregulated” enzymes such as TK as well as regulated enzymes.
Nucleotide synthesis requires pentose phosphates, which are another product of TK. Overall nucleotide levels did not decrease in the leaves of the antisense-TK transformants. In studies of transformants with decreased expression of enzymes of the nucleotide biosynthetic pathways, however, we have observed that growth is decreased even though nucleotide levels in source leaves are unaffected (R. Zrenner, unpublished results). To investigate the possible role of TK during nucleotide biosynthesis, it will therefore be necessary to extend the investigations to young growing tissues.
In conclusion, our results identify plastid TK as a key determinant of plant metabolism. First, tobacco does not possess an alternate route that can substitute for plastid TK, in striking contrast to many other processes in primary carbon metabolism, which show a high degree of redundancy (see Introduction). Second, several pathways that are directly or indirectly dependent on the substrates and products of TK, including the Calvin cycle, starch and sucrose synthesis, and the shikimate pathway and phenylpropanoid metabolism, are remarkably sensitive to small changes of plastid TK expression. Our results also raise the question of why TK expression is not greater in wild-type tobacco leaves. One answer is that greater TK activity might lead to an increase of Ery4P (see above), which would be unfavorable for starch synthesis. This points to the intriguing possibility that the ability of enzymes to differentiate between closely related sugar phosphates places a constraint on the level of expression of TK and on fluxes at this focal site in metabolism.
METHODS
Recombinant DNA Techniques
Standard procedures were used for recombinant DNA work (Sambrook et al., 1989). DNA was sequenced by the dideoxy method (Sanger et al., 1977). Agrobacterium tumefaciens strain C58C1 containing pGV2260 (Deblaere et al., 1985) was cultivated in yeast-enriched broth (YEB) medium (Vervliet et al., 1975).
cDNA Library Screening
After screening a poly(A)+ RNA in a λZAPII (Stratagene, La Jolla, CA) cDNA library from tobacco (Nicotiana tabacum cv SNN) leaves by using the heterologous probe TXT10 from resurrection plant (Craterostigma plantagineum) (Bernacchia et al., 1995), we obtained 18 positive plasmids from 2 × 105 recombinant phage. After in vivo excision, the cDNA were characterized by restriction analysis, and two were selected for sequencing. TK-23 (2629 bp) contained an open reading frame corresponding to the entire 743–amino acid protein (EMBL accession number A52295). TK-26 (1282 bp) was a partial clone corresponding to nucleotides 1329 to 2611 of TK-23 encoding amino acids 425 to 745 of the open reading frame.
Expression of Antisense TK in Transgenic Tobacco Plants
The partial cDNA TK-26 was used for antisense inhibition to allow endogenous TK mRNA to be distinguished from antisense transcript. TK-26 was isolated as an XbaI-SalI fragment from plasmid pBluescript SK− and ligated in the antisense orientation into XbaI-SalI–restricted pBinAR19 (Höfgen and Willmitzer, 1990) between the cauliflower mosaic virus 35S promoter and the octopine synthase polyadenylation signal (Figure 1A). The construct was introduced into Agrobacterium strain C58C1:pGV2260 (Höfgen and Willmitzer, 1988) and used to transform tobacco cv SNN (Rosahl et al., 1989). After regeneration on selective medium, 100 primary transformants were transferred to soil and screened for TK protein in primary leaves, and lines with decreased TK were grown to seed and rescreened.
Production of Polyclonal Antibodies against TK
Mature TK was overexpressed in Escherichia coli cells for subsequent antibody production. A polymerase chain reaction (PCR) fragment from TK-23 corresponding to nucleotides 292 to 2291 was amplified using the oligonucleotides TKA (5′-AAGTCGAACGAATTCAAAATCGAGACTGCGCTTGTTGAC-3′) and TKB (5′-TTGTCG-ACGAATTCCTAAGAAACTTGTTTAGCTGCAGC-3′). After PCR amplification, the fragment was ligated into the PCR vector pGEM-T, sequenced to exclude changes that occurred during PCR amplification, and ligated into the SalI-restricted Escherichia expression vector pQE-9 (Qiagen, Hilden, Germany). Synthesis of the TK–His fusion protein was induced, the protein was purified on nickel-agarose according to standard protocols, and polyclonal antibodies were raised in rabbits.
Plant Growth and Harvest
Seeds were germinated on quartz sand (1:1 mixture of 0.3 to 0.8 and 0.6 to 1.2 mm diameter; Dorsolit, Mannheim, Germany). After primary leaf formation, individual plantlets were transferred to 12-cm-diameter pots containing quartz sand that were placed apart to avoid mutual shading. Plants were grown in a greenhouse in Heidelberg (early autumn to late spring) with supplemental illumination to maintain a mimimum irradiance of 170 μmol m−2 sec−1, with 13 hr of light/11 hr of dark at ∼24 and 20°C, respectively, and ∼60% RH. The plants were watered daily to field capacity with nutrient solution containing 12 mM nitrate (Scheible et al., 1997). Leaf nitrate was 50 and 25 μmol/g fresh weight at the start and end of the light period, respectively, in wild-type plants and >100 μmol/g fresh weight in transformants. After 7 to 8 weeks, the youngest fully expanded leaves were harvested by transferring them under ambient light to liquid nitrogen in a shallow Dewar container and stored in liquid nitrogen at −80°C. Whole leaves were homogenized to a fine powder with a mortar and pestle precooled to liquid nitrogen temperature, and aliquots were extracted (see below) to determine individual components (Haake et al., 1998).
Protein Separation and Immunoblotting
Frozen leaf powder was extracted for 5 min at 95°C in 60 mM Tris-HCl, pH 6.8, 10% (v/v) glycerol, 2% (w/v) SDS, 0.005% (w/v) bromphenol blue, and 0.7 M β-mercaptoethanol, cooled, and centrifuged for 5 min at 14,000g. Protein was determined in the supernatant; the concentration was adjusted to 1 mg protein/mL; 5- to 20-μg protein aliquots were separated by SDS-PAGE, blotted, and transferred; and individual proteins were immunodetected using Luminol (Pierce, Rockford, IL) (Haake et al., 1998). Membranes were reversibly stained with PonceauS to confirm transfer. The polyclonal antibody against sedoheptulose-1,7-bisphosphatase was from B. Buchanan (University of Berkeley, CA).
RNA Gel Blot Analysis
Total RNA was extracted from 1 to 2 g of frozen leaf powder and separated as described by Bernacchia et al. (1995). Radioactive probes to deoxyxylulose-7-phosphate synthase (DXS) were prepared as described by Krapp et al. (1993), and digoxigenin-labeled probes (TK) were prepared as described by Haake et al. (1999). RNA separations were viewed under UV light to confirm loading and separation. Exposition times were varied to ensure that signals were not saturated. Probes used were as follows: TK (TK-26; EMBL accession number A52295) and DXS (SunGene; IPK, Gatersleben, Germany).
Protoplast Isolation and Subcellular Fractionation
Two to three grams of young, fully expanded source leaves was deribbed and placed in 15 mL of incubation medium (0.4 M mannitol and 3 mM Mes-KOH, pH 5.8), and 3-mm strips were cut with a razor blade, washed, added to 15 mL of incubation medium plus 1% (w/v) cellulase (Onuzuka R10) and 0.1% (w/v) macerozyme (Serva, Heidelberg, Germany), placed in a desiccator, evacuated five times, incubated for 4 to 6 hr at 30°C, shaken gently, filtered through Miracloth (Calbiochem, La Jolla, CA), and centrifuged for 5 min at 40g. The pellet was gently resuspended in 2 mL of medium A (0.33 M sorbitol, 2 mM NaNO3, 2 mM Na-EDTA, 1 mM MgCl2, 1 mM MnCl2, 0.5 mM KH2PO4, 2 mM Na-ascorbate, 20 mM NaCl, and 50 mM Hepes-NaOH, pH 6.7) and recentrifuged for 5 min at 40g. The sediment was resuspended in 2 mL of medium A and passed through a 14-μm-mesh nylon net to rupture the protoplasts; 1.8 mL of the filtrate was applied to the top of a sucrose density gradient consisting of a 1-mL 60% sucrose cushion, a 3-mL linear gradient from 60 to 42% sucrose, a 1-mL 42% sucrose plateau, and a 3-mL linear gradient from 42 to 30% sucrose, all in medium A. This material was centrifuged in a swing-out rotor at 10,000 rpm for 10 min, and 0.5-mL fractions were collected. Recovery of TK, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and pyrophosphate:fructose-6-phosphate phosphotransferase (PFP) was 92, 96, and 89% of the applied activity, respectively.
Determination of Chlorophyll, Carotene, Protein, and Enzyme Activities
Frozen leaf powder (∼20 mg fresh weight) was extracted (Haake et al., 1998), and ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), phosphoglycerate kinase, NADP-GAPDH, aldolase, plastid fructose-1,6-bisphosphatase (FBPase), TK, phosphoribulokinase (PRK), UGPase (Haake et al., 1998, 1999), and PFP (Hajirezaei et al., 1994) were determined immediately using optimized assays to detect maximum activity. Protein was determined according to Bradford (1976). Aliquots (100 μL) of extract were diluted to 80% (v/v) acetate to measure chlorophyll a, chlorophyll b, and total carotenoids (Porra et al., 1989). Carotenoids were analyzed by reversed phase HPLC (Flachmann, 1997).
Gas Exchange and Chlorophyll Fluorescence
Photosynthesis and transpiration were measured at ambient CO2 at 170, 220, 350, and 700 μmol photons m−2 sec−1 by using a CQP-130 porometer (Walz, Effeltrich, Germany). Measurements were made in the greenhouse on an overcast day with supplementary illumination from a diaprojector with neutral gray filters (Schott, Mainz, Germany). The intensity was determined in a measuring cuvette. Data were calculated according to von Caemmerer and Farquhar (1981). CO2-saturated photosynthesis and chlorophyll were measured as described by Quick et al. (1991). The ratio of variable to maximum fluorescence (Fv/Fm) was determined after 45 min of dark adaptation, and photochemical and nonphotochemical quenching were determined after 15 min of illumination. Chlorophyll fluorescence quenching parameters were calculated as described by von Willert et al. (1995).
Extraction and Analysis of Carbohydrates, Phosphorylated Intermediates, Nucleotides, and Amino Acids
Sugars and starch were determined in the soluble and residual fractions of an ethanol-water extract (Jelitto et al., 1992). Phosphorylated intermediates and nucleotides were extracted in trichloroacetic acid (Jelitto et al., 1992) and measured as described by Stitt et al. (1989). Total amino acids, Phe, Tyr, and Trp were determined by fluorescence after derivatization with orthophthaldialdehyde and separation by HPLC (Geiger et al., 1998). Amino acids were identified by cochromatography with authentic standards and quantified by comparison with internal standards. The reliability of the extraction and analytic techniques with tobacco leaves has been documented previously (Jelitto et al., 1992; Hajirezaei et al., 1994; Geiger et al., 1998).
Extraction and Determination of Phenols and Lignin
Soluble and cell wall–bound phenols were extracted as described by Yao et al. (1995). Phenols were analyzed using reversed phase chromatography on a 300 × 4-mm Vertex column (Nucleosid 100 C18; particle diameter of 3 μm; Knauer GmBH, Berlin, Germany) preceded by a short 40 × 5-mm precolumn (B5Y75, filled with Nucleosid 110). Soluble phenols were eluted with 30% acetonitrile (9 min at 0.6 mL/sec), followed by a linear gradient from 30 to 80% acetonitrile (3 min at 0.8 mL/sec), and 80% acetonitrile (5 min at 0.8 mL/sec). Cell wall–bound phenols (see above) were eluted with 30% acetonitrile (6 min), followed by a linear gradient from 30 to 75% acetonitrile (14 min), a linear gradient from 75 to 80% acetonitrile (9 min), and a 2-min wash with 80% acetonitrile, all at a flow rate of 0.6 mL/min. Phenolics were detected using a two-channel UV light detector (272 and 300 nm) and evaluated by comparing their retention times and A272:A300 ratios with a standard mix. To determine the reliability of the extraction and analysis, we added small amounts of phenols to the plant material before extraction was commenced. Recovery was >90% for chlorogenic acid (CGA), cinnamic acid, caffeic acid, and hydroxycinnamic acids. Lignin was extracted and determined in thioglycollate (Lange et al., 1995).
Extraction and Analysis of Tocopherol
Powdered frozen plant material (1 to 200 mg) was extracted (30 min at 50°C) in a fivefold excess of 80% (v/v) ethanol, 10 mM Hepes-NaOH, pH 7.0, and 1 mM Na-ascorbate and centrifuged. The supernatant was shaken vigorously with an equal volume of n-hexane and allowed to stand until the ethanol and hexane phases separated; the ethanol phase was reextracted with n-hexane, and the hexane phases were combined, lyophilized, stored as a powder at −20°C, and taken up in 100 μL of n-hexane immediately before reversed phase chromatography, as described for phenolic components, except that it was eluted with isocratic 100% n-hexane/0.2% 2-propanol and detected by fluorescence (excitation, 295 nm; emission, 450 nm).
Microscopy
Sections were prepared by hand from stems of similar diameters from wild-type plants and transformants that had been fixed in formalin:glacial acetic acid:ethanol:water (5:5:56:34 [v/v]), incubated for 1 min with phloroglucinol and for 1 min in concentrated HCl, and photographed in a light microscope (Olympus BX-40, Hamburg, Germany). Alternatively, stem sections were embedded in 2-hydroxyethyl methacrylate (Igersheim, 1993), and 6-μm sections were cut in a microtome (model RM2445; Leica, Bensheim, Germany) and viewed (excitation, 390 to 420 nm) in a fluorescence microscope (Zeiss Axioscope, Jena, Germany). Exposure times were adjusted automatically to allow adequate resolution.
Incubations with Specifically Labeled 14C-Glucose
Plants were transferred to darkness, and the next day leaf discs (8 mm diameter) were cut from the lamella of the youngest fully expanded leaves, rinsed in water, transferred to Warburg flasks containing 1 mL of 20 mM phosphate buffer, pH 5.2, containing 1 mM glucose labeled with 1-14C-glucose, 2-14C-glucose, or 6-14C-glucose (Boehringer Mannheim, Ingelheim, Germany) (final specific activity, 1 μCi/μmol), and incubated at 20°C in the dark with gentle shaking. Released 14CO2 was trapped in 800 μL of 1% (v/v) KOH in the side arm, from which 100-μL samples were removed after 2 and 4 hr and analyzed with a scintillation counter.
Acknowledgments
We are grateful to Christiane Börnke and Andrea Knospe for excellent technical assistance, to Prof. D. Bartels for providing the resurrection plant TK cDNA (TKT10), to Dr. C. Erbar (Institute of Systematic Botany and Biogeography, University of Heidelberg) for aid in the analysis of lignin immunofluorescence, to Christina Fritz for great assistance with the figures, and to an anonymous reviewer for perceptive comments about the implications of the results. This research was supported by BASF AG (Ludwigshafen, Germany) and the Bundesministerium für Forschung und Technologie.
References
- ap Rees, T. (1980). Assessment of the contributions of metabolic pathways to plant respiration. In The Biochemistry of Plants: A Comprehensive Treatise, Vol. 2, P.K. Stumpf and E.E. Conn, eds (London: Academic Press), pp. 1–29.
- Backhausen, J.E., Jostingmeyer, P., and Scheibe, R. (1997). Competitive inhibition of spinach leaf phosphoglucose isomerase isoenzymes by erythrose-4-phosphate. Plant Sci. 130, 121–131. [Google Scholar]
- Bate, N.J., Orr, J., Ni, W., Meromi, A., Nadler-Hassar, T., Doerner, P.W., Dixon, R.A., Lamb, C.J., and Elkind, Y. (1994). Quantitative relationship between phenylalanine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate-limiting step in natural product synthesis. Proc. Natl. Acad. Sci. USA 91, 7608–7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batz, O., Logemann, E., Reinold, S., and Hahlbrock, K. (1998). Extensive reprogramming of primary and secondary metabolism by fungal elicitor or infection in parsley cells. Biol. Chem. 379, 1127–1135. [DOI] [PubMed] [Google Scholar]
- Baucher, M., Bernard-Vailhe, M.A., Chabbert, B., Besle, J.M., Opsomer, C., and Van Montagu, M. (1999). Down-regulation of cinnamyl alcohol dehydrogenase in transgenic alfalfa and the effect on lignin composition and digestibility. Plant Mol. Biol. 39, 437–447. [DOI] [PubMed] [Google Scholar]
- Bernacchia, G., Schwall, G., Lottspeich, F., Salamini, F., and Bartels, D. (1995). The transketolase gene family of the resurrection plant Craterostigma plantagineum: Differential expression during the rehydration phase. EMBO J. 14, 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Vailhe, M.A., Besle, J.M., Mailot, M.P., Cornu, A., Halpin, C., and Knight, M. (1998). Effect of down-regulation of cinnamyl alcohol dehydrogenase on cell wall composition and on degradability of tobacco stems. J. Sci. Food Agric. 76, 505–514. [Google Scholar]
- Björkman, O., and Demmig, B. (1987). Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origins. Planta 170, 489–504. [DOI] [PubMed] [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein–dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Burrell, M.M., Mooney, P.J., Blundy, M., Carter, D., and Wilson, F. (1994). Genetic manipulation of 6-phosphofructokinase in potato tubers. Planta 194, 95–101. [Google Scholar]
- Chapple, C., and Carpita, N. (1998). Plant cell walls as targets for biotechnology. Curr. Opin. Plant Biol. 1, 179–185. [DOI] [PubMed] [Google Scholar]
- Deblaere, R., Bytebier, B., De Greve, H., Debroeck, F., Schell, J., Van Montagu, M., and Leemans, J. (1985). Efficient octopine Ti-plasmid derived vectors of Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 13, 4777–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam, P.M., and Emes, M.J. (1999). Subcellular distribution of enzymes of the oxidative pentose phosphate pathway in root and leaf tissues. J. Exp. Bot. 340, 1653–1661. [Google Scholar]
- Dennis, D.T. (1987). Energy Utilisation in Plants. (New York: Chapman and Hall).
- Dennis, D.T., Huang, Y., and Negm, F.B. (1997). Glycolysis, the pentose phosphate pathway and anaerobic respiration. In Plant Metabolism, D.T. Dennis, D.B. Layzell, D.D. Lefebvre, and D.H. Turpin, eds (Singapore: Longman Singapore Publishers), pp. 105–124.
- Dietz, K.-J., and Heber, U. (1986). Light and CO2 limitation of photosynthesis and states of reactions regenerating ribulose-1,5-bisphosphate or reducing glycerate-3-phosphate. Biochim. Biophys. Acta 848, 392–401. [Google Scholar]
- Dixon, R.A., and Paiva, N.L. (1995). Stress-induced phenylpropanoid metabolism. Plant Cell 7, 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell, D.A., and Thomas, S. (1995). Physiological control of metabolic flux: The requirement for multisite modulation. Biochem. J. 311, 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, K., Kämmerer, B., Gutensohn, M., Arbinger, B., Weber, A., Häusler, R.E., and Flügge, U.I. (1997). A new class of plastidic phosphate translocators: A putative link between primary and secondary metabolism by the phosphoenolpyruvate/phosphate antiporter. Plant Cell 9, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachmann, R. (1997). Composition of photosystem II antenna in light-harvesting complex II antisense tobacco plants at varying irradiances. Plant Physiol. 113, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechner, A., Dressen, U., Westhoff, P., Henze, K., Schnarrenberger, C., and Martin, W. (1996). Molecular characterization of transketolase (EC 2.2.1.1) active in the Calvin cycle of spinach chloroplasts. Plant Mol. Biol. 32, 475–484. [DOI] [PubMed] [Google Scholar]
- Flores, N., Xiao, J., Berry, A., Bolivar, F., and Valle, F. (1996). Pathway engineering for the production of aromatic compounds in Escherichia coli. Nat. Biotechnol. 14, 620–623. [DOI] [PubMed] [Google Scholar]
- Geiger, M., Walch-Liu, P., Engels, C., Harnecker, J., Schulze, E.-D., Ludewig, F., Sonnewald, U., Scheible, W.-R., and Stitt, M. (1998). Enhanced carbon dioxide leads to a modified diurnal rhythm of nitrate reductase activity in older plants, and a large stimulation of nitrate reductase activity and higher levels of amino acids in young tobacco plants. Plant Cell Environ. 21, 253–268. [Google Scholar]
- Gerhardt, R., Stitt, M., and Heldt, H.W. (1987). Subcellular metabolite levels in spinach leaves: Regulation of sucrose synthesis during diurnal alterations in photosynthetic partitioning. Plant Physiol. 83, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassgen, W.E., Rose, A., Madlung, J., Koch, W., Gleitz, J., and Seitz, H.U. (1998). Regulation of enzymes involved in anthrocyanin biosynthesis in carrot cell cultures in response to treatment with ultraviolet light and fungal elicitors. Planta 204, 490–498. [DOI] [PubMed] [Google Scholar]
- Gosset, G., Yong-Xiao, J., and Berry, A. (1996). A direct comparison of approaches for increasing carbon flow to aromatic biosynthesis in Escherichia coli. J. Ind. Microbiol. 17, 47–52. [DOI] [PubMed] [Google Scholar]
- Gottlob-McHugh, S.G., Sangwan, R.S., Blakeley, S.D., Vanleberghe, G.C., Ko, K.C., Turpin, D.H., Plaxton, W.C., Miki, B.L., and Dennis, D.T. (1992). Normal growth of transgenic tobacco plants in the absence of cytosolic pyruvate kinase. Plant Physiol. 100, 820–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, J.C., Paul, M.J., Barnes, S.A., Knight, J.S., Loynes, A., Habash, D., Parry, M.A.J., and Lawlor, D.W. (1995). Manipulation of phosphoribulokinase and phosphate translocator activities in transgenic tobacco plants. J. Exp. Bot. 46, 1309–1315. [Google Scholar]
- Grotewald, E., Chamberlin, M., Snook, M., Siame, B., Butler, L., Swenson, J., Maddock, S., St. Clair, G., and Bevan, B. (1998). Engineering secondary metabolism in maize cells by ectopic expression of transcription factors. Plant Cell 10, 721–740. [PMC free article] [PubMed] [Google Scholar]
- Haake, V., Zrenner, R., Sonnewald, U., and Stitt, M. (1998). A moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the levels of sugars and starch, and inhibits growth of potato plants. Plant J. 14, 147–157. [DOI] [PubMed] [Google Scholar]
- Haake, V., Geiger, M., Walch-Liu, P., Engels, C., Zrenner, R., and Stitt, M. (1999). Changes in aldolase activity in wild-type potato plants are important for acclimation to growth irradiance and carbon dioxide concentration, because plastid aldolase exerts control over the ambient rate of photosynthesis across a range of growth conditions. Plant J. 17, 479–489. [Google Scholar]
- Hajirezaei, M., Sonnewald, U., Viola, R., Carlisle, S., Dennis, D.T., and Stitt, M. (1994). Transgenic potato plants with strongly decreased expression of pyrophosphate:fructose-6-phosphate phosphotransferase show no visible phenotype and only minor changes in metabolic fluxes in their tubers. Planta 192, 16–30. [Google Scholar]
- Harrison, E.P., Willingham, N.M., Lloyd, J.C., and Raines, C.A.. (1998). Reduced sedoheptulose-1,7-bisphosphatase levels in transgenic tobacco lead to decreased photosynthetic capacity and altered carbohydrate accumulation. Planta 204, 27–36. [Google Scholar]
- Hirschberg, J. (1999). Production of high-value compounds: Carotenoids and vitamin E. Curr. Opin. Biotechnol. 10, 186–191. [DOI] [PubMed] [Google Scholar]
- Höfgen, R., and Willmitzer, L. (1988). Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 16, 9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfgen, R., and Willmitzer, L. (1990). Biochemical and genetic analysis of different patatin isoforms expressed in various organs of potato (Solanum tuberosum). Plant Sci. 66, 221–230. [Google Scholar]
- Höfgen, R., Axelsen, K.B., Kannangara, C.G., Schuettke, I., Pohlenz, H.D., Willmitzer, L., Grimm, B., and von Wettstein, D. (1994). A visible marker for antisense mRNA expression in plants: Inhibition of chlorophyll synthesis with a glutamate-1-semialdehyde aminotransferase antisense gene. Proc. Natl. Acad. Sci. USA 91, 1726–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, P., Ruban, A.V., and Walters, R.G. (1996). Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655–684. [DOI] [PubMed] [Google Scholar]
- Howles, P.A., Sewalt, V.J.H., Paiva, N.L., Elkind, Y., Bate, N.J., Lamb, C.J., and Dixon, R.A. (1996). Overexpression of l-phenylalanine ammonia lyase in transgenic tobacco plants reveals control points for flux into phenylpropanoid biosynthesis. Plant Physiol. 112, 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igersheim, A. (1993). The character states of the Caribbean monotypic endemic Strumpfia Jacq. (Rubiaceae), including a discussion of its taxonomic position. Nord. J. Bot. 13, 545–559. [Google Scholar]
- Jelitto, T., Sonnewald, U., Willmitzer, L., Hajirezeai, M., and Stitt, M. (1992). Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta 188, 238–244. [DOI] [PubMed] [Google Scholar]
- Jensen, R.A. (1985). Shikimate/arogenate pathway: Link between carbohydrate metabolism and secondary metabolism. Physiol. Plant. 66, 164–168. [Google Scholar]
- Juhnke, H., Krems, B., Kötter, P., and Entian, K.-D. (1996). Mutants that show increased sensitivity to hydrogen peroxide reveal an important role for the oxidative pentose phosphate pathway in protection of yeast against oxidative stress. Mol. Gen. Genet. 252, 456–464. [DOI] [PubMed] [Google Scholar]
- Kacser, H., and Burns, J. (1973). The control of flux. Symp. Soc. Exp. Biol. 27, 65–107. [PubMed] [Google Scholar]
- Kelly, G., and Latzko, E. (1980). Oat Avena sativa leaf phosphoglucose isomerase: Competitive inhibition by erythrose-4-phosphate. Photosynth. Res. 3, 181–188. [DOI] [PubMed] [Google Scholar]
- Koopman, E., Logemann, E., and Hahlbrock, K. (1999). Regulation and functional expression of cinnamate 4-hydroxylase from parsley. Plant Physiol. 119, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossmann, J., Sonnewald, U., and Willmitzer, L. (1994). Reduction of the chloroplastic fructose-1,6-bisphosphatase in transgenic potato plants impairs photosynthesis and plant growth. Plant J. 6, 637–650. [Google Scholar]
- Krapp, A., Hofmann, B., Schäfer, C., and Stitt, M. (1993). Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: A mechanism for the sink regulation of photosynthesis? Plant J. 3, 817–828. [Google Scholar]
- Landschütze, V., Willmitzer, L., and Müller-Röber, B. (1995). Inhibition of flower formation by antisense repression of mitochondrial citrate synthase in transgenic potato plants leads to a specific disintegration of the ovary tissues of flowers. EMBO J. 14, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, B.M., Lapierre, C., and Sandermann, H. (1995). Elicitor-induced spruce stress lignin. Plant Physiol. 108, 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D., Meyer, K., Chapple, C., and Douglas, C.J. (1997). Antisense suppression of 4-coumarate:coenzyme A ligase activity in Arabidopsis leads to altered lignin subunit composition. Plant Cell 9, 1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, N.G., and Yamamoto, E. (1990). Lignin: Occurrence, biogenesis and biodegradation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41, 455–496. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler, H.K. (1999). The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 47–65. [DOI] [PubMed] [Google Scholar]
- Logemann, E., Tavernarno, A., Schulz, A.W., Somssich, I., and Hahlbrock, K. (2000). UV light selectively coinduces supply pathways from primary metabolism and flavonoid secondary product formation in parsley. Proc. Natl. Acad. Sci. USA 97, 1903–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois, L.M., Campos, N., Putra, S.R., Danielsen, K., Rohmer, M., and Boronat, A. (1998). Cloning and characterization of a gene from Escherichia coli encoding a transketolase-like enzyme that catalyzes the synthesis of 1-deoxy-d-xylulose-5-phosphate, a common precursor for isoprenoid, thiamin and pyridoxol biosynthesis. Proc. Natl. Acad. Sci. USA 95, 2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel, M.A., Feldmann, K.A., Herrera-Estrela, L., Rocha-Sosa, M., and Leon, P. (1996). Cla1, a novel gene required for chloroplast development, is highly conserved in evolution. Plant J. 9, 649–658. [DOI] [PubMed] [Google Scholar]
- Newsholme, E.A., and Start, C. (1973). Regulation in Metabolism. (Chichester, UK: John Wiley).
- Patnaik, R., and Liao, J.C. (1994). Engineering of Escherichia coli central metabolism for aromatic metabolite production with near theoretical yield. Appl. Environ. Microbiol. 60, 3903–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, M.J., Knight, J.S., Habash, D., Parry, M.A.J., Lawlor, D.W., Barnes, S.A., Loynes, A., and Gray, J.C. (1995). Reduction in phosphoribulokinase activity by antisense RNA in transgenic tobacco: Effect on CO2 assimilation and growth in low irradiance. Plant J. 7, 535–542. [Google Scholar]
- Porra, R.J., Thompson, W.A., and Kriedemann, P.E. (1989). Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975, 384–394. [Google Scholar]
- Price, G.D., Evans, M.R., von Caemmerer, S., Yu, J.-W., and Badger, M.R. (1994). Specific reduction of chloroplast glyceraldehyde-3-phosphate dehydrogenase activity by antisense mRNA reduces CO2 assimilation via a reduction in RUBP regeneration in transgenic tobacco plants. Planta 193, 331–340. [DOI] [PubMed] [Google Scholar]
- Quick, W.P., Schurr, U., Scheibe, R., Schulze, E.D., Rodermel, S.R., Bogorad, L., and Stitt, M. (1991). Decreased ribulose-1,5-bisphosphate carboxylase/oxygenase in transgenic tobacco transformed with antisense rbcS. I. Impact on photosynthesis in ambient growth conditions. Planta 183, 542–554. [DOI] [PubMed] [Google Scholar]
- Rolleston, F.S. (1972). A theoretical background to the use of measured intermediates in the study of the control of intermediary metabolism. Curr. Top. Cell. Regul. 5, 47–75. [Google Scholar]
- Rosahl, S., Schell, J., and Willmitzer, L. (1989). Expression of a tuber-specific storage protein in transgenic tobacco plants: Demonstration of an esterase activity. EMBO J. 6, 1155–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, 2nd ed. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Sanger, F., Nicklen, S., and Coulsen, A.R. (1977). DNA-sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74, 5463–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible, W.R., Gonzalez-Fontes, A., Lauerer, M., Müller-Röber, B., Caboche, M., and Stitt, M. (1997). Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell 9, 783–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk, G., Duggleby, R.G., and Nixon, P.F. (1998). Properties and functions of the thiamin diphosphate dependent enzyme transketolase. Int. J. Biochem. Cell Biol. 30, 1297–1318. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger, C., Flechner, A., and Martin, W. (1995). Enzymatic evidence for a complete oxidative pentose phosphate pathway in chloroplasts and an incomplete pathway in the cytosol of spinach leaves. Plant Physiol. 108, 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewalt, V.J.H., Ni, W., Blount, J.W., Jung, H.G., Masoud, S.A., Howles, P.A., Lamb, C., and Dixon, R.A. (1997). Reduced lignin content and altered lignin composition in transgenic tobacco down-regulated in expression of l-phenylalanine ammonia-lyase or cinnamate 4-hydroxylase. Plant Physiol. 115, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slekar, K.H., Kosman, D.J., and Culotta, V.C. (1996). The yeast copper/zinc superoxide dismutase and the pentose phosphate pathway play overlapping roles in oxidative stress protection. J. Biol. Chem. 271, 28831–28836. [DOI] [PubMed] [Google Scholar]
- Small, J.R., and Kacser, H. (1993). Responses of metabolic systems to large changes in enzyme activities and effectors. I. The linear treatment of unbranched chains. Eur. J. Biochem. 213, 613–624. [DOI] [PubMed] [Google Scholar]
- Stitt, M. (1997). The flux of carbon between the chloroplast and cytosol. In Plant Metabolism, T.D. Dennis, D.B. Layzell, D.D. Lefebvre, and D.H. Turpin, eds (Singapore: Longman Singapore Publishers), pp. 382–400.
- Stitt, M. (1998). Pyrophosphate as an alternative energy donor in the cytosol of plant cells: An enigmatic alternative to ATP. Bot. Acta 111, 167–175. [Google Scholar]
- Stitt, M. (1999). The first will be last and the last will be first: Non-regulated enzymes call the tune? In Plant Carbohydrate Biochemistry, J.A. Burrell, M.M. Bryant, and N.J. Kruger, eds (Oxford, UK: BIOS Scientific Publishers), pp. 1–16.
- Stitt, M., and ap Rees, T. (1978). Pathways of carbohydrate oxidation in leaves of Pisum sativum and Triticum aestivum. Phytochemistry 17, 1251–1256. [Google Scholar]
- Stitt, M., and Schulze, E.-D. (1994). Does Rubisco control the rate of photosynthesis and plant growth? An exercise in molecular ecophysiology. Plant Cell Environ. 17, 465–487. [Google Scholar]
- Stitt, M., and Sonnewald, U. (1995). Regulation of metabolism in transgenic plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 341–368. [Google Scholar]
- Stitt, M., Wirtz, W., and Heldt, H.W. (1980). Metabolite levels in the chloroplast and extrachloroplast compartments of spinach protoplasts. Biochim. Biophys. Acta 593, 85–102. [DOI] [PubMed] [Google Scholar]
- Stitt, M., Lilley, R.M., Gerhardt, R., and Heldt, H.W. (1989). Determination of metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol. 174, 518–552. [Google Scholar]
- Streatfield, S.J., Weber, A., Kinsman, E.A., Häusler, R.E., Li, J., Post-Beittenmiller, D., Kaiser, W.M., Pyke, K.A., Flügge, U.I., and Chory, J. (1999). The phosphoenolpyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development, and plastid-dependent nuclear gene expression. Plant Cell 11, 1609–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone, L., Merida, A., Parr, A., Mackay, S., Culianez-Macia, F., Roberts, K., and Martin, C. (1998). The AmMYB308 and AmMYB330 transcription factors from Antirrhinum regulate phenylpropanoid and lignin biosynthesis in transgenic tobacco. Plant Cell 10, 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige, M., Kopriva, S., Bauwe, H., and Süss, K.-H. (1995). Chloroplast ribulose-5-phosphate 3-epimerase from potato: Cloning, cDNA sequence, and tissue-specific enzyme accumulation. FEBS Lett. 377, 349–352. [DOI] [PubMed] [Google Scholar]
- Vervliet, G., Holsters, M., Teuchy, H., Van Montagu, M., and Schell, J. (1975). Characterization of different plaque-forming and defective temperate phages in Agrobacterium strains. J. Gen. Virol. 26, 33–48. [DOI] [PubMed] [Google Scholar]
- von Caemmerer, S., and Farquhar, G.D. (1981). Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387. [DOI] [PubMed] [Google Scholar]
- von Willert, D.J., Matyssek, R., and Herppich, W. (1995). Experimentelle Pflanzenökologie: Grundlagen und Anwendungen. (Stuttgart, Germany: Thieme Verlag).
- Yao, K., de Luca, V., and Brisson, N. (1995). Creation of a metabolic sink for tryptophan alters the phenylpropanoid pathway and the susceptibility of potato to Phytophthora infestans. Plant Cell 7, 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]