Abstract
Plant lipoxygenases (LOXs) are a functionally diverse class of dioxygenases implicated in physiological processes such as growth, senescence, and stress-related responses. LOXs incorporate oxygen into their fatty acid substrates and produce hydroperoxide fatty acids that are precursors of jasmonic acid and related compounds. Here, we report the involvement of the tuber-associated LOXs, designated the Lox1 class, in the control of tuber growth. RNA hybridization analysis showed that the accumulation of Lox1 class transcripts was restricted to developing tubers, stolons, and roots and that mRNA accumulation correlated positively with tuber initiation and growth. In situ hybridization showed that Lox1 class transcripts accumulated in the apical and subapical regions of the newly formed tuber, specifically in the vascular tissue of the perimedullary region, the site of the most active cell growth during tuber enlargement. Suppression mutants produced by expressing antisense coding sequence of a specific tuber LOX, designated POTLX-1, exhibited a significant reduction in LOX activity in stolons and tubers. The suppression of LOX activity correlated with reduced tuber yield, decreased average tuber size, and a disruption of tuber formation. Our results indicate that the pathway initiated by the expression of the Lox1 class genes of potato is involved in the regulation of tuber enlargement.
INTRODUCTION
Tuber formation in potatoes is a complex developmental process that requires the interaction of environmental, biochemical, and genetic factors. Several important biological processes, such as carbon partitioning, signal transduction, and meristem determination are involved (Ewing and Struik, 1992). Under conditions of a short-day photoperiod and cool temperature, a transmissible signal is activated that initiates cell division and expansion and a change in the orientation of cell growth in the subapical region of the stolon tip (Ewing and Struik, 1992; Xu et al., 1998a). In this signal transduction pathway, perception of the appropriate environmental cues occurs in leaves and is mediated by phytochrome and gibberellins (van den Berg et al., 1995; Jackson and Prat, 1996; Jackson et al., 1996). van den Berg et al. (1996) detected at least 10 quanitative trait loci that control the ability to tuberize under long days, but none of these genes has been identified definitively.
Tuber development at the stolon tip consists of biochemical and morphological processes. Both processes are controlled by differential gene expression (Hannapel, 1991; Bachem et al., 1996; Macleod et al., 1999), and most of the research in this area has focused on the biochemical processes, including starch synthesis (Abel et al., 1996; Preiss, 1996; Geigenberger et al., 1998) and storage protein accumulation (Mignery et al., 1984; Hendriks et al., 1991; Suh et al., 1991). Much less is known about the morphological controls of tuberization, although it is clear that phytohormones play a prominent role (Koda et al., 1991; Xu et al., 1998b; Sergeeva et al., 2000). Several genes that are expressed during early tuber formation have been identified, including tubulins (Taylor et al., 1991), S-adenosylmethionine decarboxylase (Taylor et al., 1992), MADS box genes (Kang and Hannapel, 1996), acyl carrier protein thioesterase (Macleod et al., 1999), and lipoxygenases (LOXs; Kolomiets et al., 1996a; Royo et al., 1996). Despite considerable work on the physiology of tuber development, researchers have not identified the molecular mechanisms that control the changes in cell growth during tuberization.
Plant LOXs (EC 1.13.11.12) are dioxygenases that catalyze the oxygenation of polyunsaturated fatty acids such as linolenic and linoleic acids. Distinct LOX isozymes preferentially introduce molecular oxygen into either the C-9 or the C-13 position of linoleic and linolenic acids (Siedow, 1991). Subsequently, two distinct fatty acid monohydroperoxides are formed and directed into separate biosynthetic pathways that result in the accumulation of compounds with distinct physiological functions (Anderson, 1989; Siedow, 1991). The 13-monohydroperoxides are precursors of biologically active compounds such as traumatin, jasmonic acid, and methyl jasmonate, which serve hormonelike regulatory and defense-related roles in plants (Siedow, 1991). The 9-monohydroperoxides are converted into compounds whose physiological functions are not known, although there is evidence that some of them have antimicrobial properties (Vaughn and Gardner, 1993; Ricker and Bostock, 1994; Rusterucci et al., 1999).
Several lines of correlative evidence suggest that LOXs function in the regulation of plant growth and development. LOX isozyme profiles change quantitatively and qualitatively during soybean leaf development (Saravitz and Siedow, 1995) and during seed germination in cucumbers (Matsui et al., 1992; Feussner et al., 1996). In addition, many LOX genes are regulated differentially during Arabidopsis seedling development (Melan et al., 1994), pea nodule formation (Wisniewski et al., 1999), tomato fruit ripening (Ferrie et al., 1994; Kausch and Handa, 1997), potato tuber development (Bachem et al., 1996), and pea carpel development (Rodriguez-Concepcion and Beltran, 1995). LOX-derived products such as jasmonic acid, methyl jasmonate, and tuberonic acid may play an important role in potato tuberization. These hormonelike compounds have strong tuber-inducing activity in vitro (Pelacho and Mingo-Castel, 1991; Koda, 1992), and they may function by regulating the reorientation of microtubules that allows radial cell expansion leading to tuber enlargement (Matsuki et al., 1992; Fujino et al., 1995; Takahashi et al., 1995).
Potato LOXs are encoded by a large multigene family. Several LOX cDNAs have been isolated from potato tubers, roots, and leaves (Geerts et al., 1994; Casey, 1995; Kolomiets et al., 1996a, 1996b; Royo et al., 1996; Fidantsef and Bostock, 1998). Royo et al. (1996) proposed that potato LOX genes be divided into three classes based on their deduced amino acid sequences and their patterns of expression. Lox1 genes were expressed in tubers and roots, Lox2 genes were expressed in leaves only, and Lox3 genes were expressed in leaves and roots. LOX expression has been detected in developing tubers, and several groups have proposed that LOXs are involved in potato tuber growth (Bachem et al., 1996; Kolomiets et al., 1996a; Royo et al., 1996), but until now, there were no reports that demonstrated this involvement.
The disruption of tuber development reported here by suppression mutants of tuber-associated LOXs suggests that hydroperoxide fatty acid products (and not necessar-ily 13-hydroperoxide fatty acid products exclusively; see Hamberg, 2000) produced via this LOX pathway contribute to the control of tuber morphogenesis at the primary site of cell growth, the stolon tip. Although LOXs are known to function in diverse physiological processes, this study offers strong supporting evidence for the involvement of potato LOXs in the regulation of tuber development.
RESULTS
Sequence Analysis of POTLX-1
The tuber LOX cDNA used in this study for the antisense construct and as a probe for RNA hybridization, designated POTLX-1, was isolated from an early-stage tuber cDNA library (Kolomiets et al., 1996a). Sequence analysis revealed that this full-length clone had the greatest sequence identity to 9-type LOXs from potato tuber. The coding sequence of POTLX-1 (GenBank accession number U60200) demonstrates a >96% nucleotide identity with all of the known LOXs expressed in tubers. Most of the tuber LOX genes have a nucleotide identity of >98%. POTLX-1 has an 80.6% identity with the 9-type leaf LOX of potato, POTLX-3 (Kolomiets et al., 1996b), and 60.8 and 59% identities with the 13-LOXs of potato leaf, H1 and H3, respectively. Like other 9-LOXs of plants, POTLX-1 contains no transit peptide for chloroplast targeting and has the conserved space-saving amino acid pairing at positions 579 to 580 (Hornung et al., 1999). Protein sequence analysis of POTLX-1 revealed amino acid sequence motifs that are highly conserved among plant and mammalian LOXs. The predicted peptide of POTLX-1 contained the conserved 39–amino acid motif essential for enzyme activity, the three conserved His residues and one Ile residue implicated in iron binding, and the 11 highly conserved amino acids that constitute a cavity that accommodates the fatty acid substrate (Steczko et al., 1992; Boyington et al., 1993).
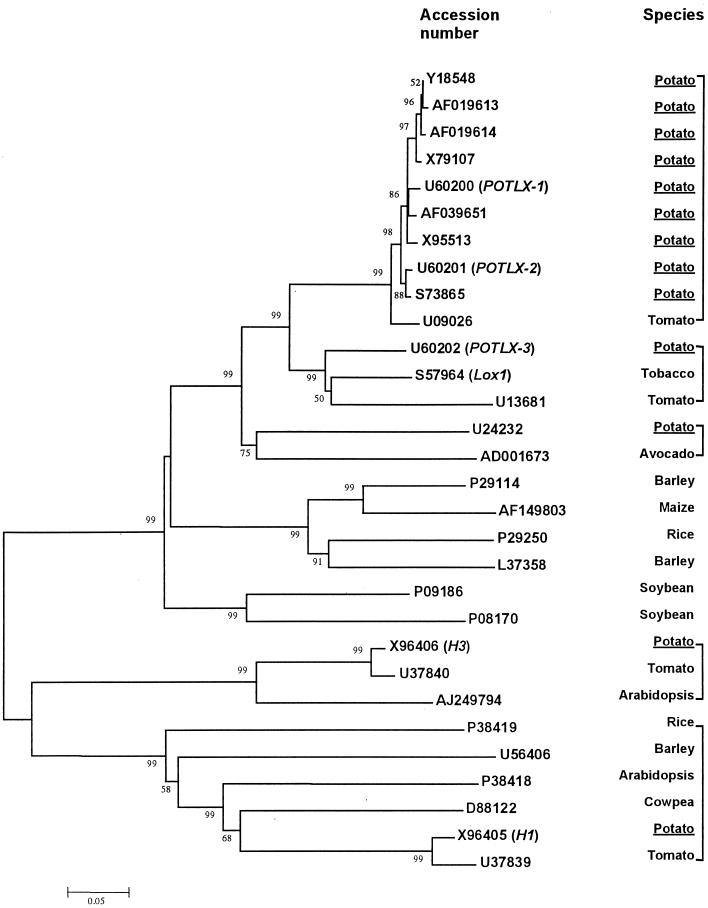
For classification, the amino acid sequences of known potato LOXs were analyzed and compared with other related LOXs of plants using the ME-Boot program of the MEGA package (Kumar et al., 1993) and the neighbor-joining program (Saitou and Nei, 1987). This phylogenetic analysis (Figure 1) showed that there are at least four classes of potato LOXs, the three identified by Royo et al. (1996) and the pathogen-induced type of leaf LOX, POTLX-3 (Kolomiets et al., 2000). POTLX-1 is grouped within the closely related cluster of tuber LOXs. As shown by boot-strapping values, the tuber LOXs are more closely related to POTLX-3 than they are to the leaf LOXs, H1 and H3. Within the tuber types, there is separation of two groups at a 98% level of significance. One group is represented by a wound-inducible LOX, STLOX1 (Geerts et al., 1994; GenBank accession number S73865) and POTLX-2 (Kolomiets et al., 1996a; GenBank accession number U60201). The other group includes the remaining tuber LOXs that have been identifed, represented by POTLX-1 in this study.
Figure 1.
Phylogenetic Tree of LOXs of Potato.
The amino acid sequences of known potato LOXs were analyzed and compared with related plant LOXs. These data were organized into a phylogenetic tree with the ME-Boot program of the MEGA package (Kumar et al., 1993) and the neighbor-joining program (Saitou and Nei, 1987). The numbers listed at the branching points are boot-strapping values, which indicate the level of significance (%) for the separation of two branches. The length of the branch line indicates the extent of difference according to the scale at the bottom. This phylogenetic analysis shows that there are at least four classes of potato LOXs, the three identified by Royo et al. (1996) and the pathogen-induced type of leaf LOX, POTLX-3 (Kolomiets et al., 2000).
Pattern of Tuber LOX mRNA Accumulation
To study tuber LOX gene expression at the RNA level, RNA gel blot hybridization analysis was conducted using a probe from the POTLX-1 cDNA coding sequence. Because of the close sequence identity of POTLX-1 with the other tuber LOXs (Figure 1), the POTLX-1 probe hybridizes to mRNA from other related Lox1 class tuber genes. In cross-hybridization experiments (data not shown), the POTLX-1 probe used in this study hybridized to the full-length insert from the tuber LOX cDNA, POTLX-2, with a 96.9% sequence identity. All other known tuber LOXs have a nucleotide sequence identity >97%. Based on these results, the mRNA detected in the RNA gel blots and in situ studies is referred to as Lox1 class transcripts, in accordance with the classification scheme of Royo et al. (1996).
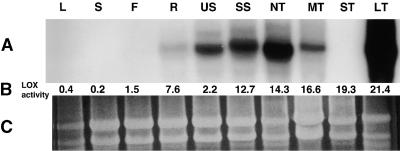
Lox1 class transcripts (∼2.8 kb in length) were detected only in underground organs (Figure 2A). The highest levels of mRNA occurred in actively growing tubers (Figure 2A, lanes NT and LT), whereas lower levels were detected in roots and nontuberizing stolons (Figure 2A, lanes R and US, respectively). The abundance of mRNA increased as tubers enlarged and reached maximum levels in large, actively growing tubers (Figure 2A, lane LT). Lox1 class mRNA accumulation decreased in mature tubers (Figure 2A, lane MT [dormant tubers stored at 21°C for 1 month]), and transcripts were not detected in mature tubers stored at 6°C for 2 months (Figure 2A, lane ST), consistent with the results of Geerts et al. (1994). Transcript accumulation was not detected in leaves, stems, or flowers (Figure 2A). LOX activity for these organs was greatest in developing and stored tubers (Figure 2B). Large, actively growing tubers had the greatest activity, whereas the lowest levels were detected in leaves, stems, and flowers. Apparently, the LOX proteins are relatively stable in some of these organs, as reflected in the high level of activity in roots, mature tubers, and stored tubers in proportion to transcript levels (Figures 2A and 2B). In addition, Lox1 class mRNA was not detected in wounded leaves or in leaves infected with the fungal pathogen Phytophthora infestans or treated with sucrose, abscisic acid, methyl jasmonate, ethylene, gibberellic acid, auxin (naphthaleneacetic acid), or cytokinin (benzyladenine) (data not shown).
Figure 2.
RNA Gel Blot Analysis of Lox1 Class mRNA and LOX Activity in Potato Organs.
Total RNA was extracted from leaves (L), stems (S), flowers (F), roots (R), unswollen stolons (US), swollen stolons (SS), and tubers in the following stages of tuber development: new tuber (NT), mature tubers stored at room temperature for 1 month (MT), mature tubers stored at 6°C for 2 months (ST), and large, actively growing tubers (⩾5 g fresh weight; LT). Equal amounts of total RNA (10 μg per lane) were subjected to electrophoresis, blotted onto a nylon membrane, and hybridized with the 32P-labeled 2.1-kb ClaI fragment of the POTLX-1 cDNA clone. Transcript size is ∼2.8 kb.
(A) Lox1 class mRNA.
(B) LOX activity.
(C) Equal loading of RNA samples in each lane was confirmed by visualizing the RNA with ethidium bromide and UV light.
LOX activity was determined as the consumption of oxygen (μmol sec−1 g−1 protein), using linolenic acid as a substrate and a Clark electrode for detection (Royo et al., 1996).
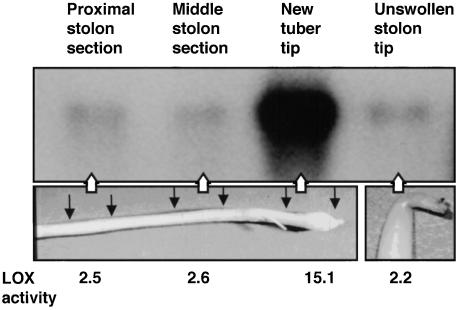
To determine in which part of a tuberizing stolon the induction of Lox1 class mRNA accumulation occurred, we isolated poly(A)+ RNA from different sections of new tubers and unswollen stolon tips. LOX activity was also assayed in these same organs. The greatest amounts of Lox1 class transcripts and LOX activity were detected in the tips of new tubers (Figure 3). Lower levels of transcript accumulation and activity were detected in proximal and middle stolon sections and in unswollen stolon tips until they were induced to tuberize.
Figure 3.
LOX Activity and RNA Gel Blot Analysis of Lox1 Class Transcripts in Stolons and New Tubers.
To determine the location of the greatest Lox1 class gene expression in developing tubers, we extracted poly(A)+ RNA from three stolon regions of developing new tubers and from unswollen stolon tips. Proximal stolon sections, middle stolon sections, new tuber tips (each ∼1 cm long), and unswollen stolon tips were used. Two micrograms of poly(A)+ RNA was loaded in each lane. Hybridization was conducted with the 32P-labeled 2.1-kb ClaI fragment of the POTLX-1 cDNA clone. Note that expression of the Lox1 class genes in the unswollen tip was approximately the same as that in other parts of the stolon until tuberization was induced (new tuber tip). Magnification is ×1.6 for the photograph of the tuberizing stolon and ×4.2 for the photograph of the unswollen stolon tip. LOX activity was determined as the consumption of oxygen (μmol sec−1 g−1 protein) using linolenic acid as a substrate and a Clark electrode for detection (Royo et al., 1996).
Localization of Lox1 Class Transcripts in a Developing Tuber
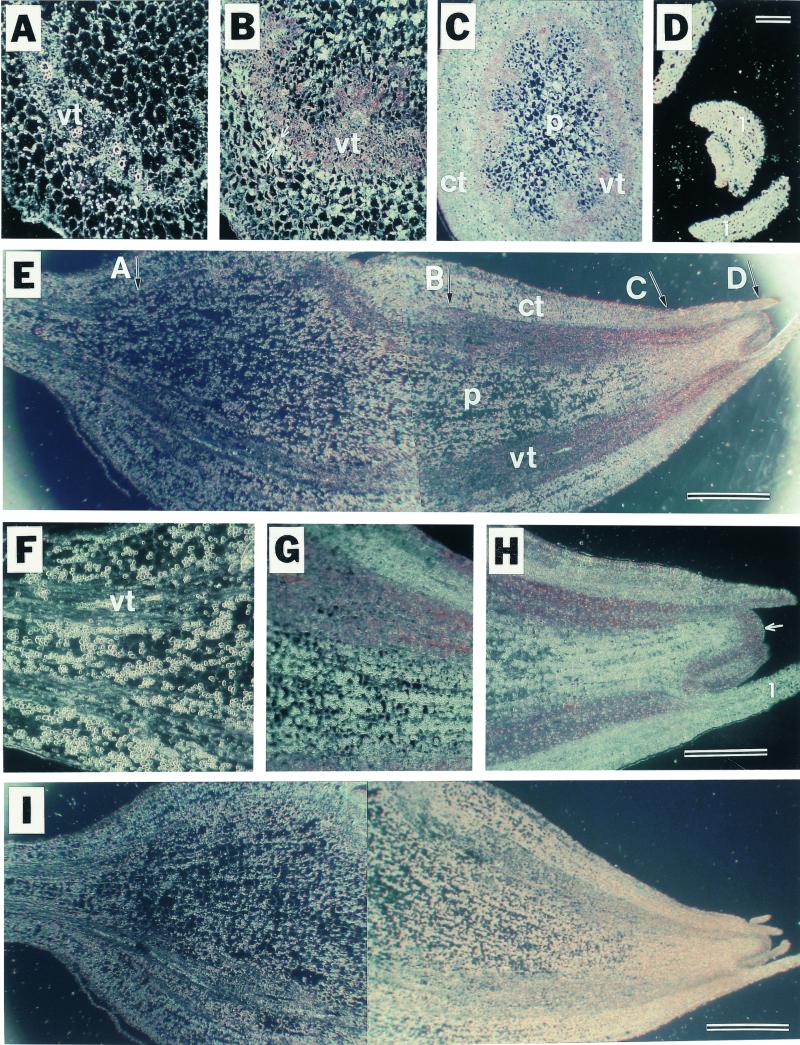
In situ hybridization was used to determine the exact location of Lox1 class mRNA accumulation in developing tubers. These localization studies were conducted on new tubers because Lox1 class mRNA is most abundant at this stage of development (Figure 3), and the organ is small enough (∼2.0 mm in diameter) to allow the use of a light microscope. Transverse (Figures 4A to 4D) and longitudinal (Figures 4E to 4H) sections through new tubers were hybridized to antisense digoxigenin-labeled POTLX-1 riboprobes. An orange-brown stain indicated a positive hybridization signal. A comparison of Figures 4A through 4D and 4F through 4H with Figure 4E clearly demonstrates that accumulation of Lox1 class transcripts occurred in the distal portion of the new tuber. Lox1 class mRNA was not detected in the proximal portion of the new tuber adjacent to the attached stolon (Figures 4A, 4E, and 4F). Figures 4E, 4G, and 4H show that mRNA accumulation was most abundant in vascular bundles and in the apical dome of the new tuber. No hybridization signal was apparent in the pith (Figures 4B, 4C, 4G, and 4H) or the lamina of primary leaves (Figure 4D). Transverse sections through the new tuber hybridized to the POTLX-1 antisense probe confirmed the location of Lox1 class transcripts in vascular bundles of the most actively growing, distal portion of the new tuber (Figures 4B and 4C). Lox1 class mRNA was not detectable in the endodermis (Figure 4B, arrows), a distinct cell layer that is present only at the early stages of tuber development (Cutter, 1978). Figure 4I shows a longitudinal section (corresponding to Figure 4E) incubated with the sense POTLX-1 probe used as a negative control. There was no background hybridization signal observed in this section. Similar results were observed consistently by using the sense probe with all other tissue sections shown here.
Figure 4.
In Situ Localization of Lox1 Class Transcripts in Developing Tubers.
Thin sections (8 μm) through a new tuber were hybridized with digoxigenin-labeled RNA probes that were synthesized from a full-size POTLX-1 cDNA in either the sense or the antisense orientation. Accumulation of Lox1 class mRNA bound to the antisense digoxigenin-labeled probe was visualized by alkaline phosphatase activity and is seen as an orange-brown stain on the dark-field micrographs. ct, cortex; l, newly formed leaf; p, pith; vt, vascular tissue.
(A) to (D) Transverse sections through a new tuber were hybridized with POTLX-1 antisense riboprobe. The approximate locations of the transverse sections correspond to the labeled arrows in (E). Hybridization signals were detected in (B) and (C) but not in (A) and (D). The arrows in (B) indicate endodermis.  (A)
(A)  (D).
(D).
(E) A longitudinal section through a new tuber was hybridized with POTLX-1 antisense probe. 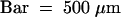 .
.
(F) Close-up micrograph of the longitudinal section hybridized to the antisense probe shown in (E). The photographed area covers the junction of the stolon and the proximal portion of the new tuber. Note that the hybridization signal is not detected in this portion of the new tuber.
(G) Close-up micrograph of the longitudinal section hybridized to the antisense probe shown in (E). The photographed area covers the subapical portion of the new tuber.
(H) Close-up micrograph of the longitudinal section hybridized to the antisense probe shown in (E). The photographed area covers the apical region of the new tuber. The arrow indicates the apical dome in which Lox1 class transcripts accumulate to high levels. 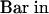 (H)
(H) 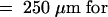 (F)
(F) 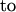 (H).
(H).
(I) In situ hybridization negative control. A longitudinal section through a new tuber was hybridized with POTLX-1 sense probe. No hybridization signal was detected. 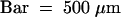 .
.
Antisense Suppression Mutants of POTLX-1 Exhibited Reduced Lox1 Class Transcript Levels and Reduced LOX Activity in Tubers and Stolons
We analyzed transformed potato plants (cv FL1607) that were downregulated for Lox1 class mRNA accumulation to examine the function of the tuber LOXs. For these experiments, we used a 1076-bp fragment of the coding sequence of POTLX-1 in an antisense orientation driven by the cauliflower mosaic virus (CaMV) 35S promoter in the binary vector pCB201 (Xiang et al., 1999). This 1076-bp sequence was compared with related sequences using a FASTA program and had a ⩾98% nucleotide sequence identity with the other known tuber LOXs. Because of this high level of match, we assumed that the POTLX-1 antisense transcripts were capable of suppressing the expression of the other known tuber LOXs. Our LOX activity assays corroborated this assumption. Using cDNA-specific primers and reverse transcription–polymerase chain reaction (PCR), levels of both POTLX-1 and POTLX-2 (98% nucleotide identity with the antisense sequence) transcripts were shown to be reduced in tubers of suppression mutant 11 relative to controls (data not shown). We even detected a three- to fourfold reduction of POTLX-3 mRNA (83.8% nucleotide identity with the antisense sequence) accumulation in leaves of POTLX-1 suppression mutants (data not shown).
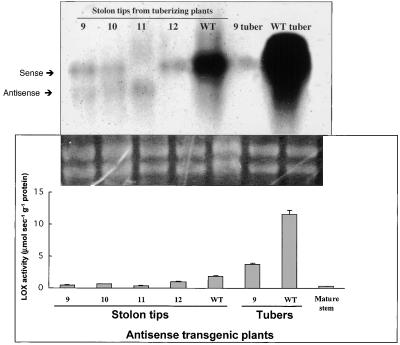
Functional transformants were identified by PCR analysis of genomic DNA and by detection of the accumulation of antisense transcripts of POTLX-1 in leaf RNA samples. From these positive transgenic plants, seven independent transformants out of ∼60 transgenic plants were selected for further analysis on the basis of reduced LOX activity in stolons or tubers. The highest expressers of POTLX-1 antisense transcripts (lines 9 to 11) had reduced sense mRNA levels and LOX activity in both stolons and tubers (Figure 5). The lower of the two RNA bands in Figure 5 (top) is the 1076-nucleotide antisense transcript. Transgenic line 11 consistently exhibited the greatest amount of antisense mRNA and the least amount of Lox1 class sense mRNA and LOX activity. These results show that suppression of Lox1 class mRNA accumulation is correlated with a reduction in LOX activity in the target organs (stolons and tubers).
Figure 5.
LOX Expression in Antisense Suppression Mutants.
Suppression mutants were produced by the introduction of antisense sequence from the LOX cDNA POTLX-1 under the control of the CaMV 35S promoter. Lines 9 to 12 exhibited high levels of sense transcript suppression (top) and reduced LOX activity (bottom). Samples for RNA and LOX activity were harvested at the same time. All four plants exhibited phenotypes characterized by a reduction in tuber yield and abnormal tuber morphology (see Results and Table 1). The lowest levels of LOX activity were detected in stolon tips from tuberizing plants of lines 9 and 11. Routine procedures were used for the RNA gel blot hybridization (top) by using 2.0 μg of total RNA per sample. The 2.8-kb insert for POTLX-1 was labeled with phosphorus-32 and used as the hybridization probe. The ethidium bromide–stained rRNA is shown as a loading control (middle). LOX activity (bottom) was determined as the consumption of oxygen (μmol sec−1 g−1 protein) using linolenic acid as a substrate and a Clark electrode for detection (Royo et al., 1996). WT, wild type.
Lox1 Class Suppression Mutants Exhibited Specific Changes in Tuber Development
Seven independent transgenic lines (9 to 12, 18, 33, and 36) with reduced LOX activity were evaluated for tuber development under long-day conditions. Average tuber yields from these primary suppression transformants were reduced almost threefold and average tuber size was reduced fourfold, whereas LOX activity was reduced 2.5-fold (Table 1, experiment 1). Yield data were evaluated a second time using vegetative clones from lines 11 and 36, representing strongly and moderately suppressed mutants, respectively. Tuber yield (tuber weight per plant) was reduced ∼20-fold and fourfold, respectively, in this second experiment, whereas average tuber size was reduced 64-fold and 13-fold (Table 1, experiment 2). Tuber number per plant was increased approximately threefold in both of these mutants compared with controls. Concomitant with this reduction in tuber yield and size, LOX activity was reduced ∼16-fold in tubers harvested from line 11 and 2.5-fold in tubers harvested from line 36 (Table 1, experiment 2). Most of the small tubers (<1 g) from line 11 had a ⩾30-fold reduction in LOX activity (data not shown).
Table 1.
Tuber Yields and LOX Activity in Lox1 Class Suppression Mutantsa
| Experiment 1
|
Experiment 2
|
||||
|---|---|---|---|---|---|
| Parameters | Control | Suppression Mutants |
Control | Suppression Mutant 11 |
Suppression Mutant 36 |
| Tuber weight per plant (g) | 126 ± 10 | 45 ± 32 | 244 ± 75 | 11.7 ± 4.1 | 67.8 ± 8.1 |
| Number of tubers per plant | 9.7 ± 3.8 | 11 ± 6.1 | 6.1 ± 3.6 | 18.3 ± 7.1 | 19.2 ± 7.2 |
| Average weight per tuber (g) | 15.8 ± 8.8 | 3.9 ± 3.3 | 49 ± 29 | 0.76 ± 0.4 | 3.8 ± 0.9 |
| LOX activity in tubers (μmol sec−1 g−1 protein) | 13.0 ± 2.2 | 5.1 ± 4.4 | 25.0 ± 5.0 | 1.5 ± 1.1 | 10.3 ± 0.8 |
Yield per plant was determined by harvesting tubers from plants grown in the greenhouse 100 days after transplanting plantlets into soil. In experiment 1, plants were grown under natural light conditions from April to July, and the data are from seven independent control transgenic plants and seven independent Lox1 class suppression mutants (lines 9, 10, 11, 12, 18, 33, and 36). In experiment 2, plants were grown under natural light supplemented by irradiance from high-pressure sodium lamps. Data for the control, mutant 11, and mutant 36 were from seven replicate plants each. LOX activity is the mean of the average of two pooled samples per plant. Controls were transgenic plants transformed with the empty binary vector pCB201. se values are shown for all data.
Many tubers produced from the antisense mutants were small, knobby, and wrinkled, clearly representing abnormal development (Figure 6). These representative tubers from lines 18 and 33 had reduced LOX activity. The smaller tuber from line 33 (Figure 6, top left) had a 76% reduction in LOX activity compared with control tubers, and the larger tuber from line 18 (Figure 6, bottom left) had a reduction of 38% in LOX activity. The proximal end of the tuber (point of attachment to the stolon; top end in Figure 6) is relatively smooth in these mutant tubers. The knobbiness and irregular growth were most obvious on the distal or apical end of the tuber (Figure 6, left). During tuber initiation, this is the location of the early stolon/tuber meristem and the site of cell growth activity (Cutter, 1978; Peterson et al., 1985; Xu et al., 1998a) and the most abundant accumulation of Lox1 class mRNA (Figure 4). The phenotype of these mutant tubers suggests that the suppression of LOX activity at an early stage in these developing stolons and tubers disrupts normal morphology as the tuber enlarges. We observed no morphological changes in the vegetative shoots of any of the suppression mutants. We did, however, observe significant phenotypic changes in tuber sprout development. Under controlled conditions, tuber sprouts from suppression mutant line 11 were reduced approximately fivefold in both sprout length and fresh weight after 2 months of growth. Line 11 sprouts produced multiple truncated, branched shoots, whereas the control sprouts were unbranched and elongate. Tuber sprouts from line 11 had a threefold reduction in LOX activity relative to control sprouts (data not shown).
Figure 6.
Malformed Tubers and Controls.
Malformed tubers produced by suppression mutants of tuber-associated LOXs are shown at left. Suppression mutants were produced by the introduction of antisense sequence from the LOX cDNA POTLX-1 under the control of the CaMV 35S promoter. LOX activity was reduced relative to the controls shown at right in both of these antisense tubers. LOX activity in the tuber from line 18 (bottom left) was reduced 38% relative to controls, whereas LOX activity in the tuber from line 33 (top left) was reduced 76%. Tubers were harvested from potted plants 4 months after transplanting in the greenhouse. The proximal end of the tuber, where it attaches to the stolon, is oriented toward the top of the figure. A remnant stolon connection can be seen on the control tuber at bottom right. The control tubers were selected to be equivalent sizes to the mutant tubers to facilitate a visual morphological comparison. The sizes of the tubers in this figure are not representative of the reduction in average tuber size exhibited by the Lox1 class suppression mutants.
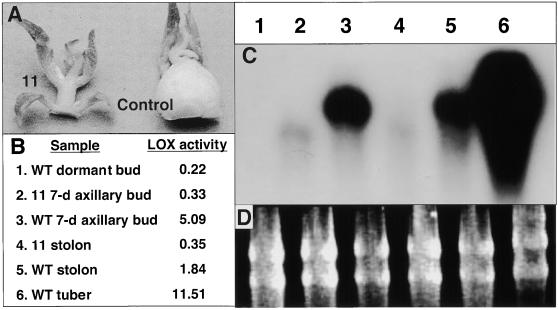
To examine the effect of suppressing Lox1 class expression on tuber formation under controlled conditions, we used a model single-node tuberization system (Ewing, 1985; Hannapel, 1991). When single-node leaf petiole stem cuttings taken from plants grown under short-day conditions are incubated in a perlite medium, the axillary buds grow out as small tubers. With similar cuttings taken from plants grown under long-day conditions, the axillary buds grow out as shoots (Paiva et al., 1982; Wheeler et al., 1988). This model system is uniform and synchronous and mimics the development of whole plant tubers (Paiva et al., 1982; Hannapel, 1991; Vreugdenhil et al., 1999). Using this model system in our study, axillary buds of cuttings from control plants grown under short-day conditions developed into tubers within 7 days (Figure 7A, right). As expected, LOX activity was high in these tubers (Figure 7B, sample 3). Axillary buds from cuttings taken from Lox1 class suppression mutant line 11 grown under short-day conditions grew out as vegetative shoots (Figure 7A, left), similar to axillary buds from long-day control plants, and had reduced LOX activity (Figure 7B, sample 2). Dormant axillary buds from a control whole plant had LOX activity of 0.22 μmol sec−1 g−1 protein. Again, Lox1 class mRNA levels were correlated closely with LOX activity in these organs. No Lox1 class mRNA was detected in the shoots produced from either short-day axillary buds of the suppression mutant (Figure 7C, lane 2) or control dormant axillary buds (Figure 7C, lane 1), whereas high levels were detected in tuberizing control buds (Figure 7C, lane 3).
Figure 7.
Tuber Development, LOX Activity, and mRNA Accumulation in Short-Day Axillary Buds of a LOX Suppression Mutant.
(A) Single-node leaf petiole cuttings taken from suppression mutant line 11 and control plants grown under a short-day (8-hr-light/16-hr-dark) photoperiod were incubated in perlite for 7 days in a growth chamber. Axillary buds from the suppression mutant grew out as shoots (left), whereas the control buds formed tubers (right).
(B) LOX activity was reduced in the axillary buds of the suppression mutant (sample 2) compared with controls (sample 3). WT, wild type.
(C) Lox1 class mRNA was also reduced in axillary buds of the suppression mutant (lane 2) compared with controls (lane 3). Lane 1 represents total RNA from dormant axillary buds harvested directly from whole control plants, lane 4 represents RNA from stolons from the suppression mutant, and lanes 5 and 6 represent stolons and tubers, respectively, from control plants. The faint hybridization signal detected in lanes 2 and 4 represents the antisense POTLX-1 transcript. Control plants were nontransgenic plants regenerated from leaf callus tissue. LOX activity (μmol sec−1 g−1 protein) was determined by measuring oxygen consumption in protein extracts from pooled samples by using a Clark electrode (Royo et al., 1996). Linolenic acid was added as a substrate.
(D) Ethidium bromide–stained rRNA is shown as a loading control.
The numbers in the activity table (B) correspond to the lane numbers in the RNA hybridization blot (C). Aliquots of plant material (10 to 20 harvested axillary buds per treatment) from the same pooled samples were used for both RNA and activity assays. Routine procedures were used for the RNA gel blot hybridization in (C), with 20 μg of total RNA loaded per sample. The 2.8-kb insert for POTLX-1 was labeled with phosphorus-32 and used as the hybridization probe.
Effect of a LOX Inhibitor on Tuber Growth in Vitro
Supportive evidence was obtained for the effect of reducing LOX activity on tuber development by using an inhibitor of LOX activity, naproxen, in an in vitro tuberization system. Naproxen is a nonsteroidal anti-inflammatory drug that was used previously to inhibit LOX activity in cultured soybean cells (Creelman et al., 1992). In a study comparing 18 nonsteroidal anti-inflammatory drugs, naproxen was the best inhibitor of purified soybean LOX, having a greater affinity for the enzyme than did the substrate (Sircar et al., 1983). In the present work, tubers were initiated after 3 weeks by both control and naproxen-treated axillary buds, but the tubers that formed did not develop at the same rate. At the end of 3 months in culture, tubers from treated axillary buds had grown to an average of only 12 mg, whereas control tubers averaged 112 mg (Table 2). LOX activity was sevenfold greater in control tubers than in LOX-inhibited tubers.
Table 2.
Effect of a LOX Inhibitor on the Development of Tubers in Vitroa
| Parameters | Control | Naproxen |
|---|---|---|
| Total fresh weight of tubers (g) | 2.571 | 0.059 |
| Fresh weight per tuber (g) | 0.112 | 0.012 |
| LOX activity (μmol sec−1 g−1 protein) | 11.94 ± 0.65 | 1.70 ± 0.15 |
Axillary buds from in vitro shoots were cultured on 8% sucrose medium in the dark, and the tubers were harvested after 3 months. Twenty-three tubers produced from 15 control axillary bud cuttings and five tubers from 10 inhibitor-treated cuttings were pooled separately and evaluated. LOX activity was inhibited by incorporating 100 μmol of naproxen, an inhibitor of LOX activity (Sircar et al., 1983), into the agar medium.
DISCUSSION
We have characterized the expression pattern of an early- stage potato tuber LOX cDNA and examined the effect of suppressing its expression during tuber development to better understand LOX involvement in potato tuberization processes. In this study, POTLX-1 was used to suppress the expression of tuber-associated Lox1 class genes. The high overall sequence match to previously characterized LOXs and the presence of the conserved amino acid motifs and residues essential for enzyme function are evidence for the identity of POTLX-1 as a LOX. POTLX-1 has a 96% amino acid sequence identity match to the tuber LOX T8 (GenBank accession number X95513). Using Escherichia coli–expressed protein, this LOX produces 96% 9-hydroperoxide fatty acids and 2% 13-hydroperoxide products (Royo et al., 1996). It is likely that POTLX-1 has similar LOX activity and is a member of the potato Lox1 class (Royo et al., 1996). Localization studies showed that Lox1 class transcripts were detected only in the most actively growing cells in a stolon tip during the early stages of tuber formation. LOX activity was downregulated in antisense plants suppressed in the expression of Lox1 class genes. These plants exhibited reduced tuber yield, reduced tuber size, and the production of malformed tubers.
Localization of Lox1 Class Transcript Accumulation
Our results show that steady state levels of Lox1 class mRNAs are under developmental control during tuber initiation and growth. Lox1 class mRNA levels increased in stolon tips when tuberization began, peaked in actively growing tubers, and decreased to minimal levels in dormant tubers (Figure 2). The induction of Lox1 class transcript accumulation occurred exclusively in the tips of tuberizing stolons, where radial cell expansion takes place to form a tuber (Figure 4). LOX enzymatic activity was greatest in newly formed and actively growing tubers. This positive correlation between LOX activity and tuber growth and development suggests that the Lox1 class genes are involved in tuber growth processes. Localization studies showed that Lox1 class transcripts accumulated most abundantly in vascular tissues in the subapical region of new tubers. Lox1 class transcripts were not detected in the vascular bundles of the most proximal part of the new tuber (immediately adjacent to the attached stolon). This finding indicates that Lox1 class function is not associated with vascular tissues in general but is specific to the processes occurring in the most actively growing part of the developing tuber. The primary sources of new cells for tuber radial expansion are the vascular bundles and parenchyma of the perimedullary zone (Cutter, 1978; Peterson et al., 1985; Xu et al., 1998a). Cell division and enlargement, which contribute to the increase in size of a young tuber, occur most abundantly in the perimedullary region of the tuberizing stolon. Cell growth in this region is also predominantly responsible for growth after a tuber reaches a diameter of 0.8 cm (Xu et al., 1998a). Therefore, the localization of Lox1 class mRNA in the perimedullary region coincides with the site of the most active cell growth during tuber formation. These findings are consistent with the possibility that the pathway activated by the Lox1 class genes could be involved in the regulation of cell growth during tuber enlargement.
Axillary buds from leaf petiole cuttings from a Lox1 class suppression mutant, grown under short-day conditions, could grow out as a vegetative shoot but not a tuber (Figure 7A). In this tuber LOX suppression mutant, axillary buds from short-day plants cannot respond to the short-day induction signal from the leaf; instead, they respond like long- day axillary buds. No POTLX-1 or other Lox1 class mRNAs were detected in poly(A)+ RNA isolated from actively growing apical shoots (data not shown). In addition, there was no detectable change in vegetative shoot development in any of the tuber LOX suppression mutants. Collectively, these results demonstrate that the expression of Lox1 class genes is associated with tuberization specifically and not with vegetative growth in general.
Suppression Mutants of Tuber LOXs Disrupt Tuber Development
Analysis of the phenotype of the Lox1 class suppression mutants strongly implicated the pathway activated by the tuber LOXs in the control of tuber enlargement. Not only did suppression mutants exhibit reduced tuber yields, but many of the tubers that formed were abnormal in shape. LOX activity was reduced in these small or malformed tubers produced from mutant plants. Under inductive conditions, numerous tubers were formed in the suppression mutant, but their average size was much less than that of controls. It is likely that this inhibition of enlargement reduced the sink strength of these individual tubers and that the mutant plants compensated in response to the inductive conditions by initiating more tubers. Consistent with observations from the antisense mutant analysis in the LOX inhibitor study (Table 2), the reduction in LOX activity was correlated with a decrease in the rate of tuber enlargement. Overall, these results suggest that members of the Lox1 class of genes may function to activate the production of oxylipin compounds that regulate tuber enlargement.
Because of the close sequence identity (>98%) of the POTLX-1 antisense transcript with the other tuber LOX genes, it is likely that all of the tuber LOXs (Figure 1, top nine accession numbers) were suppressed. Suppression was so effective that mRNA of the leaf-specific LOX POTLX-3 (Kolomiets et al., 2000), with only an 84% sequence match, was reduced threefold to fourfold in suppression mutant 11 (data not shown). A possible explanation for the existence of multiple LOX genes in tubers could be related to their function. Tuber formation is an important survival mechanism for the potato plant, and it is a conservative strategy to control this process with the contributions of several genes (van den Berg et al., 1996). Regulation of the synthesis of tuber growth compounds could be controlled more effectively by modulating the additive contributions of several individual genes to produce a dosage effect. Results from crosses between non-tuber-bearing species (which produce no stolons or tubers) and tuber-bearing species suggest that tuberization is a dosage or threshold response (Ehlenfeldt and Hanneman, 1984). As genomes from tuber-bearing species increased in the hybrids, the plants were able to produce stolons only, and then tubers, suggesting that the minimal expression of specific genes is necessary for tuberization to occur.
On the basis of our results, there are several possibilities for the function of the Lox1 class genes. One possibility is that the tuber LOX pathway may be involved in the production of jasmonic acid and tuberonic acid as a result of the residual 13-LOX activity ascribed to the Lox1 family (Royo et al., 1996). Both of these oxylipin products have been implicated as tuber-inducing compounds (Koda et al., 1991; Pelacho and Mingo-Castel, 1991), and jasmonic acid is involved in the induction of radial cell expansion in tubers (Takahashi et al., 1994) and tuber buds (Castro et al., 1999). Alternately, because the tuber LOXs have predominantly 9-LOX activity (Royo et al., 1996), it is possible that some of the 9-hydroperoxide derivatives, such as α-ketols, epoxy alcohols, or divinyl ethers, may play a specific role, as yet unclear, in the regulation of tuber growth. Hamberg (2000) has reported the discovery of a novel cyclopentenone, 10-oxo-11-phytodienoic acid, produced by 9-LOX activity from stolons and young tubers that is a regioisomer of 12-oxo-phytodienoic acid, the precursor of jasmonic acid. It is conceivable that this new 9-LOX cyclopentenone may be the precursor of an undiscovered compound, similar to tuberonic and jasmonic acids, that regulates tuber growth. Another possibility for the function of tuber LOXs can be explained by their dual enzymatic capacity. The 9-hydroperoxide products may be produced as a defense against microbial attack (Vaughn and Gardner, 1993; Ricker and Bostock, 1994; Reddy et al., 2000) in the tuber, whereas the 13-hydroperoxide products, generated at much lower levels, may act to regulate tuber growth through the control of cell expansion. Low levels of expression of the 13-LOX of potato, designated H3, were detected in tubers (Royo et al., 1996). Subsequent studies of transgenic LOX-H3 antisense plants (Royo et al., 1999), however, showed an increase in tuber yield in plants with suppressed H3 expression, indicating that LOX-H3 has no promotive role in the regulation of tuber development. Overall, these results suggest that the expression of the 9-LOX genes of potato tubers is important in controlling tuber development. Collectively, these data demonstrate that suppression of Lox1 class activity reduces tuber yields and disrupts normal morphology.
METHODS
Plant Material
Potato (Solanum tuberosum cv Superior) plants were grown in an environmentally controlled (20 to 22°C, 16-hr daylength) greenhouse under standard conditions for 1 week. Plants were then transferred to short days (8-hr daylength) for 3 to 4 weeks to induce tuberization and were harvested to obtain stolons and tubers at appropriate stages of development. Stolons not induced for tuberization were identified by a hook at their tip. Swollen stolons have begun tuberization and are characterized by a straightened hook and visible subapical swelling. Only stolons in which the swollen part was less than twice the diameter of the remainder of the stolon were selected for this stage. New tubers (⩽1 g fresh weight) have a recognizable tuber appearance and a swollen subapical region (2 to 5 mm) that is more than twice the diameter of the remainder of the stolon. Larger, actively growing tubers (⩾5 g fresh weight) were harvested from plants 5 to 6 weeks old. Mature tubers used for RNA gel blot analyses and lipoxygenase (LOX) activity assays were dormant and harvested from plants that senesced in the greenhouse and were stored in the dark at 21°C for 1 month. Stored tubers were mature tubers that had been stored at 6°C for at least 2 months. Roots, stems, leaves, and flowers were collected from flowering plants in the greenhouse. Single-node leaf cuttings were taken from potato cv FL1607 transgenic plants and controls grown under an 8-hr-light/16-hr-dark photoperiod and incubated as described by Hannapel (1991). Ten to 20 axillary buds were harvested per treatment and divided into two aliquots, one for the assay of LOX activity and one for RNA extraction.
RNA Gel Blot Analysis
Total RNA was extracted from various organs of cv Superior plants according to the phenol/chloroform extraction procedure described by Dix and Rawson (1983). Poly(A)+ RNA was obtained from total RNA by chromatography on oligo(dT)-cellulose. RNA was size fractionated electrophoretically through a 1% agarose gel that contained 5 mM methyl–mercuric hydroxide and transferred onto a MagnaGraph nylon membrane (Micron Separations, Inc., Westborough, MA). Equal loading of RNA samples and uniform transfer onto a nylon membrane were confirmed by visualizing RNA stained with ethidium bromide under UV light. Membranes were hybridized at high stringency in a 50% formamide hybridization buffer at 42°C for 48 hr. We used either a 32P-labeled 2.1-kb ClaI fragment or a 2.6-kb Xba fragment of the POTLX-1 cDNA clone as the probe for RNA gel blot analyses. Because of the high sequence match between these probes and the sequences of other known tuber LOX genes (>97% at the nucleotide level in this region), RNA detection analyses cannot discriminate between the related transcripts of the different tuber Lox1 genes. The hybridization buffer was 50% formamide, 6 × SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate), 3.3 × Denhardt's solution (1× Denhardt's solution is 0.02% Ficoll, 0.02% polyvinylpyrrolidone, and 0.02% BSA), 25 mM sodium phosphate buffer, pH 7.0, and 0.115 mg/mL salmon sperm DNA. Membranes were washed in 1 × SSC, 0.1% SDS at 23°C for 15 min, in 0.1 × SSC, 0.1% SDS at 23°C for 30 min, and in 0.1 × SSC, 0.1% SDS at 65°C for 30 min. RNA gel blots were exposed to x-ray film using intensifying screens for 2 to 4 days. The blots presented are representative examples from at least two independent experiments.
In Situ Hybridization Analysis
New tubers (≈2 mm across the swollen part) were fixed (Cañas et al., 1994), embedded in paraffin, and cut with a rotary microtome into 8-μm longitudinal and transverse sections. Sections were incubated with either sense or antisense digoxigenin–UTP-labeled RNA probes transcribed in vitro from the full-length POTLX-1 cDNA clone, according to the manufacturer's instructions (Boehringer Mannheim, Indianapolis, IN). RNA transcripts were hydrolyzed partly in 0.2 M sodium carbonate and 0.2 M sodium bicarbonate at 65°C for 1 hr. Tissue sections were deparaffinized, hybridized, and washed as described by Cañas et al. (1994). Hybridization was performed with 0.6 μg of either sense or antisense riboprobes for 24 hr. Sections were washed and incubated with antidigoxigenin antibody bound to alkaline phosphatase for immunological detection. Color reactions with 6.6 μg/mL nitroblue tetrazolium salt, an alkaline phosphatase substrate, were conducted for 4 hr. Accumulation of Lox1 class mRNA bound to the antisense probe was seen as an orange-brown stain when viewed under dark-field illumination. Micrographs (Figure 4) were obtained under dark-field illumination with a Leitz (Wetzlar, Germany) Orthoplan light microscope.
Plant Transformation
Transformation and regeneration of plants was undertaken on leaf sections from potato cv FL1607, as described by Liu et al. (1995). The antisense construct was made from the 5′ 1076-bp XbaI-SacI fragment from the POTLX-1 cDNA (Kolomiets et al., 1996a) cloned into the binary vector pCB201 (Xiang et al., 1999) and driven by the constitutive cauliflower mosaic virus (CaMV) 35S promoter. Constructs were checked by polymerase chain reaction (PCR) with clone-specific primers. Positive recombinant clones were transferred to Agrobacterium tumefaciens strain GV2260 using the procedure of direct transformation (An et al., 1988). Control plants in the yield studies were transgenic (containing the empty pCB201 cassette) and were regenerated from leaf callus tissue. Functional transformants were identified by PCR analysis of genomic DNA and by detection of the accumulation of antisense transcripts of POTLX-1 in leaf RNA samples. From these positive transgenic plants, the seven independent transformants used in this study were selected on the basis of suppressed LOX activity in stolons or tubers.
LOX Activity Assays
LOX activity in a 0.5-g tissue sample was determined as the consumption of oxygen (μmol sec−1 g−1 protein) using linolenic acid as a substrate and a Clark electrode for detection (Royo et al., 1996). Data presented here are means of two samples. Activity per sample is the average of three measurements per sample.
Phylogenetic Analysis
Phylogenetic analysis was performed on amino acid sequences of selected plant LOXs using the ME-Boot program of the MEGA package (Kumar et al., 1993) and the neighbor-joining program (Saitou and Nei, 1987).
LOX Inhibition of in Vitro Tuberization
For inhibition of LOX activity, naproxen (Sigma) at 100 μmol was incorporated into Murashige and Skoog (1962) medium supplemented with 1.5% agar and 8% sucrose. Naproxen, a nonsteroidal anti-inflammatory drug, is an effective inhibitor of plant LOX activity (Sircar et al., 1983; Creelman et al., 1992). Tuber formation was assayed in axillary buds of cuttings from nontransgenic plantlets of potato cv FL1607 grown in vitro. Cuttings were incubated at 24°C in the dark and evaluated for tuberization after 3 weeks and harvested after 3 months in culture.
Acknowledgments
We thank Jennifer Hart for her guidance with in situ hybridization techniques and Dr. Harry Horner and Tracey Pepper of the Iowa State University Bessey Microscopy Facility for their help with tissue preparation, light microscopy, and photography. We acknowledge Dr. Xun Gu and Yufeng Wang of Iowa State University for their assistance in the design of the phylogenetic tree. We also thank Drs. Kan Wang, Salomé Prat, Steve Rodermel, and José Sanchez-Serrano for critical reviews of the manuscript. This project was supported partially by funds from the Iowa Agriculture and Home Economics Experiment Station. This is journal paper No. J-18165 of the Iowa Agriculture and Home Economics Experiment Station, Ames, IA, projects 3703 and 3189.
References
- Abel, G.J., Springer, F., Willmitzer, L., and Kossmann, J. (1996). Cloning and functional analysis of a cDNA encoding a novel 139 kDa starch synthase from potato (Solanum tuberosum L.). Plant J. 10, 981–991. [DOI] [PubMed] [Google Scholar]
- An, G., Ebert, P.R., Mitra, A., and Ha, S.B. (1988). Binary vectors. In Plant Molecular Biology Manual, Vol. A3 (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 1–19.
- Anderson, J.M. (1989). Membrane derived fatty acids as precursors to second messengers. In Second Messengers in Plant Growth and Development, Vol. 6, W.F. Boss and D.J. Morre, eds (New York: Alan R. Liss), pp. 181–212.
- Bachem, C.W.B., van der Hoeven, R.S., de Bruijn, S.M., Vreugdenhil, D., Zabeau, M., and Visser, R.G.F. (1996). Visualization of differential gene expression using a novel method of RNA fingerprinting based on AFLP: Analysis of gene expression during potato tuber development. Plant J. 9, 745–753. [DOI] [PubMed] [Google Scholar]
- Boyington, J.C., Gaffney, B.J., and Amzel, M.L. (1993). The three-dimensional structure of an arachidonic acid 15-lipoxygenase. Science 260, 1482–1486. [DOI] [PubMed] [Google Scholar]
- Cañas, L.A., Busscher, M., Angenent, G.C., Beltran, J.P., and van Tunen, A.J. (1994). Nuclear localization of the petunia MADS box protein FBP1. Plant J. 6, 597–604. [Google Scholar]
- Casey, R. (1995). Sequence of a cDNA clone encoding a potato (Solanum tuberosum) tuber lipoxygenase. Plant Physiol. 107, 265–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, G., Kraus, T., and Abdala, G. (1999). Endogenous jasmonic acid and radial cell expansion in buds of potato tubers. J. Plant Physiol. 155, 706–710. [Google Scholar]
- Creelman, R.A., Bell, E., and Mullet, J.E. (1992). Involvement of a lipoxygenase-like enzyme in abscisic acid biosynthesis. Plant Physiol. 99, 1258–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter, E.G. (1978). Structure and development of the potato plant. In The Potato Crop, P.M. Harris, ed (London: Chapman and Hall), pp. 70–152.
- Dix, K.P., and Rawson, J.R.Y. (1983). In vivo transcriptional products of the chloroplast DNA of Euglena gracilis. Curr. Genet. 7, 265–272. [DOI] [PubMed] [Google Scholar]
- Ehlenfeldt, M.K., and Hanneman, R.E., Jr. (1984). The use of endosperm balance number and 2n gametes to transfer exotic germplasm in potato. Theor. Appl. Genet. 68, 155–161. [DOI] [PubMed] [Google Scholar]
- Ewing, E.E. (1985). Cuttings as simplified models of the potato plant. In Potato Physiology, P.L. Li, ed (Orlando, FL: Academic Press), pp. 154–199.
- Ewing, E.E., and Struik, P.C. (1992). Tuber formation in potato: Induction, initiation and growth. Hortic. Rev. 14, 89–198. [Google Scholar]
- Ferrie, B.J., Beaudoin, N., Burkhart, W., Bowsher, C.G., and Rohstein, S.J. (1994). The cloning of two tomato lipoxygenase genes and their differential expression during fruit ripening. Plant Physiol. 106, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feussner, I., Hause, B., Nellen, A., Wasternack, C., and Kindl, H. (1996). Lipid body lipoxygenase is expressed in cotyledons during germination prior to other lipoxygenase forms. Planta 198, 288–293. [Google Scholar]
- Fidantsef, A.L., and Bostock, R.M. (1998). Characterization of potato tuber lipoxygenase cDNAs and lipoxygenase expression in potato tubers and leaves. Physiol. Plant. 102, 257–271. [Google Scholar]
- Fujino, K., Koda, Y., and Kikuta, Y. (1995). Reorientation of cortical microtubules in the subapical region during tuberization in single-node stem segments of potato in culture. Plant Cell Physiol. 36, 891–895. [Google Scholar]
- Geerts, A., Feltkamp, D., and Rosahl, S. (1994). Expression of lipoxygenase in wounded tubers of Solanum tuberosum L. Plant Physiol. 105, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger, P., Hajirezaei, M., Geiger, M., Deiting, U., Sonnewald, U., and Stitt, M. (1998). Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose–starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta 205, 428–437. [DOI] [PubMed] [Google Scholar]
- Hamberg, M. (2000). New cyclopentenone fatty acids formed from linoleic and linolenic acids in potato. Lipids 35, 353–363. [DOI] [PubMed] [Google Scholar]
- Hannapel, D.J. (1991). Characterization of the early events of potato tuber development. Physiol. Plant. 83, 568–573. [Google Scholar]
- Hendriks, T., Vreugdenhil, D., and Stiekema, W. (1991). Patatin and four serine protease inhibitor genes are differentially expressed during potato tuber development. Plant Mol. Biol. 17, 385–394. [DOI] [PubMed] [Google Scholar]
- Hornung, E., Walther, M., Kuhn, H., and Feussner, I. (1999). Conversion of cucumber linoleate 13-lipoxygenase to a 9-lipoxygenating species by site-directed mutagenesis. Proc. Natl. Acad. Sci. USA 96, 4192–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, S.D., and Prat, S. (1996). Control of tuberisation in potato by gibberellins and phytochrome B. Physiol. Plant. 98, 407–412. [Google Scholar]
- Jackson, S.D., Heyer, A., Dietze, J., and Prat, S. (1996). Phytochrome B mediates the photoperiodic control of tuber formation in potato. Plant J. 9, 159–166. [Google Scholar]
- Kang, S.G., and Hannapel, D.J. (1996). A novel MADS-box gene of potato expressed during the early stages of tuberization. Plant Mol. Biol. 31, 379–386. [DOI] [PubMed] [Google Scholar]
- Kausch, K.D., and Handa, A.K. (1997). Molecular cloning of a ripening-specific lipoxygenase and its expression during wild-type and mutant tomato fruit development. Plant Physiol. 113, 1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda, Y. (1992). The role of jasmonic acid and related compounds in the regulation of plant development. Int. Rev. Cytol. 135, 155–199. [DOI] [PubMed] [Google Scholar]
- Koda, Y., Kikuta, Y., Tazaki, H., Tsujino, Y., Sakamura, S., and Yoshihara, T. (1991). Potato tuber-inducing activities of jasmonic acid and related compounds. Phytochemistry 30, 1435–1438. [Google Scholar]
- Kolomiets, M.V., Hannapel, D.J., and Gladon, R.J. (1996. a). Potato lipoxygenase genes expressed during the early stages of tuberization (accession nos. U60200 and U60201). Plant Physiol. 112, 445.12226401 [Google Scholar]
- Kolomiets, M.V., Hannapel, D.J., and Gladon, R.J. (1996. b). Nucleotide sequence of a cDNA clone for a lipoxygenase from abscisic acid–treated potato leaves (accession no. U60202). Plant Physiol. 112, 445.12226401 [Google Scholar]
- Kolomiets, M.V., Chen, H., Gladon, R.J., Braun, E.J., and Hannapel, D.J. (2000). A leaf lipoxygenase of potato induced specifically by pathogen infection. Plant Physiol. 124, 1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S., Tamura, K., and Nei, M. (1993). MEGA: Molecular Evolutionary Genetics Analysis, version 1.0. (University Park, PA: Pennsylvania State University).
- Liu, A.T.-H., Stephens, L.C., and Hannapel, D.J. (1995). Transformation of Solanum brevidens using Agrobacterium tumefaciens. Plant Cell Rep. 15, 196–199. [DOI] [PubMed] [Google Scholar]
- Macleod, M.R., Davies, H.V., Jarvis, S.B., and Taylor, M.A. (1999). Characterisation of genes isolated from a potato swelling stolon cDNA library. Potato Res. 42, 31–42. [Google Scholar]
- Matsui, K., Irie, M., Kajiwara, T., and Hatanaka, A. (1992). Developmental changes in lipoxygenase activity in cotyledons of cucumber seedlings. Plant Sci. 85, 23–32. [Google Scholar]
- Matsuki, T., Tazaki, H., Fujimori, T., and Hogetsu, T. (1992). The influences of jasmonic acid methyl ester on microtubules in potato cells and formation of potato tubers. Biosci. Biotech. Biochem. 56, 1329–1330. [Google Scholar]
- Melan, M.A., Enriquez, A.L.D., and Peterman, T.K. (1994). The LOX1 gene of Arabidopsis is temporally and spatially regulated in germinating seedlings. Plant Physiol. 105, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery, G., Pikaard, C.S., Hannapel, D.J., and Park, W.D. (1984). Isolation and sequence analysis of cDNAs for the major potato tuber protein, patatin. Nucleic Acids Res. 12, 7989–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Paiva, E., Lister, R.M., and Park, W.D. (1982). Comparison of the protein in axillary bud tubers and underground stolon tubers in potato. Am. Potato J. 59, 425–433. [Google Scholar]
- Pelacho, A.M., and Mingo-Castel, A.M. (1991). Jasmonic acid induces tuberization of potato stolons cultured in vitro. Plant Physiol. 97, 1253–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, L.R., Barker, G.W., and Howarth, M.J. (1985). Development and structure of tubers. In Potato Physiology, P.L. Li, ed (Orlando, FL: Academic Press), pp.124–148.
- Preiss, J. (1996). ADPglucose pyrophosphorylase: Basic science and applications in biotechnology. Biotechnol. Annu. Rev. 2, 259–279. [DOI] [PubMed] [Google Scholar]
- Reddy, P.S., Kumar, T.C., Reddy, M.N., Sarada, C., and Reddanna, P. (2000). Differential formation of octadecadienoic acid and octadecatrienoic acid products in control and injured/infected potato tubers. Biochim. Biophys. Acta 1483, 294–300. [DOI] [PubMed] [Google Scholar]
- Ricker, K.E., and Bostock, R.M. (1994). Eicosanoids in the Phytophthora infestans–potato interaction: Lipoxygenase metabolism of arachidonic acid and biological activities of selected lipoxygenase products. Physiol. Mol. Plant Pathol. 44, 65–80. [Google Scholar]
- Rodriguez-Concepcion, M., and Beltran, J.P. (1995). Repression of the pea lipoxygenase gene loxg is associated with carpel development. Plant Mol. Biol. 27, 887–899. [DOI] [PubMed] [Google Scholar]
- Royo, J., Vancanneyt, G., Perez, A.G., Sanz, C., Stormann, K., Rosahl, S., and Sanchez-Serrano, J.J. (1996). Characterization of three potato lipoxygenases with distinct enzymatic activities and different organ-specific and wound-regulated expression patterns. J. Biol. Chem. 271, 21012–21019. [DOI] [PubMed] [Google Scholar]
- Royo, J., Leon, J., Vancanneyt, G., Albar, J.P., Rosahl, S., Ortego, F., Castañera, P., and Sanchez-Serrano, J.J. (1999). Antisense-mediated depletion of a potato lipoxygenase reduces wound induction of proteinase inhibitors and increases weight gain of insect pests. Proc. Natl. Acad. Sci. USA 96, 1146–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusterucci, C., Montillet, J.L., Agnel, J.P., Battesti, C., Alonso, B., Knoll, A., Bessoule, J.J., Etienne, P., Suty, L., Blein, J.P., and Triantaphylides, C. (1999). Involvement of lipoxygenase-dependent production of fatty acid hydroperoxides in the development of the hypersensitive cell death induced by cryptogein on tobacco leaves. J. Biol. Chem. 274, 36446–36455. [DOI] [PubMed] [Google Scholar]
- Saitou, N., and Nei, M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Saravitz, D.M., and Siedow, J.N. (1995). The lipoxygenase isozymes in soybean [Glycine max (L.) Merr.] leaves: Changes during leaf development, after wounding, and following reproductive sink removal. Plant Physiol. 107, 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva, L.I., de Bruijn, S.M., Koot-Gronsveld, E.A.M., Navratil, O., and Vreugdenhil, D. (2000). Tuber morphology and starch accumulation are independent phenomena: Evidence from ipt-transgenic potato lines. Physiol. Plant. 108, 435–443. [Google Scholar]
- Siedow, J.N. (1991). Plant lipoxygenase: Structure and function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 145–188. [Google Scholar]
- Sircar, J.C., Schwender, C.F., and Johnson, E.A. (1983). Soybean lipoxygenase inhibition by nonsteroidal antiinflammatory drugs. Prostaglandins 25, 393–396. [DOI] [PubMed] [Google Scholar]
- Steczko, J., Donoho, G.P., Clemens, J.C., Dixon, J.E., and Axelrod, B. (1992). Conserved histidine residues in soybean lipoxygenase: Functional consequences of their replacement. Biochemistry 31, 4053–4057. [DOI] [PubMed] [Google Scholar]
- Suh, S.G., Stiekema, W.J., and Hannapel, D.J. (1991). Proteinase-inhibitor activity and wound-inducible expression of the 22-kDa potato-tuber proteins. Planta 184, 423–430. [DOI] [PubMed] [Google Scholar]
- Takahashi, K., Fujino, K., Kikuta, Y., and Koda, Y. (1994). Expansion of potato cells in response to jasmonic acid. Plant Sci. 100, 3–8. [Google Scholar]
- Takahashi, K., Fujino, K., Kikuta, Y., and Koda, Y. (1995). Involvement of the accumulation of sucrose and the synthesis of cell wall polysaccharides in the expansion of potato cells in response to jasmonic acid. Plant Sci. 111, 11–18. [Google Scholar]
- Taylor, M.A., Davies, H.V., and Scobie, L.A. (1991). Molecular changes associated with tuberization in Solanum tuberosum: Differential expression of α-tubulin isotypes. Physiol. Plant. 81, 244–250. [Google Scholar]
- Taylor, M.A., Mad Arif, S.A., Kumar, A., Davies, H.V., Scobie, L.A., Pearce, S.R., and Flawell, A.J. (1992). Expression and sequence analysis of cDNAs induced during the early stages of tuberization in different organs of the potato plant (Solanum tuberosum L.). Plant Mol. Biol. 20, 641–651. [DOI] [PubMed] [Google Scholar]
- van den Berg, J.H., Simko, I., Davies, P.J., Ewing, E.E., and Halinska, A. (1995). Morphology and [14C]gibberellin A12 metabolism in wild-type and dwarf Solanum tuberosum ssp. andigena grown under long and short photoperiods. J. Plant Physiol. 146, 467–473. [Google Scholar]
- van den Berg, J.H., Ewing, E.E., Plaisted, R.L., McMurry, S., and Bonierbale, M.W. (1996). QTL analysis of potato tuberization. Theor. Appl. Genet. 93, 307–316. [DOI] [PubMed] [Google Scholar]
- Vaughn, S.F., and Gardner, H.W. (1993). Lipoxygenase-derived aldehydes inhibit fungi pathogenic on soybean. J. Chem. Ecol. 19, 2337–2345. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil, D., Xu, X., Jung, C.S., van Lammeren, A.A.M., and Ewing, E.E. (1999). Initial anatomical changes associated with tuber formation on single-node potato (Solanum tuberosum L.) cuttings: A re-evaluation. Ann. Bot. 84, 675–680. [Google Scholar]
- Wheeler, R.M., Hannapel, D.J., and Tibbitts, T.W. (1988). Comparison of axillary bud growth and patatin accumulation in potato leaf cuttings as assays for tuber induction. Ann. Bot. 62, 25–30. [DOI] [PubMed] [Google Scholar]
- Wisniewski, J.P., Gardner, C.D., and Brewin, N.J. (1999). Isolation of lipoxygenase cDNA clones from pea nodule mRNA. Plant Mol. Biol. 39, 775–783. [DOI] [PubMed] [Google Scholar]
- Xiang, C., Han, P., Lutziger, I., Wang, K., and Oliver, D.J. (1999). A mini binary vector series for plant transformation. Plant Mol. Biol. 40, 711–718. [DOI] [PubMed] [Google Scholar]
- Xu, X., Vreugdenhil, D., and van Lammeren, A.A.M. (1998. a). Cell division and cell enlargement during potato tuber formation. J. Exp. Bot. 49, 573–582. [Google Scholar]
- Xu, X., van Lammeren, A.A.M., Vermeer, E., and Vreugdenhil, D. (1998. b). The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol. 117, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]