Abstract
In eukaryotic cells, the basic machinery of cell cycle control is highly conserved. In particular, many cellular events during cell cycle progression are controlled by cyclin-dependent kinases (CDKs). The cell cycle in animal early embryos, however, differs substantially from that of somatic cells or yeasts. For example, cell cycle checkpoints that ensure that the sequence of cell cycle events is correct have been described in somatic cells and yeasts but are largely absent in embryonic cells. Furthermore, the regulation of CDKs is substantially different in the embryonic and somatic cells. In this study, we address the nature of the first cell cycle in the brown alga Fucus, which is evolutionarily distant from the model systems classically used for cell cycle studies in embryos. This cycle consists of well-defined G1, S, G2, and M phases. The purine derivative olomoucine inhibited CDKs activity in vivo and in vitro and induced different cell cycle arrests, including at the G1/S transition, suggesting that, as in somatic cells, CDKs tightly control cell cycle progression. The cell cycle of Fucus zygotes presented the other main features of a somatic cell cycle, such as a functional spindle assembly checkpoint that targets CDKs and the regulation of the early synthesis of two PSTAIRE CDKs, p32 and p34, and the associated histone H1 kinase activity as well as the regulation of CDKs by tyrosine phosphorylation. Surprisingly, the synthesis after fertilization of p32 and p34 was translationally regulated, a regulation not described previously for CDKs. Finally, our results suggest that the activation of mitotic CDKs relies on an autocatalytic amplification mechanism.
INTRODUCTION
During animal early embryogenesis, the fertilized egg undergoes rapid cell cycles without growing. Interphases (S phase) and mitoses (M phase) alternate in quick succession without intervening G1 and G2 phases (King et al., 1994). The oscillation between S and M phases is driven by successive activation and inactivation of a key protein complex called the M phase–promoting factor (MPF) (Masui and Markert, 1971; Kishimoto, 1988). MPF is composed of cdc2, a cyclin-dependent kinase (CDK), and its positively regulating protein, cyclin B. Periodic synthesis and degradation of cyclin B is the major mechanism of CDK regulation in embryos (Arion et al., 1988; Dunphy and Newport, 1988). In contrast, additional mechanisms, such as phosphorylation, regulate CDK activity in somatic cells. In both embryos and somatic cells, mitotic CDKs operate at the G2/M boundary, where their activation triggers, through the phosphorylation of their substrate proteins, several major cellular events, including nuclear envelope breakdown, chromosome condensation, spindle assembly, and cytokinesis (Peter et al., 1990; Kishimoto, 1994).
In somatic cells and yeasts, however, the cell cycle appears to be more tightly controlled by CDKs than in embryos (Nurse, 1994; Chevalier and Blow, 1996). A restriction point controls cell cycle progression in G1 in response to extracellular and/or intracellular cues (Peter and Herskowitz, 1994; Planas-Silva and Weinberg, 1997; Neufeld and Edgar, 1998), and CDK activity promotes the assembly of replication complexes in S phase. In contrast, in the sea urchin zygote, DNA replication can occur while CDKs are maintained inactive (Moreau et al., 1998), suggesting that CDKs may not control all aspects of cell cycle progression in animal embryos.
The differences between cell cycle control in somatic and embryonic cells are reflected by different mechanisms of regulation of CDKs. Whereas the reentry of quiescent somatic cells into the cell cycle in G1 is mediated by the successive expression of G1 and mitotic CDKs (Elledge et al., 1992), constant levels of CDKs are detected in animal early embryos. Finally, cell cycle checkpoints of somatic cells differ greatly from those of embryonic cells. Somatic cells display stringent surveillance mechanisms that check the success of various cell cycle events such as DNA replication and the integrity of the spindle, preventing mitosis until accurate repairs have been performed (Rudner and Murray, 1996; Paulovich et al., 1997). CDKs are a major target of these checkpoints, and their regulation after checkpoint activation is usually correlated with changes in their phosphorylation status as well as with associations with regulatory molecules such as CDK inhibitors (Lew and Kornbluth, 1996; Rudner and Murray, 1996; Hardwick, 1998). These checkpoints are usually partial in or absent from early embryonic cells (reviewed in Hartwell and Weinert, 1989; Heichman and Roberts, 1994; Nurse, 1994; Edgar, 1995).
Although much less is known about the in vivo regulation of the cell cycle in plants, evidence strongly suggests that the cell cycle machinery is well conserved between plants and animals (Mironov et al., 1999). In particular, functional homologs of the yeast cdc2 gene, which contains the fully conserved PSTAIRE hallmark, have been identified in several plant species, and these are required for progression through mitosis (reviewed in Dudits et al., 1998). The lack of cell cycle mutants in plants has been partially overcome by using a family of specific inhibitors of CDKs, such as olomoucine, that specifically and reversibly inhibit cdk2 and cdc2 in many cell types (Vesely et al., 1994; Alessi et al., 1998). Olomoucine and other purine derivatives were found to arrest cell cycle progression at both the G1/S and G2/M transitions in Petunia and Arabidopsis somatic cells (Glab et al., 1994; Planchais et al., 1997). Finally, tyrosine phosphorylation has been proposed to regulate CDK activity in response to plant hormones in somatic cells (Bell et al., 1993; Zhang et al., 1996; McKibbin et al., 1998).
Microspectrophotometric analysis of the DNA content in sperm nuclei has suggested specificities of the cell cycle in higher plant gametes, such as the initiation of DNA replication before gametic fusion (Friedman, 1999). Furthermore, in in vitro–fertilized zygotes from maize, the transcripts of three cyclin genes are detected only after fertilization, suggesting that there may be different patterns of cell cycle activity in higher plant embryos (Sauter et al., 1998). However, higher plant embryos are embedded deeply in maternal tissue and are largely inaccessible to experimentation, and, to our knowledge, in vitro fertilization systems do no provide enough material for biochemical approaches. Consequently, little is known about the molecular mechanisms of cell cycle regulation in these embryos.
In fucoid algae, including the genera Fucus and Pelvetia, fertilization is external, and large populations of synchronously developing zygotes can be obtained (Brownlee and Bouget, 1998). This characteristic, coupled with the length of the first cell cycle (24 hr), makes fucoid algae powerful experimental systems for cellular and biochemical investigations of cell cycle regulation. In a previous study, we demonstrated the presence in fucoid zygotes of a DNA replication checkpoint, a common feature of somatic cells but also of a few animal embryos (Corellou et al., 2000a). To determine more precisely the nature of the first cell cycle in Fucus (embryonic versus somatic), we investigated the regulation of CDKs and their involvement in controlling the first embryonic cell cycle of Fucus zygotes during normal development and after various cell cycle arrests. Our results indicate that in Fucus zygotes, many events of the cell cycle and cell cycle arrest are tightly controlled by CDKs, which themselves appear to be regulated, as in somatic cells, both at the level of synthesis and by reversible phosphorylation. The presence of a functional spindle assembly checkpoint further confirmed that the cell cycle in Fucus is somatic in nature. Interestingly, CDKs appear to be synthesized from maternal mRNAs, suggesting a unique regulation of CDKs at the translational level. Our results also suggest the presence of a mechanism of autocatalytic amplification of mitotic cyclin/CDK activity similar to those described previously in animals and yeasts. We propose a model of the possible roles and regulations of CDKs throughout the first cell cycle of fucoid algae.
RESULTS
We studied three different aspects of the Fucus first cell cycle to determine more precisely its nature (embryonic versus somatic): (1) the requirement for CDKs for cell cycle progression, particularly at the G1/S transition; (2) the regulation of CDKs; and (3) the presence of a spindle assembly checkpoint.
Olomoucine Induces Different Cell Cycle Arrests in Fucus Zygotes
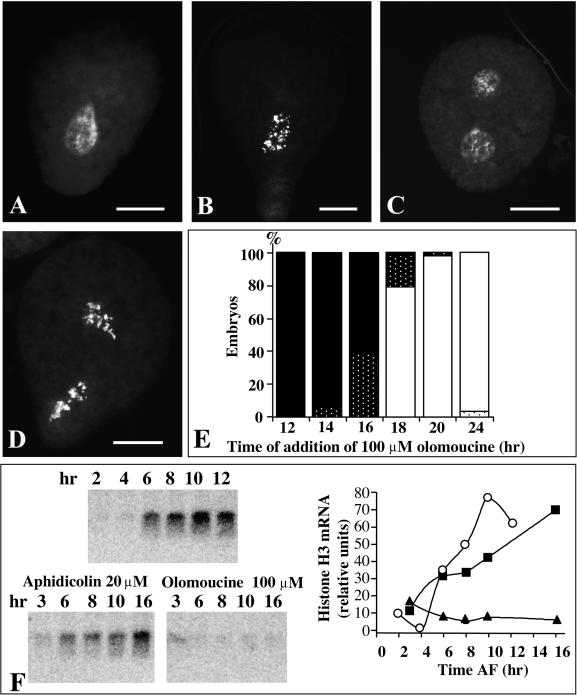
Previous results have shown that in Fucus zygotes, the purine derivative olomoucine specifically inhibits mitotic CDKs in vitro and blocks cell cycle progression from G2 to mitosis (Corellou et al., 2000a). Zygotes treated with olomoucine from 6 hr after fertilization (AF) display a decondensed nucleus and a spindle with an aligned centrosomal axis that is typical of zygotes in premitosis (Corellou et al., 2000a). Taking advantage of this specific inhibitor of CDKs, we further investigated the involvement of CDKs in the control of the Fucus first cell cycle. Zygotes were treated with various concentrations of olomoucine from 2 hr until 36 hr AF. Concentrations of ∼35 μM were required to block cytokinesis in most embryos (data not shown). At these concentrations, zygotes exhibited condensed chromosomes that were often scattered in the cytoplasm (Figure 1B). Between 50 and 100 μM olomoucine was required to arrest and maintain all zygotes with a decondensed nucleus. A concentration of 100 μM olomoucine therefore was used to block chromatin condensation (data not shown) and was added at various times AF. When added in early G2, that is, when in most cells division was no longer sensitive to the DNA replication inhibitor aphidicolin (Corellou et al., 2000a), 100 μM olomoucine blocked nuclei before mitosis, that is, at the G2/M transition (Figures 1A and 1E). In contrast, olomoucine treatments started just before mitosis, at 16 hr AF, allowed ∼40% of the chromosomes to condense (Figures 1B and 1E), like the arrest induced by continuous treatment with 35 μM olomoucine. Treatment of zygotes with olomoucine at 18 hr AF had little effect on nuclear events of the first cell cycle (Figures 1C to 1E), even though cytokinesis was still inhibited (data not shown). Olomoucine (100 μM) induced the same succession of arrests during the second cell cycle (Figures 1C to 1E) but more rapidly, consistent with the faster pace of this second cell cycle. These findings indicate that the CDK inhibitor olomoucine induces various cell cycle arrests, including one at the G2/M transition and one in mitosis, and that the targets of olomoucine display different degrees of in vivo sensitivity to this drug.
Figure 1.
Effect of Olomoucine on Cell Cycle Progression in Zygotes.
Olomoucine (100 μM) was added at various times until 36 hr AF. Cells were fixed and subsequently stained with mithramycin A ([A] to [D]).
(A) to (D) Zygotes treated with 100 μM olomoucine from 12 (A), 16 (B), 20 (C), and 24 (D) hr until 36 hr AF. 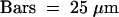 .
.
(E) Zygotes treated with 100 μM olomoucine at various times AF (x axis) displayed either one or two sets of dispersed chromosomes (white dots and black dots, respectively) or one or two decondensed nuclei (black bars and white bars, respectively). The data shown are representative of the results of two independent experiments.
(F) RNA gel blot analysis with a Fucus histone H3 DNA probe (left). Equal amounts of RNA (10 μg), extracted at the times indicated, were loaded in each lane, and RNA integrity was checked by staining with ethidium bromide (data not shown). Corresponding quantifications (right) were performed with a phosphorimager in control zygotes (open circles) as well as in zygotes treated from 3 hr AF with either 20 μM aphidicolin (closed squares) or 100 μM olomoucine (closed triangles) until the times indicated on the x axis. The data shown are representative of the results of two independent experiments. AF, after fertilization.
It proved impossible to investigate directly the effect of olomoucine on the G1/S transition by quantifying DNA levels in zygotes stained with mithramycin A or with any other DNA dye (Corellou et al., 2000a). In the one-celled zygote the nucleus is decondensed and occupies a central position, and the cell is large and filled with organelles with high autofluorescence, such as plastids and polyphenol-containing physodes. These features make it difficult to directly measure the nuclear DNA levels in zygotes; therefore, we used an indirect method to monitor the G1/S progression and the effect of cell cycle inhibitors. In most eukaryotic cells, transcription of certain histone genes, including histone H3, begins at the onset of S phase (Kapros et al., 1992; Chaubet and Gigot, 1998). We monitored histone H3 transcription as an S phase–specific marker using RNA gel blot analysis with a fragment of the coding region from a Fucus histone H3 gene. In Fucus zygotes, histone H3 transcripts were clearly detected at 6 hr AF, and they accumulated progressively until 12 hr AF (Figure 1F). This is unlikely to be due to a general increase of transcription, because the transcript of actin, a constitutively expressed gene, displays constant levels of transcript throughout the first cell cycle (Bouget et al., 1995). Although the transcription of histone H3 still occurred in zygotes in which DNA replication was blocked by aphidicolin (Figure 1F), no histone H3 transcripts were detected in zygotes incubated with 100 μM olomoucine from 3 hr AF. These results suggest that in Fucus zygotes, S phase begins at 4 to 6 hr AF, and 100 μM olomoucine induced a cell cycle arrest before the arrest induced in early S phase by aphidicolin, that is, in G1 or at the G1/S transition.
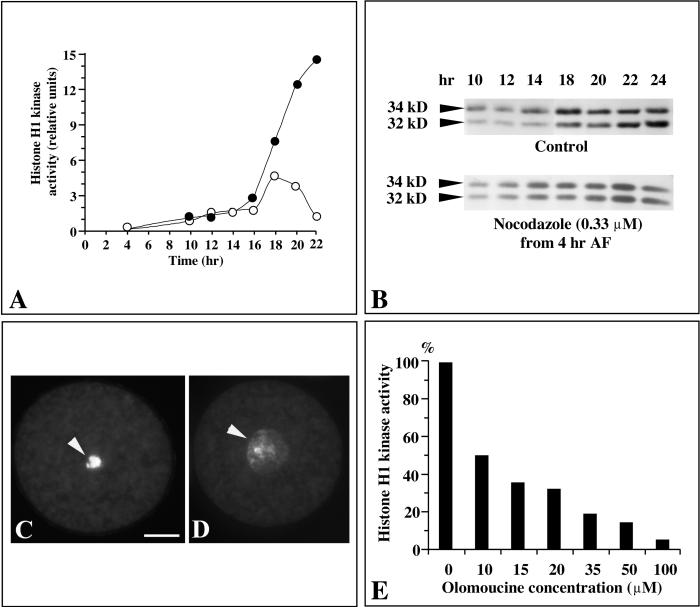
Histone H1 Kinase Activity and the Synthesis of PSTAIRE CDKs Increase Dramatically AF and Are Regulated at the Translational Level, but They Do Not Depend on Karyogamy
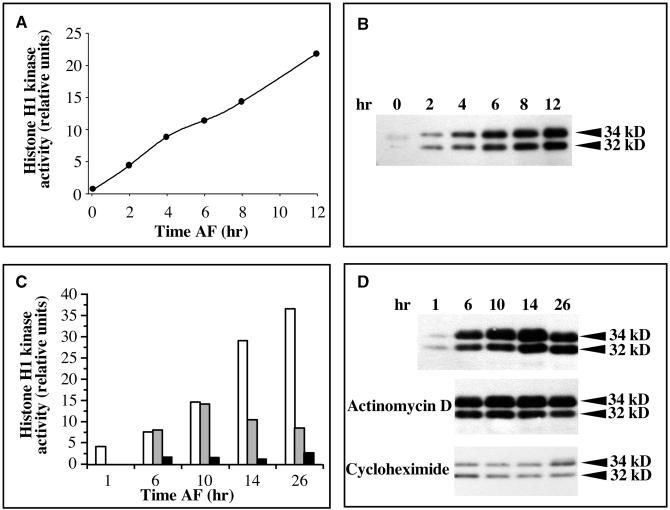
CDKs were purified using their affinity for the human suc1 homolog p9CKShs1, and their histone H1 kinase activity was measured in vitro. The levels of two PSTAIRE CDKs bound to p9CKShs1, p32 and p34, were monitored using a monoclonal anti-PSTAIRE antibody (Corellou et al., 2000a). Extracts from unfertilized eggs exhibited very low levels of histone H1 kinase activity (Figure 2A), and both p32 and p34 were not detectable or only barely detectable before fertilization (Figure 2B). Histone H1 kinase activity increased ∼10-fold between 0 and 2 hr AF (Figure 2A), which correlated with a significant increase in the synthesis of both p34 and p32 (Figure 2B). This initial increase in histone H1 kinase activity was followed by a steady and continuous increase (Figure 2A; see also controls in Figures 2C and 6A), and the synthesis of PSTAIRE CDKs increased in a similar manner (Figure 2B; see also control in Figure 2D). The specific activity of histone H1 kinase relative to total protein in crude zygote extracts gave a similar profile (data not shown), suggesting that these postfertilization changes in histone H1 kinase activity did not result from a general increase in protein synthesis.
Figure 2.
Regulation of the Expression and Activity of CDKs during Early Development.
Total proteins were extracted from Fucus zygotes at the times indicated, and CDKs were purified by affinity on p9CKShs1–Sepharose beads. The results shown are representative of the results of three independent experiments. Purified proteins were assayed for their ability to phosphorylate histone H1 in vitro ([A] and [C]). Eluted proteins were immunoblotted with an anti-PSTAIRE antibody ([B] and [D]).
(A) Histone H1 kinase activity during normal development.
(B) Synthesis of PSTAIRE CDKs during normal development.
(C) Histone H1 kinase activity in extracts from zygotes treated from 1 hr AF with either 16 μM actinomycin D (gray bars) or 0.2 μM cycloheximide (black bars), compared with control cells (white bars).
(D) Expression of PSTAIRE CDKs in extracts from zygotes treated from 1 hr AF with either 16 μM actinomycin D or 0.2 μM cycloheximide.
The requirements for the synthesis of PSTAIRE CDKs and for the associated histone H1 kinase activity were investigated using actinomycin D and cycloheximide, two inhibitors of transcription and translation, respectively, in Fucus zygotes (Quatrano, 1968). When added before 12 hr AF, actinomycin D (16 μM) and cycloheximide (0.2 μM) inhibited cell division (data not shown). When cells were treated with cycloheximide from 1 hr AF, the levels of both histone H1 kinase activity and PSTAIRE CDKs remained low for at least 26 hr (Figures 2C and 2D). In contrast, and although cell division was fully inhibited for at least 26 hr, incubations with actinomycin D from 1 hr AF did not affect the synthesis of PSTAIRE CDKs (Figure 2D), suggesting that the synthesis of CDKs is translationally regulated. In these conditions, however, histone H1 kinase activities remained at levels lower than those of 14-hr-old controls (Figure 2C), suggesting that the synthesis of mitotic CDK-activating proteins is transcriptionally regulated.
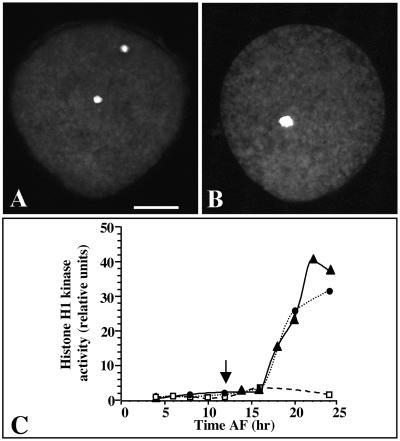
When added at 2 hr AF, the microtubule depolymerizing agent nocodazole prevented the fusion of pronuclei but had no effect on early morphogenesis (i.e., germination; Figure 3A). The male pronucleus remained condensed and always entered mitosis after the female pronucleus, as described previously in other fucoid algae (Brawley and Quatrano, 1979; Swope and Kropf, 1993; Motomura, 1995). Similarly, when nocodazole was added after pronuclei fusion, zygotes were arrested in mitosis with clustered, heavily condensed chromosomes (Figure 3B). The developmental course of histone H1 kinase activity was almost identical in zygotes treated with nocodazole before or after pronuclei fusion, and the mitotic activity was much higher than in control zygotes (Figure 3C). These results suggest that the synthesis of CDKs and the activation of their kinase activity is initially triggered by fertilization independently of karyogamy.
Figure 3.
Effect of the Inhibition of Pronuclei Fusion by Nocodazole on the Activity of CDKs.
(A) A zygote treated with 0.33 μM nocodazole from 2 to 36 hr AF and then stained with mithramycin A.
(B) A zygote treated with 0.33 μM nocodazole from 12 to 36 hr AF and then stained with mithramycin A.
(C) Histone H1 kinase activity in extracts from cells treated with 0.33 μM nocodazole from either 2 hr AF (closed circles) or 12 hr AF (closed triangles, arrow) until the times indicated on the x axis. Histone H1 kinase activity in extracts from control zygotes is represented by open squares. The data shown are representative of the results of two independent experiments.
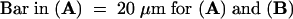 .
.
Olomoucine Inhibits Both in Vivo and in Vitro Histone H1 Kinase Activity and Induces an Accumulation of Tyrosine-Phosphorylated CDKs
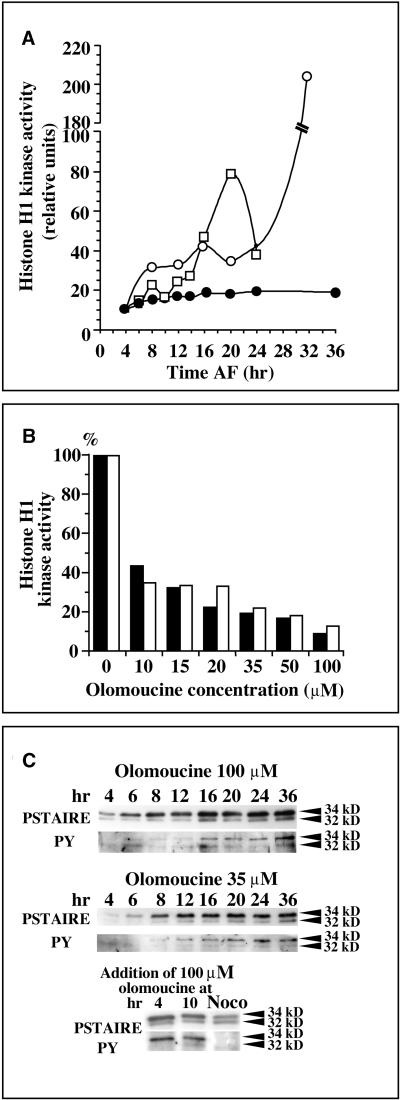
The role of tyrosine phosphorylation in CDK regulation was investigated after various cell cycle arrests. Histone H1 kinase activities were monitored after treatment of 3-hr-old zygotes with either 100 or 35 μM olomoucine for 36 hr in zygotes that were arrested before S phase and in mitosis, respectively. In control zygotes, histone H1 kinase activity doubled from 4 to 14 hr AF, and the maximal value of the mitotic peak of activity corresponded to an eightfold increase compared with the activity detected at 4 hr AF. In contrast, no peak of activity was observed when zygotes were treated with 100 μM olomoucine, and the level of H1 kinase remained similar to that of 6- to 8-hr-old control zygotes even at 36 hr AF (Figure 4A). The histone H1 kinase activity from zygotes incubated with 35 μM olomoucine increased rapidly, reaching a level similar to that of control zygotes after 14 to 16 hr. This kinase activity remained stable until 24 hr AF, increased again between 24 and 36 hr AF (a four- to 20-fold increase depending on the experiment), and finally remained stable for at least another 24 hr (Figure 4A). Staining with mithramycin A revealed that, after treatment with 35 μM olomoucine, the nuclei were decondensed at 24 hr AF, whereas condensed chromosomes were observed at 36 hr AF (data not shown).
Figure 4.
Effect of Olomoucine on Histone H1 Kinase Activity and Tyrosine Phosphorylation of CDKs.
(A) Histone H1 kinase activities in extracts from control zygotes (open squares) and zygotes treated from 3 hr AF with either 100 μM olomoucine (closed circles) or 35 μM olomoucine (open circles) until the times indicated on the x axis. The data shown are representative of the results of three independent experiments.
(B) Dose-dependent in vitro inhibition by olomoucine of histone H1 kinase activity in extracts from cells treated from 3 to 36 hr AF with either 35 μM olomoucine (black bars) or 100 μM olomoucine (white bars). For each treatment, the kinase activity is reported as a percentage of control activity (no olomoucine). The data shown are representative of the results of two independent experiments.
(C) Protein gel blot analysis of the expression and phosphorylation of PSTAIRE CDKs after treatment with olomoucine. Immunoblotting was performed with anti-PSTAIRE (PSTAIRE) or anti-phosphotyrosine (PY) antibodies. Top two panels, zygotes were treated from 3 hr AF with either 100 or 35 μM olomoucine until the times indicated (data shown are from the same experiment represented in [A]). Bottom panel, zygotes were treated from 4 or 10 hr AF with 100 μM olomoucine or with 0.33 μM nocodazole (Noco) until 36 hr AF. The data shown are representative of the results of three independent experiments.
Because olomoucine is a reversible inhibitor of CDKs, it was possible to investigate the in vitro effect of olomoucine on histone H1 kinase in cell extracts from zygotes treated previously with olomoucine (Corellou et al., 2000a). In vitro treatments with olomoucine inhibited histone H1 kinase in extracts from 36-hr-old zygotes arrested before S phase with 100 μM olomoucine or in mitosis with 35 μM olomoucine (Figure 4B). Even though the initial levels of kinase activities were dramatically different (Figure 4A), the patterns of in vitro dose-dependent inhibition were similar in both types of cell cycle arrests, suggesting that similar CDK/cyclins were targeted in vivo by olomoucine. The higher kinase activities observed after mitotic arrest with lower concentrations of olomoucine (Figure 4A) therefore may arise from a partial activation of mitotic CDKs (see Discussion).
Immunological detection of PSTAIRE CDKs in olomoucine-treated zygotes showed that until 12 to 16 hr AF, levels of both p34 and p32 increased normally as in controls (data not shown), suggesting that the synthesis of PSTAIRE CDKs was not inhibited by olomoucine regardless of the concentration of the drug (Figure 4C, which corresponds to the kinase assay shown in Figure 4A). In contrast, the immunological detection of phosphotyrosine residues revealed that tyrosine phosphorylation increased progressively on both proteins from 8 to 36 hr AF. In control zygotes, tyrosine phosphorylation of these two proteins has been shown to be cell cycle regulated, being maximal in G2 phase and absent at the time of mitosis (Corellou et al., 2000a). Zygotes treated with 100 μM olomoucine at 10 hr AF, that is, at the S/G2 transition, were arrested with a decondensed nucleus at the G2/M transition (Figure 1). In these cells, the expression patterns of both PSTAIRE and phosphotyrosine epitopes were similar to those observed in zygotes treated with 100 μM olomoucine from 3 hr AF, and the 32- and 34-kD proteins remained phosphorylated on tyrosine until at least 36 hr AF (Figure 4C). In contrast, no phosphorylation was detectable in zygotes arrested in mitosis by nocodazole (Figure 4C, bottom).
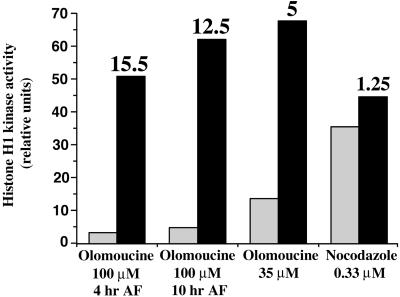
Treatments with cdc25A Phosphatase Restore Mitotic Levels of Histone H1 Kinase in Extracts from Cells Arrested by Olomoucine
Various arrests were induced during cell cycle progression by olomoucine and nocodazole (Figure 5). The earlier the cells were arrested by olomoucine during cell cycle progression, the lower their levels of histone H1 kinase activities (Figure 5; see also Figure 4A). Histone H1 kinase activities from cells arrested by 100 μM olomoucine added at 3 hr AF were similar to those obtained from zygotes blocked in early S phase by aphidicolin (Corellou et al., 2000a; data not shown). Histone H1 kinase activities were lower in extracts from zygotes arrested in mitosis by 35 μM olomoucine than in extracts from zygotes arrested in mitosis by nocodazole (Figure 5). However, treatments with the human phosphatase glutathione S-transferase (GST)–cdc25A led to a dramatic increase of histone H1 kinase activities from cells arrested by olomoucine in their cell cycle progression but not in cells arrested by nocodazole. The relative activation was higher when the zygotes were arrested early in the cell cycle by 100 μM olomoucine, that is, when the kinase activities were initially low. Although the factors of activation were variable (from 5.0 to 15.5), the final levels of kinase activity after phosphatase treatments were broadly similar to the mitotic level of histone H1 kinase observed in extracts from cells arrested by nocodazole. Together, Figures 4 and 5 demonstrate that, as in animal somatic cells, tyrosine phosphorylation is a major mechanism involved in CDK regulation in Fucus zygotes.
Figure 5.
In Vitro Activation of Histone H1 Kinase by the Human Phosphatase GST-cdc25A.
Protein extracts from zygotes treated with olomoucine or nocodazole from the times indicated until 36 hr AF were assayed for histone H1 kinase activity after treatment with 62 units of GST-cdc25A for 20 min (black bars) or after incubation in the dephosphorylation buffer (gray bars). The relative degrees of activation by GST-cdc25A are indicated above the black bars. The data shown are representative of the results of two independent experiments.
A Spindle Assembly Checkpoint Targets Mitotic CDKs in Fucus Zygotes
Nocodazole was used to inhibit mitotic spindle formation. Zygotes that were treated continuously with 0.33 μM nocodazole displayed heavily condensed chromosomes for at least 36 hr and never proceeded through mitosis (Figure 6C), suggesting the presence of a spindle assembly checkpoint. In zygotes treated with 0.33 μM nocodazole from 12 hr AF, histone H1 kinase activity increased progressively from 4 to 16 hr AF. This was followed, at the time of entry into mitosis at 16 to 18 hr AF, by a sharp increase in activity until 22 hr AF (Figure 6A), and high levels of kinase activity were observed for at least another 12 hr (data not shown). This was in contrast to the levels of histone H1 kinase activity during a normal cell cycle, which decreased after the mitotic peak at 18 hr AF (Figure 6A). The abrupt increase of histone H1 kinase activity preceded chromosome condensation in both controls (38 and 80% of cells in mitosis at 18 and 20 hr, respectively) and zygotes treated with nocodazole (22 and 68% of cells in mitosis at 18 and 20 hr, respectively), indicating that the peak or high levels of H1 kinase corresponded to kinase activities of mitotic CDKs. No significant differences in the levels of the two PSTAIRE CDKs, p32 and p34, were observed between control zygotes and zygotes arrested in mitosis by nocodazole (Corellou et al., 2000a), suggesting that the mitotic kinase activity of PSTAIRE CDKs is not regulated by the synthesis and/or degradation of these proteins (Figure 6B).
Figure 6.
A Spindle Assembly Checkpoint Targets Mitotic CDKs.
(A) Histone H1 kinase activity in extracts from either control zygotes (open circles) or cells treated with 0.33 μM nocodazole from 4 hr AF (closed circles) until the times indicated on the x axis. The proportion of cells in mitosis was determined by staining DNA with mithramycin A.
(B) Protein gel blot analysis of PSTAIRE CDKs in extracts from either control zygotes or zygotes treated with 0.33 μM nocodazole from 4 hr AF until the times indicated.
(C) A zygote arrested in mitosis with 0.33 μM nocodazole from 6 to 36 hr AF, fixed, and stained with mithramycin A. Note the highly condensed and clustered chromosomes (arrowhead).
(D) A zygote arrested in mitosis with 0.33 μM nocodazole from 6 to 36 hr AF (e.g., as in [C]), subsequently treated with both 100 μM olomoucine and 0.33 μM nocodazole for 6 hr, and finally fixed and stained with mithramycin A. Note the decondensed nucleus at 42 hr AF (arrowhead).
(E) Dose-dependent in vitro inhibition by olomoucine of histone H1 kinase activity in extracts from cells treated with 0.33 μM nocodazole from 4 to 36 hr AF. Kinase activity is represented as a percentage of control activity (no olomoucine). The data shown are representative of the results of three independent experiments.
 .
.
Zygotes arrested in mitosis by 0.33 μM nocodazole (Figure 6C) were then treated with 100 μM olomoucine for 6 hr. This allowed the chromatin to decondense in >90% of the cells (Figure 6D). Furthermore, olomoucine inhibited, in a dose-dependent manner, the high histone H1 kinase activity found in extracts from cells incubated with 0.33 μM nocodazole from 6 to 36 hr AF (Figure 6E), suggesting that CDKs present in these extracts are a target of olomoucine in vivo. Together, these results suggest strongly that in Fucus zygotes, the spindle assembly checkpoint operates by maintaining high levels of activity of mitotic CDKs. These data also suggest indirectly that the inactivation of CDKs is required for chromatin decondensation at the end of mitosis.
DISCUSSION
We have shown previously that the G2 phase in Fucus zygotes starts at 10 to 12 hr AF and ends at 16 to 18 hr AF, whereas cytokinesis is completed by 22 to 24 hr AF (Corellou et al., 2000a). Based on the increase of histone H3 transcripts, which are typically detected at the onset of S phase (Kapros et al., 1992), the S phase begins between 4 and 6 hr AF in Fucus zygotes, that is, after pronuclei fusion (3 to 4 hr AF), an event that is required for full DNA replication (Motomura, 1995). Therefore, like the cell cycles of dividing somatic cells, the first cell cycle in Fucus encompasses four well-defined phases: G1, S, G2, and M (Figure 7).
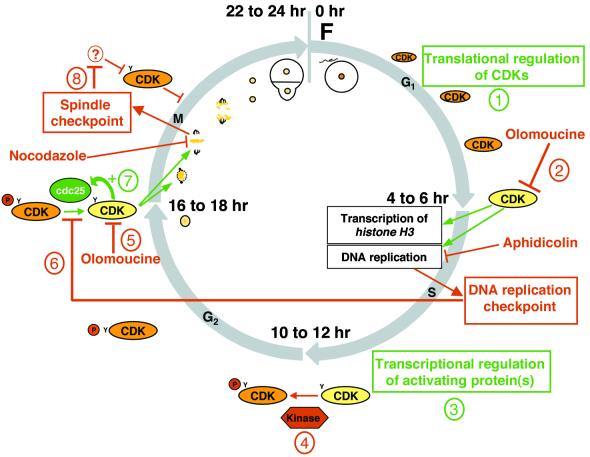
Figure 7.
The First Cell Cycle and the Possible Roles and Regulations of CDKs in Fucoid Algae.
This diagram is based on the results of this study and on those reported previously (Corellou et al., 2000a). Activating and inhibitory mechanisms are shown in red and green, respectively. Active and inactive CDKs are shown in orange and yellow, respectively. The first cell cycle comprises well-defined G1, S, G2, and M phases. (1) PSTAIRE CDKs are synthesized from maternal mRNAs after fertilization. (2) The CDK activity that is required for the G1/S transition is inhibited by olomoucine. The transcription of histone H3 in S phase is inhibited by olomoucine but not by aphidicolin. (3) Transcription, before 10 hr AF, of the genes encoding activating proteins is required for mitotic activity of CDKs. (4) CDKs are maintained inactive in G2 by inhibitory phosphorylation (P) on tyrosine residues (Y) and are activated in mitosis by a cdc25-like phosphatase. (5) Olomoucine, by inhibiting CDKs, prevents entry into and progression through mitosis. (6) A DNA replication checkpoint prevents mitosis, including chromatin condensation and spindle formation, through inactivation of mitotic CDKs by inhibitory phosphorylation. (7) The autocatalytic amplification of mitotic CDK activity (+), which may rely on the activation of a cdc25-like protein by CDKs, is inhibited by olomoucine (5). (8) A spindle assembly checkpoint prevents progression through mitosis, including chromatin decondensation, by inhibiting the inactivation of CDKs by an unknown mechanism (?). F, fertilization.
CDK Activity Is Required for S Phase Entry in Fucus Zygotes
Although in mammalian somatic cells and yeasts the onset of S phase is controlled by G1/S CDKs (reviewed in Heichman and Roberts, 1994), in animal embryos the role of G1/S CDKs in promoting DNA replication is unclear. In the sea urchin zygote, cdk2 activity is not required for DNA replication (Moreau et al., 1998). Furthermore, treatments with olomoucine do not impede DNA replication (Moreau et al., 1998). In contrast, immunodepletion of cdk2 from Xenopus egg extracts prevents DNA replication, and a peak of cyclin E/cdk2 activity correlates with the onset of S phase in frog early embryos (Fang and Newport, 1991). In Fucus zygotes, histone H3 mRNA was detected even upon inhibition of DNA replication by aphidicolin, but the transcription of histone H3 was fully inhibited by olomoucine, suggesting that this drug induces a cell cycle arrest before S phase. This cell cycle arrest was directly demonstrated in multicellular embryos by quantifying the relative DNA levels in nuclei stained with mithramycin A (data not shown). Therefore, in contrast to the observations in sea urchin zygote, CDK activity appears to be required to trigger S phase entry in the Fucus zygote.
Synthesis of PSTAIRE CDKs and Early Increase in Histone H1 Kinase Activity Are Triggered by Fertilization but Do Not Rely on Karyogamy
As illustrated in mitogen-activated quiescent human cells, which reenter the cell cycle by promoting the successive transcription of cdk2 and cdc2 genes (Pagano et al., 1993), the sequential expression of CDK genes plays an important role in regulating CDK activity and ordering cell cycle progression in animal somatic cells. In rice, the phytohormone gibberellin was reported to promote the expression of cdc2 and associated H1 kinase activity, and in both alfalfa and Arabidopsis, the expression of several cdc2-related genes is cell cycle regulated and correlates with their specific activation (Sauter et al., 1995; Magyar et al., 1997; Mironov et al., 1997). In contrast, in animal early embryos, regulation of MPF activity relies primarily on the periodic synthesis of cyclin B. Cdc2 is already present in the oocyte, and many rounds of cell division can occur when transcription is inhibited (Gerhart et al., 1984; Arion and Meijer, 1989). In Fucus eggs, the levels of both CDK activity and PSTAIRE CDKs are very low, and the postfertilization synthesis of PSTAIRE CDKs correlates with a dramatic increase of CDK activities. Although the inhibition of transcription had no effect on either the synthesis of PSTAIRE CDKs or histone H1 kinase activity until early G2 phase, the inhibition of translation completely prevented both events, suggesting an early regulation of CDK activity at the translational level. To complete the first cell cycle, however, the Fucus zygote also requires transcription for ∼10 hr AF, or until early G2 phase. Furthermore, the mitotic peak of histone H1 kinase is dependent on transcription, even though upon inhibition of transcription, the level of PSTAIRE CDKs is similar to that of mitotic controls. Therefore, it is likely that the synthesis of fully active G1/S CDK relies on maternal transcripts, whereas the transcription of proteins such as cyclin B is required to activate mitotic CDKs. Such a regulation of CDK activities represents an intermediate situation between the cell cycle control of animal early embryos and cell cycle reentry in somatic cells.
Interestingly, karyogamy is dispensable for the synthesis and activation of CDKs during the first cell cycle of Fucus zygotes, suggesting that, as in the sea urchin egg, plasmogamy is sufficient to commit the egg to enter the first cell cycle (Shatten et al., 1989). When pronuclei fusion is inhibited, DNA replication occurs in the egg pronucleus but not in the male pronucleus, which remains condensed throughout the first cell cycle of Fucus zygotes (Motomura, 1995). Furthermore, the female pronucleus always enters mitosis before the male pronucleus (Motomura, 1995). We propose that the transcription of cell cycle genes occurs only in the decondensed female pronucleus, because the transcription-dependent histone H1 kinase is still observed when pronuclei fusion is inhibited. The male pronucleus, which remains condensed at all times, would not be able to either replicate DNA or transcribe the cell cycle genes required for entry into mitosis; thus, its entry into mitosis would depend on the female pronucleus.
Tyrosine Phosphorylation Is a Major Mechanism Involved in Regulating the Mitotic Activity of CDKs in Fucus Zygotes
The regulation of cdc2 during early animal embryo cell cycles usually relies on the periodic synthesis and degradation of cyclin B. In addition to this regulatory mechanism, in fission yeast and mammalian somatic cells the activation of mitotic cdc2 operates by tyrosine dephosphorylation of cdc2 by the phosphatase cdc25 (Lew and Kornbluth, 1996). In various plant taxa, several cdc2 homologs contain a conserved tyrosine residue, most often at position 15 (Zhang et al., 1996), and in tobacco cells, tyrosine phosphorylation was shown to regulate cdc2-like kinase activity upon stimulation with cytokinin (Zhang et al., 1996). Tyrosine phosphorylation of the PSTAIRE CDKs p32 and p34 was shown to be cell cycle regulated in Fucus zygotes, and dephosphorylation of p34 and p32 correlates with entry into mitosis (Corellou et al., 2000a). Furthermore, the S/M DNA checkpoint was shown to operate by maintaining mitotic CDKs inactive by tyrosine phosphorylation (Corellou et al., 2000a), whereas after activation of the spindle assembly checkpoint, highly active mitotic CDKs were not phosphorylated on tyrosine residues. Our results indicate that the various cell cycle arrests induced by olomoucine also trigger tyrosine phosphorylation of both p32 and p34 proteins. Olomoucine treatments did not impede the synthesis of PSTAIRE CDKs, and the timing of tyrosine phosphorylation during the first cell cycle was similar in zygotes arrested in G1, at the G2/M transition, or in mitosis. Furthermore, treatment with cdc25A led to an activation of CDK activity, confirming that phosphorylation on tyrosine residues negatively regulates the activity of CDKs.
In Fucus zygotes, all of the samples treated with olomoucine (i.e., arrested in G1/S, G2/M, or mitosis) displayed, after dephosphorylation by cdc25A, kinase levels similar to the high mitotic levels observed after nocodazole treatment. Therefore, the inhibition of G1/S CDKs by olomoucine does not appear to prevent the formation of potentially active mitotic cyclin/CDK complexes, but it likely inhibits their activation by preventing their dephosphorylation on tyrosine residues. Such a downregulation of mitotic CDKs also occurs when DNA replication is inhibited (Corellou et al., 2000a). How the inhibition of G1/S CDKs leads to a downregulation of mitotic CDKs remains unclear in Fucus zygotes. As in Xenopus egg extracts, G1/S CDKs may directly and positively regulate mitotic CDKs through the reversible phosphorylation and activation of cdc25-like proteins (Guadano and Newport, 1996). Alternately, the inhibition of G1/S CDKs, by preventing DNA replication, may activate the DNA replication checkpoint, which in turn would lead to an inactivation of CDKs by tyrosine phosphorylation (Corellou et al., 2000a).
Inhibition of mitosis by nocodazole yielded maximal CDK activities associated with dephosphorylated p34 and p32, whereas zygotes arrested in mitosis by low doses of olomoucine displayed higher CDK activities than did zygotes arrested at the G2/M transition by high doses of olomoucine. Conversely, the activation by cdc25A was much higher in zygotes arrested by olomoucine at the G2/M transition than in cells arrested by olomoucine in mitosis. Together, our data suggest that a gradual dephosphorylation, or activation, occurs between G2 phase and mitosis, as reported in starfish oocytes (Borgne and Meijer, 1999). This activation seems to require CDK activity, because it is much lower after a cell cycle arrest at the G2/M transition than after an arrest in mitosis. These results suggest a possible inhibition by olomoucine of an autocatalytic amplification of mitotic cyclin/CDK activity similar to those described previously in animals and yeasts (reviewed in Lew and Kornbluth, 1996).
Fucus Zygotes Display a Functional Spindle Assembly Checkpoint
Mitotic cyclin B/cdc2 is known to promote the nucleation of the mitotic spindle (Verde et al., 1990; Kishimoto, 1994), and inactivation of the mitotic complex cyclin B/cdc2 at metaphase is required for a cell to exit from mitosis and reenter the next cell cycle (King et al., 1994). Inactivation of mitotic CDKs is first achieved by the degradation of cyclin B (Hershko, 1997). In animal somatic cells and in Schizosaccharomyces pombe, the lack of a functional spindle prevents the exit from mitosis by inhibiting the degradation of key proteins implicated in chromosome cohesion as well as that of cyclin B, thus maintaining high levels of mitotic CDK activity (Nishimoto et al., 1992). However, the early embryos of several animal species, such as Xenopus, lack a fully active spindle assembly checkpoint and undergo abnormal mitosis in the absence of a fully functional spindle (Clute and Masui, 1992; Murray, 1992; Sluder et al., 1994). Fucus zygotes lacking a functional spindle after treatment with nocodazole feature high levels of mitotic CDK. These zygotes did not divide or rereplicate DNA, indicating the presence of a fully active spindle assembly checkpoint. The fact that the inactivation of CDK activity by olomoucine triggers a decondensation of chromatin in zygotes continuously treated with nocodazole strongly suggests that inactivation of CDKs is required for the exit from mitosis and that, as in animal somatic cells, the spindle assembly checkpoint operates at least in part by hampering the inactivation of mitotic CDKs.
These findings are summarized in Figure 7, which describes the major steps of the Fucus first cell cycle together with the possible roles and regulations of CDKs. Cell cycle progression, including S phase entry, is tightly regulated by CDKs. Two functional DNA replication and spindle assembly checkpoints block cell cycle progression by altering CDK activities. CDKs are regulated at both the transcriptional and transductional levels and by phosphorylation. Together, our results indicate that in Fucus zygotes, the zygotic cell cycle resembles a somatic cell cycle more than the typical cell cycles of animal embryos in which controls are reduced.
METHODS
Culture and Inhibitors
Sexually mature receptacles of Fucus spiralis were collected at Le Dossen (Brittany, France) and stored at 4°C for up to 14 days. Gametes were released by standard osmotic shock procedures in filtered seawater for 1 hr (Corellou et al., 2000b). The time of fertilization (0 hr) was taken to be 30 min after the first eggs were released. Zygotes and embryos were grown at 14°C. Unfertilized eggs were obtained by inducing receptacles to release in high-potassium artificial seawater, according to Brawley (1987). Aphidicolin (20 μM; Sigma) was used to inhibit DNA replication and subsequent inhibition of cell division. Aphidicolin was added at various times after fertilization to determine experimentally the end of S phase, that is, the beginning of G2 phase (Corellou et al., 2000a). Olomoucine was used to inhibit cyclin-dependent kinases (CDKs) in vivo and in vitro. The structural analog iso-olomoucine had no effect on cell division at concentrations as high as 500 μM. Nocodazole (0.33 μM; Sigma) was used to prevent spindle formation, and it arrested the cells in mitosis. Actinomycin D (16 μM) and cycloheximide (0.2 μM) were used to prevent transcription and translation (Quatrano, 1968; Bouget et al., 1996). Aphidicolin, olomoucine, nocodazole, actinomycin D, and cycloheximide were stored in DMSO at 10, 300, 1, 10, and 0.5 mg/mL, respectively, and further diluted in artificial seawater before use. Control experiments were performed in filtered seawater containing the same final concentrations of DMSO.
DNA Staining and Quantification
Zygotes and embryos were fixed for 12 hr in 0.2 M citric acid and 0.2% Triton X-100 and kept in 100% methanol for long-term storage. Fixed cells were attached to poly-l-lysine–coated cover slips, processed and stained with 50 μg/mL mithramycin A, as described previously (Corellou et al., 2000a), and then observed by epifluorescence microscopy (540- to 590-nm excitation filter, 585-nm band pass, a 32-nm band pass emission filter, centered at 585 nm). As reported earlier (Corellou et al., 2000a), one-celled zygotes did not yield reproducible results. DNA was therefore quantified by measuring mithramycin A fluorescence on two-celled and older embryos. Mithramycin A fluorescence was measured either with a confocal microscope (model 1024; Bio-Rad, Hemel Hempstead, UK) or with a CCD camera (Spot-RT Slider Eurocam model 2.3.0; Diagnostic Instruments, Inc., Sterling Heights, MI). The camera was standardized with Fluoresbrite fluorescent beads (Polyscience Inc., Warrington, PA), and the images were analyzed with the Image Tool software (a freeware from UCSB computer, science supported software; www.cs.ucsb.edu). For each sample, an average of 50 to 75 nuclei were scanned. In the first experiment, the highest fluorescence value obtained in nocodazole-treated embryos was given the arbitrary value of 8.0 relative fluorescence units (RFU), and the average fluorescence value of nocodazole-treated cells was determined to be 5.7 RFU. In the following experiment, this value was used as a standard to normalize the values of both experiments. The relative fluorescence of nuclei was represented using a scale of 0 to 8.0 RFU divided into eight equal 1-unit classes. Histograms with the percentage of nuclei in each class were established. For each sample, the average relative fluorescence were also determined. The average relative fluorescence of nuclei in nocodazole-treated embryos (5.7 RFU) was close to the average value of mitotic figures in metaphase (5.3 RFU), corresponding to a 4C DNA content. The average relative fluorescence corresponding to a 2C DNA content, which was determined in derivative nuclei in anaphase (2.7 RFU), was close to the average fluorescence value of nuclei treated with aphidicolin from mitosis (2.6 RFU).
Protein Extraction, Purification of CDKs, and Protein Gel Blot Analysis
Protocols for protein extraction, CDK purification, and protein gel blot analysis have been described in detail by Corellou et al. (2000a)(2000b). Briefly, embryos were harvested, frozen in liquid nitrogen, and stored at −80°C until extraction. Frozen samples were ground in liquid nitrogen. Purification of CDKs was performed on p9CKShs1–Sepharose beads. Eluted proteins were resolved on a 10 or 12% (w/v) SDS–polyacrylamide denaturing gel and electrotransferred onto a nitrocellulose or a polyvinylidene difluoride membrane (Amersham Pharmacia Biotech, Little Chalfont, UK) for enhanced chemiluminescence (ECL) or ECL+ detection. Protein gel blotting was performed with either a monoclonal antiphosphotyrosine antibody (PY20; Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:20,000 dilution or a monoclonal anti-PSTAIRE antibody (Sigma) at a 1:3000 dilution. The antiphosphotyrosine antibody could not be detected using the classic ECL detection system. Therefore, the blots were probed first with this antibody, revealed in ECL+, and reprobed with the anti-PSTAIRE antibody, which required short times of exposure in ECL detection.
Histone H1 Kinase Activity and in Vitro Dephosphorylation of Proteins Bound to p9CKShs1
The histone H1 kinase activity of proteins bound to p9CKShs1–Sepharose beads was measured at 30°C for 30 min using γ-32P-ATP, as reported previously (Corellou et al., 2000a). Quantification of radioactive histone H1 was performed using a STORM phosphorimager and Image QuanT software (Molecular Dynamics, Sunnyvale, CA). For each lane, a protein-free region of the area under quantification was used to determine the background according to the object average method. Kinase activities are represented either as relative STORM units or as a percentage of control activity for in vitro inhibition experiments. The glutathione S-transferase (GST)–cdc25A fusion protein was overproduced in Escherichia coli and purified by affinity on glutathione–agarose beads, as described previously (Corellou et al., 2000a). Dephosphorylations were initiated by adding to the proteins bound to the p9CKShs1 beads 100 μL (62 units) of the purified GST-cdc25A in buffer B (50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 1 mM EDTA) containing 20 mM DTT. Dephosphorylation reactions were performed at 30°C for 1 hr. Control samples were treated identically but without GST-cdc25A.
Cloning of the histone H3 Gene and RNA Gel Blot Analysis
DNA was extracted from Fucus sperm using a standard protocol for brown algae (Apt and Grossman, 1993). A representative genomic library (5 × 105 clones) was established in Lambda DASH II, as recommended by the manufacturer (Stratagene, La Jolla, CA). This library was screened under low-stringency conditions (Bouget et al., 1995) with a Laminaria digitata histone H3 cDNA probe (kindly provided by Dr. Florent Crépineau, Centre National de la Recherche Scientifique Roscoff; GenBank accession number AW400773). A 300-bp exon fragment encoding a putative histone H3 (GenBank accession number AJ276797) >95% identical to the L. digitata deduced amino acid sequence of histone H3 was subcloned and used as a probe for RNA gel blot analysis as follows. RNA was extracted, electrophoresed through a 1.2% formaldehyde denaturing gel, and transferred to a Hybond N+ membrane (Bouget et al., 1996). The Fucus histone H3 DNA probe was labeled with radioactive α-32P-dCTP using a random priming labeling kit (Megaprime, Amersham Life Science). High-stringency hybridization was performed overnight at 42°C in 50% deionized formamide, 7% (w/v) SDS, 250 mM NaCl, and 120 mM Na(PO4)2, pH 7.2. Hybridization washes were performed twice at 42°C in 2 × SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate) containing 0.1% SDS and once in 1 × SSC containing 0.1% SDS. Membranes were exposed to a PhosphorScreen (Molecular Dynamics, Amersham Pharmacia Biotech) overnight. Quantification of bound radioactive probe was performed using a STORM phosphorimager and Image QuanT software.
Acknowledgments
We thank Blandine Baratte and Sophie Leclerc for producing GST-cdc25A and Florent Crépineau for providing us with the L. digitata histone H3 cDNA. Thanks also to Laurent Meijer for helpful criticism of the manuscript. F.C. was a recipient of a doctoral fellowship from the Conseil Régional de la Région Bretagne, whose help is gratefully acknowledged.
References
- Alessi, F., Quarta, S., Savio, M., Riva, F., Rossi, L., Stivala, L.A., Scovassi, A.I., Meijer, L., and Prosperi, E. (1998). The cyclin-dependent kinase inhibitors olomoucine and roscovitine arrest human fibroblasts in G1 phase by specific inhibition of CDK2 kinase activity. Exp. Cell Res. 245, 8–18. [DOI] [PubMed] [Google Scholar]
- Apt, K.E., and Grossman, A.R. (1993). Characterization and transcript analysis of the major phycobiliprotein subunit genes from Aglaothamnion neglectum (Rhodophyta). Plant Mol. Biol. 21, 27–38. [DOI] [PubMed] [Google Scholar]
- Arion, D., and Meijer, L. (1989). M-phase–specific protein kinase from mitotic sea urchin eggs: Cyclic activation depends on protein synthesis and phosphorylation but does not require DNA or RNA synthesis. Exp. Cell Res. 183, 361–375. [DOI] [PubMed] [Google Scholar]
- Arion, D., Meijer, L., Brizuela, L., and Beach, D. (1988). cdc2 is a component of the M phase–specific histone H1 kinase: Evidence for identity with MPF. Cell 55, 371–378. [DOI] [PubMed] [Google Scholar]
- Bell, M.H., Halford, N.G., Ormrod, J.C., and Francis, D. (1993). Tobacco plants transformed with cdc25, a mitotic inducer gene from fission yeast. Plant Mol. Biol. 23, 445–451. [DOI] [PubMed] [Google Scholar]
- Borgne, A., and Meijer, L. (1999). Sequential dephosphorylation of p34(cdc2) on Thr-14 and Tyr-15 at the prophase–metaphase transition. J. Biol. Chem. 271, 27847–27854. [DOI] [PubMed] [Google Scholar]
- Bouget, F.Y., Kerbouc'h, C., Liaud, M.-F., Loiseaux de Goër, S., Quatrano, R.S., Cerff, R., and Kloareg, B. (1995). Structural features and phylogeny of the actin gene of Chondrus crispus (Gigartinales, Rhodophyta). Curr. Genet. 28, 164–172. [DOI] [PubMed] [Google Scholar]
- Bouget, F.Y., Gertulla, S., Shaw, S.L., and Quatrano, R.S. (1996). Localization of actin mRNA during the establishment of cell polarity and early cell divisions in Fucus embryos. Plant Cell 8, 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley, S.H. (1987). A sodium-dependent, fast block to polyspermy occurs in eggs of fucoid algae. Dev. Biol. 124, 390–397. [DOI] [PubMed] [Google Scholar]
- Brawley, S.H., and Quatrano, R.S. (1979). Effect of microtubule inhibitors on pronuclear migration and embryogenesis in Fucus distichus (Pheophyta). J. Phycol. 15, 266–272. [Google Scholar]
- Brownlee, C., and Bouget, F.Y. (1998). Polarity determination in Fucus: From zygote to multicellular embryo. Semin. Cell. Dev. Biol. 9, 179–185. [DOI] [PubMed] [Google Scholar]
- Chaubet, N., and Gigot, C. (1998). Histone gene expression. In Plant Cell Division, Vol. 10, D. Francis, D. Dudits, and D. Inzé, eds (London: Portland Press), pp. 269–283.
- Chevalier, S., and Blow, J. (1996). Cell cycle control of replication initiation in eukaryotes. Curr. Opin. Cell Biol. 8, 815–821. [DOI] [PubMed] [Google Scholar]
- Clute, P., and Masui, Y. (1992). Development of microtubule-dependence of the chromosome cycle at the midblastula transition in Xenopus laevis embryos. Dev. Growth Differ. 34, 27–36. [DOI] [PubMed] [Google Scholar]
- Corellou, F., Bisgrove, S.R., Kropf, D.L., Meijer, L., Kloareg, B., and Bouget, F.-Y. (2000. a). A S/M DNA replication checkpoint prevents nuclear and cytoplasmic events of cell division including centrosomal axis alignment and inhibits activation of cyclin-dependent kinase-like proteins in fucoid zygotes. Development 127, 1651–1660. [DOI] [PubMed] [Google Scholar]
- Corellou, F., Potin, P., Brownlee, C., Kloareg, B., and Bouget, F.-Y. (2000. b). Inhibition of zygotic polarity by protein tyrosine kinase inhibitors leads to an alteration of embryo pattern in Fucus. Dev. Biol. 219, 165–182. [DOI] [PubMed] [Google Scholar]
- Dudits, D., Magyar, Z., Deak, M., Mészaros, P., Miskolczi, A., Brown, S., Kondorosi, E., Athanasidis, A., Pongor, S., Bako, L., Koncz, C.S., and Györgyey, J. (1998). Cyclin-dependent and calcium-dependent kinase families: Response of cell division cycle to hormone and stress signals. In Plant Cell Division, Vol. 10, D. Francis, D. Dudits, and D. Inzé, eds (London: Portland Press), pp. 21–45.
- Dunphy, W.G., and Newport, J.W. (1988). Mitosis-inducing factors are present in a latent form during interphase in the Xenopus embryo. J. Cell Biol. 106, 2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, B. (1995). Diversification of cell cycle controls in developing embryos. Curr. Opin. Cell Biol. 7, 815–824. [DOI] [PubMed] [Google Scholar]
- Elledge, S.J., Richman, R., Hall, F.L., Williams, R.T., Lodgson, N., and Harper, J.W. (1992). CDK2 encodes a 33-kDa cyclin A–associated protein kinase and is expressed before CDC2 in the cell cycle. Proc. Natl. Acad. Sci. USA 89, 2907–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, F., and Newport, J.W. (1991). Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell 66, 731–742. [DOI] [PubMed] [Google Scholar]
- Friedman, E.F. (1999). Expression of the cell cycle in sperm of Arabidopsis: Implications for understanding patterns of gametogenesis and fertilization in plants and other eukaryotes. Development 126, 1065–1075. [DOI] [PubMed] [Google Scholar]
- Gerhart, J., Wu, M., and Kirschner, M. (1984). Cell cycle dynamics of an M-phase specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J. Cell Biol. 98, 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glab, N., Labidi, B., Qin, L.X., Trehin, C., Bergounioux, C., and Meijer, L. (1994). Olomoucine, an inhibitor of the cdc2/cdk2 kinases activity, blocks plant cells at the G1 to S and G2 to M cell cycle transitions. FEBS Lett. 353, 207–211. [DOI] [PubMed] [Google Scholar]
- Guadano, T.M., and Newport, J.W. (1996). Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2–cyclin B kinase activity. Cell 84, 73–82. [DOI] [PubMed] [Google Scholar]
- Hardwick, K.G. (1998). The spindle checkpoint. Trends Genet. 14, 1–4. [DOI] [PubMed] [Google Scholar]
- Hartwell, L.H., and Weinert, T.A. (1989). Checkpoints: Controls that ensure the order of cell cycle events. Science 246, 629–634. [DOI] [PubMed] [Google Scholar]
- Heichman, K.A., and Roberts, J.M. (1994). Rules to replicate by. Cell 79, 557–562. [DOI] [PubMed] [Google Scholar]
- Hershko, A. (1997). Roles of ubiquitin-mediated proteolysin cell cycle control. Curr. Opin. Cell Biol. 9, 788–799. [DOI] [PubMed] [Google Scholar]
- Kapros, T., Bögre, L., Németh, K., Bako, L., Györgyey, J., Wu, S.C., and Dudits, D. (1992). Differential expression of histone H3 gene variants during cell cycle and somatic embryogenesis in alfalfa. Plant Physiol. 98, 621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, R.W., Jackson, P.K., and Kirschner, M.W. (1994). Mitosis in transition. Cell 79, 563–571. [DOI] [PubMed] [Google Scholar]
- Kishimoto, T. (1988). Regulation of metaphase by maturation promoting factor. Dev. Growth Differ. 30, 105–115. [DOI] [PubMed] [Google Scholar]
- Kishimoto, T. (1994). Cell reproduction: Induction of M-phase events by cyclin-dependent cdc2 kinase. Int. J. Dev. Biol. 38, 185–191. [PubMed] [Google Scholar]
- Lew, D.J., and Kornbluth, S. (1996). Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol. 8, 795–804. [DOI] [PubMed] [Google Scholar]
- Magyar, Z., et al. (1997). Cell cycle phase specificity of putative cyclin-dependent kinase variants in synchronized alfalfa cells. Plant Cell 9, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui, Y., and Markert, C.L. (1971). Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 177, 129–146. [DOI] [PubMed] [Google Scholar]
- McKibbin, R.S., Halford, N.G., and Francis, D. (1998). Expression of fission yeast cdc25 alters the frequency of lateral root formation in transgenic tobacco. Plant Mol. Biol. 36, 601–612. [DOI] [PubMed] [Google Scholar]
- Mironov, V., Van Montagu, M., and Inze, D. (1997). Regulation of cell division in plants: An Arabidopsis perspective. Prog. Cell Cycle Res. 3, 29–41. [DOI] [PubMed] [Google Scholar]
- Mironov, V., de Veylder, L., Van Montagu, M., and Inze, D. (1999). Cyclin-dependent kinases and cell division in plants: The nexus. Plant Cell 11, 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau, J.L., Marques, F., Barakat, A., Schatt, P., Lozano, J.C., Peaucellier, G., Picard, A., and Geneviere, A.M. (1998). Cdk2 activity is dispensable for the onset of DNA replication during the first mitotic cycles of the sea urchin early embryo. Dev. Biol. 200, 182–197. [DOI] [PubMed] [Google Scholar]
- Motomura, T. (1995). Premature chromosome condensation of the karyogamy-blocked sperm pronucleus in the fertilization in zygotes of the brown alga Fucus distichus. J. Phycol. 31, 108–113. [Google Scholar]
- Murray, A. (1992). Creative blocks: Cell-cycle checkpoints and feedback controls. Nature 359, 599–604. [DOI] [PubMed] [Google Scholar]
- Neufeld, T.P., and Edgar, B.A. (1998). Connections between growth and the cell cycle. Curr. Opin. Cell Biol. 10, 784–790. [DOI] [PubMed] [Google Scholar]
- Nishimoto, T., Uzawa, S., and Schlegel, R. (1992). Mitotic checkpoints. Curr. Opin. Cell Biol. 4, 174–179. [DOI] [PubMed] [Google Scholar]
- Nurse, P. (1994). Ordering S phase and M phase in the cell cycle. Cell 79, 547–550. [DOI] [PubMed] [Google Scholar]
- Pagano, M., Pepperkok, R., Lukas, J., Baldin, V., Ansorge, W., Bartek, J., and Draetta, G. (1993). Regulation of the cell cycle by the cdk2 protein kinase in cultured human fibroblasts. J. Cell Biol. 121, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulovich, A.G., Toczyski, D.P., and Hartwell, L.H. (1997). When checkpoints fail. Cell 88, 315–321. [DOI] [PubMed] [Google Scholar]
- Peter, M., and Herskowitz, I. (1994). Joining the complex: Cyclin-dependent kinase inhibitory proteins and the cell cycle. Cell 79, 181–184. [DOI] [PubMed] [Google Scholar]
- Peter, M., Nakagawa, J., Dorée, M., Labbé, J.C., and Nigg, E.A. (1990). In vitro disassembly of the nuclear lamina and M phase–specific phosphorylation of lamins by cdc2 kinase. Cell 61, 591–602. [DOI] [PubMed] [Google Scholar]
- Planas-Silva, M.D., and Weinberg, R.A. (1997). The restriction point and control of cell proliferation. Curr. Opin. Cell Biol. 9, 768–772. [DOI] [PubMed] [Google Scholar]
- Planchais, S., Glab, N., Trehin, C., Perennes, C., Bureau, J.M., Meijer, L., and Bergounioux, C. (1997). Roscovitine, a novel cyclin-dependent kinase inhibitor, characterizes restriction point and G2/M transition in tobacco BY-2 cell suspension. Plant J. 12, 191–202. [DOI] [PubMed] [Google Scholar]
- Quatrano, R.S. (1968). Rhizoid formation in Fucus zygotes: Dependence on protein and ribonucleic acid syntheses. Science 162, 468–470. [DOI] [PubMed] [Google Scholar]
- Rudner, A.D., and Murray, A.W. (1996). The spindle assembly checkpoint. Curr. Opin. Cell Biol. 8, 773–780. [DOI] [PubMed] [Google Scholar]
- Sauter, M., Mekhedov, S.L., and Kende, H. (1995). Gibberellin promotes histone H1 kinase activity and the expression of cdc2 and cyclin genes during the induction of rapid growth in deepwater rice internodes. Plant J. 7, 623–632. [DOI] [PubMed] [Google Scholar]
- Sauter, M., von Wiegen, P., Lörz, H., and Kranz, E. (1998). Cell cycle regulatory genes from maize are differentially controlled during fertilization and first embryonic cell division. Sex Plant Reprod. 11, 41–48. [Google Scholar]
- Shatten, H.C., Simerly, G., Maul, G., and Shatten, G. (1989). Microtubule assembly is required for the formation of pronuclei, nuclear lamin acquisition and DNA synthesis during mouse, but not sea urchin, fertilization. Gamete Res. 23, 309–322. [DOI] [PubMed] [Google Scholar]
- Sluder, G., Miller, F.J., Thompson, E.A., and Wolf, D.E. (1994). Feedback control of the metaphase–anaphase transition in sea urchin zygotes: Role of maloriented chromosomes. J. Cell Biol. 126, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swope, R.E., and Kropf, D.L. (1993). Pronuclear positioning and migration during fertilization in Pelvetia. Dev. Biol. 157, 269–276. [DOI] [PubMed] [Google Scholar]
- Verde, F., Labbé, J.-C., Dorée, M., and Karsenti, E. (1990). Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature 343, 233–238. [DOI] [PubMed] [Google Scholar]
- Vesely, J., Havlicek, L., Strnad, M., Blow, J.J., Donella-Deana, A., Pinna, L., Letham, D.S., Kato, J., Detivaud, L., Leclerc, S., and Meijer, L. (1994). Inhibition of cyclin-dependent kinases by purine analogues. Eur. J. Biochem. 224, 771–786. [DOI] [PubMed] [Google Scholar]
- Zhang, K., Letham, D.S., and John, P.C. (1996). Cytokinin controls the cell cycle at mitosis by stimulating the tyrosine dephosphorylation and activation of p34cdc2-like H1 histone kinase. Planta 200, 2–12. [DOI] [PubMed] [Google Scholar]