Abstract
A celery petiole phloem cDNA library was constructed and used to identify a cDNA that gives Saccharomyces cerevisiae cells the ability to grow on mannitol and transport radiolabeled mannitol in a manner consistent with a proton symport mechanism. This cDNA was named AgMaT1 (Apium graveolens mannitol transporter 1). The expression profile in source leaves and phloem was in agreement with a role for mannitol in phloem loading in celery. The identification in eukaryotes of a mannitol transporter is important because mannitol is not only a primary photosynthetic product in species such as celery but is also considered a compatible solute and antioxidant implicated in resistance to biotic and abiotic stress.
INTRODUCTION
Polyols (or sugar alcohols), the reduced form of aldoses and ketoses, can be either cyclic (cyclitols) or linear (alditols) and are present in all living forms, from bacteria to animals. Polyols are low molecular weight, highly soluble, nonreducing compounds. It has been estimated that ∼30% of the primary carbon production on earth goes through polyol synthesis in plant and algae (Bieleski, 1982). The accumulation of polyols in plants and fungi in response to abiotic stress (cold, water, or salt stress) has been taken as proof of their protective effects on metabolism. Therefore, some of the polyols (mannitol, sorbitol) are widely used as osmotica because they are considered nonpermeant and/or poorly metabolized, depending on the organism. For example, mannitol is used to maintain osmolarity during kidney and brain surgery (Poullis, 1999). Sorbitol and mannitol are commonly present at high concentrations in media designed to maintain the turgor pressure of protoplasts resulting from the degradation of the cell wall in yeast and plant cells. Polyols, notably mannitol, also are considered antioxidants that might be involved in fungal pathogenicity (Jennings et al., 1998; Hamilton and Holdom, 1999).
In some plant species, polyols are direct products of photosynthetic carbon fixation, together with sucrose. The three alditols most frequently found in angiosperms are mannitol, sorbitol, and galactitol. Sorbitol is found in several Rosaceae species, such as apple, pear, peach, and plum, and also in the Plantaginaceae (genus Plantago). Mannitol, the most widely distributed of the alditols, is present in >100 higher plant species, including members of the Rubiaceae (coffee), Oleaceae (privet, ash, olive), and Apiaceae (celery, carrot, parsley) (Lewis, 1984). Both polyols and sucrose are synthesized in autotrophic source leaves and transported inside the phloem (tissue specialized in the long-distance transport of photoassimilates) to heterotrophic sink organs to sustain plant growth and development (Loescher and Everard, 1996).
Sucrose and polyols are both nonreducing and are considered transport forms less accessible to cellular metabolism than glucose. The entry of sucrose into the conducting cells (so-called phloem loading) represents an important regulation point for plant productivity (Lalonde et al., 1999). In source leaves, phloem loading of sucrose is catalyzed by H+ cotransporters located at the plasma membrane of phloem cells. Within an organism, sucrose carriers belong to a multigene family, but the function of each member is not completely understood (Lalonde et al., 1999; Williams et al., 2000). Despite the diverse roles proposed for polyols, few data are available on the capacity of organisms to transport them, a sensitive point given their wide use as osmotica. No transport system has been described thus far for mannitol or sorbitol in eukaryotes. In prokaryotes, a mannitol phosphotransferase has been identified (Boer et al., 1994), but such systems are not found in higher organisms. In yeast, myo-inositol transporters have been cloned (Nikawa et al., 1991), and a Na+/myo-inositol transporter has been characterized in animal cells (Kwon et al., 1992). Celery is a mannitol-synthesizing plant that has been used as a model to study mannitol metabolism in relation to various stresses (Stoop et al., 1996). Moreover, the anatomy of the celery petiole allows direct access to the phloem, the tissue in which high mannitol transport activities have been described (Daie, 1986; Salmon et al., 1995). Several lines of evidence indicate that mannitol is actively loaded in the phloem of celery petioles (Daie, 1986) and that this transport step occurs by cotransport with protons (Daie, 1986; Salmon et al., 1995). In this article, we report the identification and characterization of a mannitol transporter from celery.
RESULTS
Molecular Cloning of AgMaT1
Because no sequence information for a mannitol transporter was available, we hypothesized that such a transporter would be a member of the major facilitator superfamily (MFS; Marger and Saier, 1993), which comprises a large variety of transporters for sugars and related molecules, such as myo-inositol. Comparison between various glucose transporters (MST1, GenBank accession number X66856; STP1, accession number S12042; STP4, accession number X66857; HUP1, accession number Y07520; HUP3, accession number X75440; GLUT1, accession number A27217), Lactobacillus brevis d-xylose transporter (XYLT, GenBank accession number AF045552), Escherichia coli arabinose transporter (araE, GenBank accession number Z99121), E. coli galactose transporter (GALP, GenBank accession number U28377), and yeast myo-inositol transporters (ITR1, GenBank accession number U33057; ITR2, accession number D90353) showed a conserved LLGFGVG region. This sequence was chosen as a template to design a degenerate 5′ rapid amplification of cDNA ends (RACE)–polymerase chain reaction (PCR) primer.
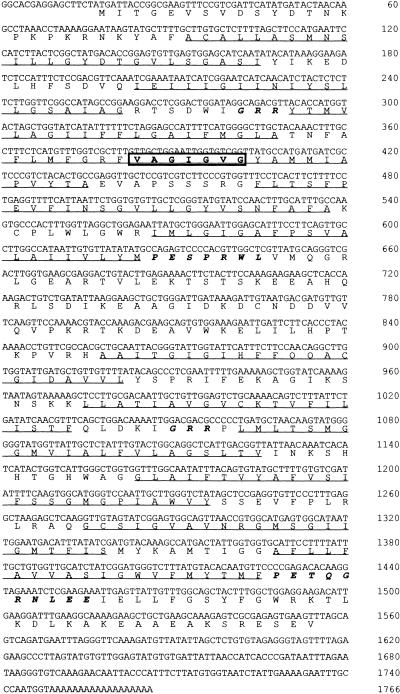
5′ RACE-PCR was run on mRNA extracted from the phloem of celery petiole. To obtain a full-length clone, we constructed a cDNA library from celery phloem tissues in λ-ZAP (Stratagene) and screened it with the 1-kb fragment obtained after 5′ RACE-PCR. Twenty-four positive clones out of 900,000 transformants were identified after three rounds of screening. Two of these clones, M7 and M22, were chosen for detailed analysis, but only M22 conferred to Saccharomyces cerevisiae MaDH4 cells the ability to grow on mannitol (see below). M22 therefore was named AgMaT1 (Apium graveolens mannitol transporter 1). The cDNA is 1766 bp long, with an open reading frame that encodes a protein of 513 amino acids and an estimated molecular mass of 56 kD (Figure 1; GenBank accession number AF215837). Hydropathy analysis of the deduced amino acid sequence (underlined in Figure 1) indicated 12 putative membrane-spanning domains, with the N and C termini and central loop located on the cytoplasmic side. In addition, the consensus sequences that are common characteristics in the sugar transporter subfamily of the MFS are present in AgMaT1 (italicized in Figure 1). PESPRXL and PETQGRXXXE are present at the ends of the sixth and 12th putative transmembrane domains, respectively, and the (R/K)XGR(R/K) motif is found between the second and third and the eighth and ninth transmembrane helices (Griffith et al., 1992). Together, these data confirmed that AgMaT1 is a member of the MFS.
Figure 1.
Nucleotide and Deduced Amino Acid Sequences of the AgMaT1 cDNA.
Putative transmembrane helices are underlined. Transmembrane regions and protein orientation were predicted using HMMTOP (Tusnady and Simon, 1998). The sequence (VAGIGVG) corresponding to LLGFGVG (used to design the initial degenerate primer for amplification) is boxed. The italicized residues correspond to the sequences conserved in the sugar transporter subfamily of the MFS.
AgMaT1 Transports Mannitol in Yeast
To demonstrate the function of AgMaT1 as a mannitol transporter, we transformed Saccharomyces cells with AgMaT1 and tested their ability to grow on mannitol as the sole carbon source and to transport radiolabeled mannitol. Saccharomyces cells are an excellent system in which to study heterologous expression of foreign genes (Frommer and Ninnemann, 1995), but previous studies had demonstrated the ability of a number of Saccharomyces strains to grow on mannitol after long-term adaptation (Quain and Boulton, 1987). This adaptation correlates with the appearance of mannitol metabolism and mannitol transport activities (N. Noiraud and R. Lemoine, unpublished data), making the expression of a plant mannitol transporter more difficult. To study the ability of a given cDNA to confer growth capacity on mannitol without interference from endogenous activities, we selected a strain unable to grow on medium containing mannitol as the sole carbon source (Σ22574d; Jauniaux et al., 1987) and crossed it with the strain Δα (Trp−; Marcireau et al., 1992) to confer a tryptophan auxotrophy. Δα cells can be grown on mannitol and thus display a detectable mannitol dehydrogenase activity (0.240 ± 0.007 μmol mannitol oxidized mg−1 protein min−1; mean ±sd of three independent experiments). Σ22574d and Δα were crossed, and microspores were then dissected and selected for tryptophan and leucine auxotrophies. From these spores, a strain called 2a was selected for its inability to grow on medium containing mannitol as the sole carbon source, with tryptophan and leucine auxotrophies. No mannitol dehydrogenase activity could be detected in 2a cells. To use yeast cells for selection of mannitol transporters, a constitutive expression of a mannitol metabolizing activity was necessary, so mannitol could be used as a carbon source by the cells. Saccharomyces cells do not express mannitol dehydrogenase unless they are induced by growth on mannitol for several days (Quain and Boulton, 1987; N. Noiraud and R. Lemoine, unpublished data). This induction period may extend the duration of the selection procedure and induce artifactual growth not due to the expression of a plant mannitol transporter. Therefore, a constitutive mannitol-hydrolyzing activity restricted to the cytoplasm of the yeast was introduced by integration of the yeast mannitol dehydrogenase gene (YEL070) amplified by PCR (see Methods) and cloned in the yeast-integrating plasmid YIP128A1 under the control of the ADH1 promoter (Riesmeier et al., 1992). The YEL070 gene was stably integrated into the leu2 gene of 2a. Several transformants showed mannitol dehydrogenase activity when grown on glucose. The strain with the highest activity (0.410 ± 0.011 μmol mannitol oxidized mg−1 protein min−1; mean ±sd of three independent experiments) was selected and named MaDH4 (ura3, trp1, LEU2, gap1-1, put4-1, uga4-1).
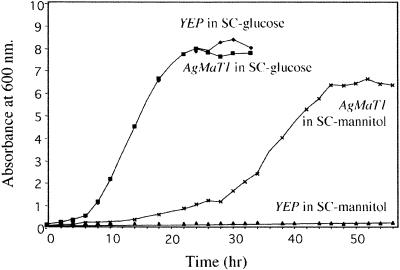
The AgMaT1 cDNA was cloned into the PstI-XhoI sites of the vector YEP112A1XE and introduced into MaDH4 cells (Dohmen et al., 1991). For a control, cells were transformed with YEP112A1XE alone. Cells were first tested for their ability to grow on mannitol as the sole carbon source. MaDH4 cells transformed with the empty plasmid did not grow on mannitol even after several days of culture, whereas cells expressing AgMaT1 grew well (Figure 2). Both strains grew equally well on glucose.
Figure 2.
Growth Curves of Transgenic Saccharomyces MaDH4 Cells in Minimal Medium Supplemented with Either 2% Glucose or 2% Mannitol.
AgMaT1 was subcloned into the PstI-XhoI sites of the yeast expression vector YEP112A1XE (YEP; Riesmeier et al., 1992). Competent MaDH4 cells were transformed with this construct (Dohmen et al., 1991), and YEP112A1XE was used as a control. YEP, MaDH4 containing the expression plasmid YEP112A1XE; AgMaT1, MaDH4 containing the AgMaT1 cDNA cloned in the PstI-XhoI sites of YEP112A1XE. SC, synthetic complete.
Uptake of 3H-Mannitol in Saccharomyces Expressing AgMaT1
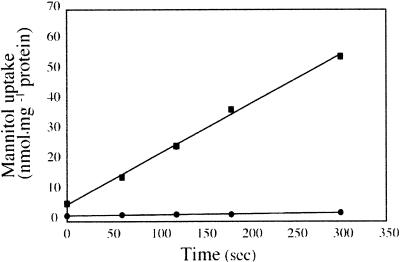
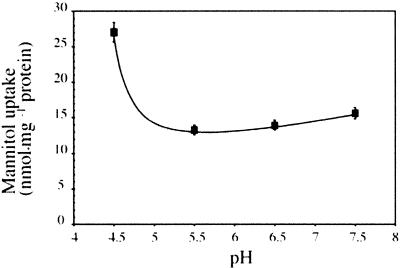
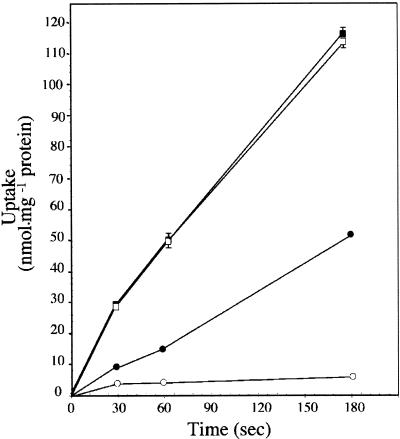
To test directly the ability of transformed cells to transport mannitol, MaDH4 cells were incubated in medium containing 3H-mannitol for selected times. Figure 3 shows that mannitol transport into control cells was negligible. However, AgMaT1-expressing yeast cells transported 3H-mannitol at high rates when grown in medium containing mannitol. Therefore, we concluded that AgMaT1 encodes a mannitol transporter. The pH dependence of mannitol transport by AgMaT1 was determined to be in the range pH 4.5 to 7.5 (Figure 4). AgMaT1 was much more active at pH 4.5, with a 50% loss of activity when the pH was increased to 5.5 (Figure 4). Moreover, a protonophore such as carbonyl cyanide m-chlorophenylhydrazone (CCCP) strongly inhibited mannitol transport catalyzed by AgMaT1 (Table 1). These data are in agreement with a mannitol/proton cotransport mechanism. Mannitol uptake was inhibited only slightly by the presence of the thiol reagent p-chloro mercury benzene sulfonic acid (PCMBS) (Table 1).
Figure 3.
Uptake of Mannitol by Transgenic Saccharomyces Cells.
Yeast cells were grown to the early logarithmic phase in minimal medium supplemented with either 2% glucose or 2% mannitol. During uptake, the mannitol concentration was 500 μM and the external pH was 4.5. Squares represent uptake by cells transformed with AgMaT1, and circles represent mannitol uptake by control cells transformed with the plasmid YEP112A1XE. The results are means ±sd of three independent experiments (four replicates per experiment).
Figure 4.
pH Dependence of Mannitol Transport in Transgenic Yeast Cells Expressing AgMaT1.
Measurements were performed as described for Figure 3 except that the incubation time was 2 min and the incubation medium was buffered at the indicated pH values with 25 mM Mes. The results are means ±sd of two independent experiments (four replicates per experiment).
Table 1.
Specificity of the AgMaT1 Mannitol Carrier a
| Added Compound | Absorption (%) |
|---|---|
| None | 100 |
| 50 μM CCCP | 14.5 ± 1.4 |
| 100 μM PCMBS | 71.6 ± 8.1 |
| Mannitol | 14.1 ± 7.1 |
| Dulcitol | 60.5 ± 6.6 |
| Sorbitol | 43.6 ± 11.0 |
| Xylitol | 41.2 ± 4.2 |
| Myo-inositol | 50.5 ± 4.9 |
| Mannose | 66.0 ± 7.5 |
| Sucrose | 86.6 ± 4.9 |
| Glucose | 22.4 ± 6.6 |
| Fructose | 35.4 ± 5.3 |
Culture of MaDH4 expressing AgMaT1 and uptake conditions are as given in Figure 3 with an incubation time of 2 min. Inhibitors (at the indicated concentration), sugars, and polyols (5.5 mM) were added 30 sec before labelled mannitol. The results are the mean ±sd of three independent experiments (four replicates per experiment).
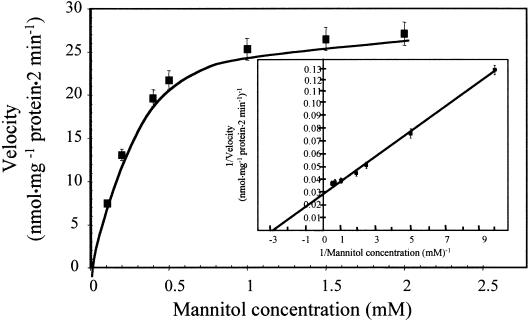
When the uptake of mannitol by AgMaT1 in yeast cells was studied for 2 min at concentrations ranging from 0.1 to 2.0 mM, a clear saturation was seen (Figure 5). The Lineweaver-Burk plot of the data (Figure 5, inset) gave an apparent affinity (Km) of 340 μM and a maximum velocity (Vmax) of 18 nmol mg−1 protein min−1.
Figure 5.
Concentration Dependence of Mannitol Transport in Transgenic Yeast Cells Expressing AgMaT1.
Culture conditions were as described in Figure 3. Mannitol uptake rates of MaDH4 control cells (with the YEP112A1XE plasmid) were subtracted from mannitol uptake rates of AgMaT1-expressing cells to determine the AgMaT1-dependent mannitol uptake rates at different mannitol concentrations. Uptake duration was 2 min. The results are means ±sd of one typical experiment (four replicates). The inset shows a Lineweaver-Burk plot of the uptake data.
Specificity of the Mannitol Carrier
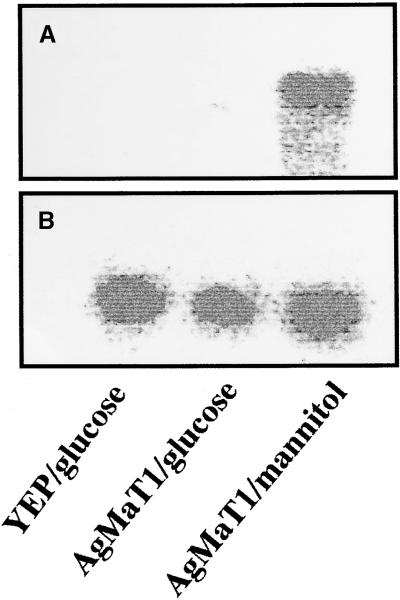
The substrate specificity of AgMaT1 was examined by measuring 3H-mannitol uptake in the presence of putative competitors at a 10-fold external concentration. The results are listed in Table 1. Sucrose had no influence on the transport rates. Other common polyols, such as dulcitol, sorbitol (both hexitols), xylitol (a pentitol), and myo-inositol (cyclic), inhibited mannitol uptake by 40 to 60%. Mannose, the ose form of mannitol, also inhibited mannitol uptake slightly. It is interesting to note that sugars stored in the parenchyma of celery petioles (glucose and fructose; Keller and Matile, 1989), and specifically glucose, were highly inhibitory to mannitol uptake. This finding would indicate a rather low specificity of AgMaT1 for mannitol. However, glucose inhibition was studied further because no marked glucose inhibition of mannitol uptake was reported in plasma membrane vesicles from celery phloem cells (Salmon et al., 1995). When MaDH4 cells expressing AgMaT1 were grown on glucose, no mannitol uptake could be measured; this was related to the absence of corresponding AgMaT1 mRNA detection (Figure 6). Repression of AgMaT1 expression by glucose (as shown for other Saccharomyces endogenous genes involved in carbon metabolism; Carlson, 1998) was demonstrated by growing cells expressing AgMaT1 on synthetic complete (SC) medium supplemented with either 2% glucose (1), 2% mannitol (2) or 3% glycerol, and 0.05% glucose (3, nonrepressing conditions; Lutfiyya et al., 1998). The corresponding mannitol uptake values after 30 sec were (1) 3.7 ± 0.7 nmol mg−1 protein for cells grown on glucose, (2) 12.7 ± 1.8 nmol mg−1 protein for cells grown on mannitol, and (3) 14.3 ± 2.5 nmol mg−1 protein for cells grown on glycerol (mean ±se of three independent experiments with four replicates per experiment). Moreover, we could not demonstrate that the inhibition of mannitol uptake by glucose was of a competitive type (L. Maurousset and R. Lemoine, unpublished data). To check whether AgMaT1 was able to transport glucose, we expressed it in the Saccharomyces strain RS453 already used to express plant glucose transporters (Sauer and Stadler, 1993) because of a rather low background of glucose uptake. AgMaT1 was cloned in the yeast plasmid pDR196 (Rentsch et al., 1995), and RS453 cells were transformed with this construct. Data presented in Figure 7 show that uptake of radiolabeled glucose was similar in RS453 cells expressing AgMaT1 and in cells transformed with the empty plasmid. Under the same conditions, uptake of radiolabeled mannitol was detected only in RS453 cells transformed with AgMaT1, a result similar to the one obtained in MaDH4 cells (Figure 3). Because mannitol uptake represented one-third of glucose uptake after 3 min, transport of glucose through AgMaT1 would have been detected, even if the basal glucose uptake was higher than mannitol uptake. Because no AgMaT1-dependent glucose uptake was measured, we therefore concluded that glucose is not transported by AgMaT1.
Figure 6.
Expression Pattern of AgMaT1 in Saccharomyces Cells.
Total RNA was extracted, and the RNA gel blot analysis was as described by Noiraud et al. (2000). Hybridization was conducted with AgMaT1 (A) or a riboprobe (B) for calibration. Level of hybridization was analyzed though radioactivity counting with an Instant Imager (Packard Instrument Co.). Saccharomyces cells were transformed either with the empty plasmid YEP and grown on glucose or with the plasmid containing AgMaT1 and grown on either glucose or mannitol.
Figure 7.
Comparison of Glucose and Mannitol Uptake by Transgenic Saccharomyces Cells.
RS453 cells were grown to the early logarithmic phase in SC medium supplemented with 3% glycerol and 0.05% glucose. Uptake of glucose (squares) and mannitol (circles) was measured as described in Figure 3. Closed symbols represent uptake by cells transformed with AgMaT1, and open symbols represent mannitol uptake by control cells transformed with the plasmid pDR196. The results are from one typical experiment (four replicates per experiment). The standard deviation is presented when larger than symbols.
Expression Profile of AgMaT1
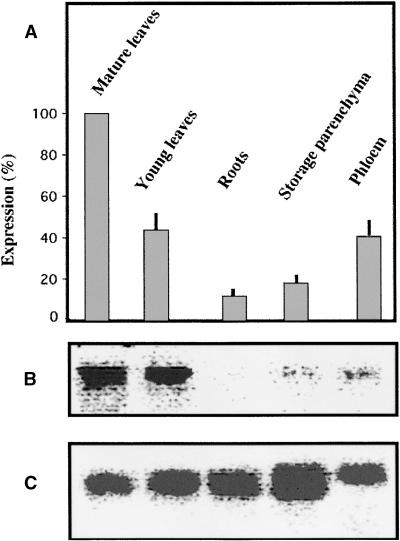
The expression of AgMaT1 was studied by RNA gel blot analysis. The results obtained were calibrated with an internal probe (Noiraud et al., 2000) and are presented in Figure 8 as a histogram showing the mean from four determinations and a typical RNA gel blot analysis. Expression was maximal in mature leaves (taken as 100% expression) and could be detected in younger leaves and phloem from petioles of mature leaves. Expression was much lower in storage parenchyma cells from mature petioles and even lower in roots.
Figure 8.
Expression Pattern of AgMaT1 in Selected Organs from Celery.
(A) Data are given as percentage of maximal expression in mature leaves and are means of four independent determinations ±se. Phloem and storage parenchyma were extracted from the petioles of mature leaves.
(B) Typical scan of the image obtained after radioactivity counted by the Instant Imager (AgMaT1 probe).
(C) Scan of the image obtained after calibration with a riboprobe (same RNA gel blot analysis as in [B]).
Total RNA was extracted, and the RNA gel blot analysis was as described by Noiraud et al. (2000). After hybridization with labeled AgMaT1 probe, the radioactivity associated with the probe bound to AgMaT1 mRNA was counted with an Instant Imager (Packard Instrument Co.). Calibration was made with a riboprobe.
Sequences Homologous with AgMaT1
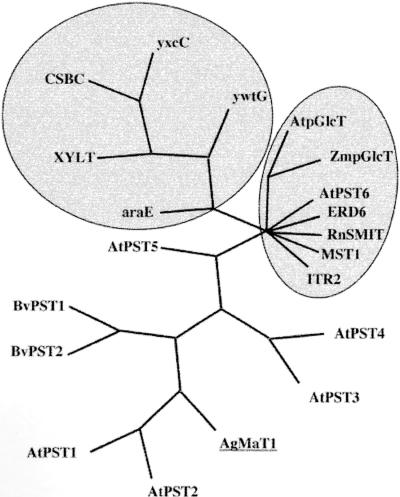
Sequences homologous with AgMaT1 were searched using the BLAST program, and a phylogenetic tree was constructed from a CLUSTAL alignment (Figure 9). The closest sequences found were all of unknown function. There were two from sugar beet (63% identity for both) and five from Arabidopsis (45 to 64% identity). Other sequences showing homologies were from bacteria, and these clustered together (Figure 9). Another related cluster was composed of polyol transporters (the myo-inositol transporter from Schizosaccharomyces pombe ITR2, the Na+/myo-inositol transporter from rat RnSMIT), ERD6 (identified in stressed Arabidopsis plants), the H+-hexose cotransporter MST1 from tobacco (the closest plant cDNA with known function), and glucose carriers from chloroplasts (pGlcTs). The identification of two proteins sharing homologies with myo-inositol transporters has been reported in the ice plant, but without sequence information (Nelson et al., 1999). Therefore, AgMaT1 does not appear to be closely related to the two major families of sugar carriers known in plants, the monosaccharide and sucrose carriers (Williams et al., 2000), and it is also distant from myo-inositol transporters.
Figure 9.
Phylogenetic Tree for AgMaT1.
Homologous sequences were obtained through the National Center for Biotechnology Information via the BLAST site (Altschul et al., 1997), alignment was performed with DNASTAR-MegAlign (Genetics Computer Group, Madison, WI), and the tree was designed with PAUP 4.0 (Sinauer Associates, Sunderland, MA). The upper left cluster includes genes from bacteria, and the cluster at right includes several glucose and myo-inositol transporters from plants, yeast, and animals. GenBank accession numbers are as follows: probable sugar transporters from sugar beet, BvPST1, U64903 and BvPST2, U64902; putative sugar transporters from Arabidopsis, AtPST1, ACOO713412; AtPST2, AC00713413; AtPST3, Z99708; AtPST4, AC006135; AtPST5, AC006234 and AtPST6, AC002335; probable metabolite transporter from Bacillus subtilis CSBC, AB005554; metabolite transport protein homolog from B. subtilis yxcC, Z99124; metabolite transport protein homolog from B. subtilis ywtG, Z92954; d-xylose proton symporter from L. brevis XYLT, AF045552; l-arabinose transporter from B. subtilis araE, Z99121H+; monosaccharide transporter from tobacco MST1, X66856; plastidic glucose transporter from Arabidopsis AtpGlcT, Af215855; plastidic glucose transporter from maize ZmpGlcT, AF251854; myo-inositol transporter 2 from S. pombe ITR2, Z95334; sodium myo-inositol transporter from rat RnSMIT, CAA04650; and Arabidopsis putative sugar transporter ERD6, D89051.
DISCUSSION
Little is known regarding the transport of polyols in polyol-synthesizing plants. However, their fate appears very similar to that of sucrose, because polyols are synthesized mainly in source tissues (mature leaves), in which the enzymes involved in synthesis have been detected, and are used in sink tissues (reviewed in Loescher and Everard, 1996). The long-distance transport of polyols was suggested after the detection of significant amounts of polyols in sieve tube exudates from a number of species (Zimmerman and Ziegler, 1975). This transport step may be highly relevant when plants that experience a stress (salt or water stress) react by synthesizing large amounts of polyols as an osmoprotectant; these polyols then have to be translocated to the different organs (Stoop et al., 1996). The metabolism of mannitol, the most common hexitol, has been studied extensively in celery, and the pathways for synthesis and degradation have been characterized down to the genes coding the major enzymes involved (Williamson et al., 1995; Everard et al., 1997). Transport of mannitol also was investigated in the same species because the phloem of the leaf petiole is easily accessible for isolation. Phloem strands have been used to demonstrate that a mannitol uptake system was present in this tissue (Daie, 1986). Later experiments on plasma membrane vesicles purified from celery phloem strands confirmed that mannitol uptake occurred by cotransport with protons (Salmon et al., 1995) and that the mannitol transport system was different from the sucrose system.
To investigate the transport of mannitol and compare it with the transport of sucrose, we chose to clone the genes coding the corresponding carriers. This was a relatively easy task in the case of the sucrose transporter (Noiraud et al., 2000) because of the high sequence conservation between species (Williams et al., 2000). However, as indicated above, no mannitol or hexitol carrier has been cloned thus far. After some unsuccessful attempts to isolate a mannitol carrier by heterologous complementation in yeast (data not shown), we hypothesized that the mannitol transporter is a member of the MFS and therefore defined a region highly conserved between several known H+/monosaccharide carriers and the myo-inositol cotransporters from yeast. This conserved region was used to design a primer for 5′ RACE-PCR. The PCR fragment obtained was used to screen a cDNA library. To compensate for the low selectivity of the conserved region, we performed all of the subsequent experiments with cDNA obtained from the phloem of celery petioles, an organ in which the presence of a mannitol transporter had been clearly demonstrated (Daie, 1987; Salmon et al., 1995).
Two cDNAs were obtained and studied, but only one passed the functional tests. AgMaT1 sequence shows the classic alternation of hydrophobic and hydrophilic segments typical for membrane proteins, with a hypothetical 12–transmembrane segment structure. All of the major signatures of members of the MFS were found in the AgMaT1 sequence. However, this finding did not indicate that AgMaT1 was able to transport mannitol. Therefore, a system was devised to demonstrate this function in yeast. Because of the long-term induction of growth on mannitol of many yeast strains (Quain and Boulton, 1987; N. Noiraud and R. Lemoine, unpublished results), a strain that did not grow on mannitol even after 2 weeks was selected and a constitutive mannitol dehydrogenase activity was introduced into it (strain MaDH4). The Saccharomyces mannitol dehydrogenase converts mannitol to fructose (a difference with the plant enzyme, which converts mannitol to mannose), which can be used directly for cell metabolism. When complemented with a mannitol carrier, this strain grew on mannitol as the sole carbon source. MaDH4 cells transformed with AgMaT1 grew on mannitol, whereas control cells transformed with the empty plasmid did not (Figure 2). Moreover, uptake of radiolabeled mannitol was measured only in cells expressing AgMaT1 (Figure 3).
The uptake characteristics determined in Saccharomyces cells were close to those determined in plants. First, the uptake seemed to occur by cotransport with protons because it was almost abolished by a protonophore (CCCP) and was maximal at acidic pH. On the other hand, the thiol reagent PCMBS had only a marginal effect on mannitol uptake, a feature already described in phloem strands (Daie, 1986) and phloem plasma membrane vesicles (Salmon et al., 1995). This is in marked contrast to the high sensitivity of the sucrose carrier to PCMBS (Noiraud et al., 2000). The kinetic parameters of AgMaT1 determined in yeast (Km = 340 μM and Vmax = 18 nmol mg−1 protein min−1) were in the same range as the values determined for the uptake of mannitol in plasma membrane vesicles purified from phloem strands of celery (Km = 644 μM and Vmax = 6.98 nmol mg−1 protein 30 sec−1; Salmon et al., 1995). Interestingly, the affinity of the sucrose transport system measured in plasma membrane vesicles was higher than that of the mannitol transport system (280 versus 644 μM; Salmon et al., 1995), and a similar relationship was found when the corresponding cDNAs were expressed in Saccharomyces cells (139 μM for sucrose and 340 μM for mannitol; Noiraud et al., 2000; this study). Therefore, all of the kinetic characteristics were in agreement with AgMaT1 corresponding to the mannitol transport activity characterized in phloem plasma membrane vesicles. This was further confirmed by the localization of AgMaT1 expression (Figure 8).
AgMaT1 was expressed mainly in mature leaves and at approximately half this level in young leaves and in phloem from petioles of mature leaves. The petioles of mature celery leaves are considered source organs, and it has been established that mannitol and sucrose are reloaded in the phloem from their transient sites of storage in the petiole (Keller and Matile, 1989). However, the most active site of phloem loading is certainly in the veins of mature leaves, where expression of AgMaT1 was higher. In fact, a similar expression pattern was observed for AgSUT1, the sucrose carrier from celery (Noiraud et al., 2000). AgMaT1 expression also was detected in storage parenchyma cells, although at a much lower level. Mannitol is stored in these cells without further metabolism (Keller and Matile, 1989), in marked contrast to sucrose, which is cleaved to hexoses. A proton motive force–dependent uptake of mannitol was detected in plasma membrane vesicles obtained from petiole storage parenchyma (Salmon et al., 1995). Therefore, the different characteristics of AgMaT1 were all in agreement with previous physiological and biochemical observations and indicated that AgMaT1 is the transporter responsible for mannitol uptake in phloem cells of celery.
Inhibitor studies (Table 1) seemed to indicate that the selectivity of AgMaT1 is rather poor because several other polyols (cyclic and linear) and mannose inhibited mannitol uptake. However, the inhibition measured was much lower than the inhibition of mannitol on its own uptake. On the other hand, the inhibition by sucrose was marginal, confirming the assumption that sucrose and mannitol uptake are mediated by two independent transport systems in celery (Daie, 1987; Salmon et al., 1995; Noiraud et al., 2000). The most surprising result was the high inhibition exerted by glucose, in contrast to data obtained in plasma membrane vesicles, in which glucose inhibition was in the same range as sorbitol inhibition (Salmon et al., 1995). However, some data indicated that this result could be an artifact linked to yeast expression. The expression of AgMaT1 was repressed when yeast cells were grown on glucose (see Results), and the inhibition of mannitol uptake by glucose was not competitive (data not shown). More convincingly, glucose was not transported by Saccharomyces expressing AgMaT1 (Figure 7).
It will be interesting to determine more precisely the specificity of AgMAT1 in another expression system, such as Xenopus oocytes. The question of the selectivity of AgMaT1 is not trivial, especially considering the homologous sequences (Figure 9). The species in which those sequences have been found (sugar beet and Arabidopsis) have not been described as mannitol producers. However, Bieleski (1982) recommended assuming the presence of a polyol in a species until evidence shows otherwise. The situation might be even more complicated, because sorbitol has been detected in germinating soybean (Kuo et al., 1990) and maize seed (Carey et al., 1982; Shaw and Dickinson, 1984) but not in adult plants. Moreover, genes homologous to mannitol dehydrogenase have been found in species that do not produce mannitol, such as Arabidopsis (Williamson et al., 1995), tobacco (Jennings et al., 1998), and tomato (Lauter, 1996). Mannitol dehydrogenase has been proposed to be involved in plant defense by degrading mannitol produced by pathogenic fungi (Jennings et al., 1998). Whether a mannitol transporter is necessary for the plant to import mannitol of fungal origin inside the cell to be degraded, or whether the mannitol dehydrogenase enzyme is excreted, remains to be determined. Therefore, AgMaT1 could represent the first member of a new plant transporter family, different from the hexose and sucrose transporter families (Williams et al., 2000), which could be involved in the uptake of polyols or related substrates. AgMaT1 is not closely related to the myo-inositol transporter from yeast (Figure 9). The cloning of myo-inositol transporters from ice plant (Nelson et al., 1999) should indicate whether there is a close relationship between those carriers and AgMaT1 when the corresponding sequences are available.
Given the number of species that transport polyols and the number of roles attributed to polyols, such as antioxidant, compatible solute, and osmoprotectant (Stoop et al., 1996), the cloning of the first mannitol carrier in eukaryotes is of major importance. Together with breakthroughs in the characterization of mannitol metabolism (Williamson et al., 1995; Everard et al., 1997), our data should help clarify why some plants synthesize and transport polyols together with sucrose. Plants have been genetically transformed to synthesize mannitol to increase their resistance to salt stress (Tarczynski et al., 1993). In those plants, mannitol was synthesized ubiquitously, a situation clearly different from that in celery, in which the synthesis and use of mannitol occur in different organs and in which phloem transport exerts some level of control (Stoop et al., 1996). Altering the expression of the genes for mannitol transport should clarify these different points and confirm unambigously the role of AgMaT1 in mannitol phloem loading, but an efficient transformation technique for celery is still lacking (Catlin et al., 1988).
METHODS
Plant Material
Celery plants (Apium graveolens var dulce cv Vert d'Elne) were grown under greenhouse conditions, as described by Davis et al. (1988). Phloem strands were isolated from petioles of mature leaves, as described by Daie (1987).
Bacteria and Yeast Strains
The following strains were used in this study: Escherichia coli strains DH5α (supE44, ΔflacU169 [φ80, lacZM15], hsdR17, recA, endA1, gyrA96, thi-1, relA1), XL1Blue MRF′ (Stratagene), and SOLR (Stratagene) were cultured according to standard techniques (Sambrook et al., 1989). The yeast strain MaDH4 (ura3, trp1, LEU2, gap1-1, put4-1, uga4-1) expresses the yeast mannitol dehydrogenase gene and was used for functional characterization of AgMaT1 (for A. graveolens mannitol transporter 1) cDNA. Strain 2a was obtained from a cross between strains Δα (MATα, ura3, trp1, leu2) and Σ22574d (MATa, ura3-1, gap1-1, put4-1, uga4-1) (Jauniaux et al., 1987). The Saccharomyces cerevisiae strain RS453 (Mata, ade2-1, trp1-1, can1-100, leu2-3, his3-1, ura3-52) was used as described by Sauer and Stadler (1993).
5′ Rapid Amplification of cDNA Ends–Polymerase Chain Reaction
Total RNA of celery phloem was isolated as described by Kay et al. (1987). First-strand cDNA was reverse transcribed from total RNA primed with a LLGFGVG (5′-CCNACNCC[G/A]AANGGNA[G/A]NA[G/A]-3′) degenerate primer using SuperScript II RNaseH− reverse transcriptase (Stratagene). After removal of the RNA template by RNaseH (Eurogentec, Seraing, Belgium), a (dC)16 anchor priming site at the 3′ end of the single-stranded cDNA was created using terminal deoxynucleotidyl transferase (Gibco BRL). By using a (dG)16 anchor primer and the LLGFGVG degenerate primer, amplification was achieved with the following conditions: 2 min at 95°C; 30 cycles of denaturation at 95°C for 2 min, annealing at 55°C for 2 min, and elongation at 72°C for 2 min; and 5 min at 72°C. The polymerase chain reaction (PCR) products were analyzed by agarose gel electrophoresis and cloned into the pGEM-T Easy vector (Promega). The 5′ rapid amplification of cDNA ends (RACE) products were sequenced in both strands.
Construction and Screening of a Phloem-Specific cDNA Library
Total RNA of phloem strands was isolated as described by Kay et al. (1987). Poly(A)+ RNA was purified from total RNA with the PolyATtract mRNA isolation system (Promega). A unidirectional EcoRI-XhoI cDNA library was constructed in a Uni-Zap XR vector (Stratagene). Recombinant phages (900,000) were screened using the 5′ RACE product as a radioactive probe according to the manufacturer's protocols (Stratagene). Hybond-N nylon filters (Amersham, Les Ulis, France) were hybridized overnight at 42°C using standard conditions (Stratagene). Filters were washed for 15 min at 42°C with 0.1% SDS and 2 × SSC (1 × SSC is 0.15 M NaCl and 0.015 M sodium citrate), for 15 min at 50°C with 0.1% SDS and 2 × SSC, and for 30 min at 50°C with 0.1% SDS and 1 × SSC. In vivo excision was performed on the 24 clones that gave positive signals during the three rounds of screening. The cDNAs were sequenced at the Eurogentec DNA sequencing department. Sequence comparisons were performed through the National Center for Biotechnology Information via the BLAST site (Altschul et al., 1997). Transmembrane regions and protein orientations were predicted using HMMTOP (Hidden Markov Model Topology Prediction; Tusnady and Simon, 1998).
Expression of AgMaT1 in S. cerevisiae
AgMaT1 cDNA was ligated into the PstI-XhoI sites of the yeast shuttle vector YEP112A1XE (Riesmeier et al., 1992). This vector allows expression of full-length cDNAs under the control of the Saccharomyces ADH1 promoter. For some experiments, AgMaT1 cDNA was ligated into the PstI-XhoI sites of pDR196 (Rentsch et al., 1995) with the Saccharomyces PMA1 promoter. Competent yeast cells were prepared and transformed according to Dohmen et al. (1991). Saccharomyces cells transformed with the corresponding empty plasmids were used as controls.
Growth Rate and Mannitol Dehydrogenase Activity Determinations
Yeast cultures were grown in synthetic complete (SC) liquid medium supplemented with 2% glucose or 2% mannitol. Aliquots of the cultures were taken periodically, and their absorbance was measured at 600 nm. Mannitol dehydrogenase activity at 30°C was determined according to Quain and Boulton (1987).
Uptake of Radiolabeled Mannitol and Glucose
Yeast cells were grown to the early logarithmic phase, washed with distilled water, and resuspended to 1% (w/v) in SC medium containing 25 mM Mes buffer, pH 4.5. The pH dependence was determined in SC medium buffered at different pH values with 25 mM Mes. Aliquots (100 μL) of the cell suspension were incubated in 100 μL of a solution containing 3H-mannitol (final specific activity, 14.8 MBq mmol−1) at 28°C for 0, 60, 120, 180, and 300 sec. For some experiments, 3H-glucose (final specific activity, 14.8 MBq mmol−1) was used. The reaction was stopped by the addition of 8 mL of ice-cold water and filtration on fiberglass filters (model 13400-25-S; Sartorius, Palaiseau, France). The radioactivity incorporated in the cells was determined with a liquid scintillation counter (Packard Instrument Co., Rungis Cedex, France). For inhibition and competition studies, the effector was added 30 sec before mannitol (p-chloro mercury benzene sulfonic acid [PCMBS]).
RNA Isolation and RNA Gel Blot Analyses
Total RNA was isolated as described by Kay et al. (1987). Twenty micrograms of total RNA was separated on 1.2% agarose gels containing formaldehyde and transferred to nylon filters, as described by Sambrook et al. (1989). Hybridization was at 65°C (Noiraud et al., 2000), and the PstI-XhoI fragment of AgMaT1 cDNA was used as a probe. The final wash was 0.1% SDS and 0.5 × SSC at 68°C for 15 min. Radioactivity was counted with an Instant Imager (Packard Instrument Co.). The amount of RNA loaded in each lane was calibrated after hybridization with a 25S riboprobe and Instant Imager counting. The values obtained with the riboprobe were used to calculate the relative intensities of the signals in each lane (Noiraud et al., 2000).
NOTE ADDED IN PROOF
AgMaTi is not closely related to MITR 1 and 2 (26 and 24% of similarity at the amino acid level, respectively), two Na+/myo-inositol transporter identified recently in Mesembryanthemum cyrstallinum (Chauhan et al. [2000]. Na+/myo-inositol symporters and Na+/H+-antiport in Mesembryanthemum cyrstallinum. Plant J. 24, 511–522).
Acknowledgments
The authors thank Dr. M. Regnacq (University of Poitiers, Poitiers, France) for help in the construction of strain MaDH4, Prof. N. Sauer (University of Erlangen, Erlangen, Germany) for providing the RS453 strain, Dr. D. Wipf (University of Tübingen, Tübingen, Germany) for the phylogenetic tree, and Prof. S. Delrot (University of Poitiers) and Prof. W. Frommer (University of Tübingen) for critical reading of the manuscript. This work was supported by the Centre National de la Recherche Scientifique, the French Ministry for Education and Research (grant to N.N.), and by Région Poitou-Charentes.
References
- Altschul, S.F., Madden, T.L., Schäffer, A.A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D.J. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski, R.L. (1982). Sugar alcohols. In Encyclopedia of Plant Physiology, Vol. 13, F. Loewus and W. Tanner, eds (Berlin: Springer-Verlag), pp. 158–192.
- Boer, H., Hoeves-Duurkens, R.H., Schuurman-Wolters, G.K., Dijkstra, A., and Robillard, G.T. (1994). Expression, purification, and kinetic characterization of the mannitol transport domain of phosphoenolpyruvate dependent mannitol phosphotransferase system of Escherichia coli. J. Biol. Chem. 269, 17863–17871. [PubMed] [Google Scholar]
- Carey, E.E., Dickinson, D.B., Wei, L.Y., and Rhodes, A.M. (1982). Occurence of sorbitol in maize. Phytochemistry 21, 1909–1911. [Google Scholar]
- Carlson, M. (1998). Regulation of glucose utilization in yeast. Curr. Opin. Genet. Dev. 8, 560–564. [DOI] [PubMed] [Google Scholar]
- Catlin, D., Ochoa, O., McCormick, S., and Quiros, C.F. (1988). Celery transformation by Agrobacterium tumefaciens: Cytological and genetic analysis of transgenic plants. Plant Cell Rep. 7, 100–103. [DOI] [PubMed] [Google Scholar]
- Daie, J. (1986). Kinetics of sugar transport in isolated vascular bundles and phloem tissue of celery. J. Am. Soc. Hortic. Sci. 111, 216–220. [Google Scholar]
- Daie, J. (1987). Sucrose uptake in isolated phloem of celery is a single saturable transport system. Planta 171, 474–482. [DOI] [PubMed] [Google Scholar]
- Davis, J.M., Fellman, J.K., and Loescher, W.H. (1988). Biosynthesis of sucrose and mannitol as a function of leaf age in celery (Apium graveolens L.). Plant Physiol. 86, 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen, R.J., Strasser, A.W.M., Höner, C.B., and Hollenberg, C.P. (1991). An efficient transformation procedure enabling long term storage of competent cells of various yeast genera. Yeast 7, 691–692. [DOI] [PubMed] [Google Scholar]
- Everard, J.D., Cantini, C., Grumet, R., Plummer, J., and Loescher, W.H. (1997). Molecular cloning of mannose-6-phosphate reductase and its developmental expression in celery. Plant Physiol. 113, 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer, W.B., and Ninnemann, O. (1995). Heterologous expression of genes in bacterial, fungal, animal, and plant cells. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 419–444. [Google Scholar]
- Griffith, J.K., Baker, M.E., Rouch, D.A., Page, M.G.P., Skurray, R.A., Paulsen, I.T., Chater, K.F., Baldwin, S.A., and Henderson, P.J.F. (1992). Membrane transport proteins: Implications of sequence comparisons. Curr. Opin. Cell Biol. 4, 684–695. [DOI] [PubMed] [Google Scholar]
- Hamilton, A.J., and Holdom, M.D. (1999). Antioxidant systems in the pathogenic fungi of man and their role in virulence. Med. Mycol. 37, 375–389. [DOI] [PubMed] [Google Scholar]
- Jauniaux, J.C., Van Den Bol, M., Visser, S., Broman, K., and Grenson, M. (1987). Nitrogen catabolite regulation of proline permease in Saccharomyces cerevisiae: Cloning of the PUT4 gene and study of PUT4 RNA levels in the wild-type and the mutant strains. Eur. J. Biochem. 164, 601–606. [DOI] [PubMed] [Google Scholar]
- Jennings, D.B., Ehrenshaft, M., Pharr, D.M., and Williamson, J.D. (1998). Roles for mannitol and mannitol dehydrogenase in active oxygen–mediated plant defense. Proc. Natl. Acad. Sci. USA 95, 15129–15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, R., Chan, A., Daly, M., and MacPherson, J. (1987). Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 236, 1299–1302. [DOI] [PubMed] [Google Scholar]
- Keller, F., and Matile, P. (1989). Storage of sugars and mannitol in petioles of celery leaves. New Phytol. 113, 291–299. [DOI] [PubMed] [Google Scholar]
- Kuo, T.M., Doehlert, D.C., and Crawford, C.G. (1990). Sugar metabolism in germinating soybean seeds: Evidence for the sorbitol pathway in soybean axes. Plant Physiol. 93, 1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, H.M., Yamauchi, A., Uchida, S., Preston, A.S., Garcia-Perez, A., Burg, M.B., and Handler, J.S. (1992). Cloning of the cDNA for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. J. Biol. Chem. 267, 6297–6301. [PubMed] [Google Scholar]
- Lalonde, S., Boles, E., Hellmann, H., Barker, L., Patrick, J.W., Frommer, W.B., and Ward, J.M. (1999). The dual function of sugar carriers: Transport and sugar sensing. Plant Cell 11, 707–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter, F.R. (1996). Root-specific expression of the LeRse-1 gene in tomato is induced by exposure of the shoot to light. Mol. Gen. Genet. 28, 751–754. [DOI] [PubMed] [Google Scholar]
- Lewis, D.H. (1984). Physiology and metabolism of alditols. In Storage Carbohydrates in Vascular Plants, D.H. Lewis, ed (Cambridge, UK: Cambridge University Press), pp. 157–179.
- Loescher, W.H., and Everard, J.D. (1996). Sugar alcohol metabolism in sinks and sources. In Photoassimilate Distribution in Plants and Crops, E. Zamski and A.A. Schaffer, eds (New York: Marcel Dekker), pp. 185–207.
- Lutfiyya, L.L., Iyer, V.R., DeRisi, J., DeVit, M.J., Brown, P.O., and Johnston, M. (1998). Characterization of three related glucose repressors and genes they regulate in Saccharomyces cerevisiae. Genetics 150, 1377–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcireau, C., Guyonnet, D., and Karst, F. (1992). Construction and growth properties of a yeast strain defective in sterol 14-reductase. Curr. Genet. 22, 267–272. [DOI] [PubMed] [Google Scholar]
- Marger, M.D., and Saier, J.M.H. (1993). A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem. Sci. 18, 13–20. [DOI] [PubMed] [Google Scholar]
- Nelson, D.E., Koukoumanos, M., and Bonhert, H.J. (1999). Myo-inositol–dependent sodium uptake in ice plant. Plant Physiol. 119, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa, J.I., Tsulkagoshi, Y., and Yamashita, S. (1991). Isolation and characterization of two distinct myo-inositol transporter genes of Saccharomyces cerevisiae. J. Biol. Chem. 266, 11184–11191. [PubMed] [Google Scholar]
- Noiraud, N., Delrot, S., and Lemoine, R. (2000). The sucrose transporter of celery: Identification and expression during salt stress. Plant Physiol. 122, 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poullis, M. (1999). Mannitol and cardiac surgery. Thorac. Cardiovasc. Surg. 47, 58–62. [DOI] [PubMed] [Google Scholar]
- Quain, D.E., and Boulton, C.A. (1987). Growth and metabolism of mannitol by strains of Saccharomyces cerevisiae. J. Gen. Bacteriol. 133, 1675–1684. [DOI] [PubMed] [Google Scholar]
- Rentsch, D., Laloi, M., Rouhara, I., Schmelzer, E., Delrot, S., and Frommer, W. (1995). NTR1 encodes a high affinity oligopeptide transporter in Arabidopsis. FEBS Lett. 370, 264–268. [DOI] [PubMed] [Google Scholar]
- Riesmeier, J.W., Willmitzer, L., and Frommer, W.B. (1992). Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 11, 4705–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon, S., Lemoine, R., Jamaï, A., Bouché-Pillon, S., and Fromont, J.C. (1995). Study of sucrose and mannitol transport in plasma-membrane vesicles from phloem and non-phloem tissues of celery (Apium graveolens L.) petioles. Planta 197, 76–83. [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Sauer, N., and Stadler, R. (1993). A sink-specific H+/monosaccharide co-transporter from Nicotiana tabacum: Cloning and heterologous expression in baker's yeast. Plant J. 4, 601–610. [DOI] [PubMed] [Google Scholar]
- Shaw, J.R., and Dickinson, D.B. (1984). Studies of sugar and sorbitol in developing corn kernels. Plant Physiol. 75, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop, J.M.H., Williamson, J.D., and Pharr, D.M. (1996). Mannitol metabolism in plants: A method for coping with stress. Trends Plant Sci. 1, 139–144. [Google Scholar]
- Tarczynski, M.C., Jensen, R.G., and Bohnert, H.J. (1993). Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259, 508–510. [DOI] [PubMed] [Google Scholar]
- Tusnady, G.E., and Simon, I. (1998). Principles governing amino acid composition of integral membrane proteins: Application to topology prediction. J. Mol. Biol. 283, 489–506. [DOI] [PubMed] [Google Scholar]
- Williams, L.E., Lemoine, R., and Sauer, N. (2000). Sugar transporter in higher plants: A diversity of roles and complex regulation. Trends Plant Sci. 5, 283–290. [DOI] [PubMed] [Google Scholar]
- Williamson, J.D., Stoop, J.M., Massel, M.O., Conkling, M.A., and Pharr, D.M. (1995). Sequence analysis of a mannitol dehydrogenase cDNA from plants reveals a function for the pathogenesis-related protein ELI3. Proc. Natl. Acad. Sci. USA 92, 7148–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, M.H., and Ziegler, H. (1975). List of sugar and sugar alcohols in sieve tube exudate. In Encyclopedia of Plant Physiology, Vol. 1, Transport in Plants, I. Phloem Transport, M.H. Zimmerman and J.A. Milburn, eds (New York: Springer-Verlag), pp. 480–503.