Abstract
Salt stress is one of the most serious environmental factors limiting the productivity of crop plants. To understand the molecular basis for salt responses, we used mutagenesis to identify plant genes required for salt tolerance in tomato. As a result, three tomato salt-hypersensitive (tss) mutants were isolated. These mutants defined two loci and were caused by single recessive nuclear mutations. The tss1 mutant is specifically hypersensitive to growth inhibition by Na+ or Li+ and is not hypersensitive to general osmotic stress. The tss2 mutant is hypersensitive to growth inhibition by Na+ or Li+ but, in contrast to tss1, is also hypersensitive to general osmotic stress. The TSS1 locus is necessary for K+ nutrition because tss1 mutants are unable to grow on a culture medium containing low concentrations of K+. Increased Ca2+ in the culture medium suppresses the growth defect of tss1 on low K+. Measurements of membrane potential in apical root cells were made with an intracellular microelectrode to assess the permeability of the membrane to K+ and Na+. K+-dependent membrane potential measurements indicate impaired K+ uptake in tss1 but not tss2, whereas no differences in Na+ uptake were found. The TSS2 locus may be a negative regulator of abscisic acid signaling, because tss2 is hypersensitive to growth inhibition by abscisic acid. Our results demonstrate that the TSS1 locus is essential for K+ nutrition and NaCl tolerance in tomato. Significantly, the isolation of the tss2 mutant demonstrates that abscisic acid signaling is also important for salt and osmotic tolerance in glycophytic plants.
INTRODUCTION
Environmental stresses are among the most limiting factors to plant productivity. Among these, salinity is one of the most detrimental (Boyer, 1982). The progressive salinization of irrigated land limits the future of agriculture in the most productive areas of the world (Ashraf, 1994). The deleterious consequences of high salt concentrations in the external solution of plant cells are hyperosmotic shock and ionic imbalance (Niu et al., 1995; Zhu et al., 1997). When salinity results from an excess of NaCl, homeostasis of not only Na+ and Cl− but also K+ and Ca2+ is disturbed (Serrano et al., 1999; Hasegawa et al., 2000; Rodriguez-Navarro, 2000). Plant survival and growth are dependent on adaptations that reestablish ionic homeostasis, thereby minimizing the duration of cellular exposure to disequilibria of ions.
Much effort has been devoted toward understanding the adaptive mechanisms of plant salt tolerance (Bohnert et al., 1995; Zhu et al., 1997). A common approach used to determine such mechanisms has been to identify cellular processes and genes whose activity or expression is regulated by salt stress (Bray, 1993; Botella et al., 1994; Zhu et al., 1997; Hasegawa et al., 2000). Identification of these salt-regulated genes has allowed a better understanding of the complexity of salt tolerance in higher plants (Cushman et al., 1990; Bray, 1993; Serrano and Gaxiola, 1994; Zhu et al., 1997; Hasegawa et al., 2000). However, even though many of these salt-regulated genes may be involved in salt tolerance, it is now clear that most of them have very small effects on tolerance, as deduced from overexpression studies. Therefore, other strategies to identify the regulatory genes that control them are needed (Zhu et al., 1997).
Recently, an alternative genetic approach was introduced for the identification of crucial genes and cellular processes involved in plant salt tolerance. Essential genes for salt tolerance could be identified by selecting and characterizing salt-hypersensitive mutants. Using this approach, three sos (for salt overly sensitive) mutants have been identified in Arabidopsis (Wu et al., 1996; Liu and Zhu, 1997; Zhu et al., 1998). Mutations in these genes render the sos plants hypersensitive to NaCl. All three SOS genes identified turned out to be involved in K+ nutrition as well, because the sos mutants are hypersensitive to growth at low concentrations of K+. Thus, the characterization of sos mutants demonstrated that K+ nutrition is a critical process for salt tolerance. In addition, the isolation of the sos3 mutant provided insight into the role of Ca2+ in salt tolerance, because the NaCl hypersensitivity of sos3 could be abolished by millimolar levels of Ca2+ (Liu and Zhu, 1997).
The molecular nature of all three SOS genes was determined recently. The SOS1 gene encodes a protein that has significant sequence similarity to plasma membrane Na+/H+ antiporters from bacteria and fungi (Shi et al., 2000). The SOS2 gene encodes a serine/threonine protein kinase with an N-terminal catalytic domain similar to that of the yeast SNF1 kinase (Liu et al., 2000). The SOS3 gene encodes a Ca2+ binding protein and has its greatest sequence homology with the yeast calcineurin B subunit and a neuronal calcium sensor, both of which are activated by Ca2+ (Liu and Zhu, 1998). Double mutant analysis showed that the three SOS genes function in a linear pathway (Liu and Zhu, 1997; Zhu et al., 1998). This is further supported by two recent findings. First, the SOS2 protein kinase interacts physically with and is activated, in the presence of calcium, by the calcium sensor SOS3 (Halfter et al., 2000). Thus, the SOS2/SOS3 kinase complex represents a regulatory pathway that, along with Ca2+, controls Na+ and K+ homeostasis and plant salt tolerance. Second, the upregulation of SOS1 in response to NaCl is reduced in sos2 and sos3 mutant plants, supporting the notion that SOS1 expression is controlled by the SOS2/SOS3 regulatory pathway (Shi et al., 2000).
Abscisic acid (ABA) plays a major role in plant adaptation to high salinity and osmotic stress (Leung and Giraudat, 1998). During vegetative growth, endogenous ABA levels increase under conditions of water stress, and the increased ABA is an essential mediator in triggering plant responses. Genetic screens have led to the isolation of several Arabidopsis mutants with altered ABA responsiveness. Mutations in the ABA-insensitive (ABI) loci ABI1 to ABI5 reduce the sensitivity of seed germination to exogenous ABA (Koornneef et al., 1984; Finkelstein, 1994). An enhanced response to ABA (era1) mutant displays hypersensitivity to ABA during germination. The abi3, abi4, and abi5 mutants do not seem to be altered in vegetative responses to ABA (Koornneef et al., 1984; Finkelstein and Somerville, 1990; Finkelstein, 1994). The proteins encoded by ABI3, ABI4, and ABI5 possess characteristics of transcriptional regulators (Giraudat et al., 1992; Finkelstein et al., 1998; Finkelstein and Lynch, 2000). However, the abi1, abi2, and era1 mutations are pleiotropic, because they affect ABA sensitivity in both seed and vegetative tissues. ERA1 encodes a farnesyl transferase that may play a role in embryonic ABA signaling (Cutler et al., 1996). ABI1 and its homolog, ABI2, encode type 2C serine/threonine phosphatase. Recently, the identification of loss-of-function mutants in the ABI1 gene demonstrated that a loss of ABI1 phosphate activity leads to enhanced responsiveness to ABA (Gosti et al., 1999). Therefore, the wild-type ABI phosphatase is a negative regulator of ABA responses and has several phenotypes in common with the era1 mutant. ABA-deficient mutants are affected in the regulation of numerous genes by drought, salt, or cold (Leung and Giraudat, 1998). Nevertheless, the adaptation to these stresses also involves ABA-independent pathways (Shinozaki and Yamaguchi-Shinozaki, 1996; Ishitani et al., 1997).
Tomato is a widely distributed annual vegetable crop adapted to a large variety of climates. However, in spite of its broad adaptation, production is concentrated in a few warm and rather dry areas (Cuartero and Fernandez-Muñoz, 1999). In these areas with an optimal climate for tomato, salinity is a serious constraint for maintaining high productivity (Szabolcs, 1994). For this reason, a large number of physiological studies of salt stress have been performed using tomato as a model plant (Cuartero and Fernandez-Muñoz, 1999). Unlike in Arabidopsis, direct studies on salinity, adaptation, and molecular changes in tomato can be assessed for crop yield. To take advantage of this wealth of information, we used a molecular genetic approach to determine which genes and cellular processes are crucial for salt tolerance in tomato. As a result, we have isolated three tomato mutants whose growth is hypersensitive to NaCl. These mutants define two genetic loci important for salt tolerance. Identification of the TSS1 locus demonstrates that K+ nutrition is essential for NaCl tolerance in tomato, as has been shown in Arabidopsis. Identification of the TSS2 locus confirms the link between NaCl tolerance and ABA signaling. Thus, physiological analyses of the tomato salt-hypersensitive mutants led us to identify mechanisms required for salt tolerance not described previously. These mutants will be a valuable tool in expanding our understanding of the critical physiological processes involved in plant salt tolerance.
RESULTS
Isolation and Genetic Analysis of Salt-Hypersensitive Tomato Mutants
Our approach to understanding the mechanisms essential for salt tolerance was to isolate tomato mutants that were hypersensitive to growth on NaCl. The growth inhibition by NaCl of tomato seedlings can be readily observed as a reduction in root elongation. We characterized the inhibition of seedling root elongation by using a modified version of the assay described by Wu et al. (1996). Tomato seedlings (cv Moneymaker) growing in the absence of salt stress were transferred to vertical agar plates containing 125 mM NaCl as a stress agent. The position of the root tip of every seedling was marked immediately after its placement, and growth was monitored for 2 days. M2 seed families from individual M1 plants were kept separate to facilitate screening and to ensure that mutants isolated from different M2 seed lots were independent. Between 30 and 40 M2 seedlings from each of the 600 M1 plants were screened for root growth under NaCl stress. M2 individuals from the families with reduced tolerance to NaCl were distinguishable 2 days after NaCl treatment and were named tomato salt-hypersensitive (tss) mutants. A NaCl-containing plate showing segregation for root growth hypersensitivity of the tss1-1 family is shown in Figure 1. Using this approach, three M2 families (0.5%) with seedlings that segregated for hypersensitivity to NaCl were identified. The details of these three families are listed in Table 1. Individual salt-hypersensitive seedlings of these families were recovered and self-pollinated. M3 seedlings obtained from the identified M2 plants were analyzed for their hypersensitivity to NaCl, and all remained hypersensitive to NaCl stress.
Figure 1.
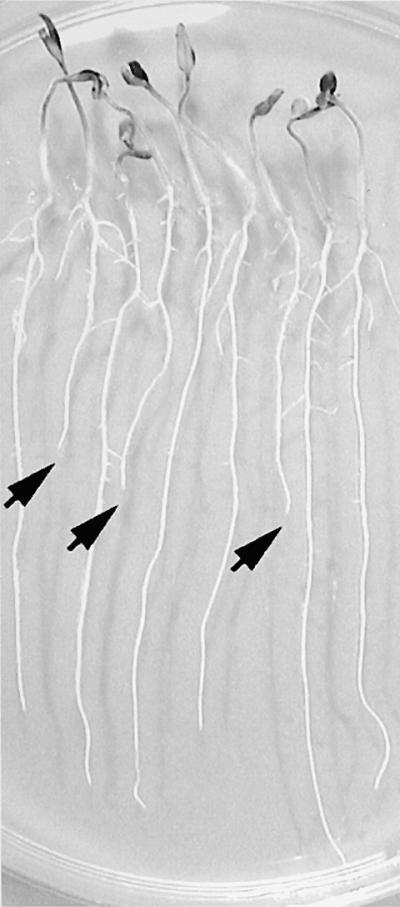
Identification of Tomato Salt-Hypersensitive Mutants.
Three-day-old tomato seedlings of the M2 tss1-1 family with 1.5-cm-long roots grown on vertical agar plates on Murashige and Skoog (MS) medium were transferred to MS plates supplemented with 125 mM NaCl and allowed to grow for another 2 days. Arrows indicate those seedlings whose growth was inhibited.
Table 1.
Quantification of Root Growth of the Wild Type, Three Salt-Hypersensitive Mutants, and Intermutant Crosses: Frequency of the Mutation in Each M2 Family
| Wild Type, Mutants, and Intermutant Crosses | Root Growtha (mm); Absolute ±sd |
Root Growth Percentage Compared with Wild Type |
Frequency of Mutantb |
χ2c |
|---|---|---|---|---|
| Wild type | 33 ± 4 | 100 | ||
| tss1-1 | 12 ± 3 | 36.4 | 36/7 | 1.75 |
| tss1-2 | 13 ± 2 | 39.4 | 32/10 | 0.032 |
| tss2 | 22 ± 4 | 66.7 | 38/8 | 1.42 |
| tss1-1 × tss1-2 | 11 ± 3 | 33.3 | 12/12 | |
| tss1-1 × tss2 | 32 ± 6 | 97.0 | 12/0 | |
| tss1-2 × tss2 | 34 ± 4 | 103.0 | 12/0 |
Root growth was determined 2 days after transfer of seedlings to 125 mM NaCl.
Frequency of segregation in M2 families: numerator, total number screened in a family or a cross; denominator, number of segregating mutants.
χ2 values represent the fit of the data to an expected 3:1 (wild type/mutant) phenotypic segregation (P > 0.05).
Analysis of the mutant segregation within each M2 family showed an ∼3:1 segregation ratio of wild type to mutant (Table 1). Thus, all of the tss mutants were caused by single recessive nuclear mutations. The F1 seedlings from the cross between the salt-hypersensitive mutants tss1-1 and tss1-2 exhibited hypersensitivity to growth in NaCl compared with wild-type seedlings (Table 1). This finding indicated that both mutations were at the same locus. The F1 seedlings from the salt-hypersensitive mutant crosses tss1-1 × tss2 and tss1-2 × tss2 exhibited wild-type growth (Table 1). These results indicated that the tss1-1 and tss1-2 mutations were in a different locus from tss2. Therefore, allelism tests showed that the mutants fell into two complementation groups and thus defined two different loci required for salt tolerance in tomato.
tss1 and tss2 Plants Exhibit Different Degrees of NaCl Hypersensitivity
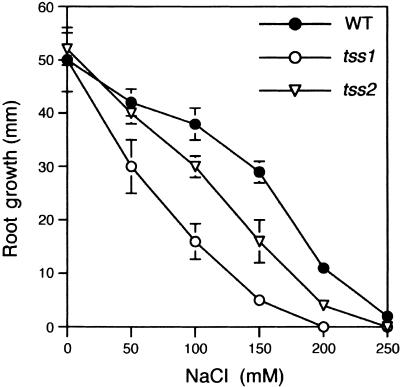
The two mutant alleles of TSS1 appeared very similar in terms of salt hypersensitivity (Table 1). Therefore, all subsequent physiological and phenotypic studies were performed using tss1-1 (hereafter referred to as tss1). Salt sensitivity of the tss1 and tss2 mutants was evaluated by measuring the root elongation of seedlings placed on agar plates containing 0 to 250 mM NaCl, as shown in Figure 2. The growth of tss1 and tss2 was identical to that of the wild type on control medium without NaCl supplementation. The concentrations of NaCl that decreased the root elongation rate by 50% relative to medium without salt (I50) for tss1, tss2, and the wild type were 68, 118, and 160 mM, respectively (Figure 2).
Figure 2.
tss1 and tss2 Seedlings Are Hypersensitive to NaCl.
Root elongation of wild-type (WT), tss1, and tss2 seedlings was measured to quantify their sensitivities to NaCl inhibition. Seed of the wild type, tss1, and tss2 were germinated and grown for 3 days on MS medium. Resulting seedlings were incubated vertically on MS medium supplemented with the indicated concentrations of NaCl, and their growth was measured 2 days later. Error bars represent the standard deviation ( ). The experiment was repeated at least three times with similar results. The measurements from one representative experiment are presented.
). The experiment was repeated at least three times with similar results. The measurements from one representative experiment are presented.
We used a very specialized set of circumstances for the determination of salt hypersensitivity, including the growth of seedlings on agar plates in sterile conditions. The gas phase alone could be very different from that in nature, with perhaps an accumulation of ethylene that would not be encountered normally by plants. Therefore, we determined whether tss1 and tss2 plants were hypersensitive to NaCl in conditions more like field conditions. Wild-type, tss1, and tss2 plants were transferred 3 days after germination in pots containing perlite. The plants were then irrigated with half-strength Hoagland nutrient solution (Jones, 1982) or nutrient solution supplemented with 250 mM NaCl. In the absence of NaCl, the growth of wild-type, tss1, and tss2 plants was very similar after 1, 7, and 14 days of NaCl treatment (Figures 3A, 3C, and 3E). However, tss1 plants started to become chlorotic after 1 day of NaCl treatment (Figure 3B). In contrast, no clear differences from the wild type were observed in tss2 plants until approximately day 11, by which time they were smaller and chlorosis began to appear (data not shown). After 14 days of treatment, the salt hypersensitivity of both tss1 and tss2 was clearly distinguishable from that of the wild type (Figure 3F). It can be concluded that the observed shoot phenotype in the plants after NaCl treatment correlates with the degree of root sensitivity to NaCl observed previously for the tss mutants.
Figure 3.
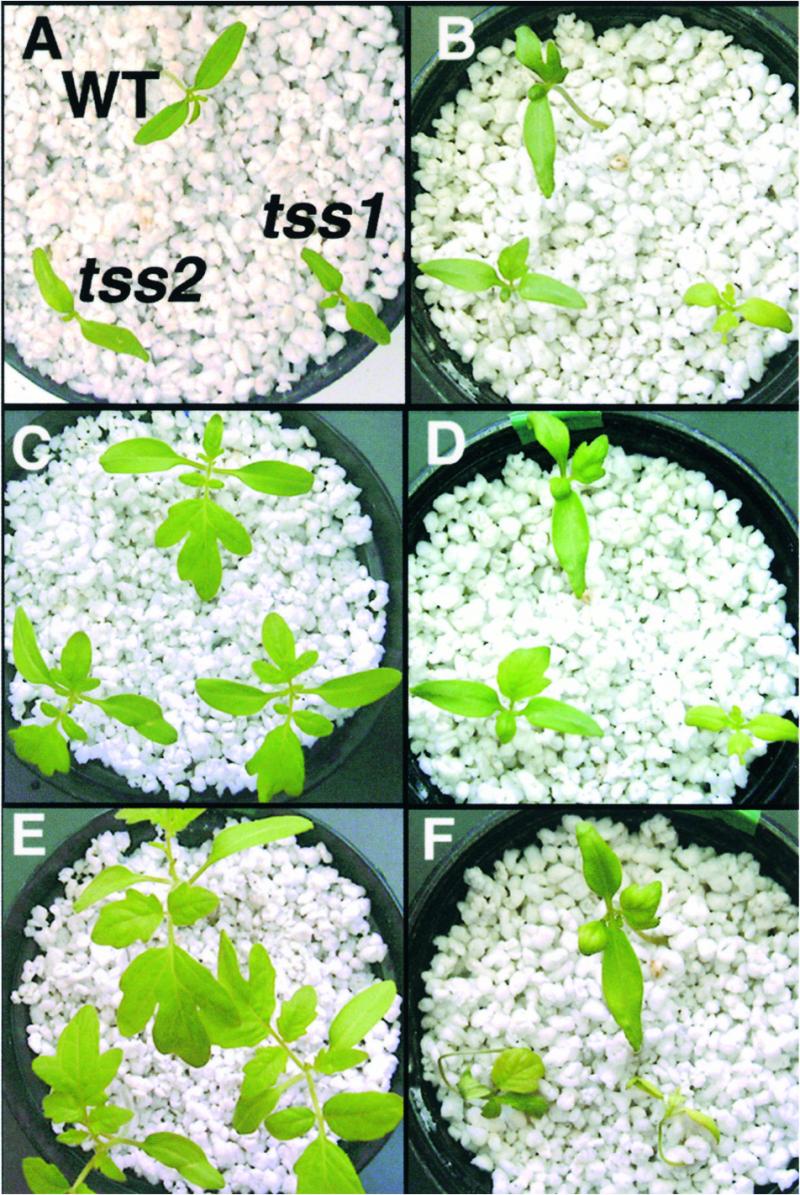
NaCl Shoot Hypersensitivity of tss1 and tss2.
Three-day-old tomato seedlings with 1.5-cm-long roots grown on vertical agar plates on MS medium were transferred to perlite. After 3 days, seedlings were flooded every day with either half-strength Hoagland nutrient solution or half-strength Hoagland nutrient solution supplemented with 250 mM NaCl. In each photograph, the wild type (WT) is at top, tss1 is at bottom right, and tss2 is at bottom left.
(A) Phenotype of plants grown in medium without NaCl stress for 1 day.
(B) Phenotype of plants exposed to NaCl stress for 1 day.
(C) Phenotype of plants grown in medium without NaCl stress for 7 days.
(D) Phenotype after 7 days of NaCl stress.
(E) Phenotype of plants grown in medium without NaCl stress for 14 days.
(F) Phenotype after 14 days of NaCl stress.
tss1 and tss2 Are Both Hypersensitive to Na+ and Li+
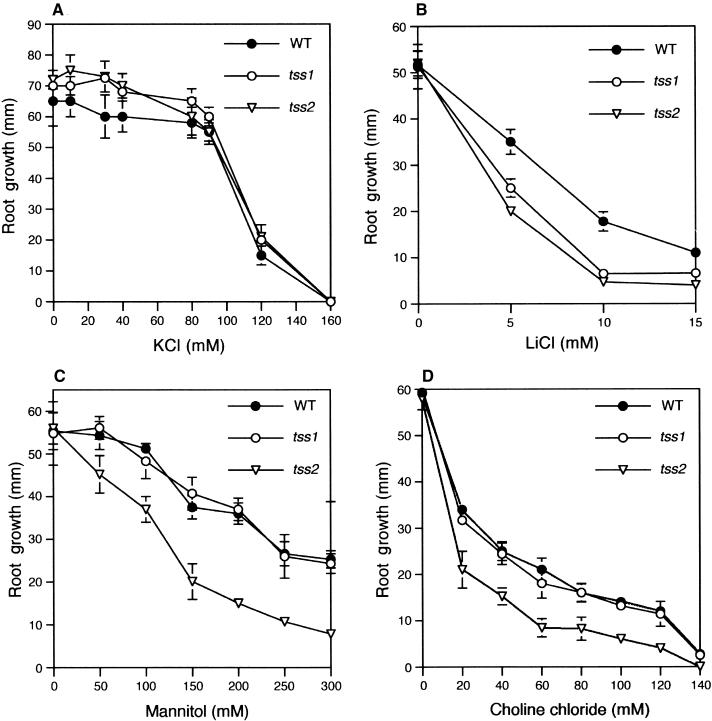
To determine if tss1 and tss2 are hypersensitive to Na+ or Cl− or both, growth curves of the wild type and the tss mutants were determined using KCl and LiCl. These showed that tss1 and tss2 were not hypersensitive to high levels of KCl (Figure 4A). In contrast, tss1 and tss2 were hypersensitive to Na2SO4 but not to MgSO4 (data not shown). Therefore, tss1 and tss2 were hypersensitive to Na+ but not to Cl−. In addition to Na+, tss1 and tss2 were both hypersensitive to Li+ (Figure 4B). Li+ is considered a more toxic analog of Na+ and has been used to mimic Na+ toxicity without the osmotic stress commonly associated with NaCl (Mendoza et al., 1994).
Figure 4.
Sensitivity of tss1 and tss2 to Ionic and Osmotic Stress.
Root elongation of wild-type (WT), tss1, and tss2 seedlings was measured to quantify their sensitivities to KCl, LiCl, mannitol, and choline chloride. Seed of the wild type, tss1, and tss2 were germinated and grown for 3 days on MS medium. Resulting seedlings were incubated vertically on MS medium supplemented with the indicated concentrations of KCl, LiCl, mannitol, or choline chloride, and their growth was measured 2 days later. Error bars represent the standard deviation ( ). The experiment was repeated at least three times with similar results. The measurements from one representative experiment are presented.
). The experiment was repeated at least three times with similar results. The measurements from one representative experiment are presented.
(A) Growth response to KCl.
(B) Growth response to LiCl.
(C) Growth response to mannitol.
(D) Growth response to choline chloride.
tss2 but Not tss1 Is Hypersensitive to Osmotic Stress
NaCl imposes both an ionic and an osmotic stress (Hasegawa et al., 2000). We determined whether tss mutants also were hypersensitive to a general osmotic stress without any ionic imbalance effect. Growth curves showed that tss1 was not hypersensitive to the osmotic stress caused by mannitol (Figure 4C). In contrast, tss2 exhibited hypersensitivity to mannitol. Experiments using sorbitol instead of mannitol produced similar results (data not shown). We also tested whether tss2 was hypersensitive to other osmotic stress agents different from a polyalcohol. For this purpose, we used choline chloride as the osmotic stress agent (Davies et al., 1990). As shown in Figure 4D, choline chloride resulted in tss2 but not tss1 showing hypersensitivity to osmotic stress.
Effect of Low Potassium on tss1 and tss2 Growth
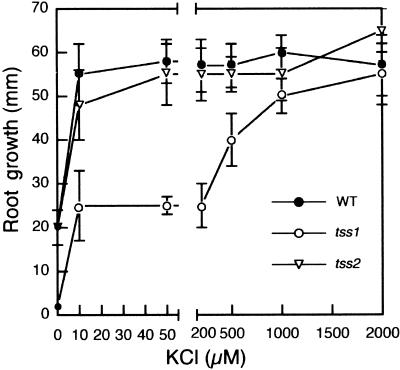
Previous studies in Arabidopsis demonstrated the importance of K+ nutrition in salt tolerance, because salt-hypersensitive sos mutants also were hypersensitive to growth at low concentrations of K+ (Wu et al., 1996; Liu and Zhu, 1997; Zhu et al., 1998). Therefore, we determined whether tss1 and tss2 also were affected in K+ nutrition. At high concentrations of K+, no differences were observed between the wild type and tss mutants (Figure 4A). However, at low K+ concentrations, clear differences were observed, as shown in Figure 5. Wild-type, tss1, and tss2 seed were grown in a medium containing very low K+ (5 μM) to allow germination but to minimize the effect of the K+ pool present in the resulting seedlings. Seedlings were then transferred to media containing different concentrations of K+. Root growth in the first 2 days was not measured so that the effect of residual K+ carried over from the germination medium was minimized. After this time, root elongation was measured 3 days later (Figure 5).
Figure 5.
tss1 Mutants but Not tss2 Mutants Are Hypersensitive to Growth on Low Potassium.
Seed of the wild type (WT), tss1, and tss2 were germinated and grown for 3 days on modified MS medium containing 5 μM K+. Resulting seedlings were incubated vertically on modified half-strength MS medium containing the indicated concentrations of KCl. Root growth in the first 2 days was not measured so that the effect of residual K+ carried over from the germination medium was minimized. After this time, root elongation was measured 3 days later. Error bars represent the standard deviation ( ). The experiment was repeated at least three times with similar results. The measurements from one representative experiment are presented.
). The experiment was repeated at least three times with similar results. The measurements from one representative experiment are presented.
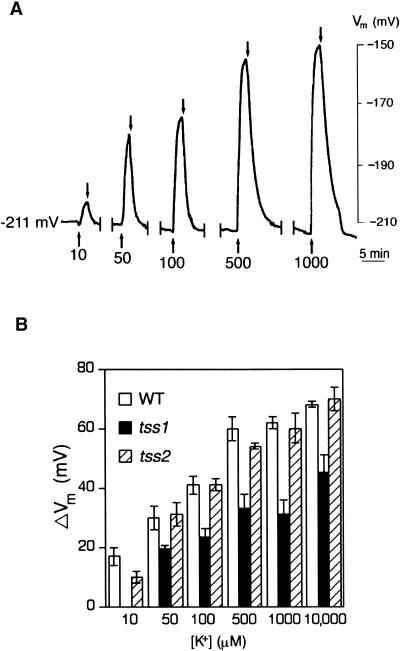
Measurements of root elongation indicated that tss1 growth was impaired on medium containing <1 mM K+, whereas no differences were observed between the wild type and tss2. Forty seedlings from both tss1-1 and tss1-2 M2 segregating populations were analyzed based on their salt sensitivity. All salt-hypersensitive seedlings were hypersensitive on 50 μM K+ as well, demonstrating that the mutations in TSS1 were responsible for both phenotypes (i.e., hypersensitivity to NaCl and inability to grow on low K+). This study also showed that the wild type and tss2 have different K+ requirements for maximal growth compared with tss1. A concentration of 10 μM K+ in the medium was enough to produce maximal growth for wild-type and tss2 seedlings. However, tss1 seedlings required ∼1 mM to reach nearly maximal growth (Figure 5). Note that some growth was maintained by the wild type and tss2 even without any added K+, probably because of tracer amounts of K+ present in the seedlings, in the agar, or in the basic medium.
Calcium Requirement of tss1
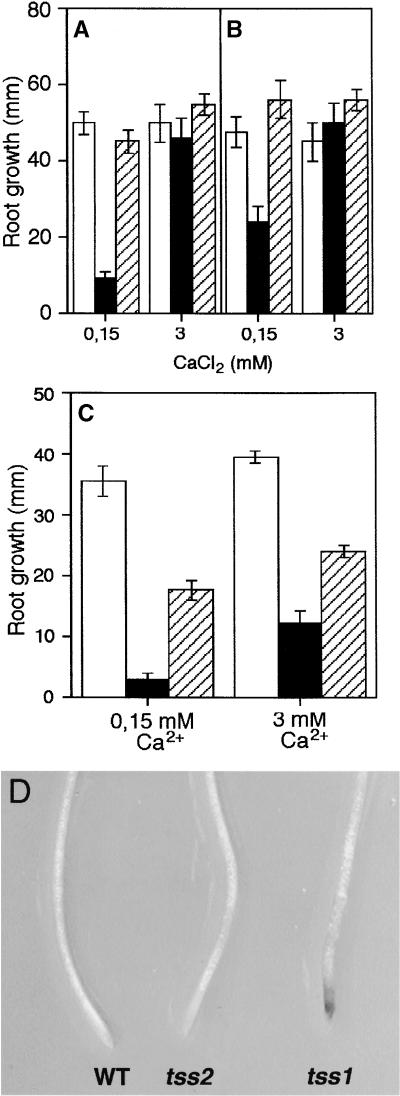
It has been shown that high external Ca2+ alleviates general ion toxicity and in particular the NaCl effect (Läuchli, 1990; Kinraide, 1998, 1999). Moreover, an Arabidopsis mutant that requires increased Ca2+ for K+ nutrition and NaCl tolerance has been identified, and the gene responsible for this mutant has been cloned (Liu and Zhu, 1997, 1998). Hence, we tested whether Ca2+ could affect the root elongation of tss1 and tss2 on low K+ and high NaCl. Wild-type, tss1, and tss2 plants were germinated and grown in a medium containing 50 μM K+ and later transferred to a medium containing 500 μM K+ and either 0.15 or 3 mM Ca2+. As shown in Figure 6A, no differences were observed between the wild type and tss2 mutants. In contrast, tss1 root growth in 0.15 mM Ca2+ was ∼20% of that in 3 mM Ca2+ (Figure 6A). Therefore, we conclude that the growth inhibition of tss1 in low-K+ medium could be corrected by increasing the Ca2+ concentration. In a second experiment, wild-type, tss1, and tss2 plants were germinated and grown in a medium containing 20 mM K+ and later transferred to a medium containing 500 μM K+ and either 0.15 or 3 mM Ca2+. The results were similar, with the only difference being that tss1 grew slightly more than in the first experiment (Figure 6B). No differences between the wild type and tss mutants were observed when the growth medium contained 0.15 mM Ca2+ and 20 mM K+ (data not shown), indicating that high Ca2+ is required only for growth at low K+. We determined whether Ca2+ could affect the growth of tss1 and tss2 seedlings at high NaCl. As shown in Figure 6C, tss1 seedlings increased their hypersensitivity to NaCl when the Ca2+ concentration was reduced from 3 to 0.15 mM in the growth media. It is noteworthy that in addition to growth inhibition, necrosis at the root tip of tss1 was observed between 3 and 5 days of growth at low Ca2+ and low K+ (Figure 6D).
Figure 6.
High Ca2+ Suppresses the Growth Defect of tss1 on Low-K+ Medium but Not on High-NaCl Medium.
(A) Plants were grown on a modified MS medium containing 50 μM K+ for 2 days and then transferred to a modified MS medium containing 500 μM K+ and either 0.15 or 3 mM Ca2+ as total Ca2+. Their growth was measured 3 days later. Open bars, wild type; closed bars, tss1; striped bars, tss2. Error bars represent the standard deviation ( ).
).
(B) Plants were grown on MS medium for 2 days and then transferred to modified MS medium containing 500 μM K+ and either 0.15 or 3 mM Ca2+ as total Ca2+. Their growth was measured 3 days later. Open bars, wild type; closed bars, tss1; striped bars, tss2. Error bars represent the standard deviation ( ).
).
(C) Plants were grown on MS medium for 2 days and then transferred to MS medium containing 125 mM NaCl and either 0.15 or 3 mM Ca2+ as total Ca2+. Their growth was measured 3 days later. Open bars, wild type; closed bars, tss1; striped bars, tss2. Error bars represent the standard deviation ( ).
).
(D) Low Ca2+ and K+ in the growth medium induces necrosis in the root tip of tss1. Root tips of wild-type (WT), tss1, and tss2 seedlings were photographed after growing for 5 days in 0.15 mM Ca2+ and 100 μM K+.
tss1 Exhibits Reduced K+ Uptake
Because tss1 is hypersensitive to growth on media with low K+, we determined whether tss1 was defective in K+ uptake by examining the K+ conductance of the plasma membrane in root cells in the absence of NH4+. Microelectrodes inserted into root hairs ∼5 to 10 mm from the apex revealed strong negative resting membrane potentials (Vm) of −212 ± 10, −190 ± 5, and −207 ± 8 mV in the wild type, tss1, and tss2, respectively. Figure 7A shows a typical recording of Vm in an impaled root cell of a wild-type tomato. In this example, after the voltage was stabilized at −211 mV for at least 30 min, the continuously flowing bathing solution without K+ was switched to solutions containing different concentrations of K+. The changes in Vm that occur in response to these shifts (ΔVm) are related to the K+ permeability of the plasma membrane (see Methods). Roots of tss1 seedlings did not show any K+ uptake when the bathing solution contained 10 μM K+ (Figure 7B). In general, K+ permeability for tss1 roots was reduced significantly at all K+ concentrations tested compared with that of the wild type and tss2 mutants. No significant differences in K+ permeability were detected between tss2 and the wild type, consistent with the similar growth of both tss2 and the wild type at different K+ concentrations.
Figure 7.
Roots of tss1 Mutants Show Reduced K+ Uptake Compared with Those of the Wild Type and tss2.
Changes in Vm of the wild type, tss1, and tss2 resulting from changes in the K+ concentration of the bathing solution are shown.
(A) Recording of Vm in a root hair 5 to 10 mm from the root tip of a wild-type tomato. From an initial Vm of −211 mV in this example, the membrane depolarized in response to increasing [K+]ext in the bathing solution. Arrows pointing up correspond to the change of a bathing solution containing the indicated K+ concentration, and arrows pointing down correspond to the change of a bathing solution without K+. This solution was kept for 10 min before the addition of a new bathing solution with K+.
(B) Magnitude of the change in steady state Vm in response to changing [K+]ext in the bathing solution without NH4+. Error bars represent the standard deviation of three assays in three independent plants (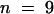 ). WT, wild type.
). WT, wild type.
Effects of NH4+ on K+ Permeability
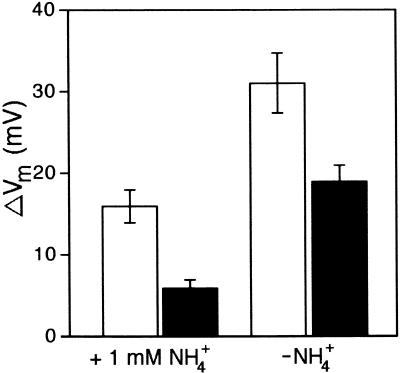
Previous studies using the Arabidopsis akt1 mutant indicated that at low K+ concentrations, K+ uptake is dependent on two classes of transport mechanisms operating in parallel (Hirsch et al., 1998; Spalding et al., 1999). The first mechanism is uninhibited by NH4+ and corresponds to the inward-rectifying K+ channel AKT1 (Sentenac et al., 1992; Spalding et al., 1999). The second mechanism is NH4+ sensitive and corresponds to non-AKT1 transporters (Spalding et al., 1999). Experiments were performed to compare the effect of NH4+ on K+ permeability in the wild type and tss1 when extracellular K+ concentration ([K+]ext) was 50 μM. For this purpose, plants were impaled, and the voltage was stabilized with a bathing solution with or without 1 mM NH4+. The use of 1 mM NH4+ allowed us to obtain values of steady state potentials very similar to those obtained without NH4+ (data not shown).
Approximately 50% of the wild-type ΔVm and ∼68% of tss1 ΔVm resulting from a change in K+ concentration from 0 to 50 μM was inhibited by 1 mM NH4+, as shown in Figure 8. The contribution of TSS1 to total K+ permeability may be inferred by subtracting the ΔVm measured in tss1 from that measured in the wild type. Thus, the K+ permeability of tss1 was ∼37% of that in the wild type in the presence of 1 mM NH4+ and ∼61% of that in the wild type in the absence of NH4+ (Figure 8). This finding suggests that in tss1, NH4+-insensitive K+ transport was more affected than was NH4+-sensitive K+ transport. Nevertheless, some depolarization occurred even in the presence of NH4+ (Figure 8).
Figure 8.
NH4+-Sensitive and NH4+-Insensitive K+ Transport in tss1.
Changes in Vm resulting from changing [K+]ext from 0 to 50 μM in wild-type and tss1 roots in the presence and absence of 1 mM NH4+. Open bars, wild type; closed bars, tss1. Error bars represent the standard deviation of three assays in three independent plants (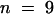 ).
).
We also determined whether tss1 was affected in Na+ permeability. A protocol similar to that used previously to determine K+ permeability was used for Na+. First, the voltage was stabilized by continuously flowing bathing solution containing 100 μM Na+ (see Methods) Then, the solution was switched to solutions containing 0.5, 1, and 10 mM Na+. Thus, the ΔVm that occurred in response to these shifts was related to the Na+ permeability of the plasma membrane. As shown in Table 2, the Na+ permeability was less than the K+ permeability. On the basis of the ΔVm resulting from the change in extracellular Na+, no differences in Na+ transport were found between the wild type, tss1, and tss2.
Table 2.
Changes in Membrane Potential Resulting from Changes in Na+ Concentrations in the Wild Type, tss1, and tss2a
| Extracellular Na+ Concentration (mM) |
Wild Type (Vm) | tss1 (Vm) | tss2 (Vm) |
|---|---|---|---|
| 0.5 | 8 ± 2 | 7 ± 2 | 9 ± 3 |
| 1 | 9 ± 3 | 8 ± 3 | 7 ± 2 |
| 10 | 22 ± 4 | 21 ± 2 | 24 ± 3 |
a Vm measured in apical root hairs sequentially bathed in flowing medium containing 0.5, 1, and 10 mM Na+. Values are averages ±sd of three assays in three independent plants (n = 9).
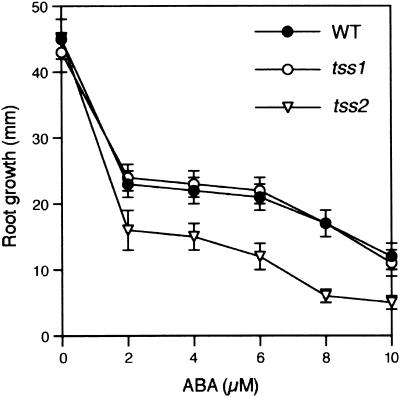
tss2 but Not tss1 Is Hypersensitive to ABA
In response to osmotic stress elicited by water deficit or conditions of high salt, there is an increase of the phytohormone ABA, which in turn induces various osmotic stress–responsive genes. We determined whether tss1 or tss2 was affected in growth at various ABA concentrations. As shown in Figure 9, growth inhibition by ABA was greater in tss2 than in the wild type and tss1, whereas no differences were observed between the wild type and tss1. All 47 NaCl-hypersensitive tss2 seedlings selected from the segregating M2 population also were hypersensitive to ABA. In contrast, all 135 seedlings selected from the M2 population that did not exhibit hypersensitivity to NaCl also were not hypersensitive to ABA. This indicated that NaCl hypersensitivity cosegregates with ABA hypersensitivity.
Figure 9.
tss2 Mutants but Not tss1 Mutants Are Hypersensitive to ABA.
Root elongation of wild-type (WT), tss1, and tss2 seedlings was measured to quantify their sensitivities to ABA. Seed of the wild type, tss1, and tss2 were germinated and grown for 3 days on ABA-free medium. Resulting seedlings were incubated vertically on medium supplemented with the indicated concentrations of ABA, and their growth was measured 2 days later. Error bars represent the standard deviation (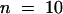 ). The experiment was repeated at least three times with similar results. The measurements from one representative experiment are presented.
). The experiment was repeated at least three times with similar results. The measurements from one representative experiment are presented.
The era1 mutant and the loss-of-function abi1 mutants of Arabidopsis show enhanced responsiveness to ABA. As a result, they exhibit increased tolerance to drought stress compared with the wild type (Cutler et al., 1996; Gosti et al., 1999). In preliminary experiments, we did not detect any differences in the drought-adaptive responses of tss1 or tss2 compared with the wild type (data not shown).
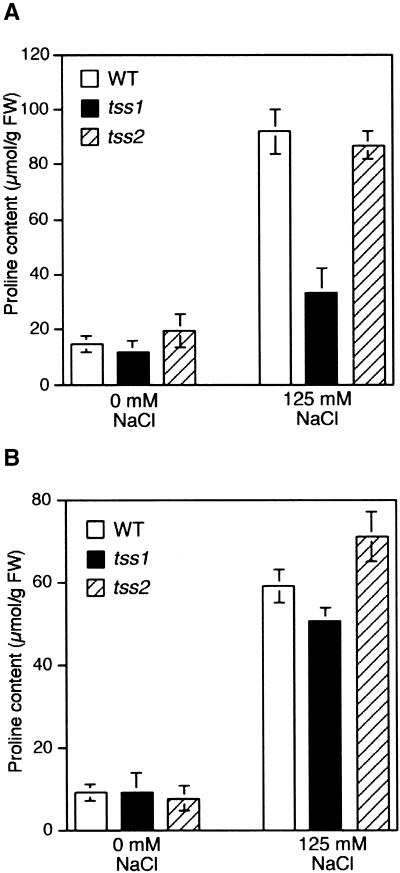
tss1 but Not tss2 Is Defective in Proline Accumulation
Proline accumulation is thought to function as an osmoregulatory solute in salt and osmotic stress adaptation in plants (Delaney and Verma, 1993). These observations suggested the possibility that decreased salt tolerance in tss1 and tss2 mutant plants might be due to decreased levels of proline. As shown in Figure 10, low levels of proline were detected in all plants cultured on control medium. Subsequent exposure to 125 mM NaCl for 3 days caused proline accumulation in the wild type, tss1, and tss2 in both roots and shoots (Figures 10A and 10B). However, diminished accumulation of proline in tss1 roots was observed after NaCl stress compared with the wild type and tss2. These results suggest that the NaCl hypersensitivity of tss2 is due to a mechanism other than a reduced accumulation of proline.
Figure 10.
Accumulation of Proline in the Wild Type, tss1, and tss2 in Roots and Shoots after NaCl Stress.
Seed of the wild type (WT), tss1, and tss2 were germinated and grown for 3 days on NaCl-free medium. Contents of proline were determined after incubating the seedlings for 3 days on control medium or medium supplemented with 125 mM NaCl. The data presented are averages of the results obtained from three independent experiments. Error bars represent the standard deviation (n = 20). The experiment was repeated twice with similar results. FW, fresh weight.
(A) Contents of proline in the root.
(B) Contents of proline in the shoot.
DISCUSSION
Tomato as a Model Crop Plant for Salt Tolerance Studies
In tomato, sources of salt tolerance have been identified among related wild species and primitive cultivars (Jones, 1986; Cuartero et al., 1992). These genetic resources have been used to improve the salt tolerance of modern tomato cultivars. However, to date, no salt-tolerant cultivars of tomato have been developed by traditional breeding programs. Probably the most limiting factor is the complexity of the trait. Classic genetic studies have demonstrated that the ability of plants to tolerate salt stress is a quantitative trait involving the action of many genes with small additive effects (Shannon et al., 1987; Cuartero and Fernandez-Muñoz, 1999). This complexity makes difficult the identification of genes and physiological processes that are critical for salt tolerance (Zhu et al., 1997; Hasegawa et al., 2000). Here, we report the isolation of three tomato salt-hypersensitive mutants that define two loci required for salt tolerance in tomato. Physiological studies of the tss mutants revealed that at least two different mechanisms are required for salt tolerance.
The tss mutants identified are slightly more sensitive to NaCl than is the wild type. The screening method we used in tomato has the advantage that it is very effective for selecting mutants showing a low reduction in salt tolerance. In fact, we put two constraints in our mutant screening. The first was that the ratio of wild-type to mutant seedlings segregating in a given family should be within the expected range for a single gene that would be responsible for the salt-hypersensitive phenotype (Table 1). This was useful in the determination of false mutants, which are abundant in this type of screening. The second constraint was that the mutant had to display normal growth in the absence of salt stress. In this way, we could identify genes required specifically for salt tolerance.
Interactions between NaCl Tolerance and ABA Signaling Defined by TSS2
The TSS2 locus is required for salt tolerance but is not required for growth on low-K+ medium. Therefore, unlike TSS1, TSS2 does not seem to be involved in K+ nutrition. tss2 mutants are hypersensitive to ionic stress, in particular to Na+ and Li+, and they are hypersensitive to general osmotic stress created by mannitol, sorbitol, and choline chloride. Surprisingly, tss2 is as resistant as the wild type and tss1 to KCl concentrations that we would expect to represent a significant osmotic stress. It is possible that at high concentrations of external K+, a high K+ loading will increase intracellular K+, and this could be used for osmotic adjustment. In addition, a high K+ loading could mask the osmotic hypersensitive phenotype due to K+ toxicity, consistent with the observation that tomato root growth is more sensitive to high levels of KCl than to high levels of NaCl. Whereas the I50 for Na+ was 160 mM, an equal concentration of KCl impaired tomato root growth completely (Figure 4A). High sensitivity of plants to moderate levels of K+ has been reported previously (Benlloch et al., 1994). This finding could be explained by the inefficient regulation of K+ homeostasis at high external concentrations, because these conditions are rarely encountered by land plants.
The TSS2 locus is required for both ionic and osmotic tolerance. tss2 is hypersensitive to ABA, a sesquiterpene produced in plants that is thought to be involved in osmotic and salt tolerance. On the basis of our analyses of the tss2 mutant, it is likely that TSS2 could encode a protein that negatively regulates ABA signaling. As deduced from Figure 8, tomato roots are extremely sensitive to low concentrations of applied ABA. Because tss2 is hypersensitive to ABA, an increase of endogenous ABA as a consequence of salt or osmotic stress could explain the hypersensitivity of tss2 under NaCl and osmotic stress. Several mutants, mostly from Arabidopsis, have been selected as being hypersensitive to ABA (Leung and Giraudat, 1998). The era1 mutant displays an enhanced response to exogenous ABA (Cutler et al., 1996). era1 plants exhibited ABA hypersensitivity of guard cell anion channel activation and stomatal closing, which in turn produced both a reduction in transpirational water loss during drought treatment and increased drought tolerance (Pei et al., 1998).
Like the era1 mutant, the loss-of-function abi1 mutants were more sensitive to ABA (Gosti et al., 1999). These mutants, in contrast to the dominant gain-of-function mutant abi1-1 isolated previously, displayed hypersensitivity of root growth to ABA. In addition, they also exhibited increases in both seed dormancy and drought-adaptive responses (Gosti et al., 1999). The era1 and abi1 mutations interact genetically, as deduced from double mutant studies (Pei et al., 1998). The tss2 mutant has a phenotype in common with era1 and abi1-1 because they all have enhanced sensitivity of root growth inhibition to applied ABA. However, in preliminary experiments, we did not find differences in the drought-adaptive responses between tss2 and the wild type (data not shown). This finding suggests that in contrast to ERA1 and ABI1, TSS2 function could be restricted to roots and may not be involved in the stomatal aperture. Hence, TSS2 could encode a negative regulator of ABA signaling specific to roots after endogenous ABA levels are increased as a consequence of salt and osmotic stress.
Interactions among Ca2+, Na+, and K+ in NaCl Tolerance Defined by TSS1
The TSS1 locus, which is defined by two independent tss mutants, is essential for salt tolerance as well as for growth on low-K+ medium. tss1 mutants are hypersensitive to ionic stress and, in particular, to Na+ and Li+ but not to general osmotic stress. The TSS1 locus is essential for normal K+ uptake because tss1 mutants have reduced K+ uptake and so exhibit slow growth on low-K+ medium compared with that of the wild type.
At low concentrations of K+, wild-type seedlings exhibited improvement in K+ transport when NH4+ was absent from the medium. This suggests that, as in Arabidopsis, two transport mechanisms are functional in tomato at low K+, one NH4+ sensitive and one NH4+ insensitive. Analysis of K+ transport altered in tss1 suggests that the NH4+-insensitive mechanism is the one controlled by TSS1.
Increased proline content confers osmotic tolerance in transgenic tobacco and freezing and salt tolerance in Arabidopsis (Kishor et al., 1995; Nanjo et al., 1999b). Moreover, transgenic lines that accumulated proline at lower levels than did wild-type plants were hypersensitive to osmotic stress (Nanjo et al., 1999a). tss1 roots showed reduced accumulation of proline after NaCl stress. It is possible that under NaCl stress the metabolism of tss1 is affected, which in turn leads to diminished proline synthesis. Therefore, the salt hypersensitivity of tss1 might be due to defects in both K+ nutrition and proline accumulation.
Increased external Ca2+ completely suppressed the growth defect on low-K+ medium and partially suppressed the salt hypersensitivity phenotype of the tss1 mutant. Ca2+ is proposed to alleviate Na+ toxicity through different mechanisms. These include the electrostatic displacement of Na+ and the replacement of essential Ca2+ at the plasma membrane if Na+ has displaced enough Ca2+ to limit growth (Kinraide, 1998, 1999). However, the factor of greatest importance is the alleviation of Na+ toxicity by reducing the effectiveness of the toxin, whether at the membrane surface or in the tissue (Kinraide, 1999), and most importantly by increasing K+/Na+ selectivity (Liu and Zhu, 1998). It is in this process that TSS1 is probably involved, that is, by increasing the efficiency of K+ nutrition at low Ca2+ concentrations.
We can speculate about the molecular nature of TSS1. tss1 mutants resemble the sos3 mutant isolated previously in Arabidopsis (Liu and Zhu, 1997, 1998). Both sos3 and tss1 plants are normal except when challenged with low potassium or salt stress. As in sos3, increased external Ca2+ suppresses the growth defect on low-K+ medium and partially suppresses the salt-hypersensitivity phenotype of tss1. Therefore, it is possible that tss1 could be the sos3 ortholog in tomato, encoding some type of Ca2+ sensor (Liu and Zhu, 1998). One difference, however, is that in sos3, no substantial differences were found in K+ uptake by using the radiolabeled K+ analog 86Rb in tracer uptake experiments (Liu and Zhu, 1997). In contrast, tss1 mutants showed differences in K+ uptake compared with the wild type by using electrophysiology. These differences may be attributable to the higher sensitivity of this method and/or the analysis of different cell populations. Reduced K+ accumulation has been described in the Arabidopsis sos mutants after NaCl stress. Furthermore, reduced Na+ content has been reported in the sos1 mutant after NaCl treatment. Surprisingly, no significant differences were found in Na+ or K+ content in either roots or shoots after salt stress between the wild type and the tss mutants (results not shown). This finding suggests that reduced K+ uptake may be limited to a particular cell type. In addition, the wild type has an active growth, thereby diluting the K+ pool present.
As reported previously in Arabidopsis (Spalding et al., 1999), in tomato there appear to be two distinct mechanisms involved in high-affinity K+ uptake. The first is a mechanism insensitive to inhibition by NH4+ that by analogy to Arabidopsis may correspond to inward-rectifying K+ channels such as the AKT1 channel. The second is a mechanism inhibited by NH4+ that may correspond to an active symporter with K+ transport coupled to the H+ electrochemical potential gradient (Spalding et al., 1999). The latter mechanism does not seem to be affected in tss1, because removing NH4+ from the medium improved K+ permeability. Moreover, the NH4+-sensitive component of the tss1 response was similar in magnitude to the NH4+-sensitive component of the wild-type response. Hence, we can speculate that the mechanism that is insensitive to NH4+ is the one primarily affected in tss1. Nevertheless, the observation of some root growth and K+ permeability in tss1 suggests that not all activity exerted by this mechanism is abolished.
Interestingly, necrosis appeared in the tss1 root tip after the plants were maintained for 5 days on low-Ca2+ and low-K+ medium. We have shown that tss1 mutant plants have a defect in K+ nutrition and impaired K+ uptake capability. The first effect of K+ deficiency is that a certain proportion of the normal K+ uptake is replaced by H+ uptake (Rodriguez-Navarro, 2000). This replacement decreases the internal pH, which in turn could produce the observed necrosis. In mammalian cells, changes in cytosolic K+ activity have been implicated in signaling programmed cell death (Hughes et al., 1997). If this mechanism is conserved between mammals and plants, then another possibility is that under these Ca2+- and K+-starved conditions, the seminal root cells of tss1 might be showing programmed cell death.
Identification of Processes Involved in Salt Tolerance Other Than K+ Nutrition
The Arabidopsis sos mutants identified to date are hypersensitive to Na+ but not to general osmotic stress. Physiological studies determined that they are specifically affected in both salt tolerance and K+ nutrition (Wu et al., 1996; Liu and Zhu, 1997; Zhu et al., 1998). The Arabidopsis mutants sos1, sos2, and sos3 are 25, 10, and 2.5 times more sensitive to NaCl, respectively, than are wild-type seedlings based on their I50 (Zhu et al., 1998). Because mostly mutants with great reductions in NaCl tolerance are isolated with the screening method developed in Arabidopsis (Zhu et al., 1998), it is tempting to speculate that mutants involved in K+ nutrition will be preferentially isolated. Because K+ is an essential element for plant processes (Rodriguez-Navarro, 2000), it is expected that disruption of K+ nutrition results in a drastic inhibition of root growth. Analysis of the mutant segregation within each M2 family allowed us to identify mutants that are moderately more sensitive to NaCl than is the wild type. On the basis of their I50, tss1 and tss2 are only 2.3 and 1.4 times more sensitive to NaCl, respectively, than is the wild type. It is possible that disruption of other genes, such as TSS2 involved in ABA signaling, that are important for salt tolerance but are not involved in K+ nutrition might result in a moderate reduction of salt tolerance.
In this study, we report the isolation and characterization of salt-hypersensitive mutants of tomato. We selected mutants that have lost certain salt tolerance mechanisms and are thus more sensitive to salt stress than is the wild type. Physiological studies of these mutants have allowed the determination of distinct mechanisms that are critical for plant salt tolerance and will provide a foundation for additional salt tolerance analyses. Furthermore, isolation of the genes identified by mutagenesis would provide an additional possibility of increasing salt tolerance by genetic engineering, circumventing the undesirable characteristics of the wild relatives used previously as genetic sources of salt tolerance.
METHODS
Plant Materials and Growth Conditions
M2 seed of the near-isogenic line of the tomato (Lycopersicon esculentum) cultivar Moneymaker homozygous for the Cf-9 resistance gene after ethyl methanesulfonate mutagenesis were kindly provided by J.D.G. Jones (Sainsbury Laboratory, John Innes Centre, Norwich, UK).
Seed were surface sterilized with 40% (v/v) commercial bleach for 30 min and washed several times with sterile water. The seed were first germinated until radicle emergence in sterile water because we found that this improved germination uniformity. The basal agar medium contained Murashige and Skoog (MS) salts (Murashige and Skoog, 1962) with 3% (w/v) sucrose and 0.7% (w/v) agar. The MS medium consisted of the following: 1690 mg/L NH4NO3, 1900 mg/L KNO3, 370 mg/L MgSO4·7H2O, 170 mg/L KHPO4, 378 mg/L CaCl2·2H2O, 27.8 mg/L FeSO4·7H2O, 37.2 mg/L disodium EDTA, 0.7495 mg/L NaI, 6.3 mg/L H3BO4, 16.9 mg/L MnSO4·H2O, 8.6 mg/L ZnSO4·7H2O, 0.25 mg/L Na2Mo4·2H2O, 0.025 mg/L CuSO4·5H2O, and 0.025 mg/L CoSO4·6H2O. The modified MS medium (free of potassium and calcium) consisted of the same ingredients with the exception that the CaCl2, KNO3, and K2HPO4 were omitted and 165 mg/L (NH4)2HPO4 was added. The potassium and calcium were supplemented in the concentrations required with KCl and CaCl2·2H2O, respectively. The various agar plates used in this work were made by adding the appropriate amount of NaCl, KCl, LiCl, choline chloride, and mannitol to the molten basal medium. When appropriate, seedlings were transferred to perlite and grown until the end of the experiment. Light provided by cool-white fluorescent bulbs was at 50 μE m−2 sec−1 with 16 hr of light at 22°C, 8 hr of dark at 18°C, and 70% relative humidity.
Isolation of Mutants and Genetic Analysis
Between 30 and 40 seed from each M2 family were screened for NaCl hypersensitivity mutants by using a modified version of the assay described by Wu et al. (1996). Three-day-old seedlings with 2-cm-long roots were transferred from vertical agar plates onto a second agar medium that was supplemented with 125 mM NaCl. To obtain specifically salt-hypersensitive mutants, only those families in which all seed grew normally in MS medium were transferred to medium with salt. Seedlings from every individual family were arranged in rows. The root tips were marked, and the plates were oriented vertically. Because we were analyzing families obtained from a single M1 seed, we expected segregation for the mutant character. The segregation ratio expected was 3:1 (wild type/mutant) for a recessive mutation and 1:3 (wild type/mutant) for a dominant mutation.
Growth Measurements
For growth measurements, the same protocol described above for mutant isolation was used. Ten seed were used per treatment, and three replicates were run for each treatment. Increases in root length were measured with a ruler after 2 days of treatment. The only modification was in the experiments with low K+, in which case the root tip was marked 2 days after transfer and growth was measured after 3 days.
Electrical and Flux Measurements
For membrane potential determination, tomato seedlings were germinated and grown for 4 days in a medium containing 5 mM Hepes–3-(cyclohexylamino)-2-hydroxy-1-propanesulfonic acid, pH 7.3, 0.1 mM CaCl2, and 0.1 mM NaCl. In this way, we obtained K+-starved plants necessary for the functioning of the high-affinity K+ uptake (Epstein, 1966). Small amounts of Ca2+ and Na+ are necessary for stabilization of the membrane to obtain the appropriate membrane potential.
Measurements of membrane potential (Vm) in root hairs to assess the permeability of the membrane to K+ were made with an intracellular microelectrode, as described previously (Felle, 1981). Shifts in the extracellular KCl concentration ([KCl]ext) were imposed on the root while Vm was recorded. Assuming that an imposed shift in [KCl]ext affects only the K+ and Cl− components and assuming a negligible Cl− conductance (based on the positive shifts in Vm shown in Figure 8), the change in Vm resulting from shifts in [KCl]ext is interpreted here as a measure of the relative K+ permeability (Spalding et al., 1999). The same reasoning can be applied to the permeability of the membrane to Na+ when changes in extracellular NaCl concentrations were imposed on the root while Vm was recorded.
Excised roots were mounted in a plexiglass chamber (volume, 1.1 mL). Continuous perfusion of the assay medium was maintained at a flux of ∼10 mL/min. Epidermal root hairs were impaled with single barrel electrodes. Microelectrodes were fixed to electrode holders containing an Ag/AgCl pellet and connected to a high-impedance differential amplifier (model FD-223; WPI, Sarasota, FL).
Determination of Proline Content
Proline was extracted and quantitated as described previously (Borsani et al., 1999).
Acknowledgments
The mutagenized M2 seed were generously supplied by Jonathan Jones. We thank Lidia Laguna and Manuel Báguena for excellent technical assistance. We also thank Alonso Rodriguez-Navarro, Des Bradley, and our laboratory colleagues for helpful discussions and critical reading of the manuscript. This work was supported by a grant from the Universidad de Málaga and Junta de Andalucía (Grant No. AGR-168).
References
- Ashraf, M. (1994). Breeding for salinity tolerance proteins in plants. Crit. Rev. Plant Sci. 13, 17–42. [Google Scholar]
- Benlloch, M., Ojeda, M.A., Ramos, J., and Rodriguez-Navarro, A. (1994). Salt sensitivity and low discrimination between potassium and sodium in bean plants. Plant Soil 166, 117–123. [Google Scholar]
- Bohnert, H.J., Nelson, D.E., and Jensen, R.G. (1995). Adaptation to environmental stress. Plant Cell 7, 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsani, O., Díaz, P., and Monza, J. (1999). Proline is involved in water stress responses of Lotus corniculatus nitrogen fixing and nitrate fed plants. J. Plant Physiol. 155, 269–273. [Google Scholar]
- Botella, M.A., Quesada, M.A., Kononowicz, A., Bressan, R.A., Hasegawa, P.M., and Valpuesta, V. (1994). Characterization and in situ localization of a salt induced tomato peroxidase gene. Plant Mol. Biol. 25, 105–114. [DOI] [PubMed] [Google Scholar]
- Boyer, J.S. (1982). Plant productivity and environment. Science 218, 443–448. [DOI] [PubMed] [Google Scholar]
- Bray, E.A. (1993). Molecular responses to water deficit. Plant Physiol. 103, 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuartero, J., and Fernandez-Muñoz, R. (1999). Tomato and salinity. Sci. Hortic. 78, 83–125. [Google Scholar]
- Cuartero, J., Yeo, A.R., and Flowers, T.J. (1992). Selection of donors for salt-tolerance in tomato using physiological traits. New Phytol. 121, 63–69. [Google Scholar]
- Cushman, J.C., DeRocher, E.J., and Bohnert, H.J. (1990). Gene expression during adaptation to salt stress. In Environmental Injury to Plants, F.J. Katerman, ed (New York: Academic Press), pp. 173–203.
- Cutler, S., Ghassemian, M., Bonetta, D., Cooney, S., and McCourt, P. (1996). A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273, 1239–1241. [DOI] [PubMed] [Google Scholar]
- Davies, J.M., Brownlee, C., and Jennings, D.H. (1990). Electrophysiological evidence for an electrogenic proton pump and the proton symport of glucose in the marine fungus Dendryphiella salina. J. Exp. Bot. 41, 449–456. [Google Scholar]
- Delaney, A.J., and Verma, D.P. (1993). Proline biosynthesis and osmoregulation in plants. Plant J. 4, 215–223. [Google Scholar]
- Epstein, E. (1966). Dual pattern of ion absorption by plant cells and by plants. Nature 212, 1324–1327. [Google Scholar]
- Felle, H. (1981). A study of the current–voltage relationships of electrogenic and passive membrane elements in Riccia fluitans. Biochim. Biophys. Acta 692, 180–195. [DOI] [PubMed] [Google Scholar]
- Finkelstein, R.R. (1994). Mutations at two new Arabidopsis ABA responsive loci are similar to the abi3 mutations. Plant J. 5, 765–771. [Google Scholar]
- Finkelstein, R.R., and Lynch, T.J. (2000). The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein, R.R., and Somerville, C.R. (1990). Three classes of abscisic acid (ABA)–insensitive mutations of Arabidopsis define genes that control overlapping subsets of ABA responses. Plant Physiol. 94, 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein, R.R., Wang, M.L., Lynch, T.J., Rao, S., and Goodman, H.M. (1998). The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA2 domain protein. Plant Cell 10, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat, J., Hauge, B.M., Valon, C., Smalle, J., Parcy, F., and Goodman, H.M. (1992). Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 4, 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti, F., Beudoin, N., Serizet, C., Webb, A.A., Vartanian, N., and Giraudat, J. (1999). ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 11, 1897–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter, U., Ishitani, M., and Zhu, J.-K. (2000). The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA 7, 3735–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa, P.M., Bressan, R.A., and Zhu, J.K. (2000). Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 463–499. [DOI] [PubMed] [Google Scholar]
- Hirsch, R.E., Lewis, B.D., Spalding, E.P., and Sussman, M.R. (1998). A role for the AKT1 potassium channel in plant nutrition. Science 280, 918–921. [DOI] [PubMed] [Google Scholar]
- Hughes, F.M., Bortner, C.D., Purdy, G.D., and Cidlowski, J.A. (1997). Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J. Biol. Chem. 272, 30567–30576. [DOI] [PubMed] [Google Scholar]
- Ishitani, M., Xiong, L., Stevenson, B., and Zhu, J.K. (1997). Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: Interactions and convergence of abscisic acid–dependent and abscisic acid–independent pathways. Plant Cell 9, 1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J.B. (1982). Hydroponics: Its history and use in plant nutrition studies. J. Plant Nutr. 5, 1005–1030. [Google Scholar]
- Jones, R.A. (1986). High salt tolerance potential in Lycopersicon species during germination. Euphytica 35, 575–582. [Google Scholar]
- Kinraide, T.B. (1998). Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol. 118, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T.B. (1999). Interactions among Ca2+, Na+ and K+ in salinity toxicity: Quantitative resolution of multiple toxic and ameliorate effects. J. Exp. Bot. 50, 1495–1505. [Google Scholar]
- Kishor, P.B., Hong, Z., Miao, G.H., Hu, C.A., and Verma, D.P. (1995). Overexpression of Δ1-pyrroline-5-carboxylase synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 108, 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M., Reuling, G., and Karssen, C.M. (1984). The isolation and characterization of abscisic acid–insensitive mutants of Arabidopsis thaliana. Physiol. Plant. 61, 377–383. [Google Scholar]
- Läuchli, A. (1990). Calcium, salinity and plasma membrane. In Calcium in Plant Growth and Development, American Society of Plant Physiologists Symposium Series, Vol 4, R.T. Leonard and P.K. Kepler, eds (Rockville, MD: American Society of Plant Physiologists), pp. 26–35.
- Leung, J., and Giraudat, J. (1998). Abscisic acid signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 199–222. [DOI] [PubMed] [Google Scholar]
- Liu, J., and Zhu, J.K. (1997). An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc. Natl. Acad. Sci. USA 94, 14960–14964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., and Zhu, J.K. (1998). A calcium sensor homolog required for plant salt tolerance. Science 280, 1943–1945. [DOI] [PubMed] [Google Scholar]
- Liu, J., Ishitani, M., Halfter, U., Kim, C.-S., and Zhu, J.-K. (2000). The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 97, 3730–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza, I., Rubio, F., Rodriguez-Navarro, A., and Pardo, J.M. (1994). The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 269, 8792–8796. [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco culture. Physiol. Plant 15, 473–497. [Google Scholar]
- Nanjo, T., Kobayashi, M., Yoshiba, Y., Sanada, Y., Wada, K., Tsukaya, H., Kakubari, Y., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1999. a). Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J. 18, 185–193. [DOI] [PubMed] [Google Scholar]
- Nanjo, T., Kobayashi, M., Yoshiba, Y., Kakubari, Y., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1999. b). Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 461, 205–210. [DOI] [PubMed] [Google Scholar]
- Niu, X., Bressan, R.A., Hasegawa, P.M., and Pardo, J.M. (1995). Ion homeostasis in NaCl stress environments. Plant Physiol. 109, 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, Z.M., Ghassemian, M., Kwak, C.M., McCourt, P., and Schroeder, J.I. (1998). Role of farnesyltransferase in ABA regulation of guard cell anion channel and plant water loss. Science 282, 287–290. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Navarro, A. (2000). Potassium transport in fungi and plants. Biochim. Biophys. Acta 1469, 1–30. [DOI] [PubMed] [Google Scholar]
- Sentenac, H., Bonneaud, N., Minet, M., Lacroute, F., Salmon, J.M., Gaymard, F., and Grignon, C. (1992). Cloning and expression in yeast of a plant potassium ion transport system. Science 256, 663–665. [DOI] [PubMed] [Google Scholar]
- Serrano, R., and Gaxiola, R. (1994). Microbial models and salt stress tolerance in plants. Crit. Rev. Plant Sci. 13, 121–138. [Google Scholar]
- Serrano, R., Mulet, J.M., Rios, G., Marquez, J.A., de Larriona, I.F., Leube, M.P., Mendizabal, I., Pascual-Ahuir, A., Proft, M., Ros, R., and Montesinos, C. (1999). A glimpse of the mechanisms of ion homeostasis during salt stress. J. Exp. Bot. 50, 1023–1036. [Google Scholar]
- Shannon, M.C., Gronwald, J.W., and Tal, M. (1987). Effects of salinity on growth and accumulation of organic and inorganic ions in cultivated and wild species. J. Am. Soc. Hortic. Sci. 112, 416–423. [Google Scholar]
- Shi, H., Ishitani, M., Kim, C., and Zhu, J.-K. (2000). The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na/H antiporter. Proc. Natl. Acad. Sci. USA 97, 6896–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki, K., and Yamaguchi-Shinozaki, K. (1996). Molecular responses to drought and cold stress. Curr. Opin. Biotechnol. 7, 161–167. [DOI] [PubMed] [Google Scholar]
- Spalding, E.P., Hirsch, R.E., Lewis, D.R., Qi, Z., Sussman, M.R., and Lewis, B.D. (1999). Potassium uptake supporting plant growth in the absence of AKT1 channel activity: Inhibition by ammonium and stimulation by sodium. J. Gen. Physiol. 113, 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabolcs, I. (1994). Soil salinization. In Handbook of Plant Crop Stress, M. Pressarkli, ed (New York: Marcel Dekker), pp. 3–11.
- Wu, S.-J., Ding, L., and Zhu, J.K. (1996). SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 8, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J.K., Hasegawa, P.M., and Bressan, R.A. (1997). Molecular aspects of osmotic stress. Crit. Rev. Plant Sci. 16, 253–277. [Google Scholar]
- Zhu, J.K., Liu, J., and Xiong, L. (1998). Genetic analysis of salt tolerance in Arabidopsis: Evidence for a critical role of potassium nutrition. Plant Cell 10, 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]