Abstract
BP-80, later renamed VSRPS-1, is a putative receptor involved in sorting proteins such as proaleurain to the lytic vacuole, with its N-terminal domain recognizing the vacuolar sorting determinant. Although all VSRPS-1 characteristics and in vitro binding properties described so far favored its receptor function, this function remained to be demonstrated. Here, we used green fluorescent protein (GFP) as a reporter in a yeast mutant strain defective for its own vacuolar receptor, Vps10p. By expressing VSRPS-1 together with GFP fused to the vacuolar sorting determinant of petunia proaleurain, we were able to efficiently redirect the reporter to the yeast vacuole. VSRPS-1 is ineffective on GFP either alone or when fused with another type of plant vacuolar sorting determinant from a chitinase. The plant VSRPS-1 therefore interacts specifically with the proaleurain vacuolar sorting determinant in vivo, and this interaction leads to the transport of the reporter protein through the yeast secretory pathway to the vacuole. This finding demonstrates VSRPS-1 receptor function but also emphasizes the differences in the spectrum of ligands between Vps10p and its plant equivalent.
INTRODUCTION
The plant secretory system is far less easy to study than its yeast counterpart, mainly due to the lack of an equivalent mutant collection. Plant and yeast cells, in opposition to mammalian cells, do not use the mannose 6-phosphate–based lysosomal sorting tag (Kornfeld, 1992); instead, the sorting information is carried by a peptidic sequence. In yeast, the two vacuolar proteins carboxypeptidase Y (CPY; Johnson et al., 1987) and proteinase A (Klionsky and Emr, 1989) both are produced with an N-terminal propeptide responsible for the vacuolar location of the mature protein. Almost 50 mutants defective for vacuolar protein sorting (vps) have allowed the identification of proteins involved in the yeast vacuolar sorting pathway. One of these proteins, Vps10p, plays a central role because it is the vacuolar receptor for CPY (Marcusson et al., 1994). Vps10p also is able to send foreign or malfolded proteins to the vacuole for degradation in a process that is believed to be independent of any sorting signal (Hong et al., 1996).
In plants, two main types of vacuolar sorting determinants (VSDs) have been well studied and are believed to correspond to two separate vacuolar sorting pathways. The first type could be defined as a sequence-specific VSD (ssVSD) and has been well studied for barley aleurain (Holwerda et al., 1992) and sweet potato sporamin A (Matsuoka and Nakamura, 1991). For these two examples, the VSD is located within an N-terminal propeptide and contains a conserved tetrapeptide NPIR, the Ile being essential (Matsuoka and Nakamura, 1999). The position of this category of VSD seems to be less important than its sequence specificity, as shown with sporamin A (Koide et al., 1997). The second type of VSD is found in C-terminal propeptides (Ct-VSD); it is rather short with no obvious sequence conservation but needs to be accessible from the C terminus of the proprotein. The best-studied examples were found in tobacco chitinase A (Neuhaus et al., 1991) and barley lectin (Bednarek and Raikhel, 1991). There is a third type of VSD that is far less characterized and internal to the polypeptide (reviewed in Neuhaus and Rogers, 1998).
Little is known about the pathway that uses Ct-VSDs besides its higher sensitivity to wortmannin, a phosphatidylinositol 3-kinase inhibitor, in the BY2 tobacco cell line (Matsuoka et al., 1995). In contrast, a good candidate for a vacuolar sorting receptor involved in an ssVSD-associated pathway has been identified. This putative receptor, BP-80 (binding protein of 80 kD), was first isolated from pea clathrin-coated vesicles (CCVs) by its ability to bind proaleurain VSD in vitro. This binding was pH sensitive because it occurred at neutral pH and was abolished at pH 4.0. Binding assays also showed the specificity of interaction between BP-80 and its ligand, because the related ssVSD from sporamin A was partially able to compete with aleurain VSD, but barley lectin Ct-VSD was not (Kirsch et al., 1994). BP-80 is a type I protein with its N-terminal domain involved in recognizing the proaleurain VSD (Kirsch et al., 1994). BP-80 was further characterized and cloned (Paris et al., 1997). In addition to CCVs, it is localized in dilated ends of the Golgi apparatus, a possible plant trans-Golgi network, as well as in small structures next to the central vacuole that are assumed to be prevacuoles (Paris et al., 1997). Several homologs were identified in different species, including Arabidopsis, rice, and maize. BP-80 therefore was renamed VSRPS-1 (vacuolar sorting receptor from Pisum sativum number 1; Paris et al., 1997).
Although all of its characteristics favor its role as a vacuolar sorting receptor, the function of VSRPS-1 has not been proven, and such a demonstration is always a challenge. Here, we set up an in vivo test in yeast to demonstrate the vacuolar sorting and targeting functions of VSRPS-1. In a yeast strain deficient for the main vacuolar sorting receptor, Vps10p, we expressed VSRPS-1 together with green fluorescent protein (GFP) fused to various VSDs. In this mutant yeast, our reporter accumulated in the vacuole exclusively when it was fused to the proaleurain VSD and expressed together with the putative receptor VSRPS-1. This not only demonstrated that VSRPS-1 interacts with aleurain VSD in vivo but also that this interaction leads to transport of the reporter through the yeast secretory system to the vacuole. This assay therefore provides a straightforward tool to address the specificity of interaction between VSRPS-1 and its ligand in vivo, and we already have found that the plant receptor has a more restricted set of ligands than its yeast equivalent Vps10p.
RESULTS
GFP Is an Appropriate Reporter in Which to Study Plant VSDs in Yeast
It has been reported that a potentially secreted form of GFP was sent to the vacuole in wild-type yeast (Kunze et al., 1999). To determine whether this was also the case in a strain with a disrupted gene for the yeast vacuolar receptor Vps10p (Δvps10), we transformed this mutant yeast with the two control constructs represented in Figure 1, a secreted GFP (Sec-GFP) and a GFP fused to the yeast CPY vacuolar signal (CPY–GFP).
Figure 1.
Constructs Used in This Study.
Sec-GFP, the secreted form of GFP in which the Arabidopsis chitinase B signal peptide (s.p.) was fused to GFP5 (GFP); CPY-GFP, the signal peptide and the N-terminal propeptide from the yeast CPY (detailed) were fused to GFP5; GFP-Chi, the Ct-VSD of the tobacco chitinase A (detailed) was fused to the secreted form of GFP (Sec-GFP); aleu-GFP, the CPY signal peptide was fused with petunia aleurain VSD (detailed) to the N terminus of GFP5. For comparison (in parentheses), the corresponding sequence of the previously described VSD from barley proaleurain (Holwerda et al., 1992) is shown. Underlined amino acids are essential for vacuolar targeting.
The potentially secreted form of the reporter, Sec-GFP, was expressed in a Δvps10 mutant strain, and the intracellular accumulation pattern of fluorescence was analyzed and compared with that found in a wild-type yeast cell. The resulting transformed strains were observed using a confocal microscope, as shown in Figure 2A (Sec-GFP). The wild-type yeast strain showed GFP fluorescence (green) accumulating intracellularly, meaning that our reporter is efficiently produced and fluoresces in yeast. The subcellular location of the reporter (green) can be clearly identified as vacuolar by using a dye specific for the vacuolar membrane, FM 4-64 (red). For more clarity, a set of four images is presented from the same cells, the third one representing a merged and pseudocolored image of GFP in green and FM 4-64 in red. Unlike the wild type, the Δvps10 strain (Figure 2A, Sec-GFP) was not able to retain the reporter Sec-GFP intracellularly because no GFP-linked fluorescence was accumulated in these cells. This result clearly showed that the absence of fluorescence in the mutant strain is the result of VPS10 disruption. We then assessed by immunoblot (Figure 2B) the ratio of GFP found in the medium to GFP retained within the cell for both strains. Some Sec-GFP was clearly found in the cell fraction (c) both from a wild-type and a mutant strain (Δvps10), but the amount of GFP was significantly lower when the gene for Vps10p was disrupted. This finding indicated, as described previously (Kunze et al., 1999), that GFP is sent to the vacuole in wild-type yeast, and our results additionally demonstrated that this is due to Vps10p activity. This is also in accord with previous observations showing a low specificity for Vps10p that can send to the vacuole for degradation many proteins that are either foreign to yeast or not properly folded (Hong et al., 1996).
Figure 2.
Vps10p-Dependent Vacuolar Localization of Sec-GFP and CPY-GFP in Yeast.
(A) The living cells were visualized with a confocal microscope after 24 hr of induction. The constructs, either a secreted form of GFP (Sec-GFP) or a GFP fused to the yeast vacuolar signal from CPY (CPY-GFP), were expressed in wild-type cells (WT) or in a yeast disrupted for the gene encoding the vacuolar receptor Vps10p (Δvps10). Each condition combines four images from the same cells: from left to right, GFP fluorescence, FM 4-64 staining of a vacuolar membrane, a pseudocolored merged image with GFP in green and FM 4-64 in red, and the transmitted light image. 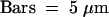 .
.
(B) After 24 hr of induction, the transformed cells were separated in two fractions: the cell extract (c) and the medium (m). Both extracts then were immunolabeled with anti-GFP antibodies. Molecular masses of standards are indicated to the left in kilodaltons.
We similarly tested how the yeast vacuolar determinant from CPY was handled by yeast when fused to GFP. We expressed the construct CPY-GFP (Figure 1) in both the wild-type and Δvps10 strains and observed the cells by confocal microscopy, as shown in Figure 2A (CPY-GFP). As expected, the CPY-GFP was found predominantly in the central vacuole of the wild-type yeast because the GFP-fluorescent structures (green) were clearly surrounded by the vacuolar membrane marker (red). In contrast with the wild type, the mutant strain (Δvps10) no longer accumulated a detectable amount of CPY-GFP in the vacuole. These results were reinforced by immunoblot analysis (Figure 2B). As seen on the CPY-GFP immunoblot, a significant proportion of reporter protein was secreted (m) by mutant cells (Δvps10) compared with wild-type cells. The amount of CPY-GFP still found intracellularly by Δvps10 cells (c) may represent a form of the reporter on its way out of the cell with a uniform and weak fluorescent pattern under confocal microscopy, as is seen occasionally in some yeast. Most importantly, GFP did not accumulate in any specific subcellular location, including the vacuole.
Although confocal microscopy leads to comparable GFP images for CPY-GFP and Sec-GFP expressed in yeast, immunoblot analysis (Figure 2B) showed that the reporter was more efficiently retained inside the wild-type cells when it carried the additional CPY vacuolar signal. This could mean that there is a signal-specific mechanism linked to the presence of the CPY determinant in addition to the retention mechanism involved in sending foreign or malfolded proteins to the vacuole. This finding suggests that, like the CPY vacuolar signal, any potential vacuolar determinant will be exposed properly to the sorting system when fused to the GFP reporter. In addition, the fact that neither Sec-GFP nor CPY-GFP was found in the vacuole of the mutant strain showed that there is no targeting machinery, independent of Vps10p, that will send GFP to the vacuole. Therefore, these control constructs allowed us to conclude that (1) GFP can be used as a reporter for vacuolar sorting in a yeast strain that lacks a functional Vps10p, and (2) GFP should properly present a given VSD to its appropriate receptor.
The Petunia Aleurain Propeptide Is Sufficient, in Plants, to Target GFP to the Vacuole
We wanted to test two types of plant signals that are presumably representative of two different vacuolar sorting pathways. The tobacco chitinase A VSD is sufficient to send GFP5 to a neutral pH vacuole in tobacco protoplasts (Di Sansebastiano et al., 1998). We also wanted to use a representative of the sequence-specific type of signal, the barley aleurain VSD (Holwerda et al., 1992). In barley proaleurain, the VSD is composed of 24 amino acids organized in three subgroups, each of which is able to partially redirect a normally secreted protein to the vacuole in protoplasts from tobacco-derived suspension cells. Unfortunately, because codon use preferences are very different between cereals and yeast, we had to replace the well-described barley aleurain VSD with an equivalent coding sequence from the dicot petunia. We took amino acids 1 to 44 from petunia preproaleurain that should contain the vacuolar sorting information equivalent to its barley counterpart. Figure 1 shows an alignment of the petunia sequence (aleu-GFP, detailed) and barley proaleurain VSD (in parentheses). Both sequences are highly homologous and contain the tetrapeptide NPIR that has been shown to be essential for targeting. Nevertheless, we had to show that this petunia aleurain sequence was sufficient for vacuolar sorting in plants by using GFP as a reporter. In our laboratory, we had already used the whole propeptide (119 amino acids) from barley aleurain fused to GFP and found that the reporter accumulated in an acidic vacuole (Di Sansebastiano et al., 2001). This necessitated the use of a more stable and brighter variant of GFP5, called GFP6, which differs from GFP5 by the mutations F64L and S65T described previously (Cormack et al., 1996).
For transient expression in plants therefore, we fused GFP6 to the first 44 amino acids from petunia preproaleurain, including the signal peptide and the potential VSD. We transformed tobacco leaf protoplasts with the GFP chimera and observed the fluorescence by using a confocal microscope after 24 hr of transient expression, as shown in Figure 3. In many cells, we found aleuPh-GFP6 fluorescence (Figure 3A, green) accumulated in the large central vacuole that occupies almost all of the cell volume (Figure 3B). This vacuolar location is easy to distinguish in cytoplasmic staining because in internal confocal sections the latter is limited to a thin layer at the periphery of the cell (Di Sansebastiano et al., 1998). In addition to the large central vacuole, occasional small GFP-labeled structures were visible that may represent intermediate transport compartments (Figure 3, cell at bottom). This result clearly showed that within petunia preproaleurain, the 24 amino acids that follow the signal peptide are sufficient for vacuolar sorting in plants, as are their barley homologs.
Figure 3.
Demonstration of the Vacuolar Sorting Properties of the Petunia Aleurain Propeptide in Tobacco Leaf Protoplasts.
Living protoplasts expressing aleuPh-GFP6 were visualized using a confocal microscope 24 hr after transformation. Two cells are shown after illumination with either the dual fluorescein isothiocyanate/tetramethylrhodamine isothiocyanate fluorescence filter (A) or transmitted light (B). The fluorescence image uses the attributed pseudocolors green for GFP and red for chloroplast autofluorescence. 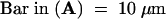 .
.
VSRPS-1 Redirects the GFP Reporter to the Yeast Vacuole When Fused to Petunia Aleurain VSD
Because we found that GFP5 is a suitable reporter for vacuolar targeting studies in a Δvps10 yeast, we fused it with each of two representative VSDs we chose from plants (Figure 1), tobacco chitinase A (GFP-Chi) and petunia aleurain (aleu-GFP). As shown in Figure 4, both constructs were efficiently expressed by a wild-type strain, because the reporter was visible and accumulated in the vacuole, as in controls. As for the secreted form of GFP, the fluorescence was no longer seen intracellularly when the same two fusion proteins with plant signals were expressed in the mutant strain (Δvps10). This means that the two plant VSDs did not lead to intracellular retention of our reporter in yeast in the absence of Vps10p. The aleurain chimera was produced and tested with the signal peptide of yeast CPY (as shown here) or with the petunia aleurain's own signal peptide with identical results (data not shown).
Figure 4.
Vps10p-Dependent Vacuolar Localization of GFP-Chi and aleu-GFP in Yeast.
The living cells were visualized with a confocal microscope after 24 hr of induction. The constructs, either a GFP fused to the tobacco chitinase A VSD (GFP-Chi) or the petunia aleurain VSD fused to GFP (aleu-GFP), were expressed in wild-type cells (WT) or a yeast disrupted for the gene encoding the vacuolar receptor Vps10p (Δvps10). Each condition combines four images from the same cells as described for Figure 2A. 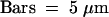 .
.
In Δvps10 yeast cells transformed by each of the four GFP fusion proteins described previously, we coexpressed the sequence coding for the putative plant vacuolar sorting receptor, VSRPS-1. As shown in Figure 5A, this coexpression did not lead to any retention of Sec-GFP, CPY-GFP, or GFP-Chi. In contrast, VSRPS-1 clearly affected aleu-GFP distribution because the reporter accumulated in the lumen of the vacuole (Figure 5A, aleu-GFP + VSRPS-1). The vacuolar location of the GFP (green) was clearly confirmed using FM 4-64 to stain the vacuolar membrane (red). The exclusive vacuolar location of aleu-GFP was even more visible in the GFP channel alone (far left). All of the confocal images presented were taken under the same conditions by using identical laser power for GFP excitation. Therefore, the GFP-linked fluorescences shown here were directly comparable to each other, meaning that a similar amount of aleu-GFP accumulated in the vacuole due to coexpression with either VSRPS-1 or Vps10p function (Figures 5A and 4, respectively).
Figure 5.
The Expression of VSRPS-1 Specifically Restores the Vacuolar Location of aleu-GFP in the Absence of the Yeast Vacuolar Sorting Receptor Vps10p.
(A) The living cells were visualized with a confocal microscope after 24 hr of induction. The Δvps10 strain was doubly transformed with the plant putative vacuolar sorting receptor VSRPS-1 and one of the four GFP fusion constructs. These GFP chimera carry no vacuolar signal (Sec-GFP), the CPY yeast vacuolar signal (CPY-GFP), the tobacco chitinase A VSD (GFP-Chi), or the petunia aleurain VSD (aleu-GFP). Each condition combines four images from the same cells as described for Figure 2A. 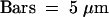 .
.
(B) Effect of VSRPS-1 on aleu-GFP localization in Δvps10 cells after 48 hr of induction. Shown are (top) a fluorescence image of a Δvps10 cell expressing both VSRPS-1 and aleu-GFP (green) and stained for the vacuolar membrane with FM 4-64 (red) and (bottom) the same cell in transmitted light. 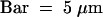 .
.
(C) Immunoblot analysis after 48 hr of induction by using anti-GFP antibodies on a cell extract from either wild-type yeast (WT) or a yeast disrupted for the gene encoding the vacuolar receptor Vps10p (Δvps10) and expressing the GFP reporter fused to either the petunia aleurain (aleu-GFP) or the chitinase A (GFP-Chi) VSD in the presence (+) or absence (−) of VSRPS-1. The top and bottom panels represent the same blots exposed for either 5 or 15 sec, respectively. Molecular masses of standards are indicated to the left in kilodaltons.
The percentage of Δvps10 cells that were fluorescent when aleu-GFP and VSRPS-1 were coexpressed reached 60%, which is close to the 70% of fluorescent wild-type cells transformed with the same aleu-GFP. Within this GFP-labeled population of Δvps10 cells, 67% accumulated aleu-GFP in a vacuolar pattern. The other, much less abundant pattern is diffuse, similar to aleu-GFP alone in a Δvps10 strain and may be due to a lack of coexpression of VSRPS-1 in those cells. This high percentage of aleu-GFP redirected to the yeast vacuole because of the putative plant receptor suggests that VSRPS-1 is as efficient as Vps10p in terms of vacuolar sorting and targeting.
As shown in Figure 5C, we performed an immunoblot analysis after 48 hr of induction. At this stage, the level of expression decreased due to the exhaustion of galactose; therefore, much of the reporter should have reached its final destination. Figure 5B shows the GFP pattern observed under confocal microscopy after this longer culture time. The fluorescence accumulation pattern is identical to that found at 24 hr, with even less residual nonvacuolar labeling. On the immunoblot at this stage, we detected less of the higher molecular weight transport intermediates than after shorter culture times (data not shown). The predominant form that we observed at 48 hr (Figure 5C, aleu-GFP) is similar in size to native GFP, suggesting that the VSD has been removed. This immunoblot analysis confirmed a significant intracellular retention of the reporter when aleu-GFP and VSRPS-1 were coexpressed in a Δvps10 strain. Compared with the signal obtained for a wild-type cell expressing the same aleu-GFP construct (left lane), VSRPS-1–mediated retention is an efficient process. In the negative control represented by GFP-Chi + VSRPS-1, the GFP signal found intracellularly was almost undetectable, and a longer exposure time was required to make it visible (Figure 5C, bottom). Most importantly, the presence of this residual GFP-Chi was not due to the presence of VSRPS-1 because a similar amount of reporter was retained as well in a mutant yeast cell.
Because the NPIR motif was shown to be a major component of the barley aleurain VSD (Holwerda et al., 1992) and to be essential for the sporamin VSD (Matsuoka and Nakamura, 1999), we performed a preliminary experiment to investigate the importance of the NPIR motif from the aleurain VSD in our assay. The Ile therefore was replaced with a glycine in the aleu-GFP chimera. When coexpressed with the plant receptor, we found an obvious diminution in the percentage of cells with a GFP-labeled vacuole as well as an overall diminution in intensity compared with the original aleu-GFP (data not shown). Although we could not express our results in a quantitative way, this finding nevertheless suggested that the sequence specificity feature of the aleurain VSD is as essential in our yeast assay as it has been shown to be in plant cells. The fact that we did obtain an intermediate answer between negative and positive control cells also is in agreement with previous work showing that the barley aleurain VSD with all four NPIR amino acids mutated was still able to target 25% of aleurain to the vacuole in tobacco culture cells (Holwerda et al., 1992).
The effect of VSRPS-1 therefore is linked exclusively to the presence of the ssVSD, as in petunia proaleurain, because the plant receptor does not retain the reporter when it carries a different type of plant VSD (GFP-Chi) and is less efficient when the essential NPIR sequence is mutated. The fact that VSRPS-1 did not affect Sec-GFP repartition also suggests that VSRPS-1 is different from Vps10p in its ability to target foreign or misfolded proteins to the yeast vacuole.
VSRPS-1 Apparently Traffics within the Yeast Secretory System Like Its Equivalent Vps10p
To assess where the plant receptor accumulated in the yeast cell, we performed immunolabeling, as shown in Figure 6. First, we labeled cells expressing the plant receptor with antibodies directed against VSRs (Figure 6A). The dot-like labeling pattern for VSRPS-1 is absent from an untransformed cell (Figure 6C, inset), and when merged with a transmitted light image it appears next to the vacuole (Figure 6A, both). This labeling shows that our plant receptor does not accumulate in the vacuolar membrane or the vacuole even when overexpressed. For comparison, we performed similar immunolabeling on wild-type yeast cells to localize the native Vps10p. As shown in Figure 6B, we also obtained dot-like labeling similar to what has been described previously (Cooper and Stevens, 1996), and again it was very close to the vacuolar membrane. This result suggests that in a mutant yeast strain the plant vacuolar receptor is located in compartments similar to those accumulating Vps10p in wild-type cells. We then performed double immunolabeling on wild-type strains expressing the plant receptor (Figure 6C). When merged (both), the two images clearly showed that all of the VSRPS-1–labeled compartments also labeled for the yeast receptor (arrowheads). This shows that the plant receptor is able to accumulate in the subcellular location where its yeast equivalent is concentrated in the latter case, as a result of controlled cycling between the late Golgi and the prevacuole.
Figure 6.
The Plant Vacuolar Sorting Receptor VSRPS-1 Is Not Vacuolar and Colocalizes with Vps10p in Yeast Cells.
Cells were fixed after 24 hr of culture and immunolabeled with anti-VSR and/or anti-Vps10p antibodies. The resulting immunostaining was visualized using a confocal microscope.
(A) A Δvps10 cell expressing VSRPS-1 was immunolabeled with anti-VSR antibodies (left). A transmitted image of the same cell (trans.) was taken and merged to the immunostaining image (both).
(B) A wild-type cell was immunolabeled with anti-Vps10p antibodies (left). A transmitted image of the same cell (trans.) was taken and merged to the immunostaining image (both).
(C) A wild-type cell expressing VSRPS-1 was immunolabeled with anti-VSR and anti-Vps10p antibodies. The immunolabeling images were pseudocolored in green for VSRPS-1 and red for Vps10p and merged (both). The inset shows the background staining with anti-VSR antibodies on untransformed wild-type cells. Arrowheads mark the location of double-labeled compartments.
 .
.
These results suggest that the accumulation of aleu-GFP in the yeast vacuole was not simply the result of one-way cotransport of the plant receptor together with its ligand from the Golgi to the vacuole. They also suggest that at least part of the overexpressed plant receptor may be able to use the yeast sequence-specific trafficking machinery.
DISCUSSION
VSRPS-1 was the first putative vacuolar sorting receptor isolated and cloned from a plant based on its hypothetical function. It binds in vitro to the VSD of barley aleurain and to related ssVSDs but not to a Ct-VSD from barley lectin (Kirsch et al., 1994). Two criteria for the receptor function are now fulfilled by our results: (1) the petunia aleurain VSD, sufficient for vacuolar targeting in plants, is recognized in vivo by VSRPS-1; and (2) this interaction causes targeting of the reporter aleu-GFP to the vacuole in Δvps10 yeast. The in vivo demonstration of VSRPS-1 function added to its previously described properties, (1) its pH-dependent binding (Kirsch et al., 1994) similar to the mammalian mannose 6-phosphate system (Kornfeld, 1992) and (2) its subcellular location in plant cells (Paris et al., 1997), allow us to propose the following model (Figure 7). At the level of the trans-Golgi (circled 1), where the pH is believed to be neutral or slightly acidic compared with that in the mammalian system, VSRPS-1 would sort proaleurain (gray squares) from other soluble proteins (white squares). More precisely (Figure 7, detail), the sorting would occur by interaction of the N-terminal domain of VSRPS-1 with the ssVSD carried by proaleurain (aleu.). Simultaneously, VSRPS-1 would expose its C-terminal domain to cytosolic proteins such as adaptins that are involved in CCV formation. The complex of VSRPS-1 and proaleurain then would be transported (circled 2) to an acidic compartment, the prevacuole. Due to the decrease of pH, the ligand then would be released from VSRPS-1 (circled 3). Finally, we propose that the free receptor would be recycled back to the Golgi (circled 4) although it is not fully demonstrated by our data. The shuttle vesicles used by the receptor must be clathrin coated at least in one direction because VSRPS-1 originally was purified from this type of vesicle (Kirsch et al., 1994).
Figure 7.
Model for VSRPS-1 Function in Plants.
The trans-Golgi (left) is the region of the secretory pathway where sorting of vacuolar soluble proteins is believed to occur. At this neutral pH, the plant vacuolar sorting receptor (brackets) sorts (step 1) proaleurain (gray squares) from other soluble proteins (white squares). The detail shows more precisely the structure of VSRPS-1, with its N-terminal domain (N) interacting with the ssVSD carried by proaleurain (aleu.). It also shows the C-terminal domain (C) of VSRPS-1 on the cytosolic side of the membrane that is available for contacts with proteins such as adaptins that are involved in CCV formation. The complex of VSRPS-1 and proaleurain (step 2) is packed into transport vesicles and targeted to an acidic compartment (right). The decrease in pH causes the ligand to be released from VSRPS-1 (step 3) within the acidic compartment, which is possibly a prevacuole. The free receptor then recycles back to the Golgi (step 4), again by means of transport vesicles. We know that CCVs are used by the receptor in plants to shuttle between the Golgi and an acidic compartment, but we do not know in which direction of transport. This model is based on our demonstration of VSRPS-1 function in yeast and on previously published characteristics (Kirsch et al., 1994; Paris et al., 1997).
The fact that VSRPS-1 functions in yeast is an argument in favor of conserved vacuolar targeting machineries between yeast and plants. VSRPS-1 must meet its ligand and therefore must accumulate to some extent in the compartment where vacuolar sorting occurs in yeast, presumably the late Golgi (Graham and Emr, 1991). Interestingly, immunostaining of VSRPS-1 always colocalized with Vps10p structures, and most importantly, the plant receptor did not show any vacuolar accumulation. These results support the hypothesis that VSRPS-1, like Vps10p, traffics from the late Golgi to the prevacuolar compartment where aleu-GFP is released and therefore does not migrate from the late Golgi together with its ligand all the way to the vacuole. However, we cannot fully rule out that the plant receptor is sent to the yeast vacuole where it is rapidly degraded, and one still may argue that the high level of expression of VSRPS-1 may lead to a constant and sufficient amount of the plant receptor within the late Golgi with no recycling requirement.
It has been shown that, when overexpressed, the yeast vacuolar receptor Vps10p devoid of its cytosolic domain still sent 35% of CPY to the vacuole in a Δvps10 strain (Cooper and Stevens, 1996). Yet, these authors also showed that, at a level of expression similar to the wild-type receptor, no residual activity was found for the same truncated receptor. In our overexpressing context, we have preliminary results suggesting that the cytosolic domain of the plant receptor is not necessary for traffic in yeast (data not shown), as was described in plants (Jiang and Rogers, 1998). The fact that Vps10p and VSRPS-1 colocalize in vivo in yeast can be interpreted as evidence for some recycling in yeast in addition to a constant flow of transport, although we would need a lower expressing system to demonstrate this hypothesis. This led to the exciting suggestion that plant trafficking signals in the cytosolic tail of VSRPS-1 could recruit some of the yeast proteins involved in vesicle formation and, more precisely, in retrograde transport to the late Golgi. The C-terminal domains of VSRPS-1 and Vps10p are extremely different in length as well as in sequence, except for a tyrosine motif that is known to interact with adaptins from CCVs. It will be interesting to pursue this comparison to identify crucial trafficking motifs that may be shared by yeast and plant cells. It also would be interesting to discover the pathway actually used by VSRPS-1 in yeast to determine, for example, which type of adaptin complex is recruited. Two secretory pathways have been shown to send proteins to the yeast vacuole: one for soluble proteins such as CPY, mediated by Vps10p, Pep12p, and AP1 adaptins, and the other for membrane proteins such as alkaline phosphatase (Stack et al., 1995). The latter pathway is independent from Vps10p and Pep12p and uses AP3 adaptins (Odorizzi et al., 1998).
Although our results suggest strong similarities between yeast and plant vacuolar trafficking machineries, we found differences in ligand preferences between the respective receptors. Unlike Vps10p, VSRPS-1 recognizes neither the GFP itself as a foreign protein nor the yeast vacuolar signal from CPY. This indicates a divergence between the two sorting systems in terms of their affinity for VSDs. We can expect such a difference because plants have developed a much more complex vacuolar system, having in some cells two (Paris et al., 1996) if not three (Jauh et al., 1999) different destinations for soluble vacuolar proteins. The Vps10p domain involved in ligand recognition may contain two binding sites, one for sequence-specific interactions and the other for unfolded structures (Jørgensen et al., 1999). Structural requirements for VSRPS-1 ligand binding have been published recently (Cao et al., 2000). The authors also found indications for two possible recognition sites in VSRPS-1, one specific for the NPIR motif and another defined as a “non-NPIR site.” Our assay would provide a useful tool to address in vivo the structural features of the N-terminal domain of VSRPS-1.
The ligand specificity for VSRPS-1 found using in vitro approaches (Kirsch et al., 1994) is now corroborated in vivo by our yeast assay. VSRPS-1 is clearly specific for the type of VSD carried by aleurain and does not recognize tobacco chitinase A VSD, a typical C-terminally required signal. The reporter GFP can efficiently expose N-terminal, C-terminal, and even internal peptides (Abedi et al., 1998) and remains fluorescent. Therefore, we can study the interaction between potential signals and VSRPS-1 in a more realistic context than with synthetic peptides linked to an affinity column. The range of ligands that are recognized by VSRPS-1 is an important issue because confusing results implicate either this receptor or members of its growing family of homologs. For example, the VSR homologs PV72 and PV82 from pumpkin recognize two peptides within pro 2S albumin, one internal and one C-terminal (Shimada et al., 1997). The ssVSD of proaleurain is clearly able to release the doublet at 72 and 82 kD that was retained on a column exposing the internal sequence of 2S albumin, which means that these VSR homologs from pumpkin are able to recognize the two types of sequences. Unfortunately, the internal sequence of pro 2S albumin used in that study has not been demonstrated to be used as a vacuolar signal in the context of the native protein. VSRPS-1, or a homolog, also recognizes the vacuolar targeting signal carried at the C terminus of 2S albumin in vitro (Kirsch et al., 1996). Finally, some tobacco VSRs interact with the C-terminal region of a Nicotiana alata protease inhibitor that is necessary for vacuolar targeting (Miller et al., 1999).
In addition to these VSRs for which ligand affinity was investigated directly, there are other VSR homologs for which we have no direct evidence in terms of vacuolar sorting function besides the high sequence similarities (Ahmed et al., 1997; Paris et al., 1997). The Arabidopsis VSR family is now represented by at least seven members, and it is not known whether all variants have ligand specificities and subcellular location identical to VSRPS-1 (Paris and Rogers, 1996). Together with the original receptor, two variants of VSRPS-1 also were cloned from the same cDNA library made from pea seed (Paris et al., 1997). It is troubling, therefore, that only one protein was isolated based on its ability to bind in vitro to the proaleurain VSD. So far, there is no available tool, such as antibodies, that would allow one to identify specifically each of these VSR variants in planta, which may explain why contradictory localization results have been published. For example, the same Arabidopsis VSR homolog was found either mainly in a PEP12 prevacuolar compartment (Sanderfoot et al., 1998) or almost exclusively in the plasma membrane (Laval et al., 1999). To date, the studies on VSR variants subcellular location are puzzling as well, and at this stage it is premature and therefore risky to conclude that all of the VSRs function exactly like VSRPS-1. It seems more reasonable to postulate that each VSR has a different spectrum of ligands and perhaps even functions in different pathways.
Our assay is now providing a clear and fast in vivo means to investigate each VSR variant in terms of ligand specificity within the limits imposed by the differences between the yeast and plant vacuolar systems. We will need a method to quantitate vacuolar targeting efficiency if we want to compare the possible differences in affinity between different members of the VSR family.
METHODS
Strains and Media
Bacterial strains were grown on standard media (Miller, 1972). Yeast strains were grown on yeast extract, peptone, dextrose medium, or synthetic dextrose (SD) medium supplemented as necessary (Sherman et al., 1979). The yeast strains SEY6210 (MATa leu2-3, 112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9) and EMY3 (SEY6210 vps10Δ1::HIS3) have been described previously (Robinson et al., 1988; Marcusson et al., 1994).
Constructs
All of the constructs used were made according to basic methods described previously (Sambrook et al., 1989). The yeast–Escherichia coli shuttle vector pGAL was used throughout this study (Blum et al., 1989). Two derivatives of pGAL were produced: (1) pGAL-BglII was obtained by removing the BglII fragment containing the TRP1 gene, and (2) pYWG1 was obtained by removing the NsiI-PstI fragment containing a part of the URA3 gene. For plant transient expression, we used the vector pGY1, which contains 35S promoter and terminator sequences (Neuhaus et al., 1991). Green fluorescent protein (GFP) constructs were derived from the previously described plasmids pSGFP5 and pSGFP5T (Di Sansebastiano et al., 1998).
For yeast expression, the construct containing the secreted GFP (Sec-GFP; Figure 1) was obtained by introducing the BamHI-SacI fragment from pSGFP5, which encodes the Arabidopsis thaliana chitinase signal peptide fused in-frame with the GFP5 sequence, in the corresponding sites of pYWG1. The construct containing the GFP fused to the C-terminal propeptide of tobacco chitinase A (GFP-Chi; Figure 1) was made by cloning the BamHI-SacI fragment from pSGFP5T, which encodes Sec-GFP5 with the C-terminal addition of the tobacco chitinase vacuolar sorting determinant (VSD), into pYWG1. For the GFP5 fused to the signal peptide and the propeptide from carboxypeptidase Y (CPY-GFP; Figure 1), we amplified by polymerase chain reaction (PCR) a fragment encoding the first 50 amino acids of preproCPY by using a plasmid encoding the CPY-invertase fusion protein (pLJL2; a kind gift from E. Marcusson, University of California–San Diego, La Jolla) as a template and two primers generating a BamHI site in 5′ and a NheI site in 3′. The PCR fragment then was used to replace the BamHI-NheI fragment from Sec-GFP5, giving CPY-GFP5. To obtain the fusion between GFP5 and the propeptide of Petunia hybrida aleurain (aleu-GFP; Figure 1), we first created a maxiprimer containing the first 20 amino acids of CPY (signal peptide) by using pLJL2 as a template and two primers generating a BamHI site in 5′ and a sequence homologous with codons 21 to 25 of the petunia preproaleurain in 3′. This maxiprimer then was used in 5′ to amplify codons 21 to 44 from preproaleurain by using pETH3 (Tournaire et al., 1996; GenBank accession number U31094) as a template and a second primer adding an NheI site in 3′. This PCR fragment then was used to replace the BamHI-NheI fragment from Sec-GFP, giving the construct aleu-GFP.
The construct containing VSRPS-1 was obtained by introducing the 2-kb XmaI-KpnI fragment from NP471 corresponding to the full coding sequence (Paris et al., 1997; GenBank accession number U79958) in the equivalent sites of pGAL-BglII. All constructs were controlled by sequencing.
For expression in tobacco protoplasts, the first 44 amino acids of preproaleurain from petunia were placed in front of a more stable and brighter derivative of GFP5, called GFP6, which contains the mutations F64L and S65T (Cormack et al., 1996). We first generated a BamHI site in 5′ and an NheI site in 3′ of the first 44 codons of petunia preproaleurain by PCR using pETH3 as a template. We then cloned the resulting fragment with the NheI-SacI GFP5 fragment from CPY-GFP between the BamHI and SacI sites of pGY1. We later introduced the two point mutations within the GFP5 sequence by PCR to give the more stable GFP6. The resulting construct, called aleuPh-GFP6, is cloned between the 35S promoter and terminator sequences carried by pGY1.
Cell Labeling and Confocal Microscopy
Transformed or cotransformed yeast cells were grown for 24 hr at 30°C in selective SD medium containing 2% galactose for induction. We then stained specifically the yeast vacuolar membrane by using the lipophilic styryl dye FM 4-64 (N-[3-triethylammoniumpropyl]- 4-[p-diethylaminophenylhexatrienyl] pyridinium dibromide; Molecular Probes, Eugene, OR; Vida and Emr, 1995). The cells were incubated for 15 min at 30°C in inducing SD medium containing 8 μM FM 4-64 and then rinsed for 90 min at 30°C in inducing SD medium. These incubation times are sufficient for the dye to reach the vacuolar membrane with no residual plasma membrane or endosomal staining. Cells were immobilized on a slide in 0.4% low-melting-point agarose for observation with a microscope.
Tobacco (Nicotiana tabacum) protoplasts were isolated from leaves of cv SR1 (Nagy and Maliga, 1976). A polyethylene glycol–based transformation protocol then was used (Negrutiu et al., 1987; Freydl et al., 1995; Di Sansebastiano et al., 1998), and the transformed protoplasts were visualized 24 hr after transformation.
Images were collected with a Leica (Wetzlar, Germany) confocal laser microscope by using a Leica TCS 4D operating system. Either a fluorescein isothiocyanate or a tetramethylrhodamine isothiocyanate filter set was used to detect GFP or FM 4-64, respectively. Digital images were pseudocolored using Adobe Photoshop version 4.0.1 (Mountain View, CA), with red attributed to FM 4-64 and green attributed to GFP. An image with transmitted light also was collected using the confocal microscope.
Immunostaining was performed on Δvps10 or wild-type cells grown overnight in inducting conditions when transformed with VSRPS-1. Cells were fixed using a modified version of the standard protocol (Pringle et al., 1991). The cells then were fixed in their growing medium for 1 hr with 3.7% formaldehyde at 30°C and for at least 12 hr at room temperature with 0.1 M KPO4, pH 6.4, containing 3.7% paraformaldehyde. They were washed three times in buffer alone and once with buffer A (1.2 M sorbitol, 0.12 M K2HPO4, and 33 mM citric acid, pH 5.9). The cell wall was digested using β-glucuronidase at 10,000 units/mL (slug juice; Sigma) and 5 mg/mL zymolyase 20T (ICN Biomedical, Costa Mesa, CA) in buffer A for 75 min at 30°C. After washing, the membranes were treated with 0.5% Triton X-100 for 5 min, and immunolabeling was performed as described previously (Paris et al., 1996). The primary antibodies used were the monoclonal 14G7 anti-VSR antibodies at a 1:100 dilution (Paris et al., 1997) and the anti-Vps10p serum (a gift from Scott Emr, University of California–San Diego, La Jolla) at a 1:100 dilution. The secondary antibodies used were anti-mouse rhodamine Red-X conjugated or anti-rabbit fluorescein conjugated (catalog numbers 115-295-100 and 711-095-152, respectively; Jackson ImmunoResearch, West Grove, PA) at a dilution of 1:100.
Protein Gel Blot Analysis
Transformed yeast cells were incubated at 30°C in inducing SD medium containing 2% galactose to an OD600 of 5 to 10. Cells equivalent to 5 OD600 units were collected and separated from the culture medium by centrifugation. The cell pellet was resuspended in SDS gel sample buffer (Laemmli, 1970), frozen in liquid nitrogen, and boiled for 10 min at 100°C. Freezing and boiling were repeated twice. The crude cellular extract then was centrifuged at 14,000g for 10 min to remove debris. Proteins were precipitated from the medium with 10% trichloroacetic acid for 15 min at −20°C and then recovered by centrifugation (10,000g, 30 min). The trichloroacetic acid pellet was washed twice in acetone and resuspended in 20 μL of SDS gel sample buffer. The cell extract and the proteins from the medium (both equivalent to 5 OD600 units) were loaded on a 12% SDS-PAGE gel. Immunoblots were prepared as described previously (Towbin et al., 1979) using anti-GFP antibodies (diluted 1:5000; Molecular Probes) and secondary goat anti-rabbit antibodies (diluted 1:30,000) coupled to alkaline phosphatase (Sigma). The bands then were visualized using the IMMUN-STAR chemiluminescent protein detection system (Bio-Rad).
Acknowledgments
We thank Prof. S.D. Emr for advice and for the gift of the yeast strains and Antje Heeze-Peck for advice on the visualization of vacuoles in yeast. We thank S. Marc-Martin and Dr. G.-P. Di Sansebastiano for their valuable help. This work was supported by a European Molecular Biology Organization long-term fellowship to N.P. (57-1996) and by a Swiss National Research Foundation grant (3100-46926.96).
References
- Abedi, M.R., Caponigro, G., and Kamb, A. (1998). Green fluorescent protein as a scaffold for intracellular presentation of peptides. Nucleic Acids Res. 26, 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, S.U., Bar-Peled, M., and Raikhel, N.V. (1997). Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein-sorting receptors of eukaryotic cells. Plant Physiol. 114, 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek, S., and Raikhel, N.V. (1991). The barley lectin carboxyl-terminal propeptide is a vacuolar protein sorting determinant in plants. Plant Cell 3, 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum, S., Mueller, M., Schmid, S.R., Linder, P., and Trachsel, H. (1989). Translation in Saccharomyces cerevisiae: Initiation factor 4A-dependent cell-free system. Proc. Natl. Acad. Sci. USA 86, 6043–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X., Rogers, S.W., Butler, J., Beevers, L., and Rogers, J.C. (2000). Structural requirements for ligand binding by a probable plant vacuolar sorting receptor. Plant Cell 12, 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, A.A., and Stevens, T.H. (1996). Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 133, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack, B.P., Valdivia, R.H., and Falkow, S. (1996). FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173, 33–38. [DOI] [PubMed] [Google Scholar]
- Di Sansebastiano, G.-P., Paris, N., Marc-Martin, S., and Neuhaus, J.-M. (1998). Specific accumulation of GFP in a non-acidic vacuolar compartment via a C-terminal propeptide-mediated sorting pathway. Plant J. 15, 449–457. [DOI] [PubMed] [Google Scholar]
- Di Sansebastiano, G.-P., Paris, N., Marc-Martin, S., and Neuhaus, J.-M. (2001). Regeneration of a lytic central vacuole and of neutral peripheral vacuoles can be visualised by GFP targeted to either type of vacuoles. Plant Physiol., in press. [DOI] [PMC free article] [PubMed]
- Freydl, E., Meins, F.J., Boller, T., and Neuhaus, J.-M. (1995). Kinetics of prolyl hydroxylation, intracellular transport and C-terminal processing of the tobacco vacuolar chitinase. Planta 147, 250–256. [Google Scholar]
- Graham, T.R., and Emr, S.D. (1991). Compartmental organisation of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J. Cell Biol. 114, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwerda, B.C., Padgett, H.S., and Rogers, J.C. (1992). Proaleurain vacuolar targeting is mediated by short contiguous peptide interactions. Plant Cell 4, 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, E., Davidson, A.R., and Kaiser, C.A. (1996). A pathway for targeting soluble misfolded proteins to the yeast vacuole. J. Cell Biol. 135, 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauh, G.-Y., Phillips, T.E., and Rogers, J.C. (1999). Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 11, 1867–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L.W., and Rogers, J.C. (1998). Integral membrane protein sorting to vacuoles in plant cells: Evidence for two pathways. J. Cell Biol. 143, 1183–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, L.M., Bankaitis, V.A., and Emr, S.D. (1987). Distinct sequence determinants direct intracellular sorting and modification of a yeast vacuolar protease. Cell 48, 875–885. [DOI] [PubMed] [Google Scholar]
- Jørgensen, M.U., Emr, S.D., and Winther, J.R. (1999). Ligand recognition and domain structure of Vps10p, a vacuolar protein sorting receptor in Saccharomyces cerevisiae. Eur. J. Biochem. 260, 461–469. [DOI] [PubMed] [Google Scholar]
- Kirsch, T., Paris, N., Butler, J.M., Beevers, L., and Rogers, J.C. (1994). Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc. Natl. Acad. Sci. USA 91, 3403–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch, T., Saalbach, G., Raikhel, N.V., and Beevers, L. (1996). Interaction of a potential vacuolar targeting receptor with amino- and carboxyl-terminal targeting determinants. Plant Physiol. 111, 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky, D.J., and Emr, S.D. (1989). Membrane protein sorting: Biosynthesis, transport and processing of yeast vacuolar alkaline phosphatase. EMBO J. 8, 2241–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide, Y., Hirano, H., Matsuoka, K., and Nakamura, K. (1997). The N-terminal propeptide of the precursor to sporamin acts as a vacuole-targeting signal even at the C-terminus of the mature part in tobacco cells. Plant Physiol. 114, 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld, S. (1992). Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 61, 307–330. [DOI] [PubMed] [Google Scholar]
- Kunze, I., Hensel, G., Adler, K., Bernard, J., Neubohn, B., Nilsson, C., Stoltenburg, R., Kohlwein, S.D., and Kunze, G. (1999). The green fluorescent protein targets secretory proteins to the yeast vacuole. Biochim. Biophys. Acta 1410, 287–298. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Laval, V., Chabannes, M., Carrière, M., Canut, H., Barre, A., Rougé, P., Pont-Lezica, R., and Galaud, J.-P. (1999). A family of Arabidopsis plasma membrane receptors presenting animal β-inte-grin domains. Biochim. Biophys. Acta 1435, 61–70. [DOI] [PubMed] [Google Scholar]
- Marcusson, E.G., Horazdovsky, B.F., Cereghino, J.L., Gharakhanian, E., and Emr, S.D. (1994). The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by VPS10 gene. Cell 77, 579–586. [DOI] [PubMed] [Google Scholar]
- Matsuoka, K., and Nakamura, K. (1991). Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc. Natl. Acad. Sci. USA 88, 834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka, K., and Nakamura, K. (1999). Large alkyl side-chains of isoleucine and leucine in the NPIRL region constitute the core of the vacuolar sorting determinant of sporamin precursor. Plant Mol. Biol. 41, 825–835. [DOI] [PubMed] [Google Scholar]
- Matsuoka, K., Bassham, D.C., Raikhel, N., and Nakamura, K. (1995). Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 130, 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E.A., Lee, M.C., and Anderson, M.A. (1999). Identification and characterization of a prevacuolar compartment in stigmas of Nicotiana alata. Plant Cell 11, 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J. (1972). Experiments in Molecular Genetics. (New York: Cold Spring Harbor Laboratory Press).
- Nagy, J.I., and Maliga, P. (1976). Callus induction and plant regeneration from mesophyll protoplasts of Nicotiana sylvestris. Z. Pflanzenphysiol. 78, 453–455. [Google Scholar]
- Negrutiu, I., Shillito, R.D., Potrikus, I., Biasini, G., and Sala, F. (1987). Hybrid genes in the analysis of transformation conditions. I. Setting up a simple method for direct gene transfer in plant protoplasts. Plant Mol. Biol. 8, 363–373. [DOI] [PubMed] [Google Scholar]
- Neuhaus, J.-M., and Rogers, J. (1998). Sorting of proteins to vacuoles in plant cells. Plant Mol. Biol. 38, 127–144. [PubMed] [Google Scholar]
- Neuhaus, J.-M., Sticher, L., Meins, F., and Boller, T. (1991). A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc. Natl. Acad. Sci. USA 88, 10362–10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi, G., Cowles, C.R., and Emr, S.D. (1998). The AP-3 complex: A coat of many colours. Trends Cell Biol. 8, 282–288. [DOI] [PubMed] [Google Scholar]
- Paris, N., and Rogers, J.C. (1996). The role of receptors in targeting soluble proteins from the secretory pathway to the vacuole. Plant Physiol. Biochem. 34, 223–227. [Google Scholar]
- Paris, N., Stanley, C.M., Jones, R.L., and Rogers, J.C. (1996). Plant cells contain two functionally distinct vacuolar compartments. Cell 85, 563–572. [DOI] [PubMed] [Google Scholar]
- Paris, N., Rogers, S.W., Jiang, L., Kirsch, T., Beevers, L., Phillips, T.E., and Rogers, J.C. (1997). Molecular cloning and further characterization of a probable plant vacuolar sorting receptor. Plant Physiol. 115, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle, J.R., Adams, A.E.M., Drubin, D.G., and Haarer, B.K. (1991). Immunofluorescence methods for yeast. Methods Enzymol. 194, 565–601. [DOI] [PubMed] [Google Scholar]
- Robinson, J.S., Klionsky, D.J., Banta, L.M., and Emr, S.D. (1988). Protein sorting in Saccharomyces cerevisiae: Isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 8, 4936–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Sanderfoot, A.A., Ahmed, S.U., Marty-Mazars, D., Rapoport, I., Kirchhausen, T., Marty, F., and Raikhel, N.V. (1998). A putative vacuolar cargo receptor partially colocalizes with atPEP12p on a prevacuolar compartment in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 95, 9920–9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, F., Fink, G.R., and Lawrence, L.W. (1979). Methods in Yeast Genetics: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Shimada, T., Kuroyanagi, M., Nishimura, M., and Hara-Nishimura, I. (1997). A pumpkin 72-kDa membrane protein of precursor-accumulating vesicles has characteristics of a vacuolar sorting receptor. Plant Cell Physiol. 38, 1414–1420. [DOI] [PubMed] [Google Scholar]
- Stack, J.H., Horazdovsky, B., and Emr, S.D. (1995). Receptor-mediated protein sorting to the vacuole in yeast: Roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu. Rev. Cell Dev. Biol. 11, 1–33. [DOI] [PubMed] [Google Scholar]
- Tournaire, C., Kushnir, S., Bauw, G., Inze, D., Serve, B., and Renaudin, J.P. (1996). A thiol protease and an anionic peroxidase are induced by lowering cytokinins during callus growth in petunia. Plant Physiol. 111, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin, H., Staehelin, T., and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida, T.A., and Emr, S.D. (1995). A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]









