Abstract
Lateral root development in Arabidopsis provides a model for the study of hormonal signals that regulate postembryonic organogenesis in higher plants. Lateral roots originate from pairs of pericycle cells, in several cell files positioned opposite the xylem pole, that initiate a series of asymmetric, transverse divisions. The auxin transport inhibitor N-1-naphthylphthalamic acid (NPA) arrests lateral root development by blocking the first transverse division(s). We investigated the basis of NPA action by using a cell-specific reporter to demonstrate that xylem pole pericycle cells retain their identity in the presence of the auxin transport inhibitor. However, NPA causes indoleacetic acid (IAA) to accumulate in the root apex while reducing levels in basal tissues critical for lateral root initiation. This pattern of IAA redistribution is consistent with NPA blocking basipetal IAA movement from the root tip. Characterization of lateral root development in the shoot meristemless1 mutant demonstrates that root basipetal and leaf acropetal auxin transport activities are required during the initiation and emergence phases, respectively, of lateral root development.
INTRODUCTION
Plants, unlike animals, use postembryonic organogenesis to elaborate their architecture. Lateral branching in root and shoot systems represents a major determinant of plant architecture. Lateral root development in Arabidopsis provides a model for the study of factors that regulate postembryonic organogenesis in higher plants. The Arabidopsis root has a relatively simple anatomy composed of single layers of epidermal, cortical, and endodermal cells surrounding the vascular tissues (Figure 1A; Dolan et al., 1993). Lateral roots in Arabidopsis are derived from pericycle founder cells positioned adjacent to the two protoxylem poles (Figure 1B; Blakely et al., 1982). Malamy and Benfey (1997) have defined seven developmental stages that precede lateral root emergence. The first periclinal division represents the most common criterion used to define the onset of lateral root formation (Esau, 1977; Lloret et al., 1989). However, in Arabidopsis, the first periclinal divisions occur within groups of eight to 10 short initial cells, indicating that founder cells first must undergo a series of transverse divisions (Malamy and Benfey, 1997). The exact nature and order of the initial transverse divisions have not been characterized in detail in Arabidopsis.
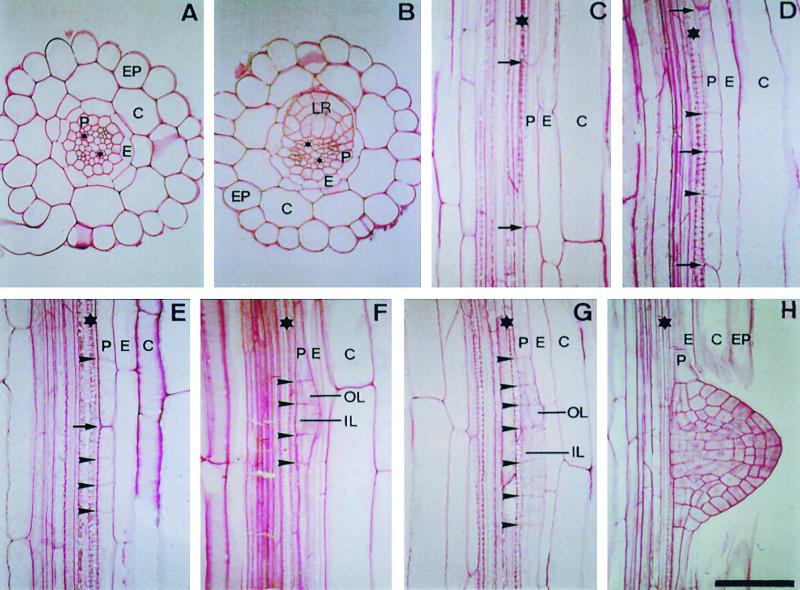
Figure 1.
Developmental Stages during Lateral Root Formation in Arabidopsis.
(A) Radial organization of the Arabidopsis primary root showing the cortical (C), endodermal (E), epidermal (EP), and pericycle (P) cell layers.
(B) Radial section of the primary root showing a lateral root primordium (LR) developing opposite the xylem pole.
(C) Longitudinal section of the primary root, with arrows indicating the cell walls of a founder pericycle cell before the first anticlinal division, initiating the formation of a lateral root primordium (stage 0).
(D) Two founder pericycle cells, each having undergone a single asymmetric anticlinal division (stage Ib primordium). Arrowheads indicate the positions of the newly formed cell walls.
(E) One founder cell has undergone three asymmetric anticlinal divisions, whereas the other has formed a single anticlinal division (stage Id).
(F) A stage II primordium that had previously undergone three asymmetric anticlinal divisions, with the newly formed inner layer (IL) and outer layer (OL) indicated.
(G) A stage II primordium that underwent six asymmetric anticlinal divisions before the first periclinal division.
(H) A newly emerged lateral root primordium.
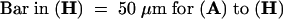 . Asterisks indicate xylem poles.
. Asterisks indicate xylem poles.
Auxin represents a key regulator of lateral root development (Blakely et al., 1982; Laskowski et al., 1995). Several auxin-related mutants have been described in Arabidopsis that arrest lateral root formation at various stages of development (Celenza et al., 1995). The alf4 mutation blocks lateral root initiation, whereas the alf3 mutation arrests organ development soon after emergence (Celenza et al., 1995). The contrasting phenotypes of the alf3 and alf4 mutants suggest that indoleacetic acid (IAA) is required at several stages of lateral root development. Laskowski et al. (1995) have proposed that IAA is initially required to establish a population of rapidly dividing pericycle cells but that their derivatives subsequently form hormone-autonomous meristems.
Before becoming hormone autonomous, developing lateral root primordia are proposed to obtain IAA via polar auxin transport (Reed et al., 1998). Polar auxin transport represents a specialized delivery system used by the plant to mobilize IAA from an auxin source in the shoot to basal sink tissues such as the root (Bennett et al., 1998). In roots, polar auxin transport has been described to move IAA to the root apex (acropetal) and toward the root–shoot junction (basipetal; Rashotte et al., 2000). To date, the functional importance of basipetal auxin transport within root apical tissues has been considered to be limited to mediating growth responses such as gravitropism (Müller et al., 1998; Marchant et al., 1999; Rashotte et al., 2000). In contrast, acropetal polar transport of shoot-derived IAA appears to be required for lateral root development (Reed et al., 1998). The authors demonstrated that localized application of the polar auxin transport inhibitor N-1-naphthylphthalamic acid (NPA) at the root–shoot junction of Arabidopsis seedlings resulted in a decrease of free IAA in roots with a corresponding reduction in the number of emerging lateral roots. However, in the absence of morphological evidence, it is difficult to assess the developmental stage at which NPA arrested lateral root formation before emergence. A comprehensive understanding of the developmental effects of NPA on lateral root formation would provide greater insight into the mode of action and transport of IAA.
Our detailed characterization of the initial stages of lateral root development has allowed us to make the novel observation that treating root tissues directly with NPA arrests lateral root development by blocking the first transverse division(s) of xylem pole pericycle cells. NPA appears to exert its developmental effects by causing IAA to accumulate in the root apex while reducing levels in basal tissues critical for lateral root initiation. This pattern of IAA redistribution is most consistent with NPA blocking basipetal IAA movement from the root tip. The conclusion that basipetal auxin transport plays an important role during lateral root development is in contrast to the conclusions of Reed et al. (1998). However, we are able to reconcile the results of both studies through our characterization of the lateral root development of the shoot meristemless1 mutant, which allows us to conclude that basipetal and acropetal polar auxin transport activities are required during the initiation and emergence phases, respectively.
RESULTS
Lateral Root Primordia Originate from Pairs of Xylem Pole Pericycle Founder Cells
The morphological events associated with the earliest stages of lateral root development were initially investigated as a basis to understand the hormonal control of lateral root initiation. Arabidopsis lateral roots were observed to emerge ∼20 mm basal to the primary root apex. In sections of the terminal 20 mm of primary roots, all stages of pericycle cell division could be visualized.
This study has concentrated on division events within a single file of xylem pole pericycle cells. However, divisions within adjacent files also contribute to the formation of the lateral root primordium (Figure 1B). The first detectable division to occur within a single file of xylem pole pericycle cells was transverse and asymmetric. Almost simultaneously, another asymmetric transverse division occurred in an adjacent xylem pole pericycle cell, creating two short cells flanked by two longer cells (Figure 1D). The consistency in pattern of the initial round of division within adjacent cells suggests that this is a highly coordinated developmental process. Daughter cells continued to divide transversely to create groups of mitotically active initial cells. Considerable plasticity in the precise order of these divisions was observed. For example, transverse divisions often occurred within derivatives of only one of the original pair of cells (Figure 1E), whereas in other cases, further divisions of both founder cells occurred. After several transverse divisions, the central short daughter cells expanded radially, then divided periclinally, giving rise to a primordium composed of inner and outer cell layers. Two-cell-layer primordia (termed stage II; Malamy and Benfey, 1997) were observed that had completed between three and 10 transverse divisions (Figures 1F and 1G), suggesting that a rigidly defined number of transverse divisions need not occur before the first periclinal division. Subsequent periclinal divisions that add additional layers within the primordia (termed stages III to VII), ultimately leading to lateral root emergence (Figure 1H), have been described in detail by Malamy and Benfey (1997).
NPA Arrests Lateral Root Development by Blocking Primordium Initiation
IAA is required to establish a population of rapidly dividing initial cells (Laskowski et al., 1995). Developing lateral root primordia are proposed to obtain IAA via polar auxin transport (Reed et al., 1998). The polar auxin transport inhibitor NPA is able to block lateral root emergence (Muday and Haworth, 1994; Reed et al., 1998), but the stage(s) at which the arrest occurs has not been described. This has prompted us to investigate the morphological effects of NPA on lateral root development as a basis for understanding the hormonal control of lateral root initiation.
Wild-type Arabidopsis seedlings were germinated on medium supplemented with NPA (0, 1, 5, or 10 μM). The numbers of lateral roots emerging from 10-day-old primary roots were scored using a dissecting microscope (Table 1). Compared with the untreated control, 1 μM NPA significantly reduced numbers of emerging lateral roots, and their development was abolished completely at concentrations of 5 μM NPA and above.
Table 1.
NPA Blocks Lateral Root Formation in Arabidopsisa
| NPA (μM) | Laterals/mm | Primary Root Length (mm) |
|---|---|---|
| 0 | 0.43 ± 0.08 | 41.8 ± 8.2 |
| 1 | 0.09 ± 0.06 | 43.5 ± 8.2 |
| 5 | 0 | 30.8 ± 5.1 |
| 10 | 0 | 27.9 ± 3.9 |
Seedlings were grown for 11 days on Murashige and Skoog agar containing 0, 1, 5, or 10 μM NPA, after which time the number of lateral roots and the primary root lengths were measured. Results shown are the averages of 20 seedlings ±sd.
To determine the precise morphological basis of our observations, we prepared sections from primary roots that had been grown in the presence of 0, 1, 5, or 10 μM NPA. The terminal 2.5- to 20-mm tissues of five primary root apices were sectioned to calculate the total number of lateral root primordia at developmental stages I to VII after these various treatments (Table 2). Despite forming a significantly reduced number of fully emerged lateral roots compared with the control (Table 1), roots grown in the presence of 1 μM NPA produced a similar number of primordia (Table 2). This disparity suggests that 1 μM NPA acts to block the developmental progression of lateral primordia from stage I onward. In contrast, roots treated with 5 μM NPA formed greatly reduced numbers of primordia that failed to develop beyond stage II. At the highest concentrations of NPA tested (10 μM), the first pericycle transverse division was blocked. These novel observations demonstrate that auxin transport activity is required during lateral root development and illustrate the fact that at the highest concentrations tested, NPA was capable of blocking the earliest stage of lateral root initiation (Tables 1 and 2).
Table 2.
NPA Causes a Block Lateral at an Early Stage of Lateral Root Developmenta
| μM NPA
|
||||
|---|---|---|---|---|
| Stage | 0 | 1 | 5 | 10 |
| I | 12 | 22 | 6 | 0 |
| II | 11 | 7 | 1 | 0 |
| III | 6 | 7 | 0 | 0 |
| IV | 6 | 2 | 0 | 0 |
| V | 9 | 3 | 0 | 0 |
| VI | 2 | 4 | 0 | 0 |
| VII | 7 | 0 | 0 | 0 |
| Total | 53 | 45 | 7 | 0 |
Seedlings were grown on Murashige and Skoog agar containing 0, 1, 5, or 10 μM NPA for 10 days, after which time the roots were collected. Roots were sectioned, and the number and stage distribution of lateral root primordia within 2.5 to 20 mm from the primary root tip were recorded. Results presented here are the total number of detected primordia at each developmental stage from five individual roots.
NPA Does Not Block Lateral Root Initiation by Respecifying Founder Cell Identity
Increased NPA levels may block lateral root initiation by respecifying the identity of its founder cells in xylem pole pericycle tissues, as has been described for non-stele tissues in the Arabidopsis primary root apex (Sabatini et al., 1999). This possibility was investigated by monitoring the expression of selected Arabidopsis GAL4 enhancer trap transactivation lines, including line J0121, which is reported to express green fluorescent protein (GFP) within files of pericycle cells (J. Haseloff, http://www.plantsci.cam ac.uk/Haseloff/DOCS/GALGFPdb.pdf). Roots from line J0121 were examined using multiphoton microscopy (Figure 2). GFP was not observed within pericycle initial cells but was first detected within xylem pole pericycle cells ∼200 μm from the root apex (data not shown). GFP was expressed within three files of pericycle cells (Figure 2A) opposite each xylem pole (Figure 2B) and was absent from all other pericycle cell files (data not shown). Our morphological studies revealed that lateral root primordia originate from pairs of founder cells (Figure 1) derived from three adjacent pericycle cell files opposite each xylem pole (I. Casimiro and P.J. Casero, unpublished data). Line J0121 therefore provides an excellent marker for the cell files from which lateral root founder cells originate.
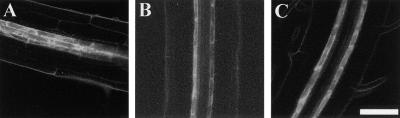
Figure 2.
Xylem Pole Pericycle Cell Identity Is Not Altered by NPA.
The GAL4-GFP enhancer trap line J0121 was grown on Murashige and Skoog agar for 7 days, and optical sections of GFP expression were imaged using multiphoton microscopy.
(A) GFP was expressed within three adjacent pericycle cell files in J0121 roots.
(B) A median longitudinal optical section showing that GFP expression was detected adjacent to both xylem poles.
(C) A median longitudinal optical section showing that seedlings grown in the presence of 10 μM NPA also show GFP expression within files of pericycle cells adjacent to the xylem pole.
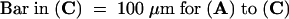 .
.
To determine whether NPA blocked lateral root development by respecifying the identity of the xylem pole pericycle founder cells, we grew J0121 seedlings in the presence or absence of 10 μM NPA on Murashige and Skoog (1962) medium for 7 days. Roots treated with the auxin transport inhibitor exhibited a GFP expression pattern (Figure 2C) identical to that of control roots (Figure 2B). Similarly, transferring J0121 roots from 0 to 10 μM NPA and vice versa failed to modify the spatial expression pattern of GFP (data not shown). The unmodified spatial expression pattern of GFP in NPA-treated roots suggests that xylem pole pericycle cells are able to maintain a developmental fate separate from that of other pericycle cell files in the presence of the auxin transport inhibitor. In contrast, parallel experiments using the root apical endodermal marker line J0571 found dramatic changes in GFP expression in the presence of NPA (data not shown), consistent with the results of Sabatini et al. (1999). Based on the J0121 expression pattern (Figure 2), we conclude that xylem pole pericycle cells retain their identity but are not able to undergo division in NPA-treated roots (Table 2).
NPA Causes Root IAA to Be Suboptimal for Lateral Root Initiation
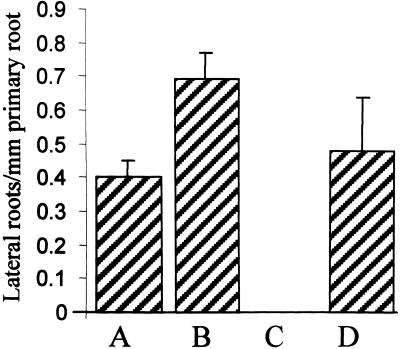
Reed et al. (1998) reported that NPA treatment resulted in reduced levels of root IAA. If NPA causes root IAA to be suboptimal for lateral root initiation, cocultivation of NPA-treated roots with auxin would be predicted to restore lateral root development. To test this model, we grew seedlings in the presence of 5 μM NPA for 9 days and then transferred them to new 5 μM NPA medium containing 0 or 10−7 M 1-naphthylacetic acid (NAA). In parallel, control seedlings were grown in the absence of NPA for 9 days and then transferred to new medium containing 0 or 10−7 M NAA. After another 3 days, the total number of emergent lateral roots was recorded (Figure 3). As expected, roots grown in the absence of NPA formed lateral roots (column A). Similarly, roots grown in the presence of exogenous auxin formed significantly more roots than did untreated control roots (column B). In contrast, roots grown continuously in the presence of NPA failed to form lateral roots (column C). However, transfer of NPA-treated roots to medium supplemented with NPA plus NAA restored lateral root formation (column D). Hence, auxin transport inhibitor–treated roots remain competent to respond to auxin and initiate lateral root development, in agreement with our J0121-based marker studies, which indicated that xylem pole pericycle cells are present but not activated (Figure 2). Moreover, our observations suggest that NPA causes endogenous root IAA to be at a level suboptimal for lateral root initiation.
Figure 3.
NAA Can Rescue the Block in Lateral Root Formation Caused by NPA.
Seedlings were grown on Murashige and Skoog agar containing either 0 μM NPA (columns A and B) or 5 μM NPA (columns C and D) for 9 days before being transferred to fresh Murashige and Skoog agar with no additions (column A) or containing 0.1 μM NAA (column B), 5 μM NPA (column C), or 5 μM NPA and 0.1 μM NAA (column D). The seedlings were allowed to grow for an additional 3 days, and the total number of roots was counted. Error bars represent the sd ( ).
).
Auxin Promotes Lateral Root Initiation in a Zone Basal to the Root Apical Meristem
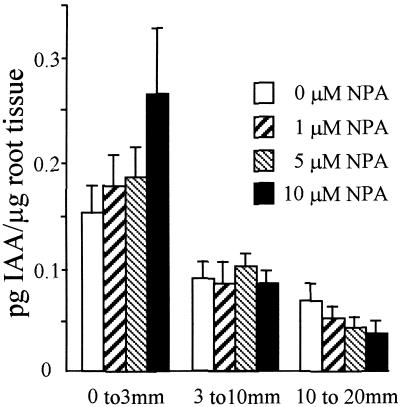
To address the effect of NPA on IAA distribution within root tissues, we used mass spectroscopy (MS) to measure IAA abundance within root segments close to the root apex. Wild-type Arabidopsis seedlings were grown in the presence of 0, 1, 5, or 10 μM NPA for 10 days. IAA abundance was determined for three regions of the primary root (0 to 3 mm, 3 to 10 mm, and 10 to 20 mm from the root tip). In control tissues, IAA was distributed asymmetrically along the apical–basal axis, with the highest levels encompassing the root tip/meristem/elongation zones followed by a significant decrease toward the next analyzed section (Figure 4; P = 0.01 [Student's t test]). Experiments using the auxin transport inhibitor demonstrate a correlation between the concentration of NPA applied and the alteration of IAA levels within the most apical region of the root (Figure 4). Significantly greater IAA levels were detected in the root tip after application of 10 μM NPA compared with levels in roots grown in the absence of NPA (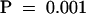 [Student's t test]), whereas the 3- to 10-mm and 10- to 20-mm segments exhibited no statistical difference between treatments.
[Student's t test]), whereas the 3- to 10-mm and 10- to 20-mm segments exhibited no statistical difference between treatments.
Figure 4.
NPA Causes a Redistribution of Root Tip IAA.
The amount of IAA was measured for three segments of the primary root between 0 and 3 mm, 3 and 10 mm, and 10 and 20 mm for seedlings grown in the presence of 0, 1, 5, or 10 μM NPA. Results are shown as pg IAA/μg root tissue. Error bars represent the sd (n = 3 to 4 pooled samples of 50 to 100 root segments). Student's t test was performed on the data to demonstrate the significance of the differences observed between IAA levels in the root tip/meristem/elongation zones and the next analyzed section (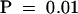 ) and after application of 10 μM NPA (
) and after application of 10 μM NPA (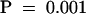 ).
).
MS measurements detected a significant increase in NPA-treated roots (Figure 4), yet our earlier observations (Figure 3) suggested that NPA causes root IAA to be suboptimal for lateral root initiation. Intuitively, auxin accumulation within root tissues would be expected to stimulate lateral root development (Laskowski et al., 1995), yet NPA caused primordia initiation to arrest (Table 2). These apparently contradictory observations could be reconciled if the site of lateral root initiation was spatially distinct from the root apical tissues that accumulate IAA in response to NPA. A greater level of spatial resolution than MS can provide was required to address this possibility, necessitating the use of markers that are expressed in response to increased auxin at the root apex or during lateral root initiation.
Transgenic Arabidopsis seedlings expressing the auxin-responsive reporter DR5::uidA (Ulmasov et al., 1997) were grown in the presence of 0, 1, 5, or 10 μM NPA for up to 10 days after germination and then histochemically stained for β-glucuronidase (GUS) activity (Figure 5). In the absence of NPA, the expression of the DR5::uidA reporter was localized to the primary root apex of 2-day-old seedlings (Figure 5A). Differential interference contrast optics showed that untreated control tissues expressed GUS within columella and lateral root cap cells (Figure 5B). Increasing concentrations of NPA enhanced histochemical staining within root apical meristem cells (Figures 5C to 5E). Our results indicate that NPA causes increased levels of endogenous IAA within root apical meristem tissues, in agreement with our MS measurements (Figure 4). Nevertheless, GUS staining remained localized to within 0.1 mm of the root tip at all NPA concentrations tested (Figures 5C to 5E), in agreement with the results of Sabatini et al. (1999).
Figure 5.
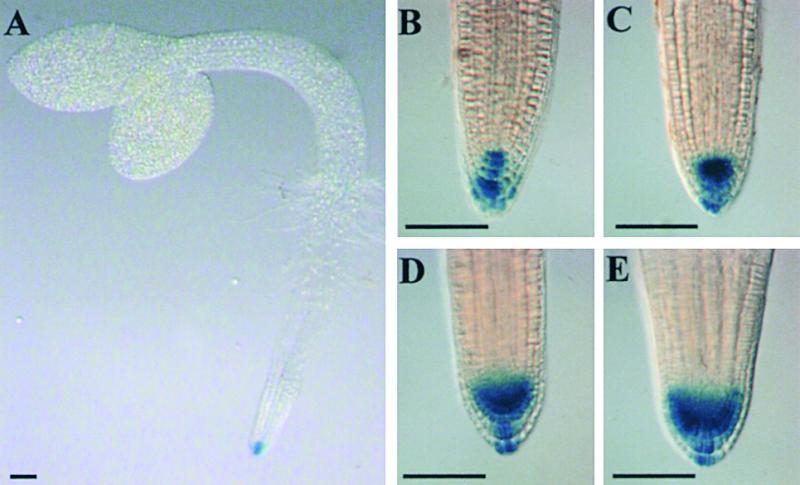
The DR5::uidA Marker Detects IAA Accumulation Close to the Tip of NPA-Treated Arabidopsis Roots.
(A) Expression of the synthetic auxin-responsive reporter DR5::uidA is localized to the primary root apex of 2-day-old seedlings grown in the absence of NPA.
(B) to (E) Differential interference contrast images of GUS-stained DR5::uidA root apical tissues grown in the absence of NPA (B) or in the presence of 1 μM NPA (C), 5 μM NPA (D), or 10 μM NPA (E).
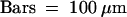 .
.
The cycB1:1::uidA reporter is a marker for early mitotic events associated with lateral root initiation (Figure 6A; Ferreira et al., 1994). Sections of GUS-stained cycB1:1::GUS seedlings demonstrated that the mitotic marker was expressed within pairs of xylem pole pericycle cells soon after the first transverse division (Figure 6B). One- to 2-day-old transgenic cycB1:1::uidA seedlings were histochemically stained (Beeckman and Engler, 1994), and the roots were inspected for lateral root primordia. Measurements were obtained for the distance from the first primordium to the root tip by using roots containing a single stained lateral root primordium, giving a mean value of 1.4 ± 0.28 mm (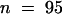 ). The nonoverlapping patterns of cycB1:1- and DR5-driven GUS expression therefore demonstrate that the site of lateral root initiation is spatially distinct from the root apical tissues that accumulate IAA in response to NPA.
). The nonoverlapping patterns of cycB1:1- and DR5-driven GUS expression therefore demonstrate that the site of lateral root initiation is spatially distinct from the root apical tissues that accumulate IAA in response to NPA.
Figure 6.
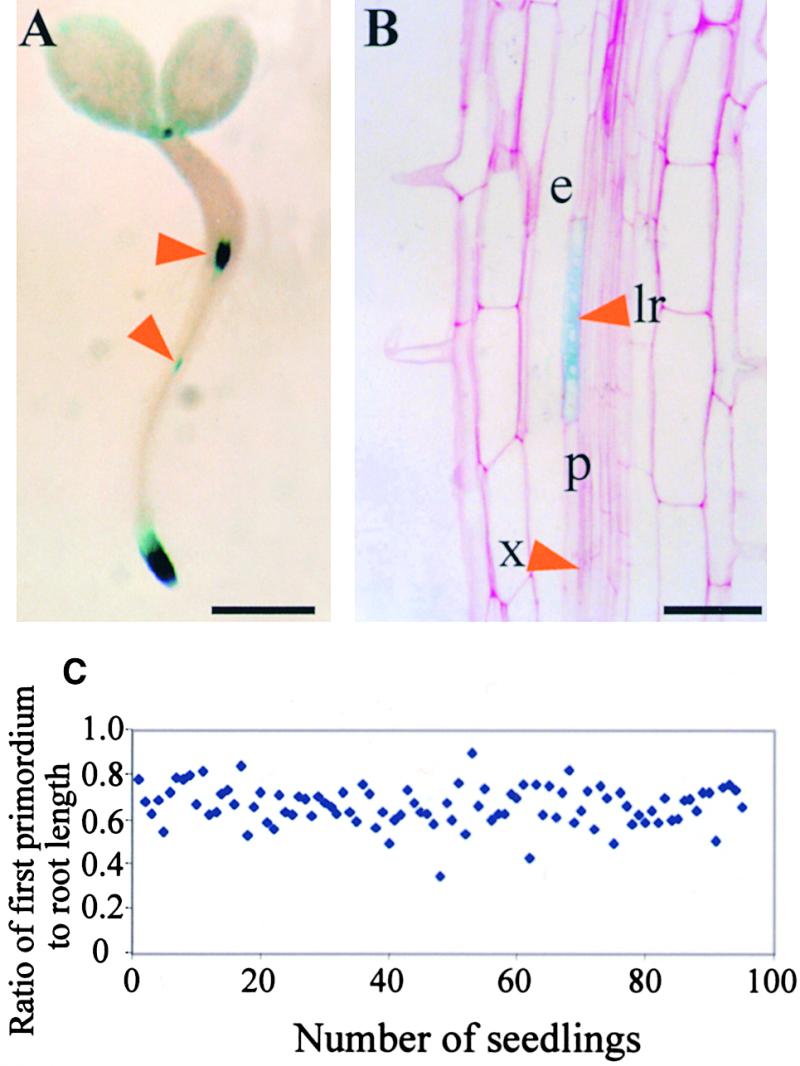
The cycB1:1::uidA Marker Reveals a Close Relationship between the Position of the First Division of Lateral Root Formation and the Root Tip.
(A) Forty-hour-old seedlings containing the cycB1:1::uidA transgene were histochemically stained for GUS activity. The top arrowhead indicates staining at the transition zone between the hypocotyl and the root, and the bottom arrowhead indicates a stained lateral root primordium. 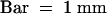 .
.
(B) A 5-μm section through a stage II primordium of a 40-hour-old seedling carrying the cycB1:1::uidA transgene that was histochemically stained for GUS activity. Arrowheads indicate the lateral root primordium (lr) and the xylem vessel (x); also indicated are the pericycle cell layer (p) and the endodermis (e). 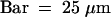 .
.
(C) Seedlings (cycB1:1::uidA) were harvested every day for 7 days and histochemically stained for GUS activity. Primary root length and the distance to the root tip from the first lateral root primordium were measured in roots containing a single primordium. The ratio of the distance between the root tip and the first primordium to the length of the root is plotted (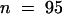 ).
).
Calculations of the ratio between the total primary root length (mean of 2.1 ± 0.36 mm; 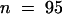 ) and the distance between the first primordium and the root tip revealed that lateral roots initiate at a predictable distance basal to the root apical meristem (Figure 6C). Previous studies have contained similar observations from other plant species (Casero et al., 1995), implying that there is a close relationship between the position of the first founder cell division and the root apex. This relationship also has been observed for subsequent lateral roots (T. Beeckman and D. Inzé, unpublished data), implying that a specialized zone of lateral root initiation is maintained during later stages of root development.
) and the distance between the first primordium and the root tip revealed that lateral roots initiate at a predictable distance basal to the root apical meristem (Figure 6C). Previous studies have contained similar observations from other plant species (Casero et al., 1995), implying that there is a close relationship between the position of the first founder cell division and the root apex. This relationship also has been observed for subsequent lateral roots (T. Beeckman and D. Inzé, unpublished data), implying that a specialized zone of lateral root initiation is maintained during later stages of root development.
Acropetal Polar Auxin Transport in the Root Is Not Required for Lateral Root Initiation
Our experimental observations suggest that NPA blocks pericycle cell division by reducing the transport of IAA to a specialized zone of lateral root initiation basal to the root apex. As an inhibitor of both acropetal and basipetal auxin transport in root tissues (Rashotte et al., 2000), NPA could disrupt the delivery of IAA to the zone of lateral root initiation by one of several possible mechanisms. First, NPA might inhibit acropetal polar auxin transport in the roots by blocking IAA movement from developing shoot apical tissues. Alternatively, NPA could block basipetal auxin transport from the primary root apex. To discriminate between these two possibilities, we examined the lateral root development of the shoot meristemless1 (stm1) mutant (Long et al., 1996).
In the absence of a shoot apical meristem, stm1 mutant seedlings fail to develop leaf primordia. Despite lacking this important IAA source, stm1 seedlings were able to initiate a wild-type number of lateral root primordia (Figure 7A). stm1 mutant seedlings are unlikely to compensate for the reduced shoot IAA levels by increasing root auxin sensitivity. Dose–response curves to assay auxin-regulated root development illustrate that stm1 roots retain a wild-type level of auxin-sensitive lateral root initiation (Figure 7A) and root elongation (data not shown). The formation of lateral root primordia by stm1 seedlings therefore implies that lateral roots are capable of initiating in the absence of IAA from developing shoot apical tissues. IAA levels appear normal in stm1 root apical tissues, based on the identical patterns of expression and staining intensity of the auxin-regulated IAA2::uidA marker compared with that of the wild type (R. Swarup and M. Bennett, unpublished results). Nevertheless, it remains possible that in the absence of leaf development, cotyledons are a source of IAA in stm1 mutant roots.
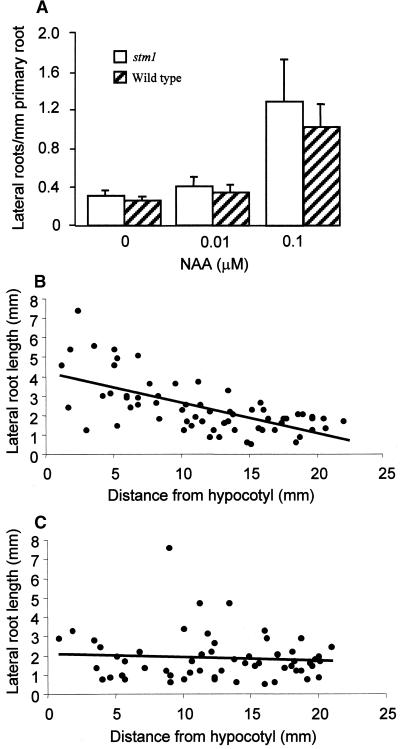
Figure 7.
stm1 Forms a Similar Number of Lateral Roots to Wild Type in Response to Auxin but Has an Altered Acropetal Development Profile.
(A) Wild-type (Landsberg erecta) and stm1 plants were grown for 10 days on Murashige and Skoog agar containing 0, 0.01, or 0.1 μM NAA. The number of lateral roots per millimeter of primary root was counted for 15 seedlings under each condition. Error bars represent the sd.
(B) and (C) Wild-type (B) and stm1 (C) seedlings were grown on Murashige and Skoog agar for 10 days, and the length of each lateral root and its distance from the hypocotyl–root junction were measured (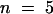 ). The values obtained were illustrated graphically using Excel software, and the trend lines (solid bars) were calculated, indicating the presence of an acropetal lateral root developmental gradient in the wild type (B) that is absent in stm1 (C).
). The values obtained were illustrated graphically using Excel software, and the trend lines (solid bars) were calculated, indicating the presence of an acropetal lateral root developmental gradient in the wild type (B) that is absent in stm1 (C).
A role for leaf-derived IAA is apparent on closer inspection of stm1 root development. The lateral root architecture of wild-type and stm1 seedlings was compared by plotting lateral root length versus position of origin on the primary root axis relative to the hypocotyl–root junction. Although wild-type lateral root growth exhibited an acropetal developmental gradient (Figure 7B), this was absent in stm1 (Figure 7C). stm1 lateral root elongation was reduced significantly and sometimes was erratic, irrespective of the position of emergence along the primary root (Figure 7C). Our observations suggest that shoot apically derived IAA may play a role in coordinating the emergence of lateral organs that create the acropetal root architecture typical of Arabidopsis.
DISCUSSION
Lateral Root Development Is Initiated by Asymmetric Divisions in Pairs of Founder Cells within Xylem Pole Pericycle Cell Files
Although periclinal division represents one of the most common criteria used to define the onset of lateral root formation (Esau, 1977; Lloret et al., 1989), periclinal division takes place within groups of initial cells that have already undergone several rounds of transverse division (Figures 1F and 1G; Malamy and Benfey, 1997). Our study has demonstrated that these early transverse divisions represent an integral part of the highly organized developmental program that ultimately gives rise to the emerging lateral root (Figure 1; Malamy and Benfey, 1997). The early patterns of cell division are unusual in several respects. First, asymmetric divisions in plants usually generate daughter cells that adopt distinct developmental fates (Scheres and Benfey, 1999). Despite the initial round of transverse division being physically asymmetric, the smaller and larger daughter cells invariably adopt the same developmental fate, acting as founder cell derivatives that give rise to initial cells within the lateral root primordium (Figure 1). Second, the initial round of mitosis involved the coordinated transverse division of pairs of founder cells (Figure 1D). The consistency of the pattern of initial transverse divisions indicates that signals are being exchanged between the pair of founder cells. The detection of rare asymmetric transverse divisions within individual xylem pole pericycle cells (data not shown) may indicate that one founder cell initiates cell division ahead of the other. In onion roots, the initial asymmetric division is preceded by nuclear migration toward the cell wall that separates the pair of founder cells (Casero et al., 1993, 1995). The direction of nuclear migration may determine whether the second member of the pair is recruited from above or below the initial founder cell.
Basipetal Auxin Transport Promotes Lateral Root Initiation in Arabidopsis
Although the nature of the patterning mechanism that determines the longitudinal spacing of root primordia in Arabidopsis is unclear at present, auxin obviously represents an important promotive factor. Our observation that NPA treatment causes IAA levels to become suboptimal for the induction of founder cell division (Figure 3) may imply that a threshold concentration of auxin is required before xylem pole pericycle cells are able to respond to a lateral root–patterning signal(s). Alternatively, auxin itself may represent the principal lateral root–patterning signal. Significantly, Reinhardt et al. (2000) recently demonstrated that auxin regulates the initiation and radial positioning of Arabidopsis leaf and floral organs, by applying microdroplets of IAA to exposed apical meristems. Similarities between the mechanisms that regulate organ initiation in roots and shoots become apparent on closer scrutiny of the results obtained in both studies. First, lateral root and leaf primordia initiate within a finite distance from their apical meristems (Figure 6C). Second, NPA is capable of arresting organ initiation in both systems (Table 2). Third, NPA reduces endogenous IAA levels, based on the ability of exogenously applied auxin to restore organ initiation (Figure 3).
Our study has demonstrated directly that IAA continues to accumulate in apical tissues of NPA-treated roots (Figure 4). This pattern of IAA redistribution is consistent with the inhibition of basipetal auxin movement from the root tip, whereas the inhibition of acropetal polar auxin transport would be expected to reduce root tip IAA levels. The accumulation of IAA in the primary root apex (Figures 3 and 5) could arise as a result of de novo synthesis or the continued delivery of IAA from the shoot via an NPA-insensitive transport route such as the phloem. Nevertheless, either hormone source still would require basipetal auxin transport to mobilize IAA to the zone of lateral root initiation. Importantly, Rashotte et al. (2000) have reported that basipetal IAA transport can be detected in root tissues up to 5 mm basal to the root apex. Hence, root tissues that mediate basipetal auxin transport overlap with the zone of lateral root initiation (1.4 ± 0.28 mm; Figure 6).
Our conclusion that basipetal auxin transport plays an important role during lateral root development is in contrast to the conclusion of Reed et al. (1998), who demonstrated that inhibition of acropetal auxin transport resulted in a reduction in the number of emerging lateral roots. However, both sets of results can be reconciled if basipetal and acropetal auxin transport regulate the initiation and emergence phases, respectively, of lateral root development. The modified root development of the stm1 mutant is consistent with this model (Figure 7). The continued formation of lateral roots by stm1 seedlings demonstrates that mutant roots remain capable of initiating primordia in the absence of IAA from developing leaves (Figure 7A). However, it remains possible that stm1 roots obtain IAA from a cotyledon source. Moreover, acropetal IAA appears capable of compensating for the loss of basipetal auxin after the genetic ablation of Arabidopsis root tip tissues (Tsugeki and Fedoroff, 1999). These observations highlight the redundancy within auxin transport–regulated root developmental processes under certain circumstances.
Basipetal Auxin Transport May Influence the Longitudinal Spacing of Primordia
Transient changes in auxin concentration within the zone of lateral root initiation could provide the basis for a patterning mechanism that influences the longitudinal spacing of lateral root primordia. Above a threshold level of auxin, xylem pole pericycle cells become founder cells committed to organogenesis, whereas below this threshold, cells lose their competence for organ initiation, defaulting to a pericycle tissue identity.
The basipetal redistribution of auxin during tropic curvature represents one mechanism that could cause periodic, asymmetric fluctuations in auxin levels close to the root apex. Interestingly, the agravitropic root mutants aux1 and axr4 form significantly reduced numbers of lateral roots (Hobbie and Estelle, 1995). The reduced number of lateral roots formed by aux1 is caused by the mutant initiating fewer lateral root primordia (A. Marchant, I. Casimiro, P.J. Casero, and M. Bennett, unpublished results), consistent with a defect in basipetal auxin transport (R. Swarup and M. Bennett, unpublished results). Although this is an attractive model, further refinements would be required to explain how basipetal auxin transport in epidermal/cortical tissues (Müller et al., 1998) could influence founder cells within xylem pole pericycle cell files (Figure 1). Moreover, the inductive effect of endogenous auxin as the patterning factor would have to be extremely focused to activate only pairs of founder cells (Figure 1). Nevertheless, the basipetal auxin transport model provides a useful basis for further studies, particularly when designing experiments to determine the signaling defect(s) that arrest lateral root initiation within Arabidopsis mutants such as aux1.
METHODS
Growth of Arabidopsis thaliana Seedlings
All seed were surface sterilized (Forsthoefel et al., 1992) and plated onto Murashige and Skoog (1962) agar (4.3 g/L Murashige and Skoog salts [Sigma, Poole, UK], 1% sucrose, and 1% bacto-agar, pH 6.0, with 1 M KOH), with the exception of transgenic CycB1:1:GUS seed, which were germinated on Hoagland medium with 0.1% sucrose (Beemster and Baskin, 1998). The plates were placed at 4°C for 48 hr and then in constant white light at 22°C.
Root Sections
Samples were fixed for 3 hr at 20°C (4% glutaraldehyde, 4% formaldehyde, and 50 mM sodium phosphate buffer, pH 7.2). Serial ethanol dehydration was then performed (30, 50, 70, 90, and 95% [twice]) at room temperature for 1 hr at each step. Samples were embedded in Technovit 7100 resin (Heraeus Kulzer, Wehrheim, Germany) according to the manufacturer's instructions. Sections were cut, dried onto glass slides, and stained for 8 min in an aqueous 0.05% ruthenium red solution. The samples were mounted in DePeX (Merck, Lutterworth, UK) before photography.
Indoleacetic Acid Quantification
Arabidopsis (ecotype Columbia) seed were surface sterilized and germinated on Murashige and Skoog agar medium containing 1% sucrose supplemented with 0, 1, 5, or 10 μM N-1-naphthylphthalamic acid (NPA) (Greyhound Chem Service, Birkenhead, UK). The plates were placed vertically, and the seedlings were grown at 22°C under 16-hr-light/8-hr-dark conditions. Root samples for indoleacetic acid (IAA) analysis were collected 12 days after germination. IAA analysis was performed on three types of sample (Figure 4): (1) 50 pooled root pieces 20 mm long including the root tip; (2) 100 pooled root pieces 10 mm long including the root tip; and (3) 100 pooled root pieces 7 mm long as in sample 2 but lacking the root tip and adjacent 3 mm. The root pieces were dissected in a humidified environment to prevent desiccation, weighed, and frozen in liquid nitrogen. Quantitative measurements of endogenous IAA were performed on 0.5 to 1 mg of pooled root segments by mass spectrometry (Edlund et al., 1995). Calculation of isotopic dilution was based on the addition of 13C6-IAA (50 pg/mg tissue). Sample preparation was performed in an ultraclean environment, and random blank samples were used to avoid the contamination problems associated with analysis of extremely low amounts of IAA. Measurements are presented as means of three or four individual samples, each consisting of material from 50 to 100 seedlings.
Expression Analysis of Arabidopsis Reporter Lines/Multiphoton Imaging of Arabidopsis Green Fluorescent Protein Lines
The GAL4 enhancer trap lines J0121 and J0571 were sown on Murashige and Skoog agar containing either 0 or 10 μM NPA and then grown vertically for 7 days under 16-hr-light/8-hr-dark cycles at 22°C. Seedlings were mounted in water on glass slides, and green fluorescent protein (GFP) expression in the root tissues was imaged using a Bio-Rad MRC1024ES multiphoton system at 810 nm.
β-Glucuronidase (GUS) expression was visualized using the protocol of Willemsen et al. (1998). After staining, seedlings were cleared and mounted according to the protocol of Malamy and Benfey (1997).
Acknowledgments
We thank Nick White from the Bio-Rad Biological Microscopy unit at the University of Oxford for help in performing the multiphoton analysis of the Arabidopsis GFP lines. We also acknowledge the Nottingham Arabidopsis Stock Centre for supplying seed for the J0121 GFP line, Tom Guilfoyle for the DR5::uidA transgenic line, and Ben Scheres for helpful discussions. We acknowledge funding from the Biotechnology and Biological Science Research Council (A.M.) and from European Community framework IV LATIN and FORMA network grants (No. PL96 0487) to I.C., P.J.C., R.P.B., G.S., and M.B. and (No. PL96 0217) T.B. and D.I.
References
- Beeckman, T., and Engler, G. (1994). An easy technique for the clearing of histochemically stained plant tissue. Plant Mol. Biol. Rep. 12, 37–42. [Google Scholar]
- Beemster, G.T.S., and Baskin, I.S. (1998). Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 116, 1515–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, M.J., Marchant, A., May, S.T., and Swarup, R. (1998). Going the distance with auxin: Unravelling the molecular basis of auxin transport. Philos. Trans. R. Soc. Lond. B 353, 1511–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely, L.M., Durham, M., Evans, T.A., and Blakely, R.M. (1982). Experimental studies on lateral root formation in radish seedling roots. I. General methods, developmental stages, and spontaneous formation of laterals. Bot. Gaz. 143, 341–352. [Google Scholar]
- Casero, P.J., Casimiro, I., Rodríguez-Gallardo, L., Martín-Partido, G., and Lloret, P.G. (1993). Lateral root initiation by means of asymmetrical transversal divisions of the pericycle cells in adventitious roots of Allium cepa. Protoplasma 176, 138–144. [Google Scholar]
- Casero, P.J., Casimiro, I., and Lloret, P.G. (1995). Lateral root initiation by means of asymmetrical transversal divisions of the pericycle cells in four different species of plants: Raphanus sativus, Helianthus annuus, Zea mays and Daucus carota. Protoplasma 188, 49–58. [Google Scholar]
- Celenza, J.L., Grisafi, P.L., and Fink, G.R. (1995). A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 9, 2131–2142. [DOI] [PubMed] [Google Scholar]
- Dolan, L., Janmaat, K., Willemsen, V., Linstead, P., Poethig, S., Roberts, K., and Scheres, B. (1993). Cellular organisation of the Arabidopsis thaliana root. Development 119, 71–84. [DOI] [PubMed] [Google Scholar]
- Edlund, A., Eklöf, S., Sundberg, B., Moritz, T., and Sandberg, G. (1995). A microscale technique for gas chromatography–mass spectrometry measurements of picogram amounts of indole-3-acetic acid in plant tissues. Plant Physiol. 108, 1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau, K. (1977). Anatomy of Seed Plants. (New York: John Wiley and Sons).
- Ferreira, P.C.G., Hemerly, A.S., de Almeida Engler, J., Van Montagu, M., Engler, G., and Inzé, D. (1994). Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell 6, 1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsthoefel, N.R., Wu, Y., Schultz, B., Bennett, M.J., and Feldmann, K.A. (1992). T-DNA insertion mutagenesis in Arabidopsis: Prospects and perspectives. Aust. J. Plant Physiol. 19, 353–366. [Google Scholar]
- Hobbie, L., and Estelle, M. (1995). The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J. 7, 211–220. [DOI] [PubMed] [Google Scholar]
- Laskowski, M.J., Williams, M.E., Nusbaum, C., and Sussex, I.M. (1995). Formation of lateral root meristems is a two-stage process. Development 121, 3303–3310. [DOI] [PubMed] [Google Scholar]
- Lloret, P.G., Casero, P.J., Pulgarín, A., and Navascués, J. (1989). The behaviour of two cell populations in the pericycle of Allium cepa, Pisum sativum and Daucus carota during early lateral root development. Ann. Bot. 63, 465–475. [Google Scholar]
- Long, J.A., Moan, E.I., Medford, J.I., and Barton, M.K. (1996). A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379, 66–69. [DOI] [PubMed] [Google Scholar]
- Malamy, J.E., and Benfey, P.N. (1997). Organisation and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124, 33–44. [DOI] [PubMed] [Google Scholar]
- Marchant, A., Kargul, J., May, S.T., Muller, P., Delbarre, A., Perrot-Rechenmann, C., and Bennett, M.J. (1999). AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 18, 2066–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday, G.K., and Haworth, P. (1994). Tomato root growth, gravitropism and lateral development: Correlation with auxin transport. Plant Physiol. Biochem. 32, 193–203. [PubMed] [Google Scholar]
- Müller, A., Guan, C., Gälweiler, L., Tänzler, P., Huijser, P., Marchant, A., Parry, G., Bennett, M., Wisman, E., and Palme, K. (1998). AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 17, 6903–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Rashotte, A.M., Brady, S.R., Reed, R.C., Ante, S.J., and Muday, G.K. (2000). Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 122, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, R.C., Brady, S.R., and Muday, G.K. (1998). Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 118, 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, D., Mandel, T., and Kuhlemeier, C. (2000). Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini, S., Beis, D., Wolkenfelt, H., Murfett, J., Guilfoyle, T., Malamy, J., Benfey, B., Leyser, H.M.O., Bechtold, N., Weisbeek, P., and Scheres, B. (1999). An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472. [DOI] [PubMed] [Google Scholar]
- Scheres, B., and Benfey, P. (1999). Asymmetric cell division in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 505–537. [DOI] [PubMed] [Google Scholar]
- Tsugeki, R., and Fedoroff, N.V. (1999). Genetic ablation of root cap cells in Arabidopsis. Proc. Natl. Acad. Sci. USA 96, 12941–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov, T., Murfett, J., Hagen, G., and Guilfoyle, T. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9, 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen, V., Wolkenfelt, H., de Vrieze, G., Weisbeek, P., and Scheres, B. (1998). The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development 125, 521–531. [DOI] [PubMed] [Google Scholar]