Abstract
Previous studies of photosynthetic carbon fixation in the marine alga Gonyaulax have shown that the reaction rates in vivo vary threefold between day and night but that the in vitro activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), which catalyzes the rate-limiting step in this process, remains constant. Using protein gel blotting, we confirm that Rubisco protein levels are constant over time. We present simultaneous measurements of the rhythms of CO2 fixation and O2 evolution and show that the two rhythms are ∼6 hr out of phase. We further show that the distribution of Rubisco within chloroplasts varies as a function of circadian time and that this rhythm in Rubisco distribution correlates with the CO2 fixation rhythm. At times of high carbon fixation, Rubisco is found in pyrenoids, regions of the chloroplasts located near the cell center, and is separated from most of the light-harvesting protein PCP (for peridinin–chlorophyll a–protein), which is found in cortical regions of the plastids. We propose that the rhythm in Rubisco distribution is causally related to the rhythm in carbon fixation and suggest that several mechanisms involving enzyme sequestration could account for the increase in the efficiency of carbon fixation.
INTRODUCTION
Circadian rhythms are daily biochemical changes that are driven by circadian clocks but are not by themselves clocks. Biological clocks are characterized by transcriptional feedback loops that maintain their own oscillation (Dunlap, 1999; Shearman et al., 2000) and that receive phasing information from changes in light intensity (Crosthwaite et al., 1997; Ceriani et al., 1999) or temperature (Liu et al., 1998) that allows them to synchronize their oscillations with natural environmental cycles. In contrast, circadian rhythms are changes in an organism's physiology or biochemistry that, although rhythmic under constant conditions, are incapable of independent oscillations and instead require the timing signals provided by circadian clocks.
A useful theoretical framework for understanding many biological rhythms is the notion that a central oscillator provides periodic regulatory signals that result in the control of a rate-limiting step (RLS) in a given biochemical pathway. Thus, understanding the regulation of the RLS is vital to understanding the clock's effect on organisms. For example, previous work on the bioluminescence rhythm in the dinoflagellate Gonyaulax has shown that both dinoflagellate luciferase (the reaction catalyst) and the luciferin (substrate) binding protein LBP contribute to the RLS of the nightly bioluminescence reaction. Cellular levels of both proteins vary seven- to 10-fold between day and night in phase with the 50- to 100-fold variations in bioluminescence capacity (Johnson et al., 1984; Morse et al., 1989a). In a vertebrate example, N-acetyltransferase catalyzes the RLS in vertebrate melatonin synthesis, and N-acetyltransferase levels correlate with the circulating melatonin rhythm (Gastel et al., 1998).
Rhythms are generally considered to reflect the differential expression of a target gene and may use components common to the clock's own transcriptional feedback loop, as with the vasopressin RNA rhythm (Jin et al., 1999), which appears linked to the rhythm in melatonin levels. On the other hand, transcriptional activators not involved directly in the clock mechanism also have been implicated in regulating target gene expression, as shown for the neuropeptide PDF (Renaud et al., 1983), which is believed to be linked to behavioral rhythms in Drosophila (Renn et al., 1999). It must be recognized, however, that changes in the amount of enzyme brought about by changes in gene expression are not the only way to regulate an enzyme's activity. In Bryophyllum, changes in phosphoenolpyruvate activity appear to be due to post-translational modification (phosphorylation) of the enzyme (Nimmo et al., 1984).
In Gonyaulax, both oxygen evolution (Sweeney, 1960) and carbon fixation (Hastings et al., 1961) are bona fide circadian rhythms. The oxygen evolution rhythm has been proposed to result from altered electron flux through photosystem II (Samuelsson et al., 1983). Although the control mechanism of this rhythm is not understood, it is possible that it may involve the soluble light-harvesting peridinin–chlorophyll a–protein (PCP), because this protein feeds energy primarily into photosystem II (Govindjee et al., 1979). Curiously, dinoflagellates are the only organisms known to use PCP in light harvesting, and the structure of this soluble protein is unlike that of any other light-harvesting protein (Norris and Miller, 1994; Hofmann et al., 1996).
In addition to the unusual light-harvesting protein PCP, dinoflagellates also are the only known organisms whose chloroplasts contain a form II ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) (Morse et al., 1995; Rowan et al., 1996). The form II enzyme is composed of only large subunits and shares limited sequence identity with the form I enzyme (Narang et al., 1984). The lack of signal on protein gel blot analyses of dinoflagellate proteins using anti-form I Rubisco and the absence of a protein at the molecular weight expected for the small Rubisco subunit show that the form II enzyme is the only form of Rubisco found in dinoflagellates (Morse et al., 1995).
The form II Rubisco, like its form I homolog, catalyzes both the first carboxylation step in the Calvin cycle and the first oxygenation step in photorespiration. Oxygen is a competitive inhibitor of CO2 binding to both forms of Rubisco, but the ratio of the reaction rate (v) with O2 as a substrate and with CO2 as a substrate (vO2/vCO2) in the form II enzyme in dinoflagellates and α-proteobacteria is five- and 10-fold greater, respectively, than the ratio in the form I enzyme in higher plants (Jordan and Ogren, 1981; Whitney and Andrews, 1998). Thus, the form II enzyme is more sensitive to competition by O2 than is the form I enzyme, and both theoretical considerations (Whitney and Andrews, 1998) and measurements of the growth of cyanobacteria engineered to express only the form II enzyme (Pierce et al., 1989) suggest that the form II Rubisco should not function efficiently in an oxygen-generating organelle.
Rubisco usually catalyzes the RLS in carbon fixation (Hartman and Harpel, 1994); thus, it would appear to be a likely target for clock control of the carbon fixation rhythm. In previous studies, the in vitro activity of Gonyaulax Rubisco was shown to be constant (Bush and Sweeney, 1972). As a caveat to this observation, however, dinoflagellate Rubisco is inactivated rapidly after extraction (Whitney and Yellowlees, 1995), which may have compromised earlier measurements of Rubisco activity. To date, there are no indications that the activity of the form II enzyme can be modulated by a Rubisco activase, as is the case with the form I homolog (Hartman and Harpel, 1994).
In the present study, we have confirmed the rhythmicity of the CO2 fixation rhythm and measured its phase with respect to the O2 evolution rhythm by measuring both simultaneously. We have used antibodies raised against dino-flagellate Rubisco to confirm that there are no changes in the cellular levels of Rubisco that could account for the rhythm of carbon fixation. We reasoned that if the amount or activity of Rubisco does not change as a function of time of extraction, differential substrate availability in vivo might be responsible for the biological rhythm. Thus, we used the anti-Rubisco and anti-PCP antibodies to document changes in the subcellular distribution of Rubisco that correlate with the rhythm in carbon fixation. The sequestration of Rubisco could produce an increase in CO2 fixation if carbon-concentrating enzymes, none of which are yet known for dinoflagellates, were colocalized along with the Rubisco, or alternatively, if separation from the site of oxygen generation decreased the effective O2 concentration near the oxygen-sensitive form II enzyme.
RESULTS
Circadian CO2 Fixation Is Phased Differently from O2 Evolution
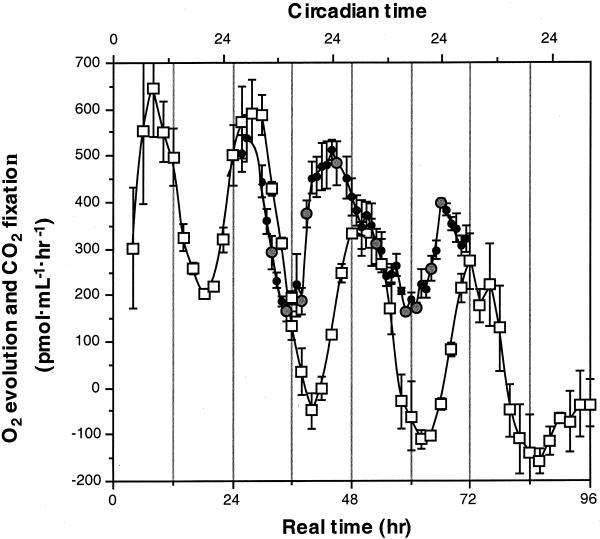
The two photosynthesis rhythms (CO2 fixation and O2 evolution) were measured simultaneously in cell cultures grown under constant conditions (Figure 1). Both rhythms continue under conditions of constant light with an approximately threefold amplitude and similar periods (∼22 hr under the light intensities used), thus representing bona fide circadian rhythms. However, the phases of the two rhythms are different, with maximum carbon fixation rates (Figure 1, circles) preceding maximum oxygen evolution rates (Figure 1, open squares) by ∼6 hr. This suggests that different regulatory mechanisms may be involved in the control of these two photosynthesis rhythms. It is also noteworthy that the waveforms of the two rhythms differ as well, with carbon fixation approximating a sawtooth wave and oxygen evolution approximating a sine wave. Waveforms are an often overlooked aspect of biological rhythms that can be informative regarding the basic biochemistry underlying the rhythm. Thus, any explanation proposed for the rhythmicity of CO2 fixation must be able to account for the sawtooth waveform.
Figure 1.
Rhythms of CO2 Fixation and O2 Evolution Are Phased Differently.
The circadian rhythms of O2 evolution (open squares) and CO2 fixation (large, shaded circles) have the same period but differ in phase and waveform. Real time (in hours from the end of the last dark period) is shown at the bottom, and circadian time (based on a 22-hr period with 0 corresponding to dawn) is shown at the top. Large shaded circles in the CO2 fixation rhythm represent times when samples of the cultures were fixed and embedded for subsequent immunolabeling experiments.
CO2 Fixation Rate Changes Are Not Due to Differing Rubisco Levels
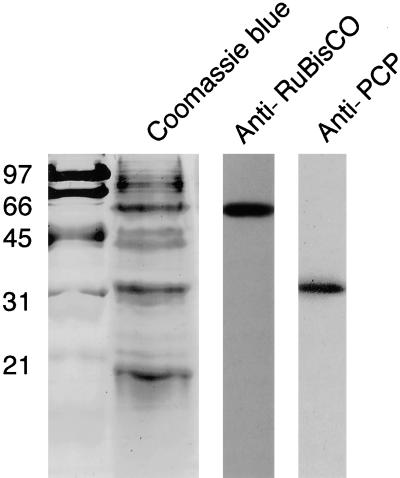
To determine if the circadian rhythm in carbon fixation was due to changes in Rubisco gene expression, measurements of Rubisco levels over a daily period were required. We raised rabbit polyclonal antibodies to the dinoflagellate Rubisco and verified that these antibodies were specific by reaction with only a single protein on protein gel (immunoblot) analyses of whole-cell extracts (Figure 2). As a control for use of the anti-Rubisco antibody in immunocytochemistry, we also prepared an antibody against the light-harvesting protein PCP. The anti-PCP antibody likewise is specific for its antigen on protein gel blot analyses. Control experiments either in the absence of primary antibody or using preimmune serum showed no reaction with Gonyaulax extracts (data not shown).
Figure 2.
Specificity of Anti-Rubisco and Anti-PCP Antibodies on Protein Gel Blots.
Crude extracts of Gonyaulax proteins stained with Coomassie Brilliant Blue after SDS-PAGE (lane second from left) show that Rubisco (55 kD) and PCP (32 kD) are major proteins in the cell and that only single proteins are recognized on protein gel blots by anti-Rubisco (Anti-RuBisCO, lane second from right) and anti-PCP (lane at right) antibodies. Molecular mass markers (in kilodaltons) are shown at left.
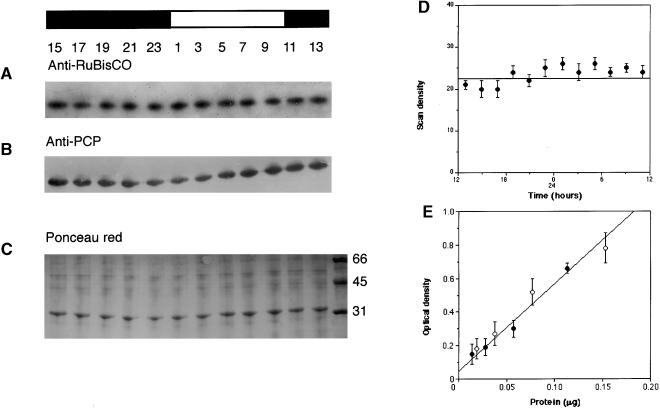
The amount of immunoreactive Rubisco extractable from the cells at different times during a light/dark cycle was first assayed using protein gel blot analysis (Figure 3A). A visual examination of the autoradiographs suggests that there are no marked changes in Rubisco protein levels, a conclusion supported by densitometric scans (Figure 3D). This conclusion is supported by measurements of Rubisco levels at times corresponding to maximum and minimum CO2 fixation using ELISA (Figure 3E). Because the amount of protein extracted for protein gel blots is approximately constant for the different times examined (Figure 3C), and because the amount of anti-Rubisco antibody per microgram of protein does not vary between periods of maximum and minimum CO2 fixation, we conclude that the threefold variation in the amplitude of the carbon fixation rhythm (Figure 1) is not due to changes in the amount of Rubisco.
Figure 3.
Rubisco Levels Are Constant during the Daily Cycle.
Protein gel blots of extracts taken from 50 mL of Gonyaulax cell culture during 1 day (closed bar, dark period; open bar, light period). Numbers correspond to the time at which protein samples were taken.
(A) to (C) Anti-Rubisco staining (A) is constant, whereas anti-PCP staining (B) shows an approximately twofold variation. Ponceau red staining (C) was used as a control for protein load. Numbers at right indicate molecular weight.
(D) Densitometric scans of the anti-Rubisco staining in (A).
(E) The cellular levels of Rubisco at times corresponding to either maximum (filled circles) or minimum (open circles) CO2 fixation rates were determined by plots of immunologically reactive protein in ELISAs as a function of total soluble protein. Only the linear region of the dilution curve is shown. Error bars in (D) and (E) indicate ±sd.
The same immunoblots also were tested for variations in PCP levels (Figure 3B). PCP levels in cells grown under light/dark cycles vary up to twofold during the course of a day and thus provide a strong contrast to the measured Rubisco levels. Neither PCP nor Rubisco levels vary in cells extracted in constant conditions (data not shown).
Plastid Morphology Changes during the Light/ Dark Cycle
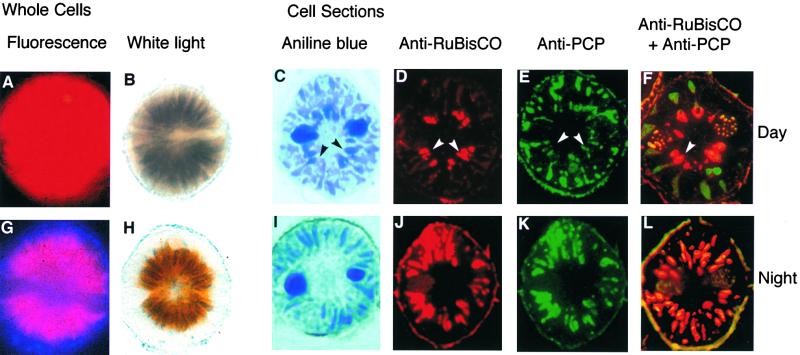
Gonyaulax plastids, viewed by light microscopy, undergo two distinct morphological changes. These changes are in (1) the proximity of the cortical end of the plastid to the plasma membrane and (2) the formation of specialized regions of the plastid, which are termed pyrenoids. The former can be observed in whole cells by using either fluorescence or visible light microscopy to visualize the endogenous fluorescence or absorbance, respectively, of chlorophyll. In cells taken in the middle of the day phase, the radially oriented chloroplasts are extended almost to the cell wall (Figures 4A and 4B). In contrast, night-phase cells have shorter, more compact plastids. The cortical ends of the plastids are seen to be withdrawn from the cell periphery by both visible (Figure 4G) and fluorescence (Figure 4H) microscopy. Fluorescence microscopy also shows that the cytoplasmic region adjacent to the cell periphery of night-phase cells contains numerous scintillons that appear blue due to the endogenous fluorescence of the bioluminescence substrate luciferin.
Figure 4.
Rubisco and PCP Distribution Is Different in Night- and Day-Phase Cells.
Cells were taken during either the middle of the light period (light dark time 6; [A] to [F]) or the middle of the night phase (light dark time 18; [G] to [L]). Whole cells were examined by either UV fluorescence ([A] and [G]) or visible light ([B] and [H]). Cell sections were stained with aniline blue ([C] and [I]), anti-Rubisco antibody ([D] and [J]), or anti-PCP antibody ([E] and [K]). Antibody binding was visualized using fluorescently labeled secondary antibodies. To visualize the distribution of both proteins, serial sections were stained with the two antibodies separately ([D] and [E], [J] and [K]). Alternatively, single sections were stained sequentially with anti-PCP antibody, an FITC-conjugated secondary antibody (green), anti-Rubisco antibody, and a Texas red–conjugated secondary antibody ([F] and [L]). A greenish yellow fluorescence due to nonspecific binding of both secondary antibodies in approximately equal amounts can be seen in the cell wall and in the permanently condensed chromosomes in both ends of the U-shaped nucleus. Arrowheads in (C) to (F) point to pyrenoid regions of the plastids. Cells are ∼35 μm in diameter.
The formation of pyrenoids cannot be observed in living intact cells and requires an examination of cell sections. To ensure that equivalent planes of sections were examined in all cases, we selected sections that contained two ends of the U-shaped nucleus. The U-shaped nucleus circles the midline of the alga, with the open end of the U corresponding to the site of flagellar attachment. The region of the cell within the U-shaped nucleus appears clear. In day-phase cells colored with aniline blue, pyrenoids are seen as slightly bulbous inner regions of the chloroplasts (Figure 4C, arrowheads) that surround this central clear area. Day-phase plastids often have a dumbbell appearance, with the inner expanded area comprising the pyrenoid; the outer expanded areas will be referred to as cortical regions. In contrast, sections from night-phase cells show none of these bulbous inner regions surrounding the central clear area (Figure 4I), and the diameter of the plastids is relatively constant from the clear central region to the cell periphery.
Rubisco Is Localized in Pyrenoids in Day-Phase Cells
In many photosynthetic algae, pyrenoids contain form I Rubisco (McKay and Gibbs, 1991). More recently, the form II Rubisco in the dinoflagellate Amphidinium also has been localized to pyrenoids (Jenks and Gibbs, 2000). Because the formation of pyrenoids in both Euglena (Osafune et al., 1990), which contains form I Rubisco, and Gonyaulax (Schmitter, 1971) is clock controlled, we reasoned that the form II Rubisco in Gonyaulax might be selectively sequestered into pyrenoids on a daily basis. Thus, we first determined whether Gonyaulax pyrenoids did, in fact, contain form II Rubisco.
Anti-Rubisco staining confirms that Rubisco is restricted to the pyrenoid region of day-phase plastids (Figure 4D, arrowheads). In contrast, when plastids from night-phase cells are labeled similarly, Rubisco staining appears uniform over the entire surface of the plastid (Figure 4J). As a control for the anti-Rubisco staining, we stained serial sections from both day-phase and night-phase cells with the anti-PCP antibody. In contrast to the anti-Rubisco staining, anti-PCP staining was less intense in the pyrenoids than in cortical regions of the day-phase chloroplasts (Figure 4E). However, in night-phase cells, anti-PCP staining was distributed evenly over the surface of the plastid, as was found with the anti-Rubisco staining (Figure 4K). No staining was observed for either day-phase or night-phase cells when preimmune serum replaced either the anti-Rubisco or the anti-PCP antibody (data not shown).
These observations suggested that the plastids of day-phase cells contained regions with different protein complements. To confirm this observation, we stained single sections sequentially with the anti-PCP antibody, a fluorescein isothiocyanate (FITC)–conjugated secondary antibody, the anti-Rubisco antibody, and a Texas red–conjugated secondary antibody. Double-labeled sections of day-phase cells show two distinctly colored regions, indicating limited overlap between Rubisco and PCP (Figure 4F). Cortical regions of the plastids are green, indicating that little Rubisco is present, whereas pyrenoids are reddish orange, indicating that some PCP is present. The yellow color of the cell wall and of the condensed chromosomes in the two ends of the U-shaped nucleus is due to nonspecific binding of approximately equal amounts of the secondary antibodies; thus, it is significant that little yellow is observed in the plastids. In contrast, when night-phase cell sections were stained sequentially with anti-Rubisco and anti-PCP antibodies, all regions of the plastids were similarly colored, and there were more regions with a yellow color than were found in day-phase plastids (Figure 4L).
Quantitative Measurements of Protein Distribution
To confirm and quantitate the unequal distribution of Rubisco and PCP in day-phase cells, we used transmission electron microscopy (TEM) to count the number of gold-labeled secondary antibodies bound to the sections. In TEM, pyrenoids can be seen to contain widely spaced (∼0.2 μm) thylakoid membranes, which appear white because the samples were not treated with osmium to preserve their antigenicity. The amount of Rubisco staining in the pyrenoids (Figure 5A), estimated by the number of gold particles per μm2, is 15- to 20-fold greater than that found in chloroplast regions near the cell periphery (Table 1). Thylakoid membranes are not visible in the cortical regions of this plastid because the plane of the section runs parallel to the thylakoids (rather than perpendicular, as in the pyrenoid region). However, this section contains a cortical region of an adjacent plastid sectioned perpendicular to the thylakoids (∼0.07 μm spacing; immediately to the right of the pyrenoid in Figure 5A). This finding confirms that the absence of Rubisco labeling is not related to the thylakoids themselves. Because pyrenoids typically represent one-third of the total area of chloroplasts, we calculate that ∼90% of the cellular Rubisco is found within the pyrenoid. In contrast, after anti-PCP staining, gold particles can be detected throughout the length of the chloroplast (Figure 5B), although the staining intensity (number of gold particles/μm2) is two- to threefold greater in the cortical regions of the chloroplasts than in the pyrenoids (Table 1). Again, taking into account the larger proportional area of the cortical regions, we calculate that these areas contain ∼80% of the total PCP. No staining is observed if preimmune serum replaces either of the two antisera (data not shown).
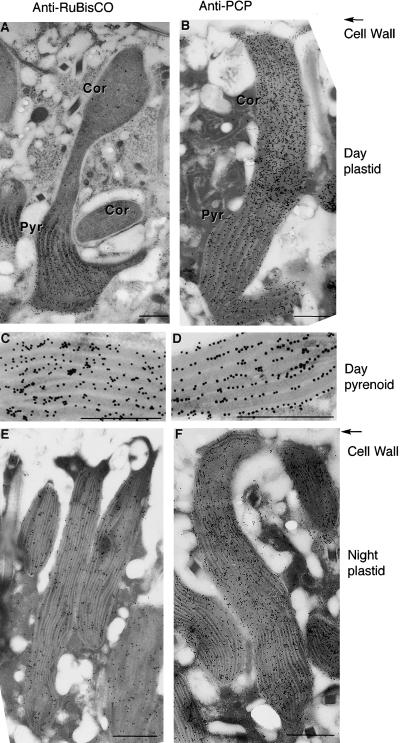
Figure 5.
Rubisco and PCP Are Found in Different Compartments.
Sections of cells from day-phase cells ([A] to [D]) and night-phase cells ([E] and [F]) were stained with anti-Rubisco antibody ([A], [C], and [E]) or anti-PCP antibody ([B], [D], and [F]). Antibody binding was visualized with gold-labeled secondary antibodies for TEM. Micrographs are oriented with the cortical (Cor) regions of the plastids at the top (Cell Wall) and the pyrenoid (Pyr) regions of day-phase plastids, with their widely spaced thylakoid membranes, at the bottom. Higher magnification of the pyrenoid regions places the anti-Rubisco labeling over the stroma (C) and the anti-PCP labeling over the thylakoids (D). Sections of night-phase cells show a uniform distribution of label after staining with either anti-Rubisco (E) or anti-PCP (F) antibody. Pyrenoid regions, with their widely spaced thylakoid membranes, are not observed in night-phase cells.  in all electron micrographs.
in all electron micrographs.
Table 1.
PCP and Rubisco Distribution within Day-Phase Plastidsa
| Label Density (Gold Particles/μm2)
|
||
|---|---|---|
| Location within Plastid | PCP | Rubisco |
| Cortex | 19 ± 6 (5998) | 3 ± 2 (1629) |
| Pyrenoid | 8 ± 0.3 (629) | 41 ± 12 (3136) |
| Pyrenoid/cortex | 0.5 ± 0.12 | 11 ± 4 |
Values shown are mean ±sd (number of gold particles).
Closer examination of the labeling patterns of anti-Rubisco and anti-PCP antibodies indicated that Rubisco was associated with the darker-stained stroma, whereas PCP was associated with the lighter-stained thylakoids. This difference in the suborganellar distribution of the two proteins was confirmed by higher magnification micrographs. In these, pyrenoid regions of day-phase plastids were examined, because the greater spacing between thylakoid membranes allowed a more accurate determination of gold particle distribution. We observed 10-fold more anti-Rubisco labeling over the stroma than over the thylakoids (Figure 5C and Table 2). This staining is in good agreement with the observation that Rubisco is found in the pyrenoid stroma in many algae (McKay and Gibbs, 1991) and with Rubisco distribution in the dinoflagellate Amphidinium (Jenks and Gibbs, 2000). In contrast, more than half of the anti-PCP staining is associated unambiguously with the thylakoid membranes, and less than one-quarter of the label is found unambiguously in the stroma (Figure 5D and Table 2). A thylakoid location of PCP is in agreement with the presence of the inner thylakoid membrane lipid digalactosyl diacyl glycerol in the crystal structure of the protein (Hofmann et al., 1996).
Table 2.
Suborganellar Distribution of PCP and Rubiscoa
| Percentage of Total Label
|
||
|---|---|---|
| Location within Plastid | PCP | Rubisco |
| Thylakoid | 54 ± 6 | 10 ± 4 |
| Undetermined | 30 ± 6 | 10 ± 4 |
| Stroma | 16 ± 4 | 80 ± 8 |
More than 1000 gold particles were counted for each antibody. The location of gold particles touching both stroma and thylakoid membranes was scored as undetermined because of the size of the primary and secondary antibodies as well as the gold particles.
There is no difference in the suborganellar distribution of the two proteins in night-phase cells (data not shown), although these measurements are more difficult due to the fact that the thylakoid membranes are generally closer together than when pyrenoids are present (see below). There is also no difference in the amount of anti-PCP label per unit length of the thylakoid membranes inside the pyrenoid or in the cortical regions of the plastid (data not shown). Together, these observations suggest a possible link between the asymmetrical distribution of Rubisco and PCP in day-phase plastids. Because thylakoid spacing is two- to threefold greater in the pyrenoids than in more cortical regions of the plastids, a thylakoid protein might be expected to decrease in labeling by the same factor, exactly as observed (Table 1). It appears that an increase in the abundance of a stromal protein such as Rubisco occurs at the expense of a thylakoidal protein such as PCP.
TEM confirms that pyrenoids, as distinguished by the widely spaced thylakoid membranes, are absent from night-phase cell plastids. Instead, thylakoid spacing is constant everywhere, and there is a uniform density of gold particles over the surface of the plastids after either anti-Rubisco (Figure 5E) or anti-PCP (Figure 5F) labeling. The plastid in Figure 5E was chosen for its similar morphology to the plastid in Figure 5F, despite a generally lower amount of labeling in this particular experiment.
Rubisco Distribution Correlates with Carbon Fixation Efficiency
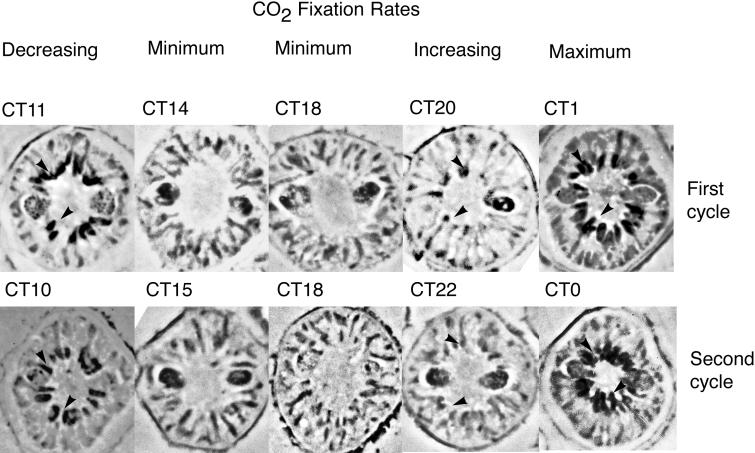
To determine if the daily changes in the distribution of Rubisco or PCP within the plastid were correlated with either CO2 fixation or O2 evolution, respectively, we examined the anti-Rubisco staining of cell sections (Figure 6) taken at different times. The specific times chosen are from the experiment shown in Figure 1 (shaded circles). The sharp increase in carbon fixation rates correlates with the movement of Rubisco toward a developing pyrenoid, whereas the maximum ability of the cells to fix carbon correlates with complete pyrenoid formation. We observe that pyrenoids also are evident in cells when carbon fixation rates are decreasing, but note that at these times, the rate of O2 evolution is increasing. These observations suggest that the increased O2 evolution may have a detrimental effect on carbon fixation. Finally, when carbon fixation rates are at a minimum, pyrenoids are not observed and Rubisco is spread out over the surface of the plastid.
Figure 6.
Rubisco Distribution in Plastids Correlates with the Circadian Rhythm in Carbon Fixation.
Cells fixed at times corresponding to decreasing, minimum, increasing, and maximum CO2 fixation rates were sectioned and stained with anti-Rubisco antibody and a peroxidase-conjugated secondary antibody. The sampling times, indicated as circadian time (CT), are those corresponding to the large, shaded circles in Figure 1. Similar circadian times from the two circadian cycles are aligned vertically. Dark areas surrounding the clear central area correspond to anti-Rubisco staining in the pyrenoids (arrowheads). The decrease in CO2 fixation rates at CT10 and CT11, despite the presence of pyrenoids, suggests that increasing O2 evolution rates (see Figure 1) are detrimental to CO2 fixation.
DISCUSSION
We have shown that in the dinoflagellate Gonyaulax, Rubisco and PCP are found in different regions of the day-phase plastid and are spread over the surface of the plastid during the night phase (Figures 4 and 5). Because PCP represents a marker for light-harvesting reactions and Rubisco represents a marker for carbon fixation, the separation of these two proteins suggests that day-phase plastids have specialized regions for the light and dark reactions. We also have shown that the sequestration of Rubisco into pyre-noids correlates with an ability of the cells to fix carbon more efficiently than when the proteins are distributed evenly over the plastids (Figures 1 and 6). Because there is no rhythm in the amount of Rubisco that can account for the CO2 fixation rhythm (Figure 3), we propose that the changes in the subcellular distribution of Rubisco might contribute to the biological rhythm of CO2 fixation. There is precedence among dinoflagellates for the idea that changes in the subcellular localization of an enzyme can regulate the rate of the reaction it catalyzes. For example, the luciferase-containing organelles responsible for bioluminescence in the dinoflagellate Pyrocystis fusiformis have been shown to move from a central location in the cytoplasm to the cell periphery, in phase with the onset of bioluminescence (Widder and Case, 1982). Interestingly, the amount of luciferase in this alga is constant over time (Knaust et al., 1998), as are the levels of Rubisco in Gonyaulax (Figure 3). However, luciferase distribution in Pyrocystis changes within the cytoplasm, whereas Rubisco localization in Gonyaulax changes within an organelle.
Many circadian changes have been noted in the ultrastructure of dinoflagellate chloroplasts. These include the packing of chloroplasts near the cell surface, the maximum length of the plastids, the spacing between thylakoid membranes in the region of the pyrenoids, and the daily formation of the bulbous centrally located pyrenoids (Herman and Sweeney, 1975; Rensing et al., 1980). Although we have not measured all of these parameters simultaneously with the measurements of the two photosynthetic rhythms, some seem more likely than others to be related to changes in O2 evolution. However, we have measured the pyrenoid formation rhythm and found a good correlation between it and the CO2 fixation rhythm. Given the presence of Rubisco in the pyrenoid and the potential of this enzyme to act as the RLS of carbon fixation, it seems likely that the microenvironment surrounding the Rubisco is important for its activity.
If Rubisco sequestration into pyrenoids is implicated in carbon fixation, is PCP abundance in cortical regions of the plastids implicated in oxygen evolution? We have observed that the rhythm in PCP distribution appears to mirror the rhythm in Rubisco distribution, presumably due to the wider spacing between thylakoids found in the pyrenoids (Figure 5) and the different suborganellar locations of the two enzymes (Table 2). We also observed that the onset of the two physiological rhythms is ∼6 hr out of phase (Figure 1). Because the phases of the PCP and Rubisco distribution rhythms are similar and the phases of the oxygen evolution and carbon fixation rhythms are different, it seems unlikely that PCP distribution plays a role in the oxygen evolution rhythm. We suggest that the changes in PCP distribution represent instead a consequence of Rubisco distribution changes.
We have shown that changes in the in vivo activity of Rubisco, as measured by the rate of carbon fixation, cannot be due to changes in the amount of protein (Figure 3). We also have shown that there are no differences in the distribution of various Rubisco isoforms, as determined by two-dimensional electrophoresis (Markovic et al., 1996), suggesting that the enzyme does not undergo post-translational modifications during the daily period. To date, there is no evidence that Rubisco activase can act on the form II Rubisco (Hartman and Harpel, 1994) or that dinoflagellates contain the form I enzyme (Morse et al., 1995). Therefore, it seems probable that the changing reaction rates in vivo reflect changes in substrate concentrations.
The metabolic consequence of the competition between O2 and CO2 for the active site of Rubisco is that the net rate of carbon fixation depends on the concentration of both rather than on the concentration of CO2 alone. In C4 plants and many algae, the vO2/vCO2 ratio is biased toward carbon fixation by CO2-concentrating mechanisms (CCM) (Badger et al., 1998). CCM in the dinoflagellate Symbiodinium depends on the energy derived from photosynthetic electron transport to move inorganic carbon into the cell against a concentration gradient (Badger et al., 1998). However, in Gonyaulax, the phase of the electron transport rhythm, as measured by O2 evolution, lags behind the CO2 fixation rhythm by 6 hr (Figure 1). Therefore, CO2 concentration by this mechanism cannot explain how CO2 fixation rates increase sharply at times when O2 evolution rates remain low (Figure 1, circadian time 20 to 22). Of course, our data do not rule out the presence of a CCM in Gonyaulax, and it is possible that carbon-concentrating enzymes may be sequestered into pyrenoids at the same time as the Rubisco. Unfortunately, there is insufficient information available regarding CCM in dinoflagellates to investigate this possibility fully. On the basis of the fact that the onset of carbon fixation precedes the onset of oxygen evolution, we conclude that the possibility that Rubisco movement and the accompanying increase in carbon fixation rates could be due to an increase in photosynthetic electron transport can be excluded.
It is also possible that the sequestration of Rubisco into pyrenoids affects its activity without involving CO2 concentrations. For example, a decrease in the relative reaction rates using either O2 or CO2 as a substrate (vO2/vCO2 ratio) could be achieved by a decrease in the oxygen tension as well as by an increase in the CO2 concentration. In this regard, it is interesting that in Amphidinium carterae, the pyrenoid persists during the dark phase (Matthys-Rochon, 1979), and that the amount of Rubisco in the pyrenoid increases as a function of the light intensity (Jenks and Gibbs, 2000). These findings suggest that Rubisco in this organism may be sequestered in response to increased oxygen tension, a situation clearly different from that in Gonyaulax, in which pyrenoid formation occurs rhythmically as a circadian clock–controlled process under constant light (Figure 6).
Is it reasonable that a spatial separation of Rubisco and PCP could decrease the local oxygen tension and thus increase the rate of carbon fixation? The net oxygen concentration in the region of the pyrenoids is not known, but it will be a function of the diffusion rate into the cell, the photosynthetic oxygen evolution rate, and the respiration rate. We have measured the mean oxygen consumption rate of cells in darkness as 62% (sd = 11, n = 15) of the oxygen evolution rate of illuminated cells (300 μE·m−2·sec−1 cool-white light), averaged from several different times during a light/dark cycle. These values represent the high end of the range reported previously for this organism (30 to 60%; Prezelin et al., 1977). Given the spherical geometry of the cells, more photosynthetically produced oxygen should diffuse out into the medium than in toward the cell center. Thus, assuming the same respiration rate in light and darkness and the same amount of oxygen diffusing inward as outward, the measured respiration rate could serve to decrease the oxygen tension in the area of the pyrenoids. Presumably, this respiration occurs in the many mitochondria that are found distributed throughout the cytoplasm from the pyrenoids out to the cell periphery (Schmitter, 1971).
What is the mechanism responsible for the separation of Rubisco and PCP? We hypothesize that aggregates of Rubisco in day-phase plastids fill the stroma and push apart the thylakoids to form pyrenoids. Because the PCP labeling along each thylakoid is constant but the thylakoids are approximately three times more densely packed in the plastid periphery, the rhythm in PCP distribution is derived directly from pyrenoid formation. One model to explain this fact could involve active recruitment of Rubisco to a developing pyrenoid, perhaps using prokaryotic-type cytoskeletal elements known to be present in plastids (Lutkenhaus and Addinall, 1997; Osteryoung et al., 1998). A second possibility is that thylakoid membranes are brought into close physical association in cortical regions of the plastid, which forces Rubisco down toward the cell center in a manner similar to the extrusion of toothpaste from a toothpaste tube. However, there is little day/night variation in the distance between thylakoids in cortical regions of the plastid (Rensing et al., 1980), suggesting that the latter possibility is unlikely.
Irrespective of the mechanism of protein sequestration, our results show that the sequestration of Rubisco into pyrenoids is the result of a bona fide circadian rhythm. This rhythm correlates with the rhythm in carbon fixation, and we propose that there is a causal link between the two. A link is plausible if the separation of Rubisco from the site of oxygen generation reduces the internal oxygen tension in the region of the pyrenoids and thus reduces competition by oxygen for the active site of the form II Rubisco in dinoflagellate plastids.
METHODS
Antibody Preparation
All antibodies were raised in rabbits by Cocalico Biologicals (Reamstown, PA), using its standard operating protocol. The ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) antigen was prepared as a His-tagged fusion protein from a Rubisco cDNA (Morse et al., 1995) cloned into the pQE31 expression vector (Qiagen, Valencia, CA). The orientation of the insert was verified by polymerase chain reaction and restriction mapping. The insert was expressed in M15 cells and purified using nickel–nitrilotriacetic acid agarose resin affinity chromatography as described by the manufacturer (Qiagen). The anti-Rubisco titer was 1:20,000, as measured by ELISA, and the antibody was used at a dilution of 1:2000 for protein gel blots and 1:100 for immunocytochemistry. For ELISA, proteins were measured as OD490 after reaction with bicinchoninic acid (Pierce Chemical Co., Rockford, IL), whereas Rubisco was measured as OD650 after reaction with anti-Rubisco antibody, a peroxidase-labeled secondary antibody (Sigma), and the chromogenic substrate 3,3′,5,5′-tetramethylbenzidine (Bio-Rad). Proteins for ELISAs were extracted in water using a beadbeater (BioSpec Products, Bartlesville, OK) and used immediately. For protein gel blots, primary antibodies were detected using either iodinated protein A (ICN Biomedicals Inc., Costa Mesa, CA) or the peroxidase-conjugated secondary antibody and a chemiluminescence substrate (Amersham).
The peridinin–chlorophyll a–protein (PCP) antigen was purified from Gonyaulax polyedra cells grown in f/2 medium (Morse et al., 1989b), using an ammonium sulfate precipitation method as described by Sharples et al. (1996), except that 100 mM Tris, pH 8, containing 10 mM EDTA and 50 mM DTT was used as the extraction buffer and a DEAE column replaced the cation exchange column. Brick-red fractions characteristic of peridinin were collected at each step of the purification, and the final DEAE eluate was purified further by preparative SDS-PAGE. The band at 32 kD was excised for immunization. The anti-PCP titer was 1:10,000, as measured by ELISA, and the antibody was used at a dilution of 1:1000 for protein gel blots and 1:100 for immunocytochemistry.
Microscopy
Cells during the middle of the day (6 hr after lights on, or light dark time 6) or night (6 hr after lights off, or light dark time 18) phase were harvested and fixed for immunocytochemistry by using standard methods. Fluorescent images of both fluorescein isothiocyanate (FITC)– and Texas red–labeled secondary antibodies (goat anti-rabbit conjugates) were captured with a Leitz Aristoplan light/fluorescence microscope (Wetzlar, Germany) equipped with a digital SPOT camera (Diagnostic Instruments, Sterling Heights, MI). Simultaneous fluorescence after double labeling was imaged through a triple filter cube (Chroma Tech, Brattleboro, VT) configured for UV (393/13 nm excitation, 458/15 nm emission), blue (485/16 nm excitation, 519/30 nm emission), and green (555/24 nm excitation, 602/40 nm emission) fluorescence. Captured images were imported directly into a personal computer operating with Windows 95 (Microsoft, Redmond, WA). Images were cropped and edited (for contrast and brightness only) in Adobe Photoshop 4.0 (Mountain View, CA). Cell samples taken in constant conditions were stained with anti-Rubisco antibody, and the position of the antibody was visualized with phase-contrast microscopy (to aid in visualization of the unstained plastids) after reaction with a peroxidase-conjugated secondary antibody (Sigma) and an enhanced 3,3′ diaminobenzidine stain (Pierce Chemical Co.). Transmission electron microscopy was performed using a JEOL JEM 100S microscope operating at 80 kV. Immunostaining was performed as described (Fritz et al., 1990), with detection using 20-nm gold–conjugated secondary antibodies (Ted Pella, Redding, CA).
Photosynthetic Oxygen Evolution
Cell cultures of Gonyaulax (strain No. CCMP407; Provasoli-Guillard National Center for the Culture of Marine Phytoplankton, Boothbay Harbor, ME) were grown under a 12-hr-light/12-hr-dark (60 μE·m−2·sec−1 white fluorescent light) cycle at 18°C in f/2 medium as described by Guillard and Ryther (1962). O2 evolution rhythms were measured from 200-mL cultures kept under constant white fluorescent light (60 μE·m−2·sec−1) by using an automated respirometer (Columbus Instruments, Columbus, OH). CO2 fixation rhythms were measured for duplicate cultures by using conversion of 14C-bicarbonate (ICN Biomedicals Inc., Costa Mesa, CA) to acid-insoluble material by five 10-mL aliquots of cell culture under saturating white light (300 μE·m−2·sec−1). Hours under constant conditions can be converted to circadian time using a period length of 22 hr under these conditions. For instantaneous measurements of O2 evolution and consumption rates, a 50-mL cell culture was concentrated to 1 mL by filtration on a 20-μm Nitex filter, and the O2 concentration was monitored with a microelectrode for 20 min in darkness and then 20 min in white light (300 μE·m−2·sec−1). Cells in the concentrate were counted with a hemocytometer to correct for differences in cell number. Zero and full-scale end points for the calibration of these O2 levels were set by a 10 mg/mL aqueous solution of sodium dithionite and by air-saturated water, respectively.
Acknowledgments
We thank L. Pelletier and T. Bertomeu for technical assistance, Drs. D. Layzell and G. Espie for helpful discussions, and Drs. S. Gibbs and M. Cappadocia for critical reviews of the manuscript. We gratefully acknowledge the financial support of the Natural Sciences and Engineering Research Council of Canada (D.M.) and Northern Arizona University organized research (L.F.).
References
- Badger, M., Andrews, T., Whitney, S., Ludwig, M., Yellowlees, D., Leggat, W., and Price, G. (1998). The diversity and coevolution of Rubisco, plastids, and chloroplast based CO2 concentrating mechanisms in algae. Can. J. Bot. 76, 1052–1071. [Google Scholar]
- Bush, K.J., and Sweeney, B.M. (1972). The activity of ribulose diphosphate carboxylase in extracts of Gonyaulax polyedra in the day and the night phases of the circadian rhythm of photosynthesis. Plant Physiol. 50, 446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriani, M., Darlington, T., Staknis, D., Mas, P., Petti, A., Weitz, C., and Kay, S. (1999). Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science 285, 553–556. [DOI] [PubMed] [Google Scholar]
- Crosthwaite, S., Dunlap, J., and Loros, J. (1997). Neurospora wc-1 and wc-2: Transcription, photoresponses, and the origins of circadian rhythmicity. Science 276, 763–769. [DOI] [PubMed] [Google Scholar]
- Dunlap, J. (1999). Molecular bases for circadian clocks. Cell 96, 271–290. [DOI] [PubMed] [Google Scholar]
- Fritz, L., Morse, D., and Hastings, J.W. (1990). The circadian bioluminescence rhythm of Gonyaulax is related to daily variations in the number of light-emitting organelles. J. Cell Sci. 95, 321–328. [DOI] [PubMed] [Google Scholar]
- Gastel, J., Roseboom, P., Rinaldi, P., Weller, J., and Klein, D. (1998). Melatonin production: Proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science 279, 1358–1360. [DOI] [PubMed] [Google Scholar]
- Govindjee, Wong, D., Prezelin, B., and Sweeney, B. (1979). Chlorophyll a fluorescence of Gonyaulax polyedra grown on a light–dark cycle and after transfer to constant light. Photochem. Photobiol. 30, 405–411. [DOI] [PubMed] [Google Scholar]
- Guillard, R.R.L., and Ryther, J.H. (1962). Studies on marine planktonic diatoms: Cyclotella nana Hufstedt and Denotula confervacea (Cleve) Gran. Can. J. Microbiol. 8, 229–239. [DOI] [PubMed] [Google Scholar]
- Hartman, F., and Harpel, M. (1994). Structure, function, regulation and assembly of d-ribulose-1,5-bisphosphate carboxylase/oxygenase. Annu. Rev. Biochem. 63, 197–234. [DOI] [PubMed] [Google Scholar]
- Hastings, J.W., Astrachan, L., and Sweeney, B.M. (1961). A persistent daily rhythm in photosynthesis. J. Gen. Physiol. 45, 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, E.M., and Sweeney, B.M. (1975). Circadian rhythm of chloroplast ultrastructure in Gonyaulax polyedra: Concentric organization around a central cluster of ribosomes. J. Ultrastruct. Res. 50, 347–354. [DOI] [PubMed] [Google Scholar]
- Hofmann, E., Wrench, P.M., Sharples, F.P., Hiller, R.G., Welte, W., and Diedrichs, K. (1996). Structural basis of light harvesting by carotenoids: Peridinin-chlorophyll-protein from Amphidinium carterae. Science 272, 1788–1791. [DOI] [PubMed] [Google Scholar]
- Jenks, A., and Gibbs, S. (2000). Immunolocalization and distribution of form II Rubisco in the pyrenoid and chloroplast stroma of Amphidinium carterae and form I Rubisco in the symbiont derived plastids of Peridinium foliaceum (Dinophyceae). J. Phycol. 36, 127–138. [Google Scholar]
- Jin, X., Shearman, L., Weaver, D., Zylka, M., De Vries, G., and Reppert, S. (1999). A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell 96, 57–68. [DOI] [PubMed] [Google Scholar]
- Johnson, C.H., Roeber, J.F., and Hastings, J.W. (1984). Circadian changes in enzyme concentration account for rhythm of enzyme activity in Gonyaulax. Science 223, 1428–1430. [DOI] [PubMed] [Google Scholar]
- Jordan, D., and Ogren, W. (1981). Species variation in the specificity of ribulose bisphosphate carboxylase/oxygenase. Nature 291, 513–515. [Google Scholar]
- Knaust, R., Urbig, T., Li, L., Taylor, W., and Hastings, J. (1998). The circadian rhythm of bioluminescence in Pyrocystis is not due to differences in the amount of luciferase: A comparative study of three bioluminescent marine dinoflagellates. J. Phycol. 34, 167–172. [Google Scholar]
- Liu, Y., Merrow, M., Loros, J., and Dunlap, J. (1998). How temperature changes reset a circadian oscillator. Science 281, 825–829. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus, J., and Addinall, S. (1997). Bacterial cell division and the Z ring. Annu. Rev. Biochem. 66, 93–116. [DOI] [PubMed] [Google Scholar]
- Markovic, P., Roenneberg, T., and Morse, D. (1996). Phased protein synthesis at several circadian times does not change protein levels in Gonyaulax. J. Biol. Rhythms 11, 57–67. [DOI] [PubMed] [Google Scholar]
- Matthys-Rochon, E. (1979). Evolution d'un dinoflagellé libre au cours d'un cycle cellulaire. Biol. Cell. 35, 313–320. [Google Scholar]
- McKay, R., and Gibbs, S. (1991). Composition and function of pyrenoids: Cytochemical and immunocytochemical approaches. Can. J. Bot. 69, 1040–1052. [Google Scholar]
- Morse, D., Milos, P.M., Roux, E., and Hastings, J.W. (1989. a). Circadian regulation of bioluminescence in Gonyaulax involves translational control. Proc. Natl. Acad. Sci. USA 86, 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse, D., Pappenheimer, A.M.J., and Hastings, J.W. (1989. b). Role of a luciferin-binding protein in the circadian bioluminescent reaction of Gonyaulax polyedra. J. Biol. Chem. 264, 11822–11826. [PubMed] [Google Scholar]
- Morse, D., Salois, P., Markovic, P., and Hastings, J.W. (1995). A nuclear encoded form II rubisco in dinoflagellates. Science 268, 1622–1624. [DOI] [PubMed] [Google Scholar]
- Narang, F., McIntosh, L., and Somerville, C. (1984). Nucleotide sequence of the ribulose bisphosphate carboxylase gene from Rhodospirillum rubrum. Mol. Gen. Genet. 193, 220–224. [Google Scholar]
- Nimmo, G., Nimmo, H., Fewson, C., and Wilkins, M. (1984). Diurnal changes in the properties of phosphoenolpyruvate carboxylase in Bryophyllum leaves: A possible covalent modification. FEBS Lett. 178, 199–203. [Google Scholar]
- Norris, B., and Miller, D. (1994). Nucleotide sequence of a cDNA clone encoding the precursor of the peridinin–chlorophyll a–binding protein from the dinoflagellate Symbiodinium. Plant Mol. Biol. 24, 673–677. [DOI] [PubMed] [Google Scholar]
- Osafune, T., Yokota, A., Sumida, S., and Hase, E. (1990). Immunogold localization of ribulose-1,5-bisphosphate carboxylase with reference to pyrenoid morphology in chloroplasts of synchronized Euglena gracilis cells. Plant Physiol. 92, 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung, K., Stokes, K., Rutherford, S., Percival, A., and Lee, W. (1998). Chloroplast division in higher plants requires members of two functionally divergent gene families with homology to bacterial ftsZ. Plant Cell 10, 1991–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, J., Carlson, T., and Williams, J. (1989). A cyanobacterial mutant requiring the expression of ribulose bisphosphate carboxylase from a photosynthetic anaerobe. Proc. Natl. Acad. Sci. USA 86, 5753–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezelin, B.B., Meeson, B.W., and Sweeney, B.M. (1977). Characterization of photosynthetic rhythms in marine dinoflagellates. I. Pigmentation, photosynthetic capacity and respiration. Plant Physiol. 60, 384–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud, F., Parisi, E., Capasso, A., and Prisco, P.D. (1983). On the pole of serotonin and 5-methoxy-tryptamine in the regulation of cell division in sea urchin eggs. Dev. Biol. 98, 37–46. [DOI] [PubMed] [Google Scholar]
- Renn, S., Park, J., Rosbash, M., Hall, J., and Taghert, P. (1999). A pdf neuropeptide gene mutation and ablation of pdf neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99, 791–802. [DOI] [PubMed] [Google Scholar]
- Rensing, L., Taylor, W.R., Dunlap, J., and Hastings, J.W. (1980). The effects of protein synthesis inhibitors on the Gonyaulax clock. II. The effect of cycloheximide on ultrastructural parameters. J. Comp. Physiol. 138, 9–18. [Google Scholar]
- Rowan, R., Whitney, S.M., Fowler, A., and Yellowlees, D. (1996). Rubisco in marine symbiotic dinoflagellates: Form II enzymes in eukaryotic oxygenic phototrophs encoded by a nuclear multigene family. Plant Cell 8, 539–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson, G., Sweeney, B.M., Matlick, H.A., and Prezelin, B.B. (1983). Changes in photosystem II account for the circadian rhythm in photosynthesis in Gonyaulax polyedra. Plant Physiol. 73, 329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter, R. (1971). The fine structure of Gonyaulax polyedra, a bioluminescent marine dinoflagellate. J. Cell Sci. 9, 147–173. [DOI] [PubMed] [Google Scholar]
- Sharples, F., Wrench, P., Ou, K., and Hiller, R. (1996). Two distinct forms of the peridinin–chlorophyll a–protein from Amphidinium carterae. Biochim. Biophys. Acta 1276, 117–123. [DOI] [PubMed] [Google Scholar]
- Shearman, L., Sriram, S., Weaver, D., Maywood, E., Chaves, I., Zheng, B., Kume, K., Lee, C., van der Horst, G., Hastings, M., and Reppert, S. (2000). Interacting molecular loops in the mammalian circadian clock. Science 288, 1013–1019. [DOI] [PubMed] [Google Scholar]
- Sweeney, B.M. (1960). The photosynthetic rhythm in single cells of Gonyaulax polyedra. Cold Spring Harbor Symp. Quant. Biol. 25, 145–148. [DOI] [PubMed] [Google Scholar]
- Whitney, S., and Andrews, T. (1998). The CO2/O2 specificity of single-subunit ribulose-bisphosphate carboxylase from the dinoflagellate Amphidinium carterae. Aust. J. Plant Physiol. 25, 131–138. [Google Scholar]
- Whitney, S., and Yellowlees, D. (1995). Preliminary investigations into the structure and activity of ribulose bisphosphate carboxylase from two photosynthetic dinoflagellates. J. Phycol. 31, 138–146. [Google Scholar]
- Widder, E., and Case, J. (1982). Distribution of subcellular bioluminescence sources in a dinoflagellate Pyrocystis fusiformis. Biol. Bull. 162, 423–428. [Google Scholar]