Abstract
The rice slender mutant (slr1-1) is caused by a single recessive mutation and results in a constitutive gibberellin (GA) response phenotype. The mutant elongates as if saturated with GAs. In this mutant, (1) elongation was unaffected by an inhibitor of GA biosynthesis, (2) GA-inducible α-amylase was produced by the aleurone layers without gibberellic acid application, and (3) endogenous GA content was lower than in the wild-type plant. These results indicate that the product of the SLR1 gene is an intermediate of the GA signal transduction pathway. SLR1 maps to OsGAI in rice and has significant homology with height-regulating genes, such as RHT-1Da in wheat, D8 in maize, and GAI and RGA in Arabidopsis. The GAI gene family is likely to encode transcriptional factors belonging to the GRAS gene superfamily. DNA sequence analysis revealed that the slr1-1 mutation is a single basepair deletion of the nuclear localization signal domain, resulting in a frameshift mutation that abolishes protein production. Furthermore, introduction of a 6-kb genomic DNA fragment containing the wild-type SLR1 gene into the slr1-1 mutant restored GA sensitivity to normal. These results indicate that the slr1-1 mutant is caused by a loss-of-function mutation of the SLR1 gene, which is an ortholog of GAI, RGA, RHT, and D8. We also succeeded in producing GA-insensitive dwarf rice by transforming wild-type rice with a modified SLR1 gene construct that has a 17–amino acid deletion affecting the DELLA region. Thus, we demonstrate opposite GA response phenotypes depending on the type of mutations in SLR1.
INTRODUCTION
Gibberellins (GAs) have an important role in the regulation of many physiologic processes in the growth and development of plants, such as seed germination, shoot/stem elongation, and flower development. Changes in both GA concentration and tissue sensitivity to GA influence these processes. The molecular mechanisms by which the GA signal is transduced into morphologic and biochemical changes in plants, however, are largely unknown.
Many studies on the detection of and response to GA are focused on identifying the mutants that affect these processes. GA response mutants isolated from various plant species fall into two phenotypic categories: elongated slender-type mutants and GA-unresponsive dwarf mutants (Hooley, 1994; Swain and Olszewski, 1996). The slender mutants have constitutive activation of their GA response, and the dwarf mutants are deficient in GA detection or signal transduction. The slender mutant of barley, which is homozygous for the recessive sln1 alleles, is characterized by a rapid growth rate and long leaf sheaths (Foster, 1977). slender barley does not respond to growth retardants such as ancymidol (Lanahan and Ho, 1988) and paclobutrazol (Croker et al., 1990). During germination, aleurone layers of slender barley synthesize α-amylase in the absence of exogenous GA (Lanahan and Ho, 1988). The mutant contains a smaller amount of active GAs compared with wild-type plants, indicating that GAs are not involved in the growth of slender barley. Thus, the slender mutation might be important for elucidating the GA signal transduction pathway.
In rice, many dwarf mutants have been characterized and classified as dwarf and GA deficient, dwarf and GA insensitive, or dwarf due to other reasons (Mitsunaga et al., 1994). A slender-type mutant has not been reported in rice, however, except for the awaodori (ao-1) mutant (Nakamura, 1992).
Several GA signaling intermediates have been identified by mutation analyses in a number of plant species (Taylor, 1998), and some corresponding genes were recently cloned. In Arabidopsis, SPINDLY (SPY) acts as a negative regulator of the GA response. The deduced amino acid sequence of SPY suggests that the protein is an O-linked N-acetylglucosamine transferase that might glycosylate other molecules involved in GA signaling (Thornton et al., 1999). A second GA signaling intermediate from Arabidopsis is encoded by the GA-INSENSITIVE (GAI) gene, which has also been cloned (Peng et al., 1997). The original gai allele causes dwarfism and behaves genetically as a gain-of-function mutation (Peng and Harberd, 1993). Recent molecular analyses confirmed that this allele encodes a constitutively active mutant protein that has apparently lost its ability to respond to GA (Peng et al., 1997). Additionally, extragenic suppressors of GA mutants, defined by rga mutants, have been identified (Silverstone et al., 1997). RGA encodes a protein similar to that of the GAI gene (Silverstone et al., 1998). The gai mutant allele that results in the GA-insensitive dwarf mutation contains a 51-bp in-frame deletion of 17 amino acids in the DELLA domain (Peng et al., 1997). Peng et al. (1999) examined GA-insensitive dwarf mutants from other species and demonstrated that the mutations of reduced height (RHT)–B1 and RHT-D1 in wheat and D8 in maize are caused by an N-terminal truncation near the DELLA domain. These results indicate that the DELLA domain is important to GA detection in the GA signal transduction pathway.
In wheat, some dwarf plants differ from tall plants in their lack of GA response. These dwarf mutants have a lodging-resistant characteristic and have resulted in increased wheat grain yields around the world since the 1960s. These so-called “green revolution” dwarf cultivars, which are derived from the Japanese variety Norin 10, have a phenotype with a reduced response to GA that is caused by mutations in the (Rht-B1 and Rht-D1) gene, which is the ortholog of GAI (Peng et al., 1999).
In this study, we isolated and characterized the slender-type mutant in rice (slr1-1). Biochemical analysis revealed that the mutation results in a constitutive GA response. Consequently, we demonstrated that the slr1-1 mutant contains a loss-of-function mutation in the SLR1 gene, which is an ortholog of GAI and RGA in Arabidopsis, RHT in wheat, and D8 in maize.
RESULTS
Isolation of slender Rice
A mutant with greatly accelerated extension growth was isolated from the rice cv Nipponbare after treatment with γ irradiation. The phenotype of the mutant, designated slender (slr1-1), has very rapid extension growth in the seedling and is sterile. M2 progeny tests of heterozygotes yielded a segregation of 311 normal and 89 slender plants (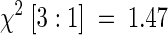 ; 0.1 < P < 0.25), indicating monofactorial recessive inheritance of the slender characteristic.
; 0.1 < P < 0.25), indicating monofactorial recessive inheritance of the slender characteristic.
slender Rice Behaves as if It Were Continually Saturated with GAs
One of the best known actions of GA is the stimulation of shoot growth in rice (Murakami, 1968; Matsukura et al., 1998). Germinating seeds of progeny from the heterozygote segregated a shoot length phenotype (Figure 1A). Shoots of the slender phenotype were more than twofold longer than those of the wild-type plant and were similar to those of wild-type plants treated with gibberellic acid (GA3; Figure 1B). Basal internodes in slender rice elongate concurrently during seedling growth, which is usually observed in the wild-type plant treated with GA3 (Figure 1C). The mutant also had a reduced number and root length compared with the wild-type plant (Figure 1D). Matsukura et al. (1998) reported that the promotion of leaf sheath growth by GA3 is due mainly to cell elongation. The average longitudinal and transverse lengths of the epidermal cells at the apical portion of the second leaf sheath were approximately twofold and 0.5-fold, respectively, the lengths of epidermal cells in the wild type (Table 1). The overall results indicate that slender rice behaves as if it were continually saturated with GAs.
Figure 1.
Phenotype of slender Rice and Its Original Wild Type, Nipponbare.
(A) slender rice was isolated as a tall mutant from rice cv Nipponbare. The plants homozygous for the recessive slender (slr1-1) gene segregated at a 1:3 ratio in 2-week-old plants.
(B) Shoot elongation of wild type treated with (middle) or without (left) 10 μM GA3 and slender without GA3 treatment (right). The photograph was taken 1 week after germination.
(C) An elongated internode of a 2-week-old slender plant. The arrowhead indicates the third internode.
(D) Root morphology of 1-week-old plants: wild type (left), slender (middle), and wild type treated with 10 μM GA3 (right).
Table 1.
Characteristics of Epidermal Cells in the Second Leaf Sheath of slender Rice and its Wild Typea
| Position | Plant | Cell Length (μm) | Cell Width (μm) | Length/Width |
|---|---|---|---|---|
| Basal | Wild type | 69.3 ± 11.8 | 19.7 ± 1.9 | 3.5 |
| slender | 88.2 ± 5.8 | 15.8 ± 1.3 | 5.7 | |
| Apical | Wild type | 55.9 ± 11.1 | 17.5 ± 2.4 | 3.2 |
| slender | 107.9 ± 10.3 | 9.2 ± 0.7 | 11.7 |
The second leaf sheath was dissected from 1-week-old slender and wild-type plants and stained with Safranin. Values are means ±se (n = 20).
The anatomic characteristics of slender rice are similar to those previously reported for the ao-1 mutant in rice (Nakamura, 1992) and the slender mutant in barley (Favret and Malvarez, 1975; Foster, 1977).
slender and wild-type plants were grown on agar plates with or without uniconazole, a GA biosynthesis inhibitor (Izumi et al., 1984; Mitsunaga and Yamaguchi, 1993), for up to 15 days. The height of wild-type plants was decreased significantly with uniconazole throughout the experimental period, but the height of slender plants was unaffected (Figure 2). This observation suggests that the elongation of slender is independent of endogenous GA levels.
Figure 2.
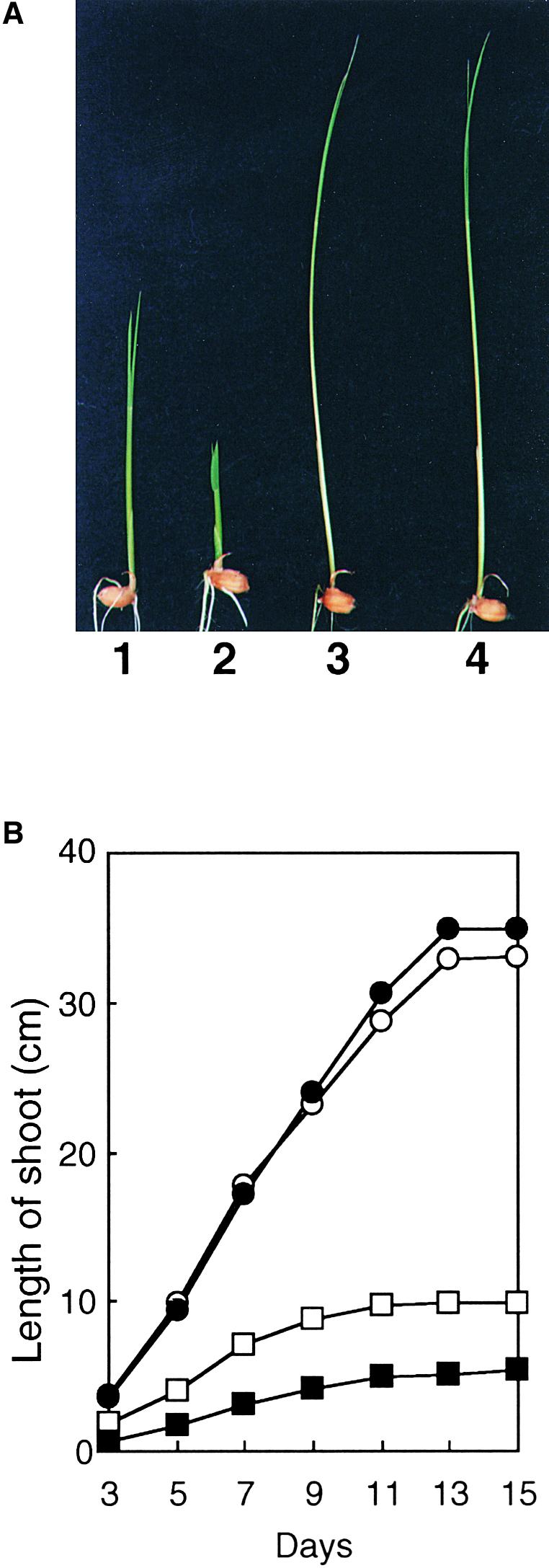
Effect of Uniconazole on Shoot Elongation in slender Rice and Its Wild Type.
(A) Seed of wild-type (1 and 2) and slender rice (3 and 4) were germinated for 6 days with (2 and 4) or without (1 and 3) 6.9 μM uniconazole.
(B) Shoots of wild-type and slender rice were measured from 3 to 15 days after germination. Results from wild-type (open squares) and slender rice (open circles) without uniconazole and wild-type (closed squares) and slender rice (closed circles) with 6.9 μM uniconazole are shown. Data are means (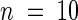 ). Variation in width was <10% of the reported data.
). Variation in width was <10% of the reported data.
slender Mutation Results in Modulation of Endogenous GAs and α-Amylase Production
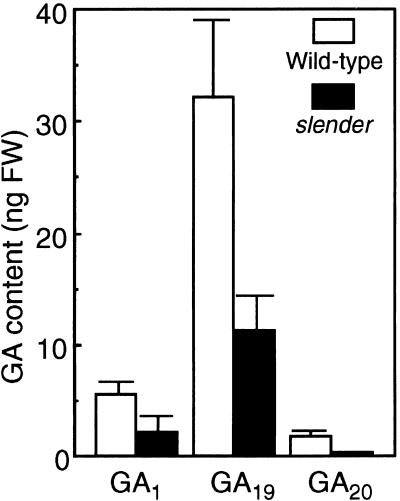
Endogenous levels of GAs were estimated using a combination of HPLC purification and gas chromatography–mass spectrometry analysis (Figure 3). The level of GA1, the active GA molecule in vegetative tissues of rice, was lower in the shoots of slender rice than in the wild type. Similarly, the levels of GA19 and GA20, both of which are inactive precursors of the GA1 molecule, were lower in slender than in the wild type (Figure 3), indicating that the mutation decreases the endogenous GA level in the shoot.
Figure 3.
GA Content of Shoots in Wild-Type and slender Rice.
Ten-day-old shoots were measured for GA content by gas chromatography–mass spectrometry analysis. Ten-day-old shoots were harvested from wild-type and slender rice and the GA content was determined. Samples were performed on 10 independent plants. FW, fresh weight. Bars indicate ±se.
For further characterization of slender rice, agar plate assays for amylases were conducted using the progeny seeds from selfing a plant heterozygous for the slender allele. The embryoless half-seeds were placed on the starch plate with or without 1 μM GA3 for 2 days, and the starch was stained with iodine (Figure 4A). Production and secretion of α-amylase from wild-type embryoless half-seeds were observed as cleared zones (plaques) only on the plate containing GA3 (Figure 4A, +GA3). Some slender half-seeds, however, produced amylase even in the absence of exogenous GA3 (Figure 4A, −GA3). The ratio of the seeds requiring GA3 for amylase secretion to seeds requiring no GA3 was less than 3:1 (30:7 actual values). This suggests that the M2 progeny were segregated into slender and normal characteristics for α-amylase production, because the aleurone layer consists of triploid tissues. These results demonstrate that exogenous GA3 is not necessary for the synthesis and secretion of amylases from the embryoless half-seeds of slender rice.
Figure 4.
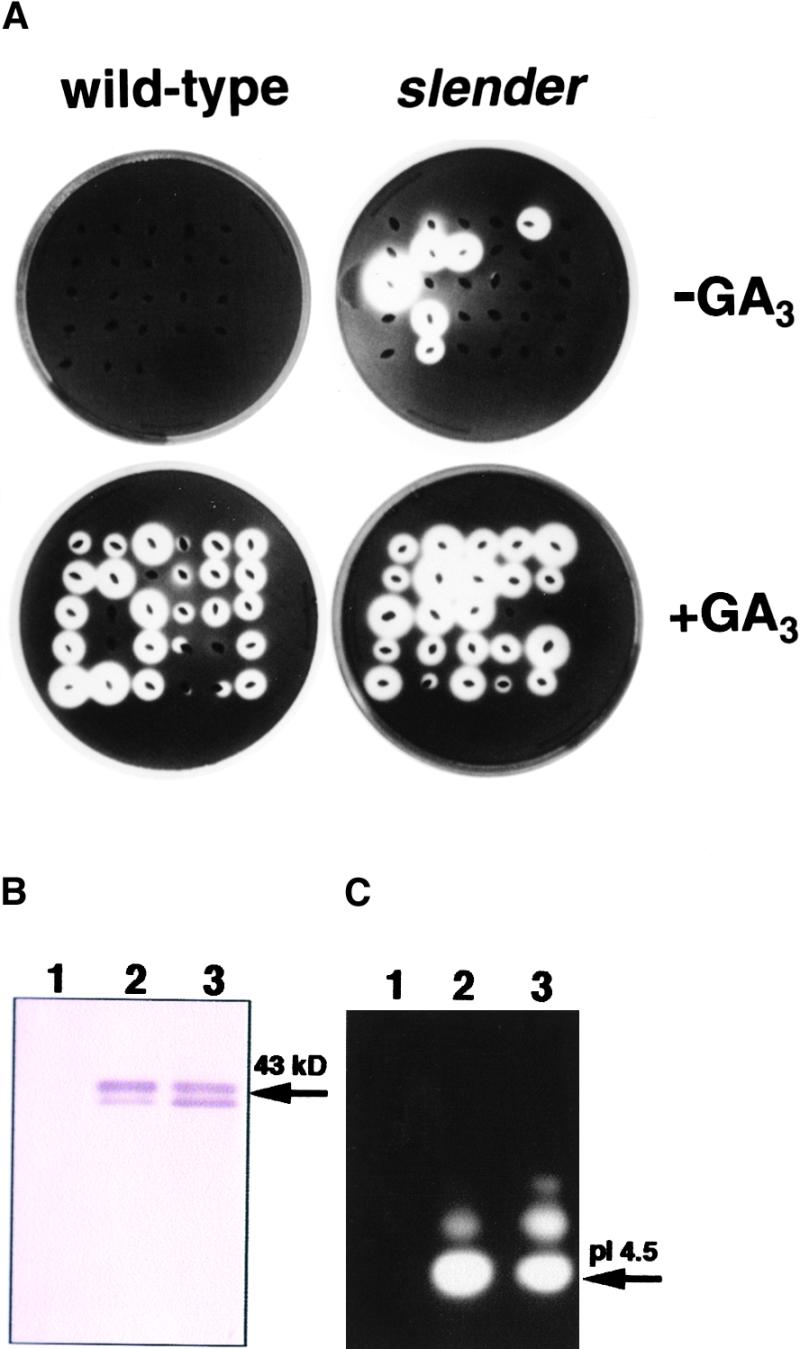
α-Amylase Production from Embryoless Half-Seeds of Wild-Type and slender Rice.
(A) Embryoless half-seeds of wild-type and slender rice were placed on starch plates containing 1 μM GA3 (+GA3) or no GA3 (−GA3) for 2 days, and starch was detected by staining with iodine.
(B) and (C) Immunoblot and zymogram pattern of α-amylase expressed in embryoless half-seeds of wild-type and slender rice. (B) shows immunochemical detection of α-amylase protein using α-amylase antibody. (C) shows zymogram pattern of α-amylase activity. Lanes 1, wild type without GA3 application; lanes 2, wild type with GA3 application; lanes 3, slender rice without GA3 application. Embryoless half-seeds of slender rice secreted isoform A (molecular mass ∼43 kD; pI 4.5), which is a GA3-inducible α-amylase in rice (Yamaguchi, 1998).
We confirmed that the secreted amylase was GA-inducible α-amylase (Figures 4B and 4C). Immunoblotting using antisera against rice α-amylase (isoform A) revealed that the amylases secreted from the embryoless half-seeds of the slender mutant without GA3 application are identical to those of wild-type seeds with GA3 application (Figure 4B, lanes 2 and 3). Isoform analysis using an isoelectric focusing gel revealed that the main amylase is an isoform A, the gene product of RAmy1A (Figure 4C; Mitsui et al., 1996; Yamaguchi, 1998). The transcript of the rice α-amylase gene RAmy1A is abundant in aleurone layer cells during germination and it is promoted by GA3 (Itoh et al., 1995; Sugimoto et al., 1998). These results indicate that α-amylase is produced independently of GAs from the aleurone layer cells of slender rice.
These findings (GA-mediated phenomena, shoot growth, and α-amylase production) suggest that slender rice (slr1-1) is a constitutive GA response mutant. A similar conclusion was reported for the barley slender mutant (Chandler, 1988; Lanahan and Ho, 1988). Based on these results, it is likely that the SLR1 gene is an intermediate of the GA signaling pathway.
Molecular Cloning of the SLR1 Gene
Nipponbare (a Japonica rice) containing slr1-1 was crossed to Kasalath (an Indica rice), and segregation of slr1-1 and OsGAI (Ogawa et al., 2000) was examined to determine if SLR1 and GAI are linked. GAI in Arabidopsis is an intermediate of the GA signaling pathway. Direct sequencing was performed to determine the difference of the GAI homolog between Nipponbare and Kasalath. Base differences at position 1025 from the translation initiation site and their phenotypes were as follows: A, Nipponbare with a slender phenotype; G, Kasalath with a normal phenotype; and a mixture of A and G in the heterozygous plant having a normal phenotype. F2 progeny tests of the heterozygotes yielded a segregation of 49 normal and 16 slender plants, and genotypes based on the base change indicated 16 Nipponbare homozygous (Japonica), 33 heterozygous (Japonica plus Indica), and 16 Kasalath homozygous (Indica) plants. These results indicate tight linkage between the slender mutation (slr1-1) and OsGAI.
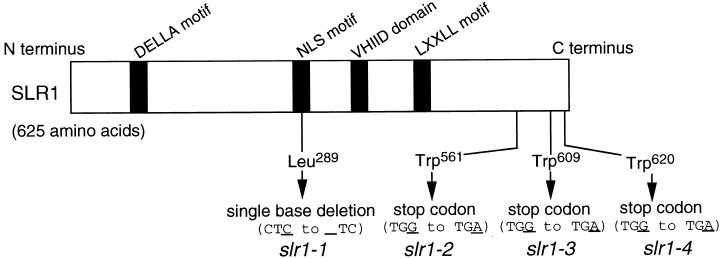
We determined the open reading frame (ORF) sequence of the rice GAI homolog (data not shown). This sequence was identical to that of OsGAI reported previously (Ogawa et al., 2000). We propose that OsGAI be renamed SLR1 after its mutant phenotype and so use the term SLR1 in place of OsGAI throughout this article. The deduced SLR1 protein shares a high overall identity with RHT-D1a in wheat (77.2%), D8 in maize (80.3%), and RGA and GAI in Arabidopsis (41.2 and 47.2%, respectively). This protein contains the DELLA motif (see Figure 5), which is believed to be a GA response domain, a putative nuclear localization signal (NLS motif), the VHIID domain, and the LXXLL motif.
Figure 5.
The Mutation Positions of the slr1 Alleles.
Scheme of the deduced amino acid sequence for SLR1. Arrows indicate mutation of the slr1 alleles in the position of the SLR1 ORF.
Molecular Analysis of the slender Mutant Alleles and Mapping of the SLR1 Gene
Three tall rice mutants (slr1-2, slr1-3, and slr1-4) were determined to be alleles of slr1-1. To identify the molecular basis of these mutations, the DNA sequences of the four mutant alleles were determined and compared with those of the SLR1 gene. The slr1-1 allele contained one base deletion at Leu289, a putative NLS region, that alters the N-terminal region of the protein that it encodes (Figure 5). The other three alleles (slr1-2, slr1-3, and slr1-4) contained premature stop codons (Figure 5). These results indicate that the SLR1 gene is associated with the slender phenotype.
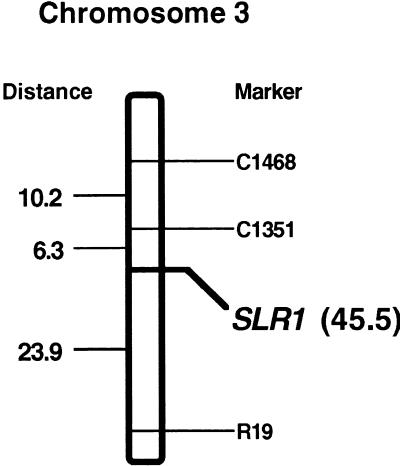
We mapped the SLR1 gene on the rice genome using restriction fragment length polymorphism analysis. Linkage analysis was performed with digested genomic DNA from recombinant inbred lines of crosses between Asominori and IR24. Consequently, chromosome 3 contained a single SLR1 gene (Figure 6). From the partial linkage maps of wheat and rice chromosomes, the rice GAI homolog was predicted to be on chromosome 3 (Peng et al., 1999). This result is consistent with the SRL1 gene being a GAI homolog.
Figure 6.
Mapping of the SLR1 Gene on Rice Chromosome 3.
The map position of the SLR1 gene is shown relative to its physical flanking markers C1351 and R19. The numbers at left are the distance of the markers (in centimorgans), and the number in parentheses denotes the position of the SLR1 gene according to the 71 recombinant inbred lines from a cross between rice cultivars Asominori (a Japonica rice) and IR24 (an Indica rice).
Molecular Complementation of the slender Mutant by the Wild-Type SLR1 Gene
To confirm that the SLR1 gene corresponds to the slender mutation, we performed complementation analysis of the slender mutant using the wild-type SLR1 gene. Transformation of the slender mutant with a control vector that carried no rice genomic DNA had no apparent effect on the phenotype (data not shown). When a 6-kb DNA fragment containing the entire wild-type SLR1 gene was introduced, however, the normal phenotype was restored in most plants that were resistant to hygromycin (Figure 7, NT). GA3 and uniconazole were applied to the transformants to determine if normal sensitivity to the GA response was restored. The application of GA3 increased and uniconazole decreased plant height (Figure 7, +GA3 and +Unico), indicating that the introduction of the wild-type SRL1 gene complemented the slender mutation. Based on these results, we concluded that the slender mutant is caused by a loss-of-function mutation of the SLR1 gene, which is identical to the rice GAI homolog.
Figure 7.

Complementation Analysis of the Slender Phenotype.
A 6-kb wild-type genomic DNA fragment containing the entire SLR1 gene was used for transformation of the slender mutant (slr1-1). The transgenic plant had a normal phenotype without hormone treatment (NT). Application of 10 μM GA3 (+GA3) led to increased height, and treatment with 6.9 μM uniconazole (+Unico) led to reduced height.
Truncation of DELLA, a Putative GA Response Motif in the SLR1 Gene, Leads to the Dwarf Phenotype
In Arabidopsis, the GA-insensitive dwarf mutation allele gai contains a 51-bp deletion of the GAI ORF. This in-frame deletion results in the absence of 17 amino acid residues in the DELLA motif of the GAI protein. We attempted to produce GA-insensitive dwarf rice using the truncated SLR1 gene construct pSLRtr, which also contains a 17–amino acid deletion (39-DELLAALGYKVRSSDMA-55) in the DELLA motif (Figure 8A). When the pSLRtr construct was introduced into the normal Japonica rice (cv Nipponbare) to produce ∼200 plants, >90% of transgenic rice resulted in a typical dwarf plant (Figure 8B, pSLRtr). The transgenic plant had retarded growth in the leaf sheath and blade and thickening in the roots, which are typical responses for a GA-deficient plant. The dwarf transgenic plant had no response, however, to exogenously applied GA3. Introduction of the truncated SLR1 gene led to a wide range of plant heights, from severe, extremely dwarf phenotypes to mild phenotypes (Figure 8C).
Figure 8.

Truncation of the DELLA Motif in SLR1 Leads to a Dwarf Phenotype.
(A) The construct pSLRtr was used for the transformation of rice plants. The Actin1 promoter (pAct1) was recovered from vector pBI101-Hm3. Transformation with the SLRtr sequence resulted in the DELLA motif truncation form (39-DELLAALGYKVRSSDMA-55) of the SLR1 ORF. nos, nopaline synthase.
(B) Construct pSLRtr (A) was introduced into the normal Japonica rice Nipponbare (Control). Introduction of the truncated SLR1 gene resulted in the typical dwarf plant (pSLRtr). The transgenic plant shows retarded growth in the leaf sheath and blade.
(C) Introduction of the truncated SLR1 gene resulted in a range of plant heights. Construct pSLRtr (A) was introduced into the wild-type cv Nipponbare (Control). The pSLRtr transgenic plants show a wide range of plant heights, from weak to severe dwarf phenotypes.
DISCUSSION
slender (slr1-1) Is a Constitutive GA Response Mutant in Rice
In this study, slender rice, which is caused by a single recessive mutation, was considered to be a constitutive GA response phenotype for the following reasons. The mutant shoot was more than twofold taller than the wild type, similar to wild-type shoots treated with high amounts of GA3. The height of slender was unaffected by the application of uniconazole, an inhibitor of GA biosynthesis, and endogenous levels of GAs were lower in slender than in the wild type. Finally, GA-inducible α-amylase, the RAmy1A protein, was produced without GA3 application.
There are similar recessive slender-type mutants in barley (slender [sln1]; Chandler, 1988; Lanahan and Ho, 1988) and in pea (la crys; Potts et al., 1985). These mutants behave as if saturated with GAs and are not responsive to either exogenous GAs or GA biosynthesis inhibitors. On the other hand, the SPY mutant in Arabidopsis (Jacobsen and Olszewski, 1993) and the pro mutant in tomato (Jones, 1987) respond to applied GAs. These two types of mutants might be due to the mutation of genes that have different functions or that encode different intermediates in the GA signal transduction pathway.
Embryoless half-seeds of slender produced GA-inducible α-amylase without GA3, indicating that the SLR1 protein might be a negative regulator that blocks transcription of the α-amylase gene (see below). The induction of α-amylase by GAs in cereal grains represents a classic model system for studying the mode of action of GAs (Jones and Jacobsen, 1991). α-Amylase induction by GA occurs mainly at the transcriptional steps, which might be mediated through abscisic acid, Ca2+ (Schuurink et al., 1996), cyclic GMP (Penson et al., 1996), or sugar signaling (Perata et al., 1997), and signals communication among them (Toyofuku et al., 2000). GA-dependent transcriptional activation of the α-amylase gene requires the GA response cis-element complex and the GAmyb protein as a trans-acting factor (Gubler et al., 1995). Further study is needed to clarify the targeting site of the SLR1 protein through the GA signaling pathway.
The SLR1 Gene Is an Ortholog of the Height-Regulating Gene GAI/RGA/RHT/D8
We succeeded in the molecular cloning of the SLENDER gene (SLR1), which was originally derived from the rice GAI homolog. Molecular analysis of slender mutant alleles revealed that four mutant alleles contain nonsense or stop codon mutations in the SLR1 gene. Introduction of a 6-kb genomic DNA fragment for the wild-type SLR1 gene into the slr1-1 mutant restored the normal phenotype and sensitivity to the GA response.
GAI, SLR1, D8, RHT-1Da, and RGA seem to be transcriptional factors belonging to the GRAS gene superfamily, which has a putative NLS, an Ser/Thr-rich repeat region, and the LXXLL motif. Indeed, in a transient assay using onion epidermal cells, RGA–GFP (green fluorescent protein) and OsGAI–GFP fusion proteins were localized exclusively in the nucleus (Silverstone et al., 1998; Ogawa et al., 2000). Transactivation assays indicate that the OsGAI protein has a transcriptional activator or coactivator (Ogawa et al., 2000).
Sequence comparison among members of the GAI family identified a putative Src homology 2 (SH2) phosphotyrosine binding domain (Peng et al., 1999). The SH2 domain is present in a family of transcription factors called STATs (signal transducers and activators of transcription) in animals (Darnell, 1997). The function of this domain is to mediate the binding of STATs to various receptor tyrosine kinases. The STATs are then activated by the receptor kinase and translocate from the cytoplasm to the nucleus. If this putative SH2 domain is functional in plants, then tyrosine phosphorylation might have a role in GA signaling (Silverstone and Sun, 2000).
The slr1-4 mutation was detected at the extreme C-terminal end (Trp620) of the SLR1 protein (deduced ORF of 625 amino acids). This mutant did not have any phenotypic differences from the other alleles. These results indicate that even the loss of six amino acid residues at the C-terminal end, which are not a conserved motif among family members, abolishes the protein function in spite of the presence of consensus motifs NLS, LXXLL, and SH2.
SLR1 Is a Negative Regulator Involved in the GA Signaling Pathway
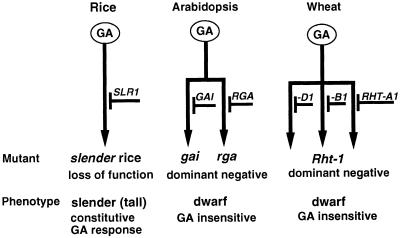
The slr1-1, slr1-2, and slr1-3 mutations truncate the protein, suggesting that these mutations are loss-of-function mutations. Therefore, SLR1 protein must be a negative regulator of the GA signaling process, because lack of its negative regulation results in a constitutive GA response phenotype (Figure 9).
Figure 9.
Model of the GA Signal Transduction Pathway in Rice, Arabidopsis, and Wheat.
Arrows indicate the GA signal transduction pathway from GA reception (GA) to various actions of GA, such as shoot elongation, α-amylase induction, and so on. SLR1 in rice, GAI and RGA in Arabidopsis, and RHT-A1, -B1, and -D1 proteins in wheat negatively regulate the GA signaling pathway.
Consistent with the similarity of the phenotypes of Rht-B1b, Rht-D1b, and D8 to the semidominant gai mutant, the genetic lesions in these mutants are also similar to those in gai (Peng et al., 1999). The lesions in three D8 mutant alleles have been identified. Both D8-1 and D8-2023 contain small internal deletions in the DELLA domain. The D8-Mpl allele has a 330-bp deletion at the N terminus that begins in the 59–untranslated amino acid region and extends through Val84. Because of the gain-of-function (semidominant) nature of this mutant, it is proposed that it still encodes a truncated D8 protein that might be initiated at Met106 (Peng et al., 1999). Rht-B1b and Rht-D1b both contain a base substitution that introduces a stop codon in the DELLA domain. Because there is a methionine a short distance after these stop codons, it is possible that translational reinitiation occurs to synthesize a protein lacking the original N terminus (Peng et al., 1999). These results support the hypothesis that the DELLA domain in the N terminus of the GAI protein family is responsible for modulating the activity of these proteins in response to the GA signal. Presumably, the effect of either the internal deletions or the N-terminal truncations is to lock the protein into a conformation that can no longer, or can only weakly, respond to the GA signal (Silverstone and Sun, 2000).
The Distinct Manner of Mutation on the GAI Ortholog Leads to Opposite Phenotypes in Terms of GA Sensitivity
In Arabidopsis, two orthologous proteins, GAI and RGA, have a redundant GA response; therefore, even if one of the proteins is inactive, the plant still has a normal phenotype (Figure 9). In this case, a dominant negative mutation such as the DELLA truncation is the only way to produce an abnormal phenotype, such as the GA-insensitive dwarf. There is a similar redundancy in wheat, with its gene duplication, because wheat possesses three sets of chromosomes (A, B, and D genomes).
In rice, the slender mutant results in a loss-of-function mutation of the SLR1 gene (Figure 9). Because the SLR1 gene is likely to be nonredundant, the loss-of-function mutation results in clearly abnormal phenotypes in terms of the GA response, that is, the slender and constitutive GA response phenotype. We demonstrated that the distinct manner of mutation on the orthologous genes leads to opposite phenotypes in terms of the GA response: slender, a constitutive GA response, and a GA-insensitive dwarf.
Dwarfism in Rice and the Alternative Green Revolution
World wheat grain yields increased substantially in the 1960s and 1970s because farmers rapidly adopted the new varieties and cultivation methods of the so-called green revolution. The new varieties are shorter (dwarf or semidwarf) and increase grain yield by means of lodging resistance. These wheat cultivars are short because they contain the DELLA-truncated RHT gene (Peng et al., 1999).
With rice, a similar green revolution was realized by the introduction of the distinct gene sd-1 from GAI/RHT. IR8 was established by crossing Dee-geo-woo-gen (sd-1) from a Chinese cultivar to Peta from an Indonesian cultivar at the International Rice Research Institute (Los Baños, Philippines; Hargrove et al., 1980). In Japan, the semidwarf cultivar Reimei (d-49; allelic to sd-1), which was derived from cv Fujiminori through γ irradiation, was released in 1966 (Futsuhara et al., 1967). Reimei was grown over wide areas of the northern part of Japan. Furthermore, it has been used as a parent strain for cross-breeding, taking advantage of its lodging resistance and high yields. We demonstrated that the introduction of the truncated SLR1 gene results in a range of plant heights (Figure 8C). DNA gel blot analysis revealed that these plants possess a single gene introduction and that the diversity in height is due mainly to the positional effect of the transgene insertion (data not shown), suggesting the potential for a plant height–regulating system. Because the SLR1 gene is distinct from sd-1 (the rice green revolution gene), it presents an alternative means to produce dwarf rice plants. Further investigation is needed to evaluate this hypothesis.
METHODS
Isolation of slender Rice
A recessive mutant, slender1-1, was isolated from the progeny of rice seeds (Oryza sativa cv Nipponbare, a Japonica rice) irradiated with 200 Gy of γ-rays (dose rate 2 Gy/min). Surface-sterilized seeds of wild-type and mutant plants were soaked in water for 3 days and then placed in artificial soil for 11 days and grown in a greenhouse. Mutant segregants were distinguished from normal segregants by the extremely extended phenotype. The other slender alleles (slr1-2, 1-3, and 1-4) were independently isolated.
Measurement of Shoot Elongation
Shoot elongation was quantified by a modification of the method described by Matsukura et al. (1998). Seeds of wild-type and mutant plants (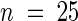 ) were surface sterilized for 30 min with a 3% NaClO solution, washed three times with sterile distilled water, soaked in the distilled water for 24 hr in the presence or absence of 6.9 μM uniconazole, and then placed in sterile distilled water for an additional 24 hr. The seeds were then placed on a 1% agar plate and grown under fluorescent lamps at 30°C. For the surface anatomy, the second leaf sheath was dissected from third leaf–stage plants and stained with Safranin (Wako Chemical, Osaka, Japan).
) were surface sterilized for 30 min with a 3% NaClO solution, washed three times with sterile distilled water, soaked in the distilled water for 24 hr in the presence or absence of 6.9 μM uniconazole, and then placed in sterile distilled water for an additional 24 hr. The seeds were then placed on a 1% agar plate and grown under fluorescent lamps at 30°C. For the surface anatomy, the second leaf sheath was dissected from third leaf–stage plants and stained with Safranin (Wako Chemical, Osaka, Japan).
Agar Plate Assay, Immunoblotting, and Zymography of α-Amylase
The agar plate assay was performed essentially as described by Lanahan and Ho (1988). Seeds were cut transversely, and the half-seed containing the embryos were planted to determine their phenotypes. The embryoless half-seeds were surface sterilized with 3% NaClO for 15 min and washed six times with sterile water. These half-seeds were then placed on 2% agar plates containing 10 mM sodium acetate and 2 mM CaCl2 at pH 5.3. GA plates were made by adding 1 μM gibberellic acid (GA3) to the cooled agar. To detect secreted α-amylase activity, we added soluble potato starch (0.2%) to the agar before autoclaving. Agar plates were developed by incubating the plates in I2 gas. Half-seeds that synthesized and secreted α-amylase had transparent halos around them resulting from the digestion of the starch by amylases.
Proteins to be examined were separated using SDS-PAGE or isoelectric focusing, transferred to a nitrocellulose membrane using the Novablot Protein Transfer Kit, and analyzed using the anti-α-amylase antibody (Mitsunaga and Yamaguchi, 1993). An alkaline phosphatase–labeled secondary antibody was used to detect the immunoreactive band.
The crude extract was examined by isoelectric focusing using Pharmacia broad range (pH 3.5 to 9.5) Ampholine Pageplates. Isoelectric focusing was performed for 1.5 hr according to the manufacturer's instructions. Samples (15 μL of crude extract) were applied to application paper placed 3 cm distant from the cathode. The application paper was removed after 45 min of electrophoresis, and the run was continued for an additional 45 min. To visualize the bands of amylolytic activities, the gel was incubated for 1 hr in 50 mM sodium acetate buffer, pH 5.2, containing 10 mM CaCl2 and 1% boiled soluble starch. After washing with distilled water, the gel was stained with 0.6% I2 and 6% KI solution (Perata et al., 1992).
Quantitative Analyses of Endogenous GAs
GAs from each sample were extracted and purified according to the method of Hisamatsu et al. (1998). As internal standards, 70 ng of 17,17,2H2-GA1, 100 ng of 17,17,2H2-GA19, and 50 ng of 17,17,2H2-GA20 were added to each sample. After several HPLC purification steps, fractions that had GA activity on a rice assay (Nishijima et al., 1992) were methylated and trimethylsilylated in glass tubes. The derived samples were analyzed using gas chromatography–mass spectrometry. The concentrations of GA1, GA19, and GA20 in the tubes were calculated from the ratios of peak areas at mass-to-charge ratios of 506:508, 434:436, and 418:420, respectively, according to the method of Gaskin and MacMillan (1991).
Screening of the SLR1 Gene from Rice Genomic Libraries
To isolate the SLR1 gene, we used the rice GAI homologous expressed sequence tag clone (accession number D39460). Genomic DNA libraries were constructed from rice. Nuclear genomic DNA was isolated from 2-week-old seedlings. The DNA was partially digested with Sau3AI and enriched for fragments of ∼20 kb on a sucrose gradient. The fragments were cloned into the BamHI site of EMBL3 (Stratagene, La Jolla, CA).
Screening by hybridization was performed in 50% formamide, 6 × SSC (1 × SSC is 0.15 M NaCl and 0.015 M sodium citrate), 5 × Denhardt's solution (1 × Denhardt's solution is 0.02% Ficoll, 0.02% polyvinylpyrrolidone, and 0.02% BSA), 0.5% SDS, and 0.1 mg/mL salmon sperm DNA at 42°C for 14 hr using the GAI homologous expressed sequence tag clone as a probe.
Mapping of the SLR1 Gene in Rice Recombinant Inbred Lines
To map the SLR1 gene, 71 recombinant inbred lines from a cross between two rice cultivars, Asominori (a Japonica rice) and IR24 (an Indica rice), were used. Restriction fragment length polymorphism analysis was performed with a probe specific for the rice GAI homologous expressed sequence tag clone. DNA gel blot hybridization was performed as described by Church and Gilbert (1984) except that membranes were hybridized at high stringency (68°C). The linkage analysis was performed using the MAPMAKER program (Whitehead Institute for Biomedical Research/Massachusetts Institute of Technology Center for Genome Research, Wilmington, MA).
Genomic DNA Isolation and Direct Genome Sequencing
Rice genomic DNA was isolated with the ISOPLANT DNA isolation kit (Nippon Gene Co., Tokyo, Japan). The coding region of the SLR1 gene was amplified by polymerase chain reaction (PCR) using specific primers S6 (5′-TCGTCGTCCTCATCGTCGTC-3′) and A6 (5′-GCAGCCGGTGCAGCTCGAAC-3′) from 300 ng of rice genomic DNA. PCR products were gel purified, and the nucleotide sequences were determined using the dideoxynucleotide chain termination method with an automated sequencing system (ABI373A; Applied Biosystem, Inc., Foster City, CA). Analysis of the nucleotide sequences was performed using DANSIS computer software (Hitachi Software Engineering, Tokyo, Japan).
Rice Transformation and Complementation of the slr1-1 Mutant
A genomic clone, pAI1, including the entire coding region and the 5′ and 3′ flanking regions, was inserted as a 6-kb restriction fragment between the SmaI sites of the hygromycin resistance binary vector pBI101-Hm3 (Sato et al., 1999). The pAI1 gene construct was introduced into the slr1-1 mutant as described above. Control plants were transformed using the vector pBI-cont.
pSLRtr was made as follows. A 123-bp fragment was amplified by PCR from 6 kb of genomic DNA containing the SLR ORF using a 5′ primer containing an additional XbaI site (5′-TCTAGAATGAAGCGCGAGTA-3′) and a 3′ primer containing an additional SalI site (5′-GTCGACGTCCTCCTCCTCCC-3′). A 1722-bp fragment was amplified by PCR from 6 kb of genomic DNA containing the SLR ORF using a 5′ primer containing an additional SalI site (5′-GTCGAC-GTCGCGCAGAAGCT-3′) and a 3′ primer containing an additional SmaI site (5′-CCCGGGTCACGCCGCGGCGA-3′). These PCR products were cloned into a pBIAct1nos vector (Yamamuro et al., 2000). pBIAct1nos containing the actin (Act1) promoter (Zhang et al., 1991) and the nopaline synthase terminator was prepared by substituting the Act1 promoter in the hygromycin resistance binary vector between the XbaI and SmaI sites. pBI-cont, which contains no insert, was used as a control vector. pSLR1tr and the control construct were introduced into rice cv Nipponbare by Agrobacterium tumefaciens–mediated transformation, as described by Hiei et al. (1994).
Acknowledgments
We are grateful to Dr. Pierdomenico Perata for critically reviewing the manuscript and Professor Kenzo Nakamura (Nagoya University) for providing us with the pBI101-Hm3 vector. This work was supported by Grants-in-Aid for Scientific Research (Grant Nos. 09660004 and 10460003) from the Ministry of Education, Science, Sports, and Culture, Japan. A.I. acknowledges a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists (2000–2001). We thank Dr. T. Sasaki of the Japanese Rice Genome Program for the expressed sequence tag clones.
References
- Chandler, P.M. (1988). Hormonal regulation of gene expression in the “slender” mutant of barley (Hordeum vulgare L.). Planta 175 115–120. [DOI] [PubMed] [Google Scholar]
- Church, G., and Gilbert, W. (1984). Genomic sequencing. Proc. Natl. Acad. Sci. USA 81 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker, S.J., Hedden, P., Lenton, J.R., and Stoddart, J.L. (1990). Comparison of gibberellins in normal and slender barley seedlings. Plant Physiol. 94 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell, J.E., Jr. (1997). STATs and gene regulation. Science 277 1630–1635. [DOI] [PubMed] [Google Scholar]
- Favret, E.A., and Malvarez, E.M. (1975). Genetic regulatory mechanisms for seedling growth in barley. Barley Genet. 3 37–43. [Google Scholar]
- Foster, C.A. (1977). Slender: An accelerated extension growth mutant of barley. Barley Genet. Newsl. 7 24–27. [Google Scholar]
- Futsuhara, Y., Toriyama, K., and Tsunoda, K. (1967). Breeding of the new rice variety, “Reimei,” through gamma-ray irradiation. Jpn. J. Breed. 17 85–90. [Google Scholar]
- Gaskin, P., and MacMillan, J. (1991). Quantitative analysis. In GC-MS of the Gibberellins and Related Compounds: Methodology and a Library of Spectra, P. Gaskin and J. MacMillan, eds (Bristol, UK: Cantock's Enterprises), pp. 117–124.
- Gubler, F., Kalla, R., Roberts, J.K., and Jacobsen, J.V. (1995). Gibberellin-regulated expression of a myb gene in barley aleurone cells: Evidence for Myb transactivation of a high-pI α-amylase gene promoter. Plant Cell 7 1879–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove, T.R., Coffman, W.R., and Cabanilla, V.L. (1980). Ancestry of improved cultivars of Asian rice. Crop Sci. 20 721–727. [Google Scholar]
- Hiei, Y., Ohta, S., Komari, T., and Kumashiro, T. (1994). Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6 271–282. [DOI] [PubMed] [Google Scholar]
- Hisamatsu, T., Koshioka, M., Kubota, S., King, R.W., and Mander, L.N. (1998). Isolation and identification of GA12 (12β-hydroxy-GA12) in Matthiola incana. Phytochemistry 47 3–6. [Google Scholar]
- Hooley, R. (1994). Gibberellins: Perception, transduction and responses. Plant Mol. Biol. 26 1529–1555. [DOI] [PubMed] [Google Scholar]
- Itoh, K., Yamaguchi, J., Huang, N., Rodriguez, R.L., Akazawa, T., and Shimamoto, K. (1995). Developmental and hormonal regulation of rice α-amylase (RAmy1A)-gusA fusion genes in transgenic rice seeds. Plant Physiol. 107 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi, K., Yamaguchi, I., Wada, A., Oshio, H., and Takahashi, N. (1984). Effects of a new plant growth retardant (E)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)-1-penten-3-ol (S-3307) on the growth and gibberellin content of rice plants. Plant Cell Physiol. 25 611–617. [Google Scholar]
- Jacobsen, S.E., and Olszewski, N.E. (1993). Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell 5 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M.G. (1987). Gibberellins and the procera mutant of tomato. Planta 172 280–284. [DOI] [PubMed] [Google Scholar]
- Jones, R.L., and Jacobsen, J.V. (1991). Regulation of synthesis and transport of secreted proteins in cereal aleurone. Int. Rev. Cytol. 126 49–88. [DOI] [PubMed] [Google Scholar]
- Lanahan, M.B., and Ho, T.-H.D. (1988). Slender barley: A constitutive gibberellin-response mutant. Planta 175 107–114. [DOI] [PubMed] [Google Scholar]
- Matsukura, C., Itoh, S., Nemoto, K., Tanimoto, E., and Yamaguchi, J. (1998). Promotion of leaf sheath growth by gibberellic acid in a dwarf mutant of rice. Planta 205 145–152. [Google Scholar]
- Mitsui, T., Yamaguchi, J., and Akazawa, T. (1996). Physicochemical and serological characterization of rice α-amylase isoforms and identification of their corresponding genes. Plant Physiol. 110 1395–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunaga, S., and Yamaguchi, J. (1993). Production of α-amylase is repressed by uniconazole, an inhibitor of the biosynthesis of gibberellin, in a dwarf mutant of rice, Waito-C. Plant Cell Physiol. 34 243–249. [Google Scholar]
- Mitsunaga, S., Tashiro, T., and Yamaguchi, J. (1994). Identification and characterization of gibberellin-insensitive mutants selected from among dwarf mutants of rice. Theor. Appl. Genet. 87 705–712. [DOI] [PubMed] [Google Scholar]
- Murakami, H. (1968). A new rice seedling test for gibberellins, ‘microdrop method’ and its use for testing extracts of rice and morning glory. Bot. Mag. Tokyo 81 33–43. [Google Scholar]
- Nakamura, I. (1992). A rice mutant showing accelerated internode overgrowth. Rice Genet. Newsl. 9 61–62. [Google Scholar]
- Nishijima, T., Koshioka, M., and Yamaji, H. (1992). A non-dwarf rice seedling bioassay for gibberellins. Plant Physiol. 98 962–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, M., Kusano, T., Katsumi, M., and Sano, H. (2000). Rice gibberellin-insensitive gene homolog, OsGAI, encodes a nuclear-localized protein capable of gene activation at the transcriptional level. Gene 245 21–29. [DOI] [PubMed] [Google Scholar]
- Peng, J., and Harberd, N.P. (1993). Derivative alleles of the Arabidopsis gibberellin-insensitive (gai) mutation confer a wild-type phenotype. Plant Cell 5 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J., Carol, P., Richards, D.E., King, K.E., Cowling, R.J., Murphy, G.P., and Harberd, N.P. (1997). The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 11 3194–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J., et al. (1999). ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400 256–261. [DOI] [PubMed] [Google Scholar]
- Penson, S.P., Schuurink, R.C., Fath, A., Gubler, F., Jacobsen, J.V., and Jones, R.L. (1996). cGMP is required for gibberellic acid–induced gene expression in barley aleurone. Plant Cell 8 2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perata, P., Pozueta-Romero, J., Akazawa, T., and Yamaguchi, J. (1992). Effect of anoxia on starch breakdown in rice and wheat seeds. Planta 188 611–618. [DOI] [PubMed] [Google Scholar]
- Perata, P., Matsukura, C., Vernieri, P., and Yamaguchi, J. (1997). Sugar repression of a gibberellin-dependent signaling pathway in barley embryos. Plant Cell 9 2197–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts, W.C., Reid, J.B., and Murfet, I.C. (1985). Internode length in Pisum: Gibberellins and the slender phenotype. Physiol. Plant. 63 357–364. [Google Scholar]
- Sato, Y., Sentoku, N., Miura, Y., Hirochika, H., Kitano, H., and Matsuoka, M. (1999). Loss of function mutations in the rice homeobox gene OSH15 affect the architecture of internodes resulting in dwarf plants. EMBO J. 18 992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurink, R.C., Chan, P.V., and Jones, R.L. (1996). Modulation of calmodulin mRNA and protein levels in barley aleurone. Plant Physiol. 111 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone, A.L., and Sun, T. (2000). Gibberellins and the green revolution. Trends Plant Sci. 5 1–2. [DOI] [PubMed] [Google Scholar]
- Silverstone, A.L., Mak, P.Y.A., Casamitjana-Martinez, E., and Sun, T.-p. (1997). The new RGA locus encodes a negative regulator of gibberellin response in Arabidopsis thaliana. Genetics 146 1087–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone, A.L., Ciampaglio, C.N., and Sun, T.-p. (1998). The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto, N., Takeda, G., Nagato, Y., and Yamaguchi, J. (1998). Temporal and spatial expression pattern of α-amylase gene during germination in rice and barley. Plant Cell Physiol. 39 323–333. [Google Scholar]
- Swain, S.M., and Olszewski, N.E. (1996). Genetic analysis of gibberellin signal transduction. Plant Physiol. 112 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, C.B. (1998). GA signaling: Genes and GTPases. Plant Cell 10 131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton, T.M., Swain, S.M., and Olszewski, N.E. (1999). Gibberellin signal transduction presents … the SPY who O-GlcNAc'd me. Trends Plant Sci. 4 424–428. [DOI] [PubMed] [Google Scholar]
- Toyofuku, K., Loreti, E., Vernieri, P., Alpi, A., Perata, P., and Yamaguchi, J. (2000). Glucose modulates the abscisic acid–inducible Rab16A gene in cereal embryos. Plant Mol. Biol. 42 451–460. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, J. (1998). Analysis of embryo-specific α-amylase using isolated mature rice embryos. Breeding Sci. 48 365–370. [Google Scholar]
- Yamamuro, C., Ihara, Y., Wu, X., Noguchi, T., Fujioka, S., Takatsuto, S., Ashikari, M., Kitano, H., and Matsuoka, M. (2000). Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12 1591–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., McElroy, D., and Wu, R. (1991). Analysis of rice Act1 5′ region activity in transgenic rice plants. Plant Cell 3 1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]