Abstract
The expression of the α and α′ subunits of β-conglycinin was suppressed by sequence-mediated gene silencing in transgenic soybean seed. The resulting seeds had similar total oil and protein content and ratio compared with the parent line. The decrease in β-conglycinin protein was apparently compensated by an increased accumulation of glycinin. In addition, proglycinin, the precursor of glycinin, was detected as a prominent polypeptide band in the protein profile of the transgenic seed extract. Electron microscopic analysis and immunocytochemistry of maturing transgenic soybean seeds indicated that the process of storage protein accumulation was altered in the transgenic line. In normal soybeans, the storage proteins are deposited in pre-existing vacuoles by Golgi-derived vesicles. In contrast, in transgenic seed with reduced β-conglycinin levels, endoplasmic reticulum (ER)–derived vesicles were observed that resembled precursor accumulating–vesicles of pumpkin seeds and the protein bodies accumulated by cereal seeds. Their ER–derived membrane of the novel vesicles did not contain the protein storage vacuole tonoplast-specific protein α-TIP, and the sequestered polypeptides did not contain complex glycans, indicating a preGolgi and nonvacuolar nature. Glycinin was identified as a major component of these novel protein bodies and its diversion from normal storage protein trafficking appears to be related to the proglycinin buildup in the transgenic seed. The stable accumulation of proteins in a protein body compartment instead of vacuolar accumulation of proteins may provide an alternative intracellular site to sequester proteins when soybeans are used as protein factories.
INTRODUCTION
Seed store members of several families of storage proteins in both specialized protein storage vacuoles (PSV) and endoplasmic reticulum (ER)–derived protein bodies (PB) (for reviews, see Herman and Larkins, 1999; Chrispeels and Herman, 2000). The storage proteins of PSVs are primarily the 7S-globulin or vicilin-type and the 11S-globulin or legumin-type proteins, and these are sequestered together with auxiliary storage proteins such as lectins, 2S albumins, various defense proteins, including protease and amylase inhibitors, and other vacuolar hydrolytic enzymes (for review, see Galili and Herman, 1996). The prevailing consensus on the mechanism of vacuolar storage protein deposition and accumulation is that the proteins are deposited in differentiated vacuoles after progression through the endomembrane system. Specific vacuolar targeting sequences and packaging in the Golgi for vesicular transport to the vacuole have been investigated intensely and many of the mechanisms have been elucidated (Dombrowski et al., 1993; Ahmed et al., 1997; Paris et al., 1997; for review and perspective, see Sanderfoot and Raikhel, 1999).
In contrast, the prolamin storage proteins of cereals are assembled into ER-derived PBs that are formed as complete mature organelles (Larkins and Hurkman, 1978; for review, see Herman and Larkins, 1999). These PBs consist of a protein core surrounded by an ER-derived membrane that retains bound ribosomes and sequesters predominantly hydrophobic prolamin storage proteins such as the zeins of maize or the gliadins of wheat. PBs lack the wide array of other auxiliary proteins and enzymes characteristic of PSVs. Such PBs appear to be inert compartments that are not osmotically active, and they have not been shown to transport or store small molecules.
PBs and their contents, however, can be sequestered in vacuoles by secondary processes that bypass the Golgi. The first naturally occurring example discovered was that of wheat PBs that are sequestered in endosperm vacuoles as the consequence of autophagy (Levanony et al., 1992). This mechanism has become a paradigm for one type of monocotyledonous seed protein accumulation.
The contrasting biology of PSVs and PBs appears to be closely related to the comparative life cycles of the cells that sequester these two organelles. PSVs are formed in maturing seed cells that survive desiccation and upon germination are reactivated to mobilize the stored proteins. In these cells, hydrolytic enzymes synthesized de novo are targeted into the PSV and degrade the storage proteins (Müntz, 1996). The resulting hydrolysis products are transported out of the seed into the developing plant by active transport processes. PBs are generally found in cereal endosperm that undergoes controlled cell death toward the end of seed maturation. Whether these PBs are formed and maintained as discrete structures that can retain a connection to the ER, as in maize (Larkins and Hurkman, 1978), or whether the PBs are sequestered into the vacuole before the end of maturation, as has been shown in wheat (Levanony et al., 1992), the final result of the prolamin deposits remaining in dead cells is the same. Mobilization of PB-stored protein reserves occurs by secretion of protein-degrading enzyme from living aleurone cells that surround the dead endosperm (Koehler and Ho, 1990), and the hydrolysis products are then absorbed by the growing seedling.
Similar ER-derived protein bodies have also been discovered in dicotyledoneous seed (maturing pumpkin) that accumulate ER-derived PBs termed precursor accumulating (PAC) vesicles (Hara-Nishimura et al., 1998). PAC vesicles sequester the precursor of the PSV-protein 2S albumin that must be deposited in the vacuole to be processed. The current model posits that PAC vesicles mediate at least part of the protein's transport to the PSV. Although PAC vesicles superficially resemble monocotyledonous PBs in structure (i.e., a protein core surrounded by a rough ER-derived membrane), these vesicles are distinct because they contain precursors of soluble vacuolar storage proteins. PAC vesicles contain complex glycans, indicating some contribution of Golgi-derived proteins. It has not been determined whether the complex glycan proteins are added during or after the PAC vesicles' formation.
The formation of cereal prolamin-containing PBs in dicotyledoneous seed cells (Bagga et al., 1995, 1997; Coleman et al., 1996), including the uptake of these PBs into the PSV, has been described in transgenic plant models. For example, tobacco endosperm coexpressing α and γ zeins are coassembled into PBs that are taken then into the PSVs via autophagy (Coleman et al., 1996). PAC vesicles also can be induced in transgenic plants by expression of a fusion protein that includes a large portion of the 2S albumin coding sequence (Hayashi et al., 1999). The interaction of PBs and PAC vesicles with the vacuole may be examples of a more broadly used system of ER-derived vesicles contributing proteins to storage and vegetative vacuoles (for additional information, see Chrispeels and Herman, 2000) that is a subset of the larger process of uptake of material into the vacuole forming diverse intravacuolar inclusions (Herman, 1994a).
In this article, we show that PB formation can be induced in transgenic soybeans in response to transgenic cosuppression of the α and α′ subunits of β-conglycinin (BCG) (a 7S storage protein). The resulting seeds compensated for the decrease of the conglycinin subunits by increased accumulation of glycinin protein (an 11S storage protein), and its precursor form, proglycinin. A significant portion of the glycinin is sequestered into ER–derived PBs. These novel structures persisted through seed maturation and are present in germinating seeds. The ability to promote the formation of a new cellular compartment that sequesters soluble proteins offers the prospect of using seed to produce large quantities of ER-synthesized novel protein products.
RESULTS
Cosuppression of α and α′ β-Conglycinin Subunits in Transgenic Soybean
The soybean line described in this study was one of a number of transgenic plant lines lacking α and/or β subunits of BCG (Kinney and Fader, 1998; Kinney and Knowlton, 1998). These lines were generated by either coding-region or 5′ untranslated leader sequence–mediated cosuppression of β-conglycinin subunits (Kinney and Fader, 1998). The event chosen lacked both the α and α′ subunits of BCG as a result of 5′ untranslated region cosuppression (Kinney and Knowlton, 1998). Because the soybean Fad 2 (fatty acid desaturase) coding sequence was fused to the BCG promoter in this line, their seed oil had an increased oleic acid content (caused by cosuppression of the Fad 2-1 gene). Because the BCG profile of other transgenic high oleate lines generated using different promoters was not affected, the change in protein profile of the chosen line had to be independent from the increase in oleic acid content.
The seed set and maturation of this line proceeded on the normal developmental schedule, and the resulting mature seed germinated normally and were also otherwise extrinsically indistinguishable from the nontransformed control plants.
The α and α′ subunits of BCG genes (ABCG) in the soybean genome constitute a small gene family of three members (Harada et al., 1989; reviewed in Nielsen and Nam, 1999). The transcript sequences of these genes are highly similar to each other (>90% identical), whereas homology with the β subunits of BCG (BBCG) is less than 70%. Thus the 5′ untranslated region sequences of α′ BCG have the potential to mediate silencing of all ABCG genes, but they are less likely to trigger suppression of transcripts of other genes in developing soybean cotyledons, including BBCG and glycinin (GY).
To determine whether the lack of ABCG subunits was due to the lack of mRNA templates, we prepared RNA gel blots from late maturation transgenic and control soybean seed (Figure 1). The blot was probed with cDNA probes of ABCG and of ω-3 fatty acid desaturase (Fad 3; Yadav et al., 1993). We have used Fad 3 as a control in a number of other experiments. In this experiment, as in previous assays, the level of Fad 3 mRNA was similar in the control and transgenic seed. In contrast, the mRNA abundance of ABCG was quite different between the control and transgenic soybean lines, with little ABCG transcript in the transgenic seed. As described elsewhere (Kinney and Knowlton, 1998), the transgenic lines also lacked Fad 2 mRNA. We concluded that decreased ABCG accumulation resulted from transcriptional-level inhibition and therefore was unlikely to be caused by obstructions during protein synthesis and trafficking or by cellular protein quality control mechanisms and protein turnover.
Figure 1.

RNA Gel Blot Shows Conglycinin Cosuppression.
RNA gel blots of mRNA purified from developing seed of transgenic line G19 cosuppressed in conglycinin (G19) and of nontransgenic control (C) are shown. The blots were probed with conglycinin and Fad 2 cDNA probes. Note the almost complete absence of conglycinin mRNA in the G19 line compared with the abundant conglycinin mRNA in the control. Fad 3 (fatty acid desaturase 3) was used as a loading control.
Conglycinin Suppression and GY Precursor Accumulation in Transgenic Soybeans
Developmental changes in the accumulation of seed proteins from wild-type control and transgenic seed were evaluated by SDS-PAGE. Two major phases of soybean seed development are distinguishable by this method. The phase within 13 to 21 days after flowering (DAF) (seed size 4 to 8 mm) represents the cell division phase and is characterized by high protein turnover and limited accumulation of seed storage proteins (Meinke et al., 1981). This is followed by the storage deposition phase, which is characterized by a rapid accumulation of seed storage proteins reaching their steady state levels between 28 and 40 DAF (seed size to 12 mm). In wild-type soybean, the first major seed storage proteins that started to accumulate were ABCG at ∼21 DAF (seed size 6 to 8 mm) followed by GY at ∼24 DAF (seed size 9 to 11 mm). The accumulation of BBCG started at later stages of development. At maturity, the final contribution to the total seed protein by GY was 35 to 40%, the contribution by ABCG was ∼25%, and the contribution by BBCG was ∼5%. Although generally similar, the protein profiles in developing transgenic seed differed noticeably in specific ways from those of wild-type seed. As previously discussed, ABCG expression never commenced. Apparently balancing for ABCG, the other seed globulins (GY and BBCG) appeared to accumulate to higher levels. However, most notably was the appearance of a novel polypeptide with an apparent molecular mass of ∼60 kD. This polypeptide persisted throughout seed development, although its level was obviously higher during midmaturation stages compared with that in the mature seed.
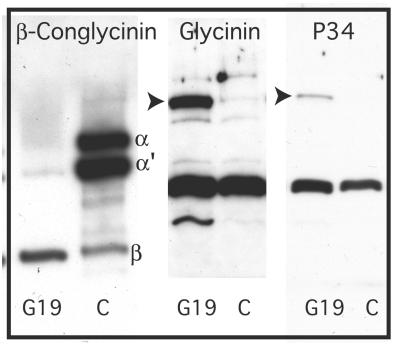
To examine the GY and ABCG polypeptides further, membrane blots of mature seed extracts fractionated by SDS-PAGE were probed with antiGY and antiBCG specific antibodies. Parallel lanes on the gel blots contained an extract of the control seed. Blots probed with BCG-specific antiserum confirmed that this protein was greatly suppressed in the transgenic seed (Figure 2), supporting the assay showing the absence of ABCG mRNA (Figure 1). A replicate blot probed with antiGY antiserum presented a very different effect of the BCG suppression. GY is produced as a larger precursor (proGY) (Nielsen et al., 1995). In the vacuole, it is post-translationally processed at conserved asparagine residues by the vacuolar processing enzyme (VPE) into two subunits that remain connected by a disulfide bond (Hara-Nishimura et al., 1995). ProGY was observed in SDS-PAGE-immunoblots of wild-type maturing seed as a minor band that is derived from protein released from the ER that has not yet progressed to the vacuole for processing. Fully mature wild-type seed do not contain precursor storage proteins because synthesis is terminated before the late maturation events, allowing the last polypeptides synthesized time to progress to the vacuole for processing. The transgenic soybeans contained abundant proGY, whereas proGY was absent in the control seed (Figure 2, glycinin; lane G19, arrowhead).
Figure 2.
Immunoblots Show the Absence of Conglycinin and the Accumulation of ProGY and P34 precursor.
Extracts of fully mature G19 and control (C) seeds were fractionated by SDS-PAGE and transferred to nitrocellulose membranes. The blot was probed with antibodies against β-conglycinin, GY, and P34. This blot shows that the G19 line contains very little conglycinin compared with the abundant level of the protein in the control. In contrast to both mature GY and mature P34, polypeptide bands appear similar in both the G19 and control samples, indicating that neither of these proteins are reduced. The G19 line contains abundant proGY (arrowhead) and proP34 (arrowhead) compared with the control.
Accumulated GY Precursor Is Primarily Group 1 Protein
To determine its N-terminal amino acid sequence, the ∼60-kD polypeptide band was subjected to protein microsequencing. Sixteen cycles of Edman degradation yielded the sequence NH2-GREQAQPNEXEIQQLN-COOH, which in a search against the SwissProt database matched most closely N termini of precursor group 1 GY subunits with their ER signal peptides detached (Nielsen et al., 1989). The apparent molecular mass of the polypeptide of 60 kD, together with the presence of an alanine at position 5 of the amino acid sequence, suggested that the sequenced band represented predominantly the unprocessed precursor of GY2 (Nielsen et al., 1989).
P34 Precursor Is Accumulated in Transgenic Soybeans
To determine whether the impairment of proGY processing was restricted to that protein, we also assayed P34. P34 is an outlying member of the papain superfamily (Kalinski et al., 1992) that is apparently a Pseudomonas defense protein (Cheng et al., 1998) and is the primary human allergen of soybean seed (Helm et al., 1998; Yaklich et al., 1999). P34 is localized in the PSVs and is accumulated simultaneously with the storage proteins (Kalinski et al., 1992). Similarly to proGY (Hara-Nishimura et al., 1995), the precursor of P34 (proP34) is post-translationally processed on the carboxyterminal side of an asparagine residue (Kalinski et al., 1992; T. Okamoto, T. Minimakawa, and E. Herman, unpublished) by VPE. Immunoblot assay of the transgenic soybean seed extract with a monoclonal antibody specific for P34 showed that proP34 was much more abundant in the transgenic soybean seed (Figure 2, P34; lane G19, arrowhead) compared with the wild-type control. This finding indicates that the process(es) that impedes GY maturation also impedes the processing of other PSV proteins.
Vacuolar Processing Enzyme Expression Is Not Altered
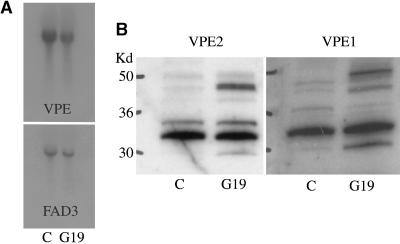
VPEs are asparagine-specific vacuolar processing enzymes that have been shown to mediate maturation of a wide variety of seed and vegetative proteins (Hara-Nishimura et al., 1993, 1995). One possible explanation for the accumulation of precursor proteins in the transgenic soybeans is that VPE expression and/or activity might be suppressed. The increase in abundance of proGY could result from suppression of this enzyme. To test whether VPE was suppressed, the mRNA level of VPE was compared in the transgenic and control late maturation seed. Figure 3A shows that VPE mRNA does not appear to be significantly suppressed, although the level in the transgenic line appeared to be slightly less than in the control line. A FAD3-labeled blot is shown as a loading control.
Figure 3.
Vacuolar Processing Enzyme (VPE) Is Not Suppressed in Conglycinin-Cosuppressing Seed.
(A) RNA gel blot shows that the G19 and control (C) seed contain very similar amounts of VPE message. Fad 3 labeling was used as a loading control as in Figure 1.
(B) SDS-PAGE immunoblots probed with VPE2 and VPE1 isoform-specific antibodies show that both forms accumulate in G19 and control seed.
The mature VPE 33-kD bands are similar in both G19 and control seeds for both VPE2 and VPE1 isoforms. The G19 seed contain abundant higher Mr precursors of both VPE2 and VPE1 isoforms that are not present in the control seed.
Soybean seed express two similar genes for VPEs (VPE1 and VPE2) for which isoform-specific antibodies are available. VPE is synthesized as a higher Mr precursor protein that is activated post-translationally in the vacuole (Hara-Nishimura et al., 1995). SDS-PAGE immunoblots of extracts from maturing wild-type and transgenic seed was probed with both of the VPE isoform-specific antibodies that demonstrated that the abundance of mature VPE 33-kD polypeptides was similar (Figure 3B). The transgenic seeds contained higher levels of VPE precursor proteins of both isoforms, indicating that, like GY and P34, a portion of the VPE was apparently diverted from the vacuole, where it would be processed to lower molecular mass mature protein.
Accumulated ProGY Is Primarily 7S to 9S
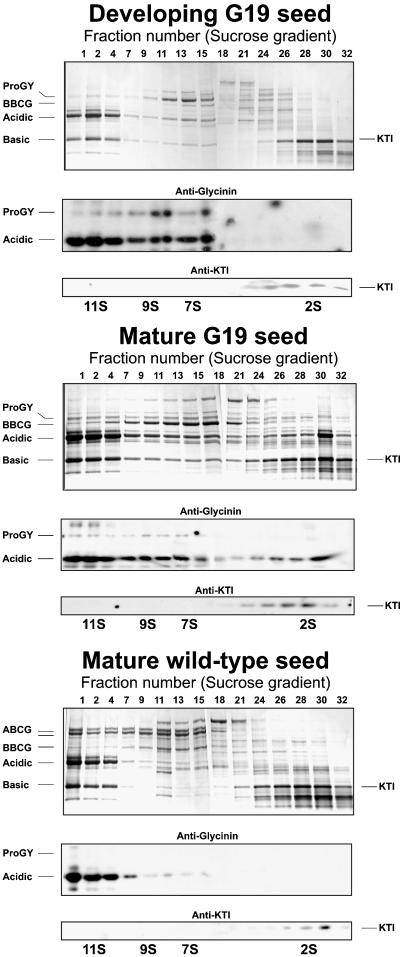
Under normal conditions, proGY subunits are present only transiently in the ER and in the endomembrane system, where they first assemble into trimeric complexes and then traverse to the PSV, respectively (Nielsen et al., 1995). In the PSV, processing into the disulfide-linked acidic and basic chains of mature GY occurs very rapidly. As a consequence, proGY extracted from developing or mature soybeans is a very minor protein fraction that is detectable only after pulse chase radiolabeling of translated seed proteins in situ and immunoprecipitation of the labeled precursor molecules. Therefore, the recognition of a proGY as a very abundant polypeptide in the transgenic soybean seed was a sign of some perturbation of the storage protein deposition process. The proGLY accumulation could, for example, result from misassembly and retention in the ER lumen due to missorting and secretion or because of a delayed processing in the PSV. To assess and compare the assembly status of seed proteins, we fractionated proteins by density centrifugation, followed by SDS-PAGE of gradient fractions (Figure 4). Upon electrophoretic separation, proteins were blotted to polyvinylidene difluoride (PVDF) membranes and sequentially probed with antibodies specific to GY and Kunitz trypsin inhibitor 3 (KTI3) (Figure 4).
Figure 4.
Assembly of GY Subunits into 11S Hexamers Is Impeded in Transgenic G19 Seed.
Sedimentation analysis of soybean seed protein fractions. Sucrose gradient fractions of protein isolated from transgenic developing G19 seed (top section), protein isolated from mature G19 seed (middle section), and protein isolated from nontransgenic mature soybean seed (bottom section). Indicated fractions were separated by SDS-PAGE (top blot in each set of sections) and probed by immunoblotting using GY-specific antibodies (middle blot in each set of sections) and Kunitz trypsin inhibitor (KTI)–specific antibodies (bottom blot in each set of sections). Fraction numbers are given at the top of the lanes; the approximate sedimentation coefficients of fractions are indicated at the bottom. In mature transgenic seed, GY chains (acidic and basic) are present in all gradient fractions, indicating only partial assembly of GY subunits into 11S hexamers.
Soybean globulins assemble into oligomers (Nielsen et al., 1995), which migrate in sucrose density gradients with characteristic sedimentation coefficients of ∼11S (mature GY hexamers), ∼9S (ProGY trimers), and ∼7S (BCG trimers). Under specific conditions (e.g., pH, ionic strength) globulin oligomers dissociate into monomers, which migrate similarly to most seed albumins (e.g., KTI3) with a sedimentation coefficient of 2S to 3S. Figure 4 (bottom section) shows the typical protein profiles of a fractionated wild-type soybean seed protein extract with GY and BCG peptides in the ∼11S and ∼7S fractions, respectively. The gradient fractions obtained from developing (Figure 4, top section) and mature (Figure 4, middle section) transgenic seed showed a different polypeptide distribution. ProGY from both developing and mature seed migrated at ∼7S to 9S, (i.e., in its majority, most likely as a trimeric complex). The bottom fraction of the gradient did not contain a detectable protein pellet; that is, no proGy (not shown). This is noteworthy, because it indicates proGY subunits folded and oligomerized correctly in the lumen of the ER and did not form aggregates. Another notable difference between wild-type (Figure 4, bottom section) and transgenic soybeans (Figure 4, middle section) was the migration pattern of mature, processed GY. In the wild-type sample, GY cross-reacting bands were detected exclusively in the ∼11S fractions, whereas in the transgenic sample only a part of the processed GY was observed in the 11S (hexamer) fraction. A substantial portion sedimented in the 7S to −9S (trimer) fractions and in the 2S (monomer) fractions (Figure 4, middle section) of the gradient. Although processing of these subunits suggested a final vacuolar destination, the assembly impairment indicated a changed microenvironment or missing factors for oligomerization.
Suppression of GY Expression Promotes Accumulation of ER-Derived Protein Bodies
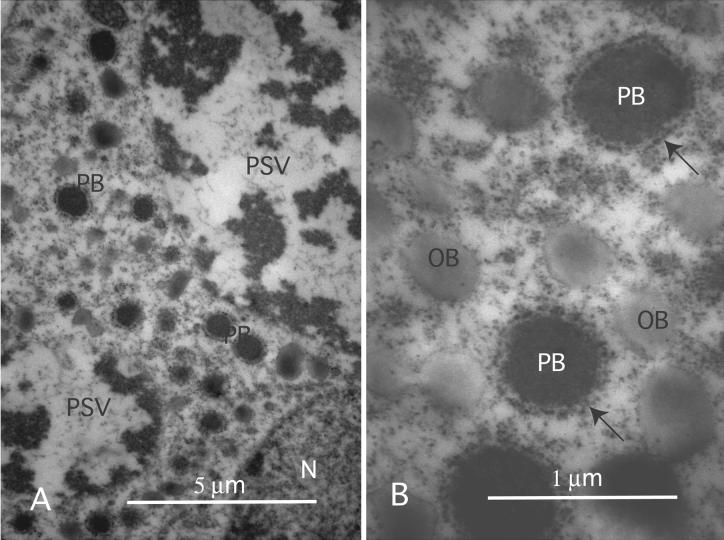
Under normal circumstances, precursor proteins can be localized in the ER and Golgi by immunocytochemistry (Herman and Shannon, 1984, 1985) or by biochemical assay in isolated ER-derived microsomes (Bollini and Chrispeels, 1979). The newly synthesized storage protein's resident half-life in the ER lumen has been shown to be ∼90 min (Chrispeels et al., 1982). On exiting from the ER, precursor proteins apparently are translocated rapidly through the Golgi (Chrispeels, 1983) and then on to the PSV, where processing to the mature form quickly occurs through the action of a processing enzyme (Hara-Nishimura et al., 1993, 1995) in the acidic conditions of the vacuolar sap. The stability of precursor forms in the transgenic soybeans suggests that these proteins must be compartmentalized in a prevacuolar compartment. Conventional electron microscope observations of transgenic seed storage parenchyma cells confirmed that there were significant structural differences between the ultrastructures of the transgenic and control storage parenchyma cells. In control soybeans, the storage proteins are accumulated in the PSVs, and except for some Golgi-derived secretion vesicles, there are no other sites that contain dense protein deposits. In contrast, the transgenic soybeans possessed a cytoplasm with abundant electron-dense PBs (Figure 5A). High-magnification examination of the PBs showed that these structures had a relatively uniform morphology featuring an amorphous electron-dense matrix surrounded by a membrane derived from the rough ER (Figure 5B).
Figure 5.
Conglycinin-Suppressing Seed Contain Abundant PBs.
(A) Examination of maturing G19 seed by conventional electron microscopy with osmium postfixation demonstrates that the cytoplasm contains numerous electron-dense vesicles.
(B) Higher magnification images of the electron-dense vesicles show that they possess an electron-dense core surrounded by a rough ER-derived membrane that is characteristic of seed PBs.
N, nucleus; OB, oil body; PB, protein body; PSV, protein storage vacuole.
PBs of Maturing Seeds Contain GY and P34
The presence of large numbers of PBs in the cells of the transgenic seeds suggests that much of the GY could be sequestered within this compartment. Electron microscope–immunogold assays using a specific antibody for GY resulted in a highly specific labeling of the PBs and PSVs (Figures 6A and 6B). The PSVs of the transgenic seed appeared to be identical to the protein-filled PSVs in the wild type in soybeans. The PSVs, in contrast to the PBs, possess a limiting membrane (tonoplast) that does not have bound ribosomes.
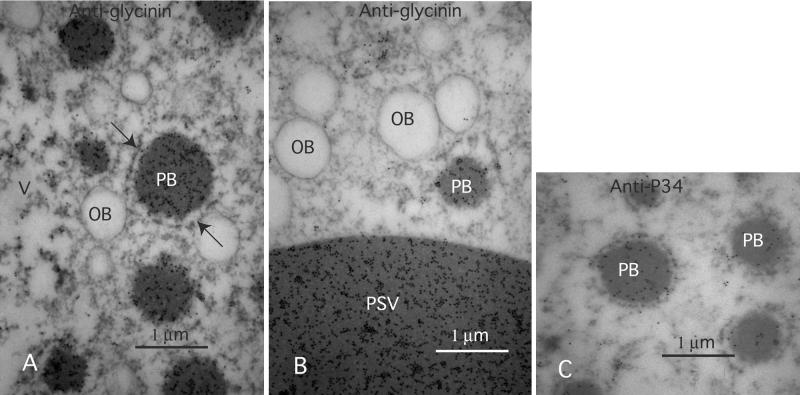
Figure 6.
PBs in Maturing Conglycinin-Cosuppressing Soybeans Contain GY and P34.
Thin sections of G19 soybeans were labeled with specific antibodies against GY and P34 to assay antigen content of the PBs.
(A) and (B) Low- and high-magnification images of antiglycinin-labeled sections. The antibody labels both the PSV and PBs with similar gold particle density (B).
(C) A monoclonal antibody against P34 also labels the PBs.
OB, oil body; PB, protein body; PSV, protein storage vacuole; V, vacuole.
Although many seeds form paracrystalline arrays from 11S proteins that are termed crystalloids (e.g., tobacco and pumpkin), soybeans normally do not contain these PSV substructures. Similarly, although the transgenic soybean PSVs contained mostly 11S GY, the PSV contents remained an amorphous matrix that did not differ in appearance from the wild-type PSV matrix. Furthermore, the PB protein deposits appeared amorphous and without substructures that could result from either paracrystalline aggregation of the storage proteins or spatial segregation of other cosequestered proteins. The latter process occurs with different zein contents in maize protein bodies (Lending and Larkins, 1989).
To test whether other PSV proteins may be cosequestered with GY in the PBs, further immunogold assays were conducted with antiP34 monoclonal antibodies. This type of assay has shown that P34 is localized in the PSV (Kalinski et al., 1992). The antiP34 antibodies labeled the protein deposits in the PBs, indicating that this protein was cosequestered with GY (Figure 6C).
GY PBs Remain in Germinated Seed
SDS-PAGE immunoblot assays of GY of extracts prepared from dry seed showed that the precursor form of GY remained after the seed was fully mature. This indicated that some of the proGY has not progressed to the vacuole for processing. To examine the precursor-containing structures that remained in mature seed, we conducted electron-microscopic analysis of germinated soybean seed. Cotyledon pieces were fixed after 18 hr of hydration to assay cells in which mobilization of reserve substances had not yet commenced. The PB structure in germinated seed was different from the PBs we observed in maturing seed. Although the PBs retained a limiting membrane, they expanded to create a much larger space between the included storage protein aggregation and the membrane (Figure 7A, arrows). This may be the consequence of the hydration of the seed in which the membrane expands because the limiting ER-derived membrane is osmotically inactive. Immunogold assays with antiGY antibodies labeled the PBs (Figure 7A). The presence of discrete PBs in germinated seeds demonstrated that the organelles are stable in soybean seed cells.
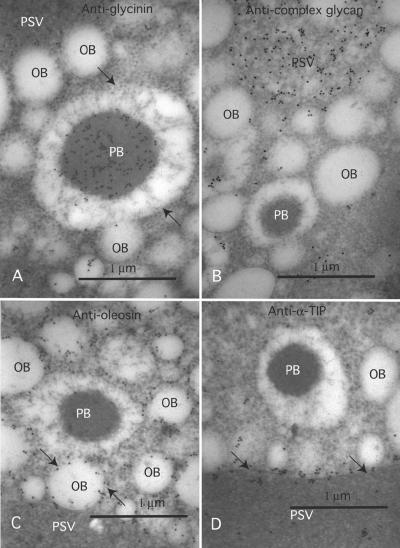
Figure 7.
PBs Remain in Germinated Seed and Do Not Contain Oil Body, Golgi-processed, or Tonoplast Proteins.
The PBs of germinated seed were assayed with antibodies against GY (A), complex glycan (B), soybean oleosin (C), and α-TIP (D). AntiGY antibodies label both the PBs and PSV (A), as in the maturing seed (A) and (B). In contrast, the anticomplex glycan antibodies label the PSV but do not label the PB. Oil bodies, like PBs are ER derived, and labeling with anti-oleosin antibodies shows that the OB proteins are restricted to the OBs (C). Although PBs contain PSV matrix proteins, they do not contain tonoplast proteins, as indicated by the lack of labeling with antibodies against the PSV tonoplast protein α-TIP (D). OB, oil body; PB, protein body; PSV, protein storage vacuole.
The Protein Bodies Do Not Contain Complex Glycans
The PSVs of soybean contain proteins that cross-react with antisera directed at xyloglycan complex glycans (Yaklich and Herman, 1995). This observation provides additional evidence for a postGolgi origin of the PSV. To determine whether PBs induced in transgenic soybeans contain complex glycans, thin sections were labeled with an antixyloglycan complex glycan antiserum and examined. The anticomplex glycan antiserum densely labeled the PSV matrix, whereas the PB matrix remained unlabeled (Figure 7B). This indicates that the one or more proteins that constitute the matrix of the PSV were processed through the Golgi apparatus, whereas the matrix protein(s) of the PBs were not modified by the Golgi. The cell wall also was labeled by the anticomplex glycan antiserum, as has been observed previously (data not shown).
PB Membranes Do Not Contain Oil Body Proteins
Soybean seeds accumulate oil in ER-derived oil bodies that possess a half-unit membrane with a characteristic intrinsic membrane protein termed oleosins (Herman, 1994b). With the induced accumulation of PBs, transgenic soybeans simultaneously assemble two structurally distinct ER-derived organelles. We tested whether oleosins might be found associated with the PBs. Immunogold assay of oleosin with a specific antibody (Herman, 1987) resulted in dense labeling of the oil body membranes (Figure 7C, arrowheads) but a lack of labeling of the PB membrane (Figure 7C).
PBs Lack α-TIP, a PSV Tonoplast Protein
Soybean PSV tonoplasts contain a characteristic abundant integral membrane protein that is an aquaporin (water channel) termed α-TIP (Johnson et al., 1990). α-TIP is accumulated in soybean PSV tonoplast during late seed maturation in conjunction with the storage proteins, and in imbibed seed it is a very abundant constituent and useful marker of the PSV tonoplast (Melroy and Herman, 1991). Immunogold assay with a specific antibody for α-TIP resulted in dense labeling of the PSV membrane (Figure 7D, arrowheads), in contrast to a complete absence of labeling on the PB limiting membrane (Figure 7D).
DISCUSSION
Cosuppression of BCG in soybean seed resulted in alteration of GY expression that compensated for the lack of BCG. The overall protein content of the transgenic seed and the ratio of protein to oil were similar to those in the nontransgenic control (Asgrow, cv A2396). Protein sequencing of the proGY polypeptide identified one specific GY gene product (GY2) as a primary component. How GY is regulated to compensate for BCG reduction is unknown; however, soybean storage protein gene expression has been shown to be regulated by both transcriptional and post-transcriptional processes (Walling et al., 1986). The transgenic BCG-cosuppressing soybean may prove to be a good experimental model with which to investigate the regulatory mechanisms that control seed storage protein composition.
The results presented here show that ER-derived PB formation can be promoted by altering the composition of storage proteins expressed in seed (Figure 8). Previous observations and controls for this project have shown that wild-type soybeans do not accumulate PBs during the course of seed maturation (for examples of electron microscopic observations of maturing soybeans, see Herman and Shannon, 1985; Melroy and Herman, 1991). Induced PB formation has been observed in transgenic tobacco seed, but this required the expression of hydrophobic prolamin storage proteins. These proteins normally are localized in ER-derived PBs in cereal seed that form by self-assembly and aggregation (reviewed in Herman and Larkins, 1999). However, the expression of a single type of prolamin during the induction of PBs results in a structure that is quite different from the more complex structure that results from the coassembly of different prolamins. The coexpression of α and γ zeins as well as β and δ zeins results in the formation of PBs that are similar to intrinsic PBs of maize (Bagga et al., 1995, 1997; Coleman et al., 1996).
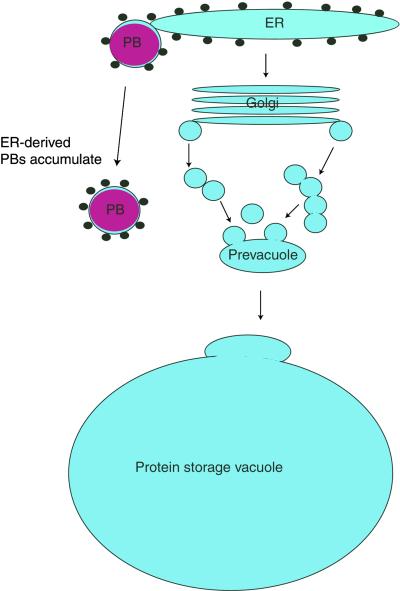
Figure 8.
Diagram of the Formation of PBs.
A diagram showing a model for the diversion of PSV matrix proteins into PBs is shown. A portion of the proteins that would otherwise progress through the endomembrane system for transport to the vacuole instead aggregate and are budded from the ER as PBs. The resulting PBs are a stable population of organelles that persist through seed maturation and remain in the dry mature seed. ER, endoplasmic reticulum; PB, protein body.
Hara-Nishimura et al. (1998) reported the presence of a variant of protein bodies (termed PACs) in maturing pumpkin seed that sequester proalbumin (2S storage protein). These PAC vesicles are structurally similar to the PBs in that they consist of a protein core surrounded by an ER-derived membrane. The PAC vesicles also appear to originate from or in cooperation with the ER. However, unlike the soybean PBs, the pumpkin PAC vesicles do contain complex glycans, indicating that these structures contain some material that has apparently been processed by the Golgi. Whether complex glycan-containing proteins are added to the pumpkin PAC vesicles during organelle assembly or in a postassembly event remains to be determined.
Seed storage proteins are synthesized from the expression of large gene families that are highly conserved among diverse species. Soybeans, like many other plants, synthesize both 7S (BCG) and 11S (GY) storage proteins in similar proportions. Other dicotyledoneous plants synthesize predominantly 7S storage proteins in conjunction with an abundant auxiliary storage protein such as a seed lectin. The common bean, Phaeolus vulgaris, which synthesizes 7S phaseolin and lectin phytohemagglutinin, is one example. Other plants, such as Brassica napus, predominantly synthesize 11S storage proteins along with abundant 2S albumins as auxilary storage proteins. The redirection of protein accumulation from endomembrane progression to the vacuole to ER-derived PBs in the transgenic soybeans described here indicates that the reduction of a 7S storage protein promotes the formation of complexes of 11S storage proteins and other vacuolar proteins in the ER.
Our results point to the possibility that glycinin deposition is partially or predominantly mediated through PBs that are subsequently either fused to or taken up by vacuoles. Such a mechanism would be an alternative pathway from the ER-Golgi-vacuole endomembrane progression trafficking that has been demonstrated in soybeans and other seeds (see Herman and Larkins, 1999). The role of the Golgi has been supported by experiments showing Golgi-mediated processing of PSV proteins and the interactions of targeting sequences on those proteins with a trans-Golgi–localized receptor (for review, see Herman and Larkins, 1999). Instead, the proteins sequestered in PBs appear to constitute a terminal trafficking step that forms an induced stable population of storage organelles (see Figure 8 for details). This is consistent with the presence of PBs in fully mature seed and with a lack of apparent autophagy of PBs by the PSVs in maturing seed. This is in contrast to the mechanism that has been shown in wheat endosperm cells by Levanony et al. (1992), who showed that the prolamin proteins are sequestered in ER-derived PBs and then deposited in the vacuole by autophagy. The wheat endosperm cells with their intravacuolar PBs undergo apoptosis after seed fill is completed, so that the resulting dead endosperm tissue is essentially equivalent to dead endosperm of other monocotyledonous seeds, such as maize. In these cases, the PBs are not sequestered in the vacuole. Similarly, Coleman et al. (1996) showed that maize α/γ-zein PBs formed in transgenic tobacco seeds are sequestered in the tobacco PSV by autophagy.
Our results suggest the possibility that BCG subunits have a functional role in maintaining transport competent proGY. In the absence of BCG, a portion of the GY assembles into complexes that promote the formation of PBs. Although the 11S storage proteins form a crystalline array in many seed, no such crystalline structure was observed in the PBs. It appears that the process that induces PB formation is not restricted to the formation of proGY complexes. The transgenic soybean seeds possess enhanced quantities of the precursor of the thiol-protease homolog P34 (Kalinski et al., 1992). This indicates that a variety of PSV precursor proteins can be cosequestered in the ER with the GY precursors into PBs. Together, this population of propolypeptides escapes processing by vacuolar endoprotease(s). In contrast to vacuolar compartments, the PBs formed in the transgenic soybeans do not contain the PSV tonoplast-specific protein α-TIP. A simple explanation is that the tonoplast proteins are transported to the PSV by processes distinct from those that affect the soluble proteins, as proposed by Gomez and Chrispeels (1993). Therefore, the induced formation of complexes between PSV proteins in the ER and the induction of PBs does not affect the trafficking membrane-bound tonoplast proteins such as α-TIP. This interpretation is consistent with observations that disrupting Golgi function with brefeldin-A inhibits the progression of storage proteins to the vacuole but does not inhibit the progression of α-TIP.
The transgenic soybeans that accumulate ER-derived PBs may prove to be a useful starting point for further alterations of soybean protein composition. The expression of foreign proteins in seed frequently results in post-translational degradation of the newly synthesized proteins. For example, a modified phaseolin, a 7S vicilin-type protein, HiMet, is post-translationally unstable (Hoffman et al., 1988), with its site of degradation being the forming PSV. The addition of ER retention sequences KDEL and HDEL to the destabilized HiMet results in increased stability of HiMet that is sequestered within the ER lumen (Pueyo et al., 1995). However, the ER is a relatively small compartment and its suitability as a place to retain otherwise unstable proteins is limited. The ER is usually degraded at the end of seed maturation and replaced by ER formed de novo after germination (Gilkes et al., 1979). Therefore, most of the protein retained and stabilized in the ER would be lost at the end of maturation. Similarly, zeins expressed in transgenic tobacco can be post-translationally unstable (Williamson et al., 1988), but coexpression of two different zeins promotes the formation of ER-derived PBs that stabilize the zeins (Coleman et al., 1996); although these PBs, too, are eventually destroyed via autophagy by the PSV. PBs can be induced in vegetative organs. Zeins expressed in tobacco leaf cells form large complex structures that appear to be stable cytoplasmic populations of organelles (Bagga et al., 1995, 1997). Expression of pea storage protein vicilin, modified to include the ER retention sequence KDEL in leaves, resulted in the formation of PBs, likely due to aggregation of the protein within the ER as a result of its retention (Wandelt et al., 1992).
Together these results indicate that the ER and ER-derived PBs are potentially protected sites for the storage of post-translationally unstable proteins. If such proteins can be cosequestered with proGY within ER-derived PBs in transgenic soybeans, this may provide a means to alter the protein composition of soybean seed and use soybeans to produce foreign proteins on an industrial scale. Perhaps by coexpressing other proteins, perhaps as a GY fusion protein with a cleavable spacer, it may be possible to configure soybeans to express and accumulate at high levels foreign proteins that require ER-mediated folding and processing events. This may open up the possibility for soybeans to accumulate novel gene products that would otherwise be difficult to produce in seeds.
METHODS
Production of Transgenic Soybean Lines
The production and characterization of transgenic soybean lines (Glycine max) with suppressed expression of the α subunits of β-conglycinin (BCG) were as described previously (Kinney, 1996; Kinney and Knowlton, 1998). A single, homozygous soybean line lacking both the α and α′ subunits of BCG was selected for analysis.
RNA Extraction
Fresh tissue was ground in a disposable pellet pestle and homogenized in RNA extraction buffer prewarmed to 80°C, 5 volumes per weight (mL/g of tissue). The tissue was further homogenized with the addition of 0.5 volume chloroform/isoamyl alcohol (24:1). The RNA was precipitated from the aqueous portion with the addition of equal volume of 4 M lithium chloride. The RNA was resuspended in RNase- free water. One tenth volume of 3 M sodium acetate was added and then the RNA was precipitated with 2.5 volumes ethanol. The RNA pellet was washed with cold 70% ethanol and resuspended in RNase-free water.
RNA Gel Blot Analysis
Thirty μg of RNA was subjected to electrophoresis in a 1% agarose gel containing 1 × formaldehyde gel running buffer and 2.2 M formaldehyde (100 milliamps for 3 hr). The gel was then rinsed in 20 × SSC (1 × SSC is 0.15 M NaCl and 0.015 M sodium citrate). The blotting membrane was prepared as per manufacturer's instruction (Immobilon N/Millipore, Bedford, MA), then rinsed in 20 × SSC. RNA gel blot capillary transfer was set up using a reservoir of 20 × SSC treated with diethyl pyrocarbonate; 20 × SSC was blotted through Whatman 3MM chromotography paper, gel, membrane, and paper towels. Transfer was continued overnight and the membrane was then air dried and baked at 80°C the next day.
Hybridization
The baked membrane was prehybridized at 65°C in hybridization solution for 2 hr. A probe was made using a gel-purified piece of DNA from restriction digest of the transformation plasmid and then labeled with 32P-dCTP using the Random Primers DNA Labeling System according to the manufacturer's instructions (Gibco BRL). The unincorporated 32P-dCTP was separated using a NICK column according to the manufacturer's instruction (Pharmacia Biotechnology). The radiolabeled probe was added to the hybridization solution at a concentration of 106 cpm/mL. The membrane was hybridized overnight at 65°C and then washed the next day in 2 × SSC and 0.5% SDS (two times for 5 min) and in 0.2 × SSC and 0.1% SDS (two times for 15 min). Kodak X-OMAT AR film was exposed to the membrane overnight at −80°C.
Protein Gels and Immunoblots
For SDS-PAGE analysis, 8 μL of (2×) loading buffer was added to 8 μL of sample extract. The (2×) loading buffer consisted of 100 mM Tris-HCl, pH 7.5, 4% SDS, 0.2% bromophenol blue, 15% glycerol, and 200 mM β-mercaptomethanol. The mixture was heated at 95°C for 4 min. Sample mixes were then microfuged (15,000g for 20 sec) and loaded onto a 10% precast Ready Gel (Bio-Rad) that was assembled into a mini-Protein II Electrophoresis Cell (Bio-Rad). Bio-Rad Tris/glycine/SDS buffer was used as the running buffer and voltage was a constant 125 V. In addition to sample extracts, each gel contained one lane with a molecular mass standard (Bio-Rad SDS-PAGE standard, low range) and one lane with total soybean seed protein extracted from commercial defatted soy flour. Upon completion, the gels were stained with Coomassie Brilliant Blue and destained to visualize proteins.
After transfer to a polyvinylidene difluoride (PVDF) membrane (Matsudaira, 1987), blots were rinsed in Tris-buffered saline for 10 min and blocked for 1 hr at room temperature in nonfat dry milk (Bio-Rad Blotting Grade Blocker 170-6404) in Tris-buffered saline plus Tween 20 (TTBS). Blots were rinsed two times for 5 min in TTBS at room temperature. The membrane was incubated in primary antibody (1:1000 dilution of antiGY or antiBCG antisera in 0.5% nonfat dry milk [Bio-Rad Blotting Grade Blocker 170-6404] in TTBS solution) for 1 hr at room temperature. The blot was rinsed (two times for 10 min in TTBS at room temperature and two times for 10 min with 0.5% nonfat dry milk in TTBS). The membrane was then incubated in secondary antibody solution (40 milliunits/mL). The secondary antibody was anti-rabbit IgG and horseradish peroxidase in 0.5% nonfat dry milk in TTBS for 30 min to 1 hr at room temperature. Blots were rinsed (four times for 15 min in TTBS at room temperature). Chemiluminescent detection was performed according to the procedure detailed in the Boehringer Mannheim Chemiluminescence Protein gel blotting kit (Catalog No. 1 520 709). The membrane was exposed to x-ray film for various times ranging from 5 sec to 5 min, until an optimal exposure was obtained.
Protein Extraction, Sucrose Density Centrifugation, Electrophoresis, and Immunodetection
Twenty seed were ground in liquid nitrogen, and the powder was lyophilized at −20°C in a model 24Dx48 specimen freeze dryer (Virtis, New York, NY). Combined fractions of seed globulin and albumin were extracted with a 20-fold (w/v) excess of 50 mM Tris-HCl, pH 8, 0.5 M NaCl, and 1 mM phenylmethylsulfonyl fluoride. The protein extracts were clarified by centrifugation followed by filtration of the supernatant through Ultrafree MC micro spin filters (Millipore Corp., Bedford, MA) and concentrated approximately fivefold using Centriprep-3 columns (Amicon, Beverly, MA). Protein concentrations were estimated according to the method of Bradford (Bio-Rad Protein Assay) with BSA (Pierce, Rockford, IL) as a standard, and protein samples were adjusted to 20 mg/mL in the extraction buffer.
Concentrated extract (200 μL) was loaded onto a linear 6 to 22% (w/v) sucrose gradient containing the extraction buffer and separated at 35,000 rpm in an SW41 rotor (Beckman Instruments) for 24 hr. Thirty-four fractions of ∼330 μL each were collected from the bottom of the gradient tube and 20 μL of each fraction was analyzed for protein content using the Bradford assay. The sedimentation coefficients of fractions were estimated using catalase (11.4S), ceruloplasmin (7.1S), hemoglobin (4.3S), and RNase A (1.6S) as standards (Sigma, St. Louis, MO). Proteins (20-μL gradient fraction per lane) were electrophoretically separated by SDS-PAGE on 4 to 20% Tris-tricine polyacrylamide gradient mini-gels (Bio-Rad). Separated polypeptides were visualized by Coomassie Brillant Blue staining in the presence of 30% trichloroacetic acid to fix the proteins in the gel matrix. Where indicated, electrophoretically separated polypeptides were transferred to PVDF membranes (Immobilon P; Millipore) using a semi-dry electroblotter (SemiPhor TE70; Hoefer, San Francisco, CA) as described (Matsudaira, 1987). Prestained molecular mass protein standards (SeeBlue; Novex, San Diego, CA) were used to monitor the electrophoresis and transfer.
The immune detection of antigens on PVDF blots was performed according to the protocol of Meyer et al. (1988). The antibodies used included a GY3-specific polyclonal antibody that binds to epitopes in the acidic chain and reacts with all members of the GY family (a gift from N. Nielsen, Purdue University, West Lafayette, IN). Antibodies specific for Kunitz trypsin inhibitor (KTI), soybean napin-type albumin, soybean vacuolar processing enzyme 1 (VPE1) (Hara-Nishimura et al., 1995), and soybean VPE2 (R. Jung, unpublished data) were produced in rabbits (Bethyl Laboratory, Montgomery, TX) with electrophoretically purified denatured antigens. The resulting polyclonal antibodies were affinity purified with antigen bound to Affigel 15 (Bio-Rad) according to standard procedures (Harlow and Lane, 1988). Immunoreacting bands were detected using the enhanced chemiluminescence kit from Amersham according to the manufacturer's instruction. After detection, membranes were stained for total protein in a 0.1% suspension of India ink in TTBS (Pelikan, Hanover, Germany) for 24 to 48 hr (Hancock and Tsang, 1983), followed by thorough washing in tap water.
Electron Microscopic Immunocytochemistry
Cotyledons of late maturation and germinated G19 high-oleic-acid transgenic soybeans were sliced into 1-mm cubes and fixed in 4% formaldehyde, 2% gluaraldehyde, and 0.1 M phosphate buffer, pH 7.4, overnight at 7°C. The samples were divided, and one portion was postfixed with aqueous 1% OsO4 for 3 hr and then rinsed with water. The tissue was dehydrated with a graded ethanol series and embedded in London White resin (Sigma). Further details can be found in a previous publication (Herman, 2000). Thin sections of the OSO4 postfixed tissue were stained with 5% (w/v) uranyl acetate before visualization. Immunogold assays of thin sections were accomplished by incubating thin sections in TTBS/FBS (Tris-buffered saline-Tween 20 and 20% fetal bovine serum) to block nonspecific sites followed by incubation dilutions of the primary antibody or antiserum in the same buffer with the exception of α-TIP, which used a specialized reaction mixture (Melroy and Herman, 1991). Antibodies used include antiGY (a gift from Dr. N. Nielsen), affinity-purified antiP34 monoclonal antibody (Kalinski et al., 1992), anti-α-TIP (a gift from Dr. M.J. Chrispeels, University of California, San Diego; Johnson et al., 1990), and anticomplex glycan (a gift from Dr. M.J. Chrispeels; Faye et al., 1993). Immunocytochemical assays with the α-TIP, P34, and complex glycans used dilutions and protocols identical to those published previously by this laboratory on maturing and germinating soybeans (Melroy and Herman, 1991; Kalinski et al., 1992, Yaklich and Herman, 1995). The antiGY antiserum was diluted 1:50 for a 30 min incubation. All antibodies were labeled indirectly with 10 nm colloidal gold coupled to either anti-rabbit or anti-mouse IgG as appropriate.
The sections were visualized with a Philips 400T electron microscope and images were obtained with an axial mounted black and white charge-coupled device camera at 1000 × 1000-bit resolution at 8-bit gray-scale and saved as TIFF files. Photographic data management was accomplished with Adobe Photoshop and Illustrator software (Mountain View, CA). See Herman (2000) for a more complete discussion of the use of digital imagery for immunocytochemical assays.
Acknowledgments
We are most grateful for the expert technical assistance of Kevin Stecca, Mary Locke, Debra Dempsey, and Craig Sanders. We thank Gary Fader and Enno Krebbers for helpful comments on the manuscript.
References
- Ahmed, S.U., Bar-Peled, M., and Raikhel, N.V. (1997). Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein-sorting receptors of eukaryotic cells. Plant Physiol. 114 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga, S., Adams, H., Kemp, J.D., and Sengupta-Gopalan, C. (1995). Accumulation of the 15-kD zein in novel protein bodies in transgenic tobacco. Plant Physiol. 107 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga, S., Adams, H.P., Rodriguez, F.D., Kemp, J.D., and Sengupta-Gopalan, C. (1997). Coexpression of the maize δ-zein and β-zein genes results in stable accumulation of δ-zein in endoplasmic reticulum–derived protein bodies formed by β-zein. Plant Cell 9 1683–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollini, R., and Chrispeels, M.J. (1979). The rough endoplasmic reticulum is the site of reserve-protein synthesis in developing Phaseolus vulgaris cotyledons. Planta 146 487–501. [DOI] [PubMed] [Google Scholar]
- Cheng, J.I., Boyd, C., Slaymaker, D., Okinaka, Y., Herman, E.M., and Keen, N.T. (1998). Purification and characterization of a 34 kDa syringolide binding protein from soybean. Proc. Natl. Acad. Sci. USA 95 3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels, M.J. (1983). The Golgi apparatus mediates the transport of phytohemagglutinin to the protein bodies in bean cotyledons. Planta 158 140–151. [DOI] [PubMed] [Google Scholar]
- Chrispeels, M.J., and Herman, E.M. (2000). Endoplasmic reticulum–derived compartments function in storage and as mediators of vacuolar remodeling via a new type of organelle, precursor protease vesicles (PPV). Plant Physiol. 123 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels, M.J., Higgins, T.J.V., Craig, S., and Spencer, D. (1982). Role of the endoplasmic reticulum in the synthesis of reserve proteins and the kinetics of their transport to protein bodies in developing pea cotyledons. J. Cell Biol. 93 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, C.E., Herman, E.M., Takasaki, K., and Larkins, B.A. (1996). γ-zein sequesters α-zein and stabilizes its accumulation in transgenic tobacco endosperm. Plant Cell 8 2335–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski, J.E., Schroeder, M.R., Bednarek, S.Y., and Raikhel, N.V. (1993). Determination of the functional elements within the vacuolar targeting signal of barley lectin. Plant Cell 5 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye, L., Gomord, V., Fitchette-Laine, A.C., and Chrispeels, M.J. (1993). Affinity purification of antibodies specific for Asn-linked glycans containing alpha 1→3 fucose or beta 1→2 xylose. Anal. Biochem. 209 104–108. [DOI] [PubMed] [Google Scholar]
- Galili, G., and Herman, E.M. (1996). Protein storage vacuoles. Adv. Bot. Res. 25 112–140. [Google Scholar]
- Gilkes, N.R., Herman, E.M., and Chrispeels, M.J. (1979). Rapid degradation and limited synthesis of phospholipids in cotyledons of mung bean seedlings. Plant Physiol. 64 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, L., and Chrispeels, M.J. (1993). Tonoplast and soluble vacuolar proteins are targeted by different mechanisms. Plant Cell 5 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, K., and Tsang, V.C.W. (1983). India ink staining of proteins on nitrocellulose paper. Anal. Biochem. 133 157–162. [DOI] [PubMed] [Google Scholar]
- Harada, J.J., Barker, S.J., and Goldberg, R.B. (1989). Soybean β-conglycinin genes are clustered in several DNA regions and are regulated by transcriptional and posttranscriptional processes. Plant Cell 1 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Takeuchi, Y., and Nishimura, M. (1993. a). Molecular characterization of a vacuolar processing enzyme related to a putative cysteine proteinase of Schistosoma mansoni. Plant Cell 5 1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Takeuchi, Y., Inoue, K., and Nishimura, M. (1993. b). Vesicle transport and processing of the precursor to 2S albumin in pumpkin. Plant J. 4 793–800. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Shimada, T., Hiraiwa, N., and Nishimura, M. (1995). Vacuolar processing enzyme responsible for maturation of seed proteins. J. Plant Physiol. 145 632–640. [Google Scholar]
- Hara-Nishimura, I., Shimada, T., Hatano, K., Takeuchi, Y., and Nishimura, M. (1998). Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Hayashi, M., Toriyama, K., Kondo, M., Hara-Nishimura, I., and Nishimura, M. (1999). Accumulation of a fusion protein containing 2S albumin induces novel vesicles in vegetative cells of Arabidopsis. Plant Cell Physiol. 40 263–272. [DOI] [PubMed] [Google Scholar]
- Helm, R.M., Cockrell, G., Herman, E., Burks, A.W., Sampson, H.A., and Bannon, G.A. (1998). Cellular and molecular characterization of a major soybean allergen. Int. Arch. Allergy Immunol. 117 29–37. [DOI] [PubMed] [Google Scholar]
- Herman, E.M. (1987). Immunogold-localization and synthesis of an oil-body membrane protein in developing soybean seeds. Planta 172 336–345. [DOI] [PubMed] [Google Scholar]
- Herman, E.M. (1994. a). Multiple origins of intravacuolar protein accumulation of plant cells. Adv. Structur. Biol. 3 243–283. [Google Scholar]
- Herman, E.M. (1994b). The cell and molecular biology of seed oil bodies. In Seed Development and Germination, J. Kigel and G. Gallili, eds (New York: Marcel Dekker), pp. 195–214.
- Herman, E.M. (2000). Electron microscopic immunogold localization. In Plant Electron Microscopy and Cytochemistry, W.V. Dashek, ed (Totowa, NJ: Humana Press Inc.), pp. 247–262.
- Herman, E.M., and Larkins, B.A. (1999). Protein storage bodies. Plant Cell 11 601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, E.M., and Shannon, L.M. (1984). Immunocytochemical localization of concanavalin A in developing jack bean cotyledons. Planta 161 97–104. [DOI] [PubMed] [Google Scholar]
- Herman, E.M., and Shannon, L.M. (1985). Accumulation and subcellular localization of α galactosidase in developing soybean cotyledons. Plant Physiol. 77 886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, L.M., Donaldson, D.D., and Herman, E.M. (1988). A modified storage protein is synthesized, processed, and degraded in seeds of transgenic plants. Plant Molec. Biol. 11 717–729. [DOI] [PubMed] [Google Scholar]
- Johnson, K.D., Hofte, H., and Chrispeels, M.J. (1990). An intrinsic tonoplast protein of protein storage vacuoles in seeds is structurally related to a bacterial solute transporter (GlpF). Plant Cell 2 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski, A.J., Melroy, D.L., Dwivedi, R.S., and Herman, E.M. (1992). A soybean vacuolar protein (P34) related to thiol proteases which is synthesized as a glycoprotein precursor during seed maturation. J. Biol. Chem. 267 12068–12076. [PubMed] [Google Scholar]
- Kinney, A.J. (1996). Development of genetically engineered soybean oils for food uses. J. Food Lipids 3 273–292. [Google Scholar]
- Kinney, A.J., and Fader, G.M (1998). Suppression of specific classes of soybean storage protein genes. Int. Patent App. WO9747731.
- Kinney, A.J., and Knowlton, S. (1998). Designer oils: The high oleic acid soybean. In Genetic Modification in the Food Industry, S. Roller and S. Harlander, eds (Blackie: London), pp. 193–213.
- Koehler, S.M., and Ho, T.H. (1990). Hormonal regulation, processing, and secretion of cysteine proteinases in barley aleurone layers. Plant Cell 2 769–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins, B.A., and Hurkman, W.J. (1978). Synthesis and deposition of zein in protein bodies of maize endosperm. Plant Physiol. 62 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lending, C.R., and Larkins, B.A. (1989). Changes in the zein composition of protein bodies during maize endosperm development. Plant Cell 1 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanony, H., Rubin, R., Altshuler, Y., and Galili, G. (1992). Evidence of a novel route of wheat storage proteins to vacuoles. J. Cell Biol. 119 1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira, P. (1987). Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem. 262 10035–10038. [PubMed] [Google Scholar]
- Meinke, D.W., Chen, J., and Beachy, R.N. (1981). Expression of storage protein genes during soybean seed development. Planta 153 130–139. [DOI] [PubMed] [Google Scholar]
- Melroy, D.L., and Herman, E.M. (1991). TIP, an integral membrane protein of the soybean seed protein storage vacuole, undergoes developmentally regulated membrane insertion and removal. Planta 184 113–122. [DOI] [PubMed] [Google Scholar]
- Meyer, D.J., Afonso, C.L., and Galbraith, D.W. (1988). Isolation and characterization of monoclonal antibodies directed against plant plasma membrane and cell wall epitopes: Identification of a monoclonal antibody that recognizes extensin and analysis of the process of epitope biosynthesis in plant tissues and cell cultures. J. Cell Biol. 107 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müntz, K. (1996). Proteases and proteolytic cleavage of storage proteins in developing and germinating dicotyledonous seeds. J. Exp. Bot. 298 605–622. [Google Scholar]
- Nielsen, N.C., and Nam, Y.-W. (1999). Soybean globulins. In Seed Proteins, P.R. Shewry and R. Casey, eds (Kluwer Academic Publishers: Dordrecht, The Netherlands), pp. 285–313.
- Nielsen, N.C., Dickinson, C.D., Cho, T.-J., Thanh, V.H., Scallon, B.J., Fischer, R.L., Sims, T.L., Drews, G.N., and Goldberg, R.B. (1989). Characterization of the glycinin gene family in soybean. Plant Cell 1 313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, N.C., Jung, R., Nam, Y., Beaman, T.W., Oliveira, L.O., and Bassuner, R.B. (1995). Synthesis and assembly of 11S globulins. J. Plant Physiol. 145 641–647. [Google Scholar]
- Paris, N., Rogers, S.W., Jiang, L.W., Kirsch, T., Beevers, L., Phillips, T.E., and Rogers, J.C. (1997). Molecular cloning and further characterization of a probable plant vacuolar sorting receptor. Plant Physiol. 115 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo, J.J., Chrispeels, M.J., and Herman, E.M. (1995). Degradation of transport-competent destabilized phaseolin with a signal for retention in the endoplasmic reticulum occurs in the vacuole. Planta 196 586–596. [DOI] [PubMed] [Google Scholar]
- Sanderfoot, A.A., and Raikhel, N.V. (1999). The specificity of vesicle trafficking: Coat proteins and SNAREs. Plant Cell 11 629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling, L., Drews, G.N., and Goldberg, R.B. (1986). Transcriptional and posttranscriptional regulation of soybean seed protein mRNA levels. Proc. Natl. Acad. Sci. USA 83 2123–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandelt, C.I., Khan, M.R.I., Craig, S., Schroeder, H.E., Spencer, D., and Higgins, T.J.V. (1992). Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants. Plant J. 2 181–192. [DOI] [PubMed] [Google Scholar]
- Williamson, J.O., Galili, G., Shaw, B.A., Larkins, B.A., and Gelvin, S.B. (1988). The synthesis of a 19 kD zein protein in transgenic petunia plants. Plant Physiol. 88 1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, N.S., Wierzbicki, A., Aegerter, M., Caster, C.S., Perez-Grau, L., Kinney, A.J., Hitz, J.R., Booth, W.D., Jr., Schweiger, B., and Stecca, K.L. (1993). Cloning of higher plant omega-3 fatty acid desaturases. Plant Physiol. 103 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaklich, B., and Herman, E.M. (1995). Protein storage vacuoles of soybean aleurone cells accumulate a unique glycoprotein as well as proteins thought to be embryo specific. Plant Sci. 107 57–67. [Google Scholar]
- Yaklich, R., Helm, R., and Herman, E. (1999). Analysis of the distribution of the major soybean allergen in a core collection of Glycine max accessions. Crop Sci. 39 1444–1447. [Google Scholar]