Abstract
Although phosphatidylinositol transfer proteins (PITPs) are known to serve critical functions in regulating a varied array of signal transduction processes in animals and yeast, the discovery of a similar class of proteins in plants occurred only recently. Here, we report the participation of Ssh1p, a soybean PITP-like protein, in the early events of osmosensory signal transduction in plants, a function not attributed previously to animal or yeast PITPs. Exposure of plant tissues to hyperosmotic stress led to the rapid phosphorylation of Ssh1p, a modification that decreased its ability to associate with membranes. An osmotic stress–activated Ssh1p kinase activity was detected in several plant species by presenting recombinant Ssh1p as a substrate in in-gel kinase assays. Elements of a similar osmosensory signaling pathway also were conserved in yeast, an observation that facilitated the identification of soybean protein kinases SPK1 and SPK2 as stress-activated Ssh1p kinases. This study reveals the activation of SPK1 and/or SPK2 and the subsequent phosphorylation of Ssh1p as two early successive events in a hyperosmotic stress–induced signaling cascade in plants. Furthermore, Ssh1p is shown to enhance the activities of a plant phosphatidylinositol 3-kinase and phosphatidylinositol 4-kinase, an observation that suggests that the ultimate function of Ssh1p in cellular signaling is to alter the plant's capacity to synthesize phosphoinositides during periods of hyperosmotic stress.
INTRODUCTION
Phosphatidylinositol transfer proteins (PITPs) were identified originally by their ability to serve as diffusible carriers of phosphatidylinositol (PtdIns) and to a lesser extent phosphatidylcholine (PtdCho) from one distinct membrane compartment to another by using an in vitro assay (Wirtz, 1991). In recent years, several intriguing and critical biological roles beyond the transfer of phospholipids have been attributed to yeast and animal PITPs. The yeast PITP (Sec14p) is an essential protein that is required for cells to properly execute the formation of secretory vesicles from the Golgi complex (Bankaitis et al., 1990). A considerable body of evidence suggests that Sec14p serves as a “molecular sensor” to monitor and regulate the levels of PtdIns, PtdCho, and potentially diacylglycerol in the Golgi complex of yeast (Skinner et al., 1995; Kearns et al., 1997). In addition, Sec14p has been implicated in modulating the activity of a PtdIns 4-kinase that regulates protein secretion (Hama et al., 1999).
An essential role for PITPs also is observed in mammals and Drosophila, in which the loss of PITP function leads to specific neurodegenerative diseases (Hamilton et al., 1997; Milligan et al., 1997). At the cellular level, the mammalian PITP is known to be required for inositol lipid signaling, secretory vesicle formation from the trans-Golgi network, and the fusion of secretory vesicles to the plasma membrane (Hay and Martin, 1993; Cunningham et al., 1995; Kauffmann-Zeh et al., 1995). Although yeast and animal PITPs are very similar in size, share identical PtdIns and PtdCho transfer properties, and are both required for secretory vesicle formation within their respective cellular envi-ronments, the two proteins display no amino acid sequence similarity (Bankaitis et al., 1989; Dickeson et al., 1989). Despite the lack of sequence conservation, a considerable degree of functional equivalence has been documented. Yeast Sec14p can substitute for mammalian PITP in stimulating the formation and fusion of secretory vesicles (Hay et al., 1995; Wiedemann et al., 1996) and in assays that measure the stimulation of inositol lipid signaling (Cunningham et al., 1996). Similarly, expression of mammalian PITPs can restore viability to yeast strains harboring a mutation in the SEC14 gene (Skinner et al., 1993; Tanaka and Hosaka, 1994).
Until recently, the question of whether higher plants possess PITP-like proteins remained unanswered. We reported the isolation, by functional complementation of a yeast strain harboring a temperature-sensitive mutation at the sec14-1 locus, of two unique soybean genes, designated SSH1 and SSH2 (Soybean SEC14 homolog 1 and 2, respectively), each encoding a distinct PITP-like protein (Kearns et al., 1998). Both soybean PITP-like proteins, designated Ssh1p and Ssh2p, exhibit limited but significant sequence homology with the yeast Sec14p. Ssh1p and Ssh2p exhibit unique lipid transfer specificities that distinguish them somewhat from their mammalian and yeast counterparts. Neither soybean protein is capable of facilitating the transfer of PtdCho in vitro, one of the hallmarks of animal and yeast PITPs. Surprisingly, Ssh1p even appears to lack in vitro PtdIns transfer function, despite its sequence similarity to yeast Sec14p and its ability to functionally complement deficiencies in yeast PITP activity; Ssh2p, in contrast, displays a robust in vitro PtdIns transfer activity (Kearns et al., 1998).
Further evidence that higher plants possess PITP-like proteins is found in the report by Jouannic et al. (1998), who similarly described the isolation of an Arabidopsis cDNA by functional complementation of the yeast sec14 mutation. The Arabidopsis PITP-like protein shows substantially greater sequence homology with soybean Ssh1p than with Ssh2p. Unlike Ssh1p, however, AtSec14p was reported to display in vitro PtdIns transfer activity.
Although the identification of soybean and Arabidopsis PITP-like cDNAs by complementation of yeast mutants confirms the existence of this class of protein within the plant kingdom, their function within the plant cell remains to be elucidated. Considering the diverse array of cellular and whole-organism phenotypes associated with PITPs in animals and yeast, establishing the cellular functions of these plant proteins is not likely to be trivial. One of the novel findings in our initial characterization of SSH1 expression in yeast was the observation that Ssh1p became phosphorylated upon exposure to hyperosmotic stresses within this heterologous system (Kearns et al., 1998). In this report, we expound on this phenomenon within higher plants. Ssh1p is rapidly phosphorylated after plant tissue is treated specifically with agents that induce a hyperosmotic stress response. We identify SPK1 and SPK2, two soybean serine/threonine protein kinases that are members of the SnRK2 subfamily of the SNF1-related family of protein kinases (Halford and Hardie, 1998), as representing hyperosmotic stress–activated Ssh1p kinases. Our findings define the soybean PITP-like protein, Ssh1p, and the SPK1 and SPK2 kinases as early components of a hyperosmotic stress signal transduction pathway in plants. Furthermore, we demonstrate that Ssh1p enhances the in vitro synthesis of the phosphoinositides PtdIns(4)P and PtdIns(3)P and propose that the cellular function of the soybean PITP-like protein is to stimulate PtdIns kinase activities.
RESULTS
Hyperosmotic Stress Induces Rapid Phosphorylation of Ssh1p in Soybean and Transgenic Tobacco
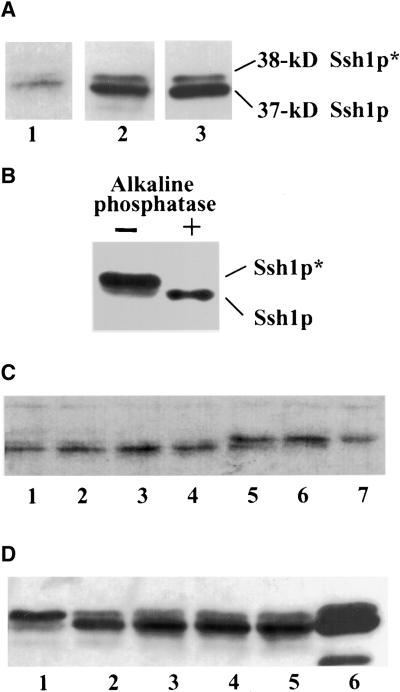
By expressing the soybean SSH1 cDNA in yeast, we were able to establish certain important biochemical characteristics of the encoded protein, such as its inability to mediate the transfer of PtdIns or PtdCho in vitro and its binding affinity toward phosphoinositides (Kearns et al., 1998). Analysis of Ssh1p within its native environment, however, proved to be considerably more difficult because of the comparatively low levels of Ssh1p accumulation observed in soybean relative to the yeast system, in which high copy vectors and strong promoters were used to facilitate gene expression. Detection of native Ssh1p in various soybean tissues by immunoblot analysis proved to be difficult, with nonspecific antibody binding often preventing adequate visualization of the presumably low levels of Ssh1p found in these tissues (data not shown). The scarcity of endogenous Ssh1p as judged by immunoblotting was consistent with our RNA gel blot results, which revealed that SSH1 transcript could be detected only by using purified mRNA preparations and not total RNA (Kearns et al., 1998). Of several soybean tissues surveyed, newly emergent soybean roots (2 to 3 cm in length) proved to be the only tissue in which Ssh1p could be detected consistently and unambiguously with our polyclonal antibody. Figure 1A shows a typical immunoblot profile of Ssh1p in young soybean roots.
Figure 1.
Increased Phosphorylation of Ssh1p Is Induced upon Exposure of Plant Tissues to Hyperosmotic Stress.
(A) Immunoblot analysis of Ssh1p in untreated soybean root (lane 1; 100 μg of protein), transgenic tobacco leaf (lane 2; 40 μg of protein), and yeast strain CTY182 expressing SSH1 (lane 3; 3 μg of protein). Sizes of the phosphorylated (Ssh1p*) and nonphosphorylated forms of Ssh1p were estimated using molecular mass standards.
(B) Immunoblot analysis of transgenic tobacco leaf extracts incubated in the presence (+) or absence (−) of calf intestinal alkaline phosphatase. Leaf discs were incubated with 1 M NaCl for 30 min before the isolation of the cell-free extracts to enhance the accumulation of the Ssh1p* species.
(C) Immunoblot analysis of soybean roots exposed to 37°C (lane 1), 4°C (lane 2), 0.1 mM abscisic acid (lane 3), water control (lane 4), desiccation (lane 5), 0.2 M NaCl (lane 6), and 50% glycerol (lane 7) for 2.5 hr.
(D) Immunoblot analysis of transgenic tobacco leaf segments exposed to 0.5 M NaCl (lane 1), water control (lane 2), 20 μM 2,4-D (lane 3), 50 μM fusicoccin (lane 4), and 5 μM gibberellic acid (lane 5) for 2.5 hr. Lane 6 represents Ssh1p produced in yeast as a control.
As an additional tool to assist our characterization of Ssh1p within plant cells, transgenic tobacco plants were generated using SSH1 gene constructs under the transcriptional control of the 35S promoter of Cauliflower mosaic virus. Soybean Ssh1p is easily visualized by immunoblot analysis in transgenic tobacco (Figure 1A). Similar to our observation of SSH1 expression in yeast (Kearns et al., 1998), Ssh1p resolves by SDS-PAGE as a 37- and 38-kD doublet (Ssh1p and Ssh1p*, respectively) both in its native environment and when expressed in transgenic tobacco. Ssh1p* in yeast was shown to represent phosphorylated Ssh1p (Kearns et al., 1998). Evidence that the 38-kD species in plants also represents phosphorylated Ssh1p includes the collapse of the 38-kD species to the 37-kD form upon treatment of clarified cell extracts with alkaline phosphatase (Figure 1B) and our characterization of a specific Ssh1p kinase in plants (see below). In all three systems in which SSH1 expression has been assayed (soybean, transgenic tobacco, and yeast), under normal growth conditions the nonphosphorylated form predominates (Figure 1A), with Ssh1p* typically constituting ≤10% of the total Ssh1p species.
An increasing body of literature has demonstrated that mammalian and fungal PITPs are critical components of certain cellular signaling pathways (reviewed in Cockcroft, 1998). This prompted us to test the possibility that the soybean Ssh1p PITP-like protein is involved in signal transduction. One approach involved subjecting soybean, transgenic tobacco, and yeast materials to an array of biotic and abiotic treatments followed by immunoblot analysis to determine whether changes had occurred in either total Ssh1p abundance or the relative ratios of Ssh1p and Ssh1p*. Young soybean roots and/or leaf segments of transgenic tobacco expressing SSH1 were exposed to the following treatments: temperature and pH extremes, wounding, desiccation, high NaCl, and high concentrations of glycerol, sorbitol, abscisic acid, gibberellic acid, salicylic acid, mastoparan analogs, gadolinium, fusicoccin, and 2,4-D. The results of some of these treatments are shown in Figures 1C and 1D. Although no obvious differences in total Ssh1p abundance (Ssh1p plus Ssh1p*) were observed in these assays, a dramatic increase in the relative amount of Ssh1p* was detected upon exposure of the plant tissues to conditions of hyperosmotic stress (i.e., desiccation and high concentrations of NaCl, glycerol, and sorbitol); no other treatment elicited a notable change in the Ssh1p*/Ssh1p ratio (Figures 1C and 1D). Concomitantly, we found a similarly dramatic increase in Ssh1p* after exposing yeast strains expressing SSH1 to hyperosmotic stress. This result was reported in our previous characterization of the properties of Ssh1p when expressed in the heterologous yeast system (Kearns et al., 1998).
To establish more completely the conditions in which Ssh1p phosphorylation becomes induced, we exposed leaf segments of SSH1-expressing transgenic tobacco plants to different concentrations of NaCl for varying lengths of time. These assays were conducted by floating leaf discs on various NaCl solutions followed by immunoblot analysis of total protein preparations. We initially speculated that vacuum infiltration of the medium into the leaf discs might result in more efficient exposure of the individual cells to the stress than would occur by merely floating the leaf segments on the solutions. Upon comparing the results of vacuum-infiltrated and noninfiltrated samples at various NaCl concentrations, however, no differences were observed in either the timing or the magnitude of the response (data not shown). Therefore, we routinely initiated the stress response by simply floating the leaf segments on the treatment solutions.
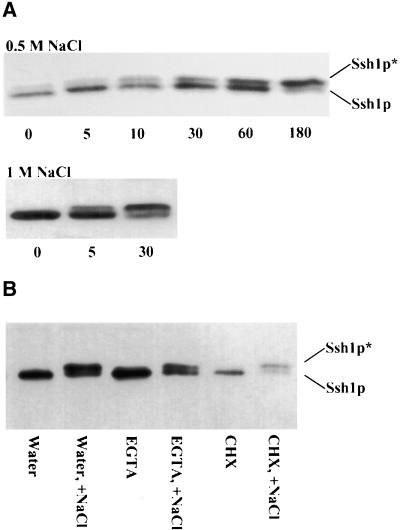
As shown in Figure 2A, a notable increase in the relative amount of Ssh1p* was observed after 10 min when transgenic tobacco leaf discs were placed on a solution of 0.5 M NaCl. By 3 hr, most of the Ssh1p protein was detected as the phosphorylated species. When 1 M NaCl was used, the results were even more dramatic. A notable increase in the amount of Ssh1p* was detected within 5 min after exposure to 1 M NaCl, and by 30 min the ratio of Ssh1p* to Ssh1p was comparable to that observed after 3 hr of treatment with 0.5 M NaCl. Although exposure of tobacco leaf segments to a NaCl concentration of 0.25 M resulted in no detectable increase in Ssh1p* accumulation during a 3-hr time course, after 6 hr a modest increase in Ssh1p phosphorylation was observed (data not shown). This observation differs somewhat from the results found when young soybean roots were treated with 0.2 M NaCl for a 2.5-hr period; this treatment was effective at inducing a dramatic shift in the Ssh1p*/Ssh1p ratio (Figure 1C, lane 6).
Figure 2.
Induced Phosphorylation of Ssh1p Increases with the Severity and Duration of the Hyperosmotic Stress and Is Not Influenced by Pretreatment with EGTA or CHX.
(A) Immunoblot analysis of protein preparations from transgenic tobacco leaf segments floated on solutions of 0.5 or 1 M NaCl. The duration of the exposure is indicated in minutes. The phosphorylated (Ssh1p*) and nonphosphorylated forms of Ssh1p are indicated.
(B) Immunoblot analysis of transgenic tobacco leaf discs treated with water (control), 50 mM EGTA, or 5 mM CHX. Lanes marked +NaCl indicate tissue that was additionally exposed to 1 M NaCl after a 2-hr incubation with the original treatment alone.
Usami et al. (1995) showed that protein synthesis can be effectively inhibited in tobacco leaf discs by incubating the tissue on a solution of 0.3 mM cycloheximide (CHX). To determine whether hyperosmotic stress–induced increases in Ssh1p* accumulation were dependent on new protein synthesis, we preexposed transgenic tobacco leaf discs to 5 mM CHX for 2 hr before treatment with 1 M NaCl for 30 min. An additional set of leaf discs was pretreated with 50 mM EGTA for 2 hr before NaCl induction to determine whether inhibiting the pool of available extracellular Ca2+ affected Ssh1p* accumulation. As shown in Figure 2B, neither pretreatment significantly altered the NaCl-induced phosphorylation of Ssh1p.
Phosphorylation of Ssh1p Coincides with a Loss of Membrane Association
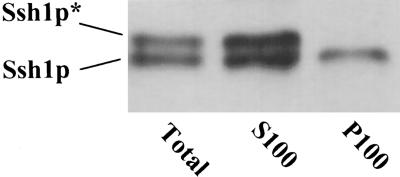
Our previous characterization of SSH1 expression in yeast included the observation that nonphosphorylated Ssh1p localized to both cellular membranes and a membrane-free supernatant fraction (Kearns et al., 1998). In contrast, Ssh1p* appeared to show no affinity for cellular membranes. Immunoblot analysis of subcellular fractions of NaCl-stressed tobacco leaf tissue suggested that this property is shared when SSH1 is expressed in plant cells. As shown in Figure 3, Ssh1p and Ssh1p* were found in both the crude homogenate fraction and the 100,000g supernatant fraction. Only nonphosphorylated Ssh1p, however, was found in association with the membrane-rich 100,000g pellet.
Figure 3.
Ssh1p* Does Not Associate with Microsomal Membrane Preparations.
Transgenic tobacco leaf segments were exposed to 0.5 M NaCl for 1 hr followed by tissue homogenization and differential centrifugation. Immunoblot analysis was conducted using a crude cellular fraction (Total) and the supernatant (S100) and pellet (P100) fractions recovered from a 100,000g centrifugation. The phosphorylated (Ssh1p*) and nonphosphorylated forms of Ssh1p are indicated.
Hyperosmotic Stress–Induced Phosphorylation of Ssh1p Is Localized to the Site of Stress Application
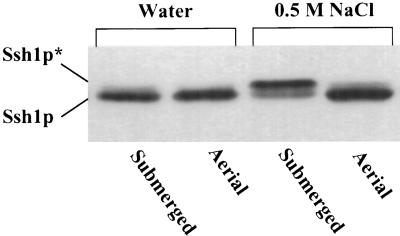
In the experiments described above, osmotic stresses were applied by directly exposing plant tissues to a hypertonic solution. To determine whether the cellular signals responsible for inducing Ssh1p phosphorylation could be relayed rapidly to plant tissue distal to the specific site of stress application, we used a detached leaf assay in which only the base of a severed leaf was submerged in a hyperosmotic solution. Immersion of the lower 1 cm of a freshly cut transgenic tobacco leaf (total length ∼6 cm) in a 0.5-M NaCl solution for 1 hr resulted in a severe loss of turgor in the aerial portion of the leaf. As shown in Figure 4, immunoblot analysis of protein extracted from the submerged leaf base revealed substantial phosphorylation of Ssh1p in contrast with a control leaf submerged in water. Despite the severely wilted phenotype displayed by the aerial portion of the leaf, no increase in Ssh1p* was detected in this tissue, even when samples as close as 0.75 cm above the site of stress application were assayed (Figure 4). Thus, within the time frame of this experiment, the induction of Ssh1p phosphorylation appeared to be confined to the tissue that was in direct contact with the stress-inducing solution.
Figure 4.
Hyperosmotic Induction of Ssh1p Phosphorylation Is Restricted to the Site of Stress Application.
The cut ends of leaves excised from a transgenic tobacco plant expressing SSH1 were placed in water or 0.5 M NaCl for 1 hr. Protein samples extracted from the portion of the leaf exposed directly to the solution (Submerged) and from a leaf segment located 0.75 cm above the submerged portion (Aerial) were subjected to immunoblot analysis. The phosphorylated (Ssh1p*) and nonphosphorylated forms of Ssh1p are indicated.
Ssh1p Is the Substrate of a Hyperosmotic Stress–Activated Ser/Thr Protein Kinase
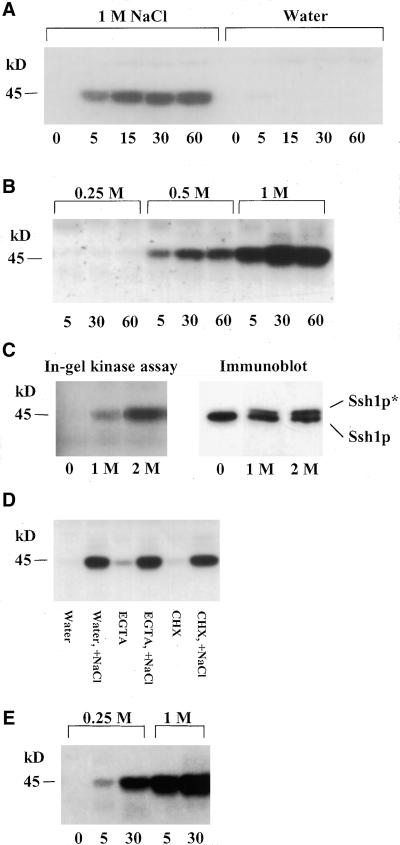
In-gel kinase assays have proven to be very useful in establishing several physiological and biochemical properties of protein kinases whose activities are capable of being restored after removal from the harsh denaturing conditions of SDS-PAGE (Zhang et al., 1993; Zhang and Klessig, 1997). Among the properties that can be assessed using this assay are the apparent molecular mass of the kinase, its substrate specificity, and activation kinetics. Total protein extracts from leaf tissue of wild-type tobacco exposed to 1 M NaCl were separated by SDS-PAGE using gels embedded with purified recombinant Ssh1p. As shown in Figure 5A, the subsequent in-gel kinase assay revealed that Ssh1p appears to be the substrate of a 45-kD NaCl-induced protein kinase. Although total protein preparations were typically used in these assays, Ssh1p kinase activity was recovered quantitatively in a membrane/organelle-free 100,000g supernatant fraction (data not shown).
Figure 5.
Ssh1p Is Phosphorylated by a Hyperosmotic Stress–Activated 45-kD Kinase.
(A) and (B) In-gel kinase assays of protein extracts from tobacco leaf segments exposed to various concentrations of NaCl or water for the indicated times (minutes). Protein samples were separated on 10% SDS–polyacrylamide gels embedded with purified recombinant Ssh1p. The size of the visualized kinase was estimated using molecular mass standards.
(C) In-gel kinase assay and immunoblot analysis of transgenic tobacco leaf discs treated with 1 and 2 M sorbitol for 30 min.
(D) In-gel kinase assay of tobacco leaf segments treated with water, 50 mM EGTA, or 5 mM CHX. Lanes marked +NaCl indicate tissue that was additionally exposed to 1 M NaCl for 30 min after a 2-hr incubation with the original treatment alone.
(E) In-gel kinase assay of total cellular protein from tobacco cell suspension cultures exposed to 0.25 or 1 M NaCl for the indicated times (minutes).
To test the specificity of the 45-kD kinase for Ssh1p, we tested three additional proteins as potential substrates in the in-gel kinase assay: recombinant Ssh2p, casein, and myelin basic protein (MBP). Furthermore, control assays were conducted using gels with no added protein to test for the possibility of autophosphorylation of the 45-kD kinase. Although the Ssh2p protein shares some sequence homology with Ssh1p and both proteins have been shown to bypass the sec14 mutation in yeast (Kearns et al., 1998), no in-gel kinase activity was detected in tobacco leaf preparations that recognized this protein as a substrate (data not shown). Because the recombinant Ssh1p and Ssh2p proteins share identical N-terminal His6 domains (derived from vector pRSET) and were purified by identical means, it is unlikely that either sequences in this N-terminal domain or minor amounts of contaminating Escherichia coli proteins could provide the substrate for the 45-kD kinase. The introduction of casein as an in-gel kinase substrate also failed to identify the 45-kD kinase, nor was this band detected in control gels without protein substrate, confirming that the signal observed (Figure 5A) is not the product of autophosphorylation (data not shown). In contrast, MBP does appear to serve as an alternative substrate for the Ssh1p kinase (see below).
Exposure of tobacco leaf discs to varying conditions of stress severity and duration revealed an activation profile for the Ssh1p kinase that correlated very well with the increases in Ssh1p* accumulation observed by immunoblot analysis. Treatment with 1 M NaCl elicited a much greater response than that with 0.5 M NaCl, whereas exposure to 0.25 M NaCl was insufficient to activate the Ssh1p kinase during the course of 1 hr (Figure 5B). Equally good correlations between Ssh1p kinase activation, as determined by in-gel kinases assays, and increased phosphorylation of Ssh1p, as judged by immunoblotting, were observed when alternative agents such as sorbitol were used to induce a hyperosmotic stress response (Figure 5C). Finally, pretreatment of the tobacco leaf discs with either EGTA or CHX failed to inhibit the NaCl-induced activation of the Ssh1p kinase (Figure 5D), a result consistent with the failure of these compounds to alter the induced accumulation of Ssh1p* in the transgenic tobacco materials (Figure 2B). Together, these observations strongly support the conclusion that the Ssh1p kinase detected by the in-gel kinase assay is the same kinase responsible for the increased phosphorylation of Ssh1p in transgenic tobacco plants in vivo.
Although the leaf disc system enabled us to compare directly the activation of the Ssh1p kinase with increases in Ssh1p phosphorylation by using identical plant materials and assay conditions, interpretation of the induction kinetics at the cellular level was hindered by the inability to apply a uniform osmotic stress to all cells when using intact tissue. Cell cultures offer a system whereby the effects of stress application at the cellular level can be controlled more precisely. As shown in Figure 5E, the activation profile of the Ssh1p kinase was somewhat altered when in-gel kinase assays were conducted using salt-stressed BY-2 tobacco culture cells; induction was apparent after exposure to 0.25 M NaCl for 5 and 30 min. Similar to the results using intact plant tissue, however, much greater activation was observed when the culture cells were exposed to 1 M NaCl for the same time periods.
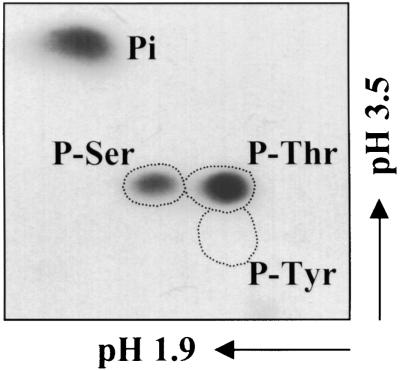
To establish which amino acid residues were substrates for the Ssh1p kinase, an in-gel kinase assay was conducted similar to that shown in Figure 5A. Subsequently, the 45-kD region of this gel was excised and subjected to acid hydrolysis, and the products were resolved using two-dimensional electrophoresis. As shown in Figure 6, serine and threonine residues, but not tyrosine residues, were radiolabeled with 32P. Quantitation of the signals revealed an approximately threefold greater incorporation of radiolabel in 32P-Thr compared with 32P-Ser.
Figure 6.
Phosphoamino Acid Analysis of Ssh1p*.
The 45-kD region of an in-gel kinase reaction similar to that shown in Figure 5A (using purified Ssh1p as the embedded substrate and NaCl-stressed tobacco leaf extracts) was excised and subjected to partial acid hydrolysis. The resulting products were analyzed using two-dimensional electrophoresis and autoradiography. Dotted regions indicate the migration of nonradiolabeled phosphoserine (P-Ser), phosphothreonine (P-Thr), and phosphotyrosine (P-Tyr) standards. Pi, inorganic phosphate.
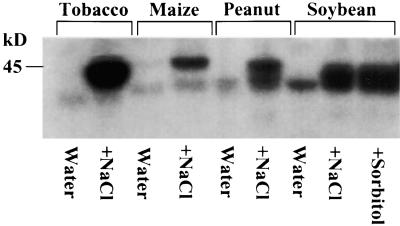
To determine whether an NaCl-inducible Ssh1p kinase activity can be detected among diverse plant species, we treated leaf discs obtained from young soybean, maize, and peanut plants with 1 M NaCl for 1 hr and subjected them to the in-gel kinase assay with embedded Ssh1p serving as the substrate. For each plant species, an inducible kinase migrating at ∼45 kD was observed, as shown in Figure 7. Soybean leaf discs also were treated with 2 M sorbitol for 1 hr, giving results similar to the 1 M NaCl treatment. Of the four species tested using this assay, we consistently observed the greatest activity from tobacco leaf segments. Particularly notable in the soybean and peanut preparations was a noninducible band that migrated slightly below the inducible species. Whether this species represents a relatively low level constitutive kinase activity capable of recognizing Ssh1p as a substrate or is merely the result of autophosphorylation has not been determined.
Figure 7.
Ssh1p Kinase Activity Is Induced by Hyperosmotic Stress in Diverse Plant Species.
Leaf discs from four plant species were exposed to either water or 1 M NaCl for 1 hr. For soybean, a 2-M sorbitol treatment (1 hr) was included as well. Each lane represents 60 μg of a crude total protein fraction that was subjected to in-gel kinase analysis using purified Ssh1p as the embedded substrate.
The Ssh1p Kinase Does Not Appear to Be a Mitogen-Activated Protein Kinase
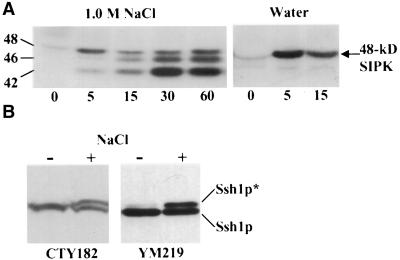
Mitogen-activated protein (MAP) kinases are well known for participating in the early stages of a variety of stress-activated signal transduction cascades in eukaryotes, including osmotic stresses (Maeda et al., 1994; Hirt, 1997; Mizoguchi et al., 1997). Because most known MAP kinases can phosphorylate MBP in vitro, it is widely used as an artificial substrate in studies designed to measure MAP kinase activities (Hirt, 1997; Mizoguchi et al., 1997). As shown in Figure 8A, when protein preparations from NaCl-stressed tobacco leaf discs were subjected to an in-gel kinase assay using MBP instead of Ssh1p as the embedded substrate, three bands were observed ranging from 42 to 48 kD. The activity of the 48-kD kinase could be induced by wounding alone, as shown by its activation in tobacco leaf segments exposed only to water. The specific immunoprecipitation of this 48-kD species from tobacco preparations by using antibodies that recognize salicylic acid–inducible protein kinase (SIPK), a 48-kD salicylic acid– and wound-inducible MAP kinase (Zhang and Klessig, 1997, 1998), verified the identity of this band (data not shown). Although recent reports suggest that SIPK also can be induced by hyperosmotic stress in tobacco cell cultures (Droillard et al., 2000; Hoyos and Zhang, 2000; Mikolajczyk et al., 2000), we did not observe any induction of SIPK activity upon exposure to hyperosmotic medium beyond what could be induced by wounding alone using the leaf disc system (Figure 8A). The identity of the NaCl-activated 46-kD kinase is unknown.
Figure 8.
Ssh1p Kinase Appears to Recognize MBP as a Substrate, but Ssh1p Phosphorylation Is Unaffected in a Yeast Strain Completely Lacking MAP Kinase Activities.
(A) In-gel kinase assay using extracts from tobacco leaf segments exposed to 1 M NaCl or water for the times indicated (minutes). Protein samples were analyzed using a 10% SDS–polyacrylamide gel embedded with MBP. Numbers indicate molecular mass estimations in kilodaltons.
(B) Immunoblot analysis of SSH1 expressed in yeast strains CTY182 (wild type) and YM219 (MAP kinase null mutant). Cellular protein was isolated from cultures treated with 0.9 M NaCl for 30 min (+) and untreated controls (−). The phosphorylated (Ssh1p*) and nonphosphorylated forms of Ssh1p are indicated.
Although the 42-kD kinase shown in Figure 8A migrates somewhat faster than does the kinase observed using Ssh1p as the substrate, its pattern of induction mimicked that of the Ssh1p kinase when the duration and magnitude of several osmotic stress conditions were assayed and compared (data not shown). Therefore, we speculated that the same hyperosmotic stress–activated kinase was capable of using both Ssh1p and MBP as substrates but that the migration of the kinase relative to protein size standards was slightly altered depending on the nature of the protein used as the embedded substrate. To test this possibility, we conducted an in-gel kinase assay in which both Ssh1p and MBP were incorporated as substrates within the same gel. If the kinases phosphorylating Ssh1p and MBP were distinct, four bands would be predicted using the gel with both substrates. Instead, a triplet similar to that shown in Figure 8A was observed, with the exception that the migration of all three bands was slower with respect to the size standards; the lowest band of the triplet migrated at 45 kD (data not shown). More direct evidence that the Ssh1p kinase recognizes MBP as an alternative substrate is presented in the yeast expression studies described below.
To determine whether the Ssh1p kinase is a MAP kinase, we made attempts to immunoprecipitate the kinase from NaCl-stressed plant tissue by using the following antibodies: (1) Ab-p48C, an antibody generated against the conserved C terminus of the tobacco SIPK that is capable of recognizing several distinct plant MAP kinases (Zhang and Klessig, 1998); (2) an anti–phospho-Tyr antibody that has been used previously to immunoprecipitate MAP kinases (Brewster et al., 1993; Zhang and Klessig, 1997; Hoyos and Zhang, 2000); and (3) six different commercially available antibodies directed against mammalian MAP kinases. Although we could quantitatively recover the 48-kD SIPK by using either Ab-p48C or the anti–phospho-Tyr antibody, in no case were we able to recover the 45-kD Ssh1p kinase (data not shown). Even more compelling evidence that Ssh1p is not the substrate of a MAP kinase comes from observations of Ssh1p phosphorylation in the heterologous yeast system. Saccharomyces cerevisiae possesses exactly six MAP kinases (Hunter and Plowman, 1997), and a yeast strain has been generated (YM219) that harbors deletion mutations in the genes encoding all six of these kinases (Madhani et al., 1997). As shown in Figure 8B, when SSH1 was expressed in YM219, the pattern of Ssh1p phosphorylation upon hyperosmotic insult was the same as that observed when SSH1 was expressed in a wild-type yeast strain.
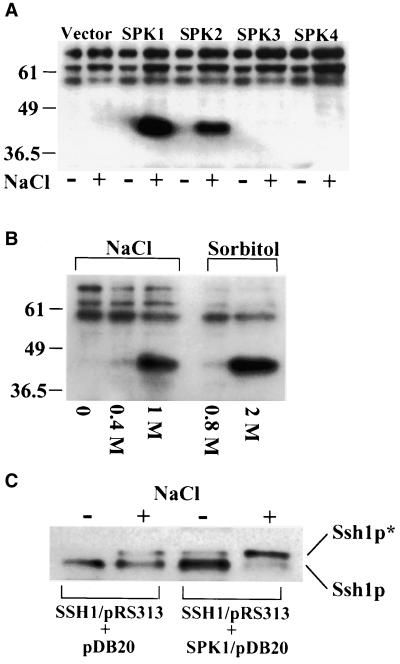
The Soybean Protein Kinases SPK1 and SPK2 Are Activated by Hyperosmotic Stress and Use Ssh1p as a Substrate
Several recent reports have documented that hyperosmotic stress rapidly activates two protein kinases in plants, the SIPK MAP kinase and another kinase that does not appear to be a member of the MAP kinase family (Munnik et al., 1999; Droillard et al., 2000; Hoyos and Zhang, 2000; Mikolajczyk et al., 2000). Estimated molecular masses of the non–MAP kinase stress-activated proteins range from 38 to 44 kD, and all were identified on the basis of their abilities to recognize MBP as an artificial substrate by using in-gel kinase assays. Mikolajczyk et al. (2000) isolated this kinase from tobacco culture cells and showed that it represented a homolog of the Arabidopsis protein kinase ASK1, a member of the SnRK2b subfamily of SNF1-related kinases. Four different protein kinases of unknown function from soybean, designated SPK1 through SPK4, also have been placed in this subfamily on the basis of sequence homology (Yoon et al., 1997; Halford and Hardie, 1998). Pairwise comparisons of the predicted amino acid sequences using the GAP program show that the SPK1 and SPK2 proteins share 95% sequence identity and the SPK3 and SPK4 enzymes are 93% identical; similar comparisons between SPK1 or SPK2 and either SPK3 or SPK4 reveal sequence identities ranging from 70 to 73%. The predicted molecular masses of the four soybean kinases are similar, ranging from 38.6 to 40.4 kD (Yoon et al., 1997; Halford and Hardie, 1998).
Because elements of the pathway leading to the phosphorylation of Ssh1p under conditions of hyperosmotic stress appear to be conserved between yeast and plants, we speculated that candidate plant Ssh1p kinases could be assayed effectively in this heterologous system. To determine whether any of the soybean kinases classified as members of the SnRK2b subfamily represent genuine Ssh1p kinases, we isolated cDNAs corresponding to all four soybean SPK proteins from young soybean leaves by using reverse transcription–polymerase chain reaction (PCR) and cloned each cDNA into a yeast expression vector. Wild-type yeast strain CTY182 was transformed with these constructs, subjected to hyperosmotic stress, and assayed using the in-gel kinase procedure with Ssh1p embedded in the gel matrix. As shown in Figure 9A, strong induction of Ssh1p kinase activity was observed when yeast cultures expressing SPK1 and SPK2 were exposed to 1 M NaCl. No significant Ssh1p kinase activity was detected in yeast harboring the SPK3 or SPK4 construct. An identical pattern was observed when MBP was used as an alternative in-gel substrate, with the exception that the inducible kinase activity migrated at a position ∼3 kD lower than was observed with gels embedded with Ssh1p (data not shown), a phenomenon we observed using tobacco leaf extracts. Interestingly, no inducible Ssh1p kinase activity was evident with the in-gel kinase assays in the absence of the soybean SPK constructs, suggesting that the endogenous yeast kinase that phosphorylates Ssh1p does not regain activity after being subjected to SDS-PAGE (or it becomes separated from essential cofactors). Three bands in the 55- to 80-kD size range were observed consistently in the in-gel assay (Figure 9A), but because they also were apparent in gels lacking added substrate (and gels embedded with MBP or BSA), they likely represent the products of autophosphorylation (data not shown).
Figure 9.
Protein Kinases SPK1 and SPK2 Phosphorylate Ssh1p When Expressed in Yeast and Activated by Hyperosmotic Stress.
(A) In-gel kinase assay of proteins recovered from yeast transformed with soybean SPK cDNAs. Protein was harvested from yeast cultures exposed to 1 M NaCl for 30 min (+) and untreated controls (−). Numbers indicate molecular mass estimations in kilodaltons. Vector, yeast strain expressing only the pDB20 expression plasmid.
(B) In-gel kinase assay of yeast expressing SPK1 after a 30-min exposure to moderate (0.4 M NaCl and 0.8 M sorbitol) and high (1 M NaCl and 2 M sorbitol) levels of hyperosmotic stress.
(C) Immunoblot analysis of Ssh1p in a yeast strain coexpressing SPK1 (SSH1/pRS313 + SPK1/pDB20) compared with coexpression with an empty vector (SSH1/pRS313 + pDB20). Cultures were treated with (+) or without (−) 1 M NaCl for 30 min. The phosphorylated (Ssh1p*) and nonphosphorylated forms of Ssh1p are indicated.
Because of the high amino acid sequence identity shared by SPK1 and SPK2 (95%) and the likelihood that they merely represent products of an equivalent gene derived from the different ancestral parents of soybean (an ancient tetraploid), we conducted additional experiments using only the SPK1 isoform. Consistent with our previous observations of Ssh1p phosphorylation in yeast (Kearns et al., 1998), substantial induction of SPK1 activity was detected only upon exposure of yeast to particularly acute levels of osmotic stress (e.g., 1 M NaCl and 2 M sorbitol; Figure 9B). Negligible SPK1 activation was observed when a more moderate osmotic stress was applied (0.4 M NaCl and 0.8 M sorbitol), conditions sufficient for activation of the high osmolarity glycerol response MAP kinase pathway (Brewster et al., 1993).
When Ssh1p is expressed in yeast by using the strong constitutive ADH1 promoter, the maximum conversion to Ssh1p* observed upon exposure to severe hyperosmotic stress is 50 to 60% (Kearns et al., 1998). If Ssh1p represents a true in vivo substrate for the SPK1 kinase, one would predict that a greater percentage of conversion to Ssh1p* would be witnessed in yeast strains producing both Ssh1p and SPK1. To test this prediction, we subcloned the cassette containing the SSH1 cDNA with the flanking ADH1 promoter and termination sequences from SSH1/pDB20 into pRS313, a yeast expression plasmid that enables selection of appropriate yeast strains on medium lacking histidine (Sikorski and Hieter, 1989). CTY182 was cotransformed with SSH1/pRS313 and SPK1/pDB20 and exposed to 1 M NaCl for 30 min. As shown in Figure 9C, a greater proportion of total Ssh1p protein was phosphorylated to Ssh1p* in yeast coexpressing SPK1 and SSH1 than the control strain cotransformed with the SSH1/pRS313 construct and pDB20 vector alone. A modest increase in the Ssh1p*/Ssh1p ratio also was observed in noninduced yeast expressing both the SPK1 and SSH1 cDNAs, suggesting that the nonactivated SPK1 (and/or SPK2) enzyme is responsible for the relatively low level of Ssh1p phosphorylation typically observed in plant cells in the absence of osmotic stress (Figure 1).
Consistent with being classified as an SNF1-related protein kinase, SPK1 showed greater amino acid sequence identity to Snf1p than any other yeast polypeptide when protein database searches were conducted using the BLAST algorithm (data not shown). Pairwise comparisons between SPK1 and Snf1p using the GAP program showed that the proteins share 32% sequence identity and 42% similarity. Although Snf1p has not been implicated in osmosensory signaling in yeast, we speculated that it might represent the endogenous yeast kinase that phosphorylates Ssh1p in response to hyperosmotic stress. However, the pattern of Ssh1p phosphorylation remained unaffected when the SSH1 cDNA was expressed not only in yeast strains possessing knockout mutations at the SNF1 locus but also in any of the five other loci that Hunter and Plowman (1997) classified as constituting the SNF1/AMPK family of yeast protein kinases (data not shown). Therefore, the hyperosmotic stress–activated yeast kinase that phosphorylates the soybean Ssh1p protein either is not a member of the SNF1/AMPK family or is redundant, necessitating mutations in two or more members of this family to eliminate Ssh1p kinase activity.
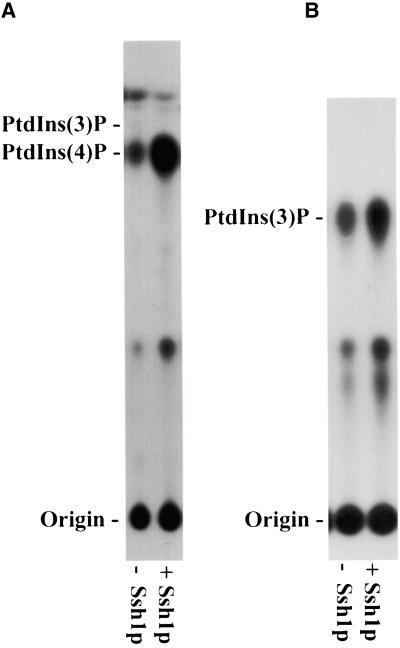
Ssh1p Stimulates the in Vitro Synthesis of PtdIns(4)P and PtdIns(3)P
Mammalian PITPs serve as cofactors for the production of phosphoinositides that participate in cellular signaling and membrane trafficking (Wirtz, 1997; Cockcroft, 1998). In particular, they have been shown to stimulate the synthesis of PtdIns(4)P (Kauffmann-Zeh et al., 1995) and PtdIns(3)P (Panaretou et al., 1997), presumably by actively presenting the PtdIns substrate to the PtdIns 4-kinase and phosphoinositide 3-kinases, respectively. To determine whether Ssh1p is similarly capable of stimulating plant phosphoinositide kinase activities, we assayed plant cell extracts for PtdIns kinase activity in the presence or absence of recombinant Ssh1p. Because E. coli does not produce phosphoinositides, any observed stimulation of PtdIns kinase activities by recombinant Ssh1p cannot be attributed to increased substrate availability by the cointroduction of a prebound PtdIns ligand. Also, Ssh1p possesses no PtdIns kinase activity per se, as evidenced by the failure of the protein to produce phosphoinositides in control assays with added PtdIns substrate (data not shown). As shown in Figure 10A, PtdIns 4-kinase activity was readily detected in cellular extracts obtained from young Arabidopsis leaves. Synthesis of PtdIns(4)P was increased greatly when purified recombinant Ssh1p was included in the assay. We estimate, on the basis of densitometry scans of the autoradiogram shown in Figure 10A and of others derived from duplicate experiments, that the in vitro synthesis of PtdIns(4)P can increase as much as sixfold by the addition of Ssh1p.
Figure 10.
Ssh1p Stimulates PtdIns Kinase Activities in Vitro.
(A) Cellular extracts from young Arabidopsis leaves were incubated with γ-32P-ATP in the presence (+) or absence (−) of purified recombinant Ssh1p. 32P-labeled products were separated by thin-layer chromatography and detected by autoradiography. The migration of PtdIns(3)P and PtdIns(4)P standards is indicated.
(B) The activity of recombinant Arabidopsis PtdIns 3-kinase expressed in E. coli was assayed in the presence (+) or absence (−) of Ssh1p. Products were analyzed by thin-layer chromatography. The migration of an authentic PtdIns(3)P standard is indicated.
Although PtdIns 3-kinases are essential for normal plant growth and development (Welters et al., 1994), their activities often are difficult to detect in whole-plant extracts. No evidence of in vitro PtdIns(3)P formation was observed using young Arabidopsis leaf extracts with or without added Ssh1p (Figure 10A), presumably because we could not detect the low activities of this enzyme (Hama et al., 2000). As a more informative means of testing whether Ssh1p also is capable of stimulating PtdIns 3-kinase activity, in vitro assays were conducted using recombinant Arabidopsis PtdIns 3-kinase produced in E. coli (see Methods). Introduction of Ssh1p resulted in a twofold increase in PtdIns 3-kinase activity (Figure 10B), an increase similar in magnitude to the in vitro stimulation of PtdIns 3-kinase activity in animal systems by the addition of mammalian PITP (Panaretou et al., 1997).
DISCUSSION
Because water availability is one the most limiting factors for terrestrial plant growth, a great deal of research has focused on the response and adaptation of plants to water deficit. Although a wealth of information has accumulated regarding the identification and characterization of genes that are transcriptionally regulated in response to osmotic stress (Ingram and Bartels, 1996; Bray, 1997; Shinozaki and Yamaguchi-Shinozaki, 1997), relatively little is known concerning the earliest steps of osmotic stress recognition and subsequent signal transduction in plants. In this report, two components of a hyperosmotic stress–induced signaling pathway are characterized that are activated (SPK1/SPK2) or phosphorylated (Ssh1p) within minutes of stress perception.
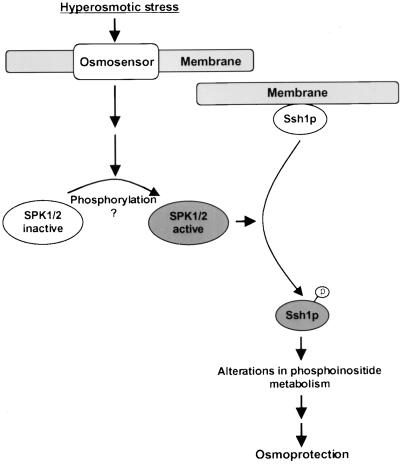
The overall conclusions derived from this study are represented by the model proposed in Figure 11. Upon the perception of hyperosmotic stress by an osmosensor (depicted as membrane bound), a signaling cascade is initiated that results in the activation of SPK1 and/or SPK2. This discovery was based on the recent report by Mikolajczyk et al. (2000) in which a hyperosmotic stress–activated tobacco kinase belonging to the SnRK2b subfamily of SNF1-related kinases was characterized that we believe shared properties with the kinase responsible for phosphorylating Ssh1p in transgenic tobacco. Our study supports the conclusion of Mikolajczyk et al. (2000) that members of the SnRK2b kinase subfamily are involved in environmental stress signaling in plants. Two other soybean kinases that belong to this subfamily, SPK3 and SPK4, also were tested in the yeast system, but we were unable to demonstrate that Ssh1p serves as a substrate for their kinase activities (Figure 9A). Because we do not possess an anti-SPK antibody that would allow us to conduct an immunoblot assay to verify that SPK3 and SPK4 accumulate in yeast to levels comparable to those observed for SPK1 and SPK2, we cannot exclude the possibility that all four proteins may represent viable Ssh1p kinases. The fact that SPK3 and SPK4 transcript accumulation increases upon exposure of soybean tissue to dehydration and high salinity suggests that these kinases may play some role in osmoprotection (Yoon et al., 1997). However, regardless of which SPK enzymes ultimately prove to function as Ssh1p kinases in the heterologous yeast system, an important goal of future studies should include verification that the same kinase/substrate relationship exists within the native plant environment.
Figure 11.
Speculative Model of the Role of SPK1 and Ssh1p in Osmosensory Signal Transduction.
Upon perception of hyperosmotic stress, a putative membrane-bound osmosensor initiates a signaling cascade that results in the activation of SPK1 and the subsequent phosphorylation of Ssh1p. Phosphorylation reduces Ssh1p's affinity for cellular membranes, potentially redirecting its PtdIns 3-kinase– and PtdIns 4-kinase–stimulating activities to a different subcellular location. Alternately, the PtdIns kinase–stimulating activities of Ssh1p per se may be modified by phosphorylation. Phosphate groups are indicated with a circled “p.”
There are seemingly contradictory reports in the literature regarding the role of phosphorylation in activating the kinase that phosphorylates Ssh1p. Two separate groups have characterized what appears to be a tobacco homolog of SPK1 by using tobacco cell cultures (and MBP as the artificial substrate). Mikolajczyk et al. (2000) reported that treatment of their 42-kD hyperosmotic stress–activated kinase with a serine/threonine protein phosphatase inactivated the enzyme, suggesting a mechanism of activation via phosphorylation. In contrast, Hoyos and Zhang (2000) found that pretreating tobacco cells with a kinase inhibitor activated what is likely to be the same kinase (termed HOSAK in their report), causing them to propose that the enzyme is inactive when phosphorylated and activated by a hyperosmotic stress– induced protein phosphatase. The specific mechanism by which SPK1 is activated, and the role that protein phosphorylation plays in this process, clearly warrants further investigation.
As shown in Figure 11, activation of SPK1 enables the recognition of Ssh1p as a substrate, leading to its phosphorylation. One probable consequence of Ssh1p phosphorylation is a reduction in its affinity for biological membranes (Figure 3). Given the ability of recombinant Ssh1p to stimulate PtdIns 3-kinase and PtdIns 4-kinase activities in vitro (Figure 10), coupled with the well-documented function of mammalian PITPs in stimulating these same phosphoinositide kinase activities both in vitro and in vivo (Panaretou et al., 1997; Cockcroft, 1998), we propose that the role of Ssh1p within this signaling cascade is to modify the cell's ability to produce phosphoinositides under conditions of hyperosmotic stress. A scenario consistent with our results shown in Figure 3 is that phosphorylation of Ssh1p results in the mobilization and redistribution of the protein, and thus its phosphoinositide-stimulating activities, within the cell.
An alternative but not necessarily mutually exclusive possibility is that the lipid binding properties and/or PtdIns 3-kinase– and PtdIns 4-kinase–stimulating abilities of Ssh1p per se may be altered by phosphorylation. PtdIns(3)P and PtdIns(4)P serve as substrates for the lipid kinases responsible for production of the bis-phosphorylated phosphoinositide species PtdIns(3,5)P2 and PtdIns(4,5)P2, respectively. The synthesis and/or turnover of both PtdIns(3,5)P2 and PtdIns(4,5)P2 have been shown to increase rapidly upon exposure of plant cells to hyperosmotic stress (Munnik et al., 1998; Meijer et al., 1999; Pical et al., 1999). Interestingly, nonphosphorylated Ssh1p binds both of these phosphoinositides with a much higher affinity than does PtdIns or any other lipid species tested (Kearns et al., 1998). An intriguing hypothesis that is consistent with the observations described above is that under normal cellular conditions, the strong binding of Ssh1p to cellular PtdIns(3,5)P2 or PtdIns(4,5)P2 may prevent it from binding PtdIns and thereby inhibit its ability to stimulate phosphoinositide production. Phosphorylation of Ssh1p could lead to a reduction in its affinity for the bis-phosphorylated PtdIns species, enabling it to bind PtdIns and stimulate the synthesis of PtdIns(3)P and PtdIns(4)P, and consequently their bis-phosphorylated derivatives through increased substrate availability, under conditions of hyperosmotic insult. The purification of Ssh1p* in quantities sufficient to enable a direct examination of its lipid binding properties and PtdIns kinase–stimulating activities should facilitate testing of the hypothesis described above and assist in elucidating the specific mechanism by which the cellular signal is perpetuated subsequent to the phosphorylation of Ssh1p.
Within the heterologous yeast system, increases in Ssh1p phosphorylation and activation of SPK1 are observed only upon exposure to particularly severe levels of osmotic stress (Kearns et al., 1998; Figure 9). The osmosensory pathway that the soybean proteins use in yeast is clearly distinct from the well-characterized high osmolarity glycerol pathway, a signaling cascade that is initiated by the Sholp and Sln1p osmosensors and mediated through the Hog1p MAP kinase after exposure to comparatively modest levels of osmotic stress (reviewed in Hohmann, 1997). The existence of a unique pathway in yeast that responds specifically to acute hyperosmotic stress has been reported, but the individual components have not been defined (Serrano et al., 1997). The involvement of phosphoinositides in this pathway is suggested by the study of Dove et al. (1997), who reported the rapid synthesis of PtdIns(3,5)P2 via a Hog1p-independent pathway when yeast cells were exposed specifically to severe hyperosmotic stress.
The matter of whether the proposed signaling pathway shown in Figure 11 also serves a unique function in response to particularly severe levels of osmotic stress in plant systems is less clear. Using leaf tissue or cell cultures, maximal Ssh1p phosphorylation and Ssh1p kinase activation were observed upon treatment with 1 M NaCl or 2 M sorbitol (Figures 2 and 5), that is, under conditions of extreme stress. Moreover, no increase in Ssh1p phosphorylation was observed in the aerial portion of a detached leaf placed in 0.5 M NaCl for 1 hr, despite the fact that the leaf was severely wilted and the submerged plant tissue showed substantial Ssh1p* induction (Figure 4). Finally, the activation in alfalfa culture cells of a 38-kD kinase whose properties resemble those of SPK1 was observed only under conditions of severe osmotic stress (>0.75 M NaCl), prompting speculation regarding a signaling pathway in plants that is elicited specifically in response to extreme hyperosmotic stress (Munnik et al., 1999). In contrast, however, we observed a very dramatic increase in Ssh1p phosphorylation when young soybean roots were exposed to only 0.2 M NaCl (Figure 1C, lane 6). Furthermore, Mikolajczyk et al. (2000) and Hoyos and Zhang (2000) reported activation of the kinase that likely represents the tobacco homolog of SPK1 using NaCl concentrations ranging from 0.1 to 0.25 M, and we also observed modest activation of the Ssh1p kinase in cell cultures using 0.25 M NaCl (Figure 5E).
Of paramount interest to future investigations of the SPK1/Ssh1p signal transduction pathway will be the definition of its physiological role at the cellular and whole-plant levels. Glycophytic plant species such as soybean and tobacco cannot survive the extreme osmotic stress treatments that were required to elicit maximal activation of the SPK1/Ssh1p pathway. Therefore, questions naturally arise regarding the utility of a signaling pathway that is most responsive to stresses that are lethal to normal plant growth and development. The biological function of the recently discovered acute hyperosmotic stress signaling pathway described in yeast is also unknown (Serrano et al., 1997). Despite these unanswered questions, it is apparent that both plants and yeast possess at least two distinct hyperosmotic stress–induced signaling pathways, one mediated through a MAP kinase cascade and another that shares features of the model presented in Figure 11.
In summary, through the characterization of a soybean PITP-like protein, we have identified Ssh1p and the SPK1 and SPK2 kinases as early components of a signaling pathway activated by hyperosmotic stress. The participation of Ssh1p in osmosensory signaling is unique among the previously identified functions of PITPs in yeast and mammalian systems. The surprising conservation between yeast and plants of a signaling pathway capable of the hyperosmotic stress–induced phosphorylation of Ssh1p reveals that elements of this pathway are shared among diverse species. Finally, the ability to reconstruct elements of this pathway in yeast promises to provide a powerful tool for further elucidating the cellular functions of Ssh1p and SPK1, facilitating the identification of additional upstream components, and understanding the biological role of this pathway within the cell.
METHODS
Generation of Transgenic Tobacco
To mediate the constitutive expression of the SSH1 cDNA in transgenic plants, we excised the β-glucuronidase open reading frame in plant expression vector pBI121 (Clontech, Palo Alto, CA) and replaced it with the SSH1 sequence. This placed the soybean (Glycine max) cDNA downstream from the constitutive 35S promoter of Cauliflower mosaic virus. The construct was transformed into Agrobacterium tumefaciens strain LBA 4404, and tobacco (Nicotiana tabacum cv SR1) leaf discs were transformed using kanamycin selection as described (Holsters et al., 1978; Horsch et al., 1988).
Plant Tissue Treatment and Homogenization
Stock solutions of the chemical agents detailed in the legend to Figure 1 were first diluted to the appropriate concentrations in water and then transferred to a Petri dish. Either young newly emerged soybean roots (2 to 3 cm in length) or transgenic tobacco leaf pieces (∼1 cm2 in area) were treated as described and incubated (with occasional agitation) for the times indicated. Desiccation was achieved by subjecting the tissue to a gentle air stream until the tissue had lost >50% of its initial volume. BY-2 tobacco cell cultures were maintained as described previously (Fontes et al., 1994).
Treated tissue was transferred to an Eppendorf tube and homogenized with a plastic pestle in a buffer containing 100 mM Tris-HCl, pH 7.5, 10 mM KCl, 1 mM EDTA, 10 mM DTT, 0.2 g/mL sucrose, 1 mM phenylmethylsulfonyl fluoride, 1 mM aprotinin, 5 μg/mL chymostatin, 1 μg/mL leupeptin, and 20 μM pepstatin A. Homogenates were centrifuged at 1000g for 5 min in a refrigerated microcentrifuge to remove large debris. Protein concentrations of the resulting supernatants were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA) with γ-globulin as the standard. Treatment of cell extracts with calf intestinal phosphatase was conducted as described previously (Kearns et al., 1998).
Immunoblot Analysis
Protein samples were separated on 10% SDS–polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Micron Separations, Westborough, MA) by using the NOVEX XCell II mini-gel system according to the manufacturer's guidelines (Invitrogen, Carlsbad, CA). Membrane blocking and detection were performed according to instructions supplied with the ECL Plus kit (Amersham, Piscataway, NJ). Polyclonal anti-Ssh1p primary antibody (described by Kearns et al., 1998) was used at a dilution of 1:3000, and a goat anti-rabbit IgG conjugated to horseradish peroxidase (Amersham) was used at a dilution of 1:30,000.
Subcellular Fractionation
Transgenic tobacco leaf tissue was exposed to 1 M NaCl for 1 hr and homogenized as described above. The homogenate was centrifuged for 5 min at 4°C and 1000g, and the supernatant (designated “total”) was centrifuged at 13,000g for 10 min at 4°C. Subsequently, the resulting supernatant was centrifuged for 1 hr at 100,000g to generate supernatant (S100) and pellet (P100) fractions.
Detached Leaf Assay
A small leaf (∼6 cm long) was cut from a transgenic tobacco plant and inserted into an Eppendorf tube filled with 0.5 M NaCl. The leaf was left undisturbed for 1 hr, at which time the entire leaf had lost turgor. The wilted leaf was removed from the solution, and samples were excised from both the submerged and aerial portions of the tissue for immunoblot analysis. A control leaf from the same plant was treated using water in place of the NaCl solution.
In-Gel Kinase Assay
In-gel kinase assays were performed as described previously (Zhang and Klessig, 1997). Briefly, total protein extracts from treated tobacco tissue or yeast were separated on 10 or 12% SDS–polyacrylamide gels previously embedded with potential protein substrates (Ssh1p, Ssh2p, myelin basic protein [MBP], or casein) at a concentration of 0.25 mg/mL. After electrophoresis, the SDS was removed from the gel, and separated proteins were allowed to renature by soaking the gel overnight at 4°C in a solution containing 25 mM Tris-HCl, pH 7.5, 1 mM DTT, 0.1 mM Na3VO4, and 5 mM NaF. The gel then was incubated for 2 hr in a reaction buffer containing 25 mM Tris-HCl, pH 7.5, 2 mM EGTA, 1 mM DTT, 12 mM MgCl2, 0.1 mM Na3VO4, and 50 μCi γ-32P-ATP. Unincorporated γ-32P-ATP was removed by five washes of 20 min each in a solution containing 5% trichloroacetic acid (w/v) and 1% NaPPi (w/v). The washed gel was dried under vacuum and exposed to Kodak XAR-5 film. Molecular mass estimations of the kinases were determined using prestained protein size markers (Gibco BRL). Expression and purification of recombinant His6-tagged Ssh1p for use in the in-gel kinase assays were performed as described previously (Kearns et al., 1998).
Phosphoamino Acid Analysis
The 45-kD region of an in-gel kinase reaction (using Ssh1p as the embedded substrate) was excised and subjected to partial acid hydrolysis according to the method of Boyle et al. (1991). The resulting products were analyzed using two-dimensional electrophoresis and autoradiography. Nonradiolabeled phosphoamino acid standards (Sigma) were run with the radiolabeled samples to identify the individual species.
Isolation of Soybean SPK cDNAs
Young soybean leaves were frozen in liquid nitrogen and ground to a fine powder using a mortar and pestle. RNA was extracted from the ground tissue using Trizol reagent as described by the manufacturer (Gibco BRL). First-strand cDNA was generated using the Superscript system (Gibco BRL), and individual soybean SPK sequences were amplified using the Expand High Fidelity polymerase chain reaction (PCR) system (Roche Diagnostics, Indianapolis, IN). Specific PCR primers corresponding to the 5′ and 3′ untranslated regions of SPK1 to SPK4 were designed based on sequence information found in GenBank (accession numbers L01453, L19360, L19361, and L38855) and synthesized by Sigma-Genosys (The Woodlands, TX). The 5′ terminal nucleotides for each primer set were specified to generate either HindIII or NotI restriction sites to facilitate cloning into the yeast expression vector pDB20 (Becker et al., 1991). The fidelity of all PCR-generated constructs was confirmed by DNA sequence analysis.
Culture, Transformation, and Hyperosmotic Stress of Yeast Strains
Expression of SSH1 in the wild-type yeast strain CTY182 using expression vector pDB20 was conducted as described previously (Kearns et al., 1998). To facilitate the expression of SSH1 in the mitogen-activated protein (MAP) kinase null yeast strain YM219 (Madhani et al., 1997) and the coexpression of SSH1 and SPK1 in strain CTY182, it was necessary to transfer the cDNA into a yeast vector that uses HIS3 as the selectable marker. A 3-kb BamHI expression cassette from SSH1/pDB20 (containing the ADH1 promoter/SSH1 cDNA/ADH1 terminator) was ligated into the BamHI restriction site of yeast vector pRS313 (Sikorski and Hieter, 1989). SSH1/pRS313 was transformed into YM219 according to the procedure described by Gietz et al. (1992), selecting for histidine auxotrophy; SSH1/pRS313 and SPK1/pDB20 were cotransformed into CTY182 followed by coselection for histidine and uracil auxotrophy. Yeast cells were subjected to hyperosmotic stress by the direct addition of NaCl or sorbitol to cultures during log phase growth. Collection and homogenization of cells were performed as described (Kearns et al., 1998). All yeast manipulations and growth conditions were conducted according to standard protocols (Guthrie and Fink, 1991).
In Vitro PtdIns Kinase Assays
In vitro phosphatidylinositol (PtdIns) kinase assays were performed as described previously (Hama et al., 2000) using protein extracts from plant tissue (nontransformed Arabidopsis thaliana ecotype Columbia) and Escherichia coli cells overexpressing the Arabidopsis Vps34 PtdIns 3-kinase. Extracts from young leaves of 4-week-old magenta box–grown Arabidopsis were prepared by powdering tissue (∼100 mg) in liquid nitrogen and suspending the powder in a minimal volume (0.25 mL) of 0.1 M KCl, 15 mM Hepes-KOH, pH 7.5, 3 mM EGTA, and 10% glycerol containing a cocktail of protease inhibitors (pepstatin, leupeptin, aprotinin, and phenylmethylsulfonyl fluoride). Extracts of mid-log phase E. coli cells were obtained from a strain expressing the Arabidopsis PtdIns 3-kinase cDNA cloned as a 2.8-kb NotI fragment (Welters et al., 1994) into the NotI site of pBluescript SK+ (Stratagene).
PtdIns kinase assays were performed in a final reaction volume of 50 μL containing 20 mM Hepes-KOH, pH 7.5, 10 mM MgCl2, 1 mM MnCl2, 0.2 mg/mL sonicated PtdIns, 60 μM ATP, and 0.2 mCi/mL γ-32P-ATP. Reactions were initiated by the addition of 5 μL of cell extract with or without 10 μg of purified His-tagged Ssh1p. After a 10-min incubation at 25°C, the reactions were terminated by the addition of 80 μL of 1 M HCl. The lipids were extracted with 160 μL of chloroform:methanol (1:1), and the products of the in vitro assays were analyzed by thin-layer chromatography. Equivalently loaded samples were spotted onto silica gel 60 thin-layer chromatography plates, developed using a borate buffer system (Walsh et al., 1991), and subjected to autoradiography. To estimate the magnitude of the increased PtdIns kinase activities attributable to the addition of Ssh1p, we conducted densitometry scans of autoradiograms using the Personal Densitometer SI (Molecular Dynamics, Sunnyvale, CA).
Acknowledgments
We are very grateful to Dr. Daniel F. Klessig for providing antibodies specific to SIPK and Ab-p48C, an antibody that recognizes the conserved domain of several plant MAP kinases. We also thank Dr. Gerald Fink for yeast strain YM219, Dr. Marian Carlson for the snf1 deletion yeast strain MCY1846, and members of the laboratory of Dr. Steve Huber for assistance with the phosphoamino acid assays. We are particularly appreciative of the expert technical assistance provided by Nancy Gillikin and Carol Griffin. This research was supported by grants from the National Science Foundation (Grant Nos. IBN-9513582 to R.E.D. and MCB-9630108 to D.B.D) and the United States Department of Agriculture (National Research Initiative Competitive Grants Program Grant No. 99-35304-8114 to D.B.D.).
References
- Bankaitis, V.A., Malehorn, D.E., Emr, S.D., and Greene, R. (1989). The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J. Cell Biol. 108 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis, V.A., Aitken, J.R., Cleves, A.E., and Dowhan, W. (1990). An essential role for a phospholipid transfer protein in yeast Golgi function. Nature 347 561–562. [DOI] [PubMed] [Google Scholar]
- Becker, D.M., Fikes, J.D., and Guarente, L. (1991). A cDNA encoding a human CCAAT binding protein cloned by functional complementation in yeast. Proc. Natl. Acad. Sci. USA 88 1968–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle, W.J., van der Greer, P., and Hunter, T. (1991). Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201 110–148. [DOI] [PubMed] [Google Scholar]
- Bray, E. (1997). Plant responses to water deficit. Trends Plant Sci. 2 48–54. [Google Scholar]
- Brewster, J.L., de Valoir, T., Dwyer, N.D., Winter, E., and Gustin, M.C. (1993). An osmosensing signal transduction pathway in yeast. Science 259 1760–1763. [DOI] [PubMed] [Google Scholar]
- Cockcroft, S. (1998). Phosphatidylinositol transfer proteins: A requirement in signal transduction and vesicle traffic. Bioessays 20 423–432. [DOI] [PubMed] [Google Scholar]
- Cunningham, E., Thomas, G.M.H., Ball, A., Hiles, I., and Cockcroft, S. (1995). Phosphatidylinositol transfer protein dictates the rate of inositol trisphosphate production by promoting synthesis of PIP2. Curr. Biol. 5 775–783. [DOI] [PubMed] [Google Scholar]
- Cunningham, E., Tan, S.K., Swigart, P., Hsuan, J., Bankaitis, V.A., and Cockcroft, S. (1996). The yeast and mammalian isoforms of phosphatidylinositol transfer protein can restore phospholipase C-mediated inositol lipid signalling in cytosol-depleted RBL-2H3 and HL60 cells. Proc. Natl. Acad. Sci. USA 93 6589–6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickeson, S.K., Lim, C.N., Schuyler, G.T., Dalton, T.P., Helmkamp, G.M., Jr., and Yarbrough, L.R. (1989). Isolation and sequence of cDNA clones encoding rat phosphatidylinositol transfer protein. J. Biol. Chem. 264 16557–16564. [PubMed] [Google Scholar]
- Dove, S.K., Cooke, F.T., Douglas, M.R., Sayers, L.G., Parker, P.J., and Michell, R.H. (1997). Osmotic stress activates phosphatidyl-inositol-3,5-bisphosphate synthesis. Nature 390 187–192. [DOI] [PubMed] [Google Scholar]
- Droillard, M.-J., Thibivilliers, S., Cazale, A.-C., Barbier-Brygoo, H., and Lauriere, C. (2000). Protein kinases induced by osmotic stresses and elicitor molecules in tobacco suspensions: Two crossroad MAP kinases and one osmoregulation-specific protein kinase. FEBS Lett. 474 217–222. [DOI] [PubMed] [Google Scholar]
- Fontes, E.P., Eagle, P.A., Sipe, P.S., Luckow, V.A., and Hanley-Bowdoin, L. (1994). Interaction between a geminivirus replication protein and origin DNA is essential for viral replication. J. Biol. Chem. 269 8459–8465. [PubMed] [Google Scholar]
- Gietz, D., St. Jean, A., Woods, R.A., and Schiestl, R.H. (1992). Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, C., and Fink, G.R., eds (1991). Methods in Enzymology, Vol. 194: Guide to Yeast Genetics and Molecular Biology. (New York: Academic Press). [PubMed]
- Halford, N.G., and Hardie, D.G. (1998). SNF1-related protein kinases: Global regulators of carbon metabolism in plants? Plant Mol. Biol. 37 735–748. [DOI] [PubMed] [Google Scholar]
- Hama, H., Schneiders, E.A., Thorner, J., Takemoto, J.Y., and DeWald, D.B. (1999). Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 274 34294–34300. [DOI] [PubMed] [Google Scholar]
- Hama, H., Takemoto, J.Y., and DeWald, D.B. (2000). Analysis of phosphoinositides in protein trafficking. Methods 20 465–473. [DOI] [PubMed] [Google Scholar]
- Hamilton, B.A., et al. (1997). The vibrator mutation causes neurodegeneration via reduced expression of PITPα: Positional complementation cloning and extragenic suppression. Neuron 18 711–722. [DOI] [PubMed] [Google Scholar]
- Hay, J.C., and Martin, T.F.J. (1993). Phosphatidylinositol transfer protein is required for ATP dependent priming of Ca2+-activated secretion. Nature 366 572–575. [DOI] [PubMed] [Google Scholar]
- Hay, J.C., Fisette, P.L., Jenkins, G.H., Fukami, K., Takenawa, T., Anderson, R.A., and Martin, T.F.J. (1995). ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature 374 173–177. [DOI] [PubMed] [Google Scholar]
- Hirt, H. (1997). Multiple roles of MAP kinases in plant signal transduction. Trends Plant Sci. 2 11–15. [Google Scholar]
- Hohmann, S. (1997). Shaping up: The response of yeast to osmotic stress. In Yeast Stress Responses, S. Hohmann and W.H. Mager, eds (Heidelberg, Germany: Springer-Verlag), pp. 101–145.
- Holsters, M., de Waele, D., Depicker, A., Messens, E., Van Montague, M., and Schell, J. (1978). Transfection and transformation of A. tumefaciens. Mol. Gen. Genet. 163 181–187. [DOI] [PubMed] [Google Scholar]
- Horsch, R.B., Fry, J., Hoffman, N., Neidermeyer, J., Rogers, S.G., and Fraley, R.T. (1988). Leaf disc transformation. In Plant Molecular Biology Manual, S.B. Gelvin and R.A. Schilperoort, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 1–9.
- Hoyos, M.E., and Zhang, S. (2000). Calcium-independent activation of salicylic acid induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic stress. Plant Physiol. 122 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, T., and Plowman, G.D. (1997). The protein kinases of budding yeast: Six score and more. Trends Biochem. Sci. 22 18–22. [DOI] [PubMed] [Google Scholar]
- Ingram, J., and Bartels, D. (1996). The molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 377–403. [DOI] [PubMed] [Google Scholar]
- Jouannic, N., Lepetit, M., Vergnolle, C., Cantrel, C., Gardies, A.M., Kader, J.C., and Arondel, V. (1998). Isolation of a cDNA from Arabidopsis thaliana that complements the sec14 mutant of yeast. Eur. J. Biochem. 258 402–410. [DOI] [PubMed] [Google Scholar]
- Kauffmann-Zeh, A., Thomas, G.M.H., Ball, A., Prosser, S., Cunningham, E., Cockcroft, S., and Hsuan, J.J. (1995). Requirement for phosphatidylinositol transfer protein in epidermal growth factor signaling. Science 268 1188–1190. [DOI] [PubMed] [Google Scholar]
- Kearns, B.G., McGee, T.P., Mayinger, P., Gedvilaite, A., Phillips, S.E., Kagiwada, S., and Bankaitis, V.A. (1997). Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature 387 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns, M.A., Monks, D.E., Fang, M., Rivas, M.P., Courtney, P.D., Chen, J., Prestwich, G.D., Theibert, A.B., Dewey, R.E., and Bankaitis, V.A. (1998). Novel developmentally regulated phosphoinositide binding proteins from soybean whose expression bypasses the requirement for an essential phosphatidylinositol transfer protein in yeast. EMBO J. 17 4004–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani, H.D., Styles, C.A., and Fink, G.R. (1997). MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell 91 673–684. [DOI] [PubMed] [Google Scholar]
- Maeda, T., Wurgler-Murphy, S.M., and Saito, H. (1994). A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature 369 242–245. [DOI] [PubMed] [Google Scholar]
- Meijer, H.J.G., Divecha, N., van den Ende, H., Musgrave, A., and Munnik, T. (1999). Hyperosmotic stress induces rapid synthesis of phosphatidyl-d-inositol 3,5-bisphosphate in plant cells. Planta 208 294–298. [Google Scholar]
- Mikolajczyk, M., Awotunde, O.S., Muszynska, G., Klessig, D.F., and Grazyna, D. (2000). Osmotic stress induces rapid activation of a salicylic acid–induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell 12 165–178. [PMC free article] [PubMed] [Google Scholar]
- Milligan, S.C., Alb, J.G., Jr., Elagina, R., Bankaitis, V.A., and Hyde, D.R. (1997). A phosphatidylinositol transfer protein domain of Drosophila retinal degeneration B protein is essential for photoreceptor cell survival and recovery from light stimulation. J. Cell Biol. 139 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi, T., Ichimura, K., and Shinozaki, K. (1997). Environmental stress response in plants: The role of mitogen-activated protein kinases. Trends Biotechnol. 15 15–19. [DOI] [PubMed] [Google Scholar]
- Munnik, T., Irvine, R.F., and Musgrave, A. (1998). Phospholipid signalling in plants. Biochim. Biophys. Acta 1389 222–272. [DOI] [PubMed] [Google Scholar]
- Munnik, T., Ligterink, W., Meskiene, I., Calderini, O., Beyerly, J., Musgrave, A., and Hirt, H. (1999). Distinct osmo-sensing protein kinase pathways are involved in signalling moderate and severe hyper-osmotic stress. Plant J. 20 381–388. [DOI] [PubMed] [Google Scholar]
- Panaretou, C., Domin, J., Cockcroft, S., and Waterfield, M.D. (1997). Characterization of p150, an adapter protein for the human phosphatidylinositol (PtdIns) 3-kinase. J. Biol. Chem. 272 2477–2485. [DOI] [PubMed] [Google Scholar]
- Pical, C., Westergren, T., Dove, S.K., Larsson, C., and Sommarin, M. (1999). Salinity and hyperosmotic stress induce rapid increases in phosphatidylinositol 4,5-bisphosphate, diacylglycerol pyrophosphate, and phosphatidylcholine in Arabidopsis thaliana cells. J. Biol. Chem. 274 38232–38240. [DOI] [PubMed] [Google Scholar]
- Serrano, R., Marquez, J.A., and Rios, G. (1997). Crucial factors in salt stress tolerance. In Yeast Stress Responses, S. Hohmann and W.H. Mager, eds (Heidelberg, Germany: Springer-Verlag), pp. 147–169.
- Shinozaki, K., and Yamaguchi-Shinozaki, K. (1997). Gene expression and signal transduction in water stress response. Plant Physiol. 115 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R.S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner, H.B., Alb, J.G., Jr., Whitters, E.A., Helmkamp, G.M., Jr., and Bankaitis, V.A. (1993). Phospholipid transfer activity is relevant to, but not sufficient for, the essential function of the yeast SEC14 gene product. EMBO J. 12 4775–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner, H.B., McGee, T.P., McMaster, C.R., Fry, M.R., Bell, R.M., and Bankaitis, V.A. (1995). Phosphatidylinositol transfer protein stimulates yeast Golgi function by inhibiting choline-phosphate cytidylyltransferase activity. Proc. Natl. Acad. Sci. USA 92 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, S., and Hosaka, K. (1994). Cloning of a cDNA encoding a second phosphatidylinositol transfer protein of rat brain by complementation of the yeast sec14 mutation. J. Biochem. 115 981–984. [DOI] [PubMed] [Google Scholar]
- Usami, S., Banno, H., Ito, Y., Nishihama, R., and Machida, Y. (1995). Cutting activates a 46-kilodalton protein kinase in plants. Proc. Natl. Acad. Sci. USA 92 8660–8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, J.P., Caldwell, K.K., and Majerus, P.W. (1991). Formation of phosphatidylinositol 3-phosphate by isomerization from phosphatidylinositol 4-phosphate. Proc. Natl. Acad. Sci. USA 88 9184–9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welters, P., Takegawa, K., Emr, S.D., and Chrispeels, M.J. (1994). AtVPS34, a phosphatidylinositol 3-kinase of Arabidopsis thaliana, is an essential protein with homology to a calcium-dependent lipid binding domain. Proc. Natl. Acad. Sci. USA 91 11398–11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann, C., Schafer, T., and Burger, M.M. (1996). Chromaffin granule-associated phosphatidylinositol 4-kinase activity is re-quired for stimulated secretion. EMBO J. 15 2094–2101. [PMC free article] [PubMed] [Google Scholar]
- Wirtz, K.W.A. (1991). Phospholipid transfer proteins. Annu. Rev. Biochem. 60 73–99. [DOI] [PubMed] [Google Scholar]
- Wirtz, K.W.A. (1997). Phospholipid transfer proteins revisited. Biochem. J. 324 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, H.W., Kim, M.C., Shin, P.G., Kim, J.S., Kim, C.Y., Lee, S.Y., Hwang, I., Bahk, J.D., Hong, J.C., Han, C., and Cho, M.J. (1997). Differential expression of two functional serine/threonine protein kinases from soybean that have an unusual acidic domain at the carboxy terminus. Mol. Gen. Genet. 255 359–371. [DOI] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1997). Salicylic acid activates a 48-kD MAP kinase in tobacco. Plant Cell 9 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998). The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK. Proc. Natl. Acad. Sci. USA 95 7225–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., Jin, C.D., and Roux, S.J. (1993). Casein kinase II-type protein kinase from pea cytoplasm and its inactivation by alkaline phosphatase in vitro. Plant Physiol. 103 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]