Abstract
The tetrapeptide KDEL is commonly found at the C terminus of soluble proteins of the endoplasmic reticulum (ER), and it contributes to their localization by interacting with a receptor that recycles between the Golgi complex and the ER. We investigated the effects of the addition of KDEL to phaseolin, a protein normally delivered from the ER to storage vacuoles via the Golgi complex. We show that KDEL prevents acquisition of trans-Golgi–specific glycan modifications and causes interactions with the chaperone BiP that are distinct from the ones between BiP and defective proteins. KDEL markedly increases the stability of phaseolin, but a small proportion of phaseolin-KDEL slowly reaches the vacuole without undergoing Golgi-mediated glycan modifications, in a process that can be inhibited by brefeldin A but not monensin. Our results indicate that KDEL can operate with high efficiency before proteins can reach the late Golgi cisternae but allows or promotes delivery to vacuoles via an alternative mechanism. However, addition of KDEL does not alter the destiny of an assembly-defective form of phaseolin, suggesting that the plant ER quality control mechanism is dominant over KDEL effects.
INTRODUCTION
The secretory pathway delivers proteins from the endoplasmic reticulum (ER) to the cell surface or the vacuoles through a flow of membrane traffic that often is mediated by the Golgi complex. Soluble proteins inserted into the ER are secreted via the Golgi complex if they do not have signals that retain, retrieve, or sort them to specific compartments of the pathway (reviewed in Vitale and Denecke, 1999).
Soluble proteins that perform their functions in the lumen of the ER, termed reticuloplasmins, usually have a C-terminal tetrapeptide that contributes to their ER localization. These proteins are very long lived, and their compartment of turnover is not known. The tetrapeptide is in most cases KDEL or HDEL. A receptor for the tetrapeptide has been cloned from yeast, mammals, and plants (Lewis and Pelham, 1990; Semenza et al., 1990; Lee et al., 1993). In mammalian cells, the receptor is present in the intermediate compartment (a transition compartment between the ER and the Golgi complex, which has never been identified in plants or yeast) and throughout the Golgi cisternae, but with a concentration that markedly decreases toward the trans side (Griffiths et al., 1994). It most probably captures reticuloplasmins when they reach the intermediate compartment or the early Golgi stacks and retrieves them into the ER through retrograde membrane traffic. The distribution of the mammalian receptor is consistent with the observation that a KDEL-containing recombinant protein escapes retrieval and is secreted if it reaches the trans-Golgi complex (Martire et al., 1996). The location of the plant receptor has not been determined, but a fusion between this and green fluorescent protein is located in the Golgi complex when expressed in plants, suggesting that the receptor itself has the same location (Boevink et al., 1998). However, unlike the mammalian receptor, the fusion protein is distributed uniformly across the different Golgi cisternae (Boevink et al., 1998).
The K/HDEL signal is sufficient to confer variable extents of accumulation in the plant ER and increased stability when added to proteins destined to the vacuole (Herman et al., 1990; Wandelt et al., 1992; Pueyo et al., 1995; Napier et al., 1997) or secretion (Conrad and Fiedler, 1998) or to cytosolic proteins introduced into the secretory pathway by the addition of a signal peptide (Denecke et al., 1992; Boevink et al., 1996). HDEL causes very efficient, albeit not complete, ER localization when fused to invertase (a secreted protein); however, the glycans of invertase-HDEL show extensive Golgi-mediated modification to complex structures, strongly suggesting that retrieval into the ER occurs in this case as far as from trans-Golgi stacks (Pagny et al., 2000). Plant calreticulin (a reticuloplasmin with HDEL C-terminal sequence) has instead only high-mannose glycans (Navazio et al., 1996; Crofts et al., 1999; Pagny et al., 2000). When overexpressed, tobacco calreticulin is in part secreted as a complex glycan-containing protein, but the glycans of the intracellular form remain almost completely unmodified, indicating that, if calreticulin traffics beyond the early Golgi cisternae, it is unavailable for retrieval into the ER (Crofts et al., 1999).
In addition, KDEL is also present in plant proteins, or protein precursors, that do not perform their function in the ER. The clearest example is SH-EP, a vacuolar protease involved in the degradation of storage proteins during seed germination. SH-EP is synthesized as a KDEL-containing precursor and is sequestered into very large vesicles that reach the vacuole from the ER via a traffic pathway that bypasses the Golgi complex (Toyooka et al., 2000). SH-EP belongs to a cysteine protease family, which has members in many plants; notably, one member is present in ricinosomes, lytic compartments that seem to derive directly from the ER during the germination of castor bean seeds (Schmid et al., 1998). The term “precursor protease vesicles” recently has been proposed to group the SH-EP–containing vesicles, ricinosomes, and other similar structures and to underline that plants may have a general mechanism, involving KDEL, for the sorting of certain proteases within the ER and their direct delivery to vacuoles (Chrispeels and Herman, 2000). Finally, HDEL fused to a secreted passenger protein is also able to deliver it in part to the vacuole. Although in this case the involvement of the Golgi complex has not been studied, it has been hypothesized that the vacuole could be the compartment for turnover of reticuloplasmins in plants (Gomord et al., 1997).
The role of KDEL/HDEL in plant cells is therefore far from being fully understood, and the system may not only contribute to ER localization but also regulate delivery to other compartments. In the present study, we have tried to determine the efficiency of the KDEL system and how KDEL influences the long-term destiny of a protein in plant cells. Reticuloplasmins seem to have additional features that favor their ER localization independently of the C-terminal tetrapeptide (Hammond and Helenius, 1995; Vitale and Denecke, 1999; Pagny et al., 2000), and therefore they are not particularly useful tools to study the KDEL/HDEL mechanism. We have thus analyzed the effect of KDEL addition to the vacuolar storage protein phaseolin. We show that in tobacco leaf cells retention/retrieval mediated by KDEL operates very efficiently before the trans-cisternae of the Golgi complex. However, a small proportion of phaseolin-KDEL reaches the vacuole via a novel route that is different from the Golgi-mediated one followed by wild-type phaseolin. This alternative route may be analogous to the one followed by SH-EP. Addition of KDEL has instead no marked effect on an assembly-defective construct of phaseolin that is subjected to ER quality control retention and degradation.
RESULTS
Phaseolin is a homotrimeric glycoprotein of the 7S class, with a subunit molecular mass ∼45 to 50 kD, which accumulates in the protein storage vacuoles of the cotyledonary cells of common bean. Phaseolin is cotranslationally inserted into the ER lumen, in which it is N-glycosylated and assembles into homotrimers. Only after assembly into trimers is phaseolin released from ER quality control (Vitale et al., 1995; Pedrazzini et al., 1997). Phaseolin trimers travel through the Golgi complex and are sorted to the vacuole in a process that is saturable and therefore probably receptor mediated (Frigerio et al., 1998).
In this work, the β-phaseolin construct T343F (Slightom et al., 1985; Pedrazzini et al., 1997) was used as a background for insertion of the KDEL sequence. T343F phaseolin (hereafter referred to as PHSL) acquires a single N-linked glycan, because the second glycosylation site normally present on wild-type phaseolin was inactivated by mutagenesis. The introduced mutation has no negative effects on the assembly and intracellular transport of phaseolin and allows following progress of phaseolin through the secretory pathway by analyzing the Golgi-mediated modification of its single glycan (Pedrazzini et al., 1997; Frigerio et al., 1998). We added the sequence encoding the tetrapeptide KDEL immediately after the last C-terminal codon of PHSL (which encodes Y421), generating the phaseolin-KDEL construct (P-KDEL).
P-KDEL Is Trimeric and Trypsin Resistant
We first verified that the introduced sequence did not interfere with the normal folding and assembly of the protein.
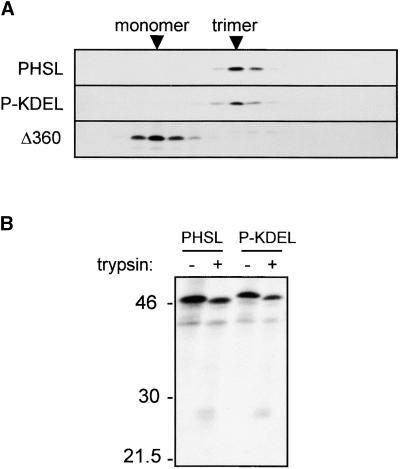
We transiently expressed in tobacco leaf protoplasts P-KDEL, PHSL, or the assembly-defective, transport-incompetent mutated construct Δ360 (Pedrazzini et al., 1997) as a control. After transformation, protoplasts were pulse-labeled for 1 hr with 35S-labeled amino acids and then homogenized, and the lysates were loaded on top of a continuous sucrose sedimentation velocity gradient. We immunoselected phaseolin from each gradient fraction with anti-phaseolin antiserum and analyzed it by SDS-PAGE and fluorography. Figure 1A shows that P-KDEL sediments in the same fractions as trimeric PHSL; in comparison, Δ360, being monomeric, sediments in fractions of lower density. We then conclude that P-KDEL rapidly and efficiently assembles into trimers.
Figure 1.
Phaseolin-KDEL Is Trimeric and Resistant to Trypsin.
(A) Tobacco leaf protoplasts were transiently transfected with plasmids encoding PHSL, P-KDEL, or the assembly-defective phaseolin mutant T343FΔ360 (Δ360). Cells were pulse-labeled for 1 hr with 35S-methionine and 35S-cysteine; cell homogenates were subjected to sedimentation velocity fractionation on a continuous 5 to 25% (w/v) sucrose gradient. Gradient fractions were immunoprecipitated with anti-phaseolin antiserum and analyzed by SDS-PAGE and fluorography. The top of the gradients is at left. Only the portions of the gels containing phaseolin are shown.
(B) Protoplasts transfected with plasmids encoding PHSL or P-KDEL were pulse-labeled for 1 hr, and cell homogenates were subjected to in vitro trypsin digestion before immunoprecipitation with anti-phaseolin antiserum. Analysis was by SDS-PAGE and fluorography. Numbers at left indicate molecular mass markers in kilodaltons.
Trimeric phaseolin is rather resistant to protease digestion in vitro, whereas monomers are completely degraded (Deshpande and Nielsen, 1987; Deshpande and Damodaran, 1989; Ceriotti et al., 1991). To establish whether P-KDEL trimers have a similar resistance to proteolysis, we subjected homogenates of transfected, pulse-labeled protoplasts to in vitro trypsin digestion, and phaseolin then was immunoselected. Figure 1B shows that incubation with trypsin resulted in a slight decrease in molecular mass of the bulk of phaseolin (intense bands at ∼46 kD; the fainter band at ∼40 kD is an endogenous tobacco polypeptide recognized by antiserum) and in the formation of discrete fragments at ∼25 kD, typical of this kind of phaseolin digestions (Deshpande and Nielsen, 1987). The pattern was the same for P-KDEL and PHSL, suggesting that P-KDEL trimers have a conformation that is very similar to that of normal phaseolin.
P-KDEL Is Stable and Is Not Secreted during Transient Expression
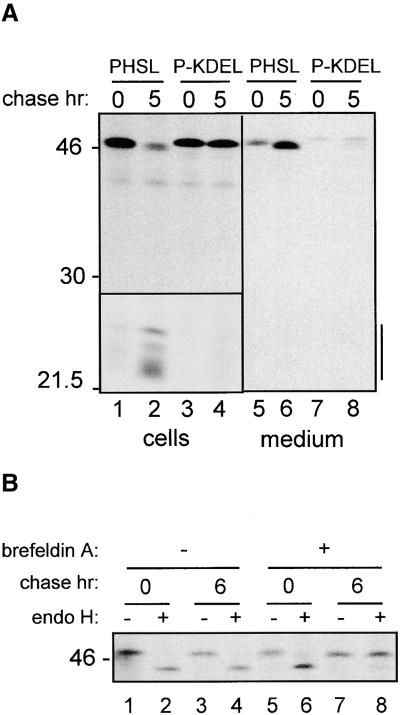
To determine whether the addition of KDEL has effects on the stability and trafficking of phaseolin, we first determined the destiny of P-KDEL during transient expression by pulse-chase analysis. In tobacco leaf protoplasts, PHSL is transported through the Golgi complex to the vacuole, and it is cleaved into fragments with molecular mass of 20 to 25 kD (Pedrazzini et al., 1997). This is shown in Figure 2A, lanes 1 and 2. This fragmentation does not occur in storage vacuoles of bean cotyledons, but it is typical of phaseolin vacuolar sorting in transgenic plants and is therefore a good indication that the protein has reached its final destination. The behavior of P-KDEL radically differs from that of PHSL: after 5-hr chase, nearly the whole of P-KDEL synthesized during the pulse was found in its intact form, and only an extremely low proportion, barely detectable in Figure 2A, was fragmented (Figure 2A, lanes 3 and 4).
Figure 2.
Phaseolin-KDEL Is Very Stable and Does Not Acquire Endo H Resistance.
(A) Tobacco protoplasts were transfected with plasmid encoding PHSL or P-KDEL and then pulse-labeled for 1 hr with 35S-methionine and 35S-cysteine and chased for the indicated periods of time. Cell homogenates or incubation media were immunoprecipitated with anti-phaseolin antiserum and analyzed by SDS-PAGE and fluorography. Vertical bar at right indicates phaseolin fragmentation products. The area containing the fragmentation products (bottom left) is taken from a threefold longer exposure of the same gel. Numbers at left indicate molecular mass markers in kilodaltons.
(B) Cells transfected with plasmid encoding phaseolin-KDEL were pulse-labeled for 1 hr in the presence (+) or in the absence (−) of brefeldin A and chased for the indicated periods of time in the same conditions. Cell homogenates were immunoprecipitated with anti-phaseolin antiserum and treated with endo H (+) or without enzyme as a control (−) and then analyzed by SDS-PAGE and fluorography.
Analysis of the extracellular medium revealed that a proportion of PHSL was secreted (Figure 2A, lanes 5 and 6); we previously have shown that this normally occurs when phaseolin is transiently expressed in tobacco protoplasts (Frigerio et al., 1998). The high expression level obtained in transient expression saturates the vacuolar sorting mechanism, causing partial default secretion that, like vacuolar delivery, is Golgi mediated (Frigerio et al., 1998). In the case of P-KDEL, virtually no secretion is observed (Figure 2A, lanes 7 and 8).
These observations strongly indicate that P-KDEL, although trimeric and therefore potentially transport competent, is very efficiently prevented from vacuolar delivery or extracellular secretion.
Intact P-KDEL Remains Sensitive to Endoglycosidase H
We wanted to establish whether the extent of recycling of P-KDEL encompasses the whole of the Golgi stack. To do so, we analyzed the single glycan of P-KDEL for resistance to in vitro digestion by endoglycosidase H (endo H). This enzyme removes from glycoproteins the N-linked glycans of the high-mannose type, but not of the complex type. The N-linked glycan of phaseolin acquires a complex structure when the protein is transported through the Golgi complex, and this makes it fully resistant to endo H (Sturm et al., 1987; Frigerio et al., 1998). The plant enzymes responsible for the acquisition of complex, endo H–resistant structures are located in the medial trans-Golgi complex (Fitchette-Lainè et al., 1994). The results of endo H treatment of P-KDEL isolated after pulse-chase are shown in Figure 2B, lanes 1 to 4. After 6-hr chase, phaseolin-KDEL was still entirely sensitive to endo H. To rule out the possibility that the absence of Golgi-mediated processing of P-KDEL was simply due to physical inaccessibility of its glycan to modifying enzymes, we performed the same experiment on protoplasts incubated in the presence of brefeldin A. This drug blocks the formation of vesicles that mediate intracellular traffic, inhibiting protein delivery to vacuoles or the cell surface and intermixing ER and Golgi complex functions (Gomez and Chrispeels, 1993; Pedrazzini et al., 1997; Staehelin and Driouich, 1997). In the presence of brefeldin A, the glycan of P-KDEL acquired, with time, endo H resistance (Figure 2B, lanes 5 to 8), indicating that it is correctly exposed and available for modification by the Golgi enzymes. Therefore, in normal conditions, P-KDEL remains unmodified because it does not reach the compartment where modifying enzymes are located.
We conclude that the mechanism of KDEL-mediated retention/retrieval operates very efficiently before the protein reaches the medial trans-Golgi cisternae.
P-KDEL Is Located in the ER and, in a Very Small Proportion, in the Vacuole
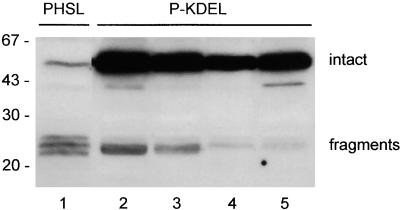
To determine the long-term destiny of P-KDEL, we generated transgenic tobacco plants expressing P-KDEL under control of the Cauliflower mosaic virus 35S promoter. We analyzed many plants by immunoblot with anti-phaseolin antiserum: four independent transformants are shown in Figure 3 and compared with a typical transgenic plant expressing PHSL. Analysis was performed on total protein extracts from small leaves. PHSL accumulates mainly as vacuolar fragments, as already reported (Pedrazzini et al., 1997). P-KDEL accumulates instead mainly in its intact form. Fragments are also detectable in the transgenic plants that express P-KDEL, but they constitute a very minor proportion of total P-KDEL. The polypeptide with molecular mass of ∼43 kD visible in lanes 2 and 5 of Figure 3 was not detected consistently in all preparations and was not analyzed further. Accumulation of intact P-KDEL is between one and two orders of magnitude higher than that of intact PHSL.
Figure 3.
P-KDEL Accumulates at High Levels in Leaves of Transgenic Tobacco.
Total protein extracts from leaves of transgenic tobacco expressing PHSL (lane 1) or P-KDEL (lanes 2 to 5, four independent transgenic plants) were analyzed by SDS-PAGE followed by protein gel blotting and immunodetection with anti-phaseolin antiserum. Numbers at left indicate molecular mass markers in kilodaltons.
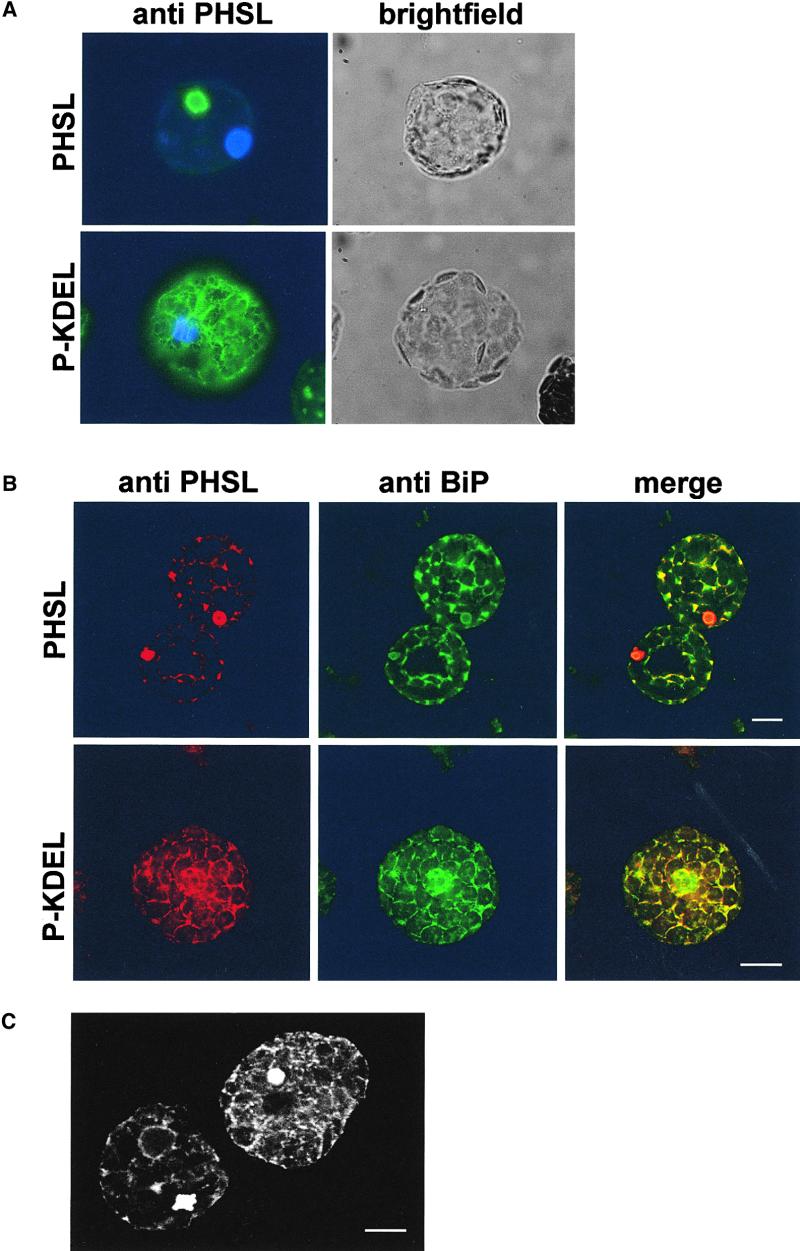
In light of the results of transient expression, the presence of vast amounts of intact P-KDEL in transgenic leaves suggests an ER localization. To test this hypothesis, we isolated protoplasts from transgenic leaves and analyzed them by immunofluorescence. Binding protein (BiP) is a major soluble resident of the ER (Vitale and Denecke, 1999) and was used as a marker for this compartment. As expected, immunolocalization of BiP shows extensive labeling of the typical plant ER network (Figure 4B). P-KDEL shows a very similar pattern (Figures 4A and 4B) and colocalizes with BiP (Figure 4B, merge). The pattern was consistently found in protoplasts, indicating that the accumulation of P-KDEL in the ER is a general feature of the protoplast population. PHSL is instead mainly detectable as aggregates and shows little ER pattern (Figures 4A and 4B). This is in agreement with our previous finding that in tobacco leaves PHSL is almost exclusively located in the vacuole in an electron-dense form (Frigerio et al., 1998); the aggregates containing vacuolar PHSL are also slightly labeled by anti-BiP antiserum, but they are clearly not a major site of BiP accumulation (Figure 4B, merge). Protoplasts from untransformed plants are not labeled at all by anti-phaseolin antiserum, indicating that the labeling is fully specific (not shown).
Figure 4.
P-KDEL Is Mainly Located in the ER.
(A) Protoplasts from transgenic tobacco plants expressing PHSL or P-KDEL were fixed, permeabilized, and subjected to immunofluorescence with rabbit anti-phaseolin antiserum, followed by secondary fluorescein isothiocyanate–conjugated (FITC) goat anti-rabbit antibody. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Cells were observed in bright field or with an FITC and UV light filter on a fluorescence microscope equipped with a digital camera.
(B) Protoplasts from transgenic tobacco plants expressing PHSL or P-KDEL were fixed, permeabilized, and subjected to immunolabeling with rabbit anti-phaseolin antiserum (detected with anti-rabbit Cy5), followed by postfixation and second immunolabeling with rabbit anti-BiP antiserum (detected with anti-rabbit FITC). Cells were observed with a confocal laser-scanning microscope. 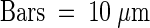 .
.
(C) P-KDEL–expressing cells present vacuolar aggregates. Protoplasts expressing P-KDEL were fixed, permeabilized, and subjected to immunolabeling with rabbit anti-phaseolin antiserum followed by anti-rabbit FITC secondary antibody. Cells were observed with a confocal laser-scanning microscope. 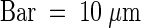 .
.
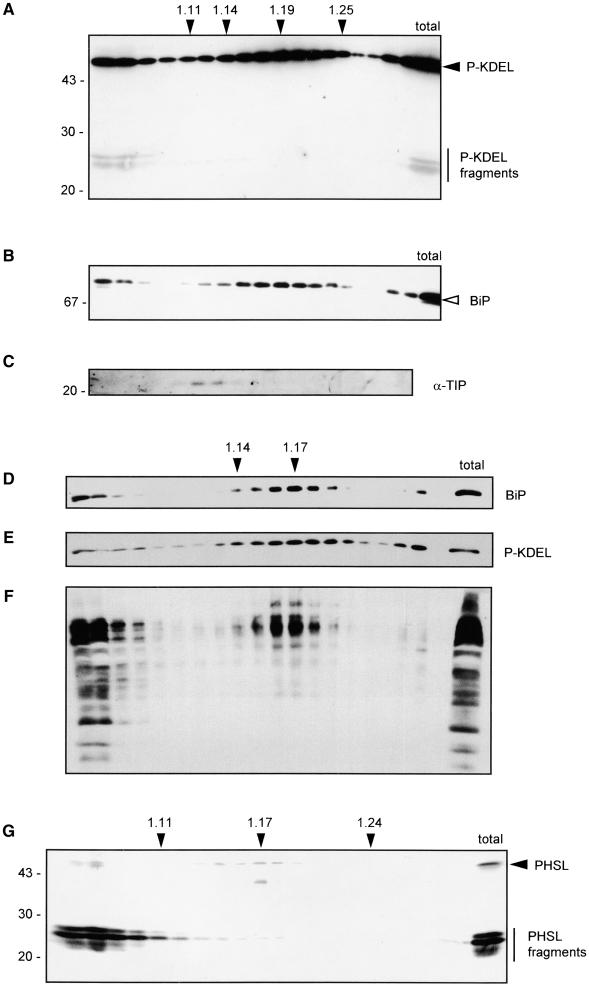
The results of immunolocalization indicate that most of P-KDEL is located in the ER; occasionally, relatively large aggregates also are detected (Figure 4C), suggesting that part of the protein also could be vacuolar. We thus verified the localization of P-KDEL by subcellular fractionation. Leaves were homogenized in buffer containing magnesium and sucrose in the absence of detergent and the subcellular compartments fractionated on isopycnic sucrose gradient. Proteins from each fraction were analyzed by SDS-PAGE followed by protein gel blot and immunodetection with different antisera. Intact P-KDEL (Figure 5A) bands at a density of ∼1.19, similarly to BiP (Figure 5B). To establish whether membranous compartments were indeed separated by our procedure, we determined the position of the tonoplast integral protein α-TIP by using specific antiserum. The result indicates that α-TIP and BiP migrate in fully distinct regions of the gradient, ruling out nonspecific membrane aggregation (Figure 5C). EDTA releases ribosomes attached to the ER. When subcellular fractionation was performed in the presence of EDTA instead of magnesium, there was a shift in the peak of P-KDEL, from density around 1.19 to density ∼1.17 (Figure 5E). The position of BiP had a similar shift (Figure 5D), confirming that most of intact P-KDEL is located in the ER. A similar analysis performed on extracts from plants expressing assembly-defective phaseolin indicated that the ER of tobacco mesophyll cells has a density of ∼1.14 to 1.15 in the presence of EDTA and 1.17 in the presence of magnesium (Pedrazzini et al., 1997). This discrepancy in compartment density between the two types of transgenic plants seems to be limited to the ER, because the Golgi complex, as revealed by an antiserum against complex glycans, peaks at density ∼1.15 to 1.16 both in P-KDEL–expressing (Figure 4F) and in defective phaseolin-expressing cells (Pedrazzini et al., 1997). We suggest that the higher density of the ER that contains P-KDEL is due to the very high levels of accumulation of the recombinant protein. A very similar change from the normal 1.14 density to 1.16 was observed for the ER of bean cotyledonary cells subjected to heat shock, a treatment that causes abnormally high accumulation of phytohemagglutinin (the second bean storage protein) in the ER (Chrispeels and Greenwood, 1987).
Figure 5.
A Very Small Proportion of P-KDEL Is Vacuolar and Fragmented.
Leaves from transgenic plants expressing P-KDEL ([A] to [F]) or PHSL (G) were homogenized in the presence of sucrose and either magnesium ([A], [B], [C], and [G]) or EDTA ([D], [E], and [F]), and the homogenate was fractionated on isopycnic sucrose gradient. Gradient fractions were analyzed by SDS-PAGE and protein immunoblotting using the antisera indicated for each panel.
(A) Anti-phaseolin.
(B) Anti-BiP.
(C) Anti-TIP.
(D) Anti-BiP.
(E) Anti-phaseolin.
(F) Anti-complex glycans.
(G) Anti-phaseolin.
In (A), (B), and (D) through (G), last lane at right contains the corresponding total unfractionated homogenate. At right, the positions of BiP (open arrowhead), intact phaseolin (filled arrowhead), and phaseolin fragments (vertical bar) are marked. Numbers at left indicate molecular mass markers in kilodaltons. Numbers on top indicate density (g mL−1).
Vacuoles completely break during the homogenation procedure used for isopycnic gradient analysis, and soluble vacuolar proteins remain in the top fractions of the gradient. This is clearly visible in Figure 5G, which shows analysis of a plant expressing PHSL in magnesium-containing buffer, using anti-phaseolin antiserum: vacuolar phaseolin fragments remain on top of the gradient, whereas the small proportion of intact phaseolin remains in the ER fraction at density 1.17, indicating that it represents the newly synthesized protein, in full agreement with the results of immunofluorescence shown in Figure 4. P-KDEL fragments are exclusively in the top fractions of the gradient (Figure 5A). A proportion of intact P-KDEL and of BiP (Figures 5A and 5B) is also in the top fractions and may represent partial release from the ER lumen during homogenation (see also the behavior of BiP in a similar experiment in Gomord et al., 1997). Most importantly, no fragmented P-KDEL is present in the ER region (Figure 5A), suggesting that intracellular traffic is necessary for its fragmentation.
We conclude that most of P-KDEL is intact in the ER and a very small proportion (see total in Figure 5A) is fragmented and located in the vacuole. Protein blot analysis of extracts from roots and stems revealed that also in those tissues most P-KDEL molecules are intact and a very small proportion is fragmented (not shown). Therefore, the minor vacuolar delivery of P-KDEL does not seem to be restricted to specific cell types. We wanted to establish whether the fragmentation products represent a proportion of P-KDEL that has escaped retrieval into the ER and has travelled through the entire Golgi complex toward the vacuole, following the normal route of wild-type phaseolin. If this were the case, the fragmentation products of P-KDEL should: (1) appear only after a lag of time necessary for trafficking to the vacuole in radioactive labeling experiments, (2) fail to appear when synthesis is allowed in the presence of inhibitors of traffic, and (3) have a Golgi-modified glycan. These hypotheses were tested with the experiments reported below.
Fragmentation of P-KDEL Is a Slow Process That Requires Vesicular Traffic
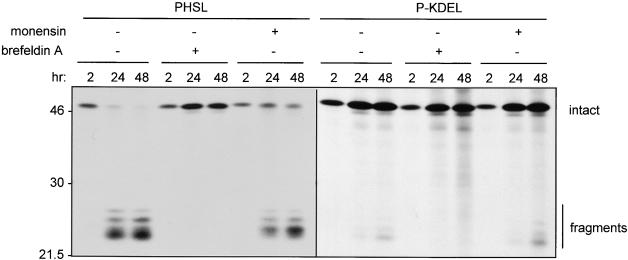
Leaf protoplasts from transgenic plants expressing P-KDEL or PHSL were subjected to radioactive labeling for 2, 24, or 48 hr, and phaseolin then was immunoprecipitated. The experiment, which is shown in Figure 6, was performed in the absence or presence of either brefeldin A or monensin. Monensin inhibits correct sorting of vacuolar proteins and leads to their partial secretion, most probably because of alterations in the pH of the Golgi complex (Gomez and Chrispeels, 1993). P-KDEL fragments cannot be detected after 2-hr labeling and begin to be detectable only after 24 hr. Even at 48 hr, they represent a very minor proportion of total newly synthesized P-KDEL. By comparison, PHSL fragments become detectable at 2 hr labeling and represent almost all of PHSL at 24 hr. Brefeldin A fully inhibits fragmentation of both PHSL and P-KDEL. Monensin partially inhibits fragmentation of PHSL but not of P-KDEL. Actually, there is a slight induction of fragmentation of P-KDEL in the experiment shown in Figure 6. The experiment was repeated three times, and we observed such a slight induction in two experiments, whereas in one there was no effect. Monensin also leads to partial secretion of intact PHSL, but does not stimulate secretion of P-KDEL (not shown). We conclude that fragmentation of P-KDEL is a very slow post-translational process that requires traffic out of the ER but is not inhibited by monensin. This satisfies the requirements in items 1 and 2 in the previous paragraph but also suggests that P-KDEL traffic may bypass at least part of the Golgi complex.
Figure 6.
P-KDEL Fragmentation Is Inhibited by Brefeldin A but Not Monensin.
Protoplasts were prepared from leaves of transgenic tobacco expressing PHSL or P-KDEL and labeled with 35S-methionine and 35S-cysteine for 2, 24, or 48 hr in the presence (+) or absence (−) of brefeldin A or monensin. Protoplast homogenates were immunoprecipitated with anti-phaseolin antiserum and analyzed by SDS-PAGE and fluorography. Numbers at left indicate molecular mass markers in kilodaltons.
The Glycan of P-KDEL Fragments Is Not Endo H Resistant
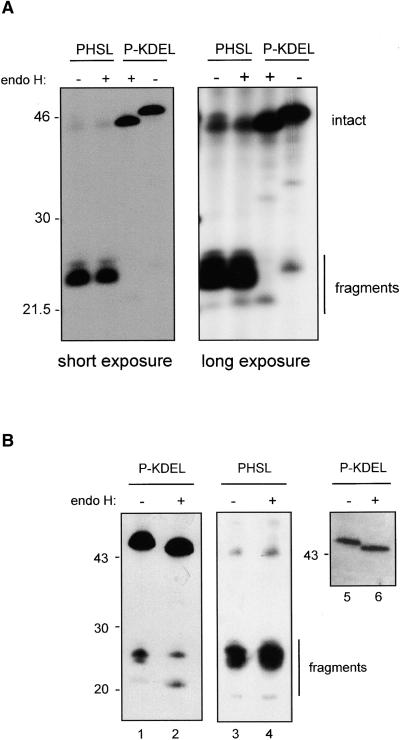
Three phaseolin fragments are clearly distinguishable in pulse-chase experiments (see, e.g., Figures 2 and 6). The largest fragment is actually a precursor to the middle one: detailed time-course analysis of PHSL fragmentation (not shown) reveals a clear precursor–product relationship between the two in a slow trimming process. Consistently, the upper fragment is a minor component after 48-hr labeling (see Figure 6), and it is not always easily detectable in protein blots. The smallest phaseolin fragment also originates from a slightly larger precursor, which is, however, a minor component already at 24-hr chase and is not always distinguishable. Therefore, after a first fragmentation event that splits the polypeptides into two approximate halves, further trimming occurs. Most importantly for this study, we previously have shown that the smallest PHSL vacuolar fragment contains the N-linked oligosaccharide, whereas the two larger ones are not glycosylated (Frigerio et al., 1998). This oligosaccharide is fully resistant to in vitro digestion by endo H, indicating that PHSL travels through the entire Golgi stacks (Frigerio et al., 1998). To verify whether the same holds true also for P-KDEL fragments, we subjected phaseolin immunoprecipitated from protoplasts labeled for 48 hr to endo H digestion, as shown in Figure 7A. Short exposure of the fluorograph indicated that, as expected, the glycosylated PHSL fragment is endo H resistant and intact P-KDEL is sensitive (Figure 7A, short exposure). Much longer exposure allowed establishing that the glycosylated P-KDEL fragment is fully sensitive to endo H (Figure 7A, long exposure). We also subjected to endo H digestion a leaf homogenate from a P-KDEL plant and analyzed phaseolin by SDS-PAGE and protein blot. The results indicate that both intact P-KDEL and the glycosylated P-KDEL fragment present in leaves at steady state levels are fully endo H sensitive (Figure 7B, lanes 1, 2, 5, and 6). When a parallel experiment was performed on extracts of a plant expressing PHSL, the fragment bearing the oligosaccharide was fully resistant to endo H, as expected (Figure 7B, lanes 3 and 4).
Figure 7.
P-KDEL Fragments Are Sensitive to Endo H.
(A) Protoplasts were prepared from leaves of transgenic tobacco expressing PHSL or P-KDEL and labeled with 35S-methionine and 35S-cysteine for 48 hr. Protoplast homogenates were immunoprecipitated with anti-phaseolin antiserum, and the immunoprecipitates were incubated in the presence (+) or absence (−) of endo H before analysis by SDS-PAGE and fluorography. Two different exposures of the same gel are shown.
(B) Total protein extracts from leaves of transgenic tobacco expressing P-KDEL or PHSL were incubated in the presence (+) or absence (−) of endo H before analysis by SDS-PAGE followed by protein gel blotting and immunodetection with anti-phaseolin antiserum. The three blots represent fully independent experiments. The blot at right (lanes 5 and 6) was exposed for a much shorter period of time than the one at left (lanes 1 and 2) to allow a better definition of the bands representing intact P-KDEL. Numbers at left indicate molecular mass markers in kilodaltons.
Therefore, even the proportion of P-KDEL that is delivered to the vacuole via vesicular traffic does not undergo the typical phaseolin Golgi-mediated modification. This does not satisfy the requirement described above in item 3, strongly suggesting that P-KDEL reaching the vacuole bypasses at least part of the Golgi cisternae.
P-KDEL Is in a Complex with BiP
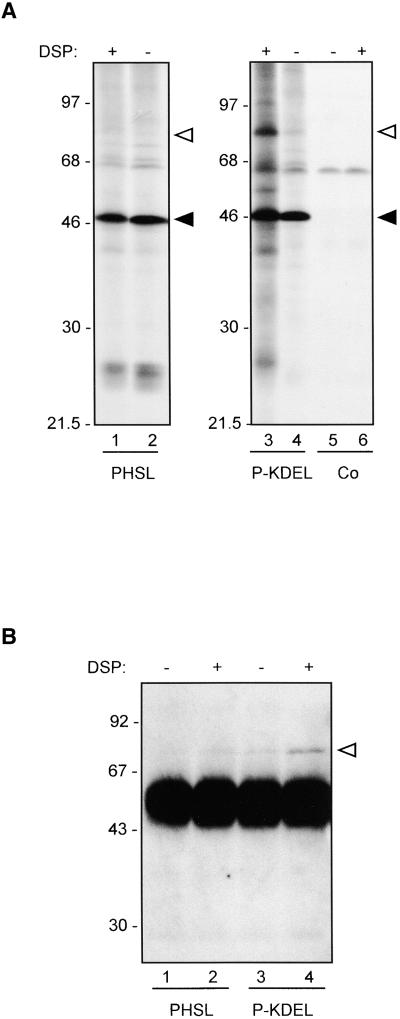
The bulk of P-KDEL is located in the ER. It has been suggested that the ER may be organized in subcompartments (Staehelin, 1997). The mechanism of ER retention/retrieval of H/KDEL-containing proteins, however, implies that such proteins must be in close proximity to each other. It actually has been shown that most of BiP is in a complex with calreticulin (Crofts et al., 1998); in addition, close association also could occur when reticuloplasmins are concentrated within the retrograde Golgi-to-ER transport vesicles. To test whether the presence of a KDEL signal is sufficient to bring a passenger protein in close vicinity to other reticuloplasmins, we performed cross-linking studies. Leaf protoplasts from transgenic plants expressing either P-KDEL or PHSL, or from control plants transformed with plasmid without insert, were radioactively labeled for 3 hr and homogenized in the absence of detergent and presence of sucrose to preserve as much as possible the lumenal ER environment but favor access of cross-linking agents. The homogenate was subjected to cross-linking with dithiobis(succinimidyl propionate) (DSP), a bifunctional cross-linker that can be cleaved by reducing agents. Phaseolin then was immunoprecipitated from DSP-treated or untreated homogenates and analyzed by SDS-PAGE and fluorography. The presence of β-mercaptoethanol in the protein denaturation buffer allows separation of cross-linked polypeptides. Upon treatment, P-KDEL, but not PHSL, was cross-linked to several tobacco polypeptides (Figure 8A, cf. lanes 1 and 3). One of them had the same molecular mass of BiP (78 kD; open triangles in Figure 8). Note that the band at ∼60 kD that is coimmunoprecipitated with phaseolin even without cross-linking is selected also from control protoplasts (Figure 8A, lanes 5 and 6), and therefore, as we already reported (Pedrazzini et al., 1997), it is an endogenous, cross-reacting tobacco polypeptide not relevant to our experiments.
Figure 8.
P-KDEL, but Not PHSL or Δ384-KDEL, Is in a Complex with BiP and Other Polypeptides.
(A) Protoplasts were prepared from leaves of transgenic tobacco expressing PHSL or P-KDEL or from tobacco transformed with empty plasmid as control (Co). Protoplasts were labeled with 35S-methionine and 35S-cysteine for 3 hr and homogenated in cross-linking buffer. The homogenates were incubated in the presence (+) or absence (−) of DSP for 30 min. After addition of glycine, the samples were immunoprecipitated with anti-phaseolin antiserum and analyzed by SDS-PAGE and fluorography. The positions of BiP (open arrowhead) and intact phaseolin (filled arrowhead) are marked at right.
(B) Protoplasts were prepared from leaves of transgenic tobacco expressing PHSL or P-KDEL and homogenated in cross-linking buffer. The homogenates were incubated in the presence (+) or absence (−) of DSP for 30 min. After addition of glycine, the samples were immunoprecipitated with anti-phaseolin antiserum. The immunoprecipitates were analyzed by SDS-PAGE followed by protein gel blotting and immunodetection with anti-BiP antiserum. The very intense bands that migrate between the 46- and 68-kD markers represent the heavy chains of the immunoglobulins used for immunoprecipitation. The position of BiP (open arrowhead) is marked at right.
Numbers at left indicate molecular mass markers in kilodaltons.
To verify the identity of the 78-kD cross-linked polypeptide, we performed cross-linking by using homogenates from unlabeled protoplasts; phaseolin then was immunoprecipitated and subjected to SDS-PAGE followed by protein gel blot probed using anti-BiP antiserum. DSP caused a marked increase in the amount BiP coimmunoprecipitated with P-KDEL, confirming that the 78-kD radioactive polypeptide is BiP (Figure 8B).
KDEL Does Not Change the Destiny of Assembly-Defective Phaseolin
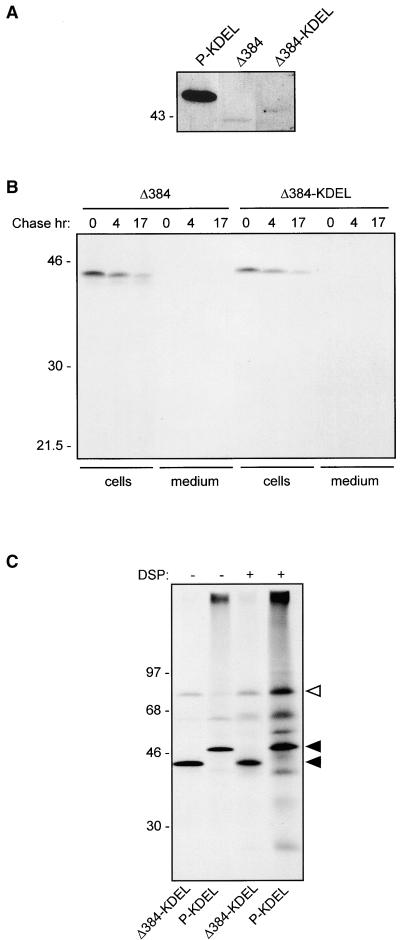
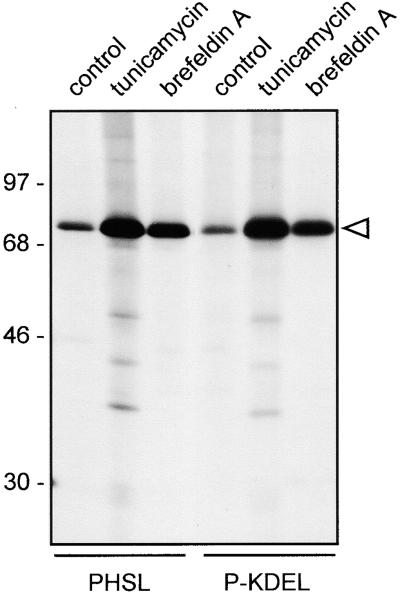
The results reported in Figures 8A and 8B indicate that at least a proportion of P-KDEL is in a complex with BiP. We have shown previously that, in the absence of cross-linking, a relevant proportion of BiP is coimmunoprecipitated with assembly-defective phaseolin constructs (Pedrazzini et al., 1997). In comparison, the amount of BiP coimmunoprecipitated with P-KDEL when protoplasts are not treated for cross-linking is not particularly high (such a comparison is not shown in the previous experiments, but see below), suggesting that the interaction of BiP with P-KDEL is different from the one with structurally immature or defective proteins. To test this hypothesis, we produced an assembly-defective construct of phaseolin with an added C-terminal KDEL sequence and compared its BiP binding with that of P-KDEL. The construct, Δ384-KDEL, was produced starting from T343F. A sequence encoding KDEL followed by a stop codon was inserted at position 384. In the encoded polypeptide, 38 amino acids at the C terminus of wild-type phaseolin are missing, including the last of the three C-terminal α-helical segments involved in assembly. As control, we also constructed Δ384, in which a stop codon was directly placed at position 384 of T343F. Transgenic tobacco plants expressing Δ384-KDEL or Δ384 were produced. The proteins are unable to form trimers and remain monomeric (not shown). Protein blot analysis of leaf extracts showed that the accumulation of Δ384-KDEL is much lower than that of P-KDEL and very similar to that of Δ384 (Figure 9A). Pulse-chase analysis indicated that the half-lives of Δ384-KDEL and Δ384 are also very similar to each other (Figure 9B). The experiment in Figure 9B was performed in the presence of brefeldin A; the same result was obtained in the absence of the inhibitor (not shown). Brefeldin A–insensitive degradation is indicative of disposal by ER quality control (Pedrazzini et al., 1997). Therefore, when added to an assembly-defective phaseolin construct, KDEL does not increase phaseolin stability and does not avoid quality control degradation. When protoplasts from transgenic plants expressing Δ384-KDEL were pulse-labeled and phaseolin immunoselected, there was a marked coselection of BiP, consistent with the defect in assembly (Figure 8C, first lane, cf. with P-KDEL in the second lane). However, cross-linking led to a very limited change in the pattern of proteins coselected with Δ384-KDEL (Figure 8C, third lane, cf. with BiP cross-linked to P-KDEL in the fourth lane). Longer exposure of the fluorograph (not shown) revealed that DSP caused cross-linking of the same polypeptides to P-KDEL and Δ384-KDEL, albeit quantitatively the effect was much lower to the latter. This strongly suggests that the different effect of DSP is not due to a marked influence of the C-terminal deletion on available cross-linking residues but rather to a much lower accumulation of Δ384-KDEL than P-KDEL in the ER.
Figure 9.
KDEL Does Not Alter the Destiny of Assembly-Defective Phaseolin.
(A) Total protein extracts from leaves of transgenic tobacco expressing P-KDEL, Δ384 or Δ384-KDEL were analyzed by SDS-PAGE followed by protein gel blotting and immunodetection with anti-phaseolin antiserum. Only the region of the blot containing phaseolin is shown.
(B) Protoplasts prepared from leaves of transgenic tobacco expressing Δ384 or Δ384-KDEL were pulse-labeled for 1 hr with 35S-methionine and 35S-cysteine and chased for the indicated periods of time, in the presence of brefeldin A. Cell homogenates or incubation media were immunoprecipitated with anti-phaseolin antiserum and analyzed by SDS-PAGE and fluorography.
(C) Protoplasts were prepared from leaves of transgenic tobacco expressing Δ384-KDEL or P-KDEL and labeled with 35S-methionine and 35S-cysteine for 3 hr and homogenated in cross-linking buffer. The homogenates were incubated in the presence (+) or absence (−) of DSP for 30 min. After addition of glycine, the samples were immunoprecipitated with anti-phaseolin antiserum and analyzed by SDS-PAGE and fluorography. The positions of BiP (open arrowhead) and the phaseolin constructs (filled arrowheads) are indicated at right.
Numbers at left indicate molecular mass markers, in kilodaltons.
We conclude that the interaction between BiP and P-KDEL revealed by cross-linking is not due to chaperone activity of the reticuloplasmin and that the KDEL tetrapeptide itself is not responsible for such interaction. KDEL must be attached to a structurally correct form of phaseolin to increase its stability, promote its extended ER residence, and allow interaction with BiP and other unknown proteins revealed by DSP treatment.
Extensive accumulation of defective proteins in the ER induces the synthesis of BiP and other chaperones through the unfolded protein response (reviewed in Gething, 1999). ER chaperone synthesis can also be induced by drugs that cause misfolding of some proteins or alter the ER structure, such as tunicamycin (reviewed in Vitale and Denecke, 1999) and brefeldin A (Liu et al., 1992). To confirm that the ER of P-KDEL plants is not subjected to severe stress caused by extensive accumulation of a misfolded protein, we therefore compared BiP synthesis, under different conditions, in protoplasts isolated from P-KDEL and PHSL plants. There was no difference between the two types of plants in the synthesis of BiP in normal conditions or in its induction by tunicamycin or brefeldin A (Figure 10). This indicates that P-KDEL does not have structural defects that can induce the unfolded protein response and that both plants have similar spare inducibility of BiP. The induction of BiP synthesis was higher in tunicamycin-treated than in brefeldin A–treated protoplasts. As expected, BiP induction by tunicamycin is accompanied by increased association of newly synthesized proteins to the chaperone, but, perhaps curiously, a similar association was not detected upon brefeldin A treatment (Figure 10).
Figure 10.
The Synthesis of P-KDEL Does Not Induce Unfolded Protein Response.
Protoplasts were prepared from leaves of transgenic tobacco expressing PHSL or P-KDEL and labeled with 35S-methionine and 35S-cysteine for 3 hr in the presence of tunicamycin or brefeldin A, or in the absence of inhibitors (control). The cell homogenates were immunoprecipitated with anti-BiP antiserum and analyzed by SDS-PAGE and fluorography. The open arrowhead marks the position of BiP. Numbers at left indicate molecular mass markers in kilodaltons.
DISCUSSION
KDEL Acts before the Trans-Cisternae of the Plant Golgi Complex
The KDEL signal has been shown previously to mediate to variable extents retention in the plant ER when fused to plant (Herman et al., 1990; Wandelt et al., 1992; Pagny et al., 2000), animal (Boevink et al., 1996), or bacterial (Denecke et al., 1992) proteins normally located in compartments other than the ER. However, these studies provided limited or contradictory information on how far the passenger proteins can travel along the plant secretory pathway before the receptor brings them back into the ER. In the case of a phytohemagglutinin–KDEL fusion protein, endo H treatment indicated that many of the polypeptides did not undergo Golgi-mediated processing, but the rather complex pattern of glycosylation of phytohemagglutinin and the absence of information on the accessibility of phytohemagglutinin–KDEL to processing enzymes left the question open (Herman et al., 1990). Overexpression of calreticulin leads to its partial secretion, and analysis of its glycans suggested that retrieval from distal Golgi cisternae is not efficient (Crofts et al., 1999). In contradiction with these results, invertase-HDEL that is retained intracellularly undergoes extensive Golgi-mediated processing, suggesting that retrieval can occur from distal Golgi cisternae (Pagny et al., 2000).
Taking advantage of the simple glycosylation pattern of T343F phaseolin and of the use of brefeldin A, we have shown here that in a plant cell retention/retrieval by KDEL can occur with high efficiency before a protein reaches the trans-Golgi cisternae. This range of KDEL action appears to be similar to the one observed in mammalian cells (Griffiths et al., 1994; Martire et al., 1996), although in plant cells the intermediate, pre-Golgi compartment has not been identified. Our conclusion is based on the observation that the oligosaccharide of phaseolin-KDEL never acquires endo H resistance and therefore is not modified by enzymes known to be located in the medial/trans-cisternae of the plant Golgi complex (Fitchette-Lainè et al., 1994). The absence of modifications is not due to a conformation of P-KDEL that prevents physical access of enzymes to its oligosaccharide, because the glycan of P-KDEL is efficiently modified when the ER and Golgi complex functions are intermixed upon brefeldin A treatment. In this respect, our results are similar to those obtained studying calreticulin (Navazio et al., 1996; Crofts et al., 1999; Pagny et al., 2000). The normal ER residents may, however, have additional features that allow their efficient ER retention, besides the C-terminal tetrapeptide (Munro and Pelham, 1987; Barlowe et al., 1994; Hammond and Helenius, 1995; Pagny et al., 2000). For the chimeric protein that we have constructed, retention instead must be exclusively due to the addition of KDEL.
Arabidopsis has more than one gene encoding for potential receptors of the tetrapeptides, and it is possible that HDEL and KDEL have different receptors with separate distributions along the Golgi complex or different affinity for their ligands, thus explaining the difference between our results and those obtained studying invertase-HDEL (Pagny et al., 2000), but this has not been proven. Alternatively, the abundance of the receptor can be tissue specific or the accessibility of the tetrapeptide can be influenced by its surface exposure in the folded passenger protein. Note that three KDEL sequences are exposed on the surface of each phaseolin trimer. We wish to point out that our results do not rule out the possibility that retrieval of P-KDEL is also possible from the trans-Golgi cisternae in which complex glycans are formed; they, however, demonstrate that the capacity of the KDEL system is so high that P-KDEL simply does not reach such a Golgi location.
KDEL Retention/Retrieval in Tobacco Leaf Cells Is Very Tight
During transient expression in leaf protoplasts, the mechanism that sorts normal phaseolin to the vacuole can be saturated when expression levels are high, leading to Golgi-mediated secretion (Frigerio et al., 1998). We were unable to saturate retention by KDEL at similar expression levels, as indicated by negligible fragmentation (indicative of vacuolar sorting) or secretion of phaseolin-KDEL. This means that in protoplasts the KDEL retention system has higher capacity than the phaseolin vacuolar sorting system. It is possible that the stress imposed onto the plant cells by protoplast preparation induces the expression of the KDEL receptor but not of the unidentified phaseolin vacuolar sorting mechanism. Indeed, transcription of genes encoding reticuloplasmins is induced by protoplast preparation (Denecke et al., 1995), and these proteins have C-terminal KDEL or HDEL; this may drive a parallel induction of the receptor. However, this has not been tested, and tunicamycin, which is the most potent inducer of reticuloplasmins, is a much weaker inducer of the KDEL receptor, suggesting that the mechanism is oversized (Bar-Peled et al., 1995). It is therefore possible that in plants the capacity of the KDEL system is constitutively higher than that of vacuolar sorting, perhaps because escape of reticuloplasmins, which are involved in the productive structural maturation of all secretory proteins (Vitale and Denecke, 1999), could be more deleterious to cell metabolism than escape of vacuolar proteins to the cell surface.
Long-Term Delivery to the Vacuole: An Alternative Mechanism
The partial vacuolar delivery of vacuolar proteins to which KDEL or HDEL were added (Herman et al., 1990; Pueyo et al., 1995), in theory, could reveal a proportion of molecules that, for undefined reasons, escape recognition by the KDEL receptor and proceed along the normal pathway of the wild-type counterparts to the vacuole. There is, however, evidence that HDEL could itself determine vacuolar sorting. This has been shown by fusing HDEL to a mutated form of sporamin (Gomord et al., 1997). Sporamin is a vacuolar protein, but the mutated form was deprived of its known vacuolar sorting signal. Without added HDEL, the mutant was secreted, whereas, with added HDEL, it was in part delivered to a compartment that is either the vacuole or an unknown compartment that copurifies with it (Gomord et al., 1997). We have shown here that in transgenic plants a very small proportion of P-KDEL was hydrolyzed into the typical vacuolar fragments. Fragmentation is a slow post-translational event that is blocked by the traffic inhibitor brefeldin A. Subcellular fractionation indicated that the fragments are vacuolar. Consistently, electron microscopy revealed aggregates of P-KDEL in the central vacuole, besides severe enlargements of the ER cisternae (not shown). However, the oligosaccharide of the fragmented protein was not of the Golgi-modified type, and fragmentation was not inhibited by monensin. The explanation we favor for these observations is that vacuolar delivery did not occur through the normal, Golgi-mediated route, again indicating a very tight block of transport through the Golgi complex due to KDEL and revealing an alternative route to the vacuole. The slight induction of fragmentation of P-KDEL observed upon monensin treatment, albeit not consistently in all repeated experiments, suggests that inhibition of Golgi-mediated delivery to the vacuole may actually stimulate the alternative route.
A small proportion of phaseolin could traffic through the entire Golgi complex to the vacuole escaping the KDEL receptor and glycan processing because it is either misfolded or aggregated. We think this is very unlikely for P-KDEL because (1) its vacuolar delivery is not inhibited by monensin, and (2) we expect a misfolded or aggregated protein to be unavailable for the precise fragmentation that occurs on P-KDEL and is typical of phaseolin trimers when they reach vacuoles of transgenic plants. Consistently, assembly-defective phaseolin and hi-met phaseolin, which have conformational defects, do not give rise to any detectable fragment before their full degradation (Hoffman et al., 1988; Pedrazzini et al., 1997).
Delivery of P-KDEL to the vacuole can occur directly from the ER itself or from the cis-medial Golgi complex; our results cannot distinguish between the two possibilities. Although to our best knowledge, it has never been demonstrated that proteins can leave the Golgi complex for the vacuole or the cell surface bypassing the trans-cisternae, a monensin-insensitive route from the cis-medial Golgi complex to the cell surface has been shown to be followed by certain polysaccharides (Moore et al., 1991).
Golgi-independent routes to the vacuole exist in plant cells and are involved in the synthesis of certain storage proteins and protease precursors (recently reviewed in Chrispeels and Herman, 2000). Protein bodies, which are formed in the ER by certain storage proteins, may undergo autophagy by vacuoles either naturally, like in wheat endosperm (Levanony et al., 1992) or, apparently, in transgenic plants such as tobacco transformed with maize zeins (Coleman et al., 1996). Pumpkin storage proteins also form large structures in the ER, termed precursor-accumulating vesicles, which are delivered to vacuoles bypassing the Golgi complex (Hara-Nishimura et al., 1998). This process is insensitive to monensin, but it is not known whether it operates through autophagy or direct fusion with the tonoplast. Thiol proteases, like SH-EP, involved in the degradation of storage proteins upon seed germination are transported from the ER to vacuoles into large vesicles that bypass the Golgi complex and seem to directly fuse with the tonoplast (Toyooka et al., 2000). These proteases are synthesized as KDEL-containing precursors, and the tetrapeptide is lost at some step before vacuolar delivery. Finally, traffic of the tonoplast protein α-TIP is brefeldin A and monensin insensitive (Gomez and Chrispeels, 1993). A well-established effect of brefeldin A in mammalian cells is the inhibition of formation of COP I vesicles, which mediate Golgi-to-ER retrograde traffic. This, in turn, causes uncontrolled intermixing of the Golgi complex with the ER and inhibition of transport out of this new supercompartment. However, brefeldin A probably also has other long-term, less characterized effects in plant cells, and because fragmentation of P-KDEL is a very slow process our assay involved prolonged incubation with the inhibitor. We therefore do not know at which step the drug blocks P-KDEL traffic.
There may be different sorting mechanisms within the ER that lead to different forms of Golgi-independent vacuolar delivery (Chrispeels and Herman, 2000), but none has been elucidated. Our results favor the hypothesis that one of these sorting mechanisms recognizes high levels of protein accumulation within the ER or a high number of cycles of retrieval from the cis-Golgi complex. This may be caused either by the particular structure of a protein, as in the case of pumpkin and wheat storage proteins, or by the presence of KDEL, as for P-KDEL and thiol proteases.
We have shown that BiP interacts with P-KDEL in a fashion that is distinct from its normal chaperone activity. BiP also has been found in precursor-accumulating vesicles (Hara-Nishimura et al., 1998). The chaperone may be nonspecifically trapped into the large structures formed by the storage proteins. A less trivial hypothesis is that prolonged ER permanence allows a protein to share other aspects of the cell biology of reticuloplasmins. Plant reticuloplasmins are very long-lived proteins (D'Amico et al., 1992; Crofts et al., 1998); however, it is obvious that they must be eventually turned over. To our knowledge, the intracellular location of their degradation has not been identified. It is possible that the slow vacuolar delivery of P-KDEL and some of the other known Golgi-independent routes to vacuoles actually represent the normal route for reticuloplasmin turnover, a hypothesis suggested when the vacuolar delivery of sporamin-HDEL was revealed (Gomord et al., 1997).
In P-KDEL, KDEL is immediately preceded by the natural C-terminal sequence of phaseolin, AFVY, which is necessary for the normal, Golgi-mediated vacuolar sorting of this protein (Frigerio et al., 1998). We do not know whether, followed by the new tetrapeptide, AFVY still can be recognized by the poorly characterized vacuolar sorting mechanism of phaseolin. However, our data clearly indicate that KDEL overrides the normal mechanism of vacuolar sorting.
A Severe Structural Defect Is Dominant over the Effect of KDEL
We previously have shown that assembly-defective phaseolin is subjected to ER quality control: it is transiently retained in the ER in which it extensively interacts with BiP before being degraded in a brefeldin A–insensitive process without the formation of detectable fragmentation products (Pedrazzini et al., 1997). In the present study, we showed that KDEL does not alter the destiny of an assembly-defective phaseolin construct. Consistently with a lack of accumulation of defective protein within the ER, cross-linking did not increase the association between BiP and Δ384-KDEL. Therefore, KDEL stabilizes phaseolin only if the storage protein has the correct trimeric structure, suggesting that quality control diverts a passenger protein from the possibility of entering the cycle of delivery to and retrieval from the Golgi complex that results in extensive accumulation in the ER. This parallels the recent observation that in COS mammalian cells a misfolded form of vesicular stomatitis virus G protein tagged with green fluorescent protein is retained in the ER without visiting post-ER compartments (Nehls et al., 2000). It therefore seems that misfolded proteins are unavailable for ER export.
METHODS
Recombinant DNA
The KDEL coding sequence was added to the 3′ end of the T343F phaseolin coding sequence by polymerase chain reaction, using the antisense oligonucleotide 5′-CTGCAGTCATAGCTCATCTTTGTACACAAATGCACCCTTTCTTCCC-3′ (bases in boldface type indicate the KDEL-coding sequence; the stop codon is underlined). Phaseolin Δ384 was obtained with the antisense oligonucleotide 5′-CTGCAGTCACCCAGAGAACGTAGCCCCAAC-3′, and the KDEL coding sequence was added to Δ384 by using the antisense oligonucleotide, 5′-CTGCAGTCATAGCTCATCTTTCCCAGAGAACGTAGCCCCAAC-3′. P-KDEL, Δ384, and Δ384-KDEL were introduced into the vector pDHA (Tabe et al., 1995) for transient expression experiments. For constitutive expression in transgenic tobacco, pDHA containing the above-mentioned phaseolin mutants was inserted into the HindIII site of the binary vector pGA470 (An et al., 1985), which then was used to transform Agrobacterium tumefaciens EHA105 (Hood et al., 1986) by electroporation.
Transient Transformation of Leaf Protoplasts and Production of Transgenic Tobacco Plants
Protoplasts were prepared from axenic leaves (4 to 7 cm long) of Nicotiana tabacum cv Petit Havana SR1. Protoplasts were subjected to polyethylene glycol–mediated transfection as described by Pedrazzini et al. (1997). The Agrobacterium containing phaseolin-KDEL (P-KDEL), Δ384, or Δ384-KDEL was used to produce transgenic plants as described (Pedrazzini et al., 1997).
In Vivo Labeling of Protoplasts and Analysis of Phaseolin
Pulse-chase labeling of protoplasts by using Pro-Mix (a mixture of 35S-methionine and 35S-cysteine; Amersham) and immunoprecipitation of phaseolin were performed as described previously using rabbit polyclonal antiserum raised against phaseolin purified from mature been seeds (Pedrazzini et al., 1997). When indicated, 10 μg mL−1 brefeldin A (Boehringer; from a 2-mg-mL−1 stock solution in ethanol), 5 μM monensin (Sigma; from a 1-mM stock in ethanol), 50 μg mL−1 tunicamycin (Boehringer; from a 5-mg-mL−1 stock in 10 mM NaOH), or equivalent amounts of the respective solvents for the controls were added to the incubation medium 45 min before labeling and maintained at the same concentration throughout pulse-chase. Unless otherwise stated, protoplast homogenation was performed by adding to frozen samples 2 volumes of ice-cold homogenation buffer (150 mM Tris-Cl, 150 mM NaCl, 1.5 mM EDTA, and 1.5% Triton X-100, pH 7.5) supplemented with Complete (Boehringer) protease inhibitor cocktail. For treatment with trypsin, protoplasts were pulse-labeled for 1 hr and homogenized with protoplast homogenization buffer without protease inhibitors. Trypsin was added to a final concentration of 10 mg mL−1, and samples were incubated at 37°C for 15 min. “Complete” protease inhibitor cocktail (Boehringer Mannheim) was added to terminate the digestion, and samples were immunoprecipitated as described (Pedrazzini et al., 1997).
Analysis of phaseolin assembly by sedimentation velocity on sucrose gradient and immunoblot analysis of extracts from small (3 to 6 cm long) leaves of tobacco were performed as described (Frigerio et al., 1998).
Endoglycosidase H (endo H) digestion of immunoprecipitated proteins was performed as described previously (Ceriotti et al., 1991). For endo H treatment of total leaf proteins, leaves from transgenic plants were homogenized as described (Frigerio et al., 1998); 0.5 volumes of denaturing buffer (0.5% SDS, 1% β-mercaptoethanol, 100 mM Tris-Cl, pH 8.0) were added to the homogenate, and the mixture was boiled for 15 min. BSA (100 mg mL−1) then was added to a final concentration of 0.8 mg/mL, and samples were incubated at 37°C for 15 min. Sodium citrate, pH 5.5, was added to a final concentration of 0.25 M. Samples were split into two tubes and incubated with 20 mU endo H (Boehringer Mannheim), or with water as a control, at 37°C for 4 hr. Total proteins then were precipitated adding 1 volume of cold 30% trichloroacetic acid, and the protein pellet was washed twice with ice-cold acetone and then dissolved in SDS-PAGE loading buffer. Samples then were analyzed by SDS-PAGE followed by immunoblot as described (Frigerio et al., 1998).
For subcellular fractionation on isopycnic sucrose gradients, small tobacco leaves were homogenized in an ice-cold mortar with ice-cold 100 mM Tris-Cl, pH 7.8, 10 mM KCl, containing 12% (w/w) sucrose and either 2 mM MgCl2 or 1 mM EDTA, using 6 mL of buffer per gram of fresh leaf tissue. The homogenate was centrifuged for 10 min at 1000g at 4°C; ∼600 μL of the supernatant were loaded on a 12-mL linear 16 to 55% (w/w) sucrose gradient made in the same buffer. After centrifugation at 35,000 rpm, 4°C for 2 hr in a Beckman SW40 rotor (154,400g average), fractions of ∼650 μL were collected. Immunoblot analysis of the fractions was performed as described (Pedrazzini et al., 1997) using anti-phaseolin antiserum, polyclonal antiserum raised against plant complex N-linked glycans (Lainé et al., 1991), polyclonal antiserum against α-TIP purified from bean cotyledons (Johnson et al., 1989), or polyclonal anti–binding protein (BiP) antiserum raised against a recombinant fusion between maltose BiP and amino acids 551 to 667 of tobacco BiP (Pedrazzini et al., 1997).
Cross-Linking
Protoplast (∼300,000, either unlabeled or radioactively labeled with ProMix for 3 hr) were pelleted by addition of 3 volumes of ice-cold W5 medium (154 mM NaCl, 5 mM KCl, 125 mM CaCl2·2H2O, 5 mM glucose) followed by centrifugation at 60g for 10 min at 4°C and washed once again with W5, leaving 50 μL to cover the pellet. All subsequent manipulations were performed on ice. The pellet was resuspended by adding 350 μL of cross-linking buffer (12% [w/w] sucrose, 200 mM Na-phosphate, pH 7.8). The resuspension was vortexed 30 sec and pipetted 15 times through a Gilson 200-μL tip. This operation was repeated three times to lyse protoplasts. Eight microliters of anhydrous DMSO supplemented or not with 12.5 mM dithiobis(succinimidyl propionate) (DSP; Sigma) were added, and the sample was incubated 30 min on ice with occasional gentle mixing. To stop the reaction, we added 20 μL of 200 mM glycine to each sample. After 30 min on ice, the sample was frozen. Homogenation and immunoprecipitation were performed as described above, using protoplast homogenation buffer and immunoprecipitation buffer at pH 8.0 instead of 7.5 to increase the stability of DSP.
Immunocytochemistry
After purification, protoplasts from plants expressing PHSL or P-KDEL, or untransformed plants were resuspended in MaCa buffer (0.5 M mannitol, 20 mM CaCl2, 0.1% MES, pH 5.7) at a concentration of 5 × 105 cells mL−1; 300 μL of cell suspension was spread onto polylysine-coated slides (Sigma), and cells were allowed to adhere for 30 min at room temperature. Cells were fixed for 30 min at room temperature in MaCa buffer containing 4% (w/v) paraformaldehyde. Cells then were permeabilized by washing three times with TSW buffer (10 mM Tris-HCl, pH 7.4, 0.9% NaCl, 0.25% gelatin, 0.02% SDS, 0.1% Triton X-100) for 10 min at room temperature. Incubation with rabbit anti-PHSL and/or anti-BiP antiserum (both at 1:1000 dilution) was in the same buffer for 1 hr at room temperature. After three washes in TSW, cells were incubated for 1 hr at room temperature with fluorescein isothiocyanate–conjugated (FITC) or Cy5-conjugated donkey anti-rabbit secondary antibody (Jackson Immunoresearch, West Grove, PA) at a dilution of 1:200. After three final washes in TSW, cells were mounted in Vectashield-DAPI (Vector Laboratories, Burlingame, CA). When two primary antibodies of the same species were used for colocalization, the procedure followed was that described by Paris et al. (1996). Cells were visualized with a Bio-Rad MRC1024 confocal laser scanning microscope equipped with a ×40 oil immersion objective and FITC/Cy5 filter sets. Thickness of the optical sections was 2 μm. For optical microscopy, cells were visualized with a Zeiss AxioScope fluorescence microscope equipped with a ×100 oil immersion objective. Images were collected with a CCD camera (Photometrics, Tucson, AZ) and visualized with SmartCapture software (Digital Scientific, Cambridge, UK).
Acknowledgments
We thank Emanuela Pedrazzini for the help with confocal microscopy, Loïc Faye for the gift of anticomplex glycan antiserum, and Maarten Chrispeels for the gift of anti-TIP antiserum. We also thank Heidi Holkeri and Emanuela Pedrazzini for critical reading of the manuscript. This work was supported in part by the European Union (Grant No. CHRX-CT94-0590), the Consiglio Nazionale de Ricerche (CNR) Target Project in Biotechnology, a CNR fellowship to L.F., and the British Council British–Italian Joint Research Program (Grant No. ROM/889/99/10).
References
- An, G., Watson, B.D., Stachel, S., Gordon, M.P., and Nester, E.W. (1985). New cloning vehicles for transformation of higher plants. EMBO J. 4 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M.F., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77 895–907. [DOI] [PubMed] [Google Scholar]
- Bar-Peled, M., da Silva Conceicão, A., Frigerio, L., and Raikhel, N.V. (1995). Expression and regulation of aERD2, a gene encoding the KDEL receptor homolog in plants, and other genes encoding proteins involved in ER-Golgi vesicular trafficking. Plant Cell 7 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boevink, P., Santa Cruz, S., Hawes, C., Harris, N., and Oparka, K.J. (1996). Virus-mediated delivery of the green fluorescent protein to the endoplasmic reticulum of plant cells. Plant J. 10 935–941. [Google Scholar]
- Boevink, P., Oparka, K., Santa Cruz, S., Martin, B., Batteridge, A., and Hawes, C. (1998). Stacks on tracks: The plant Golgi apparatus traffics on an actin/ER network. Plant J. 15 441–447. [DOI] [PubMed] [Google Scholar]
- Ceriotti, A., Pedrazzini, E., Fabbrini, M.S., Zoppè, M., Bollini, R., and Vitale, A. (1991). Expression of wild-type and mutated vacuolar storage protein phaseolin in Xenopus oocytes reveals relationships between assembly and intracellular transport. Eur. J. Biochem. 202 959–968. [DOI] [PubMed] [Google Scholar]
- Chrispeels, M.J., and Greenwood, J.S.. (1987). Heat stress enhances phytohemagglutinin synthesis but inhibits its transport out of the endoplasmic reticulum. Plant Physiol. 83 778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels, M.J., and Herman, E.M. (2000). Endoplasmic reticulum-derived compartments function in storage and as mediators of vacuolar remodeling via a new type of organelle, precursor protease vesicles. Plant Physiol. 123 1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, C.E., Herman, E.M., Takasaki, K., and Larkins, B.A. (1996). The maize γ-zein sequesters α-zein and stabilizes its accumulation in protein bodies of transgenic tobacco endosperm. Plant Cell 8 2335–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, U., and Fiedler, U. (1998). Compartment-specific accumulation of recombinant immunoglobulins in plant cells: An essential tool for antibody production and immunomodulation of physiological functions and pathogen activity. Plant Mol. Biol. 38 101–109. [PubMed] [Google Scholar]
- Crofts, A.J., Leborgne-Castel, N., Pesca, M., Vitale, A., and Denecke, J. (1998). BiP and calreticulin form an abundant complex that is independent of endoplasmic reticulum stress. Plant Cell 10 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts, A.J., Leborgne-Castel, N., Hillmer, S., Robinson, D.G., Phillipson, B., Carlsson, L.E., Ashford, D.A., and Denecke, J. (1999). Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell 11 2233–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico, L., Valsasina, B., Daminati, M.G., Fabbrini, M.S., Nitti, G., Bollini, R., Ceriotti, A., and Vitale, A. (1992). Bean homologs of the mammalian glucose regulated proteins: Induction by tunicamycin and interaction with newly synthesized storage proteins in the endoplasmic reticulum. Plant J. 2 443–455. [DOI] [PubMed] [Google Scholar]
- Denecke, J., De Rycke, R., and Botterman, J. (1992). Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 11 2345–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., Carlsson, L.E., Vidal, S., Höglund, A.-S., Ek, B., van Zeijl, M.J., Sinjorgo, K.M.C., and Palva, E.T. (1995). The tobacco homolog of mammalian calreticulin is present in protein complexes in vivo. Plant Cell 7 391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande, S.S., and Damodaran, S. (1989). Heat-induced conformational changes in phaseolin and its relation to proteolysis. Biochim. Biophys. Acta 998 179–188. [Google Scholar]
- Deshpande, S.S., and Nielsen, S.S. (1987). In vitro enzymatic hydrolysis of phaseolin, the major storage protein of Phaseolus vulgaris L. J. Food Sci. 52 1326–1329. [Google Scholar]
- Fitchette-Lainè, A.-C., Gomord, V., Chekkafi, A., and Faye, L. (1994). Distribution of xylosylation and fucosylation in the plant Golgi apparatus. Plant J. 5 673–682. [Google Scholar]
- Frigerio, L., de Virgilio, M., Prada, A., Faoro, F., and Vitale, A. (1998). Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell 10 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething, M.-J. (1999). Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 10 465–472. [DOI] [PubMed] [Google Scholar]
- Gomez, L., and Chrispeels, M.J. (1993). Tonoplast and soluble vacuolar proteins are targeted by different mechanisms. Plant Cell 5 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomord, V., Denmat, L.-A., Fitchette-Lainè, A.-C., Satiat-Jeunemaitre, B., Hawes, C., and Faye, L. (1997). The C-terminal HDEL sequence is sufficient for retention of secretory proteins in the endoplasmic reticulum (ER) but promotes vacuolar targeting of proteins that escape the ER. Plant J. 11 313–325. [DOI] [PubMed] [Google Scholar]
- Griffiths, G., Ericsson, M., Krijnse-Locker, J., Nilsson, T., Goud, B., Söling, H.-D., Tang, B.L., Wong, S.H., and Hong, W. (1994). Localization of the Lys, Asp, Glu, Leu tetrapeptide receptor to the Golgi complex and the intermediate compartment in mammalian cells. J. Cell Biol. 127 1557–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, C., and Helenius, A. (1995). Quality control in the secretory pathway. Curr. Opin. Cell Biol. 7 523–529. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Shimada, T., Hatano, K., Takeuchi, Y., and Nishimura, M. (1998). Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, E.M., Tague, B.W., Hoffman, L.M., Kjemtrup, S.E., and Chrispeels, M.J. (1990). Retention of phytohemagglutinin with carboxyterminal tetrapeptide KDEL in the nuclear envelope and the endoplasmic reticulum. Planta 182 305–312. [DOI] [PubMed] [Google Scholar]
- Hoffman, L.M., Donaldson, D.D., and Herman, E.M. (1988). A modified storage protein is synthesized, processed, and degraded in the seeds of transgenic plants. Plant Mol. Biol. 11 717–729. [DOI] [PubMed] [Google Scholar]
- Hood, E.E., Helmer, G.L., Fraley, R.T., and Chilton, M.D. (1986). The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J. Bacteriol. 168 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K.D., Herman, E.M., and Chrispeels, M.J. (1989). An abundant, highly conserved tonoplast protein in seeds. Plant Physiol. 91 1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainé, A.C., Gomord, V., and Faye, L. (1991). Xylose-specific antibodies as markers of subcompartmentation of terminal glycosylation in the Golgi apparatus of sycamore cells. FEBS Lett. 295 179–184. [DOI] [PubMed] [Google Scholar]
- Lee, H.-I., Gal, S., Newman, T.C., and Raikhel, N.V. (1993). The Arabidopsis endoplasmic reticulum retention receptor functions in yeast. Proc. Natl. Acad. Sci. USA 90 11433–11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanony, H., Rubin, R., Altschuler, Y., and Galili, G. (1992). Evidence for a novel route of wheat storage proteins to vacuoles. J. Cell Biol. 119 1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, M.J., and Pelham, H.R.B. (1990). A human homologue of the yeast HDEL receptor. Nature 348 162–163. [DOI] [PubMed] [Google Scholar]
- Liu, E.S., Ou, J.H., and Lee, A.S. (1992). Brefeldin A as a regulator of grp78 gene expression in mammalian cells. J. Biol. Chem. 267 7128–7133. [PubMed] [Google Scholar]
- Martire, G., Mottola, G., Pascale, M.C., Malagolini, N., Turrini, I., Serafini-Cessi, F., Jackson, M.R., and Bonatti, S. (1996). Different fate of a single reporter protein containing KDEL or KKXX targeting signals stably expressed in mammalian cells. J. Biol. Chem. 271 3541–3547. [DOI] [PubMed] [Google Scholar]
- Moore, P.J., Swords, K.M., Lynch, M.A., and Staehelin, L.A. (1991). Spatial organization of the assembly pathways of glycoproteins and complex polysaccharides in the Golgi apparatus of plants. J. Cell Biol. 112 589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, S., and Pelham, H.R.B. (1987). A C-terminal signal prevents secretion of luminal ER proteins. Cell 48 899–907. [DOI] [PubMed] [Google Scholar]
- Napier, J.A., Richard, G., Turner, M.F.P., and Shewry, P.R. (1997). Trafficking of wheat gluten proteins in transgenic tobacco plants: γ-Gliadin does not contain an endoplasmic reticulum–retention signal. Planta 203 488–494. [DOI] [PubMed] [Google Scholar]
- Navazio, L., Baldan, B., Mariani, P., Gerwig, G.J., and Vliegenthart, J.F.G. (1996). Primary structure of the N-linked carbohydrate chains of calreticulin from spinach leaves. Glycoconj. J. 13 977–983. [DOI] [PubMed] [Google Scholar]
- Nehls, S., Snapp, E.L., Cole, N.B., Zaal, K.J., Kenworthy, A.K., Roberts, T.H., Ellenberg, J., Presley, J.F., Siggia, E., and Lippincott-Schwartz, J. (2000). Dynamics and retention of misfolded proteins in native ER membranes. Nat. Cell Biol. 2 288–295. [DOI] [PubMed] [Google Scholar]
- Pagny, S., Cabanes-Macheteau, M., Gillikin, J.W., Leborgne-Castel, N., Lerouge, P., Boston, R.S., Faye, L., and Gomord, V. (2000). Protein recycling from the Golgi apparatus to the endoplasmic reticulum in plants and its minor contribution to calreticulin retention. Plant Cell 12 739–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris, N., Stanley, C.M., Jones, R.L., and Rogers, J.C. (1996). Plant cells contain two functionally distinct compartments. Cell 85 563–572. [DOI] [PubMed] [Google Scholar]
- Pedrazzini, E., Giovinazzo, G., Bielli, A., de Virgilio, M., Frigerio, L., Pesca, M., Faoro, F., Bollini, R., Ceriotti, A., and Vitale, A. (1997). Protein quality control along the route to the plant vacuole. Plant Cell 9 1869–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo, J.J., Chrispeels, M.J., and Herman, E.M. (1995). Degradation of transport-competent destabilized phaseolin with a signal for retention in the endoplasmic reticulum occurs in the vacuole. Planta 196 586–596. [DOI] [PubMed] [Google Scholar]
- Schmid, M., Simpson, D., Kalousek, F., and Gietl, C. (1998). A cysteine endopeptidase with a C-terminal KDEL motif isolated from castor bean endosperm is a marker enzyme for the ricinosome, a putative lytic compartment. Planta 206 466–475. [DOI] [PubMed] [Google Scholar]
- Semenza, J.C., Hardwick, K.G., Dean, N., and Pelham, H.R.B. (1990). ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell 61 1349–1357. [DOI] [PubMed] [Google Scholar]
- Slightom, J.L., Drong, R.F., Klassy, R.C., and Hoffman, L.M. (1985). Nucleotide sequences from phaseolin cDNA clones: The major storage proteins from Phaseolus vulgaris are encoded by two unique gene families. Nucleic Acids Res. 13 6483–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin, L.A. (1997). The plant ER: A dynamic organelle composed of a large number of discrete functional domains. Plant J. 11 1151–1165. [DOI] [PubMed] [Google Scholar]
- Staehelin, L.A., and Driouich, A. (1997). Brefeldin A effects in plants. Are different Golgi responses caused by different sites of action? Plant Physiol. 114 401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm, A., Van Kuik, J.A., Vliegenthart, J.F.G., and Chrispeels, M.J. (1987). Structure, position, and biosynthesis of the high mannose and the complex oligosaccharide side chains of the bean storage protein phaseolin. J. Biol. Chem. 262 13392–13403. [PubMed] [Google Scholar]
- Tabe, L.M., Wardley-Richardson, T., Ceriotti, A., Aryan, A., McNabb, W., Moore, A., and Higgins, T.J.V. (1995). A biotechnological approach to improving the nutritive value of alfalfa. J. Anim. Sci. 73 2752–2759. [DOI] [PubMed] [Google Scholar]
- Toyooka, K., Okamoto, T., and Minamikawa, T. (2000). Mass transport of proform of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuoles by endoplasmic reticulum–derived vesicle is involved in protein mobilization in germinating seeds. J. Cell Biol. 148 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, A., and Denecke, J. (1999). The endoplasmic reticulum—Gateway of the secretory pathway. Plant Cell 11 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, A., Bielli, A., and Ceriotti, A. (1995). The binding protein associates with monomeric phaseolin. Plant Physiol. 107 1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandelt, C.I., Khan, M.R.I., Craig, S., Schroeder, H.E., Spencer, D., and Higgins, T.J.V. (1992). Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants. Plant J. 2 181–192. [DOI] [PubMed] [Google Scholar]