Abstract
Sieve tubes of legumes (Fabaceae) contain characteristic P-protein crystalloids with controversial function. We studied their behavior by conventional light, electron, and confocal laser scanning microscopy. In situ, crystalloids are able to undergo rapid (<1 sec) and reversible conversions from the condensed resting state into a dispersed state, in which they occlude the sieve tubes. Crystalloid dispersal is triggered by plasma membrane leakage induced by mechanical injury or permeabilizing substances. Similarly, abrupt turgor changes imposed by osmotic shock cause crystalloid dispersal. Because chelators generally prevent the response, divalent cations appear to be the decisive factor in crystalloid expansion. Cycling between dispersal and condensation can be induced in opened cells by repetitive exchange of bathing media containing either Ca2+ or chelators. Sr2+ and Ba2+, but not Mg2+, are equally active. In conclusion, the fabacean P-protein crystalloids represent a novel class of mechanically active proteinaceous structures, which provide an efficient mechanism with which to control sieve tube conductivity.
INTRODUCTION
In higher plants, long-distance photoassimilate transport takes place through sieve tubes, which are longitudinal arrays of sieve elements (SEs; Behnke and Sjolund, 1990; Schulz, 1998). SEs develop by a unique differentiation, during which they abandon their nucleus, ribosomes, dictyosomes, and tonoplast (van Bel and Knoblauch, 2000). Plasmodesmata in the cross walls between SEs develop into large pores, thus turning these walls into sieve plates (SPs), the characteristic histological feature of the elongate adult SE (Esau and Thorsch, 1984). According to Münch's (1930) theory, transport through SEs occurs as mass flow driven by pressure gradients. This notion has long been in dispute due to the observation of structural barriers (proteinaceous plugs on the SP pores) in several electron microscopy studies (Weatherley and Johnson, 1968; Robidoux et al., 1973; Johnson et al., 1976). However, alternative hypotheses proved even more controversial, and so pressure flow has remained the most plausible mechanism for phloem transport (Evert, 1982).
The pressure flow theory has gained substantial experimental support recently from the visualization of operating SEs in vivo by confocal laser scanning microscopy (CLSM; Knoblauch and van Bel, 1998) and from structural studies based on the development of appropriate preparation techniques (Ehlers et al., 2000). As a result, it became indisputable that unimpeded mass flow occurs at velocities in the range of 1 cm min−1 in SEs of the model system broad bean (Vicia faba). Proteinaceous SP plugs occur within seconds after wounding by laser light or mechanical damage (Knoblauch and van Bel, 1998) or as artifacts caused by inadequate preparation techniques for electron microscopy (Ehlers et al., 2000). Such barriers aggregate from parietal P-proteins and the contents of phloem-specific SE-plastids, which in the functional state are located near the longitudinal walls of the SEs (Ehlers et al., 2000). Thus, at least some of the confusingly multifarious structures previously described (Cronshaw and Sabnis, 1990; Sabnis and Sabnis, 1995) actually represent the same material at different states of a cellular wound response.
However, despite recent progress, the biological role of most structural phloem-specific proteins (P-proteins) remains elusive. For example, sieve tubes of the legumes (Fabaceae) contain characteristic protein crystalloids (Lawton, 1978a; Behnke, 1991), whose function is entirely obscure. On the basis of cytological studies, some authors have suggested that these crystalline P-proteins undergo a transition from a crystalloid to a dispersed state during SE differentiation (Wergin and Newcomb, 1970; Palevitz and Newcomb, 1971). Others have argued that the apparent dispersal in the adult state is an artifact attributable to turgor loss during tissue preparation for electron microscopy (Fisher, 1975; Lawton, 1978b). Here, we demonstrate that these crystalloids represent a novel type of mechanically active intracellular structure: they reversibly plug sieve tubes by performing rapid Ca2+-controlled cycles of dispersal and reassembly in response to turgor changes.
RESULTS
Crystalloids Visible in Intact SEs
Protein crystalloids were studied in living SEs in the midrib of fully grown broad bean (V. faba) leaves. After phloem tissue had been made accessible to light microscopy by a shallow surface cut, a few crystalloids usually were visible. The number of crystalloids seemed to increase with time; in some cases, crystalloids could not be detected before 10 to 15 min after preparation. Crystalloids were usually seen first in SEs a few cell layers below the cut surface; in the uppermost layer, they regularly appeared some minutes later.
As a rule, each SE contained one elongate crystalloid. Rarely, two crystalloids were observed in the same SE. Crystalloids were mostly located close to the SP on the downstream end of the SE. Our attempts to isolate individual crystalloids failed because crystalloids vanished within a few seconds when the surrounding cell wall was damaged mechanically. Thus, it seemed that crystalloids dispersed and assembled spontaneously within the cells. Unfortunately, conventional light microscopy did not provide further insights into the mechanisms of these phenomena.
Variable Ultrastructure of Crystalloids
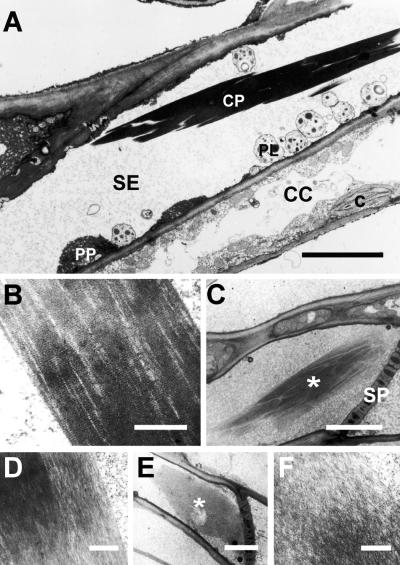
To gain information about the nature of spontaneous crystalloid assembly and dispersal, we determined which of the ultrastructural appearances of P-protein crystalloids described previously (cf. Sabnis and Sabnis, 1995) were present in virtually intact SEs (cf. Ehlers et al., 2000). In transmission electron micrographs, crystalloids from SEs of adult leaves appeared as elongate electron-dense bodies, up to 30 μm long and 2 to 6 μm wide (Figure 1A). Higher magnification showed that crystalloids consisted of coaligned fibrils with a superimposed, regularly striped pattern perpendicular to fibril orientation (Figure 1B). Occasionally, crystalloids were found in which the dense ultrastructure seen in their center became less ordered along their edges (Figures 1C and 1D). In some cases, crystalloids consisted entirely of irregularly interwoven fibrils (Figure 1F). These flakelike structures extended over the diameter of the sieve tube and usually were found in contact with a SP (Figure 1E).
Figure 1.
Ultrastructural Appearance of P-Protein Crystalloids in SEs of Adult Broad Bean Leaves.
(A) Compact crystalline P-protein body (CP) within a SE. Note undamaged SE-plastids (PL) and masses of parietal P-protein (PP; interspersed with membrane cisternae of the endoplasmic reticulum) located close to the longitudinal SE wall, indicating that the cell remained intact during preparation. c, chloroplast; CC, companion cell. Bar = 3 μm.
(B) Compact crystalline P-protein body as in (A) at higher magnification. Note the regular striped pattern perpendicular to the longitudinal fibrillar structure. Bar = 0.5 μm.
(C) Crystalline P-protein body (asterisk) located near a SP, showing the structural transition from the dense, ordered state at its center to a more fluffy appearance along its edges. Bar = 5 μm.
(D) Detail of (C) at higher magnification. Bar = 0.3 μm.
(E) Crystalline P-protein body (asterisk) with dispersed appearance. The fibrillar mass extends over almost the entire diameter of the SE, next to a SP. Bar = 5 μm.
(F) Detail of (E) at higher magnification.  .
.
Our ultrastructural findings were consistent with the idea that in living SEs, P-protein crystalloids could dissolve into filamentous masses or assemble from that material. However, due to the static nature of electron micrographs, alternative interpretations could not be excluded.
Plasma Membrane Leakage Induces Crystalloid Dispersal
To further examine the hypothesis that P-protein crystalloids transform reversibly into unordered fibrillar masses, we searched for a vital dye specific for P-protein crystalloids. We tested numerous fluorescent dyes commonly used in plant cell biology, but only one member of the dimethylfluorescein diacetate family, CDMFDA (for 5[6]-carboxy-4′,5′-dimethylfluorescein diacetate) transiently contrasted the crystalloids. When the dye was applied and washed out after 5 min, it allowed the observation of crystalloids by CLSM for a period of a few minutes.
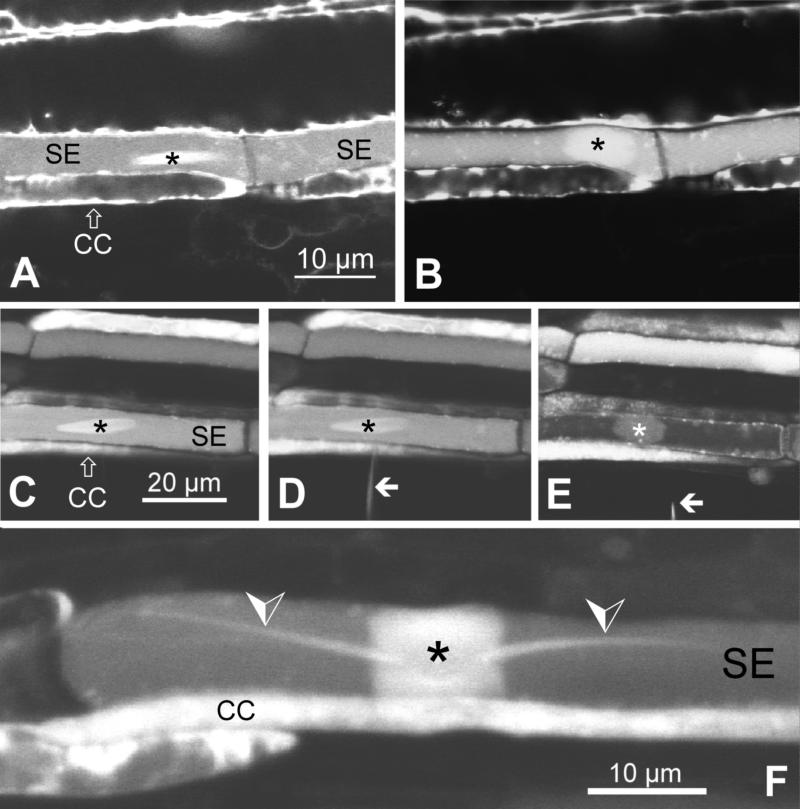
Using CLSM on CDMFDA-stained sieve tubes, we were able to verify that the apparent disappearance of crystalloids actually was a transformation of the dense elongate crystalloid structure into a less dense roundish plug that extended over the width of the SE (Figures 2A and 2B). The reaction could be triggered reliably (four repetitions) by micropipette insertion into the SEs. The transformation obviously entailed a change in refractive index that rendered the dispersed plug invisible in the conventional light microscope.
Figure 2.
Dynamics of Crystalline P-Protein Bodies Observed in CDMFDA-Stained Phloem Tissue of Broad Bean (V. faba) and Runner Bean (Phaseolus vulgaris) by CLSM.
(A) and (B) Crystalloid P-protein body in broad bean before (A) and after (B) insertion of a micropipettete (not visible; tip diameter of 2 μm). Micropipettete insertion triggers the transformation of the dense, elongate crystalloid into a roundish sieve tube plug.
(C) to (E) Dispersal of a crystalloid P-protein body in a broad bean SE in response to retraction of an inserted GEF (tip diameter of 0.1 μm). (C) In the undisturbed SE, an elongate crystalline P-protein body is visible. (D) GEF (arrow) insertion has no effect. (E) GEF (arrow) retraction triggers plug formation.
(F) Crystalline P-protein body in a sieve tube in runner bean. Crystalloid dispersal had been induced by micropipettete insertion (as in [A] and [B]). Although the main body of the crystalloid forms a sieve tube plug as in broad bean (cf. [B] and [E]), the two tail-like protrusions (arrowheads), which are characteristic for crystalline P-protein bodies in runner bean, remain unchanged.
Asterisks, crystalline P-protein bodies; CC, companion cell.
The reaction to micropipette insertion depended on the pipette tip size. With tip diameters >2 μm, crystalloids always dispersed within 1 to 3 sec (>30 experiments). Decreasing tip diameter slowed the response; 1-μm tips triggered plug formation within an average of 4 sec (n = 8). Upon insertion of galinstan-expansion-femtosyringes (GEFs; tip diameter of 0.1 μm), a pipette type developed for leak-free microinjection into high-turgor cells such as SEs (Knoblauch et al., 1999), crystalloids remained undisturbed (in six out of seven experiments; Figures 2C and 2D). However, retraction of the GEFs always caused instantaneous crystalloid dispersal (Figure 2E). Thus, membrane leakage, and not the penetration as such, triggered the response. This interpretation was corroborated by the reaction to the application of Triton X-100 and digitonin, substances that induce unspecific membrane permeability. Solutions of 1% (v/v) Triton X-100 and 1 mM digitonin induced dispersal of the crystalloids within 20 to 30 sec (two repetitions of each experiment; data not shown).
Crystalloid Dispersal Is Triggered by Turgor Changes
Crystalloid dispersal in response to membrane leakage might be triggered by turgor changes in the leaky cell. We tested this possibility by challenging SEs osmotically according to a variety of experimental protocols. Changes in extracellular osmolarity in steps <400 mosmol (equivalent to a change in osmotic pressure of 1 MPa) were ineffective, whereas changes in steps >600 mosmol (1.5 MPa) triggered crystalloid dispersal; intermediate steps yielded mixed results. These observations were independent not only of the osmolyte used (KCl, CaCl2, MgCl2, and sucrose were tested) but also of the osmolarity prevailing before the step. External osmolarity could be increased slowly from 100 to 700 mosmol in three steps of 200 mosmol each at 5-min intervals without any response of the crystalloids. Further increase from 700 to 1300 mosmol in one step triggered crystalloid dispersal in the same way that a single step from 100 to 700 mosmol did. SEs actually could be plasmolyzed with crystalloids remaining intact if extracellular osmolarity was increased stepwise. If the plasma membrane of plasmolyzed cells was damaged with micropipettes, then crystalloids dispersed within a few seconds (in both of two experiments).
The dispersal response was transient; spontaneous crystalloid reassembly usually occurred 4 to 6 min after the dispersal event. However, reassembly could not be induced by bringing external osmolarity back to the initial value immediately after the disappearance of the crystalloid. Surprisingly, decreases of extracellular osmolarity worked exactly as increases did. The reaction to a 600-mosmol step down was indistinguishable from the response to a corresponding step up.
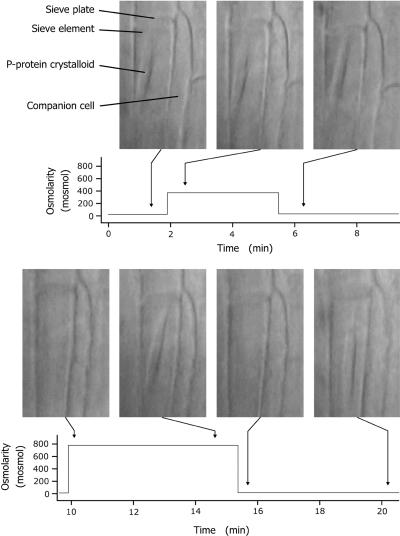
The main results of our osmolarity experiments are exemplified by a typical test shown in Figure 3; similar tests with modified treatments were performed on >20 crystalloids with consistent results.
Figure 3.
Reversible Dispersal of a Crystalline P-Protein Body in a Broad Bean SE in Response to Osmotic Shock.
The time course of extracellular osmolarity is given, with photographs of the SE taken at the times indicated by arrows. The crystalloid did not react to osmolarity steps of 350 mosmol (corresponding to a change in osmotic pressure of almost 0.9 MPa; upper row). Steps of 750 mosmol (corresponding to a change in osmotic pressure of ∼1.9 MPa) induced dispersal of the crystalline P-protein body regardless of the direction of the step (lower row). Note that the crystalloid spontaneously reassembled at 5 min after osmotic shock–induced dispersal. In this experiment, external osmolarity was adjusted with KCl. Identical results were obtained using CaCl2, MgCl2, and sucrose.
Calcium Acts as Dispersing Agent
As an alternative to changes in turgor, membrane leakage might also provoke crystalloid dispersal because of increased membrane fluxes of signaling substances. Coincidentally, we had noted that more crystalloids seemed to be visible immediately after tissue preparation if the bathing medium contained a chelator. Therefore, we studied the influence of extracellular Ca2+ on crystalloid dispersal.
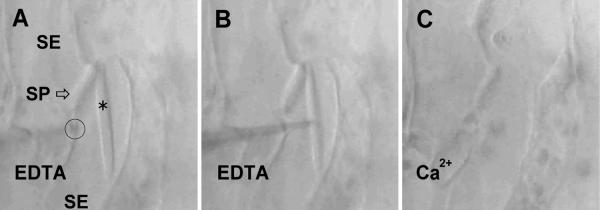
The incubation medium in all experiments described above contained 10 mM CaCl2; repetitions of these experiments with Ca2+-free standard medium always yielded identical results. However, when endogenous Ca2+ that might have originated from the cell wall was intercepted by EDTA or EGTA (≥1 mM) in the bathing solution, none of the treatments that in standard medium had triggered crystalloid dispersal (micropipette insertion, >80 repetitions; application of Triton X-100, two repetitions; changes in extracellular osmolarity, >20 repetitions) showed any effect. When holes up to 150 μm long were torn into SE walls with micropipettes in the presence of 10 mM EDTA, crystalloids remained unaffected even when agitated with micropipette tips (Figure 4). In these experiments, the addition of excess Ca2+ (12 mM) to chelator-containing media always caused rapid crystalloid dispersal. When bathing solutions containing 10 mM of either Ca2+ or EDTA were exchanged, crystalloids in opened SEs instantaneously dispersed or assembled, respectively (Figure 5; this experiment was repeated on 16 crystalloids with identical results). In individual crystalloids, such reaction cycles could be repeated at least 15 times. Crystalloid dispersal and assembly could be initiated in severed cells even after they had been stored for several hours (in all of five tests). As a corollary of the results obtained with damaged SEs, a direct involvement of the membrane potential in triggering crystalloid dispersal could be ruled out.
Figure 4.
Mechanical Robustness of a Crystalline P-Protein Body in a Severed Broad Bean SE in the Presence of a Chelator (10 mM EDTA).
(A) A crystalline P-protein body (asterisk) is seen located next to a SP in a kinked sieve tube; two SEs are visible. A micropipette tip touches a longitudinal SE wall (circle).
(B) The micropipette is inserted into the SE. The crystalloid can be pushed around and rotated along its longitudinal axis in this situation.
(C) After retraction of the micropipette, crystalloid dispersal is induced by exchanging 10 mM EDTA for 10 mM CaCl2 in the external medium.
A video showing the complete experiment is available (supplemental material under file name crystalloids movie 1.mov).
Figure 5.
Rapid Dispersal and Reassembly of a Crystalloid P-Protein Body in a Severed Broad Bean SE.
(A) A crystalline P-protein body (asterisk) is seen in a SE that has been ripped open in the presence of 10 mM EDTA.
(B) The crystalloid disperses instantaneously when EDTA is exchanged for 10 mM CaCl2 in the external medium.
(C) Instantaneous crystalloid reassembly is induced by exchanging CaCl2 for EDTA again.
A video showing the complete experiment is available (supplemental material under file name crystalloids movie 2.mov).
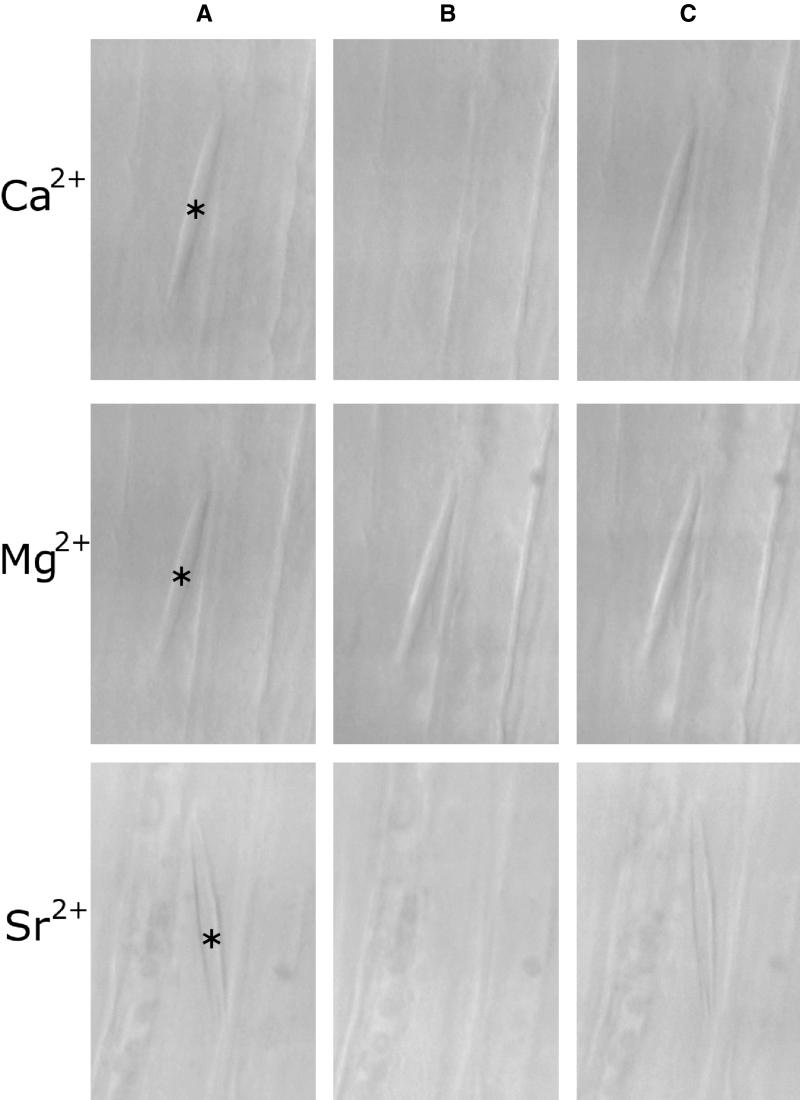
The Ca2+ effect in these experiments could be mimicked reliably by equimolar concentrations of the divalent cations Sr2+ (in all of eight crystalloids tested; Figure 6) and Ba2+ (six crystalloids; data not shown). However, Mg2+ was ineffective (13 crystalloids tested; Figure 6), as were monovalent cations (K+ and Na+) and Cl− (eight crystalloids tested; data not shown).
Figure 6.
Induction of Crystalline P-Protein Body Dispersal by Ca2+ and Sr2+ but Not by Mg2+ in Broad Bean SEs.
(A) Crystalloids (asterisks) in SEs severed in the presence of 10 mM EDTA.
(B) Effect of exchanging EDTA for 10 mM chloride salt of the divalent cation indicated to the left of each row. Crystalloid dispersal is induced by Ca2+ and Sr2+ but not by Mg2+.
(C) Crystalloid reassembly by exchanging divalent cation salts for 10 mM EDTA.
Reversible Crystalloid Dispersal Is Characteristic of Fabaceae
Elongate crystalline P-protein bodies are typical of the Fabaceae (Behnke, 1991). To establish the general validity of our findings in broad bean, we examined protein crystalloids of runner bean (Phaseolus vulgaris) and Lupinus polyphyllos. Despite subtle morphological differences between the crystalloids of the different species (such as the crystalloid “tails” in runner bean seen in Figure 2F; cf. Lawton, 1978a), sieve tube plug formation was induced by micropipette insertion (three repetitions in each species), as described above for broad bean. In all species, spontaneous crystalloid assembly occurred a few minutes after preparation of the leaves.
More or less globular P-protein bodies, which remain nondispersive during SE ontogeny and ultrastructurally resemble the fabacean P-protein crystalloids, exist in numerous angiosperm families (Behnke, 1991). We tested the reaction to wounding in Urtica dioica (Urticaceae; two repetitions) and Rubus fruticosus (Rosaceae; three repetitions). P-protein bodies in these species were truly nondispersive, that is, they failed to react even when SEs were severely damaged mechanically in the presence of free Ca2+ (data not shown).
DISCUSSION
Structural Dynamics of P-Protein Crystalloids
Crystalline P-proteins from legume SEs were first described more than a century ago (Baccarini, 1892) and have been studied ever since. However, on the basis of descriptive studies alone, the structural state of these protein bodies in transporting SEs could not be established unequivocally (reviewed in Cronshaw and Sabnis, 1990; Sabnis and Sabnis 1995). In our plant material, we were able not only to reproduce all major ultrastructural features described previously, including crystalloid, fibrillar, and intermediate states (Figure 1), but also to observe directly the transformation of crystalloids into filamentous plugs in intact SEs (Figure 2). Moreover, we established the reversibility of the structural transformation in living (Figure 3) and dead (Figures 4 to 6) cells. Our results provide conclusive evidence for the material identity of crystalloid and filamentous structures in fabacean SEs. Their remarkable structural dynamics distinguish crystalline P-proteins of the legumes from the nondispersive phloem-specific protein bodies of other families (such as the Urticaceae and Rosaceae tested here).
Control of Crystalloid Conformation by Ca2+
Because extracellular chelators prevent crystalloid dispersal in vivo, an influx of divalent cations into the SE seems a prerequisite for the response. Cytoplasmic Ca2+, typically kept to submicromolar levels in plant cells (Gilroy et al., 1993), is a well-established link of various signal transduction chains (Poovaiah and Reddy, 1993; Bush, 1995). In sieve tubes, the concentration of free Ca2+ has been reported to be 20- to 100-fold higher than it is in typical plant cells (Brauer et al., 1998). Nevertheless, the electrochemical Ca2+ gradient is directed into the SE cytoplasm, where the concentration of free Ca2+ may be controlled by sequestration in the so-called SE reticulum (Arsanto, 1986), by specific Ca2+ binding proteins (McEuen et al., 1981), and probably by Ca2+-ATPases, which seem to be ubiquitous in plant plasma membranes (Askerlund and Sommarin, 1996). Thus, changes in free Ca2+ have the potential to function as signals in SEs. It must be expected, though, that phloem-specific Ca2+-regulated proteins respond to relatively high Ca2+ concentrations in the micromolar or low millimolar range (Brauer et al., 1998).
Ca2+ controlled the conformational state of crystalloids in severely damaged cells (Figures 4 to 6). It seems inconceivable that a cellular signaling machinery should be functional under these conditions. Therefore, it is plausible that Ca2+ acts directly on P-protein crystalloids. Under the low Ca2+ conditions prevailing in the undisturbed SE, crystalloids probably exist in the condensed resting state. Presumably, they transform into plugs in direct response to surges in intracellular Ca2+ caused by Ca2+ influx from extracellular sources (Figure 7). If this idea is correct, then the reversible conformational changes would actually be controlled by those cellular activities that establish and regulate the inwardly directed electrochemical Ca2+ gradient (e.g., Ca2+-ATPase action). Generally, the action of mechanically active proteins is often controlled but not usually driven by Ca2+. To our knowledge, only spasmonems, the contractile organelles of certain ciliates, show similar behavior. Spasmonemes are regulated by the free Ca2+ concentration as controlled by storage and release from intracellular stores (Amos et al., 1976; Mahadevan and Matsudaira, 2000). It remains to be determined whether spasmonemes provide a valid model for the regulation of P-protein crystalloids in sieve tubes.
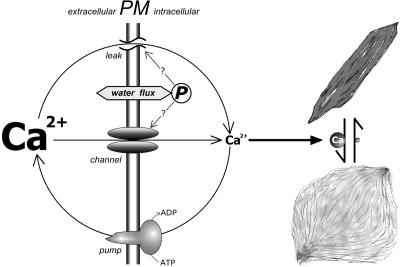
Figure 7.
Hypothetical Regulation of the Conformational State of Crystalline P-Protein Bodies in the Fabaceae.
The conformation of the crystalline P-protein is controlled directly by intracellular Ca2+. High Ca2+ levels lead to dispersal of the protein body; in the absence of Ca2+, the protein transforms into a dense “crystalloid” state. Intracellular Ca2+ is regulated by events in the plasma membrane (PM). Low Ca2+ concentrations in the cells probably are maintained by Ca2+ pumps, which establish a steep electrochemical Ca2+ gradient directed into the cells. Therefore, membrane leakage as well as Ca2+ channel activity result in Ca2+ influx, leading to dispersal of the crystalline P-protein bodies. Water fluxes over the membrane provoked by changes in external osmolarity modify turgor pressure (P). Changes in turgor may cause increases in intracellular Ca2+ by the activation of Ca2+ channels; alternatively, osmotic shock might induce membrane leakage. The resulting dispersal of crystalline P-protein bodies is fully reversible, because normal intracellular Ca2+ levels are restored by the pumps when Ca2+ channel activity ceases or membrane leaks are repaired.
P-protein crystalloids are responsive only to specific divalent cations, as demonstrated by the lack of response to Mg2+ (Figure 6). Interestingly, crystalloids respond to Sr2+ and Ba2+, which, just like Ca2+, are excluded efficiently from the sieve tubes. On the other hand, Mg2+ is taken up and transported (Ziegler, 1975). Thus, crystalline P-proteins seem adapted to detect perturbations of membrane function by responding to increasing intracellular levels of ions, which normally are kept to low concentrations in the sieve tube sap.
Stimuli Triggering Crystalloid Dispersal: Membrane Permeability and Turgor
Increased nonspecific membrane permeability, that is, leakage induced by micropipette insertion or chemical effectors, triggered crystalloid dispersal if Ca2+ was present in the extracellular medium. This is explained readily by the Ca2+ dependence of crystalloid conformation, because extracellular Ca2+ enters leaky SEs following its electrochemical gradient (Figure 7).
Turgor-dependent crystalloid dispersal as induced by sudden steps in external osmolarity also depends on extracellular Ca2+. However, it remains unclear whether Ca2+ enters the cell via nonspecific pathways (membrane leakage induced by osmotic shock) or whether the rapid change in turgor is a signal that leads to the opening of Ca2+ channels in the plasma membrane (Figure 7). The latter explanation would appear to make sense in a wider context. Cytoplasmic Ca2+ has been implicated as a signal in turgor regulation in a range of taxa (Okazaki and Tazawa, 1990). In characean algae, turgor steps in either direction trigger action potentials if they exceed a threshold of 0.2 to 0.5 MPa (Zimmermann and Beckers, 1978). Cytoplasmic Ca2+ rises sharply during these action potentials (Kikuyama and Tazawa, 1983; Thiel et al., 1997). In higher plants, similar potential waves are transmitted along sieve tubes (Eschrich et al., 1988; Fromm, 1991; Rhodes et al., 1996), where they seem to raise levels of cytoplasmic Ca2+ (Fromm and Spanswick, 1993). It seems a reasonable working hypothesis that exogenously induced turgor changes trigger membrane potential waves in SEs that are accompanied by intracellular Ca2+ surges. Increased intracellular Ca2+ then could cause reversible sieve tube occlusion by controlling P-protein crystalloid conformation (Figure 7).
Physiological Significance of Reversible Plug Formation
The appearance of the dispersed fibrillar masses by CLSM (Figure 2) and transmission electron microscopy (Figure 1) leaves little doubt that crystalline P-proteins drastically increase the hydraulic resistance of the sieve tubes when they disperse (cf. Weatherley and Johnson, 1968; Weatherley, 1972; Ehlers et al., 2000). Thus, SEs potentially control the rate of phloem flux by regulating intracellular Ca2+. Ca2+ antagonists such as EDTA have long been known to prevent sieve tube occlusion (King and Zeevaart, 1974; Fellows et al., 1978). The effect is commonly ascribed to the inhibition of Ca2+-dependent stimulation of callose synthesis (Sabnis and Sabnis, 1995). However, the moderate Ca2+-mediated increase of callose formation on SPs detectable after hours of treatment (King and Zeevaart, 1974) does not seem to provide a sufficient explanation for the dramatic stimulation of phloem exudation by chelating agents (Fellows et al., 1978; Urquhart and Joy, 1981; Schulz, 1998). Our results demonstrate that an additional Ca2+-dependent mechanism operates in the Fabaceae. The process of Ca2+-triggered crystalloid dispersal and plug formation is much more rapid than sieve tube occlusion by callose synthesis, and it is reversible within seconds. These features render crystalloid dispersal an ideal first line of defense against loss of sieve tube contents, particularly in cases of localized and transient disturbances, such as attacks of phloem-feeding aphids. Fabaceae are notoriously poorly suited for phloem exudate studies using severed aphid stylets (Fisher and Frame, 1984). The reason may be that they have evolved a unique type of cellular stopcock to control the hydraulic conductivity of their sieve tubes.
Concluding Remarks—A Wider Perspective
We have demonstrated that crystalline P-proteins of the Fabaceae occlude SEs by a rapid and reversible conformational switch in response to wounding, changes in turgor, and disturbances of membrane integrity. Available evidence suggests that the control of crystalline P-protein conformation is tightly linked with the regulation of intracellular Ca2+. Previously, several lines of indirect evidence suggested a possible relation between intracellular Ca2+-regulation and the functioning of phloem-specific proteins other than the fabacean P-protein crystalloids (Kleinig et al., 1971; McEuen et al., 1981; Arsanto, 1986). It now seems reasonable to generalize our findings and hypothesize that intracellular Ca2+ controls interconversions between different structural states of P-proteins and thereby regulates the flow through the phloem.
METHODS
Plant Material
Vicia faba cv Witkiem major (Nunhems Zaden BV, Haarlem, The Netherlands) was grown individually in pots in a greenhouse at 20°C, with 60 to 70% relative humidity, and a 14/10-hr light/dark period (daylight plus additional lamp light [model SONT Agro 400 W; Phillips, Eindhoven, The Netherlands], with a minimum irradiance of 250 mol m−2 sec−1 at plant level). Plants were used for experiments 17 to 21 days after germination. Lupinus polyphyllos, Phaseolus vulgaris, Urtica dioica, and Rubus fruticosus were collected from the Botanical Garden of Giessen University (Giessen, Germany).
Conventional Light Microscopy
Vascular tissue for microscopical in vivo observation was prepared from detached leaves by a shallow pericline cut as described previously (Knoblauch and van Bel, 1998). Specimens were covered with standard medium (10 mM KCl, 10 mM CaCl2, and 5 mM NaCl, buffered at pH 7.5 with 50 mM Hepes; in experiments in which extracellular osmolarity was varied, quarter-strength standard medium was used) or calcium-free medium (10 mM disodium-salt of EGTA or EDTA instead of CaCl2). Leaves were fixed on microscope slides with adhesive tape so that crystalloids in sieve elements (SEs) could be observed with a microscope (Leica DMLB equipped with an HCX APO L40X/0.80 W U-V-I water immersion objective; Leica, Wetzlar, Germany) in the bright-field mode. Attached was a video camera (model TK-C1360; JVC, Tokyo, Japan) connected to a Miro Video Capture Card (Pinnacle Systems, Braunschweig, Germany) to store digital images on a desktop computer. Bathing solutions were changed with laboratory pipettes and a glass capillary that was connected to a suction pump. When necessary, cells and P-protein crystalloids were agitated by micropipettes with different tip diameters mounted on four-axis micromanipulators (model MMW-204; Narishige, Tokyo, Japan). Pipettes were produced from borosilicate glass capillaries (Clark Electromedical Instruments, Reading, UK) by using a microelectrode puller (model P-200; Sutter Instruments, Novato, CA).
The effect of nonspecific membrane leakage was tested by adding either 1% (v/v) Triton X-100 or 1 mM digitonin to the standard medium. For osmolarity tests, standard medium or calcium-free medium was used; osmolarities were adjusted by adding KCl, CaCl2, MgCl2, or sucrose. To test the ion specificity of the dispersal response, standard medium was used in which CaCl2 had been exchanged for the equivalent concentrations of MgCl2, BaCl2, or SrCl2. The effects of monovalent ions were tested in calcium-free medium in which the contents of NaCl and KCl were modified to adjust the concentration of Na+, K+, or Cl−, respectively, to either 0 or 10 mM.
Confocal Laser Scanning Microscopy
Confocal laser scanning microscopy was performed using a Leica (Heidelberg, Germany) TCS 4D microscope as described in detail previously (Knoblauch and van Bel, 1998). To specifically contrast crystalloids, we used 5[6]-carboxy-4′,5′-dimethylfluorescein diacetate (CDMFDA; Molecular Probes, Eugene, OR). A droplet of 0.02‰ (w/v) CDMFDA in a solution containing 10 mM KCl, 10 mM CaCl2, and 5 mM NaCl was applied for 5 min and washed out; crystalloids then were clearly visible for a few minutes. Excitation light (488 nm) was produced with a 75-mW argon/krypton laser (Omnichrome, Chino, CA). Emitted light passed through a fluorescein band pass filter.
Electron Microscopy
To keep the SEs intact, vascular tissue was laid open in whole leaves, as described previously (Ehlers et al., 2000), and a solution of 3% (v/v) paraformaldehyde, 4% (v/v) glutaraldehyde, 50 mM sodium cacodylate buffer, and 2 mM CaCl2, pH 7.2, was applied to the exposed tissue. The solution was replaced every 30 min for 6 hr. Pieces (15 × 5 mm) were excised from the midrib and transferred to fresh solution. After 3 hr at room temperature, tissue samples were washed and incubated overnight in 50 mM sodium cacodylate buffer with 2 mM CaCl2, pH 7.2, and 1% (w/v) OsO4 at 4°C. After contrasting in 0.5% (w/v) aqueous uranyl acetate for 2 hr at 4°C, samples were dehydrated in a graded ethanol series and propylene oxide and were transferred to Spurr's epoxy resin. Ultrathin sections were cut with a diamond knife on a Reichert Om U2 ultramicrotome (Leica, Bensheim, Germany) and collected on Formvar-coated single-slot copper grids. After poststaining with 2% aqueous uranylacetate and Reynolds' lead citrate, sections were examined at 80 kV with an electron microscope (model EM 300; Phillips, Eindhoven, The Netherlands).
Supplementary Material
Acknowledgments
Helpful suggestions and comments given during the course of this study by Hubert Felle and Gerhard Thiel are gratefully acknowledged. We thank Jürgen Bereiter-Hahn, Julian Hibberd, and Gerhard Thiel for critical discussion of earlier versions of the manuscript.
Footnotes
Online version contains Web-only data.
References
- Amos, W.B., Routledge, L.M., Weis-Fogh, T., and Yew, F.F. (1976). The spasmoneme and calcium-dependent contraction in connection with specific calcium binding proteins. In Calcium in Biological Systems (Symposia of the Society for Experimental Biology 30), C.J. Duncan, ed (Cambridge, UK: Cambridge University Press), pp. 273–301.
- Arsanto, J.P. (1986). Ca2+-binding sites and phosphatase activities in sieve element reticulum and P-protein of chick-pea phloem. A cytochemical and X-ray microanalysis survey. Protoplasma 132 160–171. [Google Scholar]
- Askerlund, P., and Sommarin, M. (1996). Calcium efflux transporters in higher plants. In Membranes: Specialized Functions in Plants, M. Smallwood, J.P. Knox, and D.J. Bowles, eds (Oxford, UK: BIOS Scientific Publishers), pp. 281–299.
- Baccarini, P. (1892). Intorno ad uno particolarita dei vasi cribose nelle Papilionaceae. Malpighia 6 53–57. [Google Scholar]
- Behnke, H.D. (1991). Nondispersive protein bodies in sieve elements: A survey and review of their origin, distribution and taxonomic significance. IAWA Bull. 12 143–175. [Google Scholar]
- Behnke, H.D., and Sjolund, R.D. (1990). Sieve Elements. (Berlin: Springer-Verlag).
- Brauer, M., Zhong, W.-J., Jelitto, T., Schobert, C., Sanders, D., and Komor, E. (1998). Free calcium ion concentration in the sieve-tube sap of Ricinus communis L. seedlings. Planta 206 103–107. [Google Scholar]
- Bush, D.S. (1995). Calcium regulation in higher plant cells and its role in signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46 95–122. [Google Scholar]
- Cronshaw, J., and Sabnis, D.D. (1990). Phloem proteins. In Sieve Elements, H.D. Behnke and R.D. Sjolund, eds (Berlin: Springer-Verlag), pp. 257–283.
- Ehlers, K., Knoblauch, M., and van Bel, A.J.E. (2000). Ultrastructural features of well-preserved and injured sieve elements: Minute clamps keep the phloem transport conduits free for mass flow. Protoplasma 214 80–92. [Google Scholar]
- Esau, K., and Thorsch, J. (1984). The sieve plate of Echium (Boraginaceae): Developmental aspects and response of P-protein to protein digestion. J. Ultrastruct. Res. 86 31–45. [Google Scholar]
- Eschrich, W., Fromm, J., and Evert, R.F. (1988). Transmission of electric signals in sieve tubes of zucchini plants. Bot. Acta 101 327–331. [Google Scholar]
- Evert, R.F. (1982). Sieve-tube structure in relation to function. BioScience 32 789–795. [Google Scholar]
- Fellows, R.J., Egli, D.E., and Leggett, J.E. (1978). A pod leakage technique for phloem translocation studies in soybean (Glycine max [L.] Merr.). Plant Physiol. 62 812–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, D.B. (1975). Structure of functional soybean sieve elements. Plant Physiol. 56 555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, D.B., and Frame, J.M. (1984). A guide to the use of the exuding-stylet technique in phloem physiology. Planta 161 385–393. [DOI] [PubMed] [Google Scholar]
- Fromm, J. (1991). Control of phloem unloading by action potentials in Mimosa. Physiol. Plantarum 83 529–533. [Google Scholar]
- Fromm, J., and Spanswick, R. (1993). Characteristics of action potentials in willow (Salix viminalis L.). J. Exp. Bot. 44 1119–1125. [Google Scholar]
- Gilroy, S., Bethke, P.C., and Jones, R.L. (1993). Calcium homeostasis in plants. J. Cell. Sci. 106 453–462. [DOI] [PubMed] [Google Scholar]
- Johnson, R.P.C., Freundlich, A., and Barclay, G.F. (1976). Transcellular strands in sieve tubes: What are they? J. Exp. Bot. 101 1117–1136. [Google Scholar]
- Kikuyama, M., and Tazawa, M. (1983). Transient increase of intracellular Ca2+ during excitation of tonoplast-free Chara cells. Protoplasma 117 62–67. [Google Scholar]
- King, R.W., and Zeevaart, J.A.D. (1974). Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiol. 53 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinig, H., Dörr, I., Weber, C., and Kollmann, R. (1971). Filamentous proteins from plant sieve tubes. Nat. New Biol. 229 152–153. [DOI] [PubMed] [Google Scholar]
- Knoblauch, M., and van Bel, A.J.E. (1998). Sieve tubes in action. Plant Cell 10 35–50. [Google Scholar]
- Knoblauch, M., Hibberd, J.M., Gray, J.C., and van Bel, A.J.E. (1999). A galinstan expansion femtosyringe for microinjection of eukaryotic organells and prokaryotes. Nat. Biotechnol. 17 906–909. [DOI] [PubMed] [Google Scholar]
- Lawton, D.M. (1978. a). Ultrastructural comparison of tailed and tailless P-protein crystals respectively of runner bean (Phaseolus multiflorus) and garden pea (Pisum sativum) with tilting stage electron microscopy. Protoplasma 97 1–11. [Google Scholar]
- Lawton, D.M. (1978. b). P-protein crystals do not disperse in uninjured sieve elements in roots of runner bean (Phaseolus multiflorus) fixed with glutaraldehyde. Ann. Bot. 42 353–361. [Google Scholar]
- Mahadevan, L., and Matsudaira, P. (2000). Motility powered by supramolecular springs and ratchets. Science 288 95–99. [DOI] [PubMed] [Google Scholar]
- McEuen, A.R., Hart, J.W., and Sabnis, D.D. (1981). Calcium-binding protein in sieve tube exudate. Planta 151 531–534. [DOI] [PubMed] [Google Scholar]
- Münch, E. (1930). Die Stoffbewegung in der Pflanze. (Jena: Fischer Verlag).
- Okazaki, Y., and Tazawa, M. (1990). Calcium ion and turgor regulation in plant cells. J. Membr. Biol. 114 189–194. [DOI] [PubMed] [Google Scholar]
- Palevitz, B.A., and Newcomb, E.H. (1971). The ultrastructure and development of tubular and crystalline P-protein in the sieve elements of certain papilionaceous legumes. Protoplasma 72 399–425. [Google Scholar]
- Poovaiah, B.W., and Reddy, A.S.N. (1993). Calcium and signal transduction in plants. Crit. Rev. Plant Sci. 12 185–211. [DOI] [PubMed] [Google Scholar]
- Rhodes, J.D., Thain, J.F., and Wildon, D.C. (1996). The pathway for systemic electrical signal conduction in the wounded tomato plant. Planta 200 50–57. [Google Scholar]
- Robidoux, J., Sandborn, E.B., Fensom, D.S., and Cameron, M.L. (1973). Plasmatic filaments and particles in mature sieve elements of Heracleum sphondylium under the electron microscope. J. Exp. Bot. 79 349–359. [Google Scholar]
- Sabnis, D.D., and Sabnis, H.M. (1995). Phloem proteins: Structure, biochemistry and function. In The Cambial Derivatives, Encyclopedia of Plant Anatomy, Vol. 9, M. Iqbal, ed (Berlin: Borntraeger), pp. 271–292.
- Schulz, A. (1998). Phloem: Structure related to function. Progr. Bot. 59 429–475. [Google Scholar]
- Thiel, G., Homann, U., and Plieth, C. (1997). Ion channel activity during the action potential in Chara: New insights with new techniques. J. Exp. Bot. 48 609–622. [DOI] [PubMed] [Google Scholar]
- Urquhart, A.A., and Joy, K.W. (1981). Use of phloem exudate technique in the study of amino acid transport in plants. Plant Physiol. 68 750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bel, A.J.E., and Knoblauch, M. (2000). Sieve element and companion cell: The story of the comatose patient and the hyperactive nurse. Austr. J. Plant Physiol. 27 477–487. [Google Scholar]
- Weatherley, P.E. (1972). Translocation in sieve tubes. Some thoughts on structure and mechanism. Physiol. Vég. 10 731–742. [Google Scholar]
- Weatherley, P.E., and Johnson, R.P.C. (1968). The form and function of the sieve tube: A problem in reconciliation. Int. Rev. Cytol. 24 149–192. [DOI] [PubMed] [Google Scholar]
- Wergin, W.P., and Newcomb, E.H. (1970). Formation and dispersal of crystalline P-protein in sieve elements of soybean (Glycine max L.). Protoplasma 71 365–388. [Google Scholar]
- Ziegler, H. (1975). Nature of transported substances. In Phloem Transport, Encyclopedia of Plant Physiology, Vol. 1, M.H. Zimmermann and J.A. Milburn, eds (Berlin: Springer-Verlag), pp. 59–100.
- Zimmermann, U., and Beckers, F. (1978). Generation of action potentials in Chara corallina by turgor pressure changes. Planta 138 173–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.