Abstract
Harpin from the bean halo-blight pathogen Pseudomonas syringae pv phaseolicola (harpinPsph) elicits the hypersensitive response and the accumulation of pathogenesis-related gene transcripts in the nonhost plant tobacco. Here, we report the characterization of a nonproteinaceous binding site for harpinPsph in tobacco plasma membranes, which is assumed to mediate the activation of plant defense responses in a receptor-like manner. Binding of 125I-harpinPsph to tobacco microsomal membranes (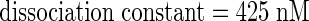 ) and protoplasts (
) and protoplasts (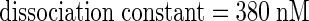 ) was specific, reversible, and saturable. A close correlation was found between the abilities of harpinPsph fragments to elicit the transcript accumulation of the pathogenesis-related tobacco gene HIN1 and to compete for binding of 125I-harpinPsph to its binding site. Another elicitor of the hypersensitive response and HIN1 induction in tobacco, the Phytophthora megasperma–derived β-elicitin β-megaspermin, failed to bind to the putative harpinPsph receptor. In contrast to activation by β-megaspermin, harpinPsph-induced activation of the 48-kD salicylic acid–responsive mitogen-activated protein kinase (MAPK) and HIN1 transcript accumulation were independent of extracellular calcium. Moreover, use of the MAPK kinase inhibitor U0126 revealed that MAPK activity was essential for pathogenesis-related gene expression in harpinPsph-treated tobacco cells. Thus, a receptor-mediated MAPK-dependent signaling pathway may mediate the activation of plant defense responses induced by harpinPsph.
) was specific, reversible, and saturable. A close correlation was found between the abilities of harpinPsph fragments to elicit the transcript accumulation of the pathogenesis-related tobacco gene HIN1 and to compete for binding of 125I-harpinPsph to its binding site. Another elicitor of the hypersensitive response and HIN1 induction in tobacco, the Phytophthora megasperma–derived β-elicitin β-megaspermin, failed to bind to the putative harpinPsph receptor. In contrast to activation by β-megaspermin, harpinPsph-induced activation of the 48-kD salicylic acid–responsive mitogen-activated protein kinase (MAPK) and HIN1 transcript accumulation were independent of extracellular calcium. Moreover, use of the MAPK kinase inhibitor U0126 revealed that MAPK activity was essential for pathogenesis-related gene expression in harpinPsph-treated tobacco cells. Thus, a receptor-mediated MAPK-dependent signaling pathway may mediate the activation of plant defense responses induced by harpinPsph.
INTRODUCTION
Phytopathogenic bacteria harbor a gene cluster (HRP, for hypersensitive reaction and pathogenicity) that controls pathogenicity in susceptible plants and the ability to elicit the hypersensitive reaction (HR) in nonhost plants or resistant cultivars of host plants (Lindgren et al., 1986; Galan and Collmer, 1999). Some HRP genes encode elements of a bacterial type III secretion system, by which effector proteins are exported and delivered into the cytosol of host plant cells (Galan and Collmer, 1999; Kjemtrup et al., 2000). Some of these effector proteins were found to interact with plant intracellular proteins and to activate the plant defense system.
Harpins constitute another group of effector proteins exported by the type III pathway of plant pathogenic Erwinia, Pseudomonas, and Ralstonia spp (Galan and Collmer, 1999). Although identified several years ago, the roles of these proteins during colonization of host plants and their site of action have remained unclear. However, when infiltrated into nonhost plants, harpins trigger disease resistance–associated responses, such as HR, transcript accumulation of pathogenesis-related (PR) genes, and systemic acquired resistance (Baker et al., 1993; He et al., 1993; Gopalan et al., 1996; Strobel et al., 1996; Dong et al., 1999; Galan and Collmer, 1999). Physiological target sites for harpin action, therefore, were suggested to reside at the plant cell surface. Immunolocalization studies revealed a Ca2+-dependent association of Pseudomonas syringae pv syringae harpin with tobacco cell walls (Hoyos et al., 1996), but harpin-induced K+/H+ exchange at the plant plasma membrane and subsequent plasma membrane depolarization (Hoyos et al., 1996; Pike et al., 1998) raised questions regarding the concept of a cell wall binding site mediating such responses. Alternatively, bacterial elicitors may be recognized by the plant just like elicitors derived from phytopathogenic fungi and oomycetes, which bind to plasma membrane proteins (Nürnberger et al., 1995; Mithöfer et al., 1996; Umemoto et al., 1997; Bourque et al., 1999). An example of this phenomenon is provided by the recent identification of a 115-kD tomato microsomal membrane protein that binds the bacterial flagellin-derived elicitor flg22 (Meindl et al., 2000). On the other hand, harpins may interact with membranes directly and trigger plant defense responses in an ionophore-like manner, because harpins from various phytopathogenic P. s. syringae pathovars were found recently to associate stably with synthetic bilayer membranes and to evoke cation currents of large unitary conductance (Lee et al., 2001).
Elicitor binding to cell surface binding sites initiates an intracellular signaling cascade that results in the activation of plant-specific defense responses (Yang et al., 1997; Scheel, 1998; Grant and Mansfield, 1999; Nürnberger, 1999). Changes in cytoplasmic free calcium concentration ([Ca2+]cyt) are implicated in elicitor-induced signal transduction chains in various plants (Yang et al., 1997; Scheel, 1998; Grant and Mansfield, 1999). Previous work had demonstrated the importance of extracellular Ca2+ for the activation of pathogen defense responses (Yang et al., 1997; Scheel, 1998; Grant and Mansfield, 1999). More recently, receptor-mediated influx of extracellular Ca2+ in elicitor-treated plant cells was shown to cause characteristic [Ca2+]cyt signatures as a prerequisite for the activation of pathogen defense (Mithöfer et al., 1999; Blume et al., 2000). In addition, mitogen-activated protein kinase (MAPK) cascades constitute another common element of intracellular signal transduction chains in eukaryotic cells (Herskowitz, 1995; Hirt, 2000). In plants, MAPK activation has been implicated in the adaptation to various environmental stimuli, including pathogen infection or treatment with pathogen-derived elicitors (Hirt, 2000). Tobacco mosaic virus infection of tobacco plants (Zhang and Klessig, 1998a) or treatment of tobacco cells with elicitins (Lebrun-Garcia et al., 1998; Zhang et al., 1998) and Trichoderma viride–derived xylanase (Suzuki et al., 1999) stimulated transient activation of a 48-kD salicylic acid–inducible MAPK (SIPK) (Zhang and Klessig, 1997, 2000). When the Cladosporium fulvum–derived race-specific elicitor AVR9 was infiltrated into tobacco plants expressing the tomato resistance gene Cf-9, both SIPK and another tobacco mosaic virus and elicitin-responsive tobacco MAPK, WIPK (for wounding-induced protein kinase) (Zhang and Klessig, 1998b), were activated (Romeis et al., 1999). Moreover, elicitor-responsive parsley SIPK and WIPK and Arabidopsis SIPK orthologous enzymes have been reported (Ligterink et al., 1997; Nühse et al., 2000; Scheel et al., 2000). In addition to specific [Ca2+]cyt signatures, differentially induced MAPK isoenzymes with characteristic activity profiles are assumed to encode signal specificity during the activation of pathogen defense in plants (Hirt, 2000).
P. s. syringae–derived harpins and elicitins from various Phytophthora species trigger the HR, PR gene expression, and systemic acquired resistance in the nonhost plant tobacco (Ricci et al., 1989; He et al., 1993; Baillieul et al., 1995; Gopalan et al., 1996). Using harpin from P. s. phaseolicola (harpinPsph) and the Phytophthora megasperma β-elicitin β-megaspermin, we have attempted a comparative analysis of signal perception and transduction mechanisms induced by bacterial and oomycete elicitors. We provide evidence that tobacco plasma membranes harbor a specific binding site for harpinPsph that does not bind β-megaspermin. This binding site is likely to mediate harpinPsph-induced expression of the PR gene HIN1 (Gopalan et al., 1996). In contrast to the β-elicitin–induced activation of HIN1, harpinPsph-induced HIN1 expression was independent of extracellular Ca2+. We further show that MAPK activity, which includes SIPK activity, is required for harpinPsph-induced HIN1 expression.
RESULTS
Tobacco Cell Responses to Treatment with HarpinPsph
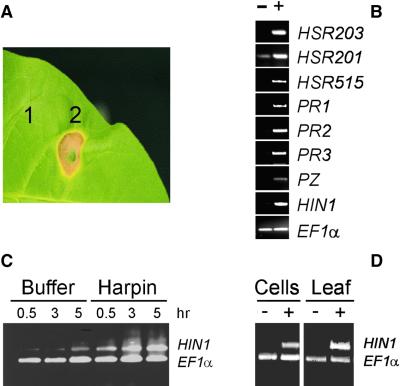
When infiltrated into tobacco leaves, purified recombinant harpinPsph elicited an HR (Figure 1A). At concentrations of 1 μM, symptoms became visible 8 hr after infiltration. In addition, treatment with harpinPsph of cultured tobacco cells resulted in transcript accumulation of the PR genes PR1, PR2, acidic chitinase (PR3), and chitinase/lysozyme (Heitz et al., 1994) (Figure 1B). Transcripts derived from genes considered HR marker genes (HSR203, HSR201, HSR515, and HIN1) (Gopalan et al., 1996; Pontier et al., 1999) also accumulated in harpinPsph-treated tobacco cells (Figure 1B).
Figure 1.
HarpinPsph-Induced Hypersensitive Cell Death and PR Gene Expression in Tobacco.
HarpinPsph (1 μM) was infiltrated into tobacco leaves or added to suspension cultured tobacco cells.
(A) Tobacco leaf treated with 5 mM Mes buffer, pH 5.5 (1), or harpinPsph (2) 2 days after infiltration.
(B) Total RNA prepared from tobacco cells treated for 3 hr with buffer (−) or harpinPsph (+) was used as a template in RT-PCR assays with DNA primers derived from the tobacco genes indicated (see text). The tobacco gene encoding EF1α served as an internal control. PZ, chitinase/lysozyme.
(C) Kinetics of HIN1 expression in tobacco cells treated with buffer or harpinPsph. Total RNA from tobacco cells was prepared at the times after infiltration indicated and used in RT-PCR to simultaneously amplify HIN1 and EF1α transcripts.
(D) HIN1 expression in tobacco cells or leaves treated for 3 hr with buffer (−) or harpinPsph (+). RT-PCR analysis was performed as described in (C).
HarpinPsph-induced HIN1 transcript accumulation was observed as early as 30 min after elicitation and persisted for a minimum of 5 hr (Figure 1C). Therefore, we chose HIN1 as an exemplary gene to analyze elicitor-induced PR gene expression by single tube multiplex reverse transcription–polymerase chain reaction (RT-PCR). A constitutively expressed gene encoding the translation elongation factor EF1α served as an internal standard in these assays. HIN1 transcript accumulation in tobacco cell cultures did not differ quantitatively from that observed in harpinPsph-infiltrated tobacco leaves (Figure 1D), thus validating the use of cell suspensions for studies of harpinPsph perception and signal transduction.
The concentration of harpinPsph required to trigger half-maximum expression (EC50) of HIN1 and HSR203 in tobacco cells was 120 and 82 nM, respectively (Table 1). This is in good agreement with concentrations of the elicitor required to stimulate a rapidly induced K+/H+ exchange and Cl− efflux (Table 1). Similar ion fluxes have been associated with pathogen resistance responses in many plant systems (Yang et al., 1997; Scheel, 1998; Grant and Mansfield, 1999) and are assumed to be involved in signaling PR gene expression and HR. Such a correlation of EC50 values strongly suggests that harpinPsph-induced cellular responses are activated upon recognition of the elicitor at a signal-specific binding site.
Table 1.
EC50 Valuesa of HarpinPsph-Induced Responses in Tobacco Cells
| Response | EC50 Value (nM) |
|---|---|
| Medium alkalinization | 100 |
| K+ efflux | 50 |
| Cl− efflux | 120 |
| Expression of HIN1b | 120 |
| Expression of HSR203b | 82 |
Concentrations of harpinPsph required to stimulate 50% of the particular plant response as derived from dose–response curves.
Transcript accumulation was quantified by phosphorimaging RNA gel blots hybridized with α-32P-dATP–labeled HIN1 and HSR203 cDNA, respectively. Hybridization of filters with α-32P-dATP–labeled rDNA was performed to normalize RNA loading.
Specific Binding of HarpinPsph to Tobacco Plasma Membranes
To characterize harpinPsph binding sites on tobacco membranes, the protein was radioiodinated (125I) at the meta position of the phenoxyl ring of a tyrosine residue. Because native harpinPsph lacked tyrosine, PCR was used to attach this amino acid to the C terminus. Expression products were nonradioactively iodinated and separated by reverse phase HPLC. Matrix-assisted laser-desorption ionization time of flight mass spectrometry analysis of the reaction products confirmed complete iodination of harpinPsph and revealed that harpinPsph was not post-translationally modified during heterologous expression. Because iodination did not affect HR- or HIN1-inducing activities of harpinPsph in tobacco leaves (data not shown), 125I-harpinPsph (specific radioactivity, 2200 Ci/mmol) was prepared and used as ligand in binding assays.
Binding of 125I-harpinPsph to tobacco microsomal membranes was investigated by filtration to separate free from bound label, which ensured that any loss of ligand caused by rapid dissociation of the receptor–ligand complex was negligible. Specific binding of 125I-harpinPsph to tobacco microsomes was not affected significantly by ionic strength (up to 1 M NaCl) and was greatest at pH 7.0 (84% at pH 6.0, 51% at pH 8.0, and 33% at pH 10.0). Therefore, binding assays were performed in neutral buffer containing 100 mM NaCl. The stability of the radioligand under binding assay conditions was confirmed by SDS-PAGE/autoradiography of aliquots taken from the binding mixture after various times of incubation (data not shown). In all experiments, specific binding constituted no more than 5% of the initially added ligand, ensuring that ligand depletion did not obscure binding assays.
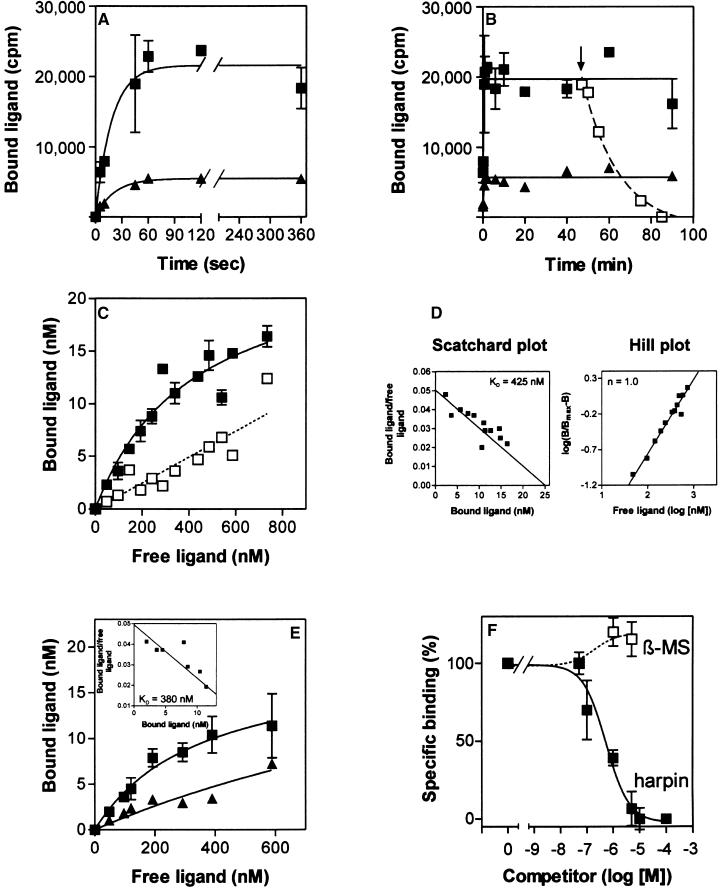
Kinetic analysis of 125I-harpinPsph binding demonstrated that ligand association with tobacco microsomal membranes was initially faster than dissociation (Figure 2A). Half-maximal binding was achieved within 15 sec after addition of the ligand, and equilibrium between association and dissociation was reached after 60 sec. Addition of a 100-fold molar excess of unlabeled harpinPsph 45 min after the addition of the radioligand to tobacco membranes resulted in the rapid dissociation of bound label (Figure 2B). Thus, binding of 125I-harpinPsph was reversible.
Figure 2.
Binding of 125I-HarpinPsph to Tobacco Membranes.
Data points represent the average of triplicate experiments. Data points representing nonspecific binding are the average of duplicate experiments. (A) Time course of binding of 50 nM 125I-harpinPsph (1:10 dilution with unlabeled harpinPsph) to tobacco microsomal membranes added at time 0. Membrane-bound radioligand was determined at the times indicated. Squares show the amount of radioligand that was bound specifically by the 125I-harpinPsph binding site. Nonspecific binding is indicated by triangles. Specific binding was obtained by subtracting nonspecific binding from total binding. Nonspecific binding was determined in the presence of 5 μM unlabeled harpinPsph.
(B) Time course of displacement of 125I-harpinPsph. 125I-harpinPsph (50 nM; 1:10 dilution with unlabeled harpinPsph) was incubated with tobacco microsomal membranes for the times indicated. To initiate the displacement of 125I-harpinPsph, a 100-fold molar excess of unlabeled harpinPsph (open squares) was added 45 min (arrow) after the radioligand. Specific binding (closed squares) and nonspecific binding (triangles) are indicated.
(C) Saturability of 125I-harpinPsph binding to tobacco microsomal membranes. Specific binding (closed squares) and nonspecific binding (open squares) were determined in the presence of a 100-fold molar excess of unlabeled harpinPsph.
(D) Scatchard plot and Hill plot of the binding data shown in (C). The binding constant (KD) and the Hill coefficient (n) were determined according to Hulme and Birdsall (1990).
(E) Saturability of 125I-harpinPsph binding to tobacco protoplasts. Graphs for specific binding (squares) and nonspecific binding (triangles) are shown. The inset shows a Scatchard plot of the binding data. The dissociation constant (KD) was determined according to Hulme and Birdsall (1990).
(F) Competitive inhibition of 125I-harpinPsph (100 nM) binding to tobacco microsomal membranes by increasing concentrations of harpinPsph or β-megaspermin (β-MS). One hundred percent specific binding corresponded to the binding detected in the absence of competitor (1,600,000 cpm), whereas 0% specific binding corresponded to the binding detected in the presence of a 100-fold molar excess of competitor (10 μM) (nonspecific binding, 374,000 cpm).
Error bars indicate standard error.
Saturation analyses with increasing concentrations of 125I-harpinPsph (50 to 700 nM) were performed. Specific binding increased exponentially at radioligand concentrations up to 250 nM. At higher concentrations, specific binding began to plateau (Figure 2C), suggesting that saturation of microsomal binding sites was approached. Nonspecific binding showed a linear increase with increasing ligand concentrations (Figure 2C). Unfortunately, experiments with higher radioligand/competitor concentrations were impeded by the tendency of harpinPsph to precipitate. Thus, the competitor concentrations required to determine the degree of nonspecific binding at higher 125I-harpinPsph levels could not be used. Linearization of the data in a Scatchard plot (Figure 2D) indicated the existence of a 125I-harpinPsph binding site in tobacco microsomal membranes with a dissociation constant (KD) of 425 nM and an apparent binding site concentration of 6.7 pmol/mg of protein. Hill plot analysis of the binding data yielded a Hill coefficient of 1 (Figure 2D), indicating that there is no cooperativity in the binding of 125I-harpinPsph to tobacco membranes.
Binding of 125I-harpinPsph to tobacco protoplasts revealed a saturable binding site with an apparent KD of 380 nM and a binding site concentration of 1.5 pmol/106 protoplasts (Figure 2E). Consistent with the experiments performed with microsomal membranes, 125I-harpinPsph binding to protoplasts did not show any cooperativity (data not shown). Because binding experiments were performed under conditions believed to prevent radioligand endocytosis (15 min at 0°C; Hulme and Birdsall, 1990), our results indicated that the harpinPsph binding site is localized predominantly in the plasma membrane.
Competition experiments with increasing concentrations of unlabeled harpinPsph yielded a concentration resulting in 50% inhibition of specific binding of 550 nM (Figure 2F). This is consistent with the KD value determined in ligand saturation analyses (Figures 2C and 2D). When added at a 50-fold molar excess over 125I-harpinPsph, unlabeled harpinPsph reduced specific binding of the radioligand by 92%. In contrast, β-megaspermin, from the phytopathogenic oomycete Phytophthora megasperma (Baillieul et al., 1995), did not compete for binding of 125I-harpinPsph when applied at the same concentration (Figure 2F). Therefore, the harpinPsph binding site and the recently characterized elicitin receptor (Bourque et al., 1998, 1999) are unlikely to be identical.
Treatment of tobacco microsomal membranes with proteinase E before binding assays did not abolish the binding of 125I-harpinPsph (data not shown). In addition, use of the radioligand in chemical cross-linking assays with the homobifunctional reagent, 3,3′-dithiobis[sulfosuccinimidyl propionate] (DTSSP), failed to identify one or more microsomal membrane proteins as possible constituents of the 125I-harpinPsph binding site (data not shown). Thus, the 125I-harpinPsph binding site may not be a protein.
HarpinPsph Fragments Are Sufficient for HIN1 Activation and Competition of 125I-HarpinPsph Binding
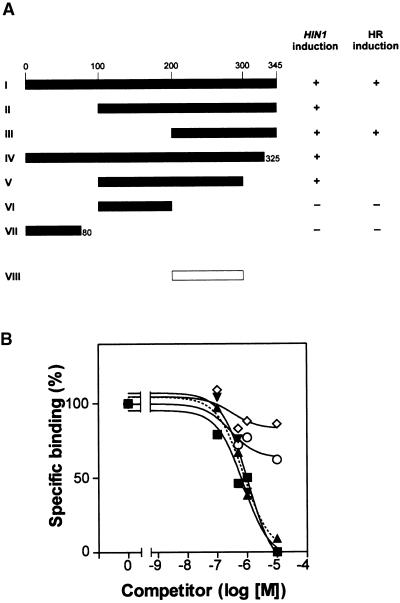
To identify the minimum portion of harpinPsph required to elicit HIN1 expression, cDNAs encoding harpinPsph or portions of the polypeptide were expressed as His10-tag fusion proteins in Escherichia coli (Figure 3A). The expression products were purified to apparent homogeneity on nickel–nitrilotriacetic acid agarose and subsequently assessed for their ability to induce HIN1 transcript accumulation in tobacco cells. Deletion of 100 or 200 N-terminal amino acid residues of a total of 345 amino acids of harpinPsph did not adversely affect HIN1-inducing activity (Figure 3A, fragments II and III). Fragment V, corresponding to amino acids 100 to 300, also was elicitor active. Further C-terminal deletion of this fragment resulted in the complete loss of elicitor activity (Figure 3A, fragment VI). In addition, an N-terminal 80–amino acid peptide was found to be devoid of HIN1-inducing activity (Figure 3A, fragment VII). Fragment III represented the smallest elicitor-active polypeptide that could be expressed in E. coli.
Figure 3.
Structure/Activity Relationship of HarpinPsph and HarpinPsph Deletion Derivatives Expressed in E. coli.
(A) cDNAs encoding harpinPsph (fragment I) and deletion derivatives (fragments II to VII) fused to a His10-encoding tag were expressed in E. coli. Expression products were purified to apparent homogeneity on nickel–nitrilotriacetic acid agarose and tested for their ability to induce HIN1 expression or the HR in tobacco cells at a concentration of 1 μM. Total RNA was prepared from tobacco cells treated with harpinPsph for 3 hr, and HIN1 expression was monitored by RT-PCR as described in the legend to Figure 1C. The white bar (fragment VIII) denotes the overlapping region of two active derivatives (fragments III and V) and defines the smallest fragment deduced to harbor elicitor activity. +, full activity relative to that of the full-length expression product; −, no detectable activity.
(B) Competitive inhibition of 125I-harpinPsph (100 nM) binding to tobacco microsomal membranes by recombinant deletion derivatives shown in (A) and harpinPss from P. s. syringae. Increasing concentrations of fragment I (closed squares), fragment III (closed triangles), harpinPss (closed inverted triangles), fragment VI (open circles), or fragment VII (open diamonds) were used as competitors of binding of 100 nM 125I-harpinPsph. The graphs show the amount of specific binding as described in the legend to Figure 2A. One hundred percent specific binding corresponded to the binding detected in the absence of competitor (1,850,000 cpm), whereas 0% specific binding corresponded to the binding detected in the presence of a 100-fold molar excess of competitor (10 μM) (nonspecific binding, 525,000 cpm). Each data point represents the average of duplicate experiments.
Together, these data suggest that the elicitor activity of harpinPsph resided in a C-terminal fragment corresponding to amino acids 200 to 300 (Figure 3A, fragment VIII). Because attempts to produce this minimal fragment in E. coli proved unsuccessful, we chemically synthesized overlapping peptides covering amino acids 174 to 345 (amino acids 174 to 218, 200 to 224, 219 to 263, 225 to 257, 236 to 280, 264 to 300, and 301 to 345). However, neither alone nor in combination did these peptides exhibit HIN1-inducing activity (data not shown). In radioligand competition assays, the elicitor-active fragment III proved to be as active as the canonical harpinPsph in blocking the binding of 125I-harpinPsph, whereas the two elicitor-inactive fragments (VI and VII) failed to do so (Figure 3B). Consistently, neither alone nor in combination did the synthetic peptides encompassing amino acids 174 to 345 compete for binding of 125I-harpinPsph as effectively as canonical harpinPsph. Hence, a qualitative and quantitative correlation was found between the abilities of harpinPsph fragments to bind to the receptor and to elicit HIN1 expression. Residual binding activity of 35 to 50% of the radioligand was detected when one of three synthetic peptides (amino acids 219 to 263, 236 to 280, or 301 to 345) was used as a competitor at a 100-fold molar excess over 125I-harpinPsph (data not shown).
Recombinant harpins from P. s. syringae (harpinPss; Figure 3B) and P. s. tomato (harpinPst; data not shown), which are structurally related to harpinPsph (77 and 53% identical at the amino acid level; GenBank accession number AF268940), exhibited competitor activity similar to that of harpinPsph, indicating that they targeted the same binding site in tobacco. HarpinPss and harpinPst also were found to induce HIN1 expression in tobacco (Gopalan et al., 1996; our unpublished data). Fragment VIII of harpinPsph (Figure 3A) exhibited 60% identity to both harpinPss and harpinPst, which is not very different from the overall identity observed between the three proteins. Thus, a secondary structure motif, rather than a highly conserved peptide fragment, is likely to represent the recognition determinant for the stimulation of HIN1 expression.
HarpinPsph Activates a Salicylic Acid–Inducible MAPK in Tobacco Cells Independent of Extracellular Calcium
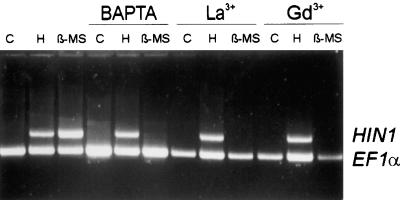
Recent studies have provided evidence that rapidly induced influxes of extracellular Ca2+ and subsequent changes in the [Ca2+]cyt contribute to the activation of defense-associated responses in various plants (Xu and Heath, 1998; Mithöfer et al., 1999; Blume et al., 2000). However, decreasing the extracellular free [Ca2+] to 20 nM using the membrane-impermeable chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N,N-tetraacetic acid (BAPTA) did not affect harpinPsph-induced HIN1 transcript accumulation (Figure 4). The Ca2+ influx inhibitors La3+ and Gd3+ also failed to block harpinPsph-induced HIN1 activation (Figure 4). In contrast, extracellular Ca2+ and Ca2+ influx proved indispensable for β-megaspermin–induced HIN1 activation, because BAPTA and lanthanides inhibited this response in elicited tobacco cells (Figure 4).
Figure 4.
HarpinPsph-Induced HIN1 Expression Is Independent of Extracellular Calcium.
Effects of Ca2+ chelator and Ca2+ channel inhibitors on elicitor-induced HIN1 expression in tobacco cells. Tobacco cells were treated for 1.5 hr with buffer (C), 1 μM harpinPsph (H), or 50 nM β-megaspermin (β-MS) in the absence or presence of BAPTA (8 mM), LaCl3 (0.25 mM; La3+), or GdCl3 (0.25 mM; Gd3+). MgSO4 (20 mM) was included in the buffer together with BAPTA to prevent membrane destabilization due to Ca2+ depletion. Total RNA prepared from elicited tobacco cells was analyzed by RT-PCR as described in the legend to Figure 1C.
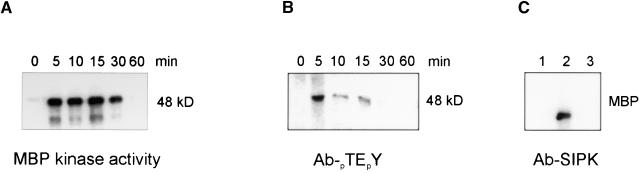
A myelin basic protein (MBP)-phosphorylating protein kinase of 48 kD was activated within 5 min after treatment of tobacco cells with harpinPsph (Figure 5A). Protein kinase activation was transient and decreased to nearly background levels within 60 min after elicitation. In immunoblot analyses performed with a MAPK-specific antibody that recognized the dually phosphorylated threonine-glutamic acid-tyrosine tripeptide motif pTEpY, a 48-kD protein was detected in protein extracts from harpinPsph-treated cells (Figure 5B). Thus, harpinPsph activated a tobacco MAPK that was likely SIPK (Zhang and Klessig, 1997). To verify this, we used a monospecific antiserum raised against a unique N-terminal peptide of SIPK (Zhang et al., 1998) for immunoprecipitation and subsequent in vitro protein kinase assay with MBP as a substrate. As shown in Figure 5C, the antiserum precipitated a MBP kinase activity from extracts of harpinPsph-induced cells (lane 2) that was not precipitated from buffer-treated cells (lane 1). Most importantly, immunoprecipitation of this kinase could be inhibited with an excess of the SIPK-specific peptide used for antibody production (lane 3). Thus, harpinPsph stimulated SIPK activity in tobacco cells.
Figure 5.
Activation of the SIPK in Tobacco Cells Treated with HarpinPsph.
Tobacco cells treated with 1 μM harpinPsph were harvested at the times after infiltration indicated and used to prepare protein extracts.
(A) Kinase activity determined by an in-gel kinase assay using MBP as the substrate.
(B) Protein extracts analyzed by immunoblotting using an antiserum (Ab) recognizing the pTEpY motif of activated MAPK.
(C) Protein extracts were prepared from tobacco cells treated with buffer (lane 1) or harpinPsph (lanes 2 and 3) for 5 min. For immunoprecipitation, the tobacco SIPK-specific antibody (Ab-p48N) (Zhang et al., 1998) was added alone (lanes 1 and 2) or together with the competitor peptide used for antibody production (lane 3). Kinase activity of immunoprecipitated material was assayed using MBP as the substrate. The phosphorylated MBP was visualized by autoradiography.
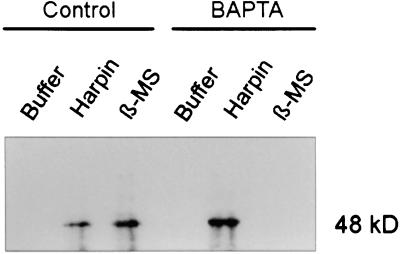
The activation of HIN1 expression by harpinPsph, but not by β-megaspermin, was independent of extracellular Ca2+ (Figure 4). Because the activation of SIPK by elicitins was reported recently for the Phytophthora parasitica–derived α-elicitin parasiticein and the Phytophthora cryptogea β-elicitin cryptogein (Zhang et al., 1998), we wondered if extracellular Ca2+ was required for SIPK activation in response to harpinPsph or β-megaspermin. Protein extracts prepared from tobacco cells treated with elicitor either in the absence or presence of BAPTA were fractionated by SDS-PAGE, blotted, and analyzed with the anti-pTEpY antiserum. HarpinPsph-mediated activation of the 48-kD MAPK (SIPK) was not affected by BAPTA treatment (Figure 6), indicating that MAPK activation was independent of extracellular Ca2+. In contrast, β-megaspermin–induced activation of this enzyme was sensitive to BAPTA treatment and thus dependent on extracellular Ca2+.
Figure 6.
HarpinPsph-Induced SIPK Activation Is Independent of Extracellular Calcium.
Protein extracts prepared from tobacco cells treated for 5 min with buffer, 1 μM harpinPsph, or 50 nM β-megaspermin (β-MS) in the absence (Control) or presence of the Ca2+ chelator BAPTA were analyzed for MAPK phosphorylation by immunoblotting using the anti-pTEpY-antiserum.
HarpinPsph-Induced MAPK Activation Is Required for PR Gene Expression in Tobacco
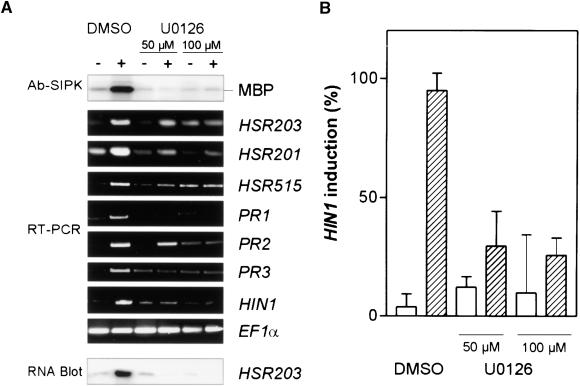
MAPK cascades are assumed to constitute an element of elicitor-induced signal transduction cascades in various plant systems (Hirt, 2000). However, the direct involvement of activated MAPK in triggering plant defense responses has yet to be demonstrated. To causally link harpinPsph-induced MAPK activity and HIN1 expression in tobacco, we used the inhibitor of MAPK kinases (MAPKK) U0126 (Favata et al., 1998). This inhibitor is believed to be more specific and more active against MAPKK than inhibitor PD98059 (Favata et al., 1998), which was reported recently to block the MAPK pathway in Arabidopsis and tobacco (Desikan et al., 1999; Romeis et al., 1999). Although PD98059 blocks MAPKK activity by binding directly to the inactive (nonphosphorylated) form of the enzyme, U0126 does not affect MAPKK activation by phosphorylation but inhibits the activated (phosphorylated) MAPKK at the catalytic site (Favata et al., 1998). When tobacco cells were pretreated with U0126, harpinPsph-mediated activation of the 48-kD MBP kinase was strongly reduced (at 50 μM) or compromised (at 100 μM) (Figure 7A, top section). SIPK activation was reduced by 50% in the presence of 5 μM U0126 (not shown). Similar U0126 concentrations (25 μM) have been found to efficiently block activation of the human MAPK, ERK2, by the MAPKK MEK1 in vitro (Favata et al., 1998; Goueli et al., 1999). Incubation of tobacco cells with U0126 for 4 hr had no apparent effect on the viability of the cells, which was determined in a combined viability/lethality assay using fluorescein diacetate and propidium iodide (Blume et al., 2000). In addition, the harpinPsph-induced oxidative burst remained unaffected in tobacco cells treated with 100 μM U0126, suggesting that MAPK activation and reactive oxygen species production are not linked functionally (not shown).
Figure 7.
The MAPKK-Specific Inhibitor U0126 Compromises Both HarpinPsph-Induced SIPK Activation and PR Gene Expression in Tobacco.
(A) Tobacco cells were treated for 3 hr with buffer (−) or 1 μM harpinPsph (+) in the absence (DMSO) or presence of 50 or 100 μM U0126, a MAPKK-specific inhibitor (Favata et al., 1998). The highest concentration of DMSO used was 0.2%. Protein extracts from elicited tobacco cells were analyzed for SIPK activity as described in the legend to Figure 5C. The expression of PR genes was analyzed by RT-PCR with total RNA and specific DNA primers for the indicated genes or, alternatively, by RNA gel blot analysis using total RNA and α-32P-dATP–labeled HSR203 cDNA.
(B) Quantification of the inhibitory effect of U0126 on harpinPsph-induced HIN1 expression. RT-PCR products obtained from three independent elicitation experiments as described in (A) were separated electrophoretically, blotted onto nylon membranes, and probed with α-32P-dATP–labeled HIN1 cDNA. The signal intensity was quantified by phosphorimaging and normalized to 100% for the maximum induction in each experiment. Buffer treatment (blank bars); harpinPsph treatment (shaded bars). Error bars indicate ±se.
The level of harpinPsph-induced HIN1 transcript accumulation in U0126-treated tobacco cells was diminished compared with that in DMSO-treated control cells (Figure 7A, bottom section). To quantify the effect of the inhibitor on harpinPsph-induced HIN1 activation, RT-PCR products (Figure 7A) were blotted for hybridization with α-32P-dATP–labeled cDNA and relative HIN1 expression levels were determined by phosphorimaging. This was necessary because the inhibitor itself slightly induced the transcript accumulation of HIN1 and other PR genes in nonelicited tobacco cells (Figure 7A). Pooled data from three independently performed experiments revealed that 100 μM U0126 reduced HIN1 expression by 78% compared with control levels (Figure 7B). Interestingly, albeit to a variable extent, transcript accumulation of PR genes such as PR1, PR2, and acidic chitinase (PR3) or of the HR marker genes HSR201, HSR515, and HSR203 also was affected by the inhibitor (Figure 7A). A similar reduction in HSR203 expression was found when RNA gel blots were probed with HSR203 cDNA (Figure 7A). Thus, a harpinPsph-activated MAPK cascade appears to be involved in signaling PR gene expression in tobacco.
DISCUSSION
A Harpin Binding Site Involved in PR Gene Expression in Tobacco
We have shown that tobacco plasma membranes harbor a binding site for P. s. syringae–derived harpins (Figures 2 and 3). Binding of 125I-harpinPsph was inhibited by excess of unlabeled harpinPsph and binding was saturable (Figure 2), as was expected of an authentic receptor (Hulme and Birdsall, 1990). Ligand saturation analyses performed with tobacco protoplasts revealed a dissociation constant of the ligand/binding site interaction of 380 nM, which is in good agreement with the EC50 value obtained for a number of harpinPsph-induced responses of tobacco cells (Figure 2D, Table 1). Moreover, use of a series of deletion derivatives of harpinPsph showed a close quantitative and qualitative correlation between the abilities of ligands to inhibit binding of the radioligand and to induce HIN1 expression (Figure 3). Thus, the harpinPsph binding site detected in the plasma membrane is likely to mediate the activation of defense responses in tobacco.
A comparably high degree of correlation between elicitor and displacement activities of ligand derivatives was found for other elicitors and their binding sites as well (Cheong and Hahn, 1991; Nürnberger et al., 1994; Bourque et al., 1998; Kooman-Gersmann et al., 1998; Meindl et al., 2000). Unfortunately, the preparation of tobacco protoplasts caused PR gene expression in the absence of harpinPsph (data not shown); hence, it was impossible to determine if protoplasts would respond to harpinPsph treatment in a manner similar to intact cells. This would have been most desirable, because Hoyos et al. (1996) reported binding of harpinPss exclusively to the uppermost layer of tobacco cell walls but not to tobacco protoplasts; this binding was detected by confocal laser microscopy using an anti-harpinPss antibody and a fluorochrome-tagged anti-IgG antibody. This association was Ca2+ dependent and detectable only at harpinPss concentrations of ∼5 μM, which exceeded by far the concentrations required for stimulation of harpinPsph-induced plant cell responses (Table 1).
Important questions regarding whether binding was saturable and reversible were not addressed in that study. Therefore, we performed radioligand saturation assays with intact tobacco cells. Scatchard plot analysis of the data yielded a scattered distribution of data points (data not shown), which prevented meaningful analysis but led us to conclude that 125I-harpinPsph may bind to tobacco cells at multiple sites with very different ligand affinities. Although it cannot be excluded that harpins may interact with the plant cell wall in addition to the plasma membrane, it is difficult to reconcile a cell wall binding site with a harpinPss-induced K+/H+ exchange response and plasma membrane depolarization (Hoyos et al., 1996; Pike et al., 1998). Signal perception remote from the plasma membrane and initiation of an intracellular signaling cascade would require the involvement of extracellular matrix receptor-like molecules, as in mammalian cells (Turner and Burridge, 1991). Although such proteins appear to exist in plants, their function has yet to be elucidated (Kohorn, 2000). Thus, it is not clear if the study by Hoyos et al. (1996) identified a physiological target implicated in the activation of plant defense responses or if this association was due to an ionic interaction with cell wall pectic polysaccharides.
Using harpinPsph fragments, we identified regions within the protein that were capable of binding to the binding site to some extent but were unable to elicit HIN1 expression in tobacco cells. When added at a 100-fold molar excess over 125I-harpinPsph, two elicitor-inactive peptide fragments (covering amino acids 219 to 280) showed residual competitor activity, suggesting that different regions of the elicitor are implicated in elicitor perception and generation of an intracellular signaling cascade. This is similar to the address-message concept according to which the yeast invertase-derived glycopeptide elicitor gp8 or the bacterial elicitor flg22 activates defense responses in tomato cells (Basse et al., 1992; Meindl et al., 2000). Similar to our findings, biologically inactive fragments of these elicitors partially retained the ability to interact with their binding sites. A somewhat surprising observation in our studies was that a peptide fragment (amino acids 301 to 345) that was apparently dispensable for the elicitor activity of harpinPsph showed partial competitor activity. However, it is possible that this region contributed only marginally to the binding of 125I-harpinPsph to tobacco membranes and that deletion of this fragment had so little impact on its elicitor activity that we did not detect it in HIN1 expression assays.
Recent studies that complement our analyses of harpinPsph-induced signal perception and transduction in tobacco cells attempted to elucidate the role of harpinPsph in bacterial pathogenicity. Like structurally related YopB from the mammalian pathogen Yersinia enterocolitica (Tardy et al., 1999), harpinPsph and homologous proteins from P. s. tomato or P. s. syringae were found to integrate into protein-free bilayer membranes and to form an ion-conducting pore in vitro (Lee et al., 2001). Binding of membrane-interacting proteins is most often mediated by specific membrane phosphoglycerolipids, such as negatively charged phosphatidic acid or neutral phosphatidylethanolamine (Thevissen et al., 2000a, and references therein). Interestingly, the association of harpinPsph to lipids was strongly enhanced in the presence of negatively charged phosphoglycerolipids and phosphatidylethanolamine (Lee et al., 2001), both of which are constituents of plant plasma membranes (Staehelin and Newcomb, 2000). Direct interaction of harpinPsph with lipids seems to be consistent with a protease-insensitive binding site detected in tobacco membranes. In addition, activation of plant defense responses by ionophore-like compounds has been reported in many plant systems (Yang et al., 1997; Scheel, 1998). Because binding of protein ligands to specific lipids may resemble very closely the molecular interactions between proteinaceous ligands and receptors, it appears likely that the harpinPsph binding site detected in tobacco plasma membranes is not a protein.
Precedent for this may be provided by high affinity binding sites for plant defensins, which were detected on fungal plasma membranes (Thevissen et al., 2000b). Defensins trigger ion fluxes similar to those observed in harpinPsph-treated tobacco cells. Recently, Thevissen et al. (2000a) reported that defensin binding to fungal plasma membranes was strongly dependent on mannose-(inositol-phosphate)2-ceramide, the major sphingolipid in membranes of Saccharomyces cerevisiae. Their data support a model in which membrane patches containing sphingolipids act as specific binding sites for defensins or, alternatively, are required to anchor membrane- or cell wall–associated proteins, which themselves interact with defensins (Thevissen et al., 2000a). Similarly, the protein antibiotic nisin Z from Lactococcus lactis binds with high affinity to the membrane-anchored cell wall precursor lipid II of Gram-positive bacteria (Breukink et al., 1999). Like harpinPsph (Lee et al., 2001), nisin Z is an amphipathic, highly charged protein. Remarkably, nisin Z exerted its biological function through high affinity binding to lipid II and subsequent formation of an ion-conducting pore (Breukink et al., 1999).
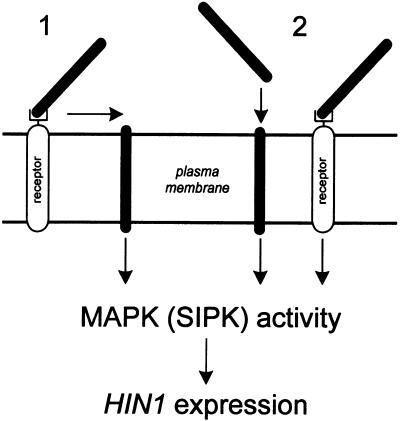
At present it is unknown if activation by harpinPsph of pathogen defense responses in tobacco is mediated by a specific (non)proteinaceous receptor, by direct insertion of the protein into membranes, or by receptor-mediated membrane insertion (Figure 8). It is also conceivable that membrane insertion and receptor-mediated recognition of harpinPsph occur independently in tobacco membranes, and that either or both pathways could lead to activation of defense responses in tobacco. The latter case seems to be supported by our observations that harpinPsph-induced ion pore formation was detectable at concentrations as low as 2 nM (Lee et al., 2001), whereas binding of harpinPsph (Figures 2D and 2E) and transcriptional activation of PR genes (Table 1) required significantly higher elicitor concentrations. On the other hand, pore formation sufficient to trigger plant defense responses may require a threshold concentration of harpinPsph higher than 2 nM. However, specific receptors would explain why plant species respond differently to harpinPsph treatment, whereas insertion into membranes may reflect the role of harpinPsph during bacterial infection of host plants.
Figure 8.
Hypothetical Model for HarpinPsph-Induced Signal Transduction in Tobacco.
Activation by harpinPsph of pathogen defense responses in tobacco may be mediated by receptor-mediated membrane insertion (1), by direct insertion of harpinPsph into membranes, or by a specific (non)proteinaceous receptor (2). Alternatively, membrane insertion and receptor-mediated recognition of harpinPsph may occur independently, with either or both pathways activating MAPK-dependent HIN1 expression in tobacco.
MAPK Activity but Not Extracellular Calcium Is Required for HarpinPsph-Induced HIN1 Expression
Extracellular Ca2+ is important for the induction of pathogen defense in various plants (Yang et al., 1997; Scheel, 1998; Grant and Mansfield, 1999). However, we found that BAPTA or lanthanide inhibitors of Ca2+ influx did not affect harpinPsph-induced HIN1 expression but abrogated β-elicitin–induced HIN1 expression (Figure 4). Thus, structurally diverse elicitors, which target different receptors (Figure 2F), trigger PR gene expression dependent on or independent of extracellular Ca2+ in the same plant. Moreover, because harpinPss-induced HR has been shown to depend on extracellular Ca2+ (He et al., 1993), several emerging signaling cascades appear to be used for the activation of a complex plant defense response triggered by a single elicitor.
We have provided evidence that harpinPsph stimulated SIPK rapidly and transiently (Figure 5). Similar activation kinetics have been reported for a 49-kD MBP-phosphorylating protein kinase that was induced in tobacco leaves upon treatment with harpinEa from Erwinia amylovora (Adam et al., 1997) and that is likely SIPK (Zhang and Klessig, 2000). In contrast, activation of SIPK by β-elicitins (Lebrun-Garcia et al., 1998; Zhang et al., 1998; Zhang and Klessig, 2000; our unpublished data) or the β-elicitin parasiticein (Zhang et al., 1998) was much more prolonged. Similarly prolonged activation of SIPK activity has been reported in tobacco cells treated with Trichoderma viride–derived xylanase (Suzuki et al., 1999) or in Cf-9–transformed tobacco cells treated with the race-specific C. fulvum elicitor AVR9 (Romeis et al., 1999). Moreover, elicitins but not harpinPsph induced prolonged activation of the 44-kD MAPK, WIPK, and another yet undefined tobacco MAPK of 40 kD (Zhang et al., 1998; Zhang and Klessig, 2000). These findings demonstrate that not only elicitor- or pathogen-induced MAPK activity but signal-specific MAPK activity profiles and isoenzyme patterns may be key features of the signal transduction cascades involved in the activation of plant pathogen defense. In addition, MAPK activity is controlled both post-translationally and transcriptionally, which is considered another regulatory mechanism through which the specificity of signal transduction cascades is maintained (Hirt, 2000).
In those cases investigated, MAPK activation by elicitor was dependent on extracellular Ca2+ (Ligterink et al., 1997; Lebrun-Garcia et al., 1998; Romeis et al., 1999; Suzuki et al., 1999; Fellbrich et al., 2000). Particularly, β-elicitin–induced SIPK activity (Lebrun-Garcia et al., 1998; Figure 6) and HIN1 expression (Figure 4) were dependent on extracellular Ca2+. In contrast, SIPK activation by harpinPsph was independent of extracellular Ca2+ and Ca2+ influx (Figures 4 and 6). Recently, Ca2+-independent activation of SIPK was observed in tobacco cells undergoing hyperosmotic stress (Hoyos and Zhang, 2000), thus exemplifying the central role of this enzyme in stress adaptation as well as its implication in differentially regulated signaling chains.
HarpinPsph-induced Ca2+-independent SIPK activation and HIN1 expression prompted us to investigate a possible causal link between the two responses. Specific inhibition of MAPKK activity by U0126 (Favata et al., 1998) not only abolished harpinPsph-induced SIPK activation but also suppressed the expression of numerous PR genes in tobacco cells (Figure 7). This is novel in that it suggests the functional involvement of MAPK (SIPK) activity in plant defense activation. Romeis et al. (1999) showed inhibition of elicitor-induced tobacco SIPK activity by another MAPKK inhibitor, PD98059, which, however, did not block the elicitation of defense-related responses, such as production of reactive oxygen species. Furthermore, PD98059 inhibited the activation of two MAPKs of 39 and 44 kD and of PR gene expression in harpinPss-treated Arabidopsis cells (Desikan et al., 1999), but because the Arabidopsis SIPK ortholog AtMPK6 is a 49-kD protein (Nühse et al., 2000), it is not certain if either one of the PD98059-sensitive MAPKs represented AtMPK6.
Taken together, harpinPsph and β-elicitin-induced HIN1 expression in tobacco cells was shown to be mediated through different receptors (Figure 2F). Subsequently, Ca2+-independent and Ca2+-dependent signaling cascades are initiated, which merge upstream of MAPK activity and give rise to expression of PR genes, such as HIN1.
METHODS
Plant Growth, Maintenance of Plant Cell Cultures, and Elicitor Application
Tobacco (Nicotiana tabacum cv Samsun NN) plants were grown in a greenhouse at 22°C with a 14-hr-light/10-hr-dark cycle. Six- to 8-week-old plants were used for infiltration experiments. Test substances were infiltrated into the apoplast using a 1-mL disposable plastic syringe. Tobacco BY2 cell lines were maintained as described (Nagata et al., 1992). For subculturing, 2 mL of cells was transferred weekly into 50 mL of fresh medium. Experiments with cultured cells were performed 3 days after subculture. To maintain uniform conditions for all experiments, cultured cells were filtered and resuspended in incubation buffer (0.5 mM Mes, 4% [v/v] B5 salts, and 3% sucrose, pH 5.7) at a density of 5 g/100 mL. Diluted cells (5 mL) were transferred into Petri dishes and equilibrated for a minimum of 1 hr at 22°C with shaking (125 rpm) before the addition of effectors. Inhibitors were added 30 min before elicitor treatment. Harpin from Pseudomonas syringae pv phaseolicola (harpinPsph) and β-megaspermin were added at final concentrations of 1 μM and 50 nM, respectively. Cells were collected by filtration 3 hr after elicitor treatment or at the indicated times and stored in liquid nitrogen. For experiments with Ca2+ channel blockers or 1,2-bis(o-aminophenoxy)ethane-N,N,N,N-tetraacetic acid, the elicitation time was reduced to 1.5 hr. Cell viability was determined routinely by fluorescein diacetate/propidium iodide staining (Blume et al., 2000). Tobacco BY2 protoplasts were prepared using the protocol described previously for the preparation of parsley protoplasts (Dangl et al., 1987).
Elicitor Preparation
For the production of recombinant harpins, a DNA fragment encoding harpinPsph (GenBank accession number AF268940) or a polymerase chain reaction (PCR) fragment of harpin from P. s. tomato amplified from genomic DNA (Preston et al., 1995) was placed under the control of a T7 promoter (pT7-7 or pJC40) (Clos and Brandau, 1994; Lee et al., 2001). For the expression of harpin from P. s. syringae, the plasmid pSYH10 was used (He et al., 1993). DNA constructs were transformed into BL21 (DE3) pLysS Escherichia coli cells, and protein expression in bacteria grown to midlogarithmic phase was induced with 1 mM isopropyl-β-d-thiogalactoside for 5 hr. Subsequently, cells were harvested and lysed by sonication in extraction buffer (50 mM Tris, 100 mM NaCl, 1 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride, pH 8.0). The sonicated material was boiled (100°C, 10 min) and centrifuged to remove denatured proteins, and heat-stable proteins were precipitated by ammonium sulfate fractionation (45% saturation). The precipitated protein was resuspended in 5 mM Mes buffer, pH 5.5, and desalted using PD-10 columns (Amersham Pharmacia, Freiburg, Germany). This procedure allowed purification to more than 95% homogeneity according to SDS-PAGE/silver staining and reverse phase HPLC analysis (Nürnberger et al., 1994).
To verify the integrity of the recombinant protein, the N terminus was sequenced using a G1050 Protein Sequencer (Hewlett-Packard, Palo Alto, CA). For the expression of truncated forms of harpinPsph, DNAs encoding the fragments shown in Figure 3A were amplified by PCR from plasmid pT7-7/hrpZ (Lee et al., 2001). Primers were designed from the harpinPsph-encoding DNA sequence and modified to construct appropriate restriction sites. NdeI–BamHI fragments (except fragment IV, which was encoded by a NdeI–EcoRI DNA fragment) were introduced into the modified pET vector pJC40 encoding an N-terminal His10 tag (Clos and Brandau, 1994). The clone encoding harpinPsph fragment VII (Figure 3A) was obtained by removal of an internal XhoI DNA fragment. Expression in BL21 (DE3) pLysS E. coli cells was initiated by 1 mM isopropyl-β-d-thiogalactoside, and expressed proteins were isolated on nickel–nitrilotriacetic acid agarose using the Qiagen (Hilden, Germany) protocol. HarpinPsph fragments were dialyzed against 5 mM Mes, pH 5.5. The homogeneity of purified recombinant harpinPsph was tested by SDS-PAGE/silver staining. β-Megaspermin was prepared as described (Baillieul et al., 1995). Protein concentrations were determined with the bicinchoninic acid assay kit (Pierce, Sankt Augustin, Germany) using BSA as the standard. Solid phase peptide synthesis with N-α-9-fluorophenylmethoxycarbonyl (Fmoc)–protected amino acids was performed with an Economy Peptide Synthesizer EPS 221 (ABIMED, Langen, Germany) according to the manufacturer's instructions. Synthesized peptides were purified to homogeneity as described (Nürnberger et al., 1994).
RNA Extraction and Analyses
RNA was extracted by the hot phenol/chloroform/LiCl precipitation method (Sambrook et al., 1989). RNA prepared from 250 mg of frozen tobacco cells was resuspended in 50 μL of water. Routinely, 1 to 2 μL of RNA solution was used in reverse transcription (RT)-PCR analyses. RT was initiated in the presence of oligo(dT) primers (42°C, 30 min), and after inactivation of the reverse transcriptase (95°C, 5 min), the appropriate primers were added for PCR cycling (25 cycles of 15 sec at 95°C, 1 min at 55°C, and 1 min at 72°C). Initially, reverse transcriptase and Taq polymerase were added separately, but subsequently, a single tube reaction was used (Ready-To-Go RT-PCR beads; Amersham Pharmacia). Each rehydrated bead was used for one reaction of 50 μL. Amplification of a constitutively expressed gene (translation elongation factor 1α [EF1α]) served as an internal control in RT-PCR assays. For analysis of HIN1 expression, multiplex PCR was used to amplify simultaneously transcripts of HIN1 and EF1α. The RT-PCR products were analyzed by agarose gel electrophoresis. For quantification, RT-PCR products were transferred to nylon membranes and hybridized to α-32P-dATP–labeled HIN1 cDNA (Sambrook et al., 1989). Signal intensity was determined by phosphorimaging.
For amplification of tobacco genes by RT-PCR, the following primers were used: HSR203 (forward, 5′-TGTACTACACTGTCTACACGC-3′; reverse, 5′-GATAAAAGCTATGTCCCACTCC-3′); HSR201 (forward, 5′-CATCACGAATACGATGAAGTACG-3′; reverse, 5′-CAGGCAAACAAATTGGAACC-3′); HSR515 (forward, 5′-AACTCTCCC-TTAAGTACGGAC-3′; reverse, 5′-CAATAGTCCATACACTCACGA-3′); PR1 (forward, 5′-GATGCCCATAACACAGCTCG-3′; reverse, 5′-TTTACAGATCCAGTTCTTCAGAGG-3′); PR2 (forward, 5′-CTGCCCTTGTACTTGTTGGG-3′; reverse, 5′-TCCAGGTTTCTTTGGAGTTCC-3′); PR3 (forward, 5′-GGTTCTATTGTAACGAGTGAC-3′; reverse, 5′-TTC-TATGTAACGAAGCCTAGC-3′); chitinase/lysozyme (forward, 5′-TCT-CATGTTTCCTTCTCCGG-3′; reverse, 5′-CAAAGTAACCTAGCA-ATCCTCTACC-3′); HIN1 (forward, 5′-GAACGGAGCCTATTATGG-CCCTTCC-3′; reverse, 5′-CATGTATATCAATGAACACTAAACGCC-GG-3′); and EF1α (forward, 5′-TCACATCAACATTGTGGTCATTGGC-3′; reverse, 5′-TTGATCTGGTCAAGAGCCTCAAG-3′). RNA gel blotting with total RNA from tobacco cells and α-32P-dATP–labeled DNA was performed as described (Sambrook et al., 1989).
Iodination of HarpinPsph, Microsomal Membrane Preparation, and Radioligand Binding Assays
A tyrosine residue was added to the C terminus of harpinPsph for radioiodination. PCR with corresponding primers was performed to change the TGA stop codon by the deletion of one nucleotide into a tyrosine-encoding TAC codon, which was followed by a TGA stop codon created by the frameshift. The introduced mutation and the integrity of the coding sequence were verified by DNA sequencing. Nonradioactive iodination of harpin was performed by the addition of IODO-BEADS (Pierce) and NaI according to the supplier's instructions. Labeling with Na125I to a specific radioactivity of 2200 Ci/mmol was performed by Biotrend Chemikalien (Köln, Germany). Tobacco BY2 cells (6 days old) were used for the preparation of microsomes as described (Nürnberger et al., 1994). The microsomal pellet recovered from 150 g of frozen tobacco cells was resuspended in 5 mL of 20 mM sodium phosphate and 100 mM NaCl, pH 7.0. Microsomal protein (200 μg) was resuspended in a total volume of 200 μL of buffer and kept on ice during the course of the experiment. If not indicated otherwise, binding was initiated by the addition of 100 nM 125I-harpinPsph. The binding reaction was terminated by adding 5 mL of ice-cold buffer, and membranes were harvested by filtration on Whatman GF/B glass filters (Maidstone, UK) preblocked with 5% BSA in binding buffer (Nürnberger et al., 1994). Subsequently, filters were subjected to γ-counting in a Wizard counter (Amersham Pharmacia). Nonspecific binding was determined in the presence of a 100-fold molar excess of unlabeled harpinPsph. Binding of 125I-harpinPsph to 2.5 × 106 tobacco protoplasts resuspended in 250 μL of Murashige and Skoog (1962) medium containing 0.4 M sucrose was performed as described above except that protoplasts were rinsed three times with 5 mL of ice-cold 0.24 M CaCl2. Appropriate dilutions of 125I-harpinPsph with unlabeled harpinPsph were used to reduce the costs and use of radioactivity.
Ion Flux Measurements and MAPK Activity Assays
Changes in ion concentrations (H+, K+, and Cl−) of elicited tobacco cells were determined as described (Nürnberger et al., 1994). Extracellular free Ca2+ concentration was calculated using MaxChelator software (version 2.5 for Windows; http://www.stanford.edu/~cpatton/) (Bers et al., 1994). Preparation of protein extracts from tobacco cells, in-gel protein kinase assays with myelin basic protein (MBP) embedded in 10% polyacrylamide gels, and immune complex kinase activity assays using the antiserum Ab-p48N were performed as described (Zhang et al., 1998). For immunoblot analyses with the antiACTIVE mitogen-activated protein kinase (MAPK) antibody (Promega, Mannheim, Germany), tobacco protein extracts (30 μg of protein) were separated on 10% SDS-polyacrylamide gels, proteins were transferred to nitrocellulose by semidry blotting, and membranes were incubated with the antiserum according to the supplier's instructions. A secondary goat anti-rabbit IgG antibody coupled to alkaline phosphatase was used to visualize immunoreactive proteins.
Acknowledgments
We thank Serge Kauffmann (Centre National de la Recherche Scientifique, Strasbourg, France) for the kind gift of purified β-megaspermin and Alan Collmer (Cornell University, Ithaca, NY) for providing plasmid pSYH10. Angela Schierhorn (Martin-Luther-University, Halle-Wittenberg, Germany) is gratefully acknowledged for matrix-assisted laser-desorption ionization time of flight analyses of iodinated harpinPsph. We thank Beatrix Blume, Dierk Scheel, and Dirk Nennstiel for critical reading of the manuscript and Jason J. Rudd for sharing with us his expertise on MAPK assays. This work was funded by grants from the European Community Biotechnology program (Grant No. BIO-CT97-2244) and the United States Department of Agriculture (Grant No. 9862200).
References
- Adam, A.L., Pike, S., Hoyos, E.M., Stone, J.M., Walker, J.C., and Novacky, A. (1997). Rapid and transient activation of a myelin basic protein kinase in tobacco leaves treated with harpin from Erwinia amylovora. Plant Physiol. 115 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillieul, F., Genetet, I., Kopp, M., Saindrenan, P., Fritig, B., and Kauffmann, S. (1995). A new elicitor of the hypersensitive response in tobacco: A fungal glycoprotein elicits cell death, expression of defence genes, production of salicylic acid, and induction of systemic acquired resistance. Plant J. 8 551–560. [DOI] [PubMed] [Google Scholar]
- Baker, C.J., Orlandi, E.W., and Mock, N.M. (1993). Harpin, an elicitor of the hypersensitive response in tobacco caused by Erwinia amylovora, elicits active oxygen production in suspension cells. Plant Physiol. 102 1341–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basse, C.W., Bock, K., and Boller, T. (1992). Elicitors and suppressors of the defense response in tomato cells: Purification and characterization of glycopeptide elicitors and glycan suppressors generated by enzymatic cleavage of yeast invertase. J. Biol. Chem. 267 10258–10265. [PubMed] [Google Scholar]
- Bers, D.M., Patton, C.W., and Nuccitelli, R. (1994). A practical guide to the preparation of Ca2+ buffers. Methods Cell. Biol. 40 3–29. [DOI] [PubMed] [Google Scholar]
- Blume, B., Nürnberger, T., Nass, N., and Scheel, D. (2000). Receptor-mediated rise in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12 1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque, S., Ponchet, M., Binet, M.N., Ricci, P., Pugin, A., and Lebrun-Garcia, A. (1998). Comparison of binding properties and early biological effects of elicitins in tobacco cells. Plant Physiol. 118 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque, S., Binet, M.-N., Ponchet, M., Pugin, A., and Lebrun-Garcia, A. (1999). Characterization of the cryptogein binding sites on plant plasma membranes. J. Biol. Chem. 274 34699–34705. [DOI] [PubMed] [Google Scholar]
- Breukink, E., Wiedemann, I., van Kraaij, C., Kuipers, O.P., Sahl, H.-G., and de Kruijff, B. (1999). Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286 2361–2364. [DOI] [PubMed] [Google Scholar]
- Cheong, J.-J., and Hahn, M.G. (1991). A specific, high-affinity binding site for the hepta-β-glucoside elicitor exists in soybean membranes. Plant Cell 3 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos, J., and Brandau, S. (1994). pJC20 and pJC40: Two high copy-number vectors for T7 RNA polymerase-dependent expression of recombinant genes in E. coli. Protein Expr. Purif. 5 133–137. [DOI] [PubMed] [Google Scholar]
- Dangl, J.L., Hauffe, K.D., Lipphardt, S., Hahlbrock, K., and Scheel, D. (1987). Parsley protoplasts retain differential responsiveness to UV light and fungal elicitor. EMBO J. 6 2551–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan, R., Clarke, A., Atherfold, P., Hancock, J.T., and Neill, S.J. (1999). Harpin induces mitogen-activated protein kinase activity during defence responses in Arabidopsis thaliana suspension cultures. Planta 210 97–103. [DOI] [PubMed] [Google Scholar]
- Dong, H., Delaney, T.P., Bauer, D.W., and Beer, S.V. (1999). Harpin induces disease resistance in Arabidopsis through the systemic acquired resistance pathway mediated by salicylic acid and the NIM1 gene. Plant J. 20 207–215. [DOI] [PubMed] [Google Scholar]
- Favata, M.F., et al. (1998). Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273 18623–18632. [DOI] [PubMed] [Google Scholar]
- Fellbrich, G., Blume, B., Brunner, F., Hirt, H., Kroj, T., Ligterink, W., Romanski, A., and Nürnberger, T. (2000). Phytophthora parasitica elicitor-induced reactions in cells of Petroselinum crispum. Plant Cell Physiol. 41 692–701. [DOI] [PubMed] [Google Scholar]
- Galan, J.E., and Collmer, A. (1999). Type III secretion machines: Bacterial devices for protein delivery into host cells. Science 284 1322–1328. [DOI] [PubMed] [Google Scholar]
- Gopalan, S., Wei, W., and He, S.Y. (1996). hrp gene–dependent induction of hin1: A plant gene activated rapidly by both harpins and the avrPto gene–mediated signal. Plant J. 10 591–600. [DOI] [PubMed] [Google Scholar]
- Goueli, S.A., Hsiao, K., Lu, T., and Simpson, D. (1999). U0126: A novel, selective and potent inhibitor of MAP kinase kinase (MEK). Promega Notes 69 6–8 (sgoueli@promega.com). [Google Scholar]
- Grant, M., and Mansfield, J. (1999). Early events in host–pathogen interactions. Curr. Opin. Plant Biol. 2 312–319. [DOI] [PubMed] [Google Scholar]
- He, S.Y., Huang, H.-C., and Collmer, A. (1993). Pseudomonas syringae pv. syringae harpinPss: A protein that is secreted by the hrp pathway and elicits the hypersensitive response in plants. Cell 73 1255–1266. [DOI] [PubMed] [Google Scholar]
- Heitz, T., Segond, S., Kauffmann, S., Geoffroy, P., Prasad, V., Brunner, F., Fritig, B., and Legrand, M. (1994). Molecular characterization of a novel tobacco pathogenesis-related (PR) protein: A new plant chitinase/lysozyme. Mol. Gen. Genet. 245 246–254. [DOI] [PubMed] [Google Scholar]
- Herskowitz, I. (1995). MAP kinase pathways in yeast: For mating and more. Cell 80 187–197. [DOI] [PubMed] [Google Scholar]
- Hirt, H. (2000). MAP kinases in plant signal transduction. Results Probl. Cell Differ. 27 1–9. [DOI] [PubMed] [Google Scholar]
- Hoyos, E., and Zhang, S. (2000). Calcium-independent activation of salicylic acid–induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic shock. Plant Physiol. 122 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos, M.E., Stanley, C.M., He, S.Y., Pike, S., Pu, X.-A., and Novacky, A. (1996). The interaction of harpinPss with plant cell walls. Mol. Plant-Microbe Interact. 9 608–616. [Google Scholar]
- Hulme, E.C., and Birdsall, N.J.M. (1990). Strategy and tactics in receptor-binding studies. In Receptor–Ligand Interactions: A Practical Approach, E.C. Hulme, ed (Oxford, UK: IRL Press), pp. 63–176.
- Kjemtrup, S., Nimchuk, Z., and Dangl, J.L. (2000). Effector proteins of phytopathogenic bacteria: Bifunctional signals in virulence and host recognition. Curr. Opin. Microbiol. 3 73–78. [DOI] [PubMed] [Google Scholar]
- Kohorn, B.D. (2000). Plasma membrane–cell wall contacts. Plant Physiol. 124 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooman-Gersmann, M., Vogelsang, R., Vossen, P., van den Hooven, H., Mahe, E., Honee, G., and de Wit, P.J.D.G. (1998). Correlation between binding affinity and necrosis-inducing activity of mutant AVR9 peptide elicitors. Plant Physiol. 117 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun-Garcia, A., Ouaked, F., Chiltz, A., and Pugin, A. (1998). Activation of MAPK homologues by elicitors in tobacco cells. Plant J. 15 773–781. [DOI] [PubMed] [Google Scholar]
- Lee, J., Klüsener, B., Tsiamis, G., Stevens, C., Neyt, C., Tampakaki, A.P., Panopoulos, N.J., Nöller, J., Weiler, E.W., Cornelis, G.R., Mansfield, J.W., and Nürnberger, T. (2001). HrpZPsph from the plant pathogen Pseudomonas syringae pv. phaseolicola binds to lipid bilayers and forms an ion-conducting pore in vitro. Proc. Natl. Acad. Sci. USA 98 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligterink, W., Kroj, T., zur Nieden, U., Hirt, H., and Scheel, D. (1997). Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science 276 2054–2057. [DOI] [PubMed] [Google Scholar]
- Lindgren, P.B., Peet, R.C., and Panopoulos, N.J. (1986). Gene cluster of Pseudomonas syringae pv. “phaseolicola” controls pathogenicity of bean plants and hypersensitivity of nonhost plants. J. Bacteriol. 168 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl, T., Boller, T., and Felix, G. (2000). The bacterial elicitor flagellin activates its receptor in tomato cells according to the address-message concept. Plant Cell 12 1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithöfer, A., Lottspeich, F., and Ebel, J. (1996). One-step purification of the beta-glucan elicitor-binding protein from soybean (Glycine max L.) roots and characterization of an anti-peptide antiserum. FEBS Lett. 381 203–207. [DOI] [PubMed] [Google Scholar]
- Mithöfer, A., Ebel, J., Bagwhat, A.A., Boller, T., and Neuhaus-Url, G. (1999). Transgenic aequorin monitors cytosolic calcium transients in soybean cells challenged with β-glucan or chitin elicitors. Planta 207 566–574. [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15 473–497. [Google Scholar]
- Nagata, T., Nemoto, Y., and Kasezawa, S. (1992). Tobacco BY2 cell line as the “Hela” cell in the cell biology of higher plants. Int. Rev. Cytol. 132 1–30. [Google Scholar]
- Nühse, T.S., Peck, S.C., Hirt, H., and Boller, T. (2000). Microbial elicitors induce activation and dual phosphorylation of the Arabidopsis thaliana MAPK 6. J. Biol. Chem. 275 7521–7526. [DOI] [PubMed] [Google Scholar]
- Nürnberger, T. (1999). Signal perception in plant pathogen defense. Cell. Mol. Life Sci. 55 167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberger, T., Nennstiel, D., Jabs, T., Sacks, W.R., Hahlbrock, K., and Scheel, D. (1994). High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell 78 449–460. [DOI] [PubMed] [Google Scholar]
- Nürnberger, T., Nennstiel, D., Hahlbrock, K., and Scheel, D. (1995). Covalent cross-linking of the Phytophthora megasperma oligopeptide elicitor to its receptor in parsley membranes. Proc. Natl. Acad. Sci. USA 92 2338–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike, S.M., Adam, A.L., Pu, X.-A., Hoyos, M.E., Laby, R.J., Beer, S.V., and Novacky, A. (1998). Effects of Erwinia amylovora harpin on tobacco leaf cell membranes are related to leaf necrosis and electrolyte leakage and distinct from perturbations caused by inoculated E. amylovora. Physiol. Mol. Plant Pathol. 53 39–60. [Google Scholar]
- Pontier, D., Gan, S., Amasino, R.M., Roby, D., and Lam, E. (1999). Markers for hypersensitive response and senescence show distinct patterns of expression. Plant Mol. Biol. 39 1243–1255. [DOI] [PubMed] [Google Scholar]
- Preston, G., Huang, H.C., He, S.Y., and Collmer, A. (1995). The HrpZ proteins of Pseudomonas syringae pvs. syringae, glycinea, and tomato are encoded by an operon containing Yersinia ysc homologs and elicit the hypersensitive response in tomato but not soybean. Mol. Plant-Microbe Interact. 8 717–732. [DOI] [PubMed] [Google Scholar]
- Ricci, P., Bonnet, P., Huet, J.-C., Sallantin, M., Beauvais-Cante, F., Bruneteau, M., Billard, V., Michel, G., and Pernollet, J.-C. (1989). Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. Eur. J. Biochem. 183 555–563. [DOI] [PubMed] [Google Scholar]
- Romeis, T., Piedras, P., Zhang, S., Klessig, D.F., Hirt, H., and Jones, J.D. (1999). Rapid Avr9- and Cf-9–dependent activation of MAP kinases in tobacco cell cultures and leaves: Convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, Vol. 2. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Scheel, D. (1998). Resistance response physiology and signal transduction. Curr. Opin. Plant Biol. 1 305–310. [DOI] [PubMed] [Google Scholar]
- Scheel, D., et al. (2000). Receptor-mediated signal transduction in plant defense. In Biology of Plant–Microbe Interactions, P.D.G.M. De Wit, T. Bisseling, and W.J. Stiekema, eds (St. Paul, MN: International Society of Plant–Microbe Interactions), pp. 131–135.
- Staehelin, L.A., and Newcomb, E.H. (2000). Membrane structure and membranous organelles. In Biochemistry and Molecular Biology of Plants, B.B. Buchanan, W. Gruissem, and R.L. Jones, eds (Rockville, MD: American Society of Plant Physiologists), pp. 2–50.
- Strobel, N.E., Gopalan, J.S., Kuc, J.A., and He, S.Y. (1996). Induction of systemic acquired resistance in cucumber by Pseudomonas syringae pv. syringae 61 hrpZPss protein. Plant J. 9 431–439. [Google Scholar]
- Suzuki, K., Yano, A., and Shinshi, H. (1999). Slow and prolonged activation of the p47 protein kinase during hypersensitive cell death in a culture of tobacco cells. Plant Physiol. 119 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardy, F., Homble, F., Neyt, C., Wattiez, R., Cornelis, G.R., Ruysschaert, J.M., and Cabiaux, V. (1999). Yersinia enterocolitica type III secretion-translocation system: Channel formation by secreted Yops. EMBO J. 18 6793–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevissen, K., Cammue, B.P.A., Lemaire, K., Winderickx, J., Dickson, R.C., Lester, R.L., Ferket, K.K.A., Van Even, F., Parret, A.H.A., and Broekaert, W.F. (2000. a). A gene encoding a sphingolipid biosynthesis enzyme determines the sensitivity of Saccharomyces cerevisiae to an antifungal plant defensin from dahlia (Dahlia merckii). Proc. Natl. Acad. Sci. USA 97 9531–9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevissen, K., Osborn, R.W., Acland, D.P., and Broekaert, W.F. (2000. b). Specific binding sites for an antifungal plant defensin from dahlia (Dahlia merckii) on fungal cells are required for antifungal activity. Mol. Plant-Microbe Interact. 13 54–61. [DOI] [PubMed] [Google Scholar]
- Turner, C.E., and Burridge, K. (1991). Transmembrane molecular assemblies in cell-extracellular matrix interactions. Curr. Opin. Cell Biol. 5 849–853. [DOI] [PubMed] [Google Scholar]
- Umemoto, N., Kakitani, M., Iwamatsu, A., Yoshikawa, M., Yamaoka, N., and Ishida, I. (1997). The structure and function of a soybean beta-glucan-elicitor-binding protein. Proc. Natl. Acad. Sci. USA 94 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H.X., and Heath, M.C. (1998). Role of calcium in signal transduction during the hypersensitive response caused by basidiospore-derived infection of the cowpea rust fungus. Plant Cell 10 585–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Shah, J., and Klessig, D.F. (1997). Signal perception and transduction in plant defense responses. Genes Dev. 11 1621–1639. [DOI] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1997). Salicylic acid activates a 48-kD MAP kinase in tobacco. Plant Cell 9 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998. a). Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 95 7433–7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998. b). The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK. Proc. Natl. Acad. Sci. USA 95 7225–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (2000). Pathogen-induced MAP kinases in tobacco. Results Probl. Cell Differ. 27 66–84. [DOI] [PubMed] [Google Scholar]
- Zhang, S., Du, H., and Klessig, D.F. (1998). Activation of the tobacco SIP kinase by both a cell wall–derived carbohydrate elicitor and purified proteinaceous elicitins from Phytophthora spp. Plant Cell 10 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]