Abstract
The geminivirus tomato golden mosaic virus (TGMV) amplifies its DNA genome in differentiated plant cells that lack detectable levels of DNA replication enzymes. Earlier studies showed that TGMV induces the accumulation of proliferating cell nuclear antigen (PCNA), the processivity factor for DNA polymerase δ, in mature cells of Nicotiana benthamiana. We sought to determine if PCNA protein accumulation reflects transcriptional activation of the host gene. RNA gel blot analysis detected an ∼1200-nucleotide PCNA transcript in young leaves. The same RNA was found in mature leaves of infected but not healthy plants. Reporter gene analysis showed that a 633-bp promoter fragment of the N. benthamiana PCNA gene supports high levels of expression in cultured cells and in young but not mature leaves of healthy transgenic plants. In contrast, PCNA promoter activity was detected in both young and mature leaves of TGMV-infected plants. Developmental studies established a strong relationship between symptom severity, viral DNA accumulation, PCNA promoter activity, and endogenous PCNA mRNA levels. Mutation of an E2F consensus element in the PCNA promoter had no effect on its activity in young leaves but increased transcription in healthy mature leaves. Unlike the wild-type PCNA promoter, TGMV infection had no detectable effect on the activity of the mutant E2F promoter. Together, these results demonstrate that geminivirus infection induces the accumulation of a host replication factor by activating transcription of its gene in mature tissues, most likely by overcoming E2F-mediated repression.
INTRODUCTION
Geminiviruses are DNA viruses that replicate their single-stranded genomes through double-stranded intermediates in nuclei of mature plant cells (reviewed in Hanley-Bowdoin et al., 1999; Gutierrez, 2000). Because of their limited coding capacity, geminiviruses supply only the factors required to initiate rolling circle replication and use plant nuclear DNA polymerases to amplify their genomes. Many geminiviruses replicate in differentiated cells that have exited the cell cycle (Coello et al., 1992; Nagar et al., 1995; Lucy et al., 1996). As a consequence, these viruses must induce the synthesis of the requisite host enzymes before replication (Nagar et al., 1995) and thus are valuable tools for studying the mechanisms that mediate and regulate DNA replication and the cell cycle in plants.
Geminiviruses are a diverse family of plant-infecting viruses that fall into three genera based on their genome structure, insect vectors, and host range (Rybicki, 1994). Tomato golden mosaic virus (TGMV), a member of the begomovirus genus, has a bipartite genome consisting of two 2.6-kb DNA components designated A and B (Bisaro et al., 1982), which together encode seven proteins. Only the AL1 protein (also designated C1 or Rep) is essential for replication, whereas AL3 (or C3) enhances viral DNA accumulation (Elmer et al., 1988a; Sunter et al., 1990). AL1 is the origin recognition protein and catalyzes DNA cleavage and ligation to initiate and terminate rolling circle replication (Fontes et al., 1994; Laufs et al., 1995; Orozco and Hanley-Bowdoin, 1996). The role of AL3 in viral replication is not well understood, and no catalytic functions have been assigned to this viral protein.
DNA replication and the corresponding enzymes are confined to meristematic and endoreduplicating tissues (Martinez et al., 1992; Staiger and Doonan, 1993). Some geminiviruses are restricted to vascular tissue (Esau, 1977; Horns and Jeske, 1991; Sanderfoot and Lazarowitz, 1996; Morra and Petty, 2000) and may replicate in vascular parenchyma cells that already contain some cell cycle components, such as the cyclin-dependent kinase cdc2a (Martinez et al., 1992; Hemerly et al., 1993). Other geminiviruses, such as TGMV, are not limited to vascular tissue; instead, they are found throughout terminally differentiated mesophyll and epidermal cells of leaves (Rushing et al., 1987; Nagar et al., 1995; Lucy et al., 1996) and in various cell types in stems and roots (S. Nagar, L. Hanley-Bowdoin, and D. Robertson, unpublished data). In animals, DNA tumor viruses, which rely on host replicative enzymes, induce their synthesis by altering cell cycle and transcriptional controls of quiescent cells (Chellappan et al., 1992; Jansen-Durr, 1996). There is evidence that TGMV also alters the cell cycle controls of its host plant, Nicotiana benthamiana. TGMV-infected cells incorporate high levels of bromodeoxyuridine into both viral and host DNA, indicative of progression into S phase and DNA replication (S. Nagar, L. Hanley-Bowdoin, and D. Robertson, unpublished data). A large fraction of TGMV-infected cells contain condensed chromatin, which is characteristic of early mitotic prophase (Bass et al., 2000). In both instances, uninfected cells immediately adjacent to cells positive for viral DNA showed no signs of reentry into the cell cycle, demonstrating that TGMV acts in a cell-autonomous manner to reprogram its host.
Clues to how TGMV alters cell cycle controls come from experiments showing that both AL1 and AL3 bind to plant homologs of the cell cycle regulator retinoblastoma (pRB; designated in plants as pRBR [retinoblastoma related]) (Ach et al., 1997; Settlage et al., 2000). By analogy with mammalian DNA viruses (Weinberg, 1995), these interactions may bypass a pRB phosphorylation requirement for cell cycle entry during geminivirus infection. Recent experiments showing that TGMV is confined to vascular cells when AL1/pRBR binding is impaired established the importance of this host–pathogen interaction for geminivirus infection (Kong et al., 2000). Other geminiviruses also encode proteins that bind to pRBR (Grafi et al., 1996; Xie et al., 1996; Horvath et al., 1998; Liu et al., 1999), providing general support for the importance of this interaction during infection. In mammalian cells, inactivation of pRB relieves E2F-mediated transcriptional repression of genes that encode proteins necessary for S phase and DNA replication (Herwig and Strauss, 1997). Recently, homologs of animal E2F transcription factors have been identified in plants (Ramirez-Parra et al., 1999; Sekine et al., 1999) and shown to regulate plant gene transcription in cultured cells (Albani et al., 2000; Chaboute et al., 2000). Like their mammalian counterparts, geminiviruses may alter host transcriptional controls through pRBR and E2F.
In a previous study, we showed that TGMV infection specifically induces the accumulation of high levels of proliferating cell nuclear antigen (PCNA), the processivity factor of host DNA polymerase δ, in differentiated cells of infected plants (Nagar et al., 1995). PCNA, albeit at lower levels, also was detected in differentiated cells of transgenic plants expressing TGMV AL1, thereby establishing that the AL1 protein alone is sufficient for PCNA accumulation. These experiments did not determine if induction occurred through transcriptional activation of the PCNA gene or at a post-transcriptional step. PCNA protein and mRNA accumulate in young but not mature tissues of rice, pea, rape, and maize (Kosugi et al., 1991; Citterio et al., 1992; Markley et al., 1993; Lopez et al., 1997). Analysis of the rice PCNA promoter showed that its activity is regulated developmentally and identified cis elements that are necessary for high-level activity in young tissues (Kosugi et al., 1991, 1995; Ohashi et al., 1992). Although these studies were performed in transgenic tobacco, they did not address how endogenous PCNA expression is regulated in dicotyledonous plants. To better understand how PCNA expression is controlled in dicots, we isolated the PCNA promoter region from N. benthamiana and characterized its activity during development and geminivirus infection.
RESULTS
PCNA mRNA Accumulates in Mature Leaves of TGMV-Infected Plants
Increased PCNA protein levels in TGMV-infected plants most likely are due to de novo mRNA synthesis. To test this hypothesis in the TGMV host N. benthamiana, we used a combination of reverse transcription–polymerase chain reaction (RT-PCR) and rapid amplification of cDNA ends to isolate cDNA clones corresponding to the endogenous PCNA mRNA. We analyzed one set of PCR products encoding amino acids 64 to 237 of N. benthamiana PCNA and two independent sets of products corresponding to the 5′ and 3′ ends of the cDNA. Sequence analysis revealed that these products represent a single mRNA (GenBank accession number AF305075) that includes a 792-bp open reading frame flanked by 110 and 170 bp of 5′ and 3′ sequences, respectively. Comparison of the N. benthamiana sequence and other plant PCNA proteins revealed >95% homology.
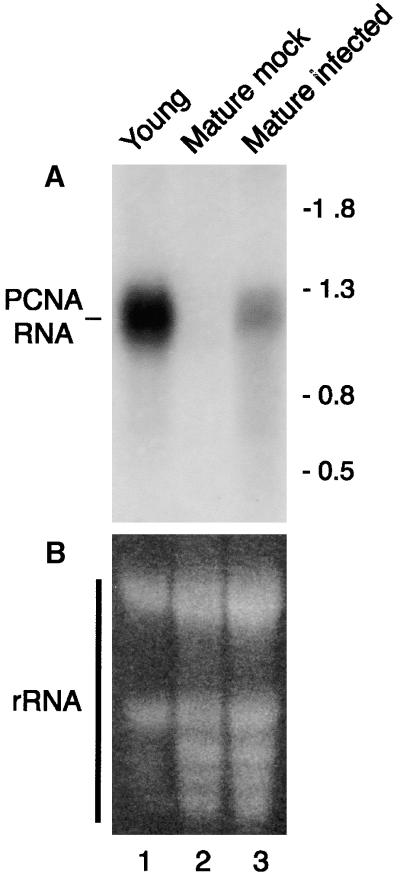
We used the N. benthamiana PCNA cDNA to probe RNA gel blots that compared mRNA levels in healthy versus infected plants. A single PCNA transcript of ∼1200 nucleotides was detected in young leaves of healthy plants (Figure 1A, lane 1). A transcript of the same size was seen in TGMV-infected mature leaves (Figure 1A, lane 3) but not in equivalent mock-infected leaves (Figure 1A, lane 2). These differences were not due to loading because the expected amounts of rRNA were seen by ethidium bromide staining in Figure 1B (one-fourth as much RNA was loaded in lane 1). Similar hybridization signals also were detected for mature leaves from mock-inoculated and infected plants using a cDNA probe for the translation initiation factor eIF4a (see Figure 5B, bottom panels). We typically observed 15- to 25-fold less PCNA mRNA in infected mature leaves than in young leaves. Together, these data established that steady state levels of N. benthamiana PCNA mRNA are regulated by development and geminivirus infection.
Figure 1.
Mature Leaves Infected with TGMV Contain PCNA mRNA.
Total RNA from young (lane 1; 15 μg), mock-inoculated mature (lane 2; 60 μg), and TGMV-infected mature (lane 3; 60 μg, 14 days after inoculation) leaves was resolved on agarose gels and transferred to nylon membranes.
(A) The blots were hybridized with 32P-labeled probe corresponding to N. benthamiana PCNA cDNA. The positions of nucleotide size markers are shown at right.
(B) Ethidium bromide staining of the same blot. The stained bands correspond to rRNA.
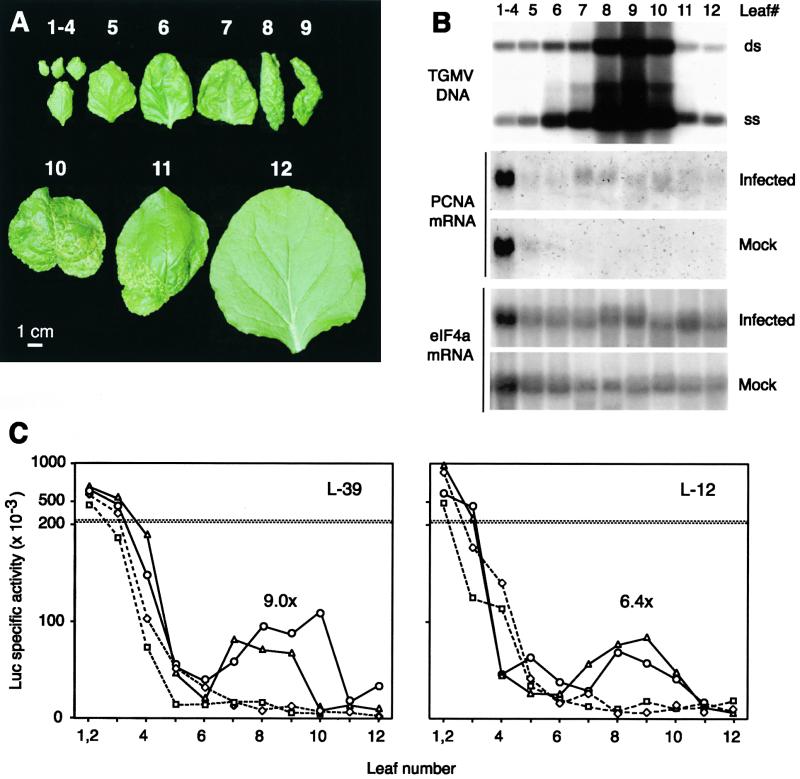
Figure 5.
TGMV Infection and Regulation of the PCNA Promoter.
(A) TGMV symptoms on an N. benthamiana plant 14 days after inoculation. Leaves are numbered consecutively from youngest to oldest.
(B) Accumulation of TGMV DNA and PCNA mRNA in individual leaves of infected and mock-inoculated plants. Top, total DNA (10 μg) was digested with BglII and analyzed on DNA gel blots using a TGMV B probe. The positions of double stranded (ds) and single stranded (ss) viral DNA are marked. Middle, total RNA (30 μg) was analyzed on RNA gel blots using an N. benthamiana PCNA probe. Bottom, the same RNA gel blots were reprobed with a tobacco eIF4a sequence. The position number of each leaf is given above the gels. It was necessary to pool leaves 1 to 4 to obtain sufficient material for analysis. The DNA and RNA samples, which were isolated 14 days after inoculation, were from different plants because of tissue limitations. The positions of the most symptomatic leaves ranged from positions 7 to 10 between individual plants.
(C) Developmental regulation of the PCNA promoter in infected and mock-inoculated transgenic plants 14 days after inoculation. Soluble protein extracts from individual leaves of infected (triangles and circles) and mock-inoculated (diamonds and squares) L-39 (left) and L-12 (right) plants carrying the −633 PCNA:luc transgene were assayed for Luc activity. Profiles from two plants are shown for each treatment. The average infected/mock-inoculated ratios for leaf 9 are given for each line above the corresponding peak.
The PCNA Promoter Is Active in Cycling Cells and Young Leaves
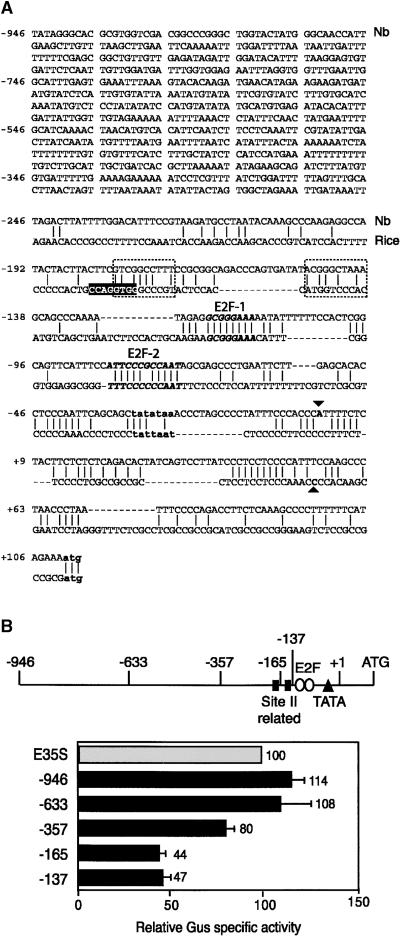
To determine if the PCNA mRNA accumulation patterns reflect changes in transcription, we developed a fully homologous system to study the regulation of the N. benthamiana PCNA promoter. Two PCNA promoter fragments were amplified independently from N. benthamiana chromosomal DNA. Both fragments contained 110 bp of leader sequence and either 946 or 633 bp of 5′ sequence. The overlapping sequences of the two promoter fragments were identical, indicating that they were derived from the same gene. Comparison of the N. benthamiana PCNA promoter sequence with the corresponding rice promoter (Kosugi et al., 1991) showed little conservation upstream of position −181 relative to the transcription start site (data not shown) and limited homology downstream of this site (Figure 2A). Position −181 is located immediately 5′ of an N. benthamiana sequence that shows identity at seven of 10 nucleotides to the site IIa element of the rice PCNA promoter. The N. benthamiana sequence displays less homology (five of 10 nucleotides) with the downstream site IIb element of the rice promoter. The site II elements have been shown to activate the rice promoter in tobacco (Kosugi et al., 1995). A TATA consensus is located 24 bp upstream of the N. benthamiana transcription start site. There also are short stretches of conservation with variable spacing in the proximal promoter and leader regions of the N. benthamiana and rice sequences. Two conserved stretches in the proximal promoter show strong homology with E2F consensus elements. The E2F-1 sequence (TTTCCCGC), which is in inverse orientation, is identical to the E2F consensus (TTTC/GC/GCGC) (Sidle et al., 1996; Herwig and Strauss, 1997). The E2F-2 sequence (ATTCCCGCCAAT) contains an exact match of the central 10 bp of an inverted repeat E2F element (TTTC/GCCGCC/GAAA) (Wade et al., 1995).
Figure 2.
The N. benthamiana PCNA Promoter Is Active in Cycling Cells.
(A) The sequence of the longest N. benthamiana (Nb) PCNA promoter fragment is shown. The transcription start site (▾) was determined by S1 nuclease protection (data not shown). The proximal sequences of the N. benthamiana and rice (Suzuka et al., 1991) PCNA promoters are compared. The TATA boxes and ATG start codons of the two genes are shown in bold lowercase letters. The G-box (white letters), the site II (dotted boxes) elements, and the transcription start site (▴) of the rice promoter (Kosugi et al., 1995) are marked. The regions of the N. benthamiana promoter that show limited homology with site II sequences are included in the dotted boxes. Two sequences that display strong homology with the E2F binding consensus and are conserved between the two promoters are shown in bold italic type.
(B) A linear diagram of the N. benthamiana promoter indicating the site II–related elements, the E2F consensus elements, the TATA box, the transcription start site (+1), and the ATG. The end points of the 5′ deletions are marked. The relative reporter activities supported by the N. benthamiana promoter fragments in transiently transfected BY-2 cells are shown below. For each experiment, the activity of the enhanced Cauliflower mosaic virus 35S (E35S) promoter was set to 100 and the activities of the PCNA:uidA constructs were standardized against it. In these experiments, an average of 722 × 103 light units/μg protein was detected for the E35S:uidA construct, whereas an average of 915 light units/μg protein was detected for a promoterless uidA cassette. Gus, β-glucuronidase. The error bars represent two standard errors.
The N. benthamiana −946 PCNA promoter fragment (designated by the position of its 5′ end relative to the transcription start site marked in Figure 2A) was fused to the β-glucuronidase (GUS) reporter gene (uidA) and analyzed in transient assays using protoplasts from log phase BY-2 cells. The −946 promoter supported high levels of GUS expression and was as active as the Cauliflower mosaic virus 35S promoter with a duplicated enhancer element (Figure 2B). Transient analysis of a −633 PCNA promoter fragment revealed that the 5′ truncation also supported high levels of reporter activity similar to the −946 promoter. Further 5′ deletions to positions −357 and −165 resulted in progressive 20 and 35% reductions in activity, whereas deletion to position −137 had no further effect. The abilities of the −165 and −137 promoters to support ∼45% of full activity were unexpected because these constructs lack one or both of the putative site II elements (Figure 2B). Although both putative E2F elements were maintained in the −165 and −137 promoter constructs, site-directed mutagenesis studies failed to uncover a role for either element during transcription in BY-2 cells (our unpublished results).
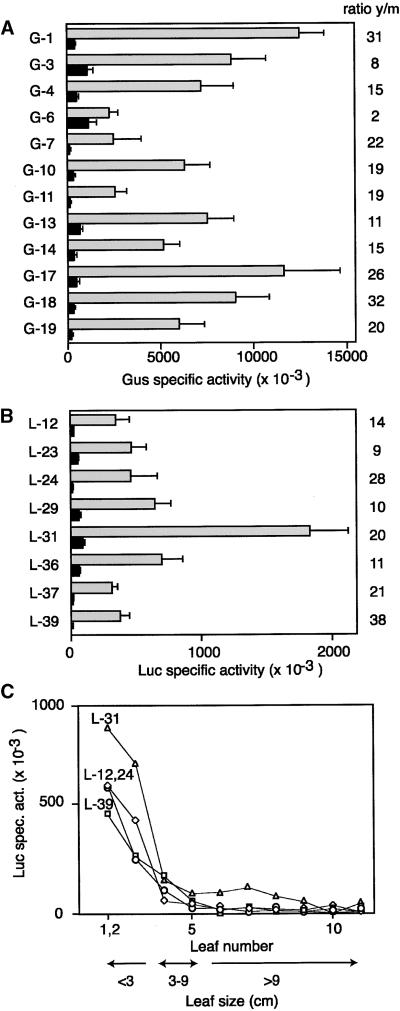
We next generated transgenic N. benthamiana carrying either uidA or the luciferase gene (luc) fused to the −633 PCNA promoter, the shortest fragment with full activity in suspension cells. Reporter activities were measured in young leaves (<2 cm in length) and fully expanded leaves (∼10 cm). To ensure uniformity, young tissue was from leaves 1 to 2 and mature tissue was from leaf 9. (Leaf 1 measured ∼1 cm at the time of harvest, and all other leaves were numbered consecutively.) In Figures 3A and 3B, all of the lines showed higher reporter activity in young than in mature leaves, with the absolute levels varying up to fivefold between lines. The ratio of young/mature activity ranged from two- to 38-fold, with the −633 PCNA:uidA and −633 PCNA:luc lines showing similar average ratios of 17- to 19-fold, indicating that the choice of reporter had no effect on the outcome. The variation in absolute activity and young/mature ratios between the different lines may be indicative of copy number and/or position effects of the transgene. However, in all cases, the N. benthamiana PCNA promoter was more active in young leaves than in mature leaves.
Figure 3.
The N. benthamiana PCNA Promoter Is Regulated Developmentally.
(A) Soluble protein extracts from young (y, gray bars) and mature (m, black bars) leaves of N. benthamiana lines (left) carrying the −633 PCNA:uidA construct were assayed for GUS (Gus) activity. Each set of bars corresponds to the mean activity/mg protein in a minimum of 10 plants. The ratio of reporter activity in young versus mature leaves (y/m) is shown at right for each line.
(B) Same as in (A) except that the N. benthamiana lines were transformed with the −633 PCNA:luc construct and assayed for Luc activity.
(C) Soluble protein extracts from individual leaves of plants representing four −633 PCNA:luc lines were assayed for Luc activity. The Luc specific activity was plotted against leaf number. The sizes of the corresponding leaves are indicated below.
The error bars indicate two standard errors.
Developmental regulation was characterized further by measuring reporter activity in individual leaves of four −633 PCNA:luc lines (Figure 3C). In all four lines, Luc activity was highest in the upper leaves (leaves 1 and 2). Expanding middle leaves (leaves 3 and 4) showed progressive loss of reporter activity, whereas lower leaves (leaves 5 to 11) had very little activity. Analysis of endogenous PCNA mRNA revealed a similar expression pattern, with high levels in young leaves, low levels in expanding leaves, and undetectable levels in mature leaves (see Figure 5B). Together, these results established that the N. benthamiana PCNA promoter is active in cultured cells and is regulated developmentally in plants.
Geminivirus Infection Activates the PCNA Promoter in Mature Leaves
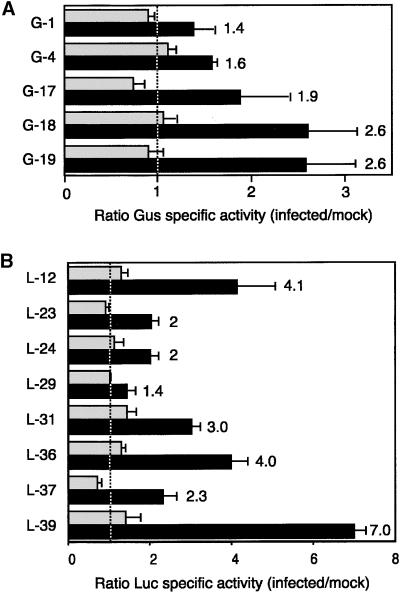
To assess the impact of TGMV infection on PCNA promoter function, we compared reporter activities in young and mature leaves of infected and mock-inoculated plants. Plants carrying the −633 PCNA:uidA or the −633 PCNA:luc fusion were inoculated with TGMV by bombardment or agroinoculation, respectively. Bombarded plants typically developed symptoms between 4 and 5 days after inoculation, whereas agroinoculated plants displayed symptoms by 5 to 6 days after inoculation. Leaves 1 to 2 (young) and leaf 9 (mature) were harvested at 14 days after inoculation, and the ratios of GUS (Figure 4A) or Luc (Figure 4B) activities for infected/mock-inoculated leaves were determined. The ratios for young leaves scattered around a value of 1 (Figure 4, dotted line), with some lines showing ratios <1 and others showing ratios slightly >1. In contrast, the ratios for mature leaves ranged from 1.4 to 7 and were always greater than those detected for young leaves of the same line. These data demonstrate that TGMV infection activates the N. benthamiana PCNA promoter in mature leaves but has no detectable effect in young leaves. However, in all cases, the absolute activity of the PCNA promoter was lower in mature than in young leaves (data not shown). This result is consistent with the lower levels of steady state PCNA mRNA in infected mature leaves than in young leaves (Figure 1).
Figure 4.
The PCNA Promoter Is Activated in Mature Leaves of TGMV-Infected Plants.
(A) Soluble protein extracts were isolated 14 days after inoculation from N. benthamiana lines (left) carrying the −633 PCNA:uidA construct and assayed for GUS (Gus) activity. The activity ratios for infected versus mock-inoculated plants are shown for young (gray bars) and mature (black bars) leaves. Each set of bars represents the mean ratios from three independent experiments. The ratio values for mature leaves are given at right. The dotted line corresponds to a ratio of 1.
(B) Same as in (A) except that the N. benthamiana lines were transformed with the −633 PCNA:luc construct and ratios of Luc activity are shown.
The error bars indicate two standard errors.
To better characterize the relationship between geminivirus and developmental regulation of the N. benthamiana PCNA promoter, we monitored symptoms, TGMV DNA accumulation, reporter activity, and endogenous PCNA mRNA levels in individual leaves of −633 PCNA:luc plants (Figure 5). For the plant depicted in Figure 5A, TGMV symptoms, which include chlorosis, leaf curling, and stunting, were most severe for leaves 8 to 10. The oldest leaves, which were fully developed at the time of agroinoculation, showed milder symptoms (leaf 11) or were free of symptoms (leaf 12). Younger leaves (leaves 1 to 7) also displayed milder symptoms. DNA gel blot analysis of TGMV B DNA showed a similar pattern, with viral DNA highest in leaves 8 to 10 (Figure 5B, top) and lower in older (leaves 11 and 12) and younger (leaves 1 to 7) leaves.
PCNA promoter activity and endogenous PCNA mRNA levels reflected the composite effect of TGMV infection and development. As shown for lines L-39 (Figure 5C, left) and L-12 (Figure 5C, right), Luc reporter activity was highest in young leaves (leaves 1 to 3) and decreased in expanding leaves (leaves 4 and 5) in both infected and mock-inoculated plants. A second, lower peak of Luc activity was detected in the most symptomatic leaves (Figure 5C, leaves 7 to 9 or leaves 7 to 10) of the infected plants. In contrast, Luc activity remained low in older leaves of the mock-inoculated plants. A similar pattern was seen for endogenous PCNA mRNA, with high levels in young leaves (leaves 1 to 4) and decreasing levels in expanding leaves (leaves 5 and 6) of both infected and mock-inoculated plants (Figure 5B, middle). In infected plants, PCNA mRNA steady state levels increased in lower leaves, with the highest amount in the most symptomatic leaves (Figure 5B, leaves 7 to 10), whereas PCNA mRNA could not be detected in mature leaves (leaves 7 to 12) of mock-inoculated plants. Together, these data demonstrate a quantitative relationship between the severity of TGMV infection, the induction of the PCNA promoter, and the accumulation of PCNA mRNA in mature leaves.
The PCNA Promoter Is Repressed through an E2F Element in Mature Leaves
Two E2F consensus sequences, which may play roles in developmental regulation and geminivirus activation, are located in the proximal promoter region of the N. benthamiana PCNA promoter. Because the E2F-2 sequence is homologous with an inverted repeat element that binds to E2F with very high affinity in animals (Wade et al., 1995), our initial studies have focused on this element. We used electrophoretic mobility shift assays to determine if the E2F-2 sequence is bound specifically by a nuclear protein(s) in vitro. A radiolabeled, double-stranded DNA (5′-gatcTCCATTCC-CGCCAATAGgatc-3′) containing 16 bp of PCNA promoter sequence (uppercase letters) with the E2F-2 motif (underlined) and 8 bp of unrelated flanking DNA (lowercase letters) was incubated with a nuclear protein extract from N. benthamiana suspension cells, and the resulting complexes were resolved by PAGE. We observed a prominent band (Figure 6A, lane 2, arrow) that was not seen in the presence of excess unlabeled E2F-2 competitor DNA (Figure 6A, lanes 3 to 5). The band was not competed by a DNA (5′-gatcTCCATTCttatCAATAGgatc-3′) altered in the central core of the E2F-2 element (Figure 6A, lanes 6 to 8) or by a DNA (5′-gatcATCCTCTCACGTACGACgatc-3′) containing a randomized sequence of equivalent nucleotide content (Figure 6A, lanes 9 to 11). In contrast, the slower migrating bands seen with the E2F-2 probe and nuclear protein extract (Figure 6A, lane 2) were competed by all three DNAs (Figure 6A, lanes 3 to 11). Together, these results demonstrated that the prominent band corresponds to a specific E2F-2 complex, whereas the slower migrating bands reflect nonspecific binding.
Figure 6.
Mutation of the E2F-2 Element Alters the Developmental Regulation and TGMV Activation of the N. benthamiana PCNA Promoter.
(A) Electrophoretic mobility shift assays with 32P-labeled E2F-2 DNA alone (lane 1) or incubated with N. benthamiana nuclear protein extract (lanes 2 to 11). Lane 2 had no added competitor DNA. Lanes 3 to 5 contained unlabeled E2F-2 DNA (wt). Lanes 6 to 8 contained unlabeled mutant E2F-2 DNA (mt). Lanes 9 to 11 contained a random control DNA (c). Competitor DNAs were used at 10-, 100-, or 250-fold molar excess relative to probe, as indicated by the triangles at the top of the gel. The shifted product corresponding to the specific E2F-2/protein complex is marked by the arrow.
(B) The ratios of Luc activities in young versus mature leaves of N. benthamiana lines (left) carrying the mutant E2F-2 −633 PCNA:luc construct are shown. The dotted line (mt) corresponds to the mean ratio for plants transformed with the mutant construct, whereas the solid line (wt) correlates with the mean ratio for plants transformed with the wild-type −633 PCNA:luc construct (Figure 3B). The error bars indicate two standard errors.
(C) Soluble protein extracts from N. benthamiana lines carrying the mutant E2F-2 −633 PCNA:luc construct were assayed for Luc activity and compared with lines carrying the wild-type −633 PCNA:luc construct shown in Figure 3B. The Luc-specific activity values from young (y) and mature (m) leaves of the mutant (mt) and wild-type (wt) constructs are compared in the scattergram. The eight independent lines given in (B) are shown for each treatment. The symbols indicate different lines.
(D) P values of t test comparisons of the data shown in (C). Statistically significant values (P < 0.05) are shown in boldface type.
(E) Developmental regulation of the mutant E2F-2 PCNA promoter in untreated, mock-inoculated, and infected plants (14 days after inoculation). Soluble protein extracts from individual leaves of untreated (crosses), mock-inoculated (open circles), and infected (closed circles) mt-16 (left) and mt-35 (right) plants carrying the mutant E2F-2 −633 PCNA:luc transgene were assayed for Luc activity. Each point represents the average of two plants. (Note the different scales relative to Figure 5C.) A gradual decrease in PCNA activity with leaf number and little effect of TGMV infection also were seen for line mt-29 (our unpublished data).
We next generated transgenic N. benthamiana carrying a −633 PCNA:luc construct with the 4-bp E2F-2 mutation that prevented competition (Figure 6A, lanes 6 to 8). Figure 6B shows Luc activity ratios in young versus mature leaves, which ranged from 1.2 to 5.2, for eight independent lines. The mutant promoter ratios were significantly lower than those calculated for plants carrying the wild-type −633 PCNA:luc construct (Figure 3B), with mean values of 3 and 19, respectively. These results established that mutation of the E2F-2 element alters developmental regulation of the PCNA promoter. Luc-specific activities in young and mature leaves of plants transformed with the wild-type and mutant promoter constructs were compared to determine how promoter function is altered during development (Figures 6C and 6D). The wild-type and mutant promoters displayed similar activities in young leaves, but the mutant promoter was significantly more active than was the wild-type promoter in mature leaves. Because of the variability between lines, it was not possible to determine from the data presented in Figures 6C and 6D whether mutation of the E2F-2 sequence is sufficient to fully activate PCNA promoter expression in mature leaves. To address this question, we monitored Luc-specific activity in individual healthy leaves of two lines, mt-16 and mt-35, both of which were transformed with the mutant E2F-2 −633:luc construct. Both lines showed a gradual decrease in PCNA promoter activity during development (Figure 6E, crosses), unlike the precipitous decrease seen in plants carrying the wild-type promoter (Figure 3C). Together, our data establish that the E2F-2 sequence acts as a negative regulatory element during development but is not solely responsible for the decrease in PCNA promoter activity in mature tissues.
Geminivirus Infection Does Not Alter the Activity of the Mutant E2F-2 Promoter
In animals, E2F acts as a transcriptional repressor by recruiting pRB and histone deacetylase to promoters (Luo et al., 1998). Mammalian DNA viruses can activate host gene expression by binding to pRB and disrupting its interaction with E2F (Weinberg, 1995). Because TGMV AL1 and AL3 both bind to the plant pRB homolog pRBR (Ach et al., 1997; Settlage et al., 2000), we sought to determine if the activity of mutant E2F-2 PCNA promoter is influenced by geminivirus infection. In Figure 6E, the developmental profiles of TGMV-infected (closed circles), mock-inoculated (open circles), and untreated (crosses) plants are shown for lines mt-16 and mt-35. For both lines, there was no detectable difference in promoter activity in mature leaves with the three treatments. This result is consistent with the two- to fivefold greater activity detected for the mutant PCNA promoter in mature leaves than for the wild-type promoter in equivalent infected leaves (cf. Figures 5C and 6E) and suggests that TGMV does not further activate the mutant E2F-2 PCNA promoter during infection.
DISCUSSION
Eukaryotic viruses with small DNA genomes replicate in nuclei using the replication machinery of their hosts. Because of their dependence on host enzymes, these viruses amplify only in cells that contain the necessary DNA polymerases and accessory factors, which generally are restricted to cycling cells. However, many small DNA viruses can overcome this constraint by inducing the synthesis of replication enzymes in terminally differentiated cells (Cheng et al., 1995). In animals, DNA tumor viruses alter replication competence by modulating host gene transcription. They activate the transcription of genes encoding cell cycle regulators and DNA replication enzymes, which in turn facilitate return to the cell cycle and progression to S phase (Ohashi et al., 1992; Ogris et al., 1993; Chen et al., 1996; Tiainen et al., 1996). In a previous study, we demonstrated that TGMV induces PCNA in mature plant cells (Nagar et al., 1995). Here, we show that TGMV infection induces the accumulation of PCNA mRNA by promoter activation. We also provide evidence that E2F transcription factors negatively regulate plant gene expression during development and that geminivirus infection may overcome E2F-mediated repression to induce PCNA expression in mature leaves. Thus, like their animal counterparts, geminiviruses alter developmental controls to activate the transcription of host genes whose products are required for viral DNA replication.
To better understand how PCNA expression is controlled in healthy and geminivirus-infected plants, we developed a fully homologous plant system for studying PCNA mRNA accumulation and promoter function. For this system, we amplified and sequenced a series of PCNA cDNA and promoter clones from N. benthamiana. The 5′ and 3′ sequences of the N. benthamiana cDNA as well as the promoter region were represented by two independent clones of identical sequence. The sequence data, combined with the detection of only one PCNA transcript on RNA gel blots and by S1 nuclease protection assays, suggested that N. benthamiana encodes a single PCNA gene. This conclusion was supported by genomic DNA hybridization experiments using probes corresponding to the 5′ and 3′ ends and the center of the N. benthamiana cDNA (data not shown). However, without intron sequence and position information, we cannot unequivocally rule out the existence of a second PCNA gene in N. benthamiana. Two PCNA genes have been identified for carrot and maize (Hata et al., 1992; Lopez et al., 1997) that display similar expression profiles (Lopez et al., 1997). As a consequence, if N. benthamiana encodes two PCNA genes, then they are likely to be regulated in parallel.
PCNA plays key roles in DNA replication, DNA repair, and cell cycle control (reviewed in Prosperi, 1997). In animals, PCNA expression is regulated tightly at the level of transcription during development and is altered by DNA virus infection (Cheng et al., 1995; Lee et al., 1995; Yamaguchi et al., 1995b). RNA gel blot analysis showed that, as in other plant species (Kosugi et al., 1991; Citterio et al., 1992; Markley et al., 1993; Lopez et al., 1997), PCNA mRNA levels in N. benthamiana are regulated developmentally. PCNA mRNA is abundant in young leaves, present in trace amounts in expanding leaves, and undetectable in fully expanded leaves. This pattern parallels the distribution of cells undergoing DNA replication during leaf development and is consistent with studies showing that PCNA mRNA is high in cycling plant cells (Kodama et al., 1991; Sekine et al., 1999). PCNA mRNA also was detected in mature leaves of TGMV-infected plants. PCNA mRNA levels were increased in leaves with the most viral DNA, indicating a relationship between PCNA accumulation—and presumably other components of the host DNA replication apparatus—and efficient viral replication in mature leaves. However, TGMV DNA levels were low in young leaves, which have the most PCNA mRNA, demonstrating that other factors in addition to replication competence influence geminivirus infection. This conclusion is supported by in situ results showing that TGMV does not accumulate in meristematic cells even though they contain plant DNA replication machinery (Nagar et al., 1995).
There is a strong correlation between PCNA mRNA levels and PCNA promoter function in healthy and geminivirus-infected plants. Analysis of transgenic N. benthamiana plants carrying the homologous PCNA promoter fused to either the uidA or luc reporter gene established that PCNA mRNA accumulation is regulated primarily at the level of transcription. Like PCNA mRNA, promoter activity is high in young leaves, decreases rapidly in expanding leaves, and is very low in mature leaves of healthy plants. The PCNA promoter also is active in mature leaves of TGMV-infected plants, with the most symptomatic leaves displaying the greatest activity. Interestingly, PCNA promoter activity does not appear to be induced in expanding leaves or the oldest leaves of infected plants, both of which contain low but detectable levels of viral DNA. The limited viral replication in expanding leaves may depend on residual replication machinery produced earlier in development, whereas viral replication in the oldest leaves may reflect the activity of preexisting DNA repair enzymes. Alternately, PCNA promoter induction may be below the limit of detection if fewer cells are infected in these leaves, or, in the case of older leaves, it may have occurred at an earlier time during the infection process. Earlier studies showed that ∼10% of the cells of a highly symptomatic leaf contain TGMV DNA, the viral replication proteins AL1 and AL3, and the PCNA protein (Nagar et al., 1995; Bass et al., 2000). This observation may account for the 15- to 25-fold difference in activity between young and infected mature leaves. However, PCNA expression is regulated with respect to the cell cycle such that not all cells in asynchronous young tissue express PCNA (Kodama et al., 1991; Sekine et al., 1999). Thus, it is likely that the difference in PCNA promoter activity in young versus highly infected mature leaves reflects both the number of cells actively transcribing the gene and the level of transcription in a given cell. The same may be true for the less symptomatic flanking leaves.
Although the N. benthamiana promoter shows weak homology with the rice PCNA promoter, the only other plant PCNA promoter that has been examined to date, several observations suggest that the two promoters are regulated differently. First, the rice PCNA promoter contains a G-box element that is required for full activity of a truncated −262 promoter (Kosugi et al., 1995). No G-box motif is found in the N. benthamiana promoter. Second, the rice promoter includes two sequences, sites IIa and IIb, that resemble auxin response elements (Kosugi et al., 1995). The N. benthamiana promoter contains sequences that show weak homology with these elements but diverges at positions that are essential for rice promoter activity and binding to the basic helix-loop-helix transcription factors PCF1 and PCF2 (Kosugi et al., 1995; Kosugi and Ohashi, 1997). Third, deletion or mutation of both site II elements inactivates the rice PCNA promoter in transgenic tobacco (Kosugi et al., 1991, 1995). Our data do not eliminate the possibility that the site II–like sequences in the N. benthamiana PCNA promoter contribute to its activity. However, the −137 PCNA:uidA reporter, which does not contain any site II–like sequences, retained 45% of full-length promoter activity. Thus, unlike in rice, these putative elements are not required for N. benthamiana PCNA promoter function.
Unlike the G-box and site II elements, the E2F-1 and E2F-2 sequences identified in Figure 2A are highly conserved in the N. benthamiana and rice PCNA promoters. An E2F-1–type element also is present in the proximal promoter region of the Arabidopsis PCNA gene (our unpublished observation), and E2F elements have been identified in Drosophila, human, and murine PCNA genes (Yamaguchi et al., 1995a; Huang and Prystowsky, 1996; Tommasi and Pfeifer, 1999), suggesting that these elements may be general features of eukaryotic PCNA genes. Analysis of transgenic plants carrying a mutation in the E2F-2 sequence of the N. benthamiana PCNA promoter demonstrated that it functions as a negative regulatory element to repress PCNA transcription in mature leaves. E2F also represses expression from the Drosophila PCNA promoter (Sawado et al., 1998). However, PCNA promoter function is complex, involving a variety of transcription factors (Yamaguchi et al., 1996; Liu et al., 1998; Hayashi et al., 1999), and E2F can activate as well as repress its activity (Sawado et al., 1998; Tommasi and Pfeifer, 1999). This complexity may explain our observation that the E2F-2 sequence acts as a negative regulatory element during development but is not solely responsible for the decrease in N. benthamiana PCNA promoter activity in mature tissues. One possibility is that the E2F-2 element does not act alone to repress PCNA promoter activity in mature tissues. A candidate for a second negative regulatory element is the E2F-1 sequence, which is under analysis. Alternately, relevant transcriptional activators may decrease during development or be replaced by less effective activators, resulting in a gradual decrease in PCNA promoter activity in the absence of active repression.
The E2F transcription factor family modulates the activities of many genes during cell division and development in animals (Dyson, 1998; Lavia and Jansen-Durr, 1999). E2F cDNAs have been reported for wheat, tobacco, and carrot (Ramirez-Parra et al., 1999; Sekine et al., 1999; Albani et al., 2000). Genomic sequence data established that Arabidopsis encodes six E2F homologs. Recently, DP1, the E2F dimerization partner, was characterized from Arabidopsis and wheat (Magyar et al., 2000; Ramirez-Parra and Gutierrez, 2000). However, little is known about the targets or mechanisms of E2F-mediated regulation of plant gene transcription. E2F has been shown to activate chimeric promoters containing E2F elements in cultured plant cells (Sekine et al., 1999; Albani et al., 2000). Recent studies of the tobacco ribonucleotide reductase small subunit gene (RNR2) indicate that its activity is regulated by E2F elements in BY-2 cells (Chaboute et al., 2000). Like the N. benthamiana PCNA promoter, the tobacco RNR2 promoter contains multiple E2F elements, two of which activate transcription and one that represses transcription outside of S phase. At present, there is no report of these elements also regulating RNR2 transcription during plant development.
TGMV may modulate PCNA promoter function by altering cell cycle controls and relieving E2F-mediated repression of PCNA transcription in differentiated cells. Three lines of evidence support this hypothesis. First, TGMV infection increases the activity of the wild-type but not the mutant E2F-2 promoter in mature leaves. Second, immunoblot analysis showed that the E2F protein is ubiquitous in carrot (Albani et al., 2000). We also detected high levels of E2F protein in mature leaves of N. benthamiana plants (our unpublished data), consistent with its negative regulation of PCNA transcription. Third, TGMV AL1 binds to the host factor, pRBR, and this interaction influences the pattern of PCNA protein accumulation during infection (Ach et al., 1997; Kong et al., 2000). Although pRBR is not well characterized in plants (de Jager and Murray, 1999), it is homologous with the animal pocket proteins pRB, p107, and p130, which repress the transcription of genes required for S phase by binding to E2F (Herwig and Strauss, 1997). In animals, DNA tumor viruses induce host gene transcription by binding to pocket proteins and disrupting their interactions with E2F (Weinberg, 1995). By analogy, AL1 may disrupt pRBR/E2F complexes that repress PCNA promoter function to induce transcription in mature leaves. Future experiments that assess the ability of wild-type AL1 and pRBR binding mutants (Kong et al., 2000) to activate the N. benthamiana PCNA promoter in mature leaves will provide insight into the roles of AL1/pRBR and pRBR/E2F interactions during geminivirus-mediated induction of host transcription and into the mechanisms that control gene expression during plant development.
METHODS
The Nicotiana benthamiana PCNA cDNA and Promoter
Total RNA was isolated from 4-week-old N. benthamiana seedlings (Hanley-Bowdoin et al., 1989) and used as a template in reverse transcription–polymerase chain reaction (RT-PCR) containing the degenerate primers 5′-GGITTYGARCAYTAYMGITGYGA-3′ and 5′-CCC-ATYTGNGCDATYTTRTAYTC-3′, which were based on carrot (GenBank accession numbers Q00268 and Q00265), rice (AAA33913), and rape (AAB27811) proliferating cell nuclear antigen (PCNA) cDNA sequences. RT-PCR was performed using the Gene-Amp RNA-PCR kit (Perkin-Elmer, Foster City, CA) using seedling RNA as a template. The sequence of the amplified product was used to design primers for subsequent rapid amplification of cDNA ends–PCR.
The 5′ end of the N. benthamiana PCNA cDNA was amplified using a 5′ rapid amplification of cDNA ends system from Gibco BRL (Grand Island, NY). The nested PCNA-specific primers were primer 1 (5′-AATCAGCAATCTTGTCTTGGGTGGGGC-3′) and primer 2 (5′-CCATGTTACCAAGGTTCATCCCCATTG-3′). Reverse transcription was performed with SuperScriptII reverse transcriptase (200 units; Gibco BRL) using primer 1 and 1 μg of N. benthamiana seedling RNA. The resulting cDNA was treated with RNase, isolated using a GlassMax spin cartridge (Gibco BRL), and extended with dCTP using terminal deoxynucleotidyl transferase. PCR was performed using the abridged universal anchor primer (Gibco BRL) and 400 nM of PCNA primer 2, and the resulting PCR product was purified and reamplified. The 3′ end of the N. benthamiana PCNA cDNA was isolated by the same procedure using the adapter primer 5′-GACTCGAGT-CGACATCGATTTTTTTTTTTTTTTTT-3′ (Genosys, The Woodlands, TX) and PCNA-specific primer 3 (5′-CAGTAATAGAGATGAATGAGCCAGTAT-3′).
The N. benthamiana PCNA promoter was amplified using the Universal Genome Walker Kit (Clontech, Palo Alto, CA) and nested PCNA-specific primers 4 (5′-CTTCCCTGAACAAGCCGTAATTCCAAC-3′) and 5 (5′-CTATGAAAAAGGGGTTTGAGAAGGTC-3′) derived from the sequence of the 5′ rapid amplification of cDNA ends product. Genomic DNA (Dellaporta et al., 1983) from young leaf tissue of 5-week-old N. benthamiana seedlings was dissolved in 1 M NaCl, purified using a Tip 500 column (Qiagen, Valencia, CA), and digested separately with EcoRV and ScaI. The fragments were purified and ligated to Clontech Universal Genome Walker Kit adapter DNA. The resulting products were amplified according to the manufacturer's instructions first using the kit AP1 primer and PCNA primer 4 and second using the kit AP2 primer and PCNA primer 5.
The various PCR products were treated with Klenow, cloned into the SmaI site of pUC119, and sequenced. β-Glucuronidase (GUS) reporter constructs containing the PCNA promoter were generated using pMON8677, which contains a Cauliflower mosaic virus E35S:uidA:rbcS E9 cassette (Coruzzi et al., 1984; Jefferson et al., 1987; Kay et al., 1987). The PCNA promoter fragments were isolated with BamHI and trimmed SacI ends and cloned in place of the E35S promoter of pMON8677 (BglII and trimmed PstI ends) to give −633 PCNA:uidA (pNSB713) and −946 PCNA:uidA (pNSB716). pNSB716 was digested with AccI and BglII, treated with Klenow, and religated to give pNSB717, the −357 PCNA:uidA construct. pNSB716 also was digested with SacII and religated to give pNSB748, the −165 PCNA:uidA construct. The −137 PCNA promoter fragment was generated by PCR using pNSB716 as a template and the primers 5′-CGTTGACTGCCTCTTCGCTG-3′ and 5′-CAGCCCAAAATAGAGGCGGG-3′. The product was treated with Klenow, digested with BstBI, and cloned into pMON8677 with BstBI and trimmed PstI ends to give pNSB1021, the −137 PCNA:uidA construct. A luciferase (Luc) reporter construct was generated by cloning the −633 promoter fragment with BamHI and trimmed SacI ends into pMON8796 (Eagle et al., 1994) with BglII and trimmed PstI ends to give pNSB931.
The E2F-2 sequence in the −633 PCNA promoter fragment was mutated by a two-step overlap extension PCR process using the universal and reverse primers (New England Biolabs, Beverly, MA) and two promoter-specific oligonucleotides, E2F-2 1 (5′-GGCTCGCTA-TTGataaGAATGGAAATGAAC-3′) and E2F-2 2 (5′-GTTCATTTCCATTCttatCAATAGCGAGCC-3′). The mutation is represented by lowercase letters. The first set of reactions used pNSB712 as a template and either E2F-2 1 and the universal primer or E2F-2 2 and the reverse primer. The resulting products were gel purified, mixed, and reamplified using the universal and reverse primers. The final product was gel purified, repaired with Klenow, cloned into pUC119 to give pNSB926, and sequenced. The mutated promoter was fused to the Luc reporter gene in pMON8796 as described above for the wild-type promoter to produce pNSB942.
Transgenic Plants
Plant transformation vectors were made by cloning NotI fragments containing the expression cassettes of pNSB713, pNSB931, and pNSB942 into pMON721 (Lanahan et al., 1994) digested with NotI. The resulting vectors contained the −633 PCNA:uidA (pNSB714), the −633 PCNA:luc (pNSB935), and the mutant E2F-2 −633 PCNA:luc (pNSB937) cassettes, respectively. pNSB714, pNSB935, and pNSB937 were introduced by direct DNA transformation (Holsters et al., 1978) into Agrobacterium tumefaciens ABI, which contains a chromosomal copy of pMP90RK (Zannis-Hadjopoulos et al., 1988). N. benthamiana seedlings grown in vitro were cocultivated with the Agrobacterium strains, and independent transformants were selected on kanamycin (300 μg/mL) and regenerated using standard procedures (Horsch et al., 1985).
Geminivirus Infection
Wild-type or transgenic N. benthamiana plants at the six-leaf stage were infected by either bombardment or agroinoculation. For bombardment, 5 μg each of tomato golden mosaic virus (TGMV) A (pMON1565) and B (pTG1.4B) DNAs cloned as partial tandem copies into pUC (Fontes et al., 1994; Orozco and Hanley-Bowdoin, 1996) were precipitated onto gold particles and inoculated as described previously (Nagar et al., 1995). For agroinoculation, overnight cultures of Agrobacterium carrying partial tandem copies of TGMV A (pMON337) or B (pMON393) in their T-DNAs (Elmer et al., 1988b) were mixed 1:1 and syringe inoculated onto the stem immediately below the apex. Infected and mock-inoculated tissues were harvested ∼2 weeks after infection using leaves 1 to 2 as young tissue and leaf 9 as mature tissue. For viral DNA analysis, total DNA was isolated from the indicated leaves at 14 days after agroinoculation (Dellaporta et al., 1983), digested with BglII, and analyzed on DNA gel blots using a TGMV B–specific probe (Fontes et al., 1994).
RNA Analysis
Total RNA was isolated from leaf tissue, resolved on 1% agarose-formaldehyde-Tris-borate/EDTA gels containing 0.4 μg/mL ethidium bromide, and transferred to Genescreen nylon membranes (DuPont–New England Nuclear, Boston, MA), as described previously (Hanley-Bowdoin et al., 1988). Blots were hybridized in 50% formamide, 5 × Denhardt's solution (1 × Denhardt's solution is 0.02% Ficoll, 0.02% polyvinylpyrrolidone, and 0.02% BSA), 1% SDS, 5 × SSPE (1 × SSPE is 0.115 M NaCl, 10 mM sodium phosphate, and 1 mM EDTA, pH 7.4), 100 μg/mL tRNA (Sigma, St. Louis, MO), and 50 μg/mL poly(A) RNA (Sigma). A 523-bp fragment from the middle of the N. benthamiana PCNA cDNA was colabeled with α-32P-dATP and α-32P-dCTP using a random-primed labeling kit (New England Biolabs) and hybridized at 106 to 3 × 106 cpm/mL. After hybridization overnight at 42°C, the membranes were washed at room temperature using the following sequence: two 30-min washes and one 60-min wash in 1 × SSPE and 0.1% SDS followed by one 30-min wash and one 60-min wash in 0.3 × SSPE and 0.1% SDS. The blots were reprobed with a 1372-bp fragment of the translation factor eIF4a from tobacco (Mandel et al., 1995) as a control for loading and transfer. Ethidium bromide staining also was used to monitor loading and transfer. RNA hybridization signals were quantified by phosphorimager analysis.
Reporter Assays
For transient assays, BY-2 protoplasts (5 × 106) were electroporated in the presence of 10 μg of expression cassette, as described previously (Eagle et al., 1994). Total soluble protein extracts were isolated ∼40 hr after transfection, and reporter activity was measured using a GUS-Light Kit (Tropix, Bedford, MA). The assays were standardized against the activity resulting from pMON8677, which contains a GUS expression cassette under the control of the Cauliflower mosaic virus E35S promoter with a duplicated enhancer. The activity of each PCNA promoter:uidA cassette was measured in triplicate in at least three independent experiments.
For plant assays, tissue (250 mg) was harvested from the indicated leaves, ground in liquid nitrogen, and mixed with 250 μL of GUS-Light Kit lysis buffer (Tropix). Extracts were centrifuged at 16,000g for 5 min, and the supernatants were recovered and monitored for reporter activity using a Monolight 3010 luminometer (Pharmingen, San Diego, CA). Reporter assays were standardized for total protein, which was measured by Bradford assays (Bio-Rad, Hercules, CA). Leaf tissue was harvested similarly for luciferase assays, which were performed as described previously (Eagle et al., 1994). Reporter activity in healthy, mock-inoculated, and infected leaves was assessed for a minimum of 10 plants for each line.
Nuclear Protein Extracts and Electrophoretic Mobility Shift Assays
Protoplasts were prepared from N. benthamiana suspension cells as described previously (Eagle et al., 1994), except that digestion was for 130 min. Nuclear protein extracts were prepared using a modified protocol based on that of Albani et al. (2000). The protoplasts (∼5 × 107) were suspended in 15 mL of resuspension buffer (0.4 M sucrose, 25 mM Tris-HCl, pH 7.6, 10 mM MgCl2, 0.3% Triton X-100, 5 mM β-mercaptoethanol, and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]) and disrupted by 12 strokes with a Dounce homogenizer. The homogenate was filtered through two layers of nylon mesh and centrifuged at 3000g for 5 min. The pellet was suspended in 5 mL of resuspension buffer, centrifuged at 3000g for 15 min, and suspended in 5 mL of wash buffer (0.4 M sucrose, 50 mM Tris-HCl, pH 7.6, 5 mM MgCl2, 20% glycerol, 5 mM β-mercaptoethanol, and 0.5 mM PMSF). The nuclei were then centrifuged at 3000g for 20 min and suspended in 1 mL of lysis buffer (25 mM Hepes, pH 7.6, 40 mM KCl, 0.5 mM EDTA, 5 mM MgCl2, 20% glycerol, 2 mM DTT, 1 mg/mL antipain, and 1 mg/mL leupeptin). After addition of 100 μL of saturated ammonium sulfate, the mixture was rocked for 30 min at 4°C. The extract was centrifuged at 200,000g in a Sorvall (Newtown, CT) 70.1 Ti rotor for 1 hr. The supernatant was recovered, precipitated by the addition of 600 μL of saturated ammonium sulfate and rocking for 1 hr at 4°C, and recentrifuged for 1 hr at 200,000g . The pellet was suspended in 800 μL of dialysis buffer (25 mM Hepes, pH 7.6, 40 mM KCl, 0.5 mM EDTA, 1 mM MgCl2, 20% glycerol, 2 mM DTT, and 0.5 mM PMSF) and dialyzed for 3 hr against 1 liter of the same buffer. The extract was divided into aliquots, frozen in liquid N2, and stored at −80°C until use.
Oligonucleotides corresponding to the wild-type E2F-2 sequence (top, 5′-gatcTCCATTCCCGCCAATAG-3′; bottom, 5′-gatcCTATT-GGCGGGAATGGA-3′), a mutated E2F-2 sequence (top, 5′-gatcTCCATTCttatCAATAG-3′; bottom, 5′-gatcCTATTGataaGAATGGA-3′), and an unrelated control sequence (top, 5′-gatcATCCTCTCACGTACGAC-3′; bottom, 5′-gatcGTCGTACGTGAGAGGAT-3′) were synthesized commercially. The lowercase letters represent 5′ overhangs or mutations in the E2F consensus. Probe for electrophoretic mobility shift assays was prepared by mixing 0.5 pmol of the wild-type E2F-2 oligonucleotides, which were then annealed and repaired using Klenow in the presence of 100 μCi of α-32P-dATP. For binding assays, 5 μL of nuclear protein extract (1.9 μg) was incubated with probe DNA (105 cpm, corresponding to ∼7 fmol) in a 20-μL reaction containing 100 ng of poly(dI-dC) and 4 μL of 5 × buffer (125 mM Hepes, pH 7.5, 0.5 M KCl, 5 mM EDTA, 5 mM MgCl2, 25% glycerol, and 50 mM DTT). The reactions were incubated at room temperature for 30 min and resolved on 4% polyacrylamide gels in 0.5 × TBE (1 × TBE is 0.9 M Tris-borate and 2 mM EDTA) for 45 min at 15 mA. For competition experiments, 10-, 100-, or 250-fold molar excess of annealed, unlabeled oligonucleotides was incubated with the reactions for 30 min on ice before probe addition.
Acknowledgments
We thank Ling-Jie Kong and Dr. Cindy Hemenway for critical reading of the manuscript. This research was supported by National Research Initiative Competitive Grants Program Grants 96-0052 and 98-01392 to L.H.-B. and D.R. from the United States Department of Agriculture.
References
- Ach, R.A., Durfee, T., Miller, A.B., Taranto, P., Hanley-Bowdoin, L., Zambriski, P.C., and Gruissem, W. (1997). RRB1 and RRB2 encode maize retinoblastoma-related proteins that interact with a plant D-type cyclin and geminivirus replication protein. Mol. Cell. Biol. 17, 5077–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albani, D., Mariconti, L., Ricagno, S., Pitto, L., Moroni, C., Helin, K., and Cella, R. (2000). DcE2F, a functional plant E2F-like transcriptional activator from Daucus carota. J. Biol. Chem. 275, 19258–19267. [DOI] [PubMed] [Google Scholar]
- Bass, H.W., Nagar, S., Hanley-Bowdoin, L., and Robertson, D. (2000). Chromosome condensation induced by geminivirus infection of mature plant cells. J. Cell Sci. 113, 1149–1160. [DOI] [PubMed] [Google Scholar]
- Bisaro, D.M., Hamilton, W.D.O., Coutts, R.H.A., and Buck, K.W. (1982). Molecular cloning and characterization of the two DNA components of tomato golden mosaic virus. Nucleic Acids Res. 10, 4913–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaboute, M.E., Clement, B., Sekine, M., Philipps, G., and Chaubet-Gigot, N. (2000). Cell cycle regulation of the tobacco ribonucleotide reductase small subunit gene is mediated by E2F-like elements. Plant Cell 12, 1987–2000. [PMC free article] [PubMed] [Google Scholar]
- Chellappan, S., Kraus, V.B., Kroger, B., Munger, K., Howley, P.M., Phelps, W.C., and Nevins, J.R. (1992). Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc. Natl. Acad. Sci. USA 89, 4549–4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H.F., Campisi, J., and Padmanabhan, R. (1996). SV40 large T antigen transactivates the human cdc2 promoter by inducing a CCAAT box binding factor. J. Biol. Chem. 271, 13959–13967. [PubMed] [Google Scholar]
- Cheng, S., Schmidt-Grimminger, D.C., Murant, T., Broker, T.R., and Chow, L.T. (1995). Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 9, 2335–2349. [DOI] [PubMed] [Google Scholar]
- Citterio, S., Sgorbati, S., Levi, M., Colombo, B.M., and Sparvoli, E. (1992). PCNA and total nuclear protein content as markers of cell proliferation in pea tissue. J. Cell Sci. 102, 71–78. [Google Scholar]
- Coello, P., Rodriguez, R., Garcia, E., and Vazquez-Ramos, J.M. (1992). A DNA polymerase from maize axes: Its purification and possible role. Plant Mol. Biol. 20, 1159–1168. [DOI] [PubMed] [Google Scholar]
- Coruzzi, G., Broglie, R., Edwards, C., and Chua, N.-H. (1984). Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 3, 1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager, S.M., and Murray, J.A.H. (1999). Retinoblastoma proteins in plants. Plant Mol. Biol. 41, 295–299. [DOI] [PubMed] [Google Scholar]
- Dellaporta, S.L., Wood, J., and Hicks, J.B. (1983). Maize DNA minipreps. Maize Genet. Coop. Newsl. 57, 26–29. [Google Scholar]
- Dyson, N. (1998). The regulation of E2F by pRB-family proteins. Genes Dev. 12, 2245–2262. [DOI] [PubMed] [Google Scholar]
- Eagle, P.A., Orozco, B.M., and Hanley-Bowdoin, L. (1994). A DNA sequence required for geminivirus replication also mediates transcriptional regulation. Plant Cell 6, 1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer, J.S., Brand, L., Sunter, G., Gardiner, W.E., Bisaro, D.M., and Rogers, S.G. (1988. a). Genetic analysis of tomato golden mosaic virus. II. Requirement for the product of the highly conserved AL1 coding sequence for replication. Nucleic Acids Res. 16, 7043–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer, J.S., Sunter, G., Gardiner, W.E., Brand, L., Browning, C.K., Bisaro, D.M., and Rogers, S.G. (1988. b). Agrobacterium-mediated inoculation of plants with tomato golden mosaic virus DNAs. Plant Mol. Biol. 10, 225–234. [DOI] [PubMed] [Google Scholar]
- Esau, K. (1977). Virus-like particles in the nuclei of phloem cells in spinach leaves infected with the curly top virus. J. Ultrastruct. Res. 61, 78–88. [DOI] [PubMed] [Google Scholar]
- Fontes, E.P.B., Gladfelter, H.J., Schaffer, R.L., Petty, I.T.D., and Hanley-Bowdoin, L. (1994). Geminivirus replication origins have a modular organization. Plant Cell 6, 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafi, G., Burnett, R.J., Helentjaris, T., Larkins, B.A., Decaprio, J.A., Sellers, W.R., and Kaelin, W.G. (1996). A maize cDNA encoding a member of the retinoblastoma protein family: Involvement in endoreduplication. Proc. Natl. Acad. Sci. USA 93, 8962–8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, C. (2000). DNA replication and cell cycle in plants: Learning from geminiviruses. EMBO J. 19, 792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley-Bowdoin, L., Elmer, J.S., and Rogers, S.G. (1988). Transient expression of heterologous RNAs using tomato golden mosaic virus. Nucleic Acids Res. 16, 10511–10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley-Bowdoin, L., Elmer, J.S., and Rogers, S.G. (1989). Functional expression of the leftward open reading frames of the A component of tomato golden mosaic virus in transgenic tobacco plants. Plant Cell 1, 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley-Bowdoin, L., Settlage, S.B., Orozco, B.M., Nagar, S., and Robertson, D. (1999). Geminiviruses: Models for plant DNA replication, transcription, and cell cycle regulation. Crit. Rev. Plant Sci. 18, 71–106. [PubMed] [Google Scholar]
- Hata, S., Tsukamoto, T., Osumi, T., Hashimoto, J., and Suzuka, I. (1992). Analysis of carrot genes for proliferating cell nuclear antigen homologs with the aid of the polymerase chain reaction. Biochem. Biophys. Res. Commun. 184, 576–581. [DOI] [PubMed] [Google Scholar]
- Hayashi, Y., Yamagishi, M., Nishimoto, Y., Taguchi, O., Matsukage, A., and Yamaguchi, M. (1999). A binding site for the transcription factor grainyhead/nuclear transcription factor-1 contributes to regulation of the Drosophila proliferating cell nuclear antigen gene promoter. J. Biol. Chem. 274, 35080–35088. [DOI] [PubMed] [Google Scholar]
- Hemerly, A.S., Ferreira, P., Engler, J.D., Van Montagu, M., Engler, G., and Inze, D. (1993). Cdc2A expression in Arabidopsis is linked with competence for cell division. Plant Cell 5, 1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig, S., and Strauss, M. (1997). The retinoblastoma protein: A master regulator of cell cycle, differentiation and apoptosis. Eur. J. Biochem. 246, 581–601. [DOI] [PubMed] [Google Scholar]
- Holsters, M., de Waele, D., Depicker, A., Messens, E., van Montagu, M., and Schell, J. (1978). Transfection and transformation of Agrobacterium tumefaciens. Mol. Gen. Genet. 163, 181–187. [DOI] [PubMed] [Google Scholar]
- Horns, T., and Jeske, H. (1991). Localization of abutilon mosaic virus (AbMV) DNA within leaf tissue by in situ hybridization. Virology 181, 580–588. [DOI] [PubMed] [Google Scholar]
- Horsch, R.B., Fry, J.E., Hoffmann, N.L., Wallroth, M., Eichholtz, D., Rogers, S.G., and Fraley, R.T. (1985). A simple and general method for transferring cloned genes into plants. Science 227, 1229–1231. [DOI] [PubMed] [Google Scholar]
- Horvath, G.V., Pettko-Szandtner, A., Nikovics, K., Bilgin, M., Boulton, M., Davies, J.W., Gutierrez, C., and Dudits, D. (1998). Prediction of functional regions of the maize streak virus replication-associated proteins by protein–protein interaction analysis. Plant Mol. Biol. 38, 699–712. [DOI] [PubMed] [Google Scholar]
- Huang, D.Y., and Prystowsky, M.B. (1996). Identification of an essential cis-element near the transcription start site for transcriptional activation of the proliferating cell nuclear antigen gene. J. Biol. Chem. 271, 1218–1225. [DOI] [PubMed] [Google Scholar]
- Jansen-Durr, P. (1996). How viral oncogenes make the cell cycle. Trends Genet. 12, 270–275. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, R., Chan, A., Daly, M., and McPherson, J. (1987). Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 236, 1299–1302. [DOI] [PubMed] [Google Scholar]
- Kodama, H., Ito, M., Ohnishi, N., Suzuka, I., and Kokamine, A. (1991). Molecular cloning of the gene for plant proliferating-cell nuclear antigen and expression of this gene during the cell cycle in synchronized cultures of Catharanthus roseus cells. Eur. J. Biochem. 197, 495–503. [DOI] [PubMed] [Google Scholar]
- Kong, L.J., Orozco, B.M., Roe, J.L., Nagar, S., Ou, S., Feiler, H.S., Durfee, T., Miller, A.B., Gruissem, W., Robertson, D., and Hanley-Bowdoin, L. (2000). A geminivirus replication protein interacts with retinoblastoma through a novel domain to determine symptoms and tissue specificity of infection in plants. EMBO J. 19, 3485–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi, S., and Ohashi, Y. (1997). PCF1 and PCF2 specifically bind to cis elements in the rice proliferating cell nuclear antigen gene. Plant Cell 9, 1607–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi, S., Suzuka, I., Ohashi, Y., Murakami, T., and Arai, Y. (1991). Upstream sequences of rice proliferating cell nuclear antigen (PCNA) gene mediate expression of PCNA–GUS chimeric gene in meristems of transgenic tobacco plants. Nucleic Acids Res. 19, 1571–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi, S., Suzuka, I., and Ohashi, Y. (1995). Two of three promoter elements identified in a rice gene for proliferating cell nuclear antigen are essential for meristematic tissue-specific expression. Plant J. 7, 877–886. [DOI] [PubMed] [Google Scholar]
- Lanahan, M.B., Yen, H.-C., Giovannoni, J.J., and Klee, H.J. (1994). The never ripe mutation blocks ethylene perception in tomato. Plant Cell 6, 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs, J., Traut, W., Heyraud, F., Matzeit, V., Rogers, S.G., Schell, J., and Gronenborn, B. (1995). In vitro cleavage and joining at the viral origin of replication by the replication initiator protein of tomato yellow leaf curl virus. Proc. Natl. Acad. Sci. USA 92, 3879–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavia, P., and Jansen-Durr, P. (1999). E2F target genes and cell-cycle checkpoint control. Bioessays 21, 221–230. [DOI] [PubMed] [Google Scholar]
- Lee, H.H., Chiang, W.H., Chiang, S.H., Liu, Y.C., Hwang, J., and Ng, S.Y. (1995). Regulation of cyclin D1, DNA topoisomerase I, and proliferating cell nuclear antigen promoters during the cell cycle. Gene Expr. 4, 95–109. [PMC free article] [PubMed] [Google Scholar]
- Liu, L., Saunders, K., Thomas, C.L., Davies, J.W., and Stanley, J. (1999). Bean yellow dwarf virus RepA, but not Rep, binds to maize retinoblastoma protein, and the virus tolerates mutations in the consensus binding motif. Virology 256, 270–279. [DOI] [PubMed] [Google Scholar]
- Liu, Y.C., Chang, H.W., Lai, Y.C., Ding, S.T., and Ho, J.L. (1998). Serum responsiveness of the rat PCNA promoter involves the proximal ATF and AP-1 sites. FEBS Lett. 441, 200–204. [DOI] [PubMed] [Google Scholar]
- Lopez, I., Khan, S., Vazquez, J., and Hussey, P.J. (1997). The proliferating cell nuclear antigen (PCNA) gene family in Zea mays is composed of two members that have similar expression programmes. Biochim. Biophys. Acta 1353, 1–6. [DOI] [PubMed] [Google Scholar]
- Lucy, A.P., Boulton, M.I., Davies, J.W., and Maule, A.J. (1996). Tissue specificity of Zea mays infection by maize streak virus. Mol. Plant-Microbe Interact. 9, 22–31. [Google Scholar]
- Luo, R.X., Postigo, A.A., and Dean, D.C. (1998). Rb interacts with histone deacetylase to repress transcription. Cell 92, 463–473. [DOI] [PubMed] [Google Scholar]
- Magyar, Z., Atanassova, A., De Veylder, L., Rombauts, S., and Inze, D. (2000). Characterization of two distinct DP-related genes from Arabidopsis thaliana. FEBS Lett. 486, 79–87. [DOI] [PubMed] [Google Scholar]
- Mandel, T., Fleming, M.T., Krahenbuhl, R., and Kuhlemeier, C. (1995). Definition of constitutive gene expression in plants: The translation initiation factor 4A gene as a model. Plant Mol. Biol. 29, 995–1004. [DOI] [PubMed] [Google Scholar]
- Markley, N.-A., Bonham-Smith, P.C., and Moloney, M.M. (1993). Molecular cloning and expression of a cDNA encoding the proliferating cell nuclear antigen from Brassica napus (oilseed rape). Genome 36, 459–466. [DOI] [PubMed] [Google Scholar]
- Martinez, M.C., Jorgensen, J.E., Lawton, M.A., Lamb, C.J., and Doerner, P.W. (1992). Spatial pattern of cdc2 expression in relation to meristem activity and cell proliferation during plant development. Proc. Natl. Acad. Sci. USA 89, 7360–7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra, M.R., and Petty, I.T. (2000). Tissue specificity of geminivirus infection is genetically determined. Plant Cell 12, 2259–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar, S., Pedersen, T.J., Carrick, K., Hanley-Bowdoin, L., and Robertson, D. (1995). A geminivirus induces expression of a host DNA synthesis protein in terminally differentiated plant cells. Plant Cell 7, 705–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogris, E., Rotheneder, H., Mudrak, I., Pichler, A., and Wintersberger, E. (1993). A binding site for transcription factor-E2F is a target for transactivation of murine thymidine kinase by polyomavirus large T-antigen and plays an important role in growth regulation of the gene. J. Virol. 67, 1765–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi, Y., Sawada, Y., Moriuchi, T., and Fujinaga, K. (1992). Analysis of the 5′ flanking region of the rat proliferating cell nuclear antigen (PCNA) gene. Biochim. Biophys. Acta 1130, 175–181. [DOI] [PubMed] [Google Scholar]
- Orozco, B.M., and Hanley-Bowdoin, L. (1996). A DNA structure is required for geminivirus origin function. J. Virol. 270, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosperi, E. (1997). Multiple roles of the proliferating cell nuclear antigen: DNA replication, repair and cell cycle control. Prog. Cell Cycle Res. 3, 193–210. [DOI] [PubMed] [Google Scholar]
- Ramirez-Parra, E., and Gutierrez, C. (2000). Characterization of wheat DP, a heterodimerization partner of the plant E2F transcription factor which stimulates E2F-DNA binding. FEBS Lett. 486, 73–78. [DOI] [PubMed] [Google Scholar]
- Ramirez-Parra, E., Xie, Q., Boniotti, M.B., and Gutierrez, C. (1999). The cloning of plant E2F, a retinoblastoma-binding protein, reveals unique and conserved features with animal G1/S regulators. Nucleic Acids Res. 27, 3527–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushing, A.E., Sunter, G., Gardiner, W.E., Dute, R.R., and Bisaro, D.M. (1987). Ultrastructural aspects of tomato golden mosaic virus infection in tobacco. Phytopathology 77, 1231–1236. [Google Scholar]
- Rybicki, E.P. (1994). A phylogenetic and evolutionary justification for three genera of Geminiviridae. Arch. Virol. 139, 49–77. [DOI] [PubMed] [Google Scholar]
- Sanderfoot, A.A., and Lazarowitz, S.G. (1996). Getting it together in plant virus movement: Cooperative interactions between bipartite geminivirus movement proteins. Trends Cell Biol. 6, 353–358. [DOI] [PubMed] [Google Scholar]
- Sawado, T., Yamaguchi, M., Nishimoto, Y., Ohno, K., Sakaguchi, K., and Matsukage, A. (1998). dE2F2, a novel E2F-family transcription factor in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 251, 409–415. [DOI] [PubMed] [Google Scholar]
- Sekine, M., Masaki, I., Uemukai, K., Maeda, Y., Nakagami, H., and Shinmyo, A. (1999). Isolation and characterization of the E2F-like gene in plants. FEBS Lett. 460, 117–122. [DOI] [PubMed] [Google Scholar]
- Settlage, S.B., Miller, A.B., and Hanley-Bowdoin, L. (2000). Dual interaction of a geminivirus replication accessory factor with a viral replication protein and a plant cell cycle regulator. Virology 279, 570–576. [DOI] [PubMed] [Google Scholar]
- Sidle, A., Palaty, C., Dirks, P., Wiggan, O., Kiess, M., Gill, R.M., Wong, A.K., and Hamel, P.A. (1996). Activity of the retinoblastoma family proteins, pRB, p107, and p130, during cellular proliferation and differentiation. Crit. Rev. Biochem. Mol. Biol. 31, 237–271. [DOI] [PubMed] [Google Scholar]
- Staiger, C., and Doonan, J. (1993). Cell division in plants. Curr. Opin. Cell Biol. 5, 226–231. [DOI] [PubMed] [Google Scholar]
- Sunter, G., Hartitz, M.D., Hormuzdi, S.G., Brough, C.L., and Bisaro, D.M. (1990). Genetic analysis of tomato golden mosaic virus: ORF AL2 is required for coat protein accumulation while ORF AL3 is necessary for efficient DNA replication. Virology 179, 69–77. [DOI] [PubMed] [Google Scholar]
- Suzuka, I., Hata, S., Matsuoka, M., Kosugi, S., and Hashimoto, J. (1991). Highly conserved structure of proliferating cell nuclear antigen (DNA polymerase auxiliary protein) gene in plants. Eur. J. Biochem. 195, 571–575. [DOI] [PubMed] [Google Scholar]
- Tiainen, M., Spitkovsky, D., Jansen-Durr, P., Sacchi, A., and Crescenzi, M. (1996). Expression of E1A in terminally differentiated muscle cells reactivates the cell cycle and suppresses tissue-specific genes by separable mechanisms. Mol. Cell. Biol. 16, 5302–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi, S., and Pfeifer, G.P. (1999). In vivo structure of two divergent promoters at the human PCNA locus: Synthesis of antisense RNA and S phase–dependent binding of E2F complexes in intron 1. J. Biol. Chem. 274, 27829–27838. [DOI] [PubMed] [Google Scholar]
- Wade, M., Blake, M.C., Jambou, R.C., Helin, K., Harlow, E., and Azizkhan, J.C. (1995). An inverted repeat motif stabilizes binding of E2F and enhances transcription of the dihydrofolate reductase gene. J. Biol. Chem. 270, 9783–9791. [DOI] [PubMed] [Google Scholar]
- Weinberg, R.A. (1995). The retinoblastoma protein and cell cycle control. Cell 81, 323–330. [DOI] [PubMed] [Google Scholar]
- Xie, Q., Sanz-Burgos, P., Hannon, G.J., and Gutierrez, C. (1996). Plant cells contain a novel member of the retinoblastoma family of growth regulatory proteins. EMBO J. 15, 4900–4908. [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, M., Hayashi, Y., and Matsukage, A. (1995. a). Essential role of E2F recognition sites in regulation of the proliferating cell nuclear antigen gene promoter during Drosophila development. J. Biol. Chem. 270, 25159–25165. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, M., Hirose, F., Nishimoto, Y., Naruge, T., Ikeda, M., Hachiya, T., Tamai, K., Kuroda, K., and Matsukage, A. (1995. b). Expression patterns of DNA replication enzymes and the regulatory factor DREF during Drosophila development analyzed with specific antibodies. Biol. Cell 85, 147–155. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, M., Hirose, F., and Matsukage, A. (1996). Roles of multiple promoter elements of the proliferating cell nuclear antigen gene during Drosophila development. Genes Cells 1, 47–58. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos, M., Frappier, L., Khoury, M., and Price, G.B. (1988). Effect of anti-cruciform DNA monoclonal antibodies on DNA replication. EMBO J. 7, 1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]