Abstract
Tie1 is an orphan receptor tyrosine kinase that is expressed almost exclusively in endothelial cells and that is required for normal embryonic vascular development. Genetic studies suggest that Tie1 promotes endothelial cell survival, but other studies have suggested that the Tie1 kinase has little to no activity, and Tie1-mediated signaling pathways are unknown. To begin to study Tie1 signaling, a recombinant glutathione S-transferase (GST)-Tie1 kinase fusion protein was produced in insect cells and found to be autophosphorylated in vitro. GST-Tie1 but not a kinase-inactive mutant associated with a recombinant p85 SH2 domain protein in vitro, suggesting that Tie1 might signal through phosphatidylinositol (PI) 3-kinase. To study Tie1 signaling in a cellular context, a c-fms-Tie1 chimeric receptor (fTie1) was expressed in NIH 3T3 cells. Ligand stimulation of fTie1 resulted in Tie1 autophosphorylation and downstream activation of PI 3-kinase and Akt. Stimulation of fTie1-expressing cells potently inhibited UV irradiation-induced apoptosis in a PI 3-kinase-dependent manner. Moreover, both Akt phosphorylation and inhibition of apoptosis were abrogated by mutation of tyrosine 1113 to phenylalanine, suggesting that this residue is an important PI 3-kinase binding site. These findings are the first biochemical demonstration of a signal transduction pathway and corresponding cellular function for Tie1, and the antiapoptotic effect of Tie1 is consistent with the results of previous genetic studies.
Tie1 is an orphan receptor tyrosine kinase (RTK) that is expressed almost exclusively in endothelial cells (29). Tie1 expression is evident during embryonic vascular development, but it is also increased in the adult during both physiological and pathological angiogenesis (6, 14, 20, 21). Genetic studies in mice have demonstrated that Tie1 is required for normal embryonic vascular development. In two independent studies, Tie1-deficient mice died late during gestation or in the immediate postnatal period with severe edema and hemorrhage, suggesting that Tie1 regulates vascular integrity (33, 36). Mice lacking Tie1 also appeared to have reduced numbers of endothelial cells, supporting a role for Tie1 in endothelial cell survival (33). In chimeric mice, endothelial cells lacking Tie1 contributed to the development of blood vessels early in gestation, but they were progressively selected against in the later stages of embryonic development and in adult animals, consistent with a role for Tie1 in the maintenance of endothelial cell survival (31). Additional studies suggest that Tie1 and the highly homologous Tie2 receptor may perform complementary functions during cardiovascular development, since Tie1 is required for the survival of mice heterozygous for Tie2 (32). Furthermore, Tie1 and the activating Tie2 ligand, angiopoietin 1 (Ang1), are both required to establish polarity of the developing venous system (23).
The cytoplasmic kinase domains of Tie1 and Tie2 are approximately 80% identical in their amino acid sequences, but their extracellular domains are more divergent, with individual domains demonstrating 24 to 59% identity between the two receptors (7, 8, 30, 35). As these differences might suggest, none of the angiopoietins binds to Tie1 (2, 24, 39) and no ligand has yet been identified for Tie1. Whereas Ang1 has been shown to activate Tie2 to promote endothelial cell sprouting, migration, and survival (13, 15, 16, 18, 27, 28, 40), the signaling pathways and cellular functions mediated by Tie1 have been difficult to study with biochemical approaches and therefore remain unknown.
Recent studies have shown that the extracellular domain of Tie1 is cleaved by an unknown protease following treatment of cultured endothelial cells with phorbol myristate acetate, vascular endothelial growth factor (VEGF), or tumor necrosis factor alpha (TNF-α), resulting in the release of soluble Tie1 into the tissue culture supernatant (41, 42). Simultaneously, an endodomain of Tie1, which likely consists of the transmembrane and kinase domains, becomes detectable in the cytosolic fraction. In cells expressing both Tie1 and Tie2, Marron et al. found that this Tie1 endodomain coimmunoprecipitated with Tie2 (25). In that study, a chimeric TrkA-Tie1 receptor lacked intrinsic tyrosine kinase activity, and ligand-induced autophosphorylation was undetectable. Based on these findings, it was suggested that a primary function of Tie1 might be to modulate Tie2 signaling and function. The Tie1 endodomain was also found to coimmunoprecipitate with the protein tyrosine phosphatase Shp2, suggesting that Tie1 might transduce signals in the absence of ligand activation (26). Taken together, these findings suggested that Tie1 might function through novel signaling mechanisms.
It was demonstrated previously that p85 associates with tyrosine 1101 (Y1101) of Tie2, resulting in activation of phosphatidylinositol (PI) 3-kinase and Akt (19), and this signaling pathway was later shown to promote endothelial cell survival (13, 16, 27, 28). At that time, we had also begun to investigate the signaling pathways downstream of Tie1. Tyrosine 1113 of Tie1 corresponds to Y1101 of Tie2, and the amino acid residues flanking this tyrosine residue comprise an ideal p85-binding site. Because genetic studies have implicated Tie1 in endothelial cell survival, we hypothesized that Tie1 might also activate the PI 3-kinase/Akt pathway to inhibit apoptosis. In addition, the studies described above raised several important questions regarding the mechanism of action of Tie1 that we sought to answer in this study: first, is Tie1 an active kinase; second, is Tie1 autophosphorylated and can it signal following ligand activation; and third, can Tie1 signal and function autonomously of Tie2?
In this study, we investigated these questions and found that the Tie1 kinase is active and that it associates with p85 in vitro in an autophosphorylation-dependent manner. With a stably expressed c-fms-Tie1 chimeric receptor (fTie1), which allowed ligand activation with colony-stimulating factor 1 (CSF-1) in a cellular environment that lacks Tie2, Tie1 induced the activation of both PI 3-kinase and Akt and potently inhibited UV irradiation-induced apoptosis. These results are the first biochemical demonstration of a signal transduction pathway and corresponding cellular function mediated by Tie1. Moreover, these findings demonstrate that Tie1 is capable of signaling autonomously of Tie2 and support the results of prior genetic analyses of Tie1.
(This work was presented in part at the 73rd Scientific Sessions of the American Heart Association, New Orleans, La., November 2000.)
MATERIALS AND METHODS
Cell lines and reagents.
NIH 3T3 cells were from the American Type Culture Collection. GP+E86 ecotrophic retroviral packaging cells were provided by Genetix Pharmaceuticals (Cambridge, Mass.). Recombinant human CSF-1 was generously provided by Genetics Institute (Cambridge, Mass.). Rat monoclonal anti-c-fms (CSF-1 receptor) antibody (Ab) (clone 3-4A4-E4) was from Oncogene Science. Rabbit polyclonal anti-Tie1 Ab (C-18, no. sc-342) was from Santa Cruz Biotechnology. The rabbit polyclonal anti-Tie2 C-tail Ab has been described previously (12). Rabbit polyclonal anti-Akt, anti-phospho-Akt (specific for phosphoserine 473), and anti-cleaved caspase 3 Abs were from New England BioLabs. Mouse monoclonal anti-p85 (clone N7B) Ab and a rabbit polyclonal anti-p85 Ab were gifts from Anke Klippel (Chiron Corporation, Emeryville, Calif.). Mouse monoclonal antiphosphotyrosine Abs were from Transduction Laboratories (clone PY20) and Santa Cruz Biotechnology (clone PY99). Rat monoclonal anti-α-tubulin was from Harlan Biochemicals. Recombinant histone H2B was from Boehringer Mannheim Biochemicals. Wortmannin, Ly294002, propidium iodide, and Hoechst 33342 were from Sigma. Protein A- and protein G-PLUS agarose was from Santa Cruz Biotechnology.
Generation of recombinant Tie1 and p85 proteins and in vitro association assays.
The murine Tie1 kinase domain (amino acid residues 783 to 1134) was obtained by reverse transcriptase PCR from total RNA from the murine microvascular endothelial cell line Py-4-1 (5) with the following primers: forward primer, 5′-CTTAAGAGAAGCTGCCTACATCGG-3′; and reverse primer, 5′-CTAGGCCTCCTCAGCTGTGGCATC-3′. To fuse the kinase domain to other upstream sequences, an AflII site was engineered into the sequence by PCR, which resulted in a conservative change of the first amino acid (residue 783) of the kinase domain from arginine to lysine. This PCR product was subcloned into the Srf I site of the pcrScript-Amp vector (Stratagene) and was verified by sequencing. A kinase-inactive Tie1 mutant was generated using the Transformer site-directed mutagenesis kit (Clontech) according to the manufacturer's instructions, using the following mutagenesis primer, which changes lysine (AAG) 866 in the ATP binding site to arginine (AGG) (K866R): 5′-GCAGCCATCAGGATGCTA-3′. The site of change is shown in boldface. The wild-type and kinase-inactive Tie1 kinase domain cDNAs were fused in frame with and downstream of a glutathione S-transferase (GST) coding sequence in the plasmid pVL-1393 (Pharmingen). This vector was cotransfected into Sf9 insect cells with Baculogold baculovirus DNA (Pharmingen) by the calcium phosphate technique, and recombinant GST-Tie1 and -K866R baculoviruses were serially amplified. Recombinant proteins were purified from lysates of infected Sf9 cells as described previously (12). Recombinant p85 SH2-C protein was generated by subcloning a cDNA encoding the C-terminal SH2 domain of p85 (19) into the EcoRI and XhoI sites of the pRSET bacterial expression vector (Invitrogen). The resulting plasmid was transformed into BL21(DE3)pLysS Escherichia coli (Novagen), and recombinant six-His-tagged protein was generated and purified as described previously (17). Recombinant p85 protein was concentrated into phosphate-buffered saline in a Centriprep centrifugal filter device (Millipore) and used in in vitro association experiments with recombinant GST-Tie1. Autophosphorylation of recombinant GST-Tie1 kinase fusion proteins and analysis of p85 association in vitro were performed essentially as described previously (19), except that recombinant kinases were incubated 2 h at 4°C with approximately 3 μg of recombinant p85 SH2-C protein and were then washed extensively with Triton lysis buffer containing 0.5 M LiCl. Tie1 kinase-associated proteins were evaluated by Western blotting with anti-p85. Because of a residual antiphosphotyrosine signal from wild-type Tie1 that gave the appearance of unequal loading, equal starting amounts of GST-Tie1 proteins purified on glutathione Sepharose were confirmed by Coomassie staining.
Generation of fTie1-expressing cells.
The LNCX-fTie2 plasmid has been described elsewhere (19). The Y1113F mutant kinase domain was generated by site-directed mutagenesis, as described above, with the following mutagenesis primer, which mutates tyrosine 1113 (TAC) to phenylalanine (TTC): 5′-AGGAAGGCCTTCGTGAACATG-3′. The site of the change is shown in boldface. To generate LNCX-fTie1, -K866R, and -Y1113F, a cDNA encoding each of these kinase domains replaced that of Tie2 in LNCX-fTie2, resulting in fusion of the Tie1 kinase domains with the extracellular and transmembrane domains of human c-fms. The resultant chimeric receptors were designated fTie1, fTie1-K866R, and fTie1-Y1113F. Recombinant ecotrophic retroviruses were generated as described previously for fTie2 (19). Briefly, LNCX-fTie1, -K866R, or -Y1113F was transfected into GP+E86 packaging cells with Lipofectamine (Life Technologies), and stable monoclonal cell lines were selected with G418 (400 μg/ml, Life Technologies) and were screened for expression of the chimeric receptor by Western blotting with anti-Tie1 Ab (1:1,000). Supernatants containing ecotrophic fTie1 retrovirus were used to infect NIH 3T3 cells in the presence of hexadimethrine bromide (Polybrene, 8 μg/ml; Sigma), and fTie1-expressing cells were selected as described above.
Immunoprecipitation and Western blotting.
fTie1-expressing cells were grown in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U of penicillin per ml and 100 μg of streptomycin (pen-strep) per ml, and 400 μg of G418/ml until they were just subconfluent and were then starved overnight in DMEM containing pen-strep but no serum. Cells were left untreated or were stimulated with CSF-1 for 10 to 30 min and were lysed as described previously (19). Total cell lysates were either analyzed by Western blotting or were immunoprecipitated with anti-c-fms or antiphosphotyrosine (PY99) (for analysis of receptor phosphorylation) or anti-Akt (for Akt kinase assays). For analysis of Akt phosphorylation, 1 mM sodium orthovanadate (vanadate) was added at the same time as CSF-1 to enhance signal detection. In other experiments, cells were pretreated for 15 min with the indicated concentrations of the selective PI 3-kinase inhibitor wortmannin or an equal volume of dimethyl sulfoxide (DMSO) prior to CSF-1 stimulation.
In vivo phospholipid analysis.
fTie1-expressing cells were labeled 72 h with 12.5 μCi of [3H]myo-inositol (10 mCi/ml; American Radiolabeled Chemicals, St. Louis, Mo.)/ml. The cells were serum starved 3 h in DMEM alone and were then either treated with 1 mM vanadate alone or with CSF-1 (500 ng/ml) plus vanadate for 2.5, 5, or 7.5 min. Cellular lipids were extracted and analyzed by high-performance liquid chromatography (HPLC), and the identity of each phospholipid peak was verified by comparison to 32P-labeled standards (kindly provided by Phil Majerus, Washington University, St. Louis, Mo.) as described previously (19). The area under individual peaks on each chromatogram was quantified and expressed relative to the total amount of PI in each sample.
Akt kinase assay.
Lysates from CSF-1-treated or untreated cells were immunoprecipitated with anti-Akt and protein A agarose. Akt immunoprecipitates were used in an in vitro kinase reaction with [γ-32P]ATP and histone H2B as a substrate, as described previously (19). Radiolabeled histone H2B was separated by sodium dodecyl sulfate (SDS)-15% polyacrylamide gel electrophoresis (PAGE) and was analyzed by autoradiography.
Apoptosis analysis.
Untransfected NIH 3T3 cells, 3T3-fTie1 cells, or 3T3-K866R cells were plated in triplicate in 60-mm-diameter dishes at 106 cells/dish in DMEM containing 10% FBS and pen-strep. The following evening, the medium was changed to DMEM without FBS but containing the indicated concentrations of CSF-1 (from 0 to 1,000 ng/ml), and the cells were incubated at 37°C for 15 h. The next morning, the cells were irradiated with UV-C light (254 nm) at 1,200 J/m2 in a Stratalinker 1800 UV Cross-linker (Stratagene). In some experiments, the cells were observed up to 9 h and were analyzed for gross morphological changes by phase-contrast microscopy using an Olympus IX70 inverted system microscope attached to an Optronics DEI-750 digital camera connected to a Power Macintosh G3 computer. Images were obtained with Adobe Premiere (version 5.2) and analyzed with Adobe Photoshop (version 5.5) software. In other experiments, nuclear morphology was evaluated by incubating the cells 3 h at 37°C following irradiation, after which they were stained with propidium iodide (2.5 μg/ml) and Hoechst 33342 (10 μg/ml) (both from Sigma) for 15 min at 37°C and visualized by fluorescence microscopy. Cells that stained with propidium iodide (pink) were considered necrotic; those that stained with Hoechst (blue) were considered viable and/or apoptotic, and nuclear condensation in these cells was evaluated as an indicator of apoptosis (22). Apoptosis was quantified by counting the number of apoptotic and total nuclei in 10 random fields in each dish. The percentage of apoptotic cells was calculated by dividing the number of apoptotic nuclei by the total number of nuclei counted. Data were expressed as the mean ± the standard error of the mean.
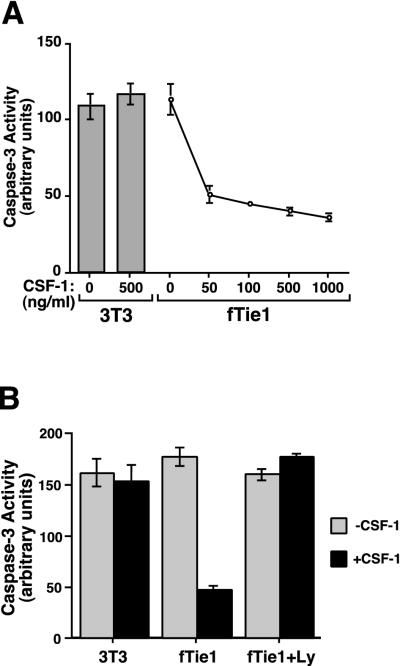
To assay caspase 3 activity, cells were plated as described above and incubated at 37°C for 3 h after UV irradiation. Floating and adherent cells were harvested by trypsinization, the trypsin was neutralized with DMEM containing 10% FBS, and cells were washed once with phosphate-buffered saline. The cells were lysed, and the lysates were used in a colorimetric assay of caspase 3 activity (ApoAlert Caspase-3 Assay Kit; Clontech) according to the manufacturer's instructions. To evaluate the effects of the selective PI 3-kinase inhibitor Ly294002 on fTie1-mediated antiapoptosis, cells were incubated overnight with or without CSF-1 in the presence of 30 μM Ly294002 or an equal volume of DMSO, and then caspase 3 activity was assayed as described.
Effects of the Y1113F mutation on apoptosis.
To evaluate the effect of the Y1113F mutation on Tie1-mediated antiapoptosis, fTie1-expressing cells were plated at 3 × 105 cells per well of a six-well plate and grown overnight in full growth medium. The following evening the medium was changed to serum-free DMEM with or without CSF-1 (500 ng/ml), and the cells were incubated an additional 16 h at 37°C. The cells were then UV irradiated as described above, incubated 2 h at 37°C, and lysed with 100 μl of Triton lysis buffer. Lysates were separated by SDS-8 to 16% or 15% PAGE and Western blotted sequentially with anti-phospho-Akt (1:1,000), anti-Akt (1:1,000), and anti-cleaved caspase 3 (1:1,000).
RESULTS
Tie1 is autophosphorylated and associates with p85 in vitro.
We demonstrated previously that the p85 subunit of PI 3-kinase associates with the endothelial RTK Tie2 at tyrosine 1101, resulting in activation of both PI 3-kinase and the downstream kinase Akt (19). Activation of this pathway was subsequently shown to prevent endothelial cell apoptosis (13, 16, 27, 28). Comparison of the primary sequences of Tie2 and Tie1 demonstrates that tyrosine 1113 of Tie1 and its adjacent residues (YVNM) match the consensus p85-binding site (Y-hydrophobic-X-M, where X = any amino acid) (38) (Fig. 1A). To investigate the potential interaction of Tie1 with p85 in vitro, recombinant GST-Tie1 kinase fusion proteins were generated in insect cells and used to evaluate Tie1 kinase autophosphorylation and p85 association. Similar amounts of wild-type GST-Tie1 and a kinase-inactive mutant (K866R) were purified on glutathione Sepharose, subjected to an in vitro kinase reaction, and then incubated with recombinant p85 SH2 domain protein. The GST fusion proteins and associated proteins were then washed extensively, eluted, and analyzed by Western blotting. In contrast to previous reports suggesting that Tie1 is an inactive kinase (25, 29), GST-Tie1 was tyrosine phosphorylated in vitro (Fig. 1B). Moreover, this effect was due to autophosphorylation, since it was abrogated by mutation of the ATP binding site lysine to arginine (K866R) (Fig. 1B). Wild-type GST-Tie1 but not the kinase-inactive mutant associated with recombinant p85 in vitro (Fig. 1B), demonstrating that Tie1 can associate with p85 in an autophosphorylation-dependent manner. In addition, these findings with recombinant proteins indicate that Tie1 can bind directly to p85/PI 3-kinase, at least in vitro.
FIG. 1.
p85 associates with Tie1 in vitro. (A) Comparison of Tie1 and Tie2 C-terminal amino acid sequences. C-terminal regions of Tie1 and Tie2 containing putative autophosphorylation sites were aligned and compared to the consensus p85-binding motif. The greatest difference in this region is in the Y + 3 position of the putative p85-binding motif. Key tyrosine residues and the putative p85-binding motif within each kinase are in boldface. Positions of tyrosine residues within each kinase are shown above and below the alignment. Solid lines, identical amino acid residues; dashed lines, conserved residues. Residues are abbreviated according to the single-letter amino acid code. (B) Tie1 is autophosphorylated and associates with p85 in vitro. Wild-type GST-Tie1 and a kinase-inactive mutant (K866R) were purified on glutathione Sepharose from Sf9 insect cell lysates infected with recombinant baculoviruses encoding each protein. As a control, an equal volume of uninfected Sf9 cell lysate was incubated with glutathione Sepharose (beads). Following an in vitro kinase reaction, the samples were incubated with recombinant C-terminal p85 SH2 domain protein. The GST kinases and associated proteins were eluted and analyzed by SDS-8 to 16% PAGE and Western blotting with anti-Tie1, antiphosphotyrosine (PTyr), and anti-p85 Abs. Similar results were obtained in three separate experiments. Note that equal starting amounts of GST-Tie1-wild type and -K866R were confirmed by Coomassie staining of glutathione Sepharose-purified Sf9 lysates.
Tie1 is autophosphorylated in vivo.
No ligands have been identified for Tie1. Therefore, to investigate the significance of the p85/Tie1 interaction in a cellular context, we generated a ligand-activatable chimeric receptor consisting of the cytoplasmic kinase domain of Tie1 and the extracellular, ligand binding domain of c-fms, the CSF-1 receptor (fTie1). This approach proved effective previously for investigating signaling by Tie2 before its ligands became available (19). Recombinant retroviruses encoding wild-type fTie1 and the kinase-inactive mutant (fTie1-K866R) were generated and used to stably express these chimeric receptors in NIH 3T3 cells (3T3-fTie1 and -K866R, respectively), and several clones of each cell line were selected.
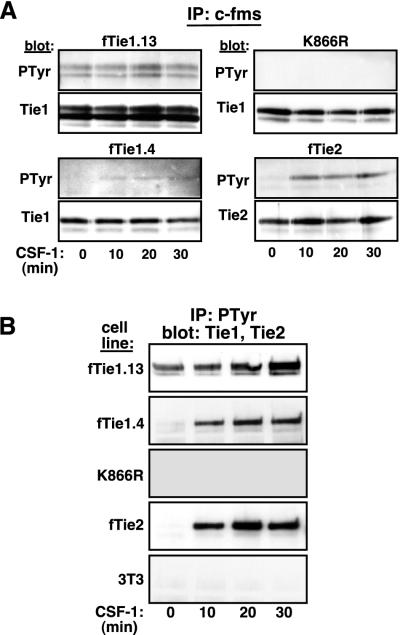
To begin to characterize signaling by Tie1, we evaluated autophosphorylation of both a low-receptor-expressing clone (clone 4) and a high-receptor-expressing clone (clone 13) of 3T3-fTie1 cells. Ligand-induced autophosphorylation of these cells and of 3T3-K866R and -fTie2 cells was evaluated simultaneously as controls. Following serum starvation, the cells were treated with CSF-1 for up to 30 min. The receptors were immunoprecipitated from cell lysates and evaluated by Western blotting sequentially with antiphosphotyrosine and anti-Tie1 or -Tie2 Abs. As with other RTKs, the fTie1 chimeric receptor migrated as a doublet (Fig. 2A), with the lower-molecular-weight band likely representing an incompletely glycosylated form of the receptor (42). In fTie1.13 cells, Tie1 was tyrosine phosphorylated at baseline, likely due to overexpression of the receptor, but this phosphorylation appeared to increase slightly after 20 min of CSF-1 stimulation (Fig. 2A). Similarly, CSF-1 stimulation of fTie1.4 cells resulted in weak but detectable tyrosine phosphorylation of Tie1 beginning at 10 min and persisting up to 30 min after stimulation. No phosphorylation of the kinase-inactive mutant, K866R, was observed either at baseline or after CSF-1 stimulation, demonstrating that Tie1 is autophosphorylated upon ligand treatment. Tie1 autophosphorylation was markedly less than that of Tie2 in the same context (Fig. 2A), which is consistent with the relative levels of autophosphorylation observed for the GST kinases in vitro.
FIG. 2.
Tie1 is autophosphorylated in vivo. (A) Autophosphorylation of Tie1 is kinase dependent. Two independent clones of NIH 3T3 cells expressing wild-type fTie1 as well as cells expressing a kinase-inactive mutant of fTie1 (K866R) or fTie2 were serum starved and were then treated with CSF-1 (500 ng/ml) for 0, 10, 20, or 30 min. Lysates from these cells were immunoprecipitated with anti-c-fms, separated by SDS-8% PAGE, and Western blotted with antiphosphotyrosine (PTyr, clone PY99), and then stripped and reprobed with anti-Tie1 or anti-Tie2. Weak but detectable increases in ligand-induced tyrosine phosphorylation were observed in both fTie1-expressing clones but not in K866R cells. As a control, fTie2 cells showed readily detectable ligand-induced autophosphorylation. (B) Tie1 is present in antiphosphotyrosine immunoprecipitates. The cells used for panel A, as well as untransfected 3T3 cells, were treated as described above, except lysates were immunoprecipitated with antiphosphotyrosine (PY99) and were then Western blotted with anti-Tie1 or (for fTie2 cell lysates) anti-Tie2. Tie1 was readily detectable in antiphosphotyrosine immunoprecipitates from both low- and high-receptor-expressing fTie1 cells but not from K866R cells or untransfected 3T3 cells. CSF-1 treatment also resulted in the presence of Tie2 in phosphotyrosine immunoprecipitates.
To confirm that Tie1 is activated in these cells following ligand stimulation, we used an alternative approach with potentially greater sensitivity for detecting Tie1 autophosphorylation. The fTie1-, K866R-, and fTie2-expressing cells, as well as untransfected NIH 3T3 cells, were again treated with CSF-1 for 10 to 30 min, but in this set of experiments the lysates were immunoprecipitated with antiphosphotyrosine Abs and Western blotted with anti-Tie1 or anti-Tie2. In both low- and high-receptor-expressing fTie1 cells, Tie1 was readily detectable in phosphotyrosine immunoprecipitates following CSF-1 stimulation (Fig. 2B). In fTie1.13 cells, which demonstrated basal fTie1 autophosphorylation, fTie1 was present in phosphotyrosine immunoprecipitates prior to CSF-1 stimulation, but this increased steadily from 10 to 30 min after CSF-1 stimulation (Fig. 2B). In cells expressing lower levels of fTie1 (fTie1.4), fTie1 immunoprecipitated with antiphosphotyrosine Abs only upon CSF-1 stimulation, and similar results were observed for fTie2. In contrast, the kinase-inactive mutant of fTie1 was undetectable in phosphotyrosine immunoprecipitates either before or after ligand stimulation. No signal for Tie1 or Tie2 was detected in phosphotyrosine immunoprecipitates from untransfected 3T3 cells (Fig. 2B and data not shown). Taken together, these findings indicate either that fTie1 undergoes autophosphorylation following CSF-1 stimulation or that ligand treatment induces the association of fTie1 with other tyrosyl phosphoproteins. In either case, however, the absence of a signal from K866R cells confirms that Tie1 kinase activity is required for this effect and therefore that Tie1 is functionally active in these cells.
Tie1 activates PI 3-kinase in vivo.
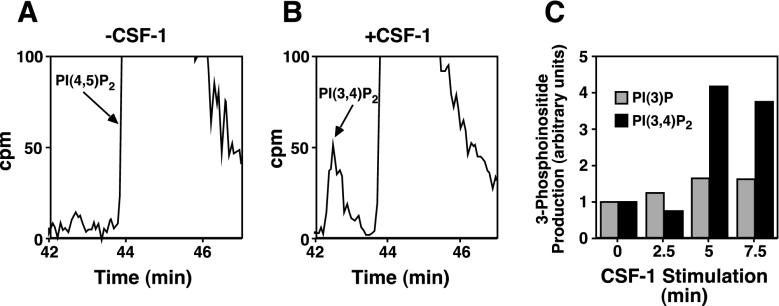
Having demonstrated that the fTie1 chimeric receptor is active in these cells, we next evaluated the association of p85 with Tie1. As was the case previously with Tie2 (19), we were unable to coimmunoprecipitate p85 and Tie1 from CSF-1-stimulated 3T3-fTie1 cells (data not shown). However, we reasoned that even weak or transient p85/Tie1 association might result in activation of PI 3-kinase with resultant accumulation of 3-phosphoinositides that might be detected more easily than the association itself. To test this possibility, 3T3-fTie1.13 cells were labeled to steady state with [3H]inositol, and phospholipids were extracted and analyzed by HPLC before and at several time points after CSF-1 stimulation. CSF-1 stimulation of 3T3-fTie1.13 cells resulted in only a 1.6-fold increase in PI 3-phosphate but a >4-fold increase in PI 3,4-bisphosphate (Fig. 3). Because PI 3-phosphate is constitutively present in mammalian cells at relatively high levels (including fTie1 cells) (34), it is more difficult to detect an appreciable increase in this phospholipid. The slight increase detected here could result from phosphorylation of PI by activated PI 3-kinase or from breakdown of PI 3,4,5-P3. In contrast, PI 3,4-bisphosphate is usually produced only following activation of class I PI 3-kinases, like p110α (34); thus, the detection of this lipid product at both 5 and 7.5 min after CSF-1 stimulation (Fig. 3B and C) demonstrates that Tie1 activates PI 3-kinase in vivo. Notably, we were unable to demonstrate changes in PI 3,4,5-P3 following activation of Tie1, which is likely due to the lower sensitivity for detecting PI 3,4,5-P3 following steady-state labeling. This finding is similar to results seen following activation of Tie2 (19).
FIG. 3.
Tie1 activates PI 3-kinase in vivo. (A and B) NIH 3T3 cells expressing fTie1 were labeled 72 h with 12.5 μCi of [3H]myo-inositol/ml and were then serum starved for 3 h. Cells were left untreated (A) or were treated with CSF-1 (500 ng/ml) for 2.5, 5, or 7.5 min (B), all in the presence of 1 mM vanadate, and lipids were extracted and separated by HPLC on a strong anion-exchange column. A peak corresponding to PI 3,4-bisphosphate [PI(3,4)P2] was detected at 5 and 7.5 min (shown) after CSF-1 treatment. The regions of the chromatograms where PI(3,4)P2 and PI(4,5)P2 eluted (between 42 and 47 min) are shown, and the peaks corresponding to PI(4,5)P2, which is much more abundant than PI(3,4)P2, are truncated at 100 cpm (maximum values were approximately 40,000 to 60,000 cpm). (C) Quantification of 3-phosphoinositide production by Tie1. On each chromatogram, total counts per minute under the peaks corresponding to PI(3)P (not shown) and PI(3,4)P2 were quantified and expressed relative to the total PI from that sample. CSF-1 induced a maximal 1.6-fold increase in PI(3)P and a 4.2-fold increase in PI(3,4)P2. Similar results were obtained in a duplicate experiment.
Tie1 induces phosphorylation and activation of Akt.
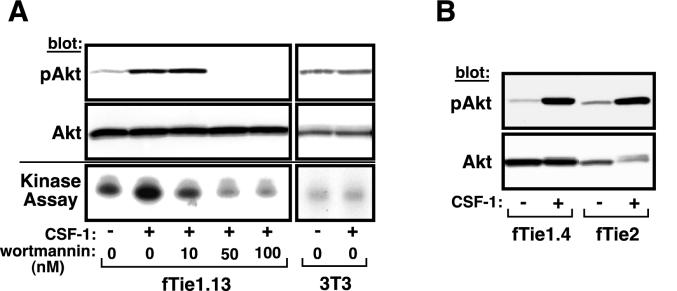
Tie2 and many other RTKs that activate PI 3-kinase also induce downstream activation of the serine/threonine kinase Akt; therefore, we next investigated whether stimulation of Tie1 results in downstream activation of Akt. Lysates from 3T3-fTie1.13 cells that had been stimulated with CSF-1 were analyzed by Western blotting with Abs specific for phosphoserine 473 of Akt, which has been shown to correlate with Akt activation (4, 19). In the absence of CSF-1, Akt was weakly phosphorylated on Ser 473 (Fig. 4A). Following CSF-1 treatment, Akt phosphorylation was markedly increased. Both CSF-1-dependent phosphorylation and basal phosphorylation of Akt were abrogated by relatively low concentrations of the selective PI 3-kinase inhibitor wortmannin (50 to 100 nM) (Fig. 4A). Akt was immunoprecipitated from the remaining cell lysate and was used in an in vitro kinase reaction with the exogenous substrate histone H2B. As with Akt phosphorylation, a low level of basal Akt kinase activity was observed in unstimulated cells, but CSF-1 stimulation markedly increased Akt activity. This activity was inhibited by 10 nM wortmannin (Fig. 4A), a concentration that is believed to be specific for PI 3-kinase (1), and higher doses of wortmannin also abrogated basal Akt kinase activity. CSF-1-dependent Akt phosphorylation was also observed in fTie1.4 cells, which express lower levels of fTie1 (Fig. 4B), but not in K866R cells (not shown). Levels of phospho-Akt produced by both fTie1-expressing cell lines were similar to those induced by fTie2, although the fTie2-expressing cells express less total Akt (Fig. 4B). No changes in Akt phosphorylation or activation were observed in untransfected NIH 3T3 cells following CSF-1 treatment (Fig. 4A), demonstrating that these effects were specific for fTie1. Together, these findings demonstrate that Tie1 induces phosphorylation and activation of Akt and that this effect is PI 3-kinase dependent.
FIG. 4.
Tie1 induces phosphorylation and activation of Akt. (A) fTie1-expressing cells or untransfected NIH 3T3 cells were serum starved overnight and were then left untreated or were treated 8 min with CSF-1 (500 ng/ml) in the presence of 1 mM vanadate and the indicated concentration of wortmannin or an equal volume of DMSO. An aliquot of each cell lysate was separated by SDS-8% PAGE and Western blotted with Abs specific for phosphoserine 473 of Akt (pAkt) or total Akt (upper panels). The remainder of each sample was immunoprecipitated with anti-Akt and was used in an in vitro kinase reaction with [γ-32P]ATP and recombinant histone H2B as a substrate. Radiolabeled histone H2B was separated by SDS-15% PAGE and evaluated by autoradiography (lower panel). (B) A second independent clone of fTie1-expressing cells as well as fTie2 and K866R cells (not shown) was treated as described for panel A, and cell lysates were Western blotted sequentially with anti-phospho-Akt and anti-Akt.
Tie1 inhibits UV irradiation-induced apoptosis.
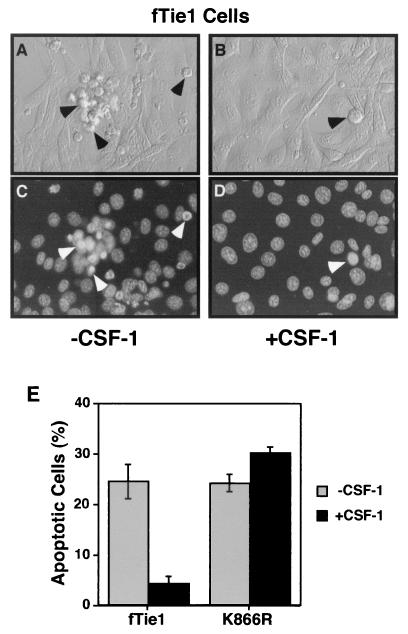
The PI 3-kinase/Akt pathway has been shown to promote cell survival downstream of numerous growth factor receptors, including Tie2 (4, 13, 16, 27). To investigate the functional significance of this pathway downstream of Tie1, apoptosis was induced in fTie1.13, K866R, and untransfected NIH 3T3 cells by UV irradiation (22), and the effects of CSF-1 treatment were evaluated. Within several hours of UV irradiation, untreated fTie1.13 cells displayed morphological characteristics of apoptosis, including membrane blebbing and nuclear condensation (Fig. 5A and C). K866R cells and untransfected 3T3 cells displayed similar morphological changes both in the absence and presence of CSF-1 (not shown). In contrast, CSF-1-treated fTie1.13 cells appeared grossly normal (Fig. 5B) and had markedly fewer apoptotic nuclei by Hoechst staining (Fig. 5D). CSF-1 treatment of fTie1.13 cells reduced the number of apoptotic cells by more than 80%, but CSF-1 had no effect on the survival of K866R cells (Fig. 5E), demonstrating that Tie1 transduces a potent survival signal that requires Tie1 kinase activity.
FIG. 5.
Tie1 blocks UV irradiation-induced apoptosis. NIH 3T3 cells expressing fTie1.13 (wild-type) or the kinase-inactive mutant (K866R) were grown 24 h in 60-mm-diameter dishes and were then serum starved 15 h in the absence or presence of CSF-1 (500 ng/ml). The cells were then UV irradiated with 1,200 J/m2, stained with Hoechst 33342 (10 μg/ml) and propidium iodide (2.5 μg/ml), and evaluated by phase-contrast (A and B) and fluorescence microscopy (C and D). Untreated fTie1 cells (A and C) began to display morphological characteristics of apoptosis, including membrane blebbing and nuclear condensation (arrowheads), as early as 1 h after UV irradiation, whereas CSF-1-treated fTie1 cells (B and D) remained viable. (E) Quantification of apoptosis following UV irradiation. The number of apoptotic nuclei was counted after Hoechst staining and expressed as the percentage of total nuclei ± standard error of the mean. CSF-1 treatment significantly decreased apoptosis of wild-type cells but not of K866R mutant cells.
Inhibition of apoptosis by Tie1 was confirmed using lysates from CSF-1-treated and untreated fTie1.13 cells in an assay of caspase 3 activity. As demonstrated morphologically, CSF-1 treatment did not protect untransfected NIH 3T3 cells from apoptosis (Fig. 6A and B). In contrast, CSF-1 treatment of fTie1-expressing cells resulted in a dose-dependent decrease in UV-induced caspase 3 activity, with a peak reduction of approximately 70% (Fig. 6A).
FIG. 6.
Tie1 inhibits UV irradiation-induced caspase 3 activation through a PI 3-kinase-dependent mechanism. (A) Untransfected NIH 3T3 cells or 3T3 cells expressing fTie1 were grown in 60-mm-diameter dishes for 24 h and then serum starved 15 h in the absence or presence of the indicated concentration of CSF-1 and were then UV irradiated as described for Fig. 5. Three hours after UV irradiation, cells were lysed and the lysates were used in a colorimetric assay of caspase 3 activity (ApoAlert; Clontech). fTie1 blocked the UV-induced increase in caspase 3 activity in a dose-dependent manner. (B) Untransfected 3T3 cells and fTie1 cells were serum starved 15 h with or without 500 ng of CSF-1/ml and Ly294002 (Ly) (30 μM) or an equal volume of DMSO. Caspase 3 activity was analyzed as for panel A. The inhibition of caspase 3 activity by Tie1 was completely reversed by Ly294002.
To determine whether PI 3-kinase plays a role in the antiapoptotic effect of Tie1, 3T3-fTie1.13 cells were treated with or without CSF-1 in the absence or presence of the reversible PI 3-kinase inhibitor Ly294002 (1, 22). The Ly compound was used in this setting because its stability is greater than that of wortmannin. Cells were UV irradiated, and lysates were used to assay caspase 3 activity. Treatment with CSF-1 in the presence of vehicle (DMSO) again reduced caspase 3 activity by about 70% (Fig. 6B). Inhibition of PI 3-kinase with Ly294002 in the absence of CSF-1 did not increase basal UV-induced caspase 3 activity, but it completely blocked the CSF-1-dependent inhibition of caspase 3 activation. Taken together, these findings demonstrate that Tie1 transduces a potent antiapoptotic signal that is PI 3-kinase dependent.
Tyrosine 1113 is required for activation of Akt and inhibition of apoptosis by Tie1.
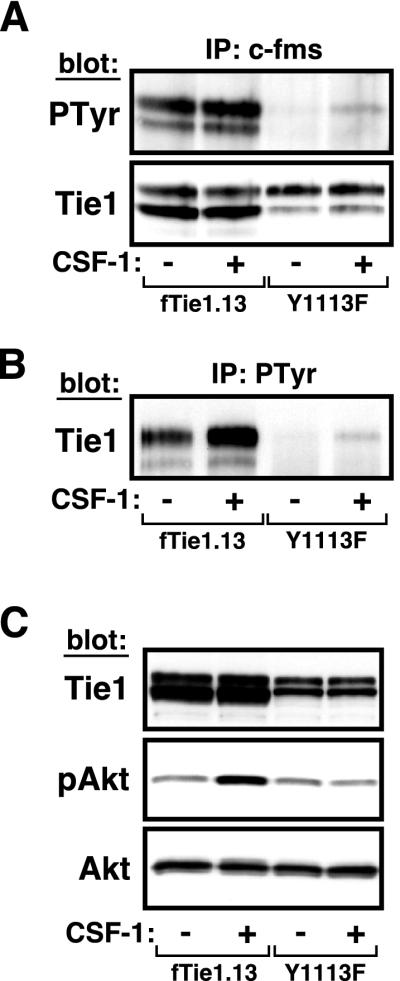
As noted, the primary structure of Tie1 reveals an ideal p85-binding site at tyrosine 1113. To determine whether this residue is required for activation of PI 3-kinase/Akt and inhibition of apoptosis by Tie1, we generated another fTie1-expressing cell line in which tyrosine 1113 was mutated to phenylalanine (Y1113F). The corresponding mutation on Tie2 was previously shown to abrogate p85 binding and downstream activation of PI 3-kinase and Akt by Tie2. To verify that the Y1113F mutant receptor was functionally active, cells expressing either wild-type fTie1 (clone fTie1.13) or fTie1-Y1113F were serum starved and were then treated with or without CSF-1 for 20 min, the time point at which Tie1 maximally coprecipitated with phosphotyrosyl proteins. Lysates from both cell lines were then immunoprecipitated with anti-c-fms and Western blotted sequentially with antiphosphotyrosine and anti-Tie1. Wild-type fTie1 was again tyrosine phosphorylated at baseline, and phosphorylation increased slightly with CSF-1 stimulation (Fig. 7A). In contrast, the Y1113F mutant receptor was phosphorylated only after activation with CSF-1, demonstrating that Y1113F is indeed active following ligand stimulation. The difference in basal receptor phosphorylation between Y1113F and wild-type fTie1 is likely due to lower levels of expression of the Y1113F receptor. To confirm the activity of the Y1113F mutant, an aliquot of each lysate was immunoprecipitated with antiphosphotyrosine and Western blotted with anti-Tie1. As demonstrated earlier, wild-type fTie1 was present in antiphosphotyrosine immunoprecipitates at baseline and increased with CSF-1 stimulation (Fig. 7B). Notably, Y1113F coimmunoprecipitated with antiphosphotyrosine only after CSF-1 treatment. Taken together, these findings demonstrate that, like wild-type fTie1, the Y1113F mutant is active following ligand activation.
FIG. 7.
Mutation of tyrosine 1113 abrogates Akt phosphorylation. (A and B) fTie1-Y1113F is functionally active. NIH 3T3 cells expressing either wild-type fTie1 (fTie1.13) or fTie1-Y1113F (Y1113F) were serum starved overnight and were then either left untreated or treated with CSF-1 (500 ng/ml) for 20 min. Receptors were immunoprecipitated from cell lysates with anti-c-fms (A) or antiphosphotyrosine (B). Proteins for panel A were Western blotted sequentially with antiphosphotyrosine and anti-Tie1, and proteins for panel B were Western blotted with anti-Tie1. Tyrosine phosphorylation of fTie1-Y1113F and its presence in phosphotyrosine immunoprecipitates were detected only after CSF-1 treatment. (C) The fTie1-Y1113F mutant fails to activate Akt. Cells expressing wild-type fTie1 or Y1113F were serum starved overnight and were then treated with or without CSF-1, all in the presence of vanadate (1 mM). An aliquot of each cell lysate was separated by SDS-8% PAGE and Western blotted with the indicated Abs. CSF-1 stimulation of Y1113F cells failed to induce Akt phosphorylation.
To investigate the effect of the Y1113F mutation on downstream activation of Akt, cells expressing both wild-type fTie1 and Y1113F were treated with or without CSF-1 in the presence of vanadate in order to increase the sensitivity for detecting changes in Akt phosphorylation. Whereas CSF-1 treatment of wild-type fTie1 induced an increase in Akt phosphorylation, no such change could be detected in Y1113F-expressing cells (Fig. 7C). Because activation and phosphorylation of Akt downstream of Tie1 are PI 3-kinase dependent, this finding suggests that tyrosine 1113 is required for PI 3-kinase binding and activation by Tie1.
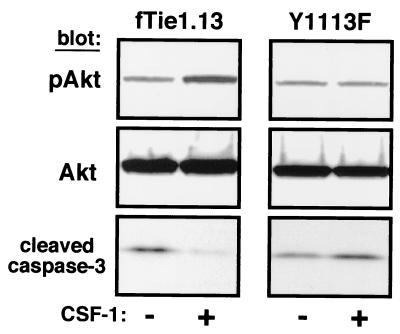
To determine whether the Y1113F mutation affects Tie1-mediated cell survival, cells expressing both wild-type fTie1 and Y1113F were serum starved and UV irradiated, and then cell lysates were evaluated for Akt phosphorylation and caspase 3 cleavage. CSF-1 treatment of wild-type fTie1-expressing cells again resulted in Akt phosphorylation and inhibition of caspase 3 cleavage (Fig. 8). In contrast, ligand treatment of Y1113F-expressing cells resulted in no change in the amount of either phospho-Akt or cleaved caspase 3. Because caspase 3 activation was inhibited by Tie1 in a PI 3-kinase-dependent manner, these findings indicate that tyrosine 1113 is required for activation of PI 3-kinase and inhibition of apoptosis by Tie1.
FIG. 8.
Mutation of Y1113 blocks the antiapoptotic effect of Tie1. Cells expressing wild-type fTie1 or Y1113F were serum starved overnight in the absence or presence of CSF-1 (500 ng/ml). The following morning the cells were UV irradiated (1,200 J/m2). Cell lysates were separated by SDS-8 to 16% PAGE and Western blotted with the indicated Abs. CSF-1 treatment induced an increase in Akt phosphorylation and a decrease in caspase 3 cleavage in wild-type fTie1 cells but not in Y1113F-expressing cells.
DISCUSSION
In this report, we have demonstrated that the endothelial RTK Tie1 is functionally active and that it can associate with the p85 regulatory subunit of PI 3-kinase in vitro in an autophosphorylation-dependent manner. Following stable expression of a chimeric Tie1 receptor in NIH 3T3 cells, ligand activation was found to induce receptor autophosphorylation that resulted in activation of both PI 3-kinase and Akt. Furthermore, Tie1 activation potently inhibited UV irradiation-induced apoptosis. Mutation of tyrosine 1113 in the Tie1 C-tail abrogated both Akt phosphorylation and inhibition of caspase 3 cleavage, suggesting that Y1113 is the preferred site of association of PI 3-kinase on Tie1. Together, these findings are the first biochemical demonstration of a signal transduction pathway and a corresponding cellular function for this orphan receptor. Moreover, these results are consistent with a role for Tie1 in endothelial cell survival that was suggested previously by the results of studies in transgenic mice (31-33).
As with other orphan receptors, the study of Tie1-mediated signal transduction pathways and cellular functions has been complicated by the lack of a known ligand. In spite of the high degree of homology between Tie1 and Tie2, none of the angiopoietins, which are ligands for Tie2, has been shown to bind to Tie1 (2, 24, 39). We circumvented this problem by stably expressing the fTie1 receptor in NIH 3T3 cells, allowing ligand activation of the Tie1 kinase and evaluation of downstream signaling and function in a cellular context. A potential limitation of this approach is that fTie1 might transduce a different set of signals than the endogenous, full-length Tie1 does. However, we used a similar approach previously to demonstrate activation of PI 3-kinase and Akt by Tie2 before its ligands became readily available (19), and these effects were subsequently confirmed in endothelial cells using the activating Tie2 ligand, Ang1 (13, 16, 27). In light of studies supporting an endothelial cell survival function for Tie1, it seems likely that the present findings will hold true in endothelial cells, but ultimate confirmation of these results will require identification of the ligand(s) for Tie1.
In a recent report, Marron et al. were unable to demonstrate autophosphorylation of a chimeric TrkA-Tie1 receptor (25). According to that report, the receptor was transiently transfected into endothelial cells, an approach that may not have yielded efficient receptor expression. In our experiments, stable expression of fTie1 likely resulted in higher levels of receptor expression and may have enhanced our ability to detect Tie1 signaling events. Although autophosphorylation of RTKs is often used as an indicator of their activation, it is possible that Tie1 is a poor substrate for itself. Similarly, phosphorylation of synthetic tyrosine-containing peptides, which Tie1 apparently fails to phosphorylate in vitro (25), may be a poor surrogate marker of Tie1 activation. Our studies suggest that this is the case; in spite of weak Tie1 autophosphorylation in fTie1 cells, we readily detected Tie1 in antiphosphotyrosine immunoprecipitates and Tie1 activation of Akt. Importantly, these effects were dependent on both ligand activation and Tie1 kinase activity, demonstrating that they were indeed mediated by Tie1. Notably, Partanen et al. found that the full-length Tie1 protein also immunoprecipitated with antiphosphotyrosine Abs in spite of undetectable tyrosine kinase activity (29). These findings suggest that Tie1 is activated out of proportion to its level of autophosphorylation.
Several reports have demonstrated that treatment of endothelial cells with phorbol myristate acetate, VEGF, or TNF-α induces cleavage of Tie1 to release a soluble extracellular domain and an endodomain fragment (41, 42), which likely consists of the transmembrane and cytoplasmic kinase domains, but whose function remains unclear. In a recent report, the Tie1 endodomain was found to coimmunoprecipitate with the protein tyrosine phosphatase Shp2 (26), although no function has been demonstrated for this interaction. Based on this finding, it was suggested that endodomain generation might constitute a potential form of ligand-independent signaling by Tie1. In fact, our results indicate that Tie1 signals effectively following ligand activation in the context of the fTie1 chimeric receptor. Because of the potential role of Tie1 in vascular maturation, it seems more likely that the purpose of Tie1 cleavage by angiogenic or inflammatory cytokines, such as VEGF and TNF-α, is to inhibit Tie1 signaling and to destabilize the mature vasculature in order to facilitate angiogenesis. Extracellular domain cleavage following ligand binding has been shown previously to be a mechanism for regulating signaling and function by other RTKs (3, 37). The presence of signaling proteins associated with cleaved Tie1 might not be unexpected following proteolytic processing and internalization of the receptor. Studies evaluating the subcellular localization of these complexes may help elucidate their true biological role.
The high degree of homology between Tie1 and Tie2, along with studies suggesting that these two receptors perform complementary functions, has led many to speculate that they heterodimerize. Hetero-oligomerization has been demonstrated for other RTKs, such as members of the EGF and VEGF receptor subfamilies, which also include receptors with weak or absent catalytic activity like Tie1 (37). Unlike these receptor subfamilies, however, a common ligand for the Tie receptors has not been identified. The recent finding that the Tie1 endodomain coimmunoprecipitates with Tie2 supports the possibility of Tie1/Tie2 heterodimerization (25), but it also raises important questions. For example, does the Tie2/Tie1 endodomain association occur at the plasma membrane or in endocytic vesicles following proteolytic processing? Furthermore, is the interaction direct, or is it mediated by a scaffold or other intermediate signaling protein? Answers to these questions will likely shed light on both the mechanism and function of Tie1/Tie2 association. Although this interaction was felt to indicate that Tie1 might modulate Tie2 signaling (25), the Tie1 endodomain was not found to be tyrosine phosphorylated in its complex with Tie2, and no effects on Tie2 have been demonstrated following interaction with Tie1. Importantly, our results demonstrate that Tie1 activates PI 3-kinase and Akt and that it transduces a potent survival signal in cells that lack Tie2. Thus, while heterodimerization with Tie2 and modulation of Tie2 signaling comprise a potential but as yet unproven function for Tie1, our results suggest that Tie1 inhibits apoptosis autonomously of Tie2.
The presence of overlapping signaling pathways and functions for Tie1 and Tie2 is suggested by their marked structural similarities, as well as by the results of genetic studies (23, 31, 32). The presence of an ideal p85 consensus binding sequence at tyrosine 1113 suggested that Tie1 could activate PI 3-kinase. Moreover, this residue corresponds to Y1101 of Tie2, which was shown to be required for activation of both PI 3-kinase and Akt (19). Ligand stimulation of Tie1 clearly activated PI 3-kinase and Akt in a cellular context, suggesting that this residue is the likely PI 3-kinase binding site. Furthermore, mutation of Y1113 abrogated two different PI 3-kinase-dependent effects of Tie1 (Akt phosphorylation and caspase 3 cleavage). Together, these results support Y1113 as the primary p85-binding site on Tie1. These findings again raise the important question of why two highly homologous receptors with overlapping functions are both required for embryonic vascular development. The answer to this question will likely provide important insights into the molecular mechanisms of angiogenesis.
Recently, there has been great interest in understanding the mechanisms of angiogenesis in order to develop both pro- and antiangiogenic therapies for a variety of diseases, from ischemic vascular diseases to cancer. Studies are currently under way to evaluate the efficacy of angiogenic growth factors like VEGF and basic fibroblast growth factor (bFGF) for nonrevascularizable coronary artery disease and peripheral vascular disease (9, 11). VEGF is required for the early steps of angiogenesis but not for subsequent vascular morphogenesis, maturation, or stabilization of the vasculature, which appears to be regulated by Ang1, Tie2, and Tie1 (10, 30, 43). A potential problem with present approaches is that it is unclear whether administration of VEGF or bFGF will initiate the subsequent steps required for proper vascular remodeling and maturation. Tie1 and Tie2 may need to be activated in an appropriate temporal and spatial context in order for nascent blood vessels to continue to develop and mature. Although much work remains to be done to elucidate the precise molecular mechanisms of angiogenesis, our identification of an antiapoptotic function of Tie1 provides an important insight into the mechanisms of vascular development. These findings and the eventual identification of a Tie1 ligand may therefore lead to improved therapeutic interventions for a variety of angiogenic diseases.
Acknowledgments
We thank Genetics Institute for providing CSF-1 and Genetix Pharmaceuticals for providing GP+E86 retroviral packaging cells.
This work was supported in part by grants from the National Institutes of Health (HL 03557 and HL 55265 to C.D.K.) and by a Career Development Award from the Duke Heart Center to C.D.K. K.G.P. is supported by the Procter & Gamble Health Care Research Center. J.D.Y. is supported by the Howard Hughes Medical Institute.
REFERENCES
- 1.Carpenter, C. L., and L. C. Cantley. 1996. Phosphoinositide kinases. Curr. Opin. Cell Biol. 8:153-158. [DOI] [PubMed] [Google Scholar]
- 2.Davis, S., T. H. Aldrich, P. F. Jones, A. Acheson, D. L. Compton, V. Jain, T. E. Ryan, J. Bruno, C. Radziejewski, P. C. Maisonpierre, and G. D. Yancopoulos. 1996. Isolation of angiopoietin-1, a ligand for the Tie-2 receptor, by secretion-trap expression cloning. Cell 87:1161-1169. [DOI] [PubMed] [Google Scholar]
- 3.Downing, J. R., M. F. Roussel, and C. J. Sherr. 1989. Ligand and protein kinase C downmodulate the colony-stimulating factor 1 receptor by independent mechanisms. Mol. Cell. Biol. 9:2890-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downward, J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10:262-267. [DOI] [PubMed] [Google Scholar]
- 5.Dubois, N. A., L. C. Kolpack, R. Wang, R. G. Azizkhan, and V. L. Bautch. 1991. Isolation and characterization of an established endothelial cell line from transgenic mouse hemangiomas. Exp. Cell Res. 196:302-313. [DOI] [PubMed] [Google Scholar]
- 6.Dumont, D. J., G. H. Fong, M. C. Puri, G. Gradwohl, K. Alitalo, and M. L. Breitman. 1995. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev. Dyn. 203:80-92. [DOI] [PubMed] [Google Scholar]
- 7.Dumont, D. J., G. J. Gradwohl, G. H. Fong, R. Auerbach, and M. L. Breitman. 1993. The endothelial-specific receptor tyrosine kinase, TEK, is a member of a new subfamily of receptors. Oncogene 8:1293-1302. [PubMed] [Google Scholar]
- 8.Dumont, D. J., T. P. Yamaguchi, R. A. Conlon, J. Rossant, and M. L. Breitman. 1992. tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene 7:1471-1480. [PubMed] [Google Scholar]
- 9.Ferrara, N., and K. Alitalo. 1999. Clinical applications of angiogenic growth factors and their inhibitors. Nat. Med. 5:1359-1364. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan, D., and J. Folkman. 1996. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86:353-364. [DOI] [PubMed] [Google Scholar]
- 11.Helisch, A., and W. Schaper. 2000. Angiogenesis and arteriogenesis—not yet for prescription. Z. Kardiol. 89:239-244. [DOI] [PubMed] [Google Scholar]
- 12.Huang, L., C. W. Turck, P. Rao, and K. G. Peters. 1995. GRB2 and SH-PTP2: potentially important endothelial signaling molecules downstream of the TEK receptor tyrosine kinase. Oncogene 11:2097-2103. [PubMed] [Google Scholar]
- 13.Jones, N., Z. Master, J. Jones, D. Bouchard, Y. Gunji, H. Sasaki, R. Daly, K. Alitalo, and D. J. Dumont. 1999. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J. Biol. Chem. 274:30896-30905. [DOI] [PubMed] [Google Scholar]
- 14.Kaipainen, A., T. Vlaykova, E. Hatva, T. Bohling, A. Jekunen, S. Pyrhonen, and K. Alitalo. 1994. Enhanced expression of the tie receptor tyrosine kinase messenger RNA in the vascular endothelium of metastatic melanomas. Cancer Res. 54:6571-6577. [PubMed] [Google Scholar]
- 15.Kim, I., H. G. Kim, S. O. Moon, S. W. Chae, J. N. So, K. N. Koh, B. C. Ahn, and G. Y. Koh. 2000. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ. Res. 86:952-959. [DOI] [PubMed] [Google Scholar]
- 16.Kim, I., H. G. Kim, J. N. So, J. H. Kim, H. J. Kwak, and G. Y. Koh. 2000. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ. Res. 86:24-29. [DOI] [PubMed] [Google Scholar]
- 17.Klinghoffer, R. A., and A. Kazlauskas. 1995. Identification of a putative Syp substrate, the PDGFβ receptor. J. Biol. Chem. 270:22208-22217. [DOI] [PubMed] [Google Scholar]
- 18.Koblizek, T. I., C. Weiss, G. D. Yancopoulos, U. Deutsch, and W. Risau. 1998. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr. Biol. 8:529-532. [DOI] [PubMed] [Google Scholar]
- 19.Kontos, C. D., T. Stauffer, W.-P. Yang, J. D. York, L. Huang, M. A. Blanar, T. Meyer, and K. G. Peters. 1998. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol. Cell. Biol. 18:4131-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korhonen, J., J. Partanen, E. Armstrong, A. Vaahtokari, K. Elenius, M. Jalkanen, and K. Alitalo. 1992. Enhanced expression of the tie receptor tyrosine kinase in endothelial cells during neovascularization. Blood 80:2548-2555. [PubMed] [Google Scholar]
- 21.Korhonen, J., A. Polvi, J. Partanen, and K. Alitalo. 1994. The mouse tie receptor tyrosine kinase gene: expression during embryonic development. Oncogene 9:395-403. [PubMed] [Google Scholar]
- 22.Kulik, G., A. Klippel, and M. J. Weber. 1997. Antiapoptotic signalling by insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol. Cell. Biol. 17:1595-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loughna, S., and T. N. Sato. 2001. A combinatorial role of angiopoietin-1 and orphan receptor TIE1 pathways in establishing vascular polarity during angiogenesis. Mol. Cell 7:233-239. [DOI] [PubMed] [Google Scholar]
- 24.Maisonpierre, P. C., C. Suri, P. F. Jones, S. Bartunkova, S. J. Wiegand, C. Radziejewski, D. Compton, J. McClain, T. H. Aldrich, N. Papadopoulos, T. J. Daly, S. Davis, T. N. Sato, and G. D. Yancopoulos. 1997. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55-60. [DOI] [PubMed] [Google Scholar]
- 25.Marron, M. B., D. P. Hughes, M. D. Edge, C. L. Forder, and N. P. Brindle. 2000. Evidence for heterotypic interaction between the receptor tyrosine kinases TIE-1 and TIE-2. J. Biol. Chem. 275:39741-39746. [DOI] [PubMed] [Google Scholar]
- 26.Marron, M. B., D. P. Hughes, M. J. McCarthy, E. R. Beaumont, and N. P. Brindle. 2000. Tie-1 receptor tyrosine kinase endodomain interaction with SHP2: potential signalling mechanisms and roles in angiogenesis. Adv. Exp. Med. Biol. 476:35-46. [DOI] [PubMed] [Google Scholar]
- 27.Papapetropoulos, A., D. Fulton, K. Mahboubi, R. G. Kalb, D. S. O'Connor, F. Li, D. C. Altieri, and W. C. Sessa. 2000. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J. Biol. Chem. 275:9102-9105. [DOI] [PubMed] [Google Scholar]
- 28.Papapetropoulos, A., G. Garcia-Cardena, T. J. Dengler, P. C. Maisonpierre, G. D. Yancopoulos, and W. C. Sessa. 1999. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab. Investig. 79:213-223. [PubMed] [Google Scholar]
- 29.Partanen, J., E. Armstrong, T. P. Makela, J. Korhonen, M. Sandberg, R. Renkonen, S. Knuutila, K. Huebner, and K. Alitalo. 1992. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol. Cell. Biol. 12:1698-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Partanen, J., and D. J. Dumont. 1999. Functions of Tie1 and Tie2 receptor tyrosine kinases in vascular development. Curr. Top. Microbiol. Immunol. 237:159-172. [DOI] [PubMed] [Google Scholar]
- 31.Partanen, J., M. C. Puri, L. Schwartz, K. D. Fischer, A. Bernstein, and J. Rossant. 1996. Cell autonomous functions of the receptor tyrosine kinase TIE in a late phase of angiogenic capillary growth and endothelial cell survival during murine development. Development 122:3013-3021. [DOI] [PubMed] [Google Scholar]
- 32.Puri, M. C., J. Partanen, J. Rossant, and A. Bernstein. 1999. Interaction of the TEK and TIE receptor tyrosine kinases during cardiovascular development. Development 126:4569-4580. [DOI] [PubMed] [Google Scholar]
- 33.Puri, M. C., J. Rossant, K. Alitalo, A. Bernstein, and J. Partanen. 1995. The receptor tyrosine kinase tie is required for integrity and survival of vascular endothelial cells. EMBO J. 14:5884-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rameh, L. E., and L. C. Cantley. 1999. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 274:8347-8350. [DOI] [PubMed] [Google Scholar]
- 35.Sato, T. N., Y. Qin, C. A. Kozak, and K. L. Audus. 1993. tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc. Natl. Acad. Sci. USA 90:9355-9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato, T. N., Y. Tozawa, U. Deutsch, K. Wolburg-Bucholz, Y. Fujiwara, M. Gendron-Maguire, T. Gridley, H. Wolburg, W. Risau, and Y. Qin. 1995. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376:70-74. [DOI] [PubMed] [Google Scholar]
- 37.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 38.Songyang, Z., S. E. Shoelson, M. Chauhuri, G. Gish, T. Pawson, W. G. Haser, F. King, T. Roberts, S. Ratnofsky, R. J. Lechleider, B. G. Neel, R. B. Birge, J. E. Fajardo, M. M. Chou, H. Hanafusa, B. Schaffhausen, and L. C. Cantley. 1993. SH2 domains recognize specific phosphopeptide sequences. Cell 72:767-778. [DOI] [PubMed] [Google Scholar]
- 39.Valenzuela, D. M., J. A. Griffiths, J. Rojas, T. H. Aldrich, P. F. Jones, H. Zhou, J. McClain, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, T. Huang, N. Papadopoulos, P. C. Maisonpierre, S. Davis, and G. D. Yancopoulos. 1999. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc. Natl. Acad. Sci. USA 96:1904-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witzenbichler, P., P. C. Maisonpierre, P. Jones, G. D. Yancopoulos, and J. M. Isner. 1998. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J. Biol. Chem. 273:18514-18521. [DOI] [PubMed] [Google Scholar]
- 41.Yabkowitz, R., S. Meyer, T. Black, G. Elliott, L. A. Merewether, and H. K. Yamane. 1999. Inflammatory cytokines and vascular endothelial growth factor stimulate the release of soluble tie receptor from human endothelial cells via metalloprotease activation. Blood 93:1969-1979. [PubMed] [Google Scholar]
- 42.Yabkowitz, R., S. Meyer, D. Yanagihara, D. Brankow, T. Staley, G. Elliott, S. Hu, and B. Ratzkin. 1997. Regulation of tie receptor expression on human endothelial cells by protein kinase C-mediated release of soluble tie. Blood 90:706-715. [PubMed] [Google Scholar]
- 43.Yancopoulos, G. D., S. Davis, N. W. Gale, J. S. Rudge, S. J. Wiegand, and J. Holash. 2000. Vascular-specific growth factors and blood vessel formation. Nature 407:242-248. [DOI] [PubMed] [Google Scholar]