Abstract
Apoliprotein J (apoJ)/clusterin has attracted considerable interest based on its inducibility in multiple injury processes and accumulation at sites of remodeling, regression, and degeneration. We therefore sought to investigate apoJ/clusterin's role in kidney aging, as this may reveal the accumulated effects of diminished protection. Aging mice deficient in apoJ/clusterin developed a progressive glomerulopathy characterized by the deposition of immune complexes in the mesangium. Up to 75% of glomeruli in apoJ/clusterin-deficient mice exhibited moderate to severe mesangial lesions by 21 months of age. Wild-type and hemizygous mice exhibited little or no glomerular pathology. In the apoJ/clusterin-deficient mice, immune complexes of immunoglobulin G (IgG), IgM, IgA, and in some cases C1q, C3, and C9 were detectable as early as 4 weeks of age. Electron microscopy revealed the accumulation of electron-dense material in the mesangial matrix and age-dependent formation of intramesangial tubulo-fibrillary structures. Even the most extensively damaged glomeruli showed no evidence of inflammation or necrosis. In young apoJ/clusterin-deficient animals, the development of immune complex lesions was accelerated by unilateral nephrectomy-induced hyperfiltration. Injected immune complexes localized to the mesangium of apoJ/clusterin-deficient but not wild-type mice. These results establish a protective role of apoJ/clusterin against chronic glomerular kidney disease and support the hypothesis that apoJ/clusterin modifies immune complex metabolism and disposal.
Apolipoprotein J (apoJ)/clusterin is a circulating glycoprotein constitutively expressed by diverse epithelial cells. The protein is induced in injured organs in various disease states, such as Alzheimer's disease, atherosclerosis, myocardial infarction, and multiple forms of acute and chronic renal disease (20, 25). Proposed functions for apoJ/clusterin include lipid transport, complement defense, regulation of apoptosis, membrane protection, and promotion of cell-cell interactions (25). ApoJ/clusterin can bind a large number of macromolecules implicated in disease initiation and progression, including immunoglobulins and complement components. Recently clusterin has been demonstrated to function as a molecular chaperone, preventing denatured protein precipitation through binding to exposed hydrophobic regions and improving high-molecular-weight complex solubility (6).
The structure of apoJ/clusterin has not provided much insight into function. Mammalian apoJ/clusterins are approximately 80-kDa heterodimers (9, 16) consisting of two 40-kDa chains joined by a unique five-disulfide-bond motif (10). The protein has limited homology to other proteins and lacks clear functional motifs (9). It does contain three putative amphipathic α-helical regions, which could allow it to interact with lipids and hydrophobic regions of other proteins (6).
We have recently shown that apoJ/clusterin-deficient mice exhibit enhanced inflammatory severity and sequelae in an autoimmune myocarditis model, suggesting that it can serve an anti-inflammatory role under some conditions (14). Given the marked upregulation of apoJ/clusterin that occurs in diverse tissue injury processes, it is likely that the effects of its absence in different models may reveal a variety of phenotypic features and manifestations. We hypothesized that if apoJ/clusterin played an important role in the management of inflammatory and apoptosis-associated protein complexes, there should be an accumulated effect of the failure to properly manage these proteins over time. Since free plasma protein and macromolecular complexes traffic through the mesangium of the kidney, this structure is particularly at risk for compromise by the absence of apoJ/clusterin. In this study, we demonstrate that the kidneys of apoJ/clusterin-deficient mice developed a progressive glomerulopathy with age, characterized by mesangial expansion and the presence of deposits of immunoglobulins and complement components. These findings implicate a role for apoJ/clusterin in the long-term health of the kidney and suggest that it participates in a biochemical system for mesangial protection.
MATERIALS AND METHODS
Generation of apoJ/clusterin-deficient mice.
apoJ/clusterin-deficient mice were generated by standard techniques of homologous recombination using the hypoxanthine phosphoribosyltransferase/thymidine kinase (HPRT/TK) selection method (14, 24). Both male and female homozygous deficient animals were fertile while young and gave birth to normal-size litters. The mice were maintained in the Swiss Black outbred background. All animals used in these studies were confirmed to lack apoJ/clusterin by Southern and PCR analysis, as previously described (14). apoJ/clusterin-deficient mice lacked constitutively expressed, immuno-detectable apoJ/clusterin in serum or liver (14) and failed to exhibit induced expression of apoJ/clusterin in kidneys following acute tubular injury induced by folic acid (data not shown). This lack of induction provides further evidence that the constitutively inactivated apoJ/clusterin gene in these mice is also uninducible. All animal studies were approved by the Institutional Review Boards at the University of Minnesota and Children's Hospital Research Foundation.
Unilateral nephrectomy.
Renal hemodynamic filtration load was increased by right unilateral nephrectomy at 3 months of age. Mice were then placed on a 40% protein diet for 3 months, at which time they were sacrificed and kidneys were examined by light, electron, and immunofluorescence microscopy.
Assessment of renal lesions. (i) Light microscopy.
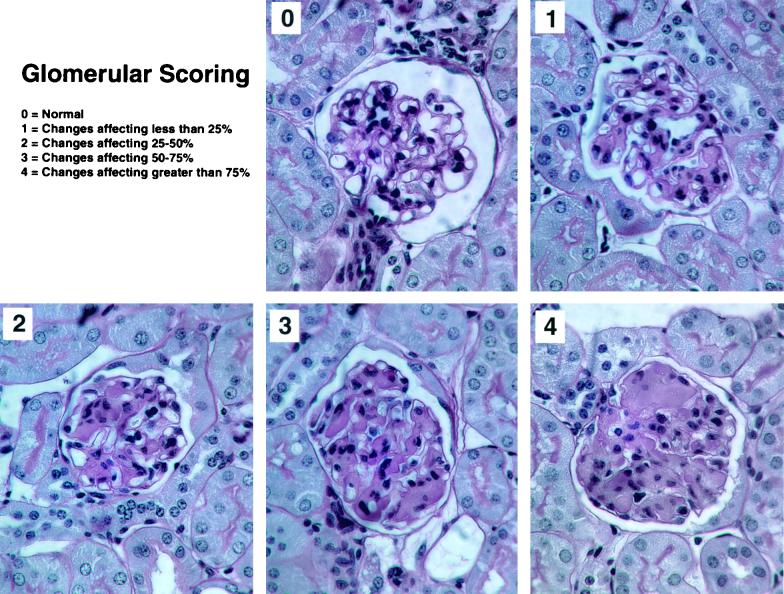
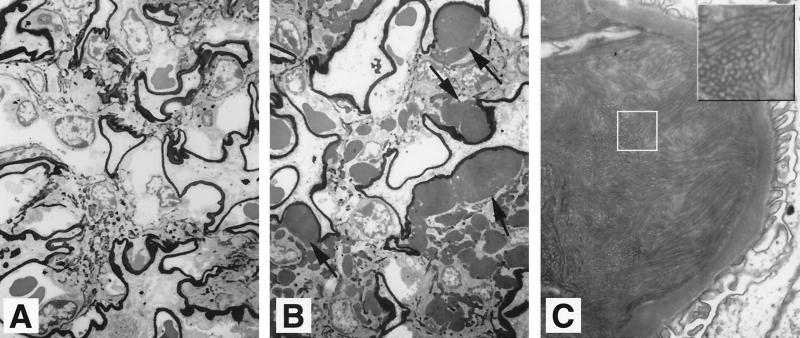
The glomerular lesions were scored using a semiquantitative scale, based on the extent of expansion of the mesangium by the hypocellular material. The following scale was used: 0 = normal; 1 = change affecting less than 25% of the glomerulus; 2 = change affecting 25 to 50%; 3 = change affecting 50 to 75%; 4 = changes affecting greater than 75% (see Fig. 1).
FIG. 1.
apoJ/clusterin-deficient mice develop glomerulopathy. Representative glomeruli are shown from a single kidney of a 21-month-old mouse to illustrate the range of affected structures and the basis of a glomerular lesion scoring system. The different panels represent scores of 0 to 4, based on the extent of expansion of the mesangium by hypocellular material, where 0 = normal, 1 = change affecting less than 25% of the glomerulus, 2 = change affecting 25 to 50%, 3 = change affecting 50 to 75%, and 4 = changes affecting greater than 75%. Magnification, ×400.
(ii) Immunofluorescence.
Tissues were harvested and snap-frozen in isopentane and liquid nitrogen. The tissues were then sectioned and fixed on glass slides. After three washes in phosphate-buffered saline, primary antibody (1:100) was applied to sections and incubated at room temperature for 1 h. If needed, secondary antibodies were applied and incubated similarly. Polyclonal fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (IgG), IgA, IgM, and complement C3 (ICN Biomedicals, Inc., Irvine, Calif.) were used as primary antibodies in all direct immunofluorescence studies. Other primary antibodies used in indirect immunofluorescence studies were a monoclonal rat anti-mouse C1q (Cedarlane Laboratories, Hornby, Ontario, Canada) and goat anti-human C9 that cross-reacted with mouse C9 (ICN Biomedicals). Secondary antibodies used in indirect immunofluorescence studies were fluorescein isothiocyanate-conjugated rabbit anti-goat and goat anti-rat IgG (ICN Biomedicals, Inc.). The glomeruli of three apoJ/clusterin deficient mice and three wild-type or hemizygous mice were stained at each time point. Lesions were quantified on a scale of 1 to 4, based on the intensity of staining, as validated previously (11).
(iii) Electron microscopy.
Kidneys were fixed in glutaraldehyde, rinsed in Cacodylate buffer, sliced into 1-mm slabs, postfixed for 2 h in 1% osmium tetroxide in 0.1 M cacodylate, dehydrated in graded ethanols, and embedded in Electron Microscopy Sciences' Embed 812. Sections 0.5-μm thick were cut and stained with Richardson's dye (1% toluidine blue) and evaluated by light microscopy. Areas with glomeruli were selected for fine trimming and thin sectioning. Sections approximately 50-nm thick were cut on a Reichert Ultracut S ultramicrotome and stained with either 50% methanolic uranyl acetate for 25 min and lead citrate for 3 min or methenamine silver (13). Grids were examined at 80 kV in a JEOL 1200EX electron microscope and photographed on Kodak Estar 4489 film.
Clearance of immune complexes.
The clearance of immune complexes was determined by their removal from blood and deposition in kidneys. Immune complexes composed of Oregon Green488-labeled goat IgG and mouse anti-goat IgG were assembled in vitro. Mouse anti-goat IgG (200 μl, 1.7 mg/ml; Jackson ImmunoResearch, West Grove, Pa.) was labeled (four to six dye molecules per immunoglobulin molecule) with FluoReporter Oregon Green488 Protein Labeling Kit (Molecular Probes, Eugene, Ore.). Equal amounts of labeled mouse antibody and goat IgG (Sigma Chemical, St. Louis, Mo.) were mixed and allowed to react at 37°C for 30 min. The size of the immune complexes was determined by gel filtration (Sephacryl S-300; PharmaciaAB, Uppsala, Sweden) (data not shown).
apoJ/clusterin-deficient (n = 7) and wild-type mice (n = 5) were injected at 5 months of age via the left internal jugular vein with 100 μg of the immune complex/30 g body weight. The mice were sacrificed 30 min after immune complex injection, and blood and kidneys were harvested. Serum fluorescence was quantified on a Perkin-Elmer LS-5B luminescence spectrophotometer using 488- and 512-nm excitation and emission, respectively. Kidney immune complex deposits were visualized by direct fluorescence of cryostat sections (5 μm) fixed in 95% ethanol for 5 min and mounted in phosphate-buffered saline-glycerol and also by the use of primary rabbit anti-goat antibody followed by Texas Red-labeled goat anti-rabbit antibody.
RESULTS
apoJ/clusterin-deficient mice develop progressive glomerulopathy.
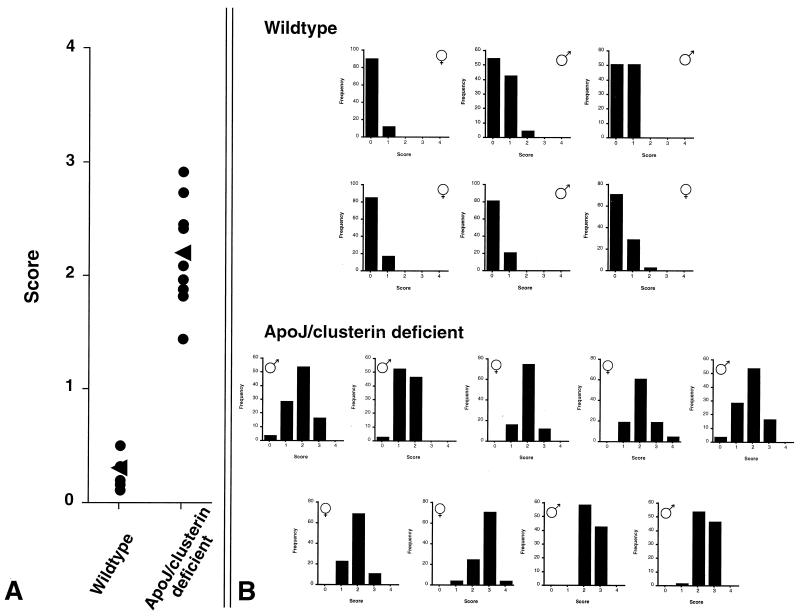
Young apoJ/clusterin-deficient mice lacked obvious pathology in the kidney or any other organ when examined by light microscopy. However, mice examined at 21 months of age exhibited a striking glomerulopathy characterized by progressive mesangial expansion and collapse of capillary lumens (Fig. 1). In contrast, kidneys of hemizygous and wild-type littermates were normal, with only occasional and limited mesangial expansion. Glomerulopathy was not associated with tubulointerstitial disease or vascular lesions. A total of 100 glomeruli in each of nine apoJ/clusterin-deficient mice and six wild-type mice were scored. Mean scores obtained by a single observer for each of the 15 mice are shown in Fig. 2A. Extensive sampling by other blinded observers produced essentially identical results. All apoJ/clusterin-deficient mice developed mesangial lesions, although the extent of involvement varied between mice. There was no gender difference in the frequency or extent of the lesions. Glomerular scores for each mouse are displayed in Fig. 2B. Consistent with the sampling shown in Fig. 1, each kidney of an apoJ/clusterin-deficient mouse contained differentially affected glomeruli.
FIG. 2.
Accumulation of damaged glomeruli in apoJ/clusterin-deficient mice. (A) Summary of average glomerular scores of wild-type and apoJ/clusterin-deficient animals. (B) Distribution of scores for 100 scored glomeruli in each wild-type and apoJ/clusterin-deficient mouse. Although all mutant mice developed glomerular lesions, there was significant heterogeneity in the extent of mesangial deposition across each kidney.
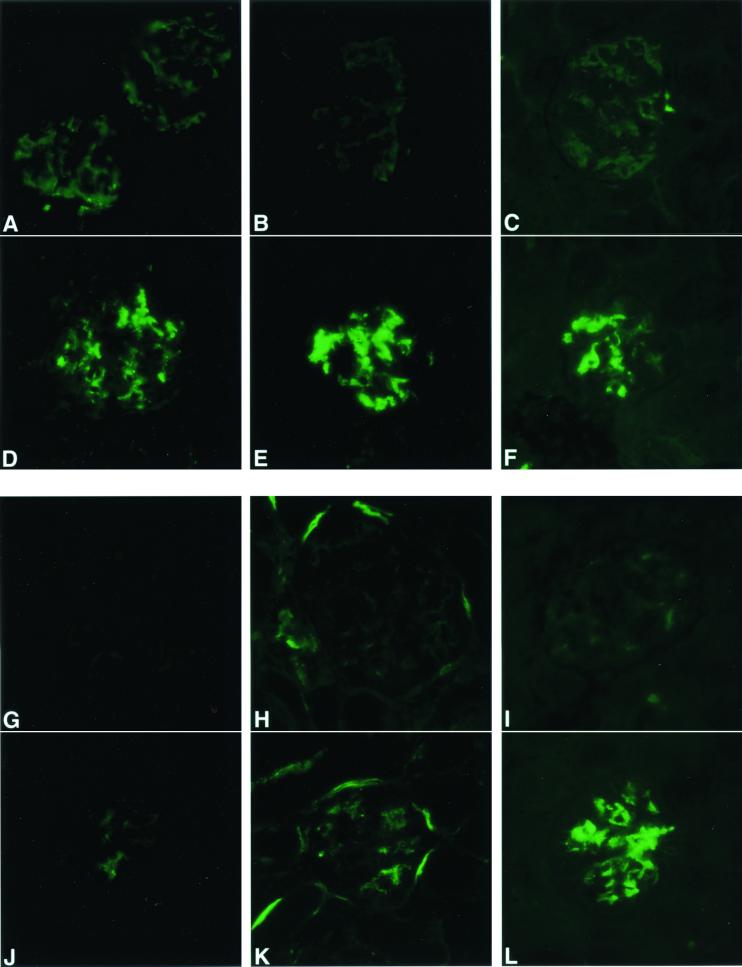
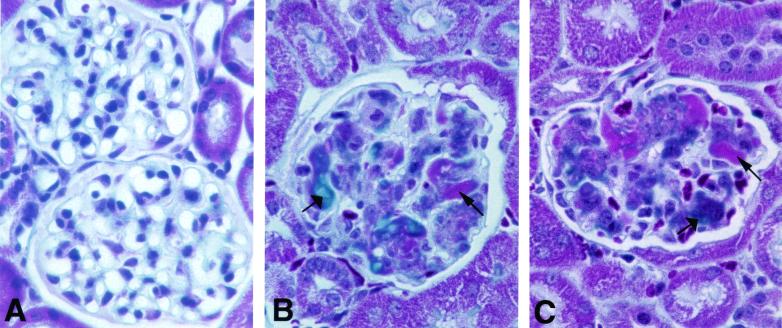
Using a series of specific immunodetection reagents, glomeruli of the apoJ/clusterin-deficient mice stained in a mesangial pattern for IgG, IgM, IgA, C1q, C3, and C9 (average scores of 2+, 3+, 2+, 1+, 1+, 3+, respectively) (Fig. 3). Glomeruli from hemizygous mice had trace staining for IgG and IgA only. Abnormal glomeruli contained Masson-Trichrome-positive blue material (Fig. 4), consistent with collagen deposition, as well as fuchsin-colored inclusions, indicative of immune deposits. In contrast, the glomeruli failed to stain with Congo Red (data not shown), suggesting the absence of amyloid deposits. Surprisingly, no inflammatory infiltrates or regions of necrosis were evident in the glomeruli, regardless of the extent of glomerulopathy.
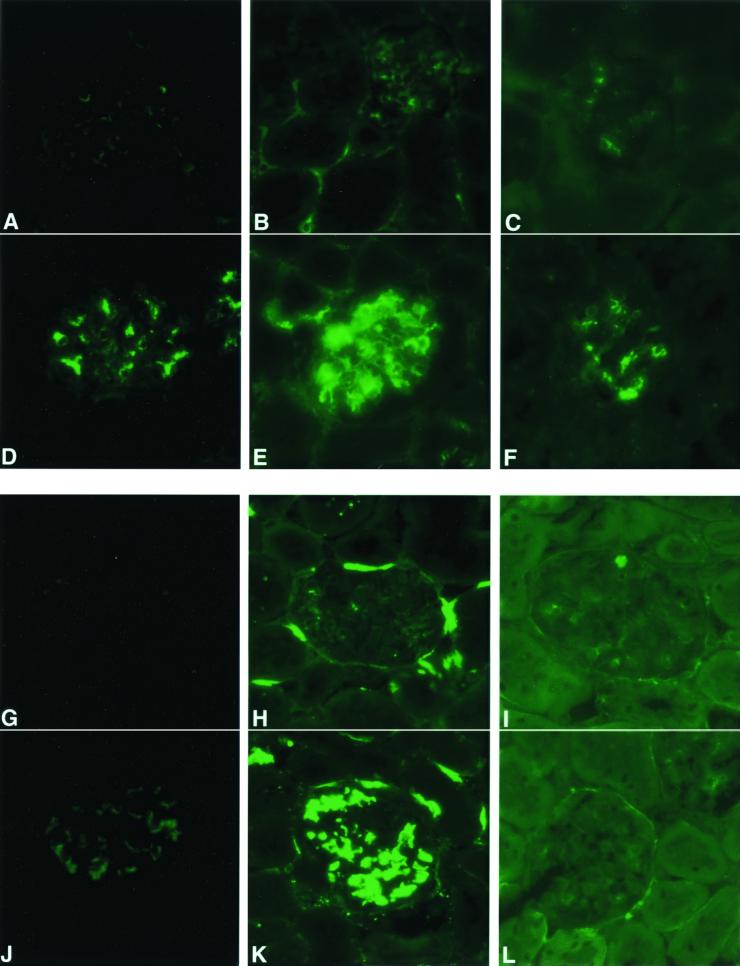
FIG. 3.
Immune complexes are deposited in glomeruli of 21-month-old apoJ/clusterin-deficient mice but not hemizygous mice. Tissue sections from hemizygous (A to C, G to I) and apoJ/clusterin-deficient mice (D to F, J to L) were analyzed with antibodies specific for IgG (A and D), IgM (B and E), IgA (C and F), C1q (G and J), C3 (H and K), and C9 (I and L). Positive staining in a mesangial pattern was seen for the apoJ/clusterin-deficient mice for all markers, with particularly strong accumulation of IgM, IgA, and C9. Magnification, ×360.
FIG. 4.
Glomerular lesions contain deposits of collagen and immune complexes. The composition of the lesions in the glomeruli of wild-type (A) and apoJ/clusterin-deficient (B and C) mice was examined by using the Masson-Trichrome stain. Blue staining (wide arrows) suggests the accumulation of collagen deposits in the glomeruli, whereas fuchsin staining (narrow arrows) indicates the deposition of immune complexes. Magnification, ×360.
Electron microscopic examination of the glomeruli of 21-month-old mice (Fig. 5) revealed numerous electron-dense mesangial deposits. At higher magnification these structures were revealed to be organized tubulo-fibrillary structures, arranged in parallel arrays, with the average tubular diameter of 80 nm. The glomerular basement membranes were normal and lacked discernible deposits. The combination of electron-dense deposits and immunoglobulin staining confirmed that the deposits were immune complexes.
FIG. 5.
Electron-dense tubulo-fibrillar aggregates in the mesangium of apoJ/clusterin-deficient glomeruli. Sections from wild-type (A) and mutant (B) mice were silver stained. Mutant mice exhibited mesangial expansion and deposits of electron-dense material. Magnification, × 2,828. (C) High-power view of the mesangial deposits from mutant mice, demonstrating the tubulo-fibrillary structures. Magnification, ×18,800. The box indicates a region where the tubulo-fibrillary structures are evident.
Despite the pronounced glomerular phenotype of apoJ/clusterin-deficient mice, there was no difference in their life span compared with that of wild-type or hemizygote mice. The deficiency of apoJ/clusterin was not associated with development of proteinuria, although an occasional mutant mouse did develop hematuria. Analysis of serum and urine proteins by gel electrophoresis failed to reveal any differences in circulating proteins between apoJ/clusterin-deficient mice and wild-type mice (data not shown).
Development of mesangial lesions.
To identify factors contributing to the formation of the mesangial deposits in old mice, kidneys of young (4 weeks, 6 months) mutant and hemizygous mice were evaluated for histologic abnormality and/or glomerular deposits. No structural changes were detected in the glomeruli at 4 weeks of age by light or electron microscopy, and there was no accumulation of complement C3. However, immunoreactive IgG, IgM, and IgA did accumulate in apoJ/clusterin-deficient mice but not in wild-type mice (Table 1).
TABLE 1.
Summary of findings with apoJ/clusterin-deficient micea
| Characteristic | Results
|
|||
|---|---|---|---|---|
| 4 wk | 6 mo | 6 mo, UNX | 21 mo | |
| Mesangial expansion | No | No | Yes | Yes |
| EM deposits | No | No | Yes | Yes |
| Immunofluorescence: | ||||
| IgG | 2+ | 2+ | 2+ | 2+ |
| IgM | 3+ | 3+ | 3+ | 3+ |
| IgA | 1+ | 1+ | 1+ | 2+ |
| C1q | ND | ND | 2+ | 1+ |
| C3 | 0 | 0 | 3+ | 1+ |
| C9 | ND | ND | 0 | 3+ |
ND, not done; UNX, uninephrectomy. n = 3 mice for each time point. See Materials and Methods for an explanation of scoring.
At 6 months of age, apoJ/clusterin-deficient mice exhibited no changes beyond those detectable at 4 weeks. However, 6-month-old mice that had undergone a unilateral nephrectomy at 3 months of age and were then fed a high-protein diet showed an exacerbated phenotype, as evidenced by mesangial expansion and hypercellularity (data not shown). Unilateral nephrectomy induced the deposition of the complement components C1q and C3 in a mesangial distribution pattern (Fig. 6; Table 1). The levels of deposited IgG, IgM, and IgA were not further increased. Only trace staining for IgM was seen in glomeruli from wild-type mice. Electron micrographs demonstrated normal glomerular basement membranes but two kinds of deposits in the mesangium (Fig. 7): large lucent deposits intimately associated with mesangial cells, and electron-dense deposits in the mesangial matrix or beneath the perimesangial basement membrane. At high power, minimal fibrillar organization was evident.
FIG. 6.
apoJ/clusterin protects glomeruli from unilateral nephrectomy-accelerated accumulation of immune complex components in the mesangium. Tissue sections from 6-month-old unilaterally nephrectomized wild-type (A to C, G to I) and apoJ/clusterin-deficient mice (D to F, J to L) were analyzed with specific antibodies to IgG (A and D), IgM (B and E), IgA (C and F), C1q (G and J), C3 (H and K), and C9 (I and L). Mesangial pattern staining was detected only in the uninephrectomized apoJ/clusterin-deficient mice. Note also that C9 did not accumulate with either genotype. Magnification, ×400.
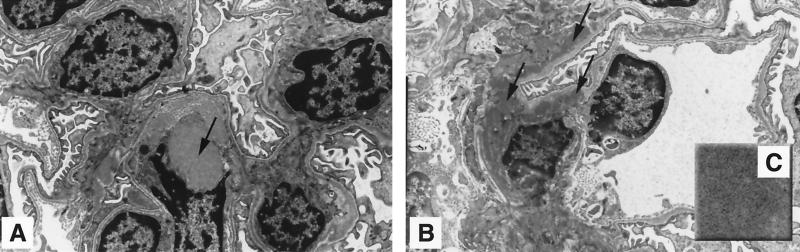
FIG. 7.
Glomeruli of uninephrectomized apoJ/clusterin-deficient mice developed two types of mesangial deposits. (A) Large lucent deposits, as indicated by the arrow, are associated with mesangial cells. (B) Electron-dense deposits (arrows) accumulated in the mesangial matrix or beneath the perimesangial basement membrane. Magnification, ×2,850. (C) The inset shows that the deposits had minimal fibrillar organization. Magnification, ×19,000.
Immune complex trafficking.
To test the ability of apoJ/clusterin to affect immune complex trafficking in the glomerulus, we administered in vitro-labeled and -assembled immune complexes intravenously. At 30 min following injection, similar levels of the immune complexes were detected in plasma (wild type, 147 ± 80, versus apoJ/clusterin-deficient, 114 ± 105 fluorescent units), suggesting that apoJ/clusterin does not play a large role in clearance of immune complexes from plasma at that time point. However, in the glomerulus, immune complexes accumulated in a mesangial distribution pattern only in apoJ/clusterin-deficient animals (Fig. 8).
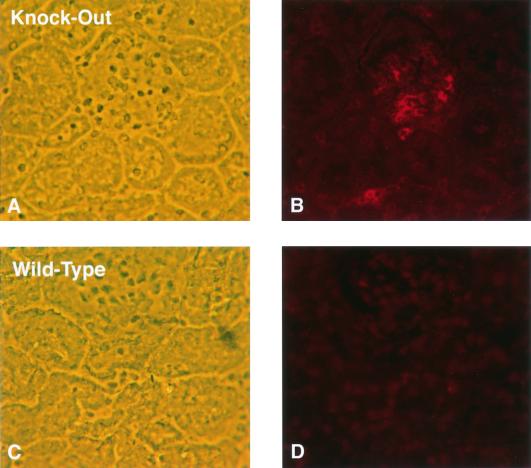
FIG. 8.
apoJ/clusterin protects the kidney from deposition of preassembled immunoglobulin complexes in the glomerulus. Phase contrast microscopy (A and C) shows comparable glomerular fields, which upon indirect fluorescence (B and D) exhibit strongly different distributions of Texas Red-labeled sheep anti-goat IgG complexes. Wild-type animals do not show any glomerular accumulation, whereas apoJ/clusterin-deficient mice do, in a patchy mesangial distribution.
DISCUSSION
We have shown here that apoJ/clusterin prevents a progressive glomerulopathy of aging in mice. apoJ/clusterin-deficient mice develop a pronounced mesangial expansion with the accumulation of electron-dense material in the matrix, which becomes highly organized into tubulo-fibrillary structures. As early as 4 weeks of age, immune material accumulates that includes IgG, IgM, and IgA. By 21 months, C1q, C3, and C9 are also deposited in the mesangium. The aging phenotype of the apoJ/clusterin-deficient mice could be accelerated in 6-month-old mice by unilateral nephrectomy combined with a high-protein diet, which increases trafficking of molecules through the mesangium (4, 23, 27). The consequent increase in mesangial trafficking led, in particular, to exacerbation of the amount of deposited material in the mesangium compared to results with similarly aged control mice. Intriguingly, in both normal and accelerated kidney aging, immune material deposition occurs without concomitant inflammation or necrosis.
The mesangial deposits associated with apoJ/clusterin deficiency may represent the products of in vivo-formed immune complexes, or they may be plasma immunoglobulins that become trapped by the mesangium as they traffic through the kidney. At this point we lack direct evidence for antigen involvement in deposit accumulation, although it is a formal possibility that the deposits are selectively those of antigen-antibody complexes. Our evidence that the mesangial deposits represent immune complexes is that there are multiple immunoglobulin isotypes present and that in late-stage deposits, complement components also are present. In addition, we showed that in vitro-assembled, intravenously injected antigen-antibody complexes became localized to the mesangium of 5-month-old apoJ/clusterin-deficient mice but not in wild-type mice to a detectable level. Nonetheless, the temporal dissociation between immunoglobulin and complement deposition suggests that apoJ/clusterin deficiency may cause an initial problem in immunoglobulin management, per se. Moreover, the absence of an elicited inflammatory response or frank and severe kidney disease elicited by the deposits suggests that the complexes are primarily sterile and may be largely devoid of antigen. At this time, the exact origin of the deposits remains uncertain.
A number of factors can influence the inflammatory processing of immune complexes. These include the degree of antigen load, immune complex physicochemical properties, site of deposition, presence of molecules that facilitate or inhibit interactions between the immune complexes, and the relative activation of additional inflammatory pathways, such as through complement. For example, immune complexes isolated from synovial fluid of patients with juvenile rheumatoid arthritis differ in their ability to induce proinflammatory events (7, 8). The immune complexes in the aging deficient mice most likely form under conditions of low antigen load, which could also be a factor responsible for the lack of observed inflammatory sequelae.
Immune complexes can activate inflammation through Fc receptor-mediated events (19). Signal transduction from Fc receptors, present on leukocytes and mesangial cells, is initiated by binding of Fc domains of immunoglobulins in immune complexes to the receptors, with their subsequent aggregation and induction of proinflammatory molecules, leukocyte recruitment, and mesangial cell activation (12, 21). This Fc receptor pathway is a critical determinant of immune complex-mediated glomerular injury, as evidenced by attenuated inflammation both in Fc receptor-deficient mice and by intravenously injected Fc receptor fragments in a number of experimental models of glomerulonephritis (1, 5, 12, 18, 26). apoJ/clusterin, by binding immunoglobulin Fc domains, potentially enhances immune complex-Fc receptor-mediated inflammatory events. If this is the case, genetic ablation of apoJ/clusterin would dissociate inflammation from immune complex deposition.
A role for apoJ/clusterin in immune complex-mediated disease was first suggested by its interaction with immunoglobulins. apoJ/clusterin can bind to the Fc and Fab regions of all isotypes of IgG, IgM and IgA by a noncovalent mechanism (28). The site of interaction is different than the Fc binding site of C1q and protein A (28). apoJ/clusterin has been shown to preferentially bind to aggregated compared to monomeric IgG (28). These results suggest that apoJ/clusterin has multiple potential binding sites through which it may interact and facilitate the clearance of polymeric IgG present in immune complexes.
In addition to its interaction with immunoglobulins, apoJ/clusterin has been found in conjunction with immune deposits in a number of immune-mediated glomerular diseases, including IgA nephropathy, membranous glomerulonephritis, and lupus nephritis (3, 15). In most cases apoJ/clusterin colocalizes with components of the membrane attack complex when these components are present with immunoglobulins but not when the membrane attack complex is found in the absence of immunoglobulins, suggesting a direct role for apoJ/clusterin in the processing of immune complexes (3, 15). apoJ/clusterin has also been localized to the glomerulus in such immune-mediated models of glomerulonephritis as Heymann nephritis and anti-Thy 1 nephritis (2, 29). In the latter model, upregulation of both clusterin mRNA and protein has been demonstrated in mesangial cells, providing evidence that clusterin can be synthesized by these cells following immune attack (29). The association of apoJ/clusterin with immune deposits in experimental and human glomerulonephritis suggests it may modulate responses to immune-complex-induced injury. Further support for this view has been provided by Saunders et al. in the isolated rat kidney model of Heymann nephritis, where these investigators demonstrated increased proteinuria, greater deposition of complement components, and greater glomerular injury when these kidneys were perfused with apoJ/clusterin-depleted serum (22).
An association between apoJ/clusterin and immune complex disease is found in patients with systemic lupus erythematosis (SLE). Levels of apoJ/clusterin in serum are lower in patients with SLE than in normal controls or patients with rheumatoid arthritis, osteoarthritis, or Sjogren's syndrome (17). In this cross-sectional study of lupus patients, serum apoJ/clusterin levels were inversely correlated with disease activity, with the lowest levels observed in those patients with the most active disease. apoJ/clusterin levels were also lower in SLE patients with proteinuria than in those without proteinuria (17). Based on these findings in SLE patients and the phenotype of apoJ/clusterin-deficient mice, apoJ/clusterin must be considered as a candidate disease modifier gene that may explain the different susceptibilities and clinical courses in human immune complex-mediated diseases.
Our results support the hypothesis that apoJ/clusterin prevents glomerular immune complex deposition of aging, and they support a role for circulating apoJ/clusterin in the clearance of immune complexes. Furthermore, these findings suggest that clusterin is part of a system that protects the mesangium from the continuous challenge of macromolecules trafficking through it. The phenotype of the apoJ/clusterin-deficient mouse may provide a clue as to the other responsibilities of apoJ/clusterin. We hypothesize that apoJ/clusterin is part of a metabolic pathway responsible for the identification and clearance of toxic and/or immunogenic macromolecules that arise during cell injury, death, and immune response processes. Disposal of bioactive material is critical for longevity in the setting of tissue turnover, inflammation, and immune response, particularly with age. This disposal function is necessary for the removal of foreign antigens, damaged endogenous macromolecules, and cellular debris generated during physiologic cell death (apoptosis) or necrosis. Failure to remove such material may contribute to progressive organ dysfunction and increase the severity of autoimmunity.
Acknowledgments
This work was supported by USPHS grants NIH/NIDDK R01DK48452 (M.E.R), NIH/NIEHS R01ES08822 (B.J.A.), a National Research Service Award (R.A.G.), a grant from the National Kidney Foundation of Minnesota (R.G.), and an American Heart Association Established Investigator grant (M.E.R.).
REFERENCES
- 1.Clynes, R., C. Dumitru, and J. Ravetch. 1998. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science 279:1052-1054. [DOI] [PubMed] [Google Scholar]
- 2.Eddy, A. A., and I. B. Fritz. 1991. Localization of clusterin in the epimembranous deposits of passive Heymann nephritis. Kidney Int. 39:247-252. [DOI] [PubMed] [Google Scholar]
- 3.French, L. E., L. L. Polla, J. Tschopp, and J. A. Schifferli. 1992. Membrane attack complex (MAC) deposits in skin are not always accompanied by S-protein and clusterin. J. Investig. Dermatol. 98:758-763. [DOI] [PubMed] [Google Scholar]
- 4.Germuth, F. G., W. A. Kelemen, and A. D. Pollack. 1967. Immune complex disease II: the role of circulatory dynamics and glomerular filtration in the development of experimental glomerulonephritis. Johns Hopkins Med. J. 120:252-257. [PubMed] [Google Scholar]
- 5.Gomez-Guerrero, C., N. Duque, M. T. Casado, C. Pastor, J. Blanco, F. Mampaso, F. Vivanco, and J. Egido. 2000. Administration of IgG Fc fragments prevents glomerular injury in experimental immune complex nephritis. J. Immunol. 164:2092-2101. [DOI] [PubMed] [Google Scholar]
- 6.Humphreys, D. T., J. A. Carver, S. B. Easterbrook-Smith, and M. R. Wilson. 1999. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J. Biol. Chem. 274:6875-6881. [DOI] [PubMed] [Google Scholar]
- 7.Jarvis, J. N., W. Wang, H. T. Moore, L. Zhao, and C. Xu. 1997. In vitro induction of proinflammatory cytokine secretion by juvenile rheumatoid arthritis synovial fluid immune complexes. Arthritis Rheum. 40:2039-2046. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis, J. N., C. Xu, W. Wang, H. R. Petty, M. Gonzalez, N. Morssy, F. Waxman, and A. Quintero del Rio. 1999. Immune complex size and complement regulate cytokine production by peripheral blood mononuclear cells. Clin. Immunol. 93:274-282. [DOI] [PubMed] [Google Scholar]
- 9.Jenne, D. E., and J. Tschopp. 1989. Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc. Natl. Acad. Sci. USA 86:7123-7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirszbaum, L., S. E. Bozas, and I. D. Walker. 1992. SP-40,40, a protein involved in the control of the complement pathway, possesses a unique array of disulphide bridges. FEBS Lett. 297:70-76. [DOI] [PubMed] [Google Scholar]
- 11.Mauer, S. M., A. J. Fish, E. B. Blau, and A. F. Michael. 1972. I. Kinetic studies of macromolecular uptake in normal and nephrotic rats. J. Clin. Investig. 51:1092-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayadas, T., A. Rosenkranz, and R. Cotran. 1999. Glomerular inflammation: use of genetically deficient mice to elucidate the roles of leukocyte adhesion molecules and Fc-gamma receptors in vivo. Curr. Opin. Nephrol. Hypertens. 8:293-298. [DOI] [PubMed] [Google Scholar]
- 13.McAdams, A. J. 1999. Glomerular capillary wall basement membrane really does have laminae lucidae: a defense. Pediatr. Dev. Pathol. 2:260-263. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin, L., M. Mistry, G. Zhu, C. Ley-Ebert, W. D. Stuart, C. J. Floria, P. A. Groen, D. Witte, T. R. Kimball, J. A. Harmony, and B. J. Aronow. 2000. Apolipoprotein J/clusterin limits the severity of autoimmune myocarditis. J. Clin. Investig. 106:1105-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy, B. F., D. J. Davies, W. Morrow, and A. J. F. d'Apice. 1989. Localization of terminal complement components, S-protein and SP-40,40 in renal biopsies. Pathology 21:275-278. [DOI] [PubMed] [Google Scholar]
- 16.Murphy, B. F., L. Kirszbaum, I. D. Walker, and A. J. F. d'Apice. 1988. SP-40,40 a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J. Clin. Investig. 81:1858-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newkirk, M. M., P. Apostolakos, C. Neville, and P. R. Fortin. 1999. Systemic lupus erythematosus, a disease associated with low levels of clusterin/apoj, an antiinflammatory protein. J. Rheumatol. 26:597-603. [PubMed] [Google Scholar]
- 18.Park, S., S. Ueda, H. Ohno, Y. Hamano, M. Tanaka, T. Shiratori, T. Yamazaki, H. Arase, N. Arase, A. Karasawa, S. Sato, B. Ledermann, Y. Kondo, K. Okumura, C. Ra, and T. Saito. 1998. Resistance of Fc receptor-deficient mice to fatal glomerulonephritis. J. Clin. Investig. 102:1229-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravetch, J. V. 1994. Fc receptors: rubor redux. Cell 78:553-560. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg, M. E., and J. Silkensen. 1995. Clusterin and the kidney. Exp. Nephrol. 3:9-14. [PubMed] [Google Scholar]
- 21.Santiago, A., J. Satriano, S. DeCandido, H. Holthofer, R. Schreiber, J. Unkeless, and D. Schlondorff. 1989. A specific Fcγ receptor on cultured rat mesangial cells. J. Immunol. 143:2575-2582. [PubMed] [Google Scholar]
- 22.Saunders, J. R., A. Aminian, J. L. McRae, K. A. O'Farrell, W. R. Adam, and B. F. Murphy. 1994. Clusterin depletion enhances immune glomerular injury in the isolated perfused kidney. Kidney Int. 45:817-827. [DOI] [PubMed] [Google Scholar]
- 23.Schultz, P. J., and L. Raij. 1991. The glomerular mesangium: role in initiation and progression of renal injury. Am. J. Kidney Dis. 27:8-14. [PubMed] [Google Scholar]
- 24.Shull, M. M., I. Ormsby, A. B. Kier, S. Pawloski, R. J. Diebold, M. Yin, R. Allen, C. Sidman, G. Proetzel, D. Calvin, N. Annunziata, and T. Doetschman. 1992. Targeted disruption of the mouse transforming growth factor-b1 gene results in multifocal inflammatory disease. Nature 359:693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silkensen, J. R., G. B. Schwochau, and M. E. Rosenberg. 1994. The role of clusterin in tissue injury. Biochem. Cell Biol. 72:483-488. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki, Y., I. Shirato, and K. Okumura. 1998. Distinct contribution of Fc receptors and angiotensin II-dependent pathways in anti-GBM glomerulonephritis. Kidney Int. 54:1166-1174. [DOI] [PubMed] [Google Scholar]
- 27.Wilson, C. B. 1996. The renal response to immunologic glomerular injury, p. 1253-1391. In B. M. Brenner and F. C. Rector (ed.), The kidney, 5th ed. Saunders, Philadelphia, Pa.
- 28.Wilson, M. R., and S. B. Easterbrook-Smith. 1992. Clusterin binds by a multivalent mechanism to the Fc and Fab regions of IgG. Biochem. Biophys. Acta 1159:319-326. [DOI] [PubMed] [Google Scholar]
- 29.Yamada, K., Y. Hori, N. Hanafusa, T. Okuda, N. Nagano, N. H. Choi-Miura, W. G. Couser, T. Miyata, K. Kurokawa, T. Fujita, and M. Nangaku. 2001. Clusterin is up-regulated in glomerular mesangial cells in complement-mediated injury. Kidney Int. 59:137-146. [DOI] [PubMed] [Google Scholar]